Linkage between STAT regulation and Epstein-Barr virus gene expression in tumors
- PMID: 11222718
- PMCID: PMC115919
- DOI: 10.1128/JVI.75.6.2929-2937.2001
Linkage between STAT regulation and Epstein-Barr virus gene expression in tumors
Abstract
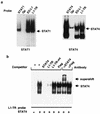
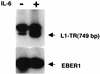
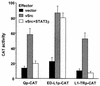
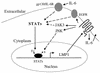
Epstein-Barr virus (EBV) latency gene expression in lymphoblastoid cell lines is regulated by EBNA2. However, the factors regulating viral expression in EBV-associated tumors that do not express EBNA2 are poorly understood. In EBV-associated tumors, EBNA1 and frequently LMP1 are synthesized. We found that an alternative latent membrane protein 1 (LMP1) promoter, L1-TR, located within the terminal repeats is active in both nasopharyngeal carcinoma and Hodgkin's disease tissues. Examination of the L1-TR and the standard ED-L1 LMP1 promoters in electrophoretic mobility shift assays revealed that both promoters contain functional STAT binding sites. Further, both LMP1 promoters responded in reporter assays to activation of JAK-STAT signaling. Cotransfection of JAK1 or v-Src or treatment of cells with the cytokine interleukin-6 upregulated expression from ED-L1 and L1-TR reporter plasmids. Cotransfection of a dominant negative STAT3 beta revealed that STAT3 is likely to be the biologically relevant STAT for EBNA1 Qp and LMP1 L1-TR promoter regulation. In contrast, LMP1 expression from ED-L1 was not abrogated by STAT3 beta, indicating that the two LMP1 promoters are regulated by different STAT family members. Taken together with the previous demonstration of JAK-STAT activation of Qp driven EBNA1 expression, this places two of the EBV genes most commonly expressed in tumors under the control of the same signal transduction pathway. Immunohistochemical analyses of nasopharyngeal carcinoma tumors revealed that STAT3, STAT5, and STAT1 are constitutively activated in these tumors while STAT3 is constitutively activated in the malignant cells of Hodgkin's disease. We hypothesize that chronic or aberrant STAT activation may be both a necessary and predisposing event for EBV-driven tumorigenesis in immunocompetent individuals.
Figures

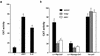


Similar articles
-
A positive autoregulatory loop of LMP1 expression and STAT activation in epithelial cells latently infected with Epstein-Barr virus.J Virol. 2003 Apr;77(7):4139-48. doi: 10.1128/jvi.77.7.4139-4148.2003. J Virol. 2003. PMID: 12634372 Free PMC article.
-
The Epstein-Barr virus latency BamHI-Q promoter is positively regulated by STATs and Zta interference with JAK/STAT activation leads to loss of BamHI-Q promoter activity.Proc Natl Acad Sci U S A. 1999 Aug 3;96(16):9339-44. doi: 10.1073/pnas.96.16.9339. Proc Natl Acad Sci U S A. 1999. PMID: 10430944 Free PMC article.
-
NF-κB Signaling Regulates Expression of Epstein-Barr Virus BART MicroRNAs and Long Noncoding RNAs in Nasopharyngeal Carcinoma.J Virol. 2016 Jun 24;90(14):6475-88. doi: 10.1128/JVI.00613-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147748 Free PMC article.
-
Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin's disease.Int J Cancer. 1996 Nov 4;68(3):285-90. doi: 10.1002/(SICI)1097-0215(19961104)68:3<285::AID-IJC3>3.0.CO;2-Y. Int J Cancer. 1996. PMID: 8903467
-
Novel roles and therapeutic targets of Epstein-Barr virus-encoded latent membrane protein 1-induced oncogenesis in nasopharyngeal carcinoma.Expert Rev Mol Med. 2015 Aug 18;17:e15. doi: 10.1017/erm.2015.13. Expert Rev Mol Med. 2015. PMID: 26282825 Review.
Cited by
-
IL-21 Stimulates the expression and activation of cell cycle regulators and promotes cell proliferation in EBV-positive diffuse large B cell lymphoma.Sci Rep. 2020 Jul 23;10(1):12326. doi: 10.1038/s41598-020-69227-0. Sci Rep. 2020. PMID: 32704112 Free PMC article.
-
Enhanced IL-6/IL-6R signaling promotes growth and malignant properties in EBV-infected premalignant and cancerous nasopharyngeal epithelial cells.PLoS One. 2013 May 1;8(5):e62284. doi: 10.1371/journal.pone.0062284. Print 2013. PLoS One. 2013. PMID: 23658720 Free PMC article.
-
Human Cytomegalovirus UL138 Interaction with USP1 Activates STAT1 in infection.bioRxiv [Preprint]. 2023 Feb 7:2023.02.07.527452. doi: 10.1101/2023.02.07.527452. bioRxiv. 2023. Update in: PLoS Pathog. 2023 Jun 8;19(6):e1011185. doi: 10.1371/journal.ppat.1011185. PMID: 36798153 Free PMC article. Updated. Preprint.
-
Signal transducer and activator of transcription 3 limits Epstein-Barr virus lytic activation in B lymphocytes.J Virol. 2013 Nov;87(21):11438-46. doi: 10.1128/JVI.01762-13. Epub 2013 Aug 21. J Virol. 2013. PMID: 23966384 Free PMC article.
-
B lymphocytes from patients with a hypomorphic mutation in STAT3 resist Epstein-Barr virus-driven cell proliferation.J Virol. 2014 Jan;88(1):516-24. doi: 10.1128/JVI.02601-13. Epub 2013 Oct 30. J Virol. 2014. PMID: 24173212 Free PMC article.
References
-
- Ambinder R F, Robertson K D, Tao Q. DNA methylation and the Epstein-Barr virus. Semin Cancer Biol. 1999;9:369–375. - PubMed
-
- Bonnet M, Guinebretiere J M, Kremmer E, Grunewald V, Benhamou E, Contesso G, Joab I. Detection of Epstein-Barr virus in invasive breast cancers. J Natl Cancer Inst. 1999;91:1376–1381. - PubMed
-
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. - PubMed
-
- Bromberg J F, Wrzeszczynska M H, Devgan G, Zhao V, Pestell R G, Albanese C, Darnell J E., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

