Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein
- PMID: 10944113
- PMCID: PMC302039
- DOI: 10.1093/emboj/19.16.4298
Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein
Abstract
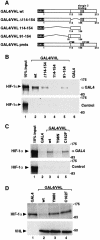
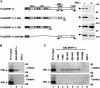
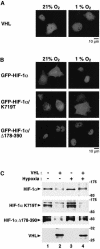
In normoxic cells the hypoxia-inducible factor-1 alpha (HIF-1 alpha) is rapidly degraded by the ubiquitin-proteasome pathway, and activation of HIF-1 alpha to a functional form requires protein stabilization. Here we show that the product of the von Hippel-Lindau (VHL) tumor suppressor gene mediated ubiquitylation and proteasomal degradation of HIF-1 alpha under normoxic conditions via interaction with the core of the oxygen-dependent degradation domain of HIF-1 alpha. The region of VHL mediating interaction with HIF-1 alpha overlapped with a putative macromolecular binding site observed within the crystal structure of VHL. This motif of VHL also represents a mutational hotspot in tumors, and one of these mutations impaired interaction with HIF-1 alpha and subsequent degradation. Interestingly, the VHL binding site within HIF-1 alpha overlapped with one of the minimal transactivation domains. Protection of HIF-1 alpha against degradation by VHL was a multistep mechanism, including hypoxia-induced nuclear translocation of HIF-1 alpha and an intranuclear hypoxia-dependent signal. VHL was not released from HIF-1 alpha during this process. Finally, stabilization of HIF-1 alpha protein levels per se did not totally bypass the need of the hypoxic signal for generating the transactivation response.
Figures
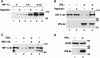



Similar articles
-
Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein.J Biol Chem. 2000 Aug 18;275(33):25733-41. doi: 10.1074/jbc.M002740200. J Biol Chem. 2000. PMID: 10823831
-
Dynamic, site-specific interaction of hypoxia-inducible factor-1alpha with the von Hippel-Lindau tumor suppressor protein.Cancer Res. 2001 May 15;61(10):4136-42. Cancer Res. 2001. PMID: 11358837
-
The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis.Nature. 1999 May 20;399(6733):271-5. doi: 10.1038/20459. Nature. 1999. PMID: 10353251
-
Regulation of HIF by the von Hippel-Lindau tumour suppressor: implications for cellular oxygen sensing.IUBMB Life. 2001 Jul;52(1-2):43-7. doi: 10.1080/15216540252774757. IUBMB Life. 2001. PMID: 11795592 Review.
-
Do VHL and HIF-1 mirror p53 and Mdm-2? Degradation-transactivation loops of oncoproteins and tumor suppressors.Oncogene. 2001 Jan 18;20(3):395-8. doi: 10.1038/sj.onc.1204055. Oncogene. 2001. PMID: 11313969 Review.
Cited by
-
Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer.J Physiol. 2013 Apr 15;591(8):2027-42. doi: 10.1113/jphysiol.2013.251470. Epub 2013 Feb 11. J Physiol. 2013. PMID: 23401619 Free PMC article. Review.
-
VHL, the story of a tumour suppressor gene.Nat Rev Cancer. 2015 Jan;15(1):55-64. doi: 10.1038/nrc3844. Nat Rev Cancer. 2015. PMID: 25533676 Review.
-
Emerging Roles of Cullin-RING Ubiquitin Ligases in Cardiac Development.Cells. 2024 Jan 26;13(3):235. doi: 10.3390/cells13030235. Cells. 2024. PMID: 38334627 Free PMC article. Review.
-
Positive feedback between oncogenic KRAS and HIF-1α confers drug resistance in colorectal cancer.Onco Targets Ther. 2015 May 26;8:1229-37. doi: 10.2147/OTT.S80017. eCollection 2015. Onco Targets Ther. 2015. PMID: 26060408 Free PMC article.
-
Distinct roles of Kaposi's sarcoma-associated herpesvirus-encoded viral interferon regulatory factors in inflammatory response and cancer.J Virol. 2013 Sep;87(17):9398-410. doi: 10.1128/JVI.03315-12. Epub 2013 Jun 19. J Virol. 2013. PMID: 23785197 Free PMC article. Review.
References
-
- Ema M., Hirota,K., Mimura,J., Abe,H., Yodoi,J., Sogawa,K., Poellinger,L. and Fujii-Kuriyama,Y. (1999) Molecular mechanisms of transcription activation by HLF and HIF1α in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J., 18, 1905–1914. - PMC - PubMed
-
- Gnarra J.R., Zhou,S., Merrill,M.J., Wagner,J.R., Krumm,A., Papavassiliou,E., Oldfield,E.H., Klausner,R.D. and Linehan,W.M. (1996) Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc. Natl Acad. Sci. USA, 93, 10589–10594. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

