Abstract
Background
Remdesivir is an antiviral medicine approved for the treatment of mild‐to‐moderate coronavirus disease 2019 (COVID‐19). This led to widespread implementation, although the available evidence remains inconsistent. This update aims to fill current knowledge gaps by identifying, describing, evaluating, and synthesising all evidence from randomised controlled trials (RCTs) on the effects of remdesivir on clinical outcomes in COVID‐19.
Objectives
To assess the effects of remdesivir and standard care compared to standard care plus/minus placebo on clinical outcomes in patients treated for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection.
Search methods
We searched the Cochrane COVID‐19 Study Register (which comprises the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase, ClinicalTrials.gov, World Health Organization (WHO) International Clinical Trials Registry Platform, and medRxiv) as well as Web of Science (Science Citation Index Expanded and Emerging Sources Citation Index) and WHO COVID‐19 Global literature on coronavirus disease to identify completed and ongoing studies, without language restrictions. We conducted the searches on 31 May 2022.
Selection criteria
We followed standard Cochrane methodology.
We included RCTs evaluating remdesivir and standard care for the treatment of SARS‐CoV‐2 infection compared to standard care plus/minus placebo irrespective of disease severity, gender, ethnicity, or setting.
We excluded studies that evaluated remdesivir for the treatment of other coronavirus diseases.
Data collection and analysis
We followed standard Cochrane methodology.
To assess risk of bias in included studies, we used the Cochrane RoB 2 tool for RCTs. We rated the certainty of evidence using the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach for outcomes that were reported according to our prioritised categories: all‐cause mortality, in‐hospital mortality, clinical improvement (being alive and ready for discharge up to day 28) or worsening (new need for invasive mechanical ventilation or death up to day 28), quality of life, serious adverse events, and adverse events (any grade).
We differentiated between non‐hospitalised individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19 and hospitalised individuals with moderate to severe COVID‐19.
Main results
We included nine RCTs with 11,218 participants diagnosed with SARS‐CoV‐2 infection and a mean age of 53.6 years, of whom 5982 participants were randomised to receive remdesivir. Most participants required low‐flow oxygen at baseline. Studies were mainly conducted in high‐ and upper‐middle‐income countries. We identified two studies that are awaiting classification and five ongoing studies.
Effects of remdesivir in hospitalised individuals with moderate to severe COVID‐19
With moderate‐certainty evidence, remdesivir probably makes little or no difference to all‐cause mortality at up to day 28 (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.81 to 1.06; risk difference (RD) 8 fewer per 1000, 95% CI 21 fewer to 6 more; 4 studies, 7142 participants), day 60 (RR 0.85, 95% CI 0.69 to 1.05; RD 35 fewer per 1000, 95% CI 73 fewer to 12 more; 1 study, 1281 participants), or in‐hospital mortality at up to day 150 (RR 0.93, 95% CI 0.84 to 1.03; RD 11 fewer per 1000, 95% CI 25 fewer to 5 more; 1 study, 8275 participants).
Remdesivir probably increases the chance of clinical improvement at up to day 28 slightly (RR 1.11, 95% CI 1.06 to 1.17; RD 68 more per 1000, 95% CI 37 more to 105 more; 4 studies, 2514 participants; moderate‐certainty evidence). It probably decreases the risk of clinical worsening within 28 days (hazard ratio (HR) 0.67, 95% CI 0.54 to 0.82; RD 135 fewer per 1000, 95% CI 198 fewer to 69 fewer; 2 studies, 1734 participants, moderate‐certainty evidence).
Remdesivir may make little or no difference to the rate of adverse events of any grade (RR 1.04, 95% CI 0.92 to 1.18; RD 23 more per 1000, 95% CI 46 fewer to 104 more; 4 studies, 2498 participants; low‐certainty evidence), or serious adverse events (RR 0.84, 95% CI 0.65 to 1.07; RD 44 fewer per 1000, 95% CI 96 fewer to 19 more; 4 studies, 2498 participants; low‐certainty evidence).
We considered risk of bias to be low, with some concerns for mortality and clinical course. We had some concerns for safety outcomes because participants who had died did not contribute information. Without adjustment, this leads to an uncertain amount of missing values and the potential for bias due to missing data.
Effects of remdesivir in non‐hospitalised individuals with mild COVID‐19
One of the nine RCTs was conducted in the outpatient setting and included symptomatic people with a risk of progression. No deaths occurred within the 28 days observation period.
We are uncertain about clinical improvement due to very low‐certainty evidence. Remdesivir probably decreases the risk of clinical worsening (hospitalisation) at up to day 28 (RR 0.28, 95% CI 0.11 to 0.75; RD 46 fewer per 1000, 95% CI 57 fewer to 16 fewer; 562 participants; moderate‐certainty evidence). We did not find any data for quality of life.
Remdesivir may decrease the rate of serious adverse events at up to 28 days (RR 0.27, 95% CI 0.10 to 0.70; RD 49 fewer per 1000, 95% CI 60 fewer to 20 fewer; 562 participants; low‐certainty evidence), but it probably makes little or no difference to the risk of adverse events of any grade (RR 0.91, 95% CI 0.76 to 1.10; RD 42 fewer per 1000, 95% CI 111 fewer to 46 more; 562 participants; moderate‐certainty evidence).
We considered risk of bias to be low for mortality, clinical improvement, and safety outcomes. We identified a high risk of bias for clinical worsening.
Authors' conclusions
Based on the available evidence up to 31 May 2022, remdesivir probably has little or no effect on all‐cause mortality or in‐hospital mortality of individuals with moderate to severe COVID‐19. The hospitalisation rate was reduced with remdesivir in one study including participants with mild to moderate COVID‐19. It may be beneficial in the clinical course for both hospitalised and non‐hospitalised patients, but certainty remains limited. The applicability of the evidence to current practice may be limited by the recruitment of participants from mostly unvaccinated populations exposed to early variants of the SARS‐CoV‐2 virus at the time the studies were undertaken.
Future studies should provide additional data on the efficacy and safety of remdesivir for defined core outcomes in COVID‐19 research, especially for different population subgroups.
Plain language summary
Remdesivir to treat people with COVID‐19
Is remdesivir (an antiviral medicine) an effective treatment for COVID‐19?
Key messages
• For adults hospitalised with COVID‐19, remdesivir probably has little or no effect on deaths up to 150 days after treatment compared with placebo (sham treatment) or usual care.
• Remdesivir probably slightly raises the chance for hospitalised patients to improve and get discharged (leave the hospital or go home). It may also decrease the risk of becoming worse (invasive ventilation through a breathing tube or death).
• Usually patients who have mild symptoms and are not hospitalised are less likely to die. Remdesivir probably reduces the risk of getting worse and being hospitalised, but we cannot say if it affects recovery (e.g. relief in symptoms).
• Future studies should investigate the impact of remdesivir on the course of COVID‐19 in different subgroups (e.g. less or more severely ill people).
What is remdesivir?
Remdesivir is a medicine that fights viruses. It has been shown to prevent the virus that causes COVID‐19 (SARS‐CoV‐2) from reproducing. Medical regulators have approved remdesivir to treat people with COVID‐19. Common reported side effects are nausea, vomiting, and headaches, as well as changes in blood tests.
What did we want to find out?
We wanted to know if remdesivir is an effective treatment for people with COVID‐19 and if it causes unwanted effects compared to placebo or usual care. Its effect could depend on how advanced the illness is when treatment begins. We therefore distinguished between hospitalised patients with moderate to severe disease (e.g. having ventilation) and non‐hospitalised people who have tested positive for COVID‐19 but have no or mild symptoms.
We were interested in the following outcomes for hospitalised patients:
• deaths in the 28 days after treatment or after more than 28 days, if available;
• deaths that occurred during hospitalisation;
• whether patients got better after treatment and were ready to be discharged;
• whether patients’ condition worsened so that they needed mechanical ventilation through a breathing tube or died;
• any unwanted effects; and
• serious unwanted effects.
We were interested in the following outcomes for non‐hospitalised patients:
• deaths in the 28 days after treatment or after more than 28 days, if available;
• whether patients got better after treatment so that they were free of symptoms;
• whether patients’ condition worsened so that they needed to be hospitalised or that they died;
• quality of life;
• any unwanted effects; and
• serious unwanted effects.
What did we do?
We searched for studies that investigated remdesivir to treat adults with COVID‐19 compared to placebo or standard care. Patients could be of any gender or ethnicity.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found eight studies with 10,656 people hospitalised with moderate to severe COVID‐19 and one study with 562 people with mild COVID‐19. Of these, 5982 people were given remdesivir. No studies evaluated people without symptoms of COVID‐19. The average age of patients was 59 years.
Main results
The included studies compared remdesivir and usual care to usual care (plus/minus placebo) in people with COVID‐19.
Hospitalised people with moderate to severe COVID‐19
Remdesivir probably makes little or no difference to deaths after 28 days, after 60 days, or to deaths in hospital during 150 days. It probably raises the chance for patients to get better slightly, and it probably lowers the risk of getting worse. The rates of unwanted effects of any severity were similar between the compared groups.
Non‐hospitalised people with mild COVID‐19
In the study with outpatients no one died during the investigation (28 days). After treatment with remdesivir, people were less likely to get worse and be hospitalised. We do not know whether remdesivir leads to more or less chance for patients to improve. Patients may suffer fewer serious unwanted effects with remdesivir than with placebo or standard care. The rates of unwanted effects of any severity were similar between the compared groups.
What are the limitations of the evidence?
We are moderately confident in the evidence for deaths and course of disease in hospitalised people. Our confidence in the evidence of all other results in this group is limited because of differences between studies and a possible influence of their methods. For non‐hospitalised people with mild COVID‐19, we are moderately confident in the evidence for worsening of patients' condition and unwanted effects. Our confidence in the evidence of all other results is limited, especially for improvement of patients' condition, for methodological reasons (e.g. measurements were carried out inadequately or are not comparable, or both) and different results between studies. The studies were conducted at a time when vaccine programmes had not been started and the virus differed from subsequent strains. Most of the people in the studies also live in high‐ and middle‐income countries. This might limit the applicability of the findings to people who are vaccinated and in low‐income countries with less access to medical care.
How up‐to‐date is this evidence?
This is an update of the initial review and the evidence is current to 31 May 2022.
Summary of findings
Summary of findings 1. Remdesivir and standard care versus standard care (plus/minus placebo) for individuals with moderate to severe COVID‐19.
| Remdesivir and standard care versus standard care (plus/minus placebo) for individuals with moderate to severe COVID‐19 | ||||||
| Patient or population: hospitalised adults with moderate to severe COVID‐19 Settings: in‐hospital Intervention: remdesivir (10 days) Comparator: placebo or standard care alone | ||||||
| Outcomes | Anticipated absolute effects | Relative effect 95% CI | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Placebo or standard care alone | Remdesivir | |||||
| All‐cause mortality at up to day 281 | 108 per 1000 | 100 per 1000 (21 fewer to 6 more) | RR 0.93 (0.81 to 1.06) | 7142 (4 RCTs) | ⊕ ⊕ ⊕ ⊖
MODERATE a |
Remdesivir probably makes little or no difference to all‐cause mortality up to 28 days. |
| All‐cause mortality at up to day 60 | 235 per 1000 | 200 per 1000 (73 fewer to 12 more) | RR 0.85 (0.69 to 1.05) | 1281 (1 RCT) | ⊕ ⊕ ⊕ ⊖ MODERATE b | Remdesivir probably makes little or no difference to all‐cause mortality up to 60 days. |
| In‐hospital mortality at up to day 150 | 156 per 1000 | 145 per 1000 (25 fewer to 5 more) | RR 0.93 (0.84 to 1.03) | 8275 (1 RCT) | ⊕ ⊕ ⊕ ⊖ MODERATE c | Remdesivir probably makes little or no difference to in‐hospital mortality up to 150 days. |
| Clinical improvement: participants alive and ready to be discharged at up to day 282 | 617 per 1000 | 685 per 1000 (37 more to 105 more) | RR 1.11 (1.06 to 1.17) | 2514 (4 RCTs) | ⊕ ⊕ ⊕ ⊖ MODERATE d | Remdesivir probably increases the chance of clinical improvement slightly. |
| Clinical worsening: time to new need for invasive mechanical ventilation or death within 28 days3 | 544 per 1000 | 409 per 1000 (198 fewer to 69 fewer) | HR 0.67 (0.54 to 0.82) | 1734 (2 RCTs) | ⊕ ⊕ ⊕ ⊖ MODERATE d | Remdesivir probably decreases the risk of clinical worsening up to day 28. |
| Adverse events (any grade) at up to day 28 | 579 per 1000 | 602 per 1000 (46 fewer to 104 more) | RR 1.04 (0.92 to 1.18) | 2498 (4 RCTs) | ⊕ ⊕ ⊖ ⊖ LOW a,e | Remdesivir may make little or no difference to the risk of adverse events (any grade). |
| Serious adverse events at up to day 28 | 273 per 1000 | 229 per 1000 (96 fewer to 19 more) | RR 0.84 (0.65 to 1.07) | 2498 (4 RCTs) | ⊕ ⊕ ⊖ ⊖ LOW a,e | Remdesivir may make little or no difference to the risk of serious adverse events. |
| CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RD: risk difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1. Time to all‐cause mortality (time‐to‐event): HR 0.88, 95% CI 0.67 to 1.16; 2 studies, 6513 participants; I² = 57%.
2. Time to clinical improvement (time‐to‐event): alive and ready to discharge: HR 1.06, 95% CI 0.93 to 1.20; 2 studies, 1225 participants; I2 = 0%.
3. Clinical worsening: new need for invasive mechanical ventilation or death: RR 0.70, 95% CI 0.52 to 0.94; RD 76 fewer per 1000, 95% CI 121 fewer to 15 fewer; 1 study, 683 participants; I² = not applicable; low‐certainty evidence.
aDowngraded one level due to serious imprecision because of wide confidence intervals in the studies and/or the 95% confidence interval includes both benefits and harms. bDowngraded one level due to serious imprecision because optimal information size not reached. cDowngraded one level due to serious risk of bias because of selective reporting. dDowngraded one level due to serious risk of bias because of lack of blinding. eDowngraded one level due to serious risk of bias because of lack of blinding and one study was stopped earlier than scheduled.
Summary of findings 2. Remdesivir and standard care versus standard care (plus/minus placebo) for individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19.
| Remdesivir and standard care versus standard care (plus/minus placebo) for individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19 | ||||||
| Patient or population: non‐hospitalised adults with mild COVID‐19 Settings: outpatient Intervention: remdesivir Comparator: placebo or standard care alone | ||||||
| Outcomes | Anticipated absolute effects | Relative effect 95% CI | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Placebo or standard care alone | Risk with remdesivir | |||||
| All‐cause mortality at up to day 28 | — | — | Not estimable | 562 (1 RCT) | — | There were no events observed, thus it was not possible to determine whether remdesivir makes a difference to 28‐day mortality. |
| Clinical improvement: participants with symptom alleviation at up to day 14 | 250 per 1000 | 333 per 1000 (61 fewer to 289 more) | HR 1.41 (0.73 to 2.71) | 126 (1 RCT) | ⊕ ⊖ ⊖ ⊖ Very lowb,c |
We are uncertain whether remdesivir increases or decreases the chance of symptom alleviation by day 14. |
| Clinical worsening: participants admitted to hospital or deceased at up to day 28 | 64 per 1000 | 18 per 1000 (57 fewer to 16 fewer) |
RR 0.28 (0.11 to 0.75) | 562 (1 RCT) | ⊕ ⊕ ⊕ ⊖ Moderatec | Remdesivir probably decreases the rate of hospitalisation or death by day 28. |
| Quality of life | — | — | — | — | — | Not reported. |
| Serious adverse events at up to day 28 | 67 per 1000 | 18 per 1000 (60 fewer to 20 fewer) |
RR 0.27 (0.10 to 0.70) | 562 (1 RCT) | ⊕ ⊕ ⊖ ⊖ Low c,d | Remdesivir may decrease the rate of serious adverse events by day 28. |
| Adverse events (any grade) at up to day 28 | 463 per 1000 | 421 per 1000 (111 fewer to 46 more) |
RR 0.91 (0.76 to 1.10) | 562 (1 RCT) | ⊕ ⊕ ⊕ ⊖ Moderatec | Remdesivir probably makes little or no difference to the risk of adverse events (any grade). |
| CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to serious imprecision because there was only one study. bDowngraded two levels due to serious risk of bias and serious indirectness because of differences in pre‐defined outcome and measurement. cDowngraded one level due to serious imprecision because of wide confidence interval and optimal information size not reached. dDowngraded one level due to serious indirectness (due to huge overlap with COVID‐19 symptoms, already considered in hospitalisation or death).
Background
This work is part of a series of Cochrane Reviews investigating treatments and therapies for coronavirus disease 2019 (COVID‐19). Reviews of this series share information in the background section and methodology based on the first published reviews about monoclonal antibodies and convalescent plasma (Kreuzberger 2021; Piechotta 2021), as well as recently published or updated reviews on Janus‐kinase inhibitors or systemic corticosteroids (Griesel 2022; Kramer 2022). They are part of the German research project “CEOsys” (COVID‐19 Evidence‐Ecosystem; CEOsys 2021).
