Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses
- PMID: 10890918
- PMCID: PMC26969
- DOI: 10.1073/pnas.150240097
Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses
Abstract
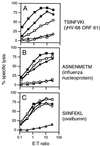
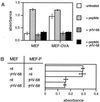
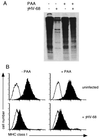
The gamma-herpesviruses, in contrast to the alpha- and beta-herpesviruses, are not known to inhibit antigen presentation to CD8(+) cytotoxic T lymphocytes (CTLs) during lytic cycle replication. However, murine gamma-herpesvirus 68 causes a chronic lytic infection in CD4(+) T cell-deficient mice despite the persistence of a substantial CTL response, suggesting that CTL evasion occurs. Here we show that, distinct from host protein synthesis shutoff, gamma-herpesvirus 68 down-regulates surface MHC class I expression on lytically infected fibroblasts and inhibits their recognition by antigen-specific CTLs. The viral K3 gene, encoding a zinc-finger-containing protein, dramatically reduced the half-life of nascent class I molecules and the level of surface MHC class I expression and was by itself sufficient to block antigen presentation. The homologous K3 and K5 genes of the related Kaposi's sarcoma-associated virus also inhibited antigen presentation and decreased cell surface expression of HLA class I antigens. Thus it appears that an immune evasion strategy shared by at least two gamma-herpesviruses allows continued lytic infection in the face of strong CTL immunity.
Figures
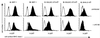



Similar articles
-
Retrovirus-induced changes in major histocompatibility complex antigen expression influence susceptibility to lysis by cytotoxic T lymphocytes.J Immunol. 1985 Oct;135(4):2287-92. J Immunol. 1985. PMID: 2411792
-
Trypanosoma cruzi infection does not impair major histocompatibility complex class I presentation of antigen to cytotoxic T lymphocytes.Eur J Immunol. 1997 Oct;27(10):2541-8. doi: 10.1002/eji.1830271012. Eur J Immunol. 1997. PMID: 9368608
-
Cytokines restore MHC class I complex formation and control antigen presentation in human cytomegalovirus-infected cells.J Gen Virol. 1995 Dec;76 ( Pt 12):2987-97. doi: 10.1099/0022-1317-76-12-2987. J Gen Virol. 1995. PMID: 8847504
-
Immune evasion by a novel family of viral PHD/LAP-finger proteins of gamma-2 herpesviruses and poxviruses.Virus Res. 2002 Sep;88(1-2):55-69. doi: 10.1016/s0168-1702(02)00120-x. Virus Res. 2002. PMID: 12297327 Review.
-
Natural history of murine gamma-herpesvirus infection.Philos Trans R Soc Lond B Biol Sci. 2001 Apr 29;356(1408):569-79. doi: 10.1098/rstb.2000.0779. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11313012 Free PMC article. Review.
Cited by
-
Murine gammaherpesvirus-68 glycoprotein B presents a difficult neutralization target to monoclonal antibodies derived from infected mice.J Gen Virol. 2006 Dec;87(Pt 12):3515-3527. doi: 10.1099/vir.0.82313-0. J Gen Virol. 2006. PMID: 17098966 Free PMC article.
-
The Kaposi's sarcoma-associated herpesvirus K5 E3 ubiquitin ligase modulates targets by multiple molecular mechanisms.J Virol. 2007 Jun;81(12):6573-83. doi: 10.1128/JVI.02751-06. Epub 2007 Apr 4. J Virol. 2007. PMID: 17409151 Free PMC article.
-
Gamma-herpesvirus latency is preferentially maintained in splenic germinal center and memory B cells.J Exp Med. 2002 Nov 18;196(10):1363-72. doi: 10.1084/jem.20020890. J Exp Med. 2002. PMID: 12438427 Free PMC article.
-
An optimized CD8+ T-cell response controls productive and latent gammaherpesvirus infection.J Virol. 2005 Feb;79(4):2573-83. doi: 10.1128/JVI.79.4.2573-2583.2005. J Virol. 2005. PMID: 15681457 Free PMC article.
-
Model for the interaction of gammaherpesvirus 68 RING-CH finger protein mK3 with major histocompatibility complex class I and the peptide-loading complex.J Virol. 2004 Aug;78(16):8673-86. doi: 10.1128/JVI.78.16.8673-8686.2004. J Virol. 2004. PMID: 15280476 Free PMC article.
References
-
- Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Nature (London) 1995;375:411–415. - PubMed
-
- Fruh K, Ahn K, Djaballah H, Sempe P, van Endert P M, Tampe R, Peterson P A, Yang Y. Nature (London) 1995;375:415–418. - PubMed
-
- Ploegh H L. Science. 1998;280:248–253. - PubMed
-
- Hengel H, Brune W, Koszinowski U H. Trends Microbiol. 1998;6:190–197. - PubMed
-
- Zeidler R, Eissner G, Meissner P, Uebel S, Tampe R, Lazis S, Hammerschmidt W. Blood. 1997;90:2390–2397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

