RANK is essential for osteoclast and lymph node development
- PMID: 10500098
- PMCID: PMC317030
- DOI: 10.1101/gad.13.18.2412
RANK is essential for osteoclast and lymph node development
Abstract
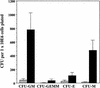
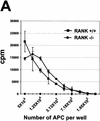
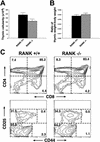
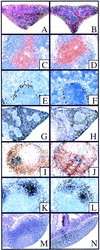
The physiological role of the TNF receptor (TNFR) family member, RANK, was investigated by generating RANK-deficient mice. RANK(-/-) mice were characterized by profound osteopetrosis resulting from an apparent block in osteoclast differentiation. RANK expression was not required for the commitment, differentiation, and functional maturation of macrophages and dendritic cells from their myeloid precursors but provided a necessary and specific signal for the differentiation of myeloid-derived osteoclasts. RANK(-/-) mice also exhibited a marked deficiency of B cells in the spleen. RANK(-/-) mice retained mucosal-associated lymphoid tissues including Peyer's patches but completely lacked all other peripheral lymph nodes, highlighting an additional major role for RANK in lymph node formation. These experiments reveal that RANK provides critical signals necessary for lymph node organogenesis and osteoclast differentiation.
Figures






Similar articles
-
OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis.Nature. 1999 Jan 28;397(6717):315-23. doi: 10.1038/16852. Nature. 1999. PMID: 9950424
-
Distinct osteoclast precursors in the bone marrow and extramedullary organs characterized by responsiveness to Toll-like receptor ligands and TNF-alpha.J Immunol. 2003 Nov 15;171(10):5130-9. doi: 10.4049/jimmunol.171.10.5130. J Immunol. 2003. PMID: 14607912
-
Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction.J Exp Med. 2000 Jan 17;191(2):275-86. doi: 10.1084/jem.191.2.275. J Exp Med. 2000. PMID: 10637272 Free PMC article.
-
Bone resorption by osteoclasts.Science. 2000 Sep 1;289(5484):1504-8. doi: 10.1126/science.289.5484.1504. Science. 2000. PMID: 10968780 Review.
-
Peyer's patch organogenesis--cytokines rule, OK?Gut. 1997 Nov;41(5):707-9. doi: 10.1136/gut.41.5.707. Gut. 1997. PMID: 9414984 Free PMC article. Review.
Cited by
-
Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer.Endocr Rev. 2013 Feb;34(1):130-62. doi: 10.1210/er.2012-1043. Epub 2013 Jan 9. Endocr Rev. 2013. PMID: 23303565 Free PMC article. Review.
-
Skeletal and extraskeletal actions of denosumab.Endocrine. 2012 Aug;42(1):52-62. doi: 10.1007/s12020-012-9696-x. Epub 2012 May 13. Endocrine. 2012. PMID: 22581255 Review.
-
Targeting the RANKL/RANK/OPG Axis for Cancer Therapy.Front Oncol. 2020 Aug 7;10:1283. doi: 10.3389/fonc.2020.01283. eCollection 2020. Front Oncol. 2020. PMID: 32850393 Free PMC article. Review.
-
Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function.J Clin Invest. 2012 Dec;122(12):4592-605. doi: 10.1172/JCI60920. Epub 2012 Nov 1. J Clin Invest. 2012. PMID: 23114597 Free PMC article.
-
RANKL cytokine: from pioneer of the osteoimmunology era to cure for a rare disease.Clin Dev Immunol. 2013;2013:412768. doi: 10.1155/2013/412768. Epub 2013 May 15. Clin Dev Immunol. 2013. PMID: 23762088 Free PMC article. Review.
References
-
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. - PubMed
-
- Brasel K, McKenna HJ, Morrissey PJ, Charrier K, Morris AE, Lee CC, Williams DE, Lyman SD. Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004–2012. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