Description of the condition
COVID‐19 is a rapidly spreading infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). On 11 March 2020, the WHO declared the current COVID‐19 outbreak as a pandemic (WHO 2020a). COVID‐19 is unprecedented compared to previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS, Table 3), with 813 and 858 deaths, respectively (WHO 2003; WHO 2019). Despite intensive international efforts to contain its spread, SARS‐CoV‐2 has resulted in an ongoing increase of new weekly cases and deaths in several regions around the globe (WHO 2022a). In the meantime, the emergence of SARS‐CoV‐2 variants with the potential for altered transmission or disease characteristics, or to impact the effectiveness of vaccines, therapeutics, diagnostics, or public health and social measures, challenges strategies to control disease spread (WHO 2022b).
1. Glossary.
| Phrase/Word | Meaning/Description |
| Acute respiratory distress syndrome (ARDS) | ARDS is characterised by a massive response of the respiratory system to a wide variety of external and internal noxious stimuli. There is a disturbance of oxygen uptake and an acute onset. ARDS is the common end route of a wide variety of diseases leading to a severe systemic inflammatory response. The condition should be distinguished from disturbances of respiration caused by cardiac diseases. |
| Adverse event | An adverse event in the context of clinical trials is an unwanted medical occurrence in patients receiving a pharmacological or non‐pharmacological treatment, or both. An adverse event may not necessarily be considered to be related to the treatment. |
| Antimicrobials | Drugs used to treat diseases caused by micro‐organisms (bacteria, fungi, viruses, parasites). |
| Antiviral (medicine) | An agent that is directed against viruses |
| Bias | (Unconscious) distortion and misinterpretation of research results, especially those obtained experimentally. The most important sources for bias are as follows.
|
| Controlled non‐randomised study | A study in which the effects of a pharmacological or non‐pharmacological measure, or both, are compared between different groups of participants. The term 'controlled' means that the measure under investigation (intervention, verum) is compared with another measure (placebo or another intervention). The group of participants receiving the intervention under study is known as the intervention group. The group of participants who do not receive the intervention is known as the control group. A controlled non‐randomised study is easier to conduct than a randomised controlled trial, but has much less power (see bias). |
| Convalescent plasma | Blood plasma from patients who have had a disease (e.g. COVID‐19). Transfer of convalescent plasma to naive patients (patients who do not have antibodies themselves) leads to an increase in the immune defence of the receiving patient because convalescent plasma contains antibodies. |
| Corticosteroids | Hormones that are mainly produced in the adrenal cortex. Corticosteroids influence many biological processes in the organism, and are in particular closely linked to the immune system. Important naturally occurring representatives are cortisone and cortisol. Examples of synthetically produced corticosteroids are dexamethasone and budesonide. |
| Dichotomous | Dichotomy describes a system that can have exactly two mutually exclusive states. Example: either one has a certain disease (state A), or one does not have this disease (state B). The co‐occurrence of state A and state B is impossible. |
| Ebola | Ebola is a viral disease that is often severe. The Ebola virus belongs to the Filoviridae (from Latin 'filum' = filamentous). There are at least six different species of the virus. Ebola virus was previously called haemorrhagic fever because it is accompanied by high fever and severe internal and external bleeding. |
| Heterogeneous | Heterogeneity can be translated as 'non‐uniformity'. It is the opposite of homogeneity. In the context of meta‐analyses, heterogeneity is a measure of the comparability of clinical trials. For example, studies that examine different populations (e.g. children versus adults) have limited comparability and can lead to misleading conclusions when the data from such studies are pooled in a meta‐analysis. |
| Hydroxychloroquine | A drug related to chloroquine, which is used mainly for the treatment of rheumatoid arthritis, lupus erythematosus, and the prevention of malaria. |
| Immunocompromised status | Immunocompromised are people who have a congenital or acquired disorder of the immune response. Examples of acquired disorders include infection with HIV. Long‐term treatment with certain drugs (e.g. corticosteroids) can also lead to disorders/weakening of the immune response. |
| Interventions | The term 'intervention' in the context of clinical trials refers to the measure whose effect (superiority, inferiority, non‐inferiority) on a specific condition is to be assessed in comparison to other measures. An intervention need not always consist of the administration of a specific drug (so‐called non‐pharmacological interventions). |
| Mechanical ventilation | Mechanical ventilation is the term used to describe a procedure in which oxygen is supplied to the patient with the aid of ventilators or other devices. This measure is very restrictive and not without risk, and is therefore used only if the patient can no longer take in enough oxygen through his or her natural breathing (spontaneous respiration). In this review, the following procedures are subsumed under the term 'mechanical ventilation'.
|
| Middle East respiratory syndrome (MERS) | MERS is a respiratory disease caused by a coronavirus (MERS‐CoV). Most cases of the disease are asymptomatic. Diarrhoea is a common accompanying symptom. In severe cases, pneumonia develops. |
| Monoclonal antibody (MAB) | Antibodies in general are produced by the organism (specifically the immune system) when it is exposed to an antigen (for example, pathogenic micro‐organisms and viruses). By reacting with specific parts of the antigen, the antibody can render it harmless. So‐called monoclonal antibodies are produced by infecting mice with an antigen, for example. The immune system (especially the B cells) of the infected mouse then produces antibodies that are specifically active against the antigen. These cells accumulate in the spleen of the infected mouse. These cells are then isolated from the animal's spleen in a complicated process and multiplied in vitro (i.e. in the test tube). The resulting monoclonal antibodies are all derived from genetically identical cells and are directed against a specific antigen. Monoclonal antibodies are administered in medicine when the patient does not produce any antibodies or produces too few of his or her own. In addition, these specific antibodies also enable the identification of antigens in the detection of various diseases. |
| Nasal prongs | Nasal prongs, or nasal cannula, is a device used to deliver low‐flow oxygen to the nose through a small plastic tube. |
| Observational study | Data collection in a specific population under a specific research question. The essential characteristic of an observational study is that no intervention/experiment is carried out. |
| Placebo | A placebo is a dummy drug that does not contain a pharmacologically active substance. |
| Randomised controlled trial | A randomised controlled trial is the best way to obtain conclusions regarding the efficacy and effectiveness of a pharmacological or non‐pharmacological intervention, or both. The term 'controlled' means that the measure under investigation (intervention, verum) is compared with another measure (placebo or another intervention). The term 'randomised' means that the participants in the study are randomly assigned to one of two or more prespecified treatment groups. The group of participants receiving the intervention under study is known as the intervention group. The group of participants who do not receive the intervention is known as the control group. |
| Severe acute respiratory syndrome (SARS) | A disease caused by SARS‐CoV, which, similar to COVID‐19, results in fever and muscle pain in combination with other flu‐like signs. In severe cases, atypical pneumonia may occur. |
| Systematic review | Scientific process of critical judgement of the data available with regard to a specific question. A 'systematic' approach is taken. This includes:
A systematic review can include a meta‐analysis, but this is not required. The aim of a systematic review is to answer the defined research question, or, if this is not possible, to identify gaps in the scientific coverage of the research question. |
The risk for severe disease mainly depends on underlying medical conditions, in addition to the serological status of the infected person and the causative virus variant. In patients without effective immunisation, such as unvaccinated or incompletely vaccinated individuals, or individuals who fail to develop an immunological response despite being fully vaccinated, the risk for severe disease is higher among individuals aged 65 years or older, smokers, and those with certain underlying medical conditions such as cancer, chronic kidney disease, chronic obstructive pulmonary disease (COPD), heart conditions, immunocompromised state, obesity, sickle cell disease, or type 2 diabetes mellitus (Huang 2020; Liang 2020; Williamson 2020a). COVID‐19 case fatality ratios vary widely between countries and reporting periods, from 0.0% to more than 18% (Johns Hopkins 2022). However, these numbers may be misleading as they tend to overestimate the infection fatality ratio due to varying testing frequency, a lack of reporting dates, and variations in case definitions, especially in the beginning of the pandemic when the main focus was on severe cases (WHO 2020b).
The median incubation time and time to symptom onset depends on the virus variant and is estimated to be three days (range zero to eight days) in the case of an infection with the Omicron SARS‐CoV‐2 variant, which is shorter compared with previous reports for the Delta SARS‐CoV‐2 variant and other previously circulating non‐Delta SARS‐CoV‐2 variants (five to six days) (Brandal 2021; Lauer 2020). Sore throat, cough, fever, headache, fatigue, and myalgia or arthralgia are the most commonly reported symptoms (Brandal 2021; Struyf 2021). Other symptoms include dyspnoea, chills, nausea or vomiting, diarrhoea, and nasal congestion (CDC 2022). The reported frequency of asymptomatic infections varies greatly, depending on the time of the investigation, the cohort investigated, and the virus variant, and ranges between approximately 14% and 50% (Buitrago‐Garcia 2022).
A smaller proportion of infected individuals are affected by severe (approximately 11% to 20%) or critical (approximately 1% to 5%) disease with hospitalisation and intensive care unit (ICU) admittance due to respiratory failure, septic shock, or multiple organ dysfunction syndrome (Funk 2021; Lewnard 2022; Nyberg 2022; Wolter 2022; Wu 2020). In a case series from 12 New York hospitals, 14% of patients hospitalised due to COVID‐19 were treated in ICU (Richardson 2020). In an observational study of 10,021 hospitalised adult patients in Germany with a confirmed COVID‐19 diagnosis, 17% received mechanical ventilation (non‐invasive and invasive). Mortality in patients not receiving mechanical ventilation was 16%, and up to 53% in ventilated patients. Mortality in patients receiving mechanical ventilation (non‐invasive and invasive) and dialysis was 73% (Karagiannidis 2020). In one systematic review and meta‐analysis of international studies, the proportion of patients who died was estimated at 34% amongst those treated in ICU, and 83% amongst those receiving invasive mechanical ventilation (Potere 2020). However, the hospitalisation and ICU treatment rates seem to depend on the virus variant.
Analyses from the United Kingdom show a significant reduction in the relative risk of hospitalisation for adult Omicron cases compared to Delta (Nyberg 2022 ). There may also have been a different threshold for admission to hospital or ICU during the course of the pandemic. Depending on the local pressure on ICU resources, some normal wards will have learned to provide continuous positive airway pressure (CPAP) therapy equivalent to ICU support in other healthcare systems. It is unclear whether triage criteria in some healthcare systems may have influenced admission to hospital or ICU (or both).
As the evidence for the treatment options for COVID‐19 that were investigated in the course of the pandemic increased, national and international guidelines emerged to support daily clinical decisions (NICE guideline 2021; NIH guideline 2022; WHO living guideline 2022). However, so far there are only a few substances with clearly proven benefits and clear recommendations as well as approval by national and international authorities for the treatment of COVID‐19 (EMA 2022; FDA 2022a; WHO living guideline 2022). In light of the extent of the COVID‐19 pandemic and the scarcity of effective treatments, there is an urgent need for effective therapies to save lives and to reduce the high burden on healthcare systems (either with a high workload caused by COVID‐19 or staff shortages due to infected health care providers), especially in the face of evolving variants of the virus with the potential for increased transmissibility and the limited global availability of vaccines.
Description of the intervention
Remdesivir (GS‐5734) is an antiviral agent derived from a small‐molecule library and designed to target the replication of pathogenic ribonucleic acid (RNA) viruses (Siegel 2017). It showed a broad‐spectrum in vitro efficacy against various emerging viruses, such as Filoviridae (e.g. Ebolavirus and Marburgvirus), Pneumoviridae (respiratory syncytial virus), and Coronaviridae (MERS‐CoV, SARS‐CoV)) (Choy 2020; Sheahan 2017). Initially developed for the treatment of Ebola virus disease, studies on animals showed effective reduction in virus replication and clinical improvement for MERS as well as SARS infection (Sheahan 2020; Williamson 2020a; de Wit 2020).
During the course of the COVID‐19 pandemic, the antiviral agent was initially administered to hospitalised patients with COVID‐19 in a compassionate‐use attempt. The Adaptive COVID‐19 Treatment Trial (ACTT‐1) was one of the first multicentre RCTs to report a shortened time to recovery in hospitalised COVID‐19 patients compared to standard care (Beigel 2020). Shortly after its publication, the US Food and Drug Administration (FDA) released an Emergency Use Authorisation on 1 May 2020 (EUA 2021). Based on the recommendation of the European Medicines Agency, the European Union Commission followed in July 2020 with the authorisation of remdesivir as the first treatment option in patients at least 12 years of age with COVID‐19 pneumonia and the need for supplementary oxygen (EUA 2020). Later that year, the Committee for Medicinal Products for Human Use narrowed the indication to patients with low‐ or high‐flow oxygen or other non‐invasive ventilation (EMA 2020). Recently, the FDA expanded approval to paediatric patients of at least 28 days of age with a minimal weight of three kilograms who are hospitalised, or not hospitalised with mild‐to‐moderate COVID‐19 and a high risk for progression to severe COVID‐19, including hospitalisation or death (FDA 2022b). However, supporting data have not been published to date and the paediatric trial by the manufacturer, Gilead Science, is still ongoing (NCT04431453). The recommended regimen has been changed to an intravenous route of three days instead of 10. Proposed dosing is 200 mg intravenously (loading dose), followed by 100 mg for adults and 5 mg/kg followed by 2.5 mg/kg for children of 3.5 kg to less than 40 kg (EUA 2022). To date, the available data revealed good tolerability and safety of intravenous administration of remdesivir in healthy individuals. Reported common side effects include nausea, headache, rash, as well as transient increase in transaminases, prothrombin time, and blood glucose in laboratory findings (NIH guideline 2022).
Meanwhile, further RCTs have added to the evidence of the efficacy and safety of remdesivir application in adolescent and adult COVID‐19 patients. Amongst them were the interim results of the WHO Solidarity trial, which could not find a benefit for time to clinical improvement, need for mechanical ventilation, or mortality (WHO Solidarity Trial Consortium 2022). Based on a meta‐analysis of four RCTs, including the preprint data from the aforementioned trial, the WHO COVID‐19 treatment guideline recommended against the use of remdesivir in hospitalised patients in November 2020 (WHO 2022c). As the first RCT evaluating remdesivir usage in an outpatient setting, the PINETREE trial showed a reduction in hospitalisation in ambulatory patients with symptomatic COVID‐19 (Gottlieb 2021). This led the guideline development group to an update in April 2022, suggesting treatment with remdesivir for patients with non‐severe illness at highest risk of hospitalisation (WHO 2022c).
How the intervention might work
Remdesivir (GS‐5734) is a mono phosphoramidate nucleoside prodrug, which inhibits the synthesis of viral RNA. By competing with its natural analogue adenosine triphosphate, it blocks the RNA‐polymerase and leads to delayed chain termination, hence inhibiting the virus replication (Siegel 2017). The addition of the monophosphate prodrug improves the intracellular uptake, where phosphorylation turns it into its active metabolite (Lo 2017; McGuigan 2006).
In the early stage of a SARS‐CoV‐2‐associated pneumonia, the reduction of the viral load is postulated to prevent a systemic inflammatory reaction and, in particular, alveolar damage. The clinical presentation of COVID‐19 in the late pulmonary phase as well as in the hyper inflammatory phase is dominated by immunological processes, so that antiviral therapy strategies are no longer likely to be effective (Gautret 2020).
In summary, the broad‐spectrum nucleoside analogue remdesivir could be beneficial in the early stages of SARS‐CoV‐2‐infection by inhibiting virus replication. This hypothesis is supported by promising in vitro and animal experiments (Choy 2020; Wang 2020; Williamson 2020b), and could be the rationale for the current recommendation of early application to prevent disease progression. However, a new laboratory study shows that mutations in the viral polymerase can lead to partial resistance to remdesivir (Stevens 2022). This highlights the importance of targeted use in patients with the highest expected benefit as well as re‐evaluation of its effect in virus variants.
Why it is important to do this review
There is a clear and urgent need for more evidence‐based information to guide clinical decision‐making for COVID‐19 patients. Current standard care consists of supportive care with oxygen supply in moderately severe cases, and non‐invasive ventilation or invasive mechanical ventilation and extracorporeal membrane oxygenation (ECMO) in severe cases. Systematic corticosteroids were the first formulation to show a reduction in mortality as well as risk for disease progression and became recommended standard care, however solely for severe or critical COVID‐19 (Wagner 2021; WHO 2022c). To date, there have been applications for emergency use authorisation for several drugs. Few of them have been approved for the treatment of COVID‐19 in the European Union (such as monoclonal antibodies) and international guidelines on their clinical implementation are constantly updated (EMA 2022). Remdesivir remains the only fully FDA‐approved drug for usage in COVID‐19, in particular for early‐stage disease, with widespread implementation.
