Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles
- PMID: 10982339
- PMCID: PMC102091
- DOI: 10.1128/jvi.74.19.8953-8965.2000
Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles
Abstract
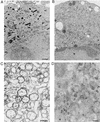
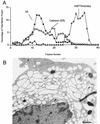
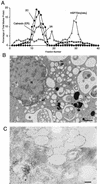
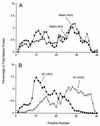
All positive-strand RNA viruses of eukaryotes studied assemble RNA replication complexes on the surfaces of cytoplasmic membranes. Infection of mammalian cells with poliovirus and other picornaviruses results in the accumulation of dramatically rearranged and vesiculated membranes. Poliovirus-induced membranes did not cofractionate with endoplasmic reticulum (ER), lysosomes, mitochondria, or the majority of Golgi-derived or endosomal membranes in buoyant density gradients, although changes in ionic strength affected ER and virus-induced vesicles, but not other cellular organelles, similarly. When expressed in isolation, two viral proteins of the poliovirus RNA replication complex, 3A and 2C, cofractionated with ER membranes. However, in cells that expressed 2BC, a proteolytic precursor of the 2B and 2C proteins, membranes identical in buoyant density to those observed during poliovirus infection were formed. When coexpressed with 2BC, viral protein 3A was quantitatively incorporated into these fractions, and the membranes formed were ultrastructurally similar to those in poliovirus-infected cells. These data argue that poliovirus-induced vesicles derive from the ER by the action of viral proteins 2BC and 3A by a mechanism that excludes resident host proteins. The double-membraned morphology, cytosolic content, and apparent ER origin of poliovirus-induced membranes are all consistent with an autophagic origin for these membranes.
Figures





Similar articles
-
Effects of foot-and-mouth disease virus nonstructural proteins on the structure and function of the early secretory pathway: 2BC but not 3A blocks endoplasmic reticulum-to-Golgi transport.J Virol. 2005 Apr;79(7):4382-95. doi: 10.1128/JVI.79.7.4382-4395.2005. J Virol. 2005. PMID: 15767438 Free PMC article.
-
Cellular origin and ultrastructure of membranes induced during poliovirus infection.J Virol. 1996 Oct;70(10):6576-88. doi: 10.1128/JVI.70.10.6576-6588.1996. J Virol. 1996. PMID: 8794292 Free PMC article.
-
Inhibition of endoplasmic reticulum-to-Golgi traffic by poliovirus protein 3A: genetic and ultrastructural analysis.J Virol. 1997 Dec;71(12):9054-64. doi: 10.1128/JVI.71.12.9054-9064.1997. J Virol. 1997. PMID: 9371562 Free PMC article.
-
Potential subversion of autophagosomal pathway by picornaviruses.Autophagy. 2008 Apr;4(3):286-9. doi: 10.4161/auto.5377. Epub 2007 Dec 5. Autophagy. 2008. PMID: 18094610 Review.
-
Endoplasmic Reticulum: The Favorite Intracellular Niche for Viral Replication and Assembly.Viruses. 2016 Jun 7;8(6):160. doi: 10.3390/v8060160. Viruses. 2016. PMID: 27338443 Free PMC article. Review.
Cited by
-
Tropism Analysis of Two Coxsackie B5 Strains Reveals Virus Growth in Human Primary Pancreatic Islets but not in Exocrine Cell Clusters In Vitro.Open Virol J. 2013 Apr 5;7:49-56. doi: 10.2174/1874357901307010049. Print 2013. Open Virol J. 2013. PMID: 23723955 Free PMC article.
-
Imaging-Based Reporter Systems to Define CVB-Induced Membrane Remodeling in Living Cells.Viruses. 2020 Sep 25;12(10):1074. doi: 10.3390/v12101074. Viruses. 2020. PMID: 32992749 Free PMC article.
-
Viruses and the autophagy pathway.Virology. 2015 May;479-480:450-6. doi: 10.1016/j.virol.2015.03.042. Epub 2015 Apr 6. Virology. 2015. PMID: 25858140 Free PMC article. Review.
-
Opportunistic intruders: how viruses orchestrate ER functions to infect cells.Nat Rev Microbiol. 2016 Jul;14(7):407-420. doi: 10.1038/nrmicro.2016.60. Epub 2016 Jun 6. Nat Rev Microbiol. 2016. PMID: 27265768 Free PMC article. Review.
-
Autophagy inhibition plays the synergetic killing roles with radiation in the multi-drug resistant SKVCR ovarian cancer cells.Radiat Oncol. 2012 Dec 17;7:213. doi: 10.1186/1748-717X-7-213. Radiat Oncol. 2012. PMID: 23244773 Free PMC article.
References
-
- Aldabe R, Carrasco L. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem Biophys Res Commun. 1995;206:64–76. - PubMed
-
- Bienz K, Egger D, Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987;160:220–226. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

