Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex
- PMID: 10973499
- PMCID: PMC27041
- DOI: 10.1073/pnas.190332597
Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex
Abstract
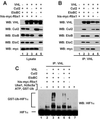
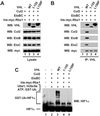
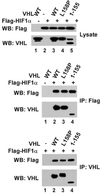
Mutations in the VHL tumor suppressor gene result in constitutive expression of many hypoxia-inducible genes, at least in part because of increases in the cellular level of hypoxia-inducible transcription factor HIF1alpha, which in normal cells is rapidly ubiquitinated and degraded by the proteasome under normoxic conditions. The recent observation that the VHL protein is a subunit of an Skp1-Cul1/Cdc53-F-box (SCF)-like E3 ubiquitin ligase raised the possibility that VHL may be directly responsible for regulating cellular levels of HIF1alpha by targeting it for ubiquitination and proteolysis. In this report, we test this hypothesis directly. We report development of methods for production of the purified recombinant VHL complex and present direct biochemical evidence that it can function with an E1 ubiquitin-activating enzyme and E2 ubiquitin-conjugating enzyme to activate HIF1alpha ubiquitination in vitro. Our findings provide new insight into the function of the VHL tumor suppressor protein, and they provide a foundation for future investigations of the mechanisms underlying VHL regulation of oxygen-dependent gene expression.
Figures


Similar articles
-
A molecular basis for stabilization of the von Hippel-Lindau (VHL) tumor suppressor protein by components of the VHL ubiquitin ligase.J Biol Chem. 2002 Aug 16;277(33):30388-93. doi: 10.1074/jbc.M203344200. Epub 2002 Jun 4. J Biol Chem. 2002. PMID: 12048197
-
Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein.EMBO J. 2000 Aug 15;19(16):4298-309. doi: 10.1093/emboj/19.16.4298. EMBO J. 2000. PMID: 10944113 Free PMC article.
-
Role of exon 2-encoded beta -domain of the von Hippel-Lindau tumor suppressor protein.J Biol Chem. 2001 Jan 12;276(2):1407-16. doi: 10.1074/jbc.M008295200. J Biol Chem. 2001. PMID: 11024059
-
The von Hippel-Lindau tumor suppressor gene.Exp Cell Res. 2001 Mar 10;264(1):117-25. doi: 10.1006/excr.2000.5139. Exp Cell Res. 2001. PMID: 11237528 Review.
-
The von Hippel-Lindau tumor suppressor, hypoxia-inducible factor-1 (HIF-1) degradation, and cancer pathogenesis.Semin Cancer Biol. 2003 Feb;13(1):83-9. doi: 10.1016/s1044-579x(02)00103-7. Semin Cancer Biol. 2003. PMID: 12507560 Review.
Cited by
-
Regulation of Bone Marrow Angiogenesis by Osteoblasts during Bone Development and Homeostasis.Front Endocrinol (Lausanne). 2013 Jul 10;4:85. doi: 10.3389/fendo.2013.00085. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23847596 Free PMC article.
-
HIF-α Prolyl Hydroxylase Inhibitors and Their Implications for Biomedicine: A Comprehensive Review.Biomedicines. 2021 Apr 24;9(5):468. doi: 10.3390/biomedicines9050468. Biomedicines. 2021. PMID: 33923349 Free PMC article. Review.
-
Dysregulated glycolysis as an oncogenic event.Cell Mol Life Sci. 2015 May;72(10):1881-92. doi: 10.1007/s00018-015-1840-3. Epub 2015 Jan 22. Cell Mol Life Sci. 2015. PMID: 25609364 Free PMC article. Review.
-
Somatic VHL gene alterations in MEN2-associated medullary thyroid carcinoma.BMC Cancer. 2006 May 17;6:131. doi: 10.1186/1471-2407-6-131. BMC Cancer. 2006. PMID: 16707008 Free PMC article.
-
Analysis of the hypoxia-sensing pathway in Drosophila melanogaster.Biochem J. 2006 Jan 15;393(Pt 2):471-80. doi: 10.1042/BJ20050675. Biochem J. 2006. PMID: 16176182 Free PMC article.
References
-
- Latif F, Tory K, Gnarra J, Yao M, Duh F M, Orcutt M L, Stackhouse T, Kuzmin I, Modi W, Geil L, et al. Science. 1993;260:1317–1320. - PubMed
-
- Gnarra J R, Duan D R, Weng Y, Humphrey J S, Chen D Y, Lee S, Pause A, Dudley C F, Latif F, Kuzmin I, et al. Biochim Biophys Acta. 1996;1242:201–210. - PubMed
-
- Siemeister G, Weindel K, Mohrs K, Barleon B, Martiny-Baron G, Marme D. Cancer Res. 1996;56:2299–2301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