The first version of this review represents the difficulty in comparing available data due to inconsistent endpoint definitions (Ansems 2021). We included five RCTs with 7452 participants and concluded with moderate certainty that remdesivir probably has little or no effect on all‐cause mortality at up to 28 days in hospitalised adults with SARS‐CoV‐2 infection. However, when it comes to analysis of patient subgroups by disease severity at baseline, as well as reduction of symptom severity and disease progression, the evidence left us uncertain about its efficacy. The publication of further trials, assessing this lack of evidence, led to a change from recommendation against its application to conditional recommendation for certain patients. Additionally, the reduced susceptibility to monoclonal antibodies of the Omicron variant of concern calls for a re‐evaluation, considering that remdesivir is believed to remain active against variants in cell cultures (Vangeel 2022).
This first update of this systematic review aims to fill current gaps by identifying, describing, evaluating, and synthesising all evidence for remdesivir on clinical outcomes in COVID‐19. There is a need for a thorough understanding and an extensive review of the current body of evidence regarding the use of remdesivir for the treatment of COVID‐19. The primary goal of this update is to provide practising clinicians, healthcare providers, and interested laypersons with reliable and evidence‐based information that will lead to improvement in the treatment of COVID‐19.
Objectives
To assess the effects of remdesivir and standard care compared to standard care plus/minus placebo on clinical outcomes in patients treated for SARS‐CoV‐2 infection.
Methods
Criteria for considering studies for this review
Types of studies
The main description of methods is based on a template from the Cochrane Haematology working group in line with the series of Cochrane Reviews investigating treatments and therapies for COVID‐19. We made specific adaptations related to the research question where necessary. The protocol for this review was registered with PROSPERO on 26 February 2021 (CRD42021238065).
To assess the effects of remdesivir for treatment in COVID‐19, we included randomised controlled trials (RCTs), as this study design, if performed appropriately, provides the best evidence for experimental therapies in highly controlled therapeutic settings. We used the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). We had planned to also accept non‐standard RCT designs, such as cluster‐randomised trials (methods as recommended in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions) and cross‐over trials (Higgins 2022b). We would only have considered results from the first period for cross‐over trials, because COVID‐19 is not a chronic condition, and its exact course and long‐term effects have yet to be defined.
We excluded controlled non‐randomised studies of the intervention and observational studies. We also excluded animal studies, pharmacokinetic studies, and in vitro studies.
We included the following formats, if sufficient information was available on study design, characteristics of participants, interventions, and outcomes.
Full‐text publications
Preprint articles
Abstract publications
Results published in trials registries
Personal communication with investigators
We included preprints and conference abstracts to have a complete overview of the ongoing research activity, especially for tracking newly emerging studies about remdesivir in COVID‐19. We did not apply any limitation with respect to length of follow‐up.
Types of participants
We included adults with a confirmed diagnosis of SARS‐CoV‐2 infection (as described in the study) and did not exclude any studies based on gender, ethnicity, disease severity, or setting.
We excluded studies that evaluated remdesivir for the treatment of other coronavirus diseases such as SARS or MERS, or other viral diseases, such as Ebola. We planned that if studies enrolled populations with or who were exposed to mixed viral diseases, we would only include these if the trial authors provided subgroup data for SARS‐CoV‐2 infection.
Types of interventions
We included the following interventions, independent of dose, frequency, and duration:
Remdesivir and standard care for the treatment of SARS‐CoV‐2 infection.
We included the following control groups:
Standard care (plus/minus placebo).
Types of outcome measures
We evaluated core outcomes based on the Core Outcome Measures in Effectiveness Trials (COMET) initiative for people with COVID‐19 (COMET 2020), and additional outcomes that have been prioritised by consumer representatives and the German guideline panel for therapy of people with SARS‐CoV‐2 infection.
We defined outcome sets with primary and secondary outcomes for two populations:
hospitalised individuals with moderate to severe COVID‐19 (defined as participants with SARS‐CoV‐2 detection and need for inpatient medical care plus/minus need for respiratory support with low‐flow oxygen, high‐flow oxygen, non‐invasive mechanical ventilation, invasive mechanical ventilation (plus/minus ECMO) due to COVID‐19); and
non‐hospitalised individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19 (defined as participants with SARS‐CoV‐2 detection plus/minus symptoms of COVID‐19 without need for inpatient medical care or respiratory support).
Primary outcomes were used to inform the summary of findings tables.
Hospitalised individuals with moderate to severe COVID‐19
Primary outcomes
All‐cause mortality at day 28.
All‐cause mortality at day 60 and up to longest follow‐up.
In‐hospital mortality at up to longest follow‐up.
Clinical improvement: proportion of participants alive and ready to be discharged at up to day 28, up to longest follow‐up, and time‐to‐event. Participants should be discharged without clinical deterioration or death.
Clinical worsening: proportion of participants with new need for invasive mechanical ventilation or deceased within 28 days, up to longest follow‐up, and time‐to‐event.
Adverse events (any grade) during the study period, defined as number of participants with any event.
Serious adverse events during the study period, defined as number of participants with any event.
Secondary outcomes
All‐cause mortality, time‐to‐event.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHO Quality of Life 100‐question patient‐reported questionnaire (WHOQOL‐100)) at up to seven days, up to 28 days, and longest follow‐up available.
Adverse events grades 3 and 4.
Ventilator‐free days (defined as days alive and free from mechanical ventilation).
Non‐hospitalised individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
All‐cause mortality at day 28, up to longest follow‐up, and time‐to‐event.
Clinical improvement: proportion of participants with symptom resolution (all symptoms resolved) at up to day 14, day 28, up to longest follow‐up, and time‐to‐event.
Clinical worsening: proportion of participants admitted to the hospital or deceased within 14 days, 28 days, up to longest follow‐up, and time‐to‐event.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to seven days, up to 28 days, and longest follow‐up available.
Serious adverse events during the study period, defined as number of participants with any event.
Adverse events (any grade) during the study period, defined as number of participants with any event.
Timing of outcome measurement
In the case of time‐to‐event analysis (e.g. for time to discharge from hospital and time to mortality), we included the outcome measure based on the longest follow‐up time. We also collected information on outcomes from all other time points reported in the publications.
Search methods for identification of studies
Electronic searches
Our Information Specialist (MIM) conducted systematic searches in the following sources from the inception of each database to 31 May 2022 (date of last search for all databases), placing no restrictions on the language of publication.
-
Cochrane COVID‐19 Study Register (CCSR) (covid-19.cochrane.org/) comprising:
Cochrane Central Register of Controlled Trials (CENTRAL), monthly updates;
PubMed, weekly updates;
Embase.com, weekly updates;
ClinicalTrials.gov (www.clinicaltrials.gov), daily updates;
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (trialsearch.who.int), weekly updates;
medRxiv (www.medrxiv.org), weekly updates.
-
Web of Science Clarivate:
Science Citation Index Expanded (1945 to 31 May 2022);
Emerging Sources Citation Index (2015 to 31 May 2022).
WHO COVID‐19 Global literature on coronavirus disease (search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/).
We did not conduct separate searches of the databases as required by the MECIR standards (Higgins 2022a), since these databases are regularly searched in the production of the CCSR.
For detailed search strategies, see Appendix 1.
Searching other resources
We identified other potentially eligible studies or ancillary publications by searching the reference lists of included studies, systematic reviews, and meta‐analyses. In addition, we contacted investigators of the included studies to obtain additional information on the retrieved studies.
Data collection and analysis
Selection of studies
Four review authors (FG, KA, KD, VT) independently screened the results of the search strategies for eligibility by reading the titles and abstracts using Covidence software (Covidence 2021). We coded the abstracts as either 'include' or 'exclude'. In the case of disagreement, or if it was unclear whether the abstract should be retrieved, we obtained the full‐text publication for further discussion. Several review authors (FG, KA, KD, VT) assessed the full‐text articles of the selected studies. If two review authors were unable to reach a consensus, they consulted a third review author to reach a final decision.
As recommended in the PRISMA statement (Moher 2009), we documented the study selection process in a flow chart showing the total numbers of retrieved references and the numbers of included and excluded studies. We listed all studies excluded after full‐text assessment and the reasons for their exclusion in the Excluded studies section.
Data extraction and management
We conducted data extraction according to the guidelines proposed by Cochrane (Li 2020). Several review authors (FG, KA, KD, VT, AM, NS) extracted data independently and in duplicate, using a customised data extraction form developed in Microsoft Excel (Microsoft 2018). Any disagreements were resolved by discussion or by consulting a third review author if necessary.
Two out of several review authors (FG, KA, KD, AM, VT, MG, NS) independently assessed the included studies for methodological quality and risk of bias. If the review authors were unable to reach a consensus, a third review author was consulted.
We extracted the following information, where reported.
General information: author, title, source, publication date, country, language, duplicate publications.
Study characteristics: trial design, setting, and dates, source of participants, inclusion/exclusion criteria, comparability of groups, treatment cross‐overs, compliance with assigned treatment, length of follow‐up.
Participant characteristics: age, gender, ethnicity, number of participants recruited/allocated/evaluated, additional diagnoses, severity of disease, previous treatments, concurrent treatments, comorbidities (e.g. diabetes, respiratory disease, hypertension, immunosuppression, obesity, heart failure).
Interventions: dosage, frequency, timing, duration and route of administration, setting, duration of follow‐up.
Control interventions (placebo or standard care alone): dosage, frequency, timing, duration and route of administration, setting, duration of follow‐up.
Outcomes: as specified in Types of outcome measures section.
Risk of bias assessment: randomisation process, deviations from the intended interventions, missing outcome data, measurement of the outcome, selection of the reported result.
Assessment of risk of bias in included studies
We used the RoB 2 tool (beta version 7) to analyse the risk of bias of the included studies (Sterne 2019). Of interest in this review was the effect of the assignment to the intervention (the intention‐to‐treat effect), thus we performed all assessments with RoB 2 on this effect. The outcomes that we assessed are those specified for inclusion as described in the Methods section.
Two out of several review authors (FG, KA, KD, AM, VT, MG, NS) independently assessed the risk of bias for each outcome using the RoB 2 Excel tool to manage and record assessments. In case of discrepancies amongst judgements and inability to reach consensus, a third review author was consulted reach a final decision. We assessed the following types of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022c).
Bias arising from the randomisation process
Bias due to deviations from the intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
For cluster‐RCTs, we had planned to add a domain to assess bias arising from the timing of identification and recruitment of participants in relation to timing of randomisation, as recommended in the archived RoB 2 guidance for cluster‐randomised trials and in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Eldridge 2016; Higgins 2022b).
To address these types of bias, we used the signalling questions recommended in RoB 2 and made a judgement according to the following options.
'Yes': if there is firm evidence that the question is fulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'Probably yes': a judgement has been made that the question is fulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'No': if there is firm evidence that the question is unfulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'Probably no': a judgement has been made that the question is unfulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'No information': if the study report does not provide sufficient information to permit a judgement.
We used the algorithms proposed by RoB 2 to assign each domain one of the following levels of bias.
Low risk of bias
Some concerns
High risk of bias
We subsequently derived an overall risk of bias rating for each prespecified outcome in each study in accordance with the following suggestions.
'Low risk of bias': we judge the trial to be at low risk of bias for all domains for the result.
'Some concerns': we judge the trial to raise some concerns in at least one domain for the result, but not to be at high risk of bias for any domain.
'High risk of bias': we judge the trial to be at high risk of bias in at least one domain for the result, or we judge the trial to have some concerns for multiple domains in a way that substantially lowers our confidence in the results.
We used the RoB 2 Excel tool to implement RoB 2 (beta version 7, available from riskofbias.info), and stored and presented our detailed RoB 2 assessments in the analyses section and as supplementary online material.
For domain three of the tool ('bias due to missing outcome data'), we considered death as a competing risk factor, especially for dichotomous clinical progression outcomes. We judged improvement to be at high risk of bias due to missing data because it is likely that death during follow‐up impeded liberation from respiratory support, and hence missing data on improvement depends on its true value.
Measures of treatment effect
For continuous outcomes, we recorded the mean, standard deviation, and total number of participants in both the treatment and control groups. Where continuous outcomes used the same scale, we performed analyses using the mean difference (MD) with 95% confidence intervals (CIs). For continuous outcomes measured with different scales, we performed analyses using the standardised mean difference (SMD). In our interpretation of SMDs, we re‐expressed SMDs in the original units of a particular scale with the most clinical relevance and impact (e.g. clinical symptoms with the WHO Clinical Progression Scale) (WHO 2020c).
For dichotomous outcomes, we recorded the number of events and the total number of participants in both the treatment and control groups. We reported the pooled risk ratio (RR) with its associated 95% CI, and risk difference (RD) with its associated 95% CI (Deeks 2020).
If sufficient information was available, we extracted and reported hazard ratios (HRs) for time‐to‐event outcomes (e.g. time to mortality). If HRs were not available, we made every effort to estimate the HR as accurately as possible from available data using the methods proposed by Parmar and Tierney (Parmar 1998; Tierney 2007). If a sufficient number of studies provided HRs, we used HRs rather than RRs or MDs in a meta‐analysis, as they provide more information.
Unit of analysis issues
The aim of this review was to summarise trials that analyse data at the level of the individual. We would also have accepted cluster‐randomised trials for inclusion had any been identified. We collated multiple reports of a given study so that each study, rather than each report, was the unit of analysis.
Studies with multiple treatment groups
As recommended in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022d), for studies with multiple treatment groups of the same intervention (i.e. dose, route of administration), we planned to evaluate if study arms were sufficiently homogeneous to be combined. We planned that if study arms could not be pooled, we would compare each arm with the common comparator separately. For pair‐wise meta‐analysis, we planned to split the ‘shared’ group into two or more groups with a smaller sample size, and include two or more (reasonably independent) comparisons. For this purpose, both the number of events and the total number of participants would have been divided for dichotomous outcomes, and the total number of participants would have been divided with unchanged means and standard deviations for continuous outcomes.
One study included in the review had multiple treatment arms of the same intervention (5‐day course of remdesivir versus 10‐day course of remdesivir) (Spinner 2020). Given the small number of participants in this study, we did not perform meta‐analysis, but have reported the results for each treatment arm narratively in our subgroup analysis (see Effects of interventions, Duration of remdesivir application).
Dealing with missing data
In Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions, a number of potential sources for missing data are suggested, which we took into account: at study level, at outcome level, and at summary data level (Deeks 2020). At all levels, it is important to differentiate between data 'missing at random', which may often be unbiased, and 'not missing at random', which may bias the study and in turn the review results.
In the case of missing data, we requested this information from the principal investigators; details are provided in the Included studies section. Beigel 2020 and Spinner 2020 provided additional data on all‐cause mortality at up to day 28 for subgroups of respiratory support, and Spinner 2020 provided data on clinical course.
If, after this, data were still missing, we consulted with content experts to judge whether data were missing at random (e.g. if missing outcomes were balanced across study arms, reasons for loss to follow‐up were common and reasonable). If we judged data to be missing at random, we performed a complete case analysis. When we judged data to be not missing at random, and we identified no supporting evidence that the results were not biased by missing outcome data, we did not make any assumptions about the missing outcome data. We had planned to conduct sensitivity analyses to assess the impact of missing data on the overall effect (excluding studies with more than 10% missing outcome data), however none of the included studies had more than 10% of missing outcome data. In future updates, we will discuss the potential impact of missing data on results.
Assessment of heterogeneity
We assessed heterogeneity of treatment effects between trials using a Chi² test with a significance level of P < 0.1. We used the I² statistic, Higgins 2003, and visual examination of the forest plot, to assess possible heterogeneity (I² > 30% to signify moderate heterogeneity, I² > 75% to signify considerable heterogeneity) (Deeks 2020). We planned that if the I2 was above 80%, we would explore possible causes of heterogeneity through sensitivity analyses. If we could not find a reason for heterogeneity, we would not perform a meta‐analysis, but instead would comment on the results from all studies and present these in tables.
Assessment of reporting biases
As mentioned above, we searched the trials registries to identify completed trials that have not been published elsewhere, to minimise publication bias or determine publication bias. We intended to explore potential publication bias by generating a funnel plot and statistically testing this by conducting a linear regression test for meta‐analyses involving at least 10 trials (Sterne 2019). We would consider P < 0.1 as significant for this test.
Data synthesis
If the clinical and methodological characteristics of individual studies were sufficiently homogeneous, we pooled the data in meta‐analysis. We performed analyses according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020). We planned to treat placebo and no treatment as the same intervention, as well as standard care at different institutions and time points.
We used the Review Manager Web (RevMan Web) software for analyses (RevMan Web 2021). One review author entered the data into the software, and a second review author checked the data for accuracy. We used the random‐effects model for all analyses, as we anticipated that true effects would be related but not the same for included studies. We planned that if meta‐analysis was not possible, we would comment on the results narratively with the results from all studies, and present these in tables. If meta‐analysis was possible, we would assess the effects of potential biases in sensitivity analyses (see Sensitivity analysis). For binary outcomes, we based the estimation of the between‐study variance using the Mantel‐Haenszel method. We used the inverse‐variance method for continuous outcomes, outcomes that included data from cluster‐RCTs, or outcomes where HRs were available.
