Suppression of arthritic bone destruction by adenovirus-mediated csk gene transfer to synoviocytes and osteoclasts
- PMID: 10411542
- PMCID: PMC408475
- DOI: 10.1172/JCI6093
Suppression of arthritic bone destruction by adenovirus-mediated csk gene transfer to synoviocytes and osteoclasts
Abstract
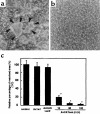
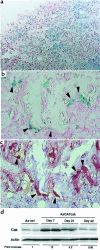
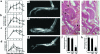
Rheumatoid arthritis (RA) is characterized by a chronic inflammation of the synovial joints resulting from hyperplasia of synovial fibroblasts and infiltration of lymphocytes, macrophages, and plasma cells, all of which manifest signs of activation. Recent studies have revealed the essential role of osteoclasts in joint destruction in RA. Src family tyrosine kinases are implicated in various intracellular signaling pathways, including mitogenic response to growth factors in fibroblasts, activation of lymphocytes, and osteoclastic bone resorption. Therefore, inhibiting Src activity can be a good therapeutic strategy to prevent joint inflammation and destruction in RA. We constructed an adenovirus vector carrying the csk gene, which negatively regulates Src family tyrosine kinases. Csk overexpression in cultured rheumatoid synoviocytes remarkably suppressed Src kinase activity and reduced their proliferation rate and IL-6 production. Bone-resorbing activity of osteoclasts was strongly inhibited by Csk overexpression. Furthermore, local injection of the virus into rat ankle joints with adjuvant arthritis not only ameliorated inflammation but suppressed bone destruction. In conclusion, adenovirus-mediated direct transfer of the csk gene is useful in repressing bone destruction and inflammatory reactions, suggesting the involvement of Src family tyrosine kinases in arthritic joint breakdown and demonstrating the feasibility of intervention in the kinases for gene therapy in RA. off
Figures
Similar articles
-
Suppression of arthritic bone destruction by adenovirus-mediated dominant-negative Ras gene transfer to synoviocytes and osteoclasts.Arthritis Rheum. 2003 Sep;48(9):2682-92. doi: 10.1002/art.11214. Arthritis Rheum. 2003. PMID: 13130489
-
In vitro and in vivo suppression of osteoclast function by adenovirus vector-induced csk gene.J Bone Miner Res. 2000 Jan;15(1):41-51. doi: 10.1359/jbmr.2000.15.1.41. J Bone Miner Res. 2000. PMID: 10646113
-
Retinoblastoma suppression of matrix metalloproteinase 1, but not interleukin-6, through a p38-dependent pathway in rheumatoid arthritis synovial fibroblasts.Arthritis Rheum. 2004 Jan;50(1):78-87. doi: 10.1002/art.11482. Arthritis Rheum. 2004. PMID: 14730602
-
The SCID mouse model: novel therapeutic targets - lessons from gene transfer.Springer Semin Immunopathol. 2003 Aug;25(1):65-78. doi: 10.1007/s00281-003-0126-2. Springer Semin Immunopathol. 2003. PMID: 12904892 Review.
-
The role of c-Src kinase in the regulation of osteoclast function.Mod Rheumatol. 2006;16(2):68-74. doi: 10.1007/s10165-006-0460-z. Mod Rheumatol. 2006. PMID: 16633924 Review.
Cited by
-
The inhibition of Src kinase suppresses the production of matrix metalloproteinases in from synovial fibroblasts and inhibits MAPK and STATs pathways.Turk J Med Sci. 2021 Aug 30;51(4):2142-2149. doi: 10.3906/sag-2008-274. Turk J Med Sci. 2021. PMID: 33714238 Free PMC article.
-
In vitro and in vivo assays for osteoclast apoptosis.Biol Proced Online. 2005;7:48-59. doi: 10.1251/bpo105. Epub 2005 May 9. Biol Proced Online. 2005. PMID: 16136224 Free PMC article.
-
Distinct roles of Smad pathways and p38 pathways in cartilage-specific gene expression in synovial fibroblasts.J Clin Invest. 2004 Mar;113(5):718-26. doi: 10.1172/JCI19899. J Clin Invest. 2004. PMID: 14991070 Free PMC article.
-
Effect of Wenhua Juanbi recipe on proliferation and apoptosis of synoviocytes in rats with collagen-inducing arthritis.Chin J Integr Med. 2013 Jun;19(6):453-8. doi: 10.1007/s11655-011-0753-8. Epub 2011 Jun 29. Chin J Integr Med. 2013. PMID: 21717160
-
Interaction between the immune system and bone metabolism: an emerging field of osteoimmunology.Proc Jpn Acad Ser B Phys Biol Sci. 2007 Jun;83(5):136-43. doi: 10.2183/pjab.83.136. Proc Jpn Acad Ser B Phys Biol Sci. 2007. PMID: 24019592 Free PMC article. Review.
References
-
- Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–1790. - PubMed
-
- Müller-Ladner U, Gay RE, Gay S. Cellular pathways of joint destruction. Curr Opin Rheumatol. 1997;9:213–220. - PubMed
-
- Fassbender HG. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983;3:141–155. - PubMed
-
- Hamilton JA. Hypothesis: in vitro evidence for the invasive and tumor-like properties of the rheumatoid pannus. J Rheumatol. 1983;10:845–851. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

