8-nitroguanosine formation in viral pneumonia and its implication for pathogenesis
- PMID: 12522148
- PMCID: PMC141057
- DOI: 10.1073/pnas.0235623100
8-nitroguanosine formation in viral pneumonia and its implication for pathogenesis
Abstract
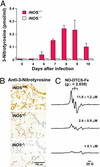
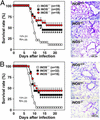
For many diseases, mediation of pathogenesis by nitric oxide (NO) has been suggested. In this study, we explored NO-induced viral pathogenesis with a focus on nucleic acid damage as evidenced by 8-nitroguanosine formation in vivo. Wild-type mice and littermate mice deficient in inducible NO synthase (iNOS) were infected with influenza or Sendai virus. Formation of 8-nitroguanosine in virus-infected lungs was assessed immunohistochemically with an antibody specific for 8-nitroguanosine. Extensive nitration of RNA either treated with peroxynitrite or obtained from cultured RAW 264 cells expressing iNOS was readily detected by this antibody. Strong 8-nitroguanosine immunostaining was evident primarily in the cytosol of bronchial and bronchiolar epithelial cells of virus-infected wild-type mice but not iNOS-deficient mice. This staining colocalized with iNOS immunostaining in the lung. 8- Nitroguanosine staining disappeared after addition of exogenous authentic 8-nitroguanosine during the antibody reaction and after pretreatment of tissues with sodium hydrosulfite, which reduces 8-nitroguanosine to 8-aminoguanosine. NO was generated in excess in lungs of wild-type mice but was eliminated in iNOS-deficient mice after virus infection; this result also correlated well with formation of 8-nitroguanosine and 3-nitrotyrosine. One consequence of the lack of iNOS expression was marked improvement in histopathological changes in the lung and the lethality of the infection without effects on cytokine responses and viral clearance. It is intriguing that 8-nitroguanosine markedly stimulated superoxide generation from cytochrome P450 reductase and iNOS in vitro. The present data constitute a demonstration of 8-nitroguanosine formation in vivo and suggest a potential role for NO-induced nitrative stress in viral pathogenesis.
Figures




Similar articles
-
Nitric oxide as an endogenous mutagen for Sendai virus without antiviral activity.J Virol. 2004 Aug;78(16):8709-19. doi: 10.1128/JVI.78.16.8709-8719.2004. J Virol. 2004. PMID: 15280479 Free PMC article.
-
Type 2 nitric oxide synthase and protein nitration in chronic lung infection.J Pathol. 2003 Jan;199(1):122-9. doi: 10.1002/path.1256. J Pathol. 2003. PMID: 12474235
-
Expression of inducible nitric oxide synthase and nitrotyrosine during the evolution of experimental pulmonary tuberculosis.Exp Toxicol Pathol. 2001 Sep;53(4):257-65. doi: 10.1078/0940-2993-00182. Exp Toxicol Pathol. 2001. PMID: 11665849
-
Nitric oxide and oxygen radicals in infection, inflammation, and cancer.Biochemistry (Mosc). 1998 Jul;63(7):854-65. Biochemistry (Mosc). 1998. PMID: 9721338 Review.
-
Management of the virulent influenza virus infection by oral formulation of nonhydrolized carnosine and isopeptide of carnosine attenuating proinflammatory cytokine-induced nitric oxide production.Am J Ther. 2012 Jan;19(1):e25-47. doi: 10.1097/MJT.0b013e3181dcf589. Am J Ther. 2012. PMID: 20841992 Review.
Cited by
-
Impact of Protein Nitration on Influenza Virus Infectivity and Immunogenicity.Microbiol Spectr. 2022 Dec 21;10(6):e0190222. doi: 10.1128/spectrum.01902-22. Epub 2022 Oct 31. Microbiol Spectr. 2022. PMID: 36314966 Free PMC article.
-
The link between infection and cancer: tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect.Cancer Sci. 2013 Jul;104(7):779-89. doi: 10.1111/cas.12152. Epub 2013 Apr 22. Cancer Sci. 2013. PMID: 23495730 Free PMC article. Review.
-
Nitric oxide in dengue and dengue haemorrhagic fever: necessity or nuisance?FEMS Immunol Med Microbiol. 2009 Jun;56(1):9-24. doi: 10.1111/j.1574-695X.2009.00544.x. Epub 2009 Feb 23. FEMS Immunol Med Microbiol. 2009. PMID: 19239490 Free PMC article. Review.
-
Clinical Features and Short-Term Outcomes of Bariatric Surgery in Morbidly Obese Patients: Institutional Experience at a Rural Hospital.Bariatr Surg Pract Patient Care. 2021 Mar 1;16(1):61-67. doi: 10.1089/bari.2020.0110. Epub 2021 Mar 15. Bariatr Surg Pract Patient Care. 2021. PMID: 33763312 Free PMC article.
-
Host nutritional status: the neglected virulence factor.Trends Microbiol. 2004 Sep;12(9):417-23. doi: 10.1016/j.tim.2004.07.007. Trends Microbiol. 2004. PMID: 15337163 Free PMC article. Review.
References
-
- Akaike T, Maeda H. In: Nitric Oxide: Biology and Pathobiology. Ignarro L J, editor. San Diego: Academic; 2000. pp. 733–745.
-
- Estévez A G, Crow J P, Sampson J B, Reiter C, Zhuang Y, Richardson G J, Tarpey M M, Barbeito L, Beckman J S. Science. 1999;286:2498–2500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