Subgroup analysis and investigation of heterogeneity
In the first version of this review we conducted subgroup analyses for all‐cause mortality at up to day 28 exclusively. Additional analyses were performed where longer follow‐up data on mortality were available.
In the case of sufficient data, we performed subgroup analyses of the following characteristics for remdesivir and standard care versus standard care plus/minus placebo.
Age of participants (divided into applicable age groups, e.g. 18 to 65 years, 65 to 79 years, 80 years and older).
Pre‐existing conditions (e.g. diabetes, respiratory disease, hypertension, immunosuppression, obesity, cardiac injury).
Timing of first dose administration with illness onset.
-
Severity of condition, based on respiratory support at baseline:
No oxygen versus low‐flow oxygen versus low‐flow or high‐flow oxygen versus mechanical ventilation (including high‐flow oxygen, non‐invasive ventilation, invasive mechanical ventilation, and extracorporeal membrane oxygenation).
-
Duration of remdesivir application:
5‐day course of remdesivir versus 10‐day course of remdesivir.
We used the tests for interaction to test for differences between subgroup results.
Sensitivity analysis
We performed sensitivity analysis of the following study characteristics for our prioritised outcomes, as described in the Types of outcome measures section.
Risk of bias assessment components (studies with a low risk of bias or some concerns versus studies with a high risk of bias).
Comparison of preprints versus peer‐reviewed articles.
Comparison of premature termination of studies with completed studies.
Summary of findings and assessment of the certainty of the evidence
We created Table 1 and Table 2 and evaluated the certainty of the evidence using the GRADE approach for interventions evaluated in RCTs.
Summary of findings
We used MAGICapp software to create summary of findings tables (MAGICapp). For time‐to‐event outcomes, we calculated absolute effects at specific time points, as recommended in the GRADE guidance 27 (Skoetz 2020).
Chapter 14 of the updated Cochrane Handbook for Systematic Reviews of Interventions specifies that the “most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes” should be included in the summary of findings table(s) (Schünemann 2021). We included our primary outcomes prioritised according to the Core Outcome Set for intervention studies, COMET 2020, and patient relevance; these are listed below.
Hospitalised individuals with moderate to severe COVID‐19
All‐cause mortality: all‐cause mortality at up to day 28 and longest follow‐up available.
In‐hospital mortality: in‐hospital mortality at up to longest follow‐up available.
Clinical improvement: proportion of participants alive and ready to be discharged at up to day 28, up to longest follow‐up, and time‐to‐event. Participants should be discharged without clinical deterioration or death.
Clinical worsening: proportion of participants with new need for invasive mechanical ventilation or deceased within 28 days, up to longest follow‐up, and time‐to‐event.
Adverse events (any grade).
Serious adverse events.
Non‐hospitalised individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
All‐cause mortality: all‐cause mortality at up to day 28 and longest follow‐up available.
Clinical improvement: proportion of participants with symptom resolution (all symptoms resolved) at up to day 14, day 28, up to longest follow‐up, and time‐to‐event.
Clinical worsening: proportion of participants admitted to the hospital or deceased within 14 days, 28 days, up to longest follow‐up, and time‐to‐event.
Quality of life: quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to seven days, up to 28 days, and longest follow‐up available.
Adverse events (any grade).
Serious adverse events.
Assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence for the outcomes listed above.
The GRADE approach uses five domains (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each prioritised outcome.
We downgraded the certainty of the evidence for:
serious (−1) or very serious (−2) risk of bias;
serious (−1) or very serious (−2) inconsistency;
serious (−1) or very serious (−2) uncertainty about directness;
serious (−1) or very serious (−2) imprecise or sparse data;
serious (−1) or very serious (−2) probability of reporting bias.
The GRADE system uses the following criteria for assigning grades of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
We followed the current GRADE guidance for these assessments in its entirety as recommended in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021).
We used the overall risk of bias judgement, derived from the RoB 2 Excel tool, to inform our decision on downgrading the certainty of the evidence for risk of bias. We phrased the findings and certainty of the evidence as suggested in the informative statement guidance (Santesso 2020).
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
In the primary review (Ansems 2021) we included 42 records (five studies: Beigel 2020; Mahajan 2021; Spinner 2020; Wang 2020; WHO Solidarity Trial Consortium 2022) in our narrative analysis and 41 records (four studies: Beigel 2020; Spinner 2020; Wang 2020; WHO Solidarity Trial Consortium 2022) in our meta‐analyses. Two studies were listed as ongoing (NCT04252664; NCT04596839).
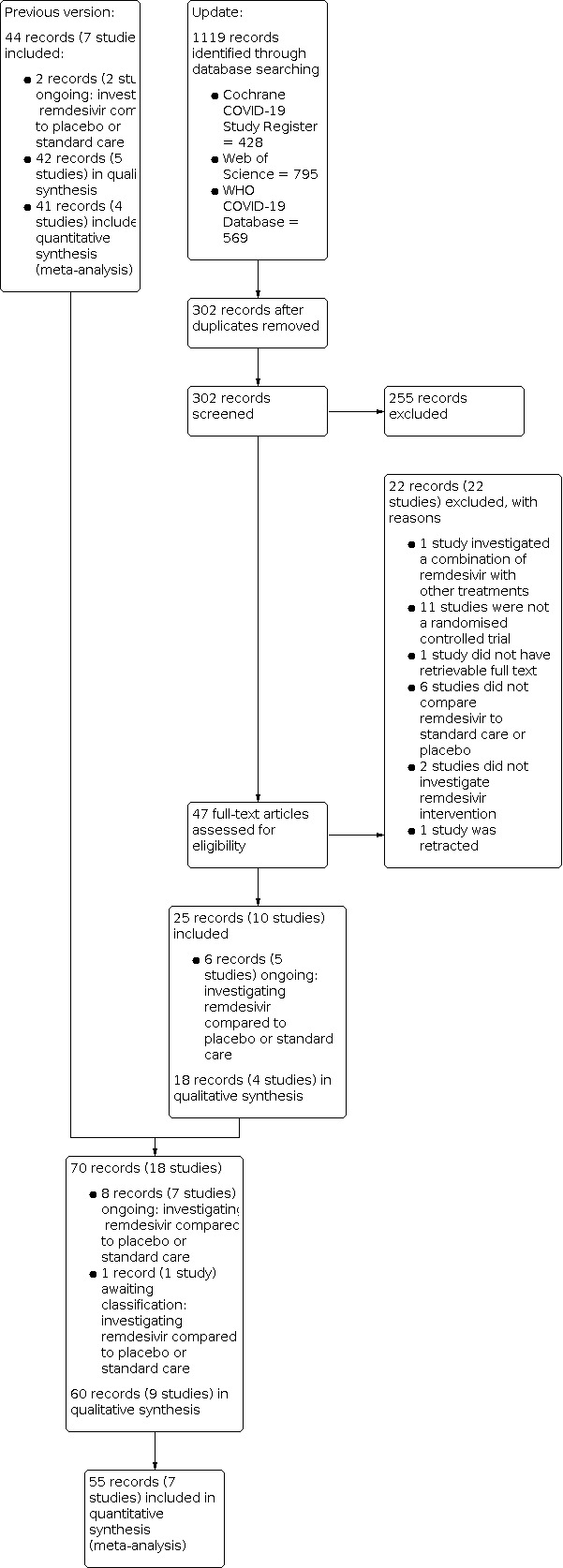
We performed update searches on 31 May 2022 and identified 1119 records. After removing duplicates, we screened 302 records based on title and abstract, of which 255 studies did not meet the prespecified inclusion criteria and were excluded. We screened the full texts or, if these were not available, the trial register entries, of the remaining 47 references. Reasons for exclusion at full‐text stage are listed in Characteristics of excluded studies. One ongoing study moved to awaiting classification because, although it was registered as completed, there are no data available yet (NCT04596839).
We identified six additional ongoing records (five studies: IRCT20210709051824N1; NCT04351724; NCT04843761; NCT04978259; REDPINE 2022; Characteristics of ongoing studies; Table 4). Overall, we included 60 records (nine studies) in our narrative analyses and 55 records (seven studies) in our meta‐analyses. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1).
2. Characteristics of ongoing studies.
| Study ID | Comparison | Aimed enrolment (n) | Expected completion date |
| NCT04252664 | Remdesivir compared to placebo | 308 | Recruiting suspended, no publication available yet |
| NCT04351724 | Remdesivir compared to standard care | NR (remdesivir arm); 500 (all study arms) |
Recruiting |
| NCT04978259 | Remdesivir compared to standard care | 202 | Recruiting |
| NCT04843761 | Remdesivir compared to placebo | 640 | Active, not recruiting |
NR = not reported
1.
Included studies
We included nine RCTs with 11,218 participants with symptomatic SARS‐CoV‐2 infection (Beigel 2020; Gottlieb 2021; Mahajan 2021; Spinner 2020; Wang 2020; WHO Solidarity Canada 2022; WHO Solidarity France 2021; WHO Solidarity Norway 2021; WHO Solidarity Trial Consortium 2022). Three studies were national add‐on trials to the WHO Solidarity Trial Consortium 2022, of which two recruited additional participants, not reported in the WHO Solidarity trial (WHO Solidarity Canada 2022; WHO Solidarity France 2021). In our meta‐analyses we only included participants of the WHO Solidarity Trial Consortium 2022, and its add‐on trials, if there was no overlap between participants.
One study was performed in an outpatient setting, including participants with mild SARS‐CoV‐2 infection (Gottlieb 2021). The other eight studies included hospitalised patients with COVID‐19. The included participants in the outpatient setting (mean age 50 years, 52.10% male), as well as the included participants in the hospitalised setting (mean age 60.9 years, 65.0% male) presented with symptomatic SARS‐CoV‐2 infection and were randomly assigned to receive either remdesivir or placebo in addition to local standard care. The majority of included studies were conducted in high‐ and upper‐middle‐income countries; the only reported lower‐middle‐income countries were Egypt, Honduras, India, and the Philippines. A detailed overview of the characteristics of included studies is provided in Characteristics of included studies and Table 5.
3. Overview of included studies.
| NA | Beigel 2020a | Spinner 2020 | Wang 2020 | WHO Solidarity Trial Consortium 2022 | Mahajan 2021 | Gottlieb 2021 | WHO Solidarity Canada 2022 | WHO Solidarity France 2021 | WHO Solidarity Norway 2021 |
| (By date of publication) | |||||||||
| Setting |
|
|
|
|
|
|
|
|
|
| Design |
|
|
|
|
|
|
|
|
|
| Study protocol | Reported | Reported | Reported | Reported | Not reported | Reported | Reported (WHO Trial Consortium) | Reported (WHO Trial Consortium) | Reported (WHO Trial Consortium) |
| Statistical analysis plan | Reported | Reported | Reported | Reported | Not reported | Reported | Reported (WHO Trial Consortium) | Reported (WHO Trial Consortium) | Reported (WHO Trial Consortium) |
|
Intervention (remdesivir) (duration of application (days)) |
10 | 5 or 10 | 10 | 10 | 5 | 3 | 10 | 10 | 10 |
| Control | SoC | Placebo + SoC | Placebo + SoC | SoC | SoC | SoC | SoC | SoC | SoC |
| Allocated participants (n) | 1062 | 596 | 236 | 8320 | 82 | 584 | 1282 | 857 | 101 |
|
Number of participants per trial arm (allocated/evaluated) |
Intervention: 541/541 Placebo + SoC: 521/521 |
5‐day intervention: 199/191 10‐day intervention: 197/193 SoC: 200/200 |
Intervention: 158/158 Placebo + SoC: 78/78 |
Intervention: 4169/4146 SoC: 4151/4129 |
Intervention: 41/34 SoC: 41/36 |
Intervention: 292/279 Placebo: 292/283 |
Intervention: 634/634 SoC: 648/647 Participants enrolled separate from WHO Solidarity Trial : Intervention: 170 SoC: 153 |
Intervention: 429/414 SoC: 428/418 Participants enrolled separate from WHO Solidarity Trial: Intervention: 210 SoC: 207 |
Intervention: 43/42 SoC: 58/57 No participants enrolled separate from WHO Solidarity Trial |
| Severity of condition according to the level of respiratory support at baseline (n/N (%)) | |||||||||
| Ambulatory, symptomatic disease | NA | NA | NA | NA | NA | Intervention: 279 (100) Placebo: 283 (100) |
NA | NA | NA |
| Hospitalised, without oxygen support | Intervention: 75 (13.9) Placebo: 63 (12.1) |
Not requiring ongoing medical care 5‐day intervention: 0 (0.0) 10‐day intervention: 6 (3.2) SoC: 2 (1.0) Requiring ongoing medical care 5‐day intervention: 160 (83.8) 10‐day intervention: 163 (84.5) SoC: 160 (80.0) |
Intervention: 0 (0.0) Placebo + SoC: 3 (3.8) |
Intervention: 869 (21) SoC: 861 (20.9) |
NA | NA | Intervention: 71/634 (11.2) SoC: 54/647 (8.4) |
Intervention: 6/414 (1.4) SoC: 6/418 (1.4) |
NA |
| Low‐flow supplemental oxygen | Intervention: 232 (42.9) Placebo: 203 (39.0) |
5‐day intervention: 29 (15.2) 10‐day intervention: 23 SoC: 36 (18.0) |
Intervention: 129 (81.6) Placebo + SoC: 65 (83.3) |
Low‐flow and high‐flow oxygen Intervention: 2918 (70.4) SoC: 2921 (70.7) |
Intervention: 27 (79.4) SoC: 26 (72.2) |
NA | Intervention: 334/634 (52.7) SoC: 363/647 (56.2) |
Intervention: 247/414 (59.6) SoC: 245/418 (58.6) |
NA |
| High‐flow oxygen or non‐invasive mechanical ventilation | Intervention: 95 (17.6) Placebo: 98 (18.8) |
5‐day intervention: 2 (1.0) 10‐day intervention: 1 (0.5) SoC: 2 (1.0) |
Intervention: 28 (17.2) Placebo + SoC: 9 (11.5) |
NA | Intervention: 7 (20.6) SoC: 10 (27.8) |
NA | Intervention: 171/634 (27.0) SoC: 176/647 (27.3) |
Intervention: 86/414 (20.7) SoC: 93/418 (22.2) |
NA |
| Invasive mechanical ventilation | Intervention: 131 (24.2) Placebo: 154 (29.6) |
NA | Intervention: 0 (0) Placebo + SoC: 1 (1.3) |
Non‐invasive and invasive mechanical ventilation Intervention: 359 (8.7) SoC: 347 (8.4) |
NA | NA | Intervention: 58/634 (9.1) SoC: 54/647 (8.3) |
Intervention: 75/414 (18.1) SoC: 74/418 (17.7) |
NA |
| Demographics | |||||||||
| Age (years) |
Mean (SD) Intervention: 58.6 (14.6) Placebo: 59.2 (15.4) |
Median (IQR) 5‐day intervention: 58 (48 to 66) 10‐day intervention: 56 (45 to 66) SoC: 57 (45 to 66) |
Median (IQR) Intervention: 66 (57 to 73) Placebo: 64 (53 to 70) |
n/Total < 50 Intervention: 1310 (31.6) SoC: 1326 (32.1) 50 to 69 Intervention: 1920 (46.3) SoC: 1908 (46.2) ≧ 70 Intervention: 916 (22.1) SoC: 895 (21.7) |
Mean (SD) Intervention: 58.09 (12.1) SoC: 57.41 (14.1) |
Mean (SD) Intervention: 50 (15) Placebo: 51 (15) |
Median (IQR) Intervention: 65 (53 to 77) SoC: 66 (54 to 74) |
Median (IQR) Intervention: 63 (55 to 73) SoC: 64 (54 to 72) |
Mean (SD) Intervention: 59.7 (± 16.5) SoC: 58.1 (15.7) |
| Gender (male (n(%))) | Intervention: 352 (65.1) Placebo: 332 (63.7) |
5‐day intervention: 114 (59.7) 10‐day intervention: 118 (61.1) SoC: 125 (62.5) |
Intervention: 89 (56.3) Placebo: 51 (65.4) |
Intervention: 2601 (62.7) SoC: 2639 (63.9) |
Intervention: 21 (61.8) SoC: 27 (75.0) |
Intervention: 148 (53) Placebo: 145 (51.2) |
Intervention: 374/634 (59) SoC: 392/647 (60.6) |
Intervention: 291/414 (70.3) SoC: 288/418 (68.9) |
Intervention: 29(69.0) SoC: 43(75.4) |
| Participants with a PCR confirmed SARS‐CoV‐2 infection | NA | NA | NA | NA | NA | Intervention: 215 of 279 patients (77.1%) Placebo: 213 of 283 patients (75.3%) |
NA | NA | NA |
| Comorbidities at baseline (n (%)) | |||||||||
| Diabetes | Intervention: 164 (30.8) Placebo: 158 (30.4) |
5‐day intervention: 71 (37) 10‐day intervention: 85 (44) SoC: 76/200 (38) |
Intervention: 40 (25) Placebo: 16 (21) |
Intervention: 1129 (27.2) SoC: 1120 (27.1) |
Intervention: 21 (62) SoC: 21 (58) |
Intervention: 173 (62) Placebo: 173 (61.1) |
Intervention: 155/634 (33.6) SoC: 188 /647 (38.4) |
Intervention: 104/414 (26) SoC: 113/418 (27) |
Intervention: 9(22) SoC: 9 (15.8) |
| Hypertension | Intervention: 269 (50.6) Placebo: 264 (50.9) |
5‐day intervention: 82 (43) 10‐day intervention: 85 (44) SoC: 81 (41) |
Intervention: 73 (46) Placebo: 30 (38) |
