A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin
- PMID: 11160133
- PMCID: PMC198872
- DOI: 10.1172/JCI8525
A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin
Abstract
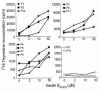
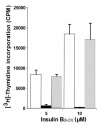
The 9-23 amino acid region of the insulin B chain (B9-23) is a dominant epitope recognized by pathogenic T lymphocytes in nonobese diabetic mice, the animal model for type 1 diabetes. We describe herein similar (B9-23)-specific T-cell responses in peripheral lymphocytes obtained from patients with recent-onset type 1 diabetes and from prediabetic subjects at high risk for disease. Short-term T-cell lines generated from patient peripheral lymphocytes showed significant proliferative responses to (B9-23), whereas lymphocytes isolated from HLA and/or age-matched nondiabetic normal controls were unresponsive. Antibody-mediated blockade demonstrated that the response was HLA class II restricted. Use of the highly sensitive cytokine-detection ELISPOT assay revealed that these (B9-23)-specific cells were present in freshly isolated lymphocytes from only the type 1 diabetics and prediabetics and produced the proinflammatory cytokine IFN-gamma. This study is, to our knowledge, the first demonstration of a cellular response to the (B9-23) insulin epitope in human type 1 diabetes and suggests that the mouse and human diseases have strikingly similar autoantigenic targets, a feature that should facilitate development of antigen-based therapeutics.
Figures

Similar articles
-
T cell autoreactivity to insulin in diabetic and related non-diabetic individuals.J Immunol. 1988 Apr 15;140(8):2569-78. J Immunol. 1988. PMID: 2451692
-
Detection of autoreactive T cells in type 1 diabetes using coded autoantigens and an immunoglobulin-free cytokine ELISPOT assay: report from the fourth immunology of diabetes society T cell workshop.Ann N Y Acad Sci. 2004 Dec;1037:10-5. doi: 10.1196/annals.1337.002. Ann N Y Acad Sci. 2004. PMID: 15699487
-
Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope.Scand J Immunol. 2006 Jan;63(1):59-69. doi: 10.1111/j.1365-3083.2005.01705.x. Scand J Immunol. 2006. PMID: 16398702 Clinical Trial.
-
Natural history and prediction of type 1 diabetes.Ann Ig. 1992 May-Jun;4(3):153-7. Ann Ig. 1992. PMID: 1284514 Review. No abstract available.
-
Immunologic implications of insulin delivery and the role of immune complexes in diabetic sequelae.Diabetes Care. 1982 May-Jun;5 Suppl 1:88-92. Diabetes Care. 1982. PMID: 6765123 Review.
Cited by
-
Regulatory vs. inflammatory cytokine T-cell responses to mutated insulin peptides in healthy and type 1 diabetic subjects.Proc Natl Acad Sci U S A. 2015 Apr 7;112(14):4429-34. doi: 10.1073/pnas.1502967112. Epub 2015 Mar 23. Proc Natl Acad Sci U S A. 2015. PMID: 25831495 Free PMC article.
-
Immune Intervention and Preservation of Pancreatic Beta Cell Function in Type 1 Diabetes.Curr Diab Rep. 2016 Oct;16(10):97. doi: 10.1007/s11892-016-0793-8. Curr Diab Rep. 2016. PMID: 27558810 Free PMC article. Review.
-
Insulin as an autoantigen in NOD/human diabetes.Curr Opin Immunol. 2008 Feb;20(1):111-8. doi: 10.1016/j.coi.2007.11.005. Curr Opin Immunol. 2008. PMID: 18178393 Free PMC article. Review.
-
Analysis of T cell receptor beta chains that combine with dominant conserved TRAV5D-4*04 anti-insulin B:9-23 alpha chains.J Autoimmun. 2009 Aug;33(1):42-9. doi: 10.1016/j.jaut.2009.02.003. Epub 2009 Mar 16. J Autoimmun. 2009. PMID: 19286348 Free PMC article.
-
MAS-1 adjuvant immunotherapy generates robust Th2 type and regulatory immune responses providing long-term protection from diabetes in late-stage pre-diabetic NOD mice.Autoimmunity. 2014 Aug;47(5):341-50. doi: 10.3109/08916934.2014.910768. Epub 2014 May 1. Autoimmunity. 2014. PMID: 24783965 Free PMC article.
References
-
- Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–1436. - PubMed
-
- Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD [erratum 1998, 8:531] Immunity. 1997;7:727–738. - PubMed
-
- Durinovic-Bello I. Autoimmune diabetes: the role of T cells, MHC molecules and autoantigens. Autoimmunity. 1998;27:159–177. - PubMed
-
- Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol. 1994;24:1853–1857. - PubMed
-
- Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25:1056–1062. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

