Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis
- PMID: 11257138
- PMCID: PMC2193420
- DOI: 10.1084/jem.193.6.713
Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis
Abstract
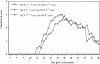
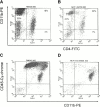
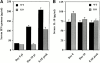
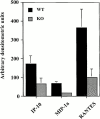
Monocyte chemoattractant protein (MCP)-1 plays a critical role in innate immunity by directing the migration of monocytes into inflammatory sites. Recent data indicated a function for this chemokine in adaptive immunity as a regulator of T cell commitment to T helper cell type 2 (Th2) effector function. Studies in a Th1-dependent animal model, experimental autoimmune encephalomyelitis (EAE), showed that MCP-1 was highly expressed in the central nervous system (CNS) of affected rodents, and MCP-1 antibodies could block relapses of the disease. Mice deficient for the major MCP-1 receptor, CC chemokine receptor (CCR)2, did not develop EAE after active immunization but generated effector cells that could transfer the disease to naive wild-type recipients. We analyzed EAE in mice deficient for MCP-1 to define the relevant ligand for CCR2, which responds to murine MCP-1, MCP-2, MCP-3, and MCP-5. We found that C57BL/6 MCP-1-null mice were markedly resistant to EAE after active immunization, with drastically impaired recruitment of macrophages to the CNS, yet able to generate effector T cells that transferred severe disease to naive wild-type recipients. By contrast, adoptive transfer of primed T cells from wild-type mice into naive MCP-1-null recipients did not mediate clinical EAE. On the SJL background, disruption of the MCP-1 gene produced a milder EAE phenotype with diminished relapses that mimicked previous findings using anti-MCP-1 antibodies. There was no compensatory upregulation of MCP-2, MCP-3, or MCP-5 in MCP-1-null mice with EAE. These results indicated that MCP-1 is the major CCR2 ligand in mice with EAE, and provided an opportunity to define the role of MCP-1 in EAE. Compared with wild-type littermates, MCP-1-/- mice exhibited reduced expression of interferon gamma in draining lymph node and CNS and increased antigen-specific immunoglobulin G1 antibody production. Taken together, these data demonstrate that MCP-1 is crucial for Th1 immune responses in EAE induction and that macrophage recruitment to the inflamed CNS target organ is required for primed T cells to execute a Th1 effector program in EAE.
Figures

Similar articles
-
Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2.J Exp Med. 2000 Oct 2;192(7):1075-80. doi: 10.1084/jem.192.7.1075. J Exp Med. 2000. PMID: 11015448 Free PMC article.
-
Modulation of experimental autoimmune encephalomyelitis: effect of altered peptide ligand on chemokine and chemokine receptor expression.J Neuroimmunol. 2000 Oct 2;110(1-2):195-208. doi: 10.1016/s0165-5728(00)00351-9. J Neuroimmunol. 2000. PMID: 11024550
-
Effects of cytokine deficiency on chemokine expression in CNS of mice with EAE.J Neurosci Res. 2002 Mar 1;67(5):680-8. doi: 10.1002/jnr.10156. J Neurosci Res. 2002. PMID: 11891780
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
-
Using EAE to better understand principles of immune function and autoimmune pathology.J Autoimmun. 2013 Sep;45:31-9. doi: 10.1016/j.jaut.2013.06.008. Epub 2013 Jul 9. J Autoimmun. 2013. PMID: 23849779 Free PMC article. Review.
Cited by
-
Myelin basic protein induces inflammatory mediators from primary human endothelial cells and blood-brain barrier disruption: implications for the pathogenesis of multiple sclerosis.Neuropathol Appl Neurobiol. 2013 Apr;39(3):270-83. doi: 10.1111/j.1365-2990.2012.01279.x. Neuropathol Appl Neurobiol. 2013. PMID: 22524708 Free PMC article.
-
Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice.J Cereb Blood Flow Metab. 2010 Apr;30(4):769-82. doi: 10.1038/jcbfm.2009.262. Epub 2009 Dec 23. J Cereb Blood Flow Metab. 2010. PMID: 20029451 Free PMC article.
-
The chemokine receptor antagonist, TAK-779, decreased experimental autoimmune encephalomyelitis by reducing inflammatory cell migration into the central nervous system, without affecting T cell function.Br J Pharmacol. 2009 Dec;158(8):2046-56. doi: 10.1111/j.1476-5381.2009.00528.x. Br J Pharmacol. 2009. PMID: 20050195 Free PMC article.
-
Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment.J Exp Med. 2001 Nov 5;194(9):1375-84. doi: 10.1084/jem.194.9.1375. J Exp Med. 2001. PMID: 11696601 Free PMC article.
-
CSF-1 maintains pathogenic but not homeostatic myeloid cells in the central nervous system during autoimmune neuroinflammation.Proc Natl Acad Sci U S A. 2022 Apr 5;119(14):e2111804119. doi: 10.1073/pnas.2111804119. Epub 2022 Mar 30. Proc Natl Acad Sci U S A. 2022. PMID: 35353625 Free PMC article.
References
-
- Rollins B.J. Chemokines. Blood. 1997;90:909–928. - PubMed
-
- Foti M., Granucci F., Aggujaro D., Liboi E., Luini W., Minardi S., Mantovani A., Sozzani S., Ricciardi-Castagnoli P. Upon dendritic cell (DC) activation chemokines and chemokine receptor expression are rapidly regulated for recruitment and maintenance of DC at the inflammatory site. Int. Immunol. 1999;11:979–986. - PubMed
-
- Sallusto F., Palermo B., Lenig D., Miettinen M., Matikainen S., Julkunen I., Forster R., Burgstahler R., Lipp M., Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999;29:1617–1625. - PubMed
-
- Maghazachi A.A., Al-Aoukaty A., Schall T.J. C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J. Immunol. 1994;153:4969–4977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