Not reported | Intervention: 15 (44) SoC: 17 (47) |
Intervention: 138 (49.5) Placebo: 130 (45.9) |
Not reported | Not reported | Intervention: 15 (36.6) SoC: 14 (24.6) |
| Cardiovascular or cerebrovascular disease | Not reported | 5‐day intervention: 111 (58) 10‐day intervention: 111 (58) SoC: 107 (54) |
Intervention: 15 (9) Placebo: 2 (3) |
Heart disease Intervention: 929 (22.4) SoC: 935 (22.6) |
Intervention: 4 (12) SoC: 5 (14) |
Intervention: 20 (7.2) Placebo: 24 (8.5) |
Intervention: 120/634 (26) SoC: 135/647 (27.6) |
Intervention: 111/414 (27) SoC: 118/418 (28) |
Chronic cardiac disease Intervention: 6 (14.6) SoC: 12 (21.1) |
| Chronic lung disease | Not reported | Not reported | Not reported | Intervention: 284 (6.9) SoC: 281 (6.8) |
Not reported | Intervention: 67 (24) Placebo: 68 (24) |
Intervention: 67/634 (14.5) SoC: 65/647 (13.3) |
Intervention: 71/414 (17) SoC: 75/418 (18) |
Intervention: 4 (9.8) SoC: 3 (5.3) |
| Asthma | Not reported | 5‐day intervention: 22 (12) 10‐day intervention: 31 (16) SoC: 28 (14) |
Not reported | Intervention: 247 (6) SoC: 242 (5.9) |
Intervention: 1 (3) SoC: 0 (0) |
Not reported | Intervention: 49/634 (10.6) SoC: 55/647 (11.2) |
Not reported | Not reported |
| Obesity | Intervention: 242 (46) Placebo: 234 (45) |
BMI (median (IQR)) 5‐day intervention: 27 (24 to 30) 10‐day intervention: 28 (25 to 32) SoC: 27 (24 to 31) |
Not reported | Not reported | Not reported | Intervention: 154 (55.2) Placebo: 156 (55.1) |
Not reported | Intervention: 138/414 (34) SoC: 140/418 (34) |
Intervention: 11 (28.9) SoC: 9 (18.4) |
| CLD | Not reported | Not reported | Not reported | Intervention: 57 (1.4) SoC: 72 (1.7) |
Not reported | Intervention: 1 (0.4) Placebo: 1 (0.4) |
Intervention: 8/634 (1.7) SoC: 19/647 (3.9) |
Intervention: 15/414 (4) SoC: 15/418 (4) |
Not reported |
| CKD | Not reported | Not reported | Not reported | Not reported | Intervention: 2 (6) SoC: 1 (3) |
Intervention: 7 (2.5) Placebo: 11 (3.9) |
Not reported | Intervention: 19/414 (5) SoC: 32/418 (8) |
Not reported |
| Other | Not reported | Not reported | Not reported | Not reported |
Hyperlipidaemia Intervention: 4 (12) SoC: 3 (8) Hypothyroidism Intervention: 4 (12) SoC: 3 (8) |
Current cancer Intervention: 12 (4.3) Placebo: 18 (6.4) Immune compromise Intervention: 14 (5) Placebo: 9 (3.2) |
HIV positive Intervention: 1/634 (0.2) SoC: 1/647 (0.2) |
Auto‐inflammatory disease Intervention: 17/414 (4) SoC: 24/418 (6) Malignant haemopathy Intervention: 16/414 (4) SoC: 19/418 (5) Chronic neurological disorder including dementia Intervention: 18/414 (4) SoC: 16/418 (4) Active malignant neoplasm Intervention: 13/414 (3) SoC: 15/418 (4) Transplantation Intervention: 2/414 (< 1) SoC: 9/418 (2) Asplenia Intervention: 1/414 (< 1) SoC: 3 /418(1) AIDS/HIV not on ART Intervention: 0/414 SoC: 2/418 (< 1) |
Not reported |
| Concomitant medications (n(%)) | |||||||||
| Corticosteroids | Intervention: 115 (21.6) Placebo: 126 (24.4) |
5‐day intervention: 33 (17) 10‐day intervention: 29 (15) SoC: 38 (19) |
Intervention: 60 (38) Placebo: 31 (40) |
Intervention: 2782 (67.1) SoC: 2820 (68.3) |
Not reported | Not reported | Intervention: 553/634 (87.2) SoC: 564/647 (87.2) |
General route Intervention: 164/414 (39.6) SoC: 169/418 (40.4) Inhaled route Intervention: 27/414 (6.5) SoC: 35/418 (8.4) |
Intervention: 1 (2.4) SoC: 2 (3.6) |
| HCQ/CQ | Intervention: 184 (35) Placebo: 189 (37) |
5‐day intervention: 16 (8) 5‐day intervention: 22 (11) SoC: 89 (45) |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Lopinavir‐ritonavir | Not reported | 5‐day intervention: 10 (5) 10‐day intervention: 11 (6) SoC: 43 (22) |
Intervention: 27 (17) Placebo: 31 (40) |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| MAB (e.g. interleukin 6) | Intervention: 23 (4.3) Placebo: 26 (5.0) |
5‐day intervention: 1 (1) 10‐day intervention: 1 (1) SoC: 10 (5) |
Not reported | Intervention: 174 (4.2) SoC: 199 (4.8) |
Not reported | Not reported |
Tocilizumab Intervention: 14/634 (2.2) SoC: 5/647 (0.8) |
Tocilizumab Intervention: 5/414 (1.2) SoC: 2/418 (0.5) |
Not reported |
| Azithromycin | Not reported | 5‐day intervention: 35 (18) 10‐day intervention: 41 (21) SoC: 62 (31) |
Not reported | Not reported | Not reported | Not reported | Not reported | Intervention: 11/414 (2.7) SoC: 4/418 (1) |
Not reported |
| Other |
Antibiotics Intervention: 420 (79) Placebo: 443 (86) Vasopressors Intervention: 147 (28) Placebo: 195 (38) Other anti‐inflammatory medications Intervention: 42 (8) Placebo: 37 (7) Other biologic therapies Intervention: 21 (4) Placebo: 13 (3) Other putative SARS‐CoV‐2 medications Intervention: 8 (2) Placebo: 14 (3) Other antiviral medications Intervention: 10 (2) Placebo: 8 (2) |
Not reported |
Antibiotics Intervention: 121 (77) Placebo: 63 (81) Interferon alfa‐2b Intervention: 29 (18) Placebo: 15 (19) |
Convalescent plasma Intervention: 125 (3) SoC: 151 (3.7) Non‐trial interferon Intervention: 5 (0.1) SoC: 30 (0.7) Non‐trial antiviral Intervention: 115 (2.8) SoC: 262 (6.4) |
Not reported | Not reported | Not reported |
Antibiotics Intervention: 178/414 (43) SoC: 166/418 (39.7) Interleukin‐1 inhibitors Intervention: 3/414 (0.7) SoC: 1/418 (0.2) Angiotensin‐receptor blockers Intervention: 33/414 (8) SoC: 42/418 (10) Anticoagulants Intervention: 212/414 (51.2) SoC: 224/418 (53.6) Vasopressors Intervention: 107/414 (25.8) SoC: 124/418 (29.7) NMBA Intervention: 97/414 (23.4) SoC: 113/418 (27) Inhaled nitric oxide Intervention: 15/414 (3.6) SoC: 17/418 (4.1) |
Other immunomodulatory
drugs Intervention: 1 (2.4) SoC: 1 (1.8) ACE inhibitors Intervention: 2 (4.9) SoC: 4 (7.1) Angiotensin II receptor blockers Intervention: 11 (26.8) SoC: 7 (12.5) |
aMissing data at baseline (n/N): intervention: 8/541, placebo: 3/521.
ACE = angiotensin converting enzyme
BMI = body mass index
CKD = chronic kidney disease
CLD = chronic liver disease
COPD = chronic obstructive pulmonary disease
HCQ/CQ = hydroxychlorquine/chloroquine
IQR = interquartile range
MAB = monoclonal antibodies
NA = not available/not applicable
NMBA = neuromuscular blocking agent(s)
RDV = remdesivir
SD = standard deviation
SoC = standard of care
WHO = World Health Organization
Study design and control
All included RCTs used a parallel‐group design. Six studies had an open‐label design with comparison of remdesivir to standard care alone (Mahajan 2021; Spinner 2020; WHO Solidarity Canada 2022; WHO Solidarity France 2021; WHO Solidarity Norway 2021; WHO Solidarity Trial Consortium 2022), whereas three studies were double‐blinded and placebo‐controlled (Beigel 2020; Gottlieb 2021; Wang 2020).
In one study (Beigel 2020), participants in the control arm received a lyophilised placebo identical in physical appearance to the active lyophilised formulation and containing the same inactive ingredients; alternatively, a normal saline of equal volume was given if there were limitations on matching placebo supplies. Gottlieb 2021 and Wang 2020 were provided with a placebo drug by Gilead Science. Notably, three studies did not provide details on standard care (Gottlieb 2021; Mahajan 2021; Wang 2020). The other studies performed non‐specified standard care according to local guidelines (Beigel 2020; Spinner 2020; WHO Solidarity Canada 2022; WHO Solidarity France 2021; WHO Solidarity Norway 2021; WHO Solidarity Trial Consortium 2022).
The WHO Solidarity Trial Consortium 2022 evaluated several possible COVID‐19 treatment options. If a participant was allocated to one control group in a study site with more than one study drug available, he or she could have also been allocated to another control arm, creating a partial overlap between control groups.
Intervention
A total of 5982 participants of the included RCTs were randomised to receive remdesivir. The treatment regimen in the interventional arm consisted of standard care plus 200 mg remdesivir intravenously as a loading dose on day 1, followed by 100 mg daily. The majority of included studies applied a 10‐day course of remdesivir (Beigel 2020; Spinner 2020; Wang 2020; WHO Solidarity Canada 2022; WHO Solidarity France 2021; WHO Solidarity Norway 2021; WHO Solidarity Trial Consortium 2022). Spinner 2020 (additionally) and Mahajan 2021 (solely) reported outcomes also for a five‐day treatment course. The participants in the outpatient study received remdesivir for three days (Gottlieb 2021). Participants in the WHO Solidarity Trial Consortium 2022 were randomly assigned to receive either remdesivir (n = 4169), hydroxychloroquine (n = 956), lopinavir (n = 1414), or interferon beta‐1a (n = 2154).
Setting
Five studies were multicentre studies performed in several countries (Beigel 2020 in 73 sites in Denmark, Germany, Greece, Japan, Korea, Mexico, Singapore, Spain, the UK, and the USA; Gottlieb 2021 in 64 centres in the United States, Spain, Denmark, and the United Kingdom; Spinner 2020 in 105 hospitals in Asia, Europe, and the USA; WHO Solidarity Trial Consortium 2022 in 454 hospitals in Albania, Argentina, Austria, Belgium, Brazil, Canada, Colombia, Egypt, Ethiopia, Finland, France, Georgia, Germany, Honduras, India, Indonesia, Iran, Ireland, Israel, Italy, Kuwait, Kenya, Lebanon, Malaysia, Mali, North Macedonia, Norway, Oman, Pakistan, Peru, the Philippines, Portugal, Qatar, Saudi Arabia, South Africa, Spain, Switzerland, and Thailand; WHO Solidarity France 2021 in 48 centres in France, Belgium, Austria, Portugal, and Luxembourg). Three studies were multicentre studies performed in one country (Wang 2020 in 10 centres in China; WHO Solidarity Canada 2022 in 52 Canadian hospitals; WHO Solidarity Norway 2021 in 23 sites in Norway). Mahajan 2021 performed a single‐centre study in India.
One study was performed in an outpatient setting (Gottlieb 2021), and eight studies included hospitalised patients with COVID‐19 (Beigel 2020; Mahajan 2021; Spinner 2020; Wang 2020; WHO Solidarity Canada 2022; WHO Solidarity France 2021; WHO Solidarity Norway 2021; WHO Solidarity Trial Consortium 2022).
Participants
All studies included individuals with symptomatic SARS‐CoV‐2 infection. The majority of included participants in the outpatient as well as inpatient setting were middle‐aged and male (mean age 50 years, 52.10% male; mean age 60.9 years, 65.0% male). Notably, two studies included adolescents younger than 18 years: Gottlieb 2021 with eight (1.423%) and Spinner 2020 with one (0.171%) of the recruited participants. Frequent comorbidities reported by some RCTs involved obesity, diabetes, and hypertension. Full details on comorbidities are provided in Table 5.
Diagnosis of SARS‐CoV‐2 infection
Whereas most studies required a positive polymerase chain reaction (PCR) test prior to inclusion, WHO Solidarity Trial Consortium 2022 stated that diagnosis of COVID‐19 was made "in the view of the responsible physician; PCR confirmation was not required". Four studies additionally instructed clinical or radiological signs of pneumonia (Beigel 2020; Mahajan 2021; Spinner 2020; Wang 2020). In Gottlieb 2021 and WHO Solidarity France 2021 a SARS‐CoV‐2 antigen rapid test was considered equal to PCR, as well as other commercial or public health assay in any specimen in WHO Solidarity Canada 2022. Gottlieb 2021 provided details about the proportion of PCR‐confirmed SARS‐CoV‐2 patients at baseline: 215 of 279 participants (77.1%) in the remdesivir group and 213 of 283 participants (75.3%) in the placebo group. Wang 2020 reported "viral positive population" in their supplements: 131 of 158 participants in the remdesivir group (83%) and 65 of 78 participants in the placebo group (83%).
Severity of illness
Severity of disease, interpreted by extent of respiratory support, was reported in different terms and definitions throughout all studies. The PINETREE trial included ambulatory patients without need for oxygen and at least one pre‐existing risk factor for progression to severe COVID‐19 (Gottlieb 2021). In Beigel 2020, participants were considered to have severe disease if they required mechanical ventilation; if the oxygen saturation as measured by pulse oximetry (SpO2) was 94% or lower whilst they were breathing ambient air; or if they had tachypnoea (respiratory rate ≥ 24 breaths per minute). The majority of participants in this study met the aforementioned criteria and needed supplemental oxygen (intervention 42.9%, control 39.0%), non‐invasive ventilation or high‐flow oxygen (intervention 17.6%, control 18.8%), or invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) (intervention 24.2%, control 29.6%). Only 13.9% of participants in the intervention arm and 12.1% of participants in the control arm were hospitalised without requiring supplemental oxygen. Mahajan 2021 classified participants in both groups as “highest disease severity”. However, participants were excluded if receiving invasive mechanical ventilation or if having multi‐organ failure. The majority (79.4%) of participants in the intervention group versus 72.2% of participants in the control group received low‐flow supplemental oxygen, and 20.6% versus 27.8% received non‐invasive ventilation or high‐flow oxygen, respectively. In Spinner 2020, the majority (84%) of participants in the intervention group versus 80% of participants in the control group did not require supplemental oxygen. Although measured SpO2 at screening was above 94% whilst breathing room air, 13% of participants in the intervention group and 19% in the control group used supplemental oxygen because of deteriorating clinical status or for breathing comfort. Wang 2020 defined severe COVID‐19 as SpO2 of 94% or lower on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen of 300 mmHg or less. The majority of participants in this study needed oxygen supplementation (intervention 82%, control 83%), whilst non‐invasive ventilation or high‐flow oxygen was necessary in 18% and 12% of participants, respectively. Invasive mechanical ventilation or ECMO was only required in 1% of the control group and none in the intervention group.
