Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells
- PMID: 10736308
- PMCID: PMC2233759
- DOI: 10.1085/jgp.115.4.405
Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells
Abstract
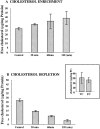
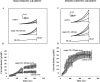
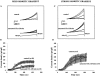
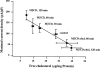
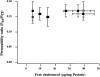
Activation of volume-regulated anion current (VRAC) plays a key role in the maintenance of cellular volume homeostasis. The mechanisms, however, that regulate VRAC activity are not fully understood. We have examined whether VRAC activation is modulated by the cholesterol content of the membrane bilayer. The cholesterol content of bovine aortic endothelial cells was increased by two independent methods: (a) exposure to a methyl-beta-cyclodextrin saturated with cholesterol, or (b) exposure to cholesterol-enriched lipid dispersions. Enrichment of bovine aortic endothelial cells with cholesterol resulted in a suppression of VRAC activation in response to a mild osmotic gradient, but not to a strong osmotic gradient. Depletion of membrane cholesterol by exposing the cells to methyl-beta-cyclodextrin not complexed with cholesterol resulted in an enhancement of VRAC activation when the cells were challenged with a mild osmotic gradient. VRAC activity in cells challenged with a strong osmotic gradient were unaffected by depletion of membrane cholesterol. These observations show that changes in membrane cholesterol content shift VRAC sensitivity to osmotic gradients. Changes in VRAC activation were not accompanied by changes in anion permeability ratios, indicating that channel selectivity was not affected by the changes in membrane cholesterol. This suggests that membrane cholesterol content affects the equilibrium between the closed and open states of VRAC channel rather than the basic pore properties of the channel. We hypothesize that changes in membrane cholesterol modulate VRAC activity by affecting the membrane deformation energy associated with channel opening.
Figures
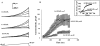
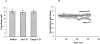

Similar articles
-
Evidence for the role of cell stiffness in modulation of volume-regulated anion channels.Acta Physiol (Oxf). 2006 May-Jun;187(1-2):285-94. doi: 10.1111/j.1748-1716.2006.01555.x. Acta Physiol (Oxf). 2006. PMID: 16734765
-
Dual effect of fluid shear stress on volume-regulated anion current in bovine aortic endothelial cells.Am J Physiol Cell Physiol. 2002 Apr;282(4):C708-18. doi: 10.1152/ajpcell.00247.2001. Am J Physiol Cell Physiol. 2002. PMID: 11880259
-
Cholesterol modulates the volume-regulated anion current in Ehrlich-Lettre ascites cells via effects on Rho and F-actin.Am J Physiol Cell Physiol. 2006 Oct;291(4):C757-71. doi: 10.1152/ajpcell.00029.2006. Epub 2006 May 10. Am J Physiol Cell Physiol. 2006. PMID: 16687471
-
The endothelial volume-regulated anion channel, VRAC.Cell Physiol Biochem. 2000;10(5-6):313-20. doi: 10.1159/000016364. Cell Physiol Biochem. 2000. PMID: 11125211 Review.
-
Molecular Biology and Physiology of Volume-Regulated Anion Channel (VRAC).Curr Top Membr. 2018;81:177-203. doi: 10.1016/bs.ctm.2018.07.005. Epub 2018 Aug 14. Curr Top Membr. 2018. PMID: 30243432 Free PMC article. Review.
Cited by
-
Cholesterol sensitivity of KIR2.1 depends on functional inter-links between the N and C termini.Channels (Austin). 2013 Jul-Aug;7(4):303-12. doi: 10.4161/chan.25437. Epub 2013 Jun 27. Channels (Austin). 2013. PMID: 23807091 Free PMC article.
-
Lipid lowering and HDL raising gene transfer increase endothelial progenitor cells, enhance myocardial vascularity, and improve diastolic function.PLoS One. 2012;7(10):e46849. doi: 10.1371/journal.pone.0046849. Epub 2012 Oct 4. PLoS One. 2012. PMID: 23056485 Free PMC article.
-
Cellular cholesterol controls TRPC3 function: evidence from a novel dominant-negative knockdown strategy.Biochem J. 2006 May 15;396(1):147-55. doi: 10.1042/BJ20051246. Biochem J. 2006. PMID: 16448384 Free PMC article.
-
The Role of Membrane Lipids in Light-Activation of Drosophila TRP Channels.Biomolecules. 2022 Feb 28;12(3):382. doi: 10.3390/biom12030382. Biomolecules. 2022. PMID: 35327573 Free PMC article. Review.
-
Membrane cholesterol regulates TRPV4 function, cytoskeletal expression, and the cellular response to tension.J Lipid Res. 2021;62:100145. doi: 10.1016/j.jlr.2021.100145. Epub 2021 Oct 25. J Lipid Res. 2021. PMID: 34710431 Free PMC article.
References
-
- Andersen O.S., Nielsen C., Maer A.M., Lundbaek J.A., Goulian M., Koeppe H. Ion channels as tools to monitor lipid bilayer-membrane protein interactionsgramicidin channels as molecular force transducers. Methods Enzymol. 1999;294:208–224. - PubMed
-
- Banderali U., Roy G. Anion channels for amino acids in MDCK cells. Am. J. Physiol. Cell Physiol. 1992;263:C1200–C1207. - PubMed
-
- Bolotina V., Omelyanenko V., Heyes B., Ryan U., Bregestovski P. Variations of membrane cholesterol alter the kinetics of Ca2+-dependent K+ channels and membrane fluidity in vascular smooth muscle cells. Pflügers Arch. 1989;415:262–268. - PubMed
-
- Catterall W.A. Voltage-dependent gating of sodium channelscorrelating structure and function. Trends Neurosci. 1986;9:7–10.
-
- Chang H.M., Reitstetter R., Mason R.P., Gruener R. Attenuation of channel kinetics and conductance by cholesterolan interpretation using structural stress as a unifying concept. J. Membr. Biol. 1995;143:51–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

