The death substrate Gas2 binds m-calpain and increases susceptibility to p53-dependent apoptosis
- PMID: 11387205
- PMCID: PMC125501
- DOI: 10.1093/emboj/20.11.2702
The death substrate Gas2 binds m-calpain and increases susceptibility to p53-dependent apoptosis
Abstract
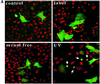
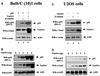
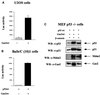
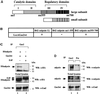
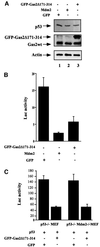
Gas2 is a caspase-3 substrate that plays a role in regulating microfilament and cell shape changes during apoptosis. Here we provide evidence that overexpression of Gas2 efficiently increases cell susceptibility to apoptosis following UV irradiation, etoposide and methyl methanesulfonate treatments, and that these effects are dependent on increased p53 stability and transcription activity. To investigate possible pathways linking Gas2 to p53, a yeast two-hybrid screen swas performed, indicating m-calpain as a strong Gas2- interacting protein. Moreover, we demonstrate that Gas2 physically interacts with m-calpain in vivo and that recombinant Gas2 inhibits calpain-dependent processing of p53. Importantly, the Gas2 dominant-negative form (Gas2171-314) that binds calpain but is unable to inhibit its activity abrogates Gas2's ability to stabilize p53, to enhance p53 transcriptional activity and to induce p53-dependent apoptosis. Finally, we show that Gas2 is able to regulate the levels of p53 independently of Mdm2 status, suggesting that, like calpastatin, it may enhance p53 stability by inhibiting calpain activity.
Figures




Similar articles
-
Truncated HBx-dependent silencing of GAS2 promotes hepatocarcinogenesis through deregulation of cell cycle, senescence and p53-mediated apoptosis.J Pathol. 2015 Sep;237(1):38-49. doi: 10.1002/path.4554. Epub 2015 May 28. J Pathol. 2015. PMID: 25925944
-
Caspase-3 and caspase-7 but not caspase-6 cleave Gas2 in vitro: implications for microfilament reorganization during apoptosis.J Cell Sci. 1999 Dec;112 ( Pt 23):4475-82. doi: 10.1242/jcs.112.23.4475. J Cell Sci. 1999. PMID: 10564664
-
Growth arrest-specific gene 2 suppresses hepatocarcinogenesis by intervention of cell cycle and p53-dependent apoptosis.World J Gastroenterol. 2019 Aug 28;25(32):4715-4726. doi: 10.3748/wjg.v25.i32.4715. World J Gastroenterol. 2019. PMID: 31528096 Free PMC article.
-
GAS2-Calpain2 axis contributes to the growth of leukemic cells.Acta Biochim Biophys Sin (Shanghai). 2015 Oct;47(10):795-804. doi: 10.1093/abbs/gmv080. Epub 2015 Sep 9. Acta Biochim Biophys Sin (Shanghai). 2015. PMID: 26358320
-
Growth arrest-specific 2 protein family: Structure and function.Cell Prolif. 2021 Jan;54(1):e12934. doi: 10.1111/cpr.12934. Epub 2020 Oct 25. Cell Prolif. 2021. PMID: 33103301 Free PMC article. Review.
Cited by
-
Calpain is required for macroautophagy in mammalian cells.J Cell Biol. 2006 Nov 20;175(4):595-605. doi: 10.1083/jcb.200601024. Epub 2006 Nov 13. J Cell Biol. 2006. PMID: 17101693 Free PMC article.
-
Changes in calpain-2 expression during glioblastoma progression predisposes tumor cells to temozolomide resistance by minimizing DNA damage and p53-dependent apoptosis.Cancer Cell Int. 2023 Mar 17;23(1):49. doi: 10.1186/s12935-023-02889-8. Cancer Cell Int. 2023. PMID: 36932402 Free PMC article.
-
The calpain system and cancer.Nat Rev Cancer. 2011 May;11(5):364-74. doi: 10.1038/nrc3050. Nat Rev Cancer. 2011. PMID: 21508973 Review.
-
CRTH2 promotes endoplasmic reticulum stress-induced cardiomyocyte apoptosis through m-calpain.EMBO Mol Med. 2018 Mar;10(3):e8237. doi: 10.15252/emmm.201708237. EMBO Mol Med. 2018. PMID: 29335338 Free PMC article.
-
Genomic analysis of the function of the transcription factor gata3 during development of the mammalian inner ear.PLoS One. 2009 Sep 23;4(9):e7144. doi: 10.1371/journal.pone.0007144. PLoS One. 2009. PMID: 19774072 Free PMC article.
References
-
- Atencio I., Ramachandra,M., Shabram,P. and Demers,G. (2000) Calpain inhibitor 1 activates p53-dependent apoptosis in tumor cell lines. Cell Growth Differ., 11, 247–253. - PubMed
-
- Brancolini C. and Schneider,C. (1997) Cut and die: proteolytic cascades regulating apoptosis. Adv. Clin. Pathol., 3, 177–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