Protocol for recruitment was identical for all studies contributing to the WHO Solidarity trial (WHO Solidarity Canada 2022; WHO Solidarity France 2021; WHO Solidarity Norway 2021; WHO Solidarity Trial Consortium 2022). Disease severity was not protocol‐defined, and baseline respiratory support was broadly divided into “no supplemental oxygen”, “supplemental oxygen”, and “mechanical ventilation” (including non‐invasive and invasive mechanical ventilation). After the Solidarity interim analysis was published in February 2021, recruitment preferentially focused on patients who were not mechanically ventilated. The majority of participants in WHO Solidarity Trial Consortium 2022 received supplemental oxygen (70.4% of the intervention group, 70.7% of the control group), whilst no supplemental oxygen was needed in 21% and 20.9%. The minority of participants received mechanical ventilation at entry: 8.7% and 8.4%, respectively. WHO Solidarity Canada 2022 and WHO Solidarity France 2021 further divided baseline respiratory support. In WHO Solidarity Canada 2022, the majority of participants in the intervention group (52.7%) and the control group (56.2%) received low‐flow oxygen at entry, whereas no supplemental oxygen was needed in 11.2% and 8.4% of participants; high‐flow oxygen via nasal cannula in 23.5% and 23.7%; non‐invasive ventilation in 3.5% and 3.6%; and invasive ventilation in 9.1% and 8.3%, respectively. In WHO Solidarity France 2021, the majority of participants in the intervention group (60%) and the control group (59%) received supplemental oxygen at entry, whereas no supplemental oxygen was needed in 1% and 1%; high‐flow nasal cannula in 17% and 18%; non‐invasive ventilation in 4% and 4%; invasive mechanical ventilation in 18% and 17%; and ECMO in 0% and < 1%, respectively. In WHO Solidarity Norway 2021, severity of condition at baseline was divided into "admitted to ward" or "admitted to ICU". The majority of participants in the intervention group (92.9%) and control group (98.2%) were admitted to ward compared to participants admitted to ICU: 7.1% and 1.8%, respectively.
Concomitant medications
For an overview of concomitant medications and their distribution between groups, see Table 5.
Gottlieb 2021 did not report concomitant medication, but stated in the protocol the prohibition of combination of "investigational agents for COVID‐19 including approved HIV protease inhibitors such as lopinavir/RTV, chloroquine, interferon, etc.; use of hydroxychloroquine or chloroquine within 7 days of randomization; strong inducers of P‐glycoprotein (e.g., rifampin or herbal medications)" with remdesivir. Concomitant medication was restricted to heparin and corticosteroids in one study (Mahajan 2021). Wang 2020 reported baseline receipt of lopinavir–ritonavir, interferon, antibiotics, and corticosteroids. Considering that Beigel 2020 was one of the early trials in the pandemic, they solely prohibited "other experimental treatment or off‐label use of marketed medications intended as specific treatment for Covid‐19". They listed antibiotics, vasopressors, corticosteroids, other anti‐inflammatory medications, monoclonal antibodies targeting cytokines, other biological therapies, hydroxychloroquine, and other putative SARS‐CoV‐2 and antiviral medication, with antibiotics being the most frequently used. In WHO Solidarity Trial Consortium 2022, non‐study drugs (corticosteroids, convalescent plasma, anti‐interleukin‐6 medication, non‐trial‐interferon, non‐trial antiviral) were balanced between groups. Additional therapy with traditional herbs including sho‐saiko‐to (or Xiao‐Shai‐Hu‐Tang) or investigational agents with putative antiviral activity against COVID‐19 was prohibited by protocol for participants receiving remdesivir in one study (Spinner 2020). However, concomitant use of lopinavir‐ritonavir, hydroxychloroquine/chloroquine, interferon, steroids, tocilizumab, and azithromycin was reported for all participants, predominantly in the control arm. In WHO Solidarity Canada 2022, the decisions for all other care were left to the treating clinicians, including co‐medication, such as dexamethasone or tocilizumab or both for eligible patients, depending on time period, hospital setting, and participation in other RCTs. In WHO Solidarity France 2021, patients received dexamethasone (added to the standard care on 1 October 2020), other immunomodulatory agents (at investigator's discretion), and prophylactic or therapeutic anticoagulation. In WHO Solidarity Norway 2021, the concomitant medication included in addition to systemic steroids (given as standard care for severe and critical COVID‐19 from 4 September 2020) and other immunomodulatory drugs, angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers.
Outcomes
Primary outcomes differed between included RCTs. Studies contributing to the WHO Solidarity trial all reported in‐hospital mortality up to day 28, as pre‐defined by the core protocol. As the time point of measurement was not pre‐defined, some additionally reported mortality up to day 60 (WHO Solidarity Canada 2022; WHO Solidarity Norway 2021) and day 150 (WHO Solidarity Trial Consortium 2022). WHO Solidarity France 2021 selected clinical status at day 15 as primary outcome. Within aforementioned studies are cross‐references and synonymous use of the term all‐cause mortality. WHO Solidarity Trial Consortium 2022 used direct comparison through meta‐analyses with other RCTs reporting all‐cause mortality, like the ACTT‐1 trial (Beigel 2020). The latter prioritised time to recovery, defined as first day on which a participant was ready to be discharged. Further primary outcomes were: improvement in clinical outcomes (Mahajan 2021); clinical status on day 11 (Spinner 2020); and time to clinical improvement within 28 days (Wang 2020). As the only outpatient trial, Gottlieb 2021 provided a composite of hospitalisation related to COVID‐19 (as determined by site investigators, who were unaware of trial‐group assignments, and defined as ≥ 24 hours of acute care) or death from any cause by day 28.
Common safety end points included incidence of any adverse event, treatment‐emergent adverse events, adverse events grade three or four, and serious adverse events. Specific safety analyses regarded discontinuation of infusion (Beigel 2020; Wang 2020), changes in laboratory values (Beigel 2020; Mahajan 2021; Spinner 2020; Spinner 2020), grade changes in the biological and inflammatory patterns of participants over time (WHO Solidarity France 2021), new hepatic dysfunction, and renal replacement therapy (WHO Solidarity Canada 2022).
Awaiting classification
We classified two randomised controlled trials as 'awaiting classification': one open‐label trial, comparing the effects of remdesivir to standard care alone (NCT04596839), and one double‐blinded trial, comparing the effects of remdesivir to placebo (REDPINE 2022). Both of them are multicentre studies, performed in hospitalised patients with severe COVID‐19 in Bangladesh (NCT04596839), or in 63 study centres in the United States, in the United Kingdom, in Portugal, Brazil, South Africa, and Spain (REDPINE 2022). NCT04596839 is already completed (completion data: 30 April 2021) and recruited 60 participants, according to the information in the study register. Recruitment in REDPINE 2022 was currently terminated after enrolment of 249 from 1116 estimated participants due to "study enrollment feasibility".
Ongoing studies
An overview of the characteristics of ongoing studies is provided in Characteristics of ongoing studies and Table 4. We identified five records of ongoing studies comparing the effects of remdesivir with placebo (IRCT20210709051824N1; NCT04252664; NCT04843761) or standard care alone (NCT04351724; NCT04978259). The majority performed intervention with remdesivir for 10 days. IRCT20210709051824N1 performed an intervention with remdesivir for five days and NCT04351724 for either five or 10 days. All studies were performed among hospitalised patients with COVID‐19 and aimed to enrol a total of 1750 participants (IRCT20210709051824N1 n = 100, NCT04252664 n = 308, NCT04351724 n = 500, NCT04843761 n = 640, NCT04978259 n = 202). IRCT20210709051824N1 was expected to be completed on 20 February 2022; the last update of the registry was performed on 11 January 2022. NCT04252664 was discontinued: “The epidemic of COVID‐19 has been controlled well at present, no eligible patients can be recruited”. NCT04351724 is a platform trial investigating different interventions with an estimated enrolment of 500 participants in all study arms, without specifying the planned number of enrolments in the remdesivir arm and was expected to be completed in 2022. The last registry update was posted on 2 March 2021. NCT04843761 is active, not recruiting, and was expected to be completed in 2023. NCT04978259 is recruiting, and was expected to be completed in 2023.
Excluded studies
In the primary review (Ansems 2021) we excluded 57 references (57 studies) that did not match our inclusion criteria. In this update we excluded 22 records (22 studies).
One reference investigated a combination of remdesivir with other treatments.
11 studies were not randomised controlled trials.
One study did not have retrievable full text.
Six studies did not compare remdesivir to standard care or placebo.
Two studies did not investigate remdesivir intervention.
One study, included in the first version of this review, was retracted.
Risk of bias in included studies
We assessed risk of bias for the results within the nine included RCTs (Beigel 2020; Gottlieb 2021; Mahajan 2021; Spinner 2020; Wang 2020; WHO Solidarity Canada 2022; WHO Solidarity France 2021; WHO Solidarity Norway 2021; WHO Solidarity Trial Consortium 2022), using the RoB 2 tool, as recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022c; Sterne 2019). Outlined below are outcomes according to our updated set (Table 6). The completed RoB 2 tool with responses to all assessed signalling questions is available online at https://doi.org/10.5281/zenodo.5101320.
4. Outcomes.
| Primary outcomes (included in summary of findings) | ||
| Review version 1 | Review update 1 | |
| Hospitalised | Non‐hospitalised | |
| All‐cause mortality at day 28, day 60, time‐to‐event, and at hospital discharge |
All‐cause mortality atday 28, day 60, and up to longest follow‐up In‐hospital mortality at up to longest follow‐up |
All‐cause mortality at day 28, up to longest follow‐up, and time‐to‐event |
Clinical status, at day 28, day 60, and up to longest follow‐up, including:
|
Clinical status atday 28, up to longest follow‐up, andtime‐to‐event including:
|
Clinical status atday 14, day 28, up to longest follow‐up, andtime‐to‐event including:
|
| Quality of life | — | Quality of life |
| Serious adverse events | Serious adverse events | Serious adverse events |
Adverse events
|
Adverse events (any grade) | Adverse events (any grade) |
| Secondary outcomes | ||
| Review version 1 | Review update 1 | |
| Hospitalised | Non‐hospitalised | |
Moved from primary to secondary outcome:
|
— | |
| Ventilator‐free days | — | |
| Need for dialysis at up to 28 days | — | — |
| Need for admission to intensive care unit (ICU) | — | — |
| Duration of ICU length of stay, or time to discharge from ICU | — | — |
| Duration of hospitalisation, or time to discharge from hospital | — | — |
| Viral clearance | — | — |
Remdesivir plus standard care versus standard care (plus/minus placebo)
Individuals with moderate to severe COVID‐19
All‐cause mortality
Four studies reported this outcome (see Table 19). Overall, we rated the risk of bias for mortality to be low for three studies (Beigel 2020; Spinner 2020; WHO Solidarity Trial Consortium 2022), and as 'some concerns' for one study (Wang 2020). We assessed this outcome on a study level at up to day 28 and as time‐to‐event, if provided (see Table 21), as well as for our subgroup analyses (see Table 30; Table 31; Table 32; Table 34). For one study, there were some concerns arising from baseline differences in gender distribution, respiratory status, comorbidities, and time from symptom onset, suggesting a possible problem with the block wise and stratified randomisation process (Wang 2020). We did not identify any concerns that could have biased the reported outcome in three studies, and therefore judged the risk of bias to be low (Beigel 2020; Spinner 2020; WHO Solidarity Trial Consortium 2022).
Risk of bias for analysis 1.1 All‐cause mortality at up to day 28.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beigel 2020 | Low risk of bias | Randomisation was stratified by study site and disease severity in a 1:1 ratio and the allocation sequence was probably concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were adequately blinded. The analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Outcome was available for all participants. | Low risk of bias | Measuring of the outcome was appropriate. The outcome was assessed using standardised methods and assessors were unaware of the treatment assignments. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Low risk of bias | Overall judged low risk of bias. The method of the randomisation process was not provided, but there were no baseline imbalances that would suggest a problem with randomisation. Blinding was appropriate, and outcome measurement was according to a pre‐specified protocol. |
| Spinner 2020 | Low risk of bias | Block‐randomisation was not stratified and web‐based. Sites did not have access to the randomisation list. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were aware of the assigned intervention. There was a neglectable number of participants with deviations from the intended intervention and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the treatment assignments. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The trial protocol provided details of time points and analysis. Mortality was analysed in accordance with a prespecified analysis plan. | Low risk of bias | Overall judged low risk of bias. Details about randomisation, blinding of outcome assessors and a protocol were provided. It was an open‐label study, but this could not have affected the outcome measurement. |
| Wang 2020 | Some concerns | Randomisation was carried out 2:1 and in stratified blocks. Concealed envelopes were prepared. There were baseline differences in gender distribution, respiratory status at baseline, co‐morbidities and time from symptom onset suggesting possible randomisation problems. | Low risk of bias | The study was carried out double blinded. Analysis was done appropriately. | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were unaware of the treatment assignments and could not have been affected by knowledge of intervention. | Low risk of bias | The trial protocol provided details of time points and analysis. However, the statistical analysis plan could not be found. | Some concerns | Overall judged some concerns for risk of bias. Details about randomisation, blinding of outcome assessors and a protocol were provided. There were baseline differences between intervention and control group suggesting a problem with block wise randomisation process. A statistical analysis plan could not be found. |
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. Multiple analyses were reported according to a pre‐defined protocol. | Low risk of bias | Overall judged low risk of bias. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were carried out according to a pre‐defined study protocol. |
Risk of bias for analysis 1.4 All‐cause mortality (time‐to‐event).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beigel 2020 | Low risk of bias | Randomisation was stratified by study site and disease severity in a 1:1 ratio and the allocation sequence was probably concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were adequately blinded. The analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Outcome available for all participants. | Low risk of bias | Measuring of the outcome was appropriate. The outcome was assessed using standardised methods and assessors were unaware of the treatment assignments. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Low risk of bias | Overall judged low risk of bias. The method of the randomisation process was not provided, but there were no baseline imbalances that would suggest a problem with randomisation. Blinding was appropriate, and outcome measurement was according to a pre‐specified protocol. |
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. Multiple analyses were reported according to a pre‐defined protocol. | Low risk of bias | Overall judged low risk of bias. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were carried out according to a pre‐defined study protocol. |
Risk of bias for analysis 2.1 All‐cause mortality at up to day 28.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.1.1 Age < 50 years | ||||||||||||
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. Multiple analyses were reported according to a pre‐defined protocol. | Low risk of bias | Overall judged low. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were carried out according to a pre‐defined study protocol. |
| Subgroup 2.1.2 Age 50 to 69 years | ||||||||||||
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. Multiple analyses were reported according to a pre‐defined protocol. | Low risk of bias | Overall judged low. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were carried out according to a pre‐defined study protocol. |
| Subgroup 2.1.3 Age > 69 years | ||||||||||||
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. Multiple analyses were reported according to a pre‐defined protocol. | Low risk of bias | Overall judged low. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were carried out according to a pre‐defined study protocol. |
Risk of bias for analysis 3.1 All‐cause mortality at up to day 28.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.1.1 ≤ 10 days of symptom onset | ||||||||||||
| Wang 2020 | Some concerns | Randomisation was carried out 2:1 and in stratified blocks. Concealed envelopes were prepared. There were baseline differences in gender distribution, respiratory status at baseline, co‐morbidities and time from symptom onset suggesting possible randomisation problems. | Low risk of bias | The study was carried out double blinded. Analysis was done appropriately. | Low risk of bias | Data for this outcome was available for nearly all participants randomised. | Low risk of bias | Outcome assessors were unaware of the treatment assignments and could not have been affected by knowledge of intervention. | Low risk of bias | The trial protocol provided details of time points and analysis. However, the statistical analysis plan could not be found. | Some concerns | Details about randomisation, blinding of outcome assessors and a protocol were provided. There were baseline differences between intervention and control group. |
| Subgroup 3.1.2 > 10 days of symptom onset | ||||||||||||
| Wang 2020 | Some concerns | Randomisation was carried out 2:1 and in stratified blocks. Concealed envelopes were prepared. There were baseline differences in gender distribution, respiratory status at baseline, co‐morbidities and time from symptom onset suggesting possible randomisation problems. | Low risk of bias | The study was carried out double blinded. Analysis was done appropriately. | Low risk of bias | Data for this outcome was available for nearly all participants randomised. | Low risk of bias | Outcome assessors were unaware of the treatment assignments and could not have been affected by knowledge of intervention. | Low risk of bias | The trial protocol provided details of time points and analysis. However, the statistical analysis plan could not be found. | Some concerns | Details about randomisation, blinding of outcome assessors and a protocol were provided. There were baseline differences between intervention and control group. |
Risk of bias for analysis 4.1 All‐cause mortality at up to day 28.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 4.1.1 No oxygen at baseline | ||||||||||||
| Beigel 2020 | Low risk of bias | Randomisation was stratified by study site and disease severity in a 1:1 ratio and the allocation sequence was probably concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were adequately blinded. The analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Outcome was available for all participants. | Low risk of bias | Measuring of the outcome was appropriate. The outcome was assessed using standardised methods and assessors were unaware of the treatment assignments. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Low risk of bias | Overall judged low risk of bias. Information on randomisation process is not provided, but blinding was appropriate, and outcome measurement was according to a pre‐specified protocol. |
| Spinner 2020 | Low risk of bias | Block‐randomisation was not stratified and web‐based. Sites did not have access to the randomisation list. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were aware of the assigned intervention. There was a neglectable number of participants with deviations from the intended intervention and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the treatment assignments. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The trial protocol provided details of time points and analysis. Mortality was analysed in accordance with a prespecified analysis plan. | Low risk of bias | Overall judged low risk of bias. Details about randomisation, blinding of outcome assessors and a protocol were provided. It was an open‐label study, but this could not have affected the outcome measurement. |
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. Multiple analyses were reported according to a pre‐defined protocol. | Low risk of bias | Overall judged low risk of bias. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were carried out according to a pre‐defined study protocol. |
| Subgroup 4.1.2 Low‐flow oxygen at baseline | ||||||||||||
| Beigel 2020 | Low risk of bias | Randomisation was stratified by study site and disease severity in a 1:1 ratio and the allocation sequence was probably concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were adequately blinded. The analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Outcome was available for all participants. | Low risk of bias | Measuring of the outcome was appropriate. The outcome was assessed using standardised methods and assessors were unaware of the treatment assignments. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Low risk of bias | Overall judged low risk of bias. Information on randomisation process is not provided, but blinding was appropriate, and outcome measurement was according to a pre‐specified protocol. |
| Subgroup 4.1.3 Low‐flow or high‐flow oxygen at baseline | ||||||||||||
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. Multiple analyses were reported according to a pre‐defined protocol. | Low risk of bias | Overall judged low risk of bias. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were carried out according to a pre‐defined study protocol. |
| Subgroup 4.1.4 Mechanical ventilation at baseline | ||||||||||||
| Beigel 2020 | Low risk of bias | Randomisation was stratified by study site and disease severity in a 1:1 ratio and the allocation sequence was probably concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were adequately blinded. The analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Outcome was available for all participants. | Low risk of bias | Measuring of the outcome was appropriate. The outcome was assessed using standardised methods and assessors were unaware of the treatment assignments. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Low risk of bias | Overall judged low risk of bias. Information on randomisation process is not provided, but blinding was appropriate, and outcome measurement was according to a pre‐specified protocol. |
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. Multiple analyses were reported according to a pre‐defined protocol. | Low risk of bias | Overall judged low risk of bias. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were carried out according to a pre‐defined study protocol. |
Risk of bias for analysis 5.1 All‐cause mortality at up to day 28.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 5.1.1 5‐day remdesivir | ||||||||||||
| Spinner 2020 | Low risk of bias | Block‐randomisation was not stratified and web‐based. Sites did not have access to the randomisation list. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were aware of the assigned intervention. There was a neglectable number of participants with deviations from the intended intervention and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the treatment assignments. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The trial protocol provided details of time points and analysis. Mortality was analysed in accordance with a prespecified analysis plan. | Low risk of bias | Overall judged low. Details about randomisation, blinding of outcome assessors and a protocol were provided. It was an open‐label study, but this could not have affected the outcome measurement. |
| Subgroup 5.1.2 10‐day remdesivir | ||||||||||||
| Spinner 2020 | Low risk of bias | Block‐randomisation was not stratified and web‐based. Sites did not have access to the randomisation list. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were aware of the assigned intervention. There was a neglectable number of participants with deviations from the intended intervention and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the treatment assignments. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The trial protocol provided details of time points and analysis. Mortality was analysed in accordance with a prespecified analysis plan. | Low risk of bias | Overall judged low. Details about randomisation, blinding of outcome assessors and a protocol were provided. It was an open‐label study, but this could not have affected the outcome measurement. |
In‐hospital mortality
One study reported this outcome for the longest follow‐up available, 150 days (see Table 20). We rated the risk of bias for in‐hospital mortality as 'some concerns', because outcome measurement and analyses were appropriate but not pre‐defined with a risk of selective reporting. This could not be clarified after contacting the authors.
