Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence
- PMID: 12584338
- PMCID: PMC149745
- DOI: 10.1128/jvi.77.5.3131-3147.2003
Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence
Abstract
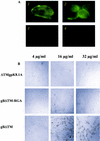
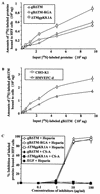
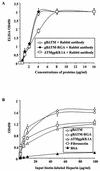
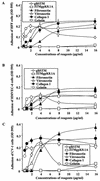
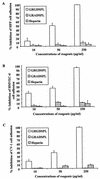
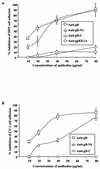
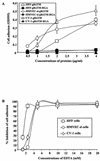
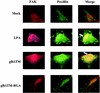
Human herpesvirus 8 (HHV-8) or Kaposi's sarcoma-associated herpesvirus, implicated in the pathogenesis of Kaposi's sarcoma, utilizes heparan sulfate-like molecules to bind the target cells via its envelope-associated glycoproteins gB and gpK8.1A. HHV-8-gB possesses the Arg-Gly-Asp (RGD) motif, the minimal peptide region of many proteins known to interact with subsets of host cell surface integrins. HHV-8 utilizes alpha3beta1 integrin as one of the receptors for its entry into the target cells via its gB interaction and induces the activation of focal adhesion kinase (FAK) (S. M. Akula, N. P. Pramod, F.-Z. Wang, and B. Chandran, Cell 108:407-419, 2002). Since FAK activation is the first step in the outside-in signaling necessary for integrin-mediated cytoskeletal rearrangements, cell adhesions, motility, and proliferation, the ability of HHV-8-gB to mediate the target cell adhesion was examined. A truncated form of gB without the transmembrane and carboxyl domains (gBdeltaTM) and a gBdeltaTM mutant (gBdeltaTM-RGA) with a single amino acid mutation (RGD to RGA) were expressed in a baculovirus system and purified. Radiolabeled HHV-8-gBdeltaTM, gBdeltaTM-RGA, and deltaTMgpK8.1A proteins bound to the human foreskin fibroblasts (HFFs), human dermal microvascular endothelial (HMVEC-d) cells, human B (BJAB) cells, and Chinese hamster ovary (CHO-K1) cells with equal efficiency, which was blocked by preincubation of proteins with soluble heparin. Maxisorp plate-bound gBdeltaTM protein induced the adhesion of HFFs and HMVEC-d and monkey kidney epithelial (CV-1) cells in a dose-dependent manner. In contrast, the gBdeltaTM-RGA and DeltaTMgpK8.1A proteins did not mediate adhesion. Adhesion mediated by gBdeltaTM was blocked by the preincubation of target cells with RGD-containing peptides or by the preincubation of plate-bound gBdeltaTM protein with rabbit antibodies against gB peptide containing the RGD sequence. In contrast, adhesion was not blocked by the preincubation of plate-bound gBdeltaTM protein with heparin, suggesting that the adhesion is mediated by the RGD amino acids of gB, which is independent of the heparin-binding domain of gB. Integrin-ligand interaction is dependent on divalent cations. Adhesion induced by the gBdeltaTM was blocked by EDTA, thus suggesting the role of integrins in the observed adhesions. Focal adhesion components such as FAK and paxillin were activated by the binding of gBdeltaTM protein to the target cells but not by gBdeltaTM-RGA protein binding. Inhibition of FAK phosphorylation by genistein blocked gBdeltaTM-induced FAK activation and cell adhesion. These findings suggest that HHV-8-gB could mediate cell adhesion via its RGD motif interaction with the cell surface integrin molecules and indicate the induction of cellular signaling pathways, which may play roles in the infection of target cells and in Kaposi's sarcoma pathogenesis.
Figures

Similar articles
-
Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements.J Virol. 2004 Apr;78(8):4207-23. doi: 10.1128/jvi.78.8.4207-4223.2004. J Virol. 2004. PMID: 15047836 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity.J Virol. 2003 Jan;77(2):1524-39. doi: 10.1128/jvi.77.2.1524-1539.2003. J Virol. 2003. PMID: 12502866 Free PMC article.
-
Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate.J Virol. 2001 Aug;75(16):7517-27. doi: 10.1128/JVI.75.16.7517-7527.2001. J Virol. 2001. PMID: 11462024 Free PMC article.
-
Integrins in cell adhesion and signaling.Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review.
-
Early events in Kaposi's sarcoma-associated herpesvirus infection of target cells.J Virol. 2010 Mar;84(5):2188-99. doi: 10.1128/JVI.01334-09. Epub 2009 Nov 18. J Virol. 2010. PMID: 19923183 Free PMC article. Review.
Cited by
-
Immune evasion by Kaposi's sarcoma-associated herpesvirus.Future Microbiol. 2010 Sep;5(9):1349-65. doi: 10.2217/fmb.10.105. Future Microbiol. 2010. PMID: 20860481 Free PMC article. Review.
-
Cell membrane-bound Kaposi's sarcoma-associated herpesvirus-encoded glycoprotein B promotes virus latency by regulating expression of cellular Egr-1.J Biol Chem. 2010 Nov 26;285(48):37491-502. doi: 10.1074/jbc.M110.159103. Epub 2010 Sep 23. J Biol Chem. 2010. PMID: 20864524 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection.J Virol. 2008 Dec;82(24):12126-44. doi: 10.1128/JVI.01146-08. Epub 2008 Oct 1. J Virol. 2008. PMID: 18829766 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus-positive primary effusion lymphoma tumor formation in NOD/SCID mice is inhibited by neomycin and neamine blocking angiogenin's nuclear translocation.J Virol. 2013 Nov;87(21):11806-20. doi: 10.1128/JVI.01920-13. Epub 2013 Aug 28. J Virol. 2013. PMID: 23986578 Free PMC article.
-
Kaposi's Sarcoma Associated Herpesvirus Entry into Target Cells.Front Microbiol. 2012 Jan 20;3:6. doi: 10.3389/fmicb.2012.00006. eCollection 2012. Front Microbiol. 2012. PMID: 22319516 Free PMC article.
References
-
- Akiyama, T., J. Ishida, S. Nakagawa, H. Ogawara, S. Watanabe, N. Itoh, M. Shibuya, and Y. Fukami. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 262:5592-5595. - PubMed
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. - PubMed
-
- Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282:245-255. - PubMed
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

