Recognition of the core RNA promoter for minus-strand RNA synthesis by the replicases of Brome mosaic virus and Cucumber mosaic virus
- PMID: 11044076
- PMCID: PMC110906
- DOI: 10.1128/jvi.74.22.10323-10331.2000
Recognition of the core RNA promoter for minus-strand RNA synthesis by the replicases of Brome mosaic virus and Cucumber mosaic virus
Abstract
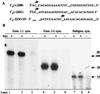
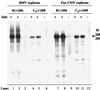
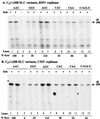
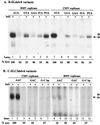
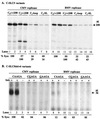
Replication of viral RNA genomes requires the specific interaction between the replicase and the RNA template. Members of the Bromovirus and Cucumovirus genera have a tRNA-like structure at the 3' end of their genomic RNAs that interacts with the replicase and is required for minus-strand synthesis. In Brome mosaic virus (BMV), a stem-loop structure named C (SLC) is present within the tRNA-like region and is required for replicase binding and initiation of RNA synthesis in vitro. We have prepared an enriched replicase fraction from tobacco plants infected with the Fny isolate of Cucumber mosaic virus (Fny-CMV) that will direct synthesis from exogenously added templates. Using this replicase, we demonstrate that the SLC-like structure in Fny-CMV plays a role similar to that of BMV SLC in interacting with the CMV replicase. While the majority of CMV isolates have SLC-like elements similar to that of Fny-CMV, a second group displays sequence or structural features that are distinct but nonetheless recognized by Fny-CMV replicase for RNA synthesis. Both motifs have a 5'CA3' dinucleotide that is invariant in the CMV isolates examined, and mutational analysis indicates that these are critical for interaction with the replicase. In the context of the entire tRNA-like element, both CMV SLC-like motifs are recognized by the BMV replicase. However, neither motif can direct synthesis by the BMV replicase in the absence of other tRNA-like elements, indicating that other features of the CMV tRNA can induce promoter recognition by a heterologous replicase.
Figures
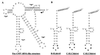

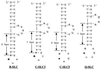
Similar articles
-
Brome mosaic virus RNA syntheses in vitro and in barley protoplasts.J Virol. 2003 May;77(10):5703-11. doi: 10.1128/jvi.77.10.5703-5711.2003. J Virol. 2003. PMID: 12719563 Free PMC article.
-
Sequences 5' of the conserved tRNA-like promoter modulate the initiation of minus-strand synthesis by the brome mosaic virus RNA-dependent RNA polymerase.Virology. 1998 Dec 20;252(2):458-67. doi: 10.1006/viro.1998.9473. Virology. 1998. PMID: 9878626
-
Replicase-binding sites on plus- and minus-strand brome mosaic virus RNAs and their roles in RNA replication in plant cells.J Virol. 2004 Dec;78(24):13420-9. doi: 10.1128/JVI.78.24.13420-13429.2004. J Virol. 2004. PMID: 15564452 Free PMC article.
-
Non-canonical substrates of aminoacyl-tRNA synthetases: the tRNA-like structure of brome mosaic virus genomic RNA.Biochimie. 1993;75(12):1143-57. doi: 10.1016/0300-9084(93)90014-j. Biochimie. 1993. PMID: 8199250 Review.
-
The brome mosaic virus 3' untranslated sequence regulates RNA replication, recombination, and virion assembly.Virus Res. 2015 Aug 3;206:46-52. doi: 10.1016/j.virusres.2015.02.007. Epub 2015 Feb 14. Virus Res. 2015. PMID: 25687214 Review.
Cited by
-
Multiple Levels of Triggered Factors and the Obligated Requirement of Cell-to-Cell Movement in the Mutation Repair of Cucumber Mosaic Virus with Defects in the tRNA-like Structure.Biology (Basel). 2022 Jul 13;11(7):1051. doi: 10.3390/biology11071051. Biology (Basel). 2022. PMID: 36101429 Free PMC article.
-
The Strange Lifestyle of Multipartite Viruses.PLoS Pathog. 2016 Nov 3;12(11):e1005819. doi: 10.1371/journal.ppat.1005819. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27812219 Free PMC article. Review.
-
Efficient in vitro system of homologous recombination in brome mosaic bromovirus.J Virol. 2006 Jun;80(12):6182-7. doi: 10.1128/JVI.02447-05. J Virol. 2006. PMID: 16731958 Free PMC article.
-
Stable RNA structures can repress RNA synthesis in vitro by the brome mosaic virus replicase.RNA. 2003 May;9(5):555-65. doi: 10.1261/rna.2190803. RNA. 2003. PMID: 12702814 Free PMC article.
-
Factors regulating template switch in vitro by viral RNA-dependent RNA polymerases: implications for RNA-RNA recombination.Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):4972-7. doi: 10.1073/pnas.081077198. Epub 2001 Apr 17. Proc Natl Acad Sci U S A. 2001. PMID: 11309487 Free PMC article.
References
-
- Adkins S, Kao C C. Subgenomic RNA promoters dictate the mode of recognition by bromoviral RNA-dependent RNA polymerase. Virology. 1998;252:1–8. - PubMed
-
- Ahlquist P. Bromovirus RNA replication and transcription. Curr Opin Genet Dev. 1992;2:71–76. - PubMed
-
- Ahlquist P, Dasgupta R, Kaesberg P. Near identity of 3′ RNA secondary structure in bromo-viruses and cucumber mosaic virus. Cell. 1981;23:183–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