Risk of bias for analysis 1.3 In‐hospital mortality at up to day 150.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no relevant deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Some concerns | There was no statistical analyses plan and pre‐defined protocol did not state the form of analysis. Reported analyses are appropriate but could have been selected. | Some concerns | Overall judged some concerns. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were appropriate but not pre‐defined with a risk of selective reporting. |
Improvement of clinical status
Four studies reported this outcome (see Table 22). We assessed this outcome by survival of participants, who are ready to be discharged from the hospital at up to day 28 and as time‐to‐event, if provided (see Table 23). Overall, we rated the risk of bias for clinical improvement to be low for two studies (Spinner 2020; Beigel 2020), and as 'some concerns' for two studies (Wang 2020; WHO Solidarity France 2021). Concerns arose because of baseline differences between groups (Wang 2020), and lack of blinding of participants and assessors, which could have influenced the assessment and caused the small amount of deviations from intended interventions (WHO Solidarity France 2021).
Risk of bias for analysis 1.5 Clinical improvement: alive and ready to discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beigel 2020 | Low risk of bias | Randomisation was stratified by study site and disease severity in a 1:1 ratio and the allocation sequence was probably concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were not aware of the assigned intervention, because of the use of matching placebo or opaque bags and tubing covers. The analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Measuring of the outcome was appropriate, the outcome was binary and assessed using standardised methods. Outcome assessors were probably unaware of the treatment assignments. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Low risk of bias | Overall judged low risk of bias. The method of the randomisation process was not provided, but there were no baseline imbalances that would suggest a problem with randomisation. Blinding was appropriate, and outcome measurement was according to a pre‐specified protocol. |
| Spinner 2020 | Low risk of bias | Block‐randomisation was not stratified and web‐based. Sites did not have access to the randomisation list. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were aware of the assigned intervention. There was a neglectable number of participants with deviations from the intended intervention and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised to the interventions, as seen in figure 1. | Low risk of bias | Outcome assessors were aware of the treatment assignments. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Low risk of bias | Overall judged low. Details about randomisation, blinding of outcome assessors and a protocol were provided. It was an open‐label study, but this could not have affected the outcome measurement. |
| Wang 2020 | Some concerns | Randomisation was carried out 2:1 and in stratified blocks. Concealed envelopes were prepared. There were baseline differences in gender distribution, respiratory status at baseline, co‐morbidities and time from symptom onset suggesting possible randomisation problems. | Low risk of bias | The study was carried out double‐blinded. Analysis was done appropriately. | Low risk of bias | Data for this outcome was available for nearly all participants randomised. | Low risk of bias | Outcome assessors were unaware of the treatment assignments and could not have been affected by knowledge of intervention. | Low risk of bias | The trial protocol provided details of time points and analysis. However, the statistical analysis plan could not be found. | Some concerns | Overall judged some concerns. Details about randomisation, blinding of outcome assessors and a protocol were provided. There were baseline differences between intervention and control group. |
| WHO Solidarity France 2021 | Low risk of bias | Randomisation appears appropriate with concealed allocation. There is an underrepresentation of female participants. | Low risk of bias | The study was open‐label and deviation from intented intervention could be because of experimental context. However, the small amount of deviation could be random and an appropriate analysis was used. | Low risk of bias | Data was available for all participants. | Some concerns | Outcome measurement was carried out according to pre‐defined protocol but assessment could have been influenced by the knowledge of received intervention. | Low risk of bias | Results were measured, analysed and reported according to a pre‐defined protocol. | Some concerns | Overall judged some concerns. Randomisation, measurement, analysis and reporting of results were appropriate. The open‐label design could have influenced the assessment and could have caused deviations in the intented intervention. |
Risk of bias for analysis 1.6 Clinical improvement: alive and ready to discharge (time‐to‐event).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Spinner 2020 | Low risk of bias | Block‐randomisation was not stratified and web‐based. Sites did not have access to the randomisation list. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were aware of the assigned intervention. There was a neglectable number of participants with deviations from the intended intervention and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised to the interventions, as seen in figure 1. | Low risk of bias | Outcome assessors were aware of the treatment assignments. Knowledge of intervention received could not have affected the outcome measurement. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Low risk of bias | Overall judged low. Details about randomisation, blinding of outcome assessors and a protocol were provided. It was an open‐label study, but this could not have affected the outcome measurement. |
| WHO Solidarity France 2021 | Low risk of bias | Randomisation appears appropriate with concealed allocation. There is an underrepresentation of female participants. | Low risk of bias | The study was open‐label and deviation from intended intervention could be because of experimental context. However, the small amount of deviation could be random, and an appropriate analysis was used. | Low risk of bias | Data was available for all participants. | Some concerns | Outcome measurement was carried out according to pre‐defined protocol, but assessment could have been influenced by the knowledge of received intervention. | Low risk of bias | Results were measured, analysed and reported according to a pre‐defined protocol. | Some concerns | Overall judged some concerns. Randomisation, measurement, analysis and reporting of results were appropriate. The open‐label design could have influenced the assessment and could have caused deviations in the intended intervention. |
Worsening of clinical status
One study reported this outcome (see Table 24), and two studies reported this outcome as time‐to‐event (see Table 25). Overall, we rated the risk of bias for clinical worsening as 'some concerns' in one study, providing data for both analyses (WHO Solidarity France 2021). Concerns arose due to lack of blinding of participants and assessors, which could have influenced the assessment and caused the small amount of deviations from intended interventions. One study, providing a hazard ratio over time, was a post hoc analysis of the ACTT‐1 trial (Beigel 2020). It therefore had a high risk for selective reporting.
Risk of bias for analysis 1.7 Clinical worsening: new need for invasive mechanical ventilation or death.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| WHO Solidarity France 2021 | Low risk of bias | Randomisation appears appropriate with concealed allocation. There is an underrepresentation of female participants. | Some concerns | It was an open‐label study with few deviations from the intended intervention. However, it is unlikely that they have affected the outcome, since an appropriate analysis was used. | Low risk of bias | There was no missing data. | Some concerns | Measurement of the outcome was performed according to a pre‐defined protocol. Knowledge of the intervention could have influenced the assessment. | Low risk of bias | There was a published protocol which did not match the version of the reported results. However, there were no deviations that would suggest selective reporting. | Some concerns | Overall judged some concerns for risk of bias due to the possible influence of the open‐label design on assessment of the outcome and few deviations from the intended intervention. |
Risk of bias for analysis 1.8 Clinical worsening: new need for invasive mechanical ventilation or death (time‐to‐event).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beigel 2020 | Low risk of bias | Randomisation was stratified by study site and disease severity in a 1:1 ratio and the allocation sequence was probably concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were not aware of the assigned intervention, because of the use of matching placebos or opaque bags and tubing covers. The analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Measuring of the outcome was appropriate, the outcome was binary and assessed using standardised methods. Outcome assessors were probably unaware of the treatment assignments. | High risk of bias | The data was post‐hoc analysed. | High risk of bias | Overall judged high risk. Not enough details about randomisation and allocation concealment. Blinding was appropriate. The outcome was post‐hoc analysed. |
| WHO Solidarity France 2021 | Low risk of bias | Randomisation appears appropriate with concealed allocation. There is an underrepresentation of female participants. | Some concerns | It was an open‐label study with few deviations from the intended intervention. However, it is unlikely that they have affected the outcome, since an appropriate analysis was used. | Low risk of bias | There was no missing data. | Some concerns | Measurement of the outcome was performed according to a pre‐defined protocol. Knowledge of the intervention could have influenced the assessment. | Low risk of bias | There was a published protocol which did not match the version of the reported results. However, there were no deviations that would suggest selective reporting. | Some concerns | Overall judged some concerns for risk of bias due to the possible influence of the open‐label design on assessment of the outcome and few deviations from the intented intervention. |
Adverse events (any grade)
Four studies reported this outcome (see Table 26). We identified concerns for risk of bias in all of the contributing studies. Reasons were inappropriate analysis and population selection (Beigel 2020), differences in baseline characteristics (Wang 2020), and the open‐label study design in Spinner 2020 and WHO Solidarity France 2021, particularly in the reporting of lower‐grade adverse events in participants who were aware of the intervention. We judged missing outcome data as 'some concerns' in all studies due to competing risk of death without evidence, that missing outcome data does not depend on its true value.
Risk of bias for analysis 1.9 Adverse events, any grade.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beigel 2020 | Low risk of bias | Randomisation was stratified by study site and disease severity in a 1:1 ratio and the allocation sequence was probably concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Some concerns | Participants and care takers were adequately blinded. The analysis was inappropriate (as‐treated population). The deviation from an appropriate analysis population is small so that it probably had no substantial impact on the outcome results. | Some concerns | There is an uncertain amount of missings due to competing risk of death. It is uncertain, whether the result is biased or not. | Low risk of bias | Measuring of the outcome was appropriate. The outcome was assessed using standardised methods and assessors were unaware of the treatment assignments. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Some concerns | Overall judged some concerns due to inappropriate analyses and non‐plausible participant selection. The method of the randomisation process was not provided, but there were no baseline imbalances that would suggest a problem with randomisation. Blinding was appropriate, and outcome measurement was according to a pre‐specified protocol. |
| Spinner 2020 | Low risk of bias | Block‐randomisation was not stratified and web‐based. Sites did not have access to the randomisation list. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were aware of the assigned intervention. There was a neglectable number of participants with deviations from the intended intervention and the analysis was appropriate (intention‐to‐treat population). | Some concerns | There is an uncertain amount of missing data due to competing risk of death. It is uncertain, whether the result is biassed or not. | Some concerns | Outcome assessors were aware of the treatment assignments. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | Data was reported according to a study protocol providing details of time points, analyses and methods for categorisation and registration of adverse events. | Some concerns | Overall judged some concerns for risk of bias. Details about randomisation, blinding of outcome assessors and a protocol were provided. It was an open‐label study, and knowledge of intervention received could have affected outcome measurement to some degree. There is an uncertain amount of missing data due to competing risk of death. |
| Wang 2020 | Some concerns | Randomisation was carried out 2:1 and in stratified blocks. Concealed envelopes were prepared. There were baseline differences in gender distribution, respiratory status at baseline, co‐morbidities and time from symptom onset suggesting possible randomisation problems. | Low risk of bias | The study was carried out double‐blinded and the outcome was assessed by safety set. | Some concerns | There is an uncertain amount of missing data due to competing risk of death. It is uncertain, whether the result is biassed or not. | Low risk of bias | Outcome assessors were unaware of the treatment assignments and could not have been affected by knowledge of intervention. | Low risk of bias | The trial protocol provided details of time points and analysis. The statistical analysis plan could not be found. | Some concerns | Overall judged some concerns for risk of bias. Details about randomisation, blinding of outcome assessors and a protocol were provided. There were baseline differences between intervention and control group suggesting a problem with block wise randomisation process. There is an uncertain amount of missing data due to competing risk of death. |
| WHO Solidarity France 2021 | Low risk of bias | Randomisation appears appropriate with concealed allocation. There is an underrepresentation of female participants. | Low risk of bias | The study was open‐label and deviation from intended intervention could be because of experimental context. However, the small amount of deviation could be random, and an appropriate analysis was used. | Low risk of bias | Data was available for all participants. | Some concerns | Recording and assessment of seriousness of adverse events in an unblinded trial could be influenced by the knowledge of treatment group by the assessors, but direction of bias remains unclear and high number of trial centres with many different assessors should rule out systematic bias. | Some concerns | The outcome was reported additionally to a pre‐defined outcome set. Bias in result selection seems unlikely, since the reported outcome is common in safety sets and does not change the safety profile. | Some concerns | Overall judged some concerns. Randomisation was appropriate. The open‐label design could have influenced the assessment and could have caused deviations in the intended intervention. The outcome was reported additionally to a pre‐defined outcome set but selection bias. |
Serious adverse events
Four studies reported this outcome (see Table 28) and we judged these as 'some concerns'. The judgement in Beigel 2020 was based on inappropriate analyses and selection of participants, which did not comply with the appropriate safety population. We assessed Wang 2020 as 'some concerns' due to baseline differences between the intervention and control group. For Spinner 2020 and WHO Solidarity France 2021, there was a low risk arising from the awareness of the assigned intervention (open‐label), which is unlikely to have affected the outcome measurement. However, we judged missing outcome data as 'some concerns' of bias in all studies due to competing risk of death without evidence, that missing outcome data does depend on its true value.
