Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha
- PMID: 11381086
- PMCID: PMC2174339
- DOI: 10.1083/jcb.153.5.1011
Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha
Abstract
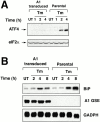
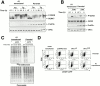
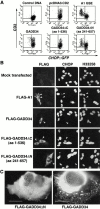
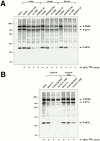
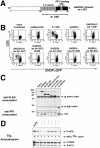
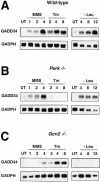
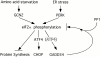
Phosphorylation of the alpha subunit of eukaryotic translation initiation factor 2 (eIF2alpha) on serine 51 integrates general translation repression with activation of stress-inducible genes such as ATF4, CHOP, and BiP in the unfolded protein response. We sought to identify new genes active in this phospho-eIF2alpha-dependent signaling pathway by screening a library of recombinant retroviruses for clones that inhibit the expression of a CHOP::GFP reporter. A retrovirus encoding the COOH terminus of growth arrest and DNA damage gene (GADD)34, also known as MYD116 (Fornace, A.J., D.W. Neibert, M.C. Hollander, J.D. Luethy, M. Papathanasiou, J. Fragoli, and N.J. Holbrook. 1989. Mol. Cell. Biol. 9:4196-4203; Lord K.A., B. Hoffman-Lieberman, and D.A. Lieberman. 1990. Nucleic Acid Res. 18:2823), was isolated and found to attenuate CHOP (also known as GADD153) activation by both protein malfolding in the endoplasmic reticulum, and amino acid deprivation. Despite normal activity of the cognate stress-inducible eIF2alpha kinases PERK (also known as PEK) and GCN2, phospho-eIF2alpha levels were markedly diminished in GADD34-overexpressing cells. GADD34 formed a complex with the catalytic subunit of protein phosphatase 1 (PP1c) that specifically promoted the dephosphorylation of eIF2alpha in vitro. Mutations that interfered with the interaction with PP1c prevented the dephosphorylation of eIF2alpha and blocked attenuation of CHOP by GADD34. Expression of GADD34 is stress dependent, and was absent in PERK(-)/- and GCN2(-)/- cells. These findings implicate GADD34-mediated dephosphorylation of eIF2alpha in a negative feedback loop that inhibits stress-induced gene expression, and that might promote recovery from translational inhibition in the unfolded protein response.
Figures

Similar articles
-
Regulated translation initiation controls stress-induced gene expression in mammalian cells.Mol Cell. 2000 Nov;6(5):1099-108. doi: 10.1016/s1097-2765(00)00108-8. Mol Cell. 2000. PMID: 11106749
-
Nck in a complex containing the catalytic subunit of protein phosphatase 1 regulates eukaryotic initiation factor 2alpha signaling and cell survival to endoplasmic reticulum stress.J Biol Chem. 2006 Sep 8;281(36):26633-44. doi: 10.1074/jbc.M513556200. Epub 2006 Jul 11. J Biol Chem. 2006. PMID: 16835242
-
Stress-induced gene expression requires programmed recovery from translational repression.EMBO J. 2003 Mar 3;22(5):1180-7. doi: 10.1093/emboj/cdg112. EMBO J. 2003. PMID: 12606582 Free PMC article.
-
Pausing to decide.Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12396-7. doi: 10.1073/pnas.250476097. Proc Natl Acad Sci U S A. 2000. PMID: 11058174 Free PMC article. Review. No abstract available.
-
Coping with stress: eIF2 kinases and translational control.Biochem Soc Trans. 2006 Feb;34(Pt 1):7-11. doi: 10.1042/BST20060007. Biochem Soc Trans. 2006. PMID: 16246168 Review.
Cited by
-
Pediatric glioblastoma cells are sensitive to drugs that inhibit eIF2α dephosphorylation and its phosphomimetic S51D variant.Front Oncol. 2022 Aug 26;12:959133. doi: 10.3389/fonc.2022.959133. eCollection 2022. Front Oncol. 2022. PMID: 36091130 Free PMC article.
-
Endoplasmic reticulum quality control and systemic amyloid disease: Impacting protein stability from the inside out.IUBMB Life. 2015 Jun;67(6):404-13. doi: 10.1002/iub.1386. Epub 2015 May 26. IUBMB Life. 2015. PMID: 26018985 Free PMC article. Review.
-
Identification of 5-nitrofuran-2-amide derivatives that induce apoptosis in triple negative breast cancer cells by activating C/EBP-homologous protein expression.Bioorg Med Chem. 2015 Aug 1;23(15):4514-4521. doi: 10.1016/j.bmc.2015.06.011. Epub 2015 Jun 14. Bioorg Med Chem. 2015. PMID: 26116180 Free PMC article.
-
CPR5 modulates salicylic acid and the unfolded protein response to manage tradeoffs between plant growth and stress responses.Plant J. 2017 Feb;89(3):486-501. doi: 10.1111/tpj.13397. Epub 2017 Feb 3. Plant J. 2017. PMID: 27747970 Free PMC article.
-
Proteostasis control by the unfolded protein response.Nat Cell Biol. 2015 Jul;17(7):829-38. doi: 10.1038/ncb3184. Nat Cell Biol. 2015. PMID: 26123108 Free PMC article. Review.
References
-
- Berlanga J.J., Santoyo J., De Haro C. Characterization of a mammalian homologue of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur. J. Biochem. 1999;265:754–762. - PubMed
-
- Bertolotti A., Zhang Y., Hendershot L., Harding H., Ron D. Dynamic interaction of BiP and the ER stress transducers in the unfolded protein response. Nat. Cell Biol. 2000;2:326–332. - PubMed
-
- Brostrom C.O., Brostrom M.A. Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. Mol. Biol. 1998;58:79–125. - PubMed
-
- Camps M., Nichols A., Arkinstall S. Dual specificity phosphatasesa gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

