Multilineage embryonic hematopoiesis requires hypoxic ARNT activity
- PMID: 10521392
- PMCID: PMC317070
- DOI: 10.1101/gad.13.19.2478
Multilineage embryonic hematopoiesis requires hypoxic ARNT activity
Abstract
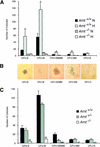
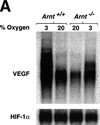
Although most cells undergo growth arrest during hypoxia, endothelial cells and placental cytotrophoblasts proliferate in response to low O(2). We demonstrate that proliferation of embryonic multilineage hematopoietic progenitors is also regulated by a hypoxia-mediated signaling pathway. This pathway requires HIF-1 (HIF-1alpha/ARNT heterodimers) because Arnt(-/-) embryoid bodies fail to exhibit hypoxia-mediated progenitor proliferation. Furthermore, Arnt(-/-) embryos exhibit decreased numbers of yolk sac hematopoietic progenitors. This defect is cell extrinsic, is accompanied by a decrease in ARNT-dependent VEGF expression, and is rescued by exogenous VEGF. Therefore, "physiologic hypoxia" encountered by embryos is essential for the proliferation or survival of hematopoietic precursors during development.
Figures
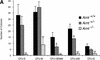
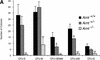



Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Functional importance of Dicer protein in the adaptive cellular response to hypoxia.J Biol Chem. 2012 Aug 17;287(34):29003-20. doi: 10.1074/jbc.M112.373365. Epub 2012 Jun 28. J Biol Chem. 2012. PMID: 22745131 Free PMC article.
-
HIF-1 is essential for multilineage hematopoiesis in the embryo.Adv Exp Med Biol. 2000;475:275-84. doi: 10.1007/0-306-46825-5_26. Adv Exp Med Biol. 2000. PMID: 10849668 No abstract available.
-
Hypoxia-induced overexpression of DEC1 is regulated by HIF-1α in hepatocellular carcinoma.Oncol Rep. 2013 Dec;30(6):2957-62. doi: 10.3892/or.2013.2774. Epub 2013 Oct 1. Oncol Rep. 2013. Retraction in: Oncol Rep. 2025 Feb;53(2):17. doi: 10.3892/or.2024.8850 PMID: 24100543 Retracted.
-
Adipose Tissue Hypoxia in Obesity: Clinical Reappraisal of Hypoxia Hypothesis.Adv Exp Med Biol. 2024;1460:329-356. doi: 10.1007/978-3-031-63657-8_11. Adv Exp Med Biol. 2024. PMID: 39287857 Review.
Cited by
-
HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth.Genes Dev. 2006 Mar 1;20(5):557-70. doi: 10.1101/gad.1399906. Genes Dev. 2006. PMID: 16510872 Free PMC article.
-
Metastasis and stem cell pathways.Cancer Metastasis Rev. 2007 Jun;26(2):261-71. doi: 10.1007/s10555-007-9053-3. Cancer Metastasis Rev. 2007. PMID: 17647111 Free PMC article. Review.
-
The t(1;12)(q21;p13) translocation of human acute myeloblastic leukemia results in a TEL-ARNT fusion.Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6757-62. doi: 10.1073/pnas.120162297. Proc Natl Acad Sci U S A. 2000. PMID: 10829078 Free PMC article.
-
Methyl tert butyl ether targets developing vasculature in zebrafish (Danio rerio) embryos.Aquat Toxicol. 2011 Sep;105(1-2):29-40. doi: 10.1016/j.aquatox.2011.05.006. Epub 2011 May 13. Aquat Toxicol. 2011. PMID: 21684239 Free PMC article.
-
Metabolic circuits in neural stem cells.Cell Mol Life Sci. 2014 Nov;71(21):4221-41. doi: 10.1007/s00018-014-1686-0. Epub 2014 Jul 19. Cell Mol Life Sci. 2014. PMID: 25037158 Free PMC article. Review.
References
-
- An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-iducible factor 1 alpha. Nature. 1998;392:405–408. - PubMed
-
- Bashan N, Burdett E, Hundal HS, Klip A. Regulation of glucose transport and GLUT1 glucose transporter expression by O2 in muscle cells in culture. Am J Physiol. 1992;262:C682–690. - PubMed
-
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. - PubMed
-
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshet E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature. 1998;394:485–490. - PubMed
-
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
