Insulin-like growth factor-1 fails to enhance central nervous system myelin repair during autoimmune demyelination
- PMID: 10980132
- PMCID: PMC1885703
- DOI: 10.1016/S0002-9440(10)64606-8
Insulin-like growth factor-1 fails to enhance central nervous system myelin repair during autoimmune demyelination
Abstract
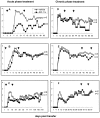
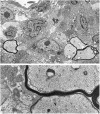
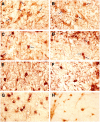
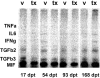
Previous studies have shown that insulin-like growth factor-1 (IGF-1) has beneficial effects, both clinically and histopathologically, on experimental autoimmune encephalomyelitis (EAE), although results vary depending on species and treatment regimen. The present study investigated whether IGF-1, delivered at different time points during the acute and chronic phases of adoptively transferred EAE in SJL mice, had the ability to affect or enhance myelin regeneration. Central nervous system tissue sampled at different stages of treatment was subjected to detailed neuropathological, immunocytochemical and molecular analysis. The results revealed some transient clinical amelioration and low level remyelination after IGF-1 administration during the acute phase of EAE. However, central nervous system tissue from acute phase treated animals sampled at chronic time points and from animals given IGF-1 during the chronic phase revealed no enhancing effect on remyelination in comparison to vehicle-treated controls. Examination of oligodendrocyte progenitor populations also revealed no differences between IGF-1- and vehicle-treated groups. At the cytokine level, the immunomodulatory molecules TGF-beta2 and TGF-beta3 displayed significant decreases that may have contributed to the transient nature of the effect of IGF-1 on EAE. Together with evidence from previous studies, it appears doubtful that IGF-1 is a good candidate for treatment in multiple sclerosis, for which EAE serves as a major model.
Figures


Similar articles
-
Regulation of experimental autoimmune encephalomyelitis with insulin-like growth factor (IGF-1) and IGF-1/IGF-binding protein-3 complex (IGF-1/IGFBP3).J Clin Invest. 1998 Apr 15;101(8):1797-804. doi: 10.1172/JCI1486. J Clin Invest. 1998. PMID: 9541512 Free PMC article.
-
Neuregulin in neuron/glial interactions in the central nervous system. GGF2 diminishes autoimmune demyelination, promotes oligodendrocyte progenitor expansion, and enhances remyelination.Adv Exp Med Biol. 1999;468:283-95. Adv Exp Med Biol. 1999. PMID: 10635037
-
Targeted expression of IGF-1 in the central nervous system fails to protect mice from experimental autoimmune encephalomyelitis.J Neuroimmunol. 2005 Nov;168(1-2):40-5. doi: 10.1016/j.jneuroim.2005.06.033. Epub 2005 Aug 24. J Neuroimmunol. 2005. PMID: 16120466
-
The effect of insulin-like growth factors on brain myelination and their potential therapeutic application in myelination disorders.Eur J Paediatr Neurol. 1997;1(4):91-101. doi: 10.1016/s1090-3798(97)80039-6. Eur J Paediatr Neurol. 1997. PMID: 10728202 Review.
-
Insulin-like growth factors in the treatment of neurological disease.Endocr Dev. 2005;9:135-159. doi: 10.1159/000085763. Endocr Dev. 2005. PMID: 15879695 Review.
Cited by
-
Growth factor regulation of remyelination: behind the growing interest in endogenous cell repair of the CNS.Future Neurol. 2007 Nov;2(6):689-697. doi: 10.2217/14796708.2.6.689. Future Neurol. 2007. PMID: 19079759 Free PMC article.
-
Pharmacological approaches to intervention in hypomyelinating and demyelinating white matter pathology.Neuropharmacology. 2016 Nov;110(Pt B):605-625. doi: 10.1016/j.neuropharm.2015.06.008. Epub 2015 Jun 24. Neuropharmacology. 2016. PMID: 26116759 Free PMC article. Review.
-
mCSF-Induced Microglial Activation Prevents Myelin Loss and Promotes Its Repair in a Mouse Model of Multiple Sclerosis.Front Cell Neurosci. 2018 Jul 3;12:178. doi: 10.3389/fncel.2018.00178. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30018535 Free PMC article.
-
Differential effects of Th1, monocyte/macrophage and Th2 cytokine mixtures on early gene expression for glial and neural-related molecules in central nervous system mixed glial cell cultures: neurotrophins, growth factors and structural proteins.J Neuroinflammation. 2007 Dec 18;4:30. doi: 10.1186/1742-2094-4-30. J Neuroinflammation. 2007. PMID: 18088439 Free PMC article.
-
Insulin-like growth factor-1 receptor controls the function of CNS-resident macrophages and their contribution to neuroinflammation.Acta Neuropathol Commun. 2023 Mar 8;11(1):35. doi: 10.1186/s40478-023-01535-8. Acta Neuropathol Commun. 2023. PMID: 36890580 Free PMC article.
References
-
- Raine CS: Demyelinating diseases. Davis RL Robertson DM eds. Textbook of Neuropathology. 1990, :pp 535-620 Williams & Wilkins, Baltimore
-
- Reder AT, Arnason BGW: Immunology of MS. Handbook of Clinical Neurology, 1985, vol. 3:pp 337-395 Elsevier, (47): Demyelinating Diseases. Edited by JC Koetsier. Amsterdam
-
- Cannoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL: GGF/Neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron 1996, 17:229-243 - PubMed
-
- Cannella B, Hoban CJ, Gao Y-L, Garcia-Arenas R, Lawson D, Marchionni M, Gwynne D, Raine CS: The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proc Natl Acad Sci USA 1998, 95:10100-10105 - PMC - PubMed
-
- McMorris FA, Dubois-Dalcq M: Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J Neurosci Res 1988, 21:199-209 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

