c-Myc functionally cooperates with Bax to induce apoptosis
- PMID: 12167710
- PMCID: PMC133996
- DOI: 10.1128/MCB.22.17.6158-6169.2002
c-Myc functionally cooperates with Bax to induce apoptosis
Abstract
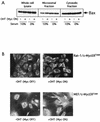
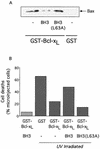
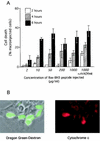
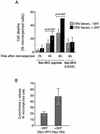
c-Myc promotes apoptosis by destabilizing mitochondrial integrity, leading to the release of proapoptotic effectors including holocytochrome c. Candidate mediators of c-Myc in this process are the proapoptotic members of the Bcl-2 family. We show here that fibroblasts lacking Bak remain susceptible to c-Myc-induced apoptosis whereas bax-deficient fibroblasts are resistant. However, despite this requirement for Bax, c-Myc activation exerts no detectable effects on Bax expression, localization, or conformation. Moreover, susceptibility to c-Myc-induced apoptosis can be restored in bax-deficient cells by ectopic expression of Bax or by microinjection of a peptide comprising a minimal BH3 domain. Microinjection of BH3 peptide also restores sensitivity to c-Myc-induced apoptosis in p53-deficient primary fibroblasts that are otherwise resistant. By contrast, there is no synergy between BH3 peptide and c-Myc in fibroblasts deficient in both Bax and Bak. We conclude that c-Myc triggers a proapoptotic mitochondrial destabilizing activity that cooperates with proapoptotic members of the Bcl-2 family.
Figures





Similar articles
-
Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release.Cancer Res. 2003 Apr 1;63(7):1712-21. Cancer Res. 2003. PMID: 12670926
-
Minimal BH3 peptides promote cell death by antagonizing anti-apoptotic proteins.J Biol Chem. 2003 May 23;278(21):19426-35. doi: 10.1074/jbc.M209472200. Epub 2003 Mar 17. J Biol Chem. 2003. PMID: 12642586
-
c-Myc sensitization to oxygen deprivation-induced cell death is dependent on Bax/Bak, but is independent of p53 and hypoxia-inducible factor-1.J Biol Chem. 2004 Feb 6;279(6):4305-12. doi: 10.1074/jbc.M312241200. Epub 2003 Nov 19. J Biol Chem. 2004. PMID: 14627695
-
Mitochondrial membrane permeabilisation by Bax/Bak.Biochem Biophys Res Commun. 2003 May 9;304(3):455-61. doi: 10.1016/s0006-291x(03)00617-x. Biochem Biophys Res Commun. 2003. PMID: 12729579 Review.
-
Mechanisms of cytochrome c release by proapoptotic BCL-2 family members.Biochem Biophys Res Commun. 2003 May 9;304(3):437-44. doi: 10.1016/s0006-291x(03)00615-6. Biochem Biophys Res Commun. 2003. PMID: 12729577 Review.
Cited by
-
Persianolide-A, an eudesmanolide-type sesquiterpene lactone from Artemisia kopetdaghensis, induces apoptosis by regulating ERK signaling pathways.Res Pharm Sci. 2024 Jul 1;19(3):328-337. doi: 10.4103/RPS.RPS_175_23. eCollection 2024 Jun. Res Pharm Sci. 2024. PMID: 39035813 Free PMC article.
-
c-Myc primed mitochondria determine cellular sensitivity to TRAIL-induced apoptosis.EMBO J. 2007 Feb 21;26(4):1055-67. doi: 10.1038/sj.emboj.7601551. Epub 2007 Feb 1. EMBO J. 2007. PMID: 17268552 Free PMC article.
-
Myc is required for activation of the ATM-dependent checkpoints in response to DNA damage.PLoS One. 2010 Jan 27;5(1):e8924. doi: 10.1371/journal.pone.0008924. PLoS One. 2010. PMID: 20111719 Free PMC article.
-
Expression of HER-2 in MCF-7 breast cancer cells modulates anti-apoptotic proteins Survivin and Bcl-2 via the extracellular signal-related kinase (ERK) and phosphoinositide-3 kinase (PI3K) signalling pathways.BMC Cancer. 2008 May 2;8:129. doi: 10.1186/1471-2407-8-129. BMC Cancer. 2008. PMID: 18454859 Free PMC article.
-
Respiratory complex I regulates dendritic cell maturation in explant model of human tumor immune microenvironment.J Immunother Cancer. 2024 Apr 11;12(4):e008053. doi: 10.1136/jitc-2023-008053. J Immunother Cancer. 2024. PMID: 38604809 Free PMC article.
References
-
- Bissonnette, R., F. Echeverri, A. Mahboubi, and D. Green. 1992. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature 359:552-554. - PubMed
-
- Cheng, E. H., M. C. Wei, S. Weiler, R. A. Flavell, T. W. Mak, T. Lindsten, and S. J. Korsmeyer. 2001. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8:705-711. - PubMed
-
- Cosulich, S. C., V. Worrall, P. J. Hedge, S. Green, and P. R. Clarke. 1997. Regulation of apoptosis by BH3 domains in a cell-free system. Curr. Biol. 7:913-920. - PubMed
-
- Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