Risk of bias for analysis 1.11 Serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beigel 2020 | Low risk of bias | Randomisation was stratified by study site and disease severity in a 1:1 ratio and the allocation sequence was probably concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Some concerns | Participants and care takers were adequately blinded. The analysis was inappropriate (as‐treated population). | Some concerns | Selection of included participants is not plausible and there is an uncertain amount of missing data due to competing risk of death. It is uncertain, whether the result is biassed or not. | Low risk of bias | Measuring of the outcome was appropriate. The outcome was assessed using standardised methods and assessors were unaware of the treatment assignments. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Some concerns | Overall judged some concerns for risk of bias due to inappropriate analyses and non‐plausible participant selection. Information on randomisation process is not provided, but blinding was appropriate, and outcome measurement was according to a pre‐specified protocol. |
| Spinner 2020 | Low risk of bias | Block‐randomisation was not stratified and web‐based. Sites did not have access to the randomisation list. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were aware of the assigned intervention. There was a neglectable number of participants with deviations from the intended intervention and the analysis was appropriate (intention‐to‐treat population). | Some concerns | There is an uncertain amount of missing data due to competing risk of death. It is uncertain, whether the result is biassed or not. | Low risk of bias | Outcome assessors were aware of the treatment assignments. Serious adverse events are unlikely to be influenced by diagnostic bias due to their severity. | Low risk of bias | Data was reported according to a study protocol providing details of time points, analyses and methods for categorisation and registration of adverse events. | Some concerns | Overall judged some concerns for risk of bias. Details about randomisation, blinding of outcome assessors and a protocol were provided. Impact of missing data due to competing risk of death is uncertain. It was an open‐label study, but it was unlikely to affect the outcome measurement. |
| Wang 2020 | Some concerns | Randomisation was carried out 2:1 and in stratified blocks. Concealed envelopes were prepared. There were baseline differences in gender distribution, respiratory status at baseline, co‐morbidities and time from symptom onset suggesting possible randomisation problems. | Low risk of bias | The study was carried out double‐blinded and the outcome was assessed by safety set. | Some concerns | There is an uncertain amount of missing data due to competing risk of death. | Low risk of bias | Outcome assessors were unaware of the treatment assignments and could not have been affected by knowledge of intervention. | Low risk of bias | The trial protocol provided details of time points and analysis. The statistical analysis plan could not be found. | Some concerns | Overall judged some concerns for risk of bias. Details about randomisation, blinding of outcome assessors and a protocol were provided. There were baseline differences between intervention and control group. There is an uncertain amount of missing data due to competing risk of death. |
| WHO Solidarity France 2021 | Low risk of bias | Randomisation appears appropriate with concealed allocation. There is an underrepresentation of female participants. | Low risk of bias | The study was open‐label and deviation from intended intervention could be because of experimental context. However, the small amount of deviation could be random, and an appropriate analysis was used. | Low risk of bias | Data was available for all participants. | Some concerns | Recording and assessment of seriousness of adverse events in an unblinded trial could be influenced by the knowledge of treatment group by the assessors, but direction of bias remains unclear and high number of trial centres with many different assessors should rule out systematic bias. | Low risk of bias | Results were measured, analysed and reported according to a pre‐defined protocol. | Some concerns | Overall judged some concerns. Randomisation, measurement, analysis and reporting of results were appropriate. The open‐label design could have influenced the assessment and could have caused deviations in the intended intervention. |
Individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
Data solely arose from one trial in the ambulatory setting (Gottlieb 2021). All judgements refer to this study.
All‐cause mortality
We judged risk of bias for all‐cause mortality to be low (see Table 35). We did not identify any concerns that could have biased the reported outcome.
Risk of bias for analysis 6.1 All‐cause mortality at up to day 28.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gottlieb 2021 | Low risk of bias | Judged low risk of bias. Randomly assigned in a 1:1 ratio, stratified according to residence in a skilled nursing facility, age (< 60 years, >= 60 years), location (US or outside US). There are only baseline differences that arose by chance and do not indicate a problem in the randomisation process. | Low risk of bias | All participants and trial personnel were unaware if the group assignments. The analysis was appropriate. ITT population was analysed. | Low risk of bias | Data were available for all participants. | Low risk of bias | As the blinding process was maintained until analysis there is likely no bias. | Low risk of bias | Outcome reported as pre‐planned | Low risk of bias | Blinding was maintained until trial termination and data were available for all participants. |
Improvement of clinical status
We judged risk of bias for clinical improvement to be high (see Table 36). There were a large number of missing values and analyses were not performed as pre‐defined by protocol, with a high risk of selective reporting. Additionally, measurement of the outcome had limited validity.
Risk of bias for analysis 6.2 Clinical improvement: symptom alleviation at up to day 14.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gottlieb 2021 | Low risk of bias | Judged low risk of bias. Randomly assigned in a 1:1 ratio, stratified according to residence in a skilled nursing facility, age (< 60 years, >= 60 years), location (US or outside US). There are only baseline differences that arose by chance and do not indicate a problem in the randomisation process. | Low risk of bias | Judged low risk of bias. Participants and trial personnel were blinded. The analysis was appropriate. | High risk of bias | Judged high risk of bias due to large number of missing values. | High risk of bias | The questionnaire is not validated for COVID‐19. Therefore, the validity is limited. It is unclear whether the FLU‐PRO questions are also applicable to COVID19 or not. | Some concerns | Analysis was performed in accordance to the pre‐specified analysis plan (Section 8 of Trial Protocol). No further information available. | High risk of bias | Judged high risk of bias due to missing data. Furthermore, it is unclear whether the FLU‐PRO questionnaire is applicable to COVID19 or not. |
Worsening of clinical status
We judged risk of bias for clinical worsening to be low (see Table 37). We did not identify any concerns that could have biased the reported outcome.
Risk of bias for analysis 6.3 Clinical worsening: admission to hospital or death at up to day 28.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gottlieb 2021 | Low risk of bias | Judged low risk of bias. Randomly assigned in a 1:1 ratio, stratified according to residence in a skilled nursing facility, age (< 60 years, >= 60 years), location (US or outside US). There are only baseline differences that arose by chance and do not indicate a problem in the randomisation process. | Low risk of bias | All participants and trial personnel were unaware if the group assignments. The analysis was appropriate. ITT population was analysed. | Low risk of bias | Data were available for all participants. | Low risk of bias | Dichotomous outcome: There is only one way to measure the outcome. All trial personnel ware unaware of the assignment. | Low risk of bias | Analysis was performed in accordance to the pre‐specified analysis plan (Section 8 of Trial Protocol). | Low risk of bias | Randomly assigned in a 1:1 ratio, stratified according to residence in a skilled nursing facility, age (< 60 years, >= 60 years), location (US or outside US). There are only baseline differences that arose by chance and do not indicate a problem in the randomisation process. All participants and trial personnel were unaware if the group assignments. The analysis was appropriate. ITT population was analysed. Data were available for all participants. There is only one way to measure the outcome. Analysis was performed in accordance to the pre‐specified analysis plan (Section 8 of Trial Protocol). |
Quality of life
There were no data available for this outcome, thus risk of bias could not be judged.
Adverse events (any grade)
We judged risk of bias for any adverse events to be low (see Table 39). We did not identify any concerns that could have biased the reported outcome.
Risk of bias for analysis 6.5 Adverse events, any grade.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gottlieb 2021 | Low risk of bias | Judged low risk of bias. Randomly assigned in a 1:1 ratio, stratified according to residence in a skilled nursing facility, age (< 60 years, >= 60 years), location (US or outside US). There are only baseline differences that arose by chance and do not indicate a problem in the randomisation process. | Low risk of bias | Participants and trial personnel were blinded. The analysis was appropriate. | Low risk of bias | Data were available for all participants (ITT). | Low risk of bias | There is only one way to measure the outcome. Double blinding was maintained until finalisation of the data. | Low risk of bias | Judged low risk of bias. Analysis was performed in accordance to the pre‐specified analysis plan (Section 8 of Trial Protocol). There is only one way to measure the outcome. AEs are defined in study protocol. | Low risk of bias | Overall judged low risk of bias. Randomly assigned, only baseline differences that arose by chance, analysis was appropriate, data were available for al participants, double blinding was maintained until finalisation of the data, analysis was performed in accordance to a pre‐specified analysis plan. |
Serious adverse events
We judged risk of bias for serious adverse events to be low (see Table 38). We did not identify any concerns that could have biased the reported outcome.
Risk of bias for analysis 6.4 Serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gottlieb 2021 | Low risk of bias | Judged low risk of bias. Randomly assigned in a 1:1 ratio, stratified according to residence in a skilled nursing facility, age (< 60 years, >= 60 years), location (US or outside US). There are only baseline differences that arose by chance and do not indicate a problem in the randomisation process. | Low risk of bias | Judged low risk of bias. Participants and trial personnel were blinded. The analysis was appropriate. | Low risk of bias | Judged low risk if bias. Data were available for all participants (ITT). | Low risk of bias | There is only one way to measure the outcome. Double blinding was maintained until finalisation of the data. | Low risk of bias | Judged low risk of bias. Analysis was performed in accordance to the pre‐specified analysis plan (Section 8 of Trial Protocol). There is only one way to measure the outcome. SAEs are defined in study protocol. | Low risk of bias | Overall judged low risk of bias. Randomly assigned in a 1:1 ratio, stratified according to residence in a skilled nursing facility, age (< 60 years, >= 60 years), location (US or outside US). There are only baseline differences that arose by chance and do not indicate a problem in the randomisation process. The analysis was appropriate. Data were available for all participants (ITT). There is only one way to measure the outcome. Double blinding was maintained until finalisation of the data. Analysis was performed in accordance to the pre‐specified analysis plan (Section 8 of Trial Protocol). There is only one way to measure the outcome. SAEs are defined in study protocol. |
Effects of interventions
See: Table 1: Remdesivir and standard care versus standard care (plus/minus placebo) for individuals with moderate to severe COVID‐19; Table 2 Remdesivir and standard care versus standard care (plus/minus placebo) for individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19. Adaptions made in our outcome set compared to the first version of this review are outlined in Differences between protocol and review and Table 6.
Remdesivir plus standard care versus standard care (plus/minus placebo)
Individuals with moderate to severe COVID‐19
We have presented the summary of findings and the certainty of the evidence for adult in‐hospital participants with moderate to severe COVID‐19, comparing a 10‐day course of remdesivir to placebo or standard care alone.
Primary outcomes
All‐cause mortality
We assessed all‐cause mortality at up to day 28, day 60 (longest follow‐up available), and as time‐to‐event (secondary outcome).
All‐cause mortality at up to day 28
Four studies reported this outcome for 7142 participants (see Analysis 1.1). In the control group an estimated 108 of 1000 participants died up to day 28. Remdesivir probably makes little or no difference to all‐cause mortality at up to day 28 (estimated 100 per 1000 participants; 95% confidence interval (CI) 21 fewer to 6 more per 1000) compared to placebo or standard care alone (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.81 to 1.06; 4 studies, 7142 participants; I² = 0%; moderate‐certainty evidence). Our main reasons for downgrading the certainty of the evidence were serious imprecision because of wide confidence intervals in the studies, and the 95% confidence interval includes both benefits and harms.
1.1. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 1: All‐cause mortality at up to day 28
All‐cause mortality at up to day 60
One study reported this outcome for 1281 participants (see Analysis 1.2). In the control group an estimated 236 of 1000 participants died up to day 60. Remdesivir probably makes little or no difference to all‐cause mortality at up to day 60 (estimated 200 per 1000 participants; 95% CI 73 fewer to 12 more per 1000) compared to placebo or standard care alone (RR 0.85, 95% CI 0.69 to 1.05; 1 study, 1281 participants; I² = not applicable; moderate‐certainty evidence). Our main reasons for downgrading the certainty of the evidence were serious imprecision because the optimal information size was not reached. WHO Solidarity Norway 2021 solely provided the 60‐day mortality rate as a percentage: 7.1% (95% CI 1.8 to 17.5) versus 5.3% (95% CI 1.3 to 13.1) for remdesivir and standard care alone; estimated marginal risk difference in percentage points: 1.9 (95% CI ‐7.8 to 11.6).
1.2. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 2: All‐cause mortality at up to day 60
In‐hospital mortality
We assessed in‐hospital mortality at the longest follow‐up available, which was 150 days.
In‐hospital mortality at up to day 150
One study reported this outcome for 8275 participants (see Analysis 1.3). In the control group an estimated 156 of 1000 participants died in hospital at up to day 150. Remdesivir probably makes little or no difference to in‐hospital mortality up to 150 days (estimated 145 per 1000 participants; 95% CI 25 fewer to 5 more per 1000) compared to placebo or standard care alone (RR 0.93, 95% CI 0.84 to 1.03; 1 study, 8275 participants; I² = not applicable; moderate‐certainty evidence). We downgraded the certainty of the evidence because of serious risk of bias due to selective reporting.
1.3. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 3: In‐hospital mortality at up to day 150
Clinical status
We assessed clinical status at day 28 by improvement of clinical status (participants alive and ready to be discharged) and worsening of clinical status (composite of participants with new need for invasive mechanical ventilation or death). Where available, we assessed time‐to‐event data for this endpoint. We did not find data for clinical improvement or worsening beyond day 28.
Improvement of clinical status (alive and ready to discharge at up to day 28)
Four studies reported this outcome for 2514 participants (see Analysis 1.5). When treated without remdesivir, 617 per 1000 participants experienced clinical status improvement within 28 days. Remdesivir probably increases the chance of clinical improvement slightly (estimated 685 per 1000 participants; 95% CI 37 more to 105 more per 1000) compared to placebo or standard care alone (RR 1.11, 95% CI 1.06 to 1.17; 4 studies, 2514 participants; I² = 0%; moderate‐certainty evidence). Our main reasons for downgrading the certainty of the evidence were risk of bias because of lack of blinding and one study was stopped earlier than scheduled.
1.5. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 5: Clinical improvement: alive and ready to discharge
Two studies reported time‐to‐event data for this outcome for 1225 participants (see Analysis 1.6). Treatment with remdesivir probably makes little or no difference to the chance of clinical improvement compared to placebo or standard care alone when measured over time (hazard ratio (HR) 1.06, 95% CI 0.93 to 1.20; 2 studies, 1225 participants; I² = 0%).
1.6. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 6: Clinical improvement: alive and ready to discharge (time‐to‐event)
Worsening of clinical status (new need for invasive mechanical ventilation or death at up to day 28)
Two studies reported time‐to‐event data for this outcome for 1734 participants (see Analysis 1.8). When treated without remdesivir, 544 per 1000 participants experienced clinical status worsening within 28 days. Remdesivir probably decreases the risk of clinical worsening (estimated 409 per 1000 participants; 95% CI 198 fewer to 69 fewer per 1000) compared to placebo or standard care over time (HR 0.67, 95% CI 0.54 to 0.82; 2 studies, 1734 participants; I² = 0%; moderate‐certainty evidence). Our main reason for downgrading the certainty of the evidence was very serious risk of bias because of lack of blinding and retrospective analyses of RCT data with a high risk for selective reporting. One study reported this composite outcome for 683 participants (see Analysis 1.7): RR 0.70, 95% CI 0.52 to 0.94; risk difference (RD) 76 fewer per 1000, 95% CI 121 fewer to 15 fewer; 1 study, 683 participants; I² = not applicable; low‐certainty evidence. Our main reasons for downgrading the certainty of the evidence were serious imprecision because the optimal information size was not reached, and serious risk of bias because of lack of blinding. One study reported a composite of progression to mechanical ventilation or death for 7569 participants, but did not differ between non‐invasive and invasive ventilation (WHO Solidarity Trial Consortium 2022). Therefore, we could not include these data in the meta‐analysis. Progression or death occurred in 744 of 3787 cases (19.6%) in the remdesivir group and 851 of 3782 cases (22.5%) in the control group (rate ratio 0.84, 95% CI 0.75 to 0.93; P value = 0.001; 1 study, 7569 participants; I² = not applicable).
1.8. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 8: Clinical worsening: new need for invasive mechanical ventilation or death (time‐to‐event)
1.7. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 7: Clinical worsening: new need for invasive mechanical ventilation or death
Adverse events (any grade at up to day 28)
Four studies reported this outcome for 2498 participants (see Analysis 1.9). In the control group, adverse events of any grade occurred in an estimated 579 per 1000 people. Remdesivir may make little or no difference to the risk of adverse events within 28 days (estimated 602 per 1000 participants; 95% CI 46 fewer to 104 more per 1000) when compared to placebo or standard care alone (RR 1.04, 95% CI 0.92 to 1.18; 4 studies, 2498 participants; I² = 68%; low‐certainty evidence). Our main reasons for downgrading the certainty of the evidence were serious imprecision because of wide confidence intervals in the studies and/or the 95% confidence interval includes both benefits and harms and serious risk of bias because of lack of blinding, and one study was stopped earlier than scheduled.
1.9. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 9: Adverse events, any grade
Serious adverse events (at up to day 28)
Four studies reported this outcome for 2498 participants (see Analysis 1.11). In the control group, serious adverse events occurred in an estimated 273 per 1000 people. Remdesivir may make little or no difference to the risk of serious adverse events within 28 days (estimated 229 per 1000 participants; 95% CI 96 fewer to 19 more per 1000) when compared to placebo or standard care alone (RR 0.84, 95% CI 0.65 to 1.07; 4 studies, 2498 participants; I² = 59%; low‐certainty evidence). Our main reasons for downgrading the certainty of the evidence were serious imprecision because of wide confidence intervals in the studies and/or the 95% confidence interval includes both benefits and harm. We also downgraded for serious risk of bias because of lack of blinding, and one study was stopped earlier than scheduled.
1.11. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 11: Serious adverse events
Secondary outcomes
All‐cause mortality at up to day 28 (time‐to‐event)
Two studies reported this outcome for 6513 participants (see Analysis 1.4). Treatment with remdesivir resulted in no difference in mortality when measured over time (HR 0.88, 95% CI 0.67 to 1.16; 2 studies, 6513 participants; I² = 57%). One study reported median number of days and interquartile range (IQR) from randomisation to death for 236 participants (Wang 2020): 9.5 days (IQR 6.0 to 18.5) for 158 participants in the remdesivir group and 11.0 days (IQR 7.0 to 18.0) for 78 participants in the control group. A Kaplan‐Meier curve was not provided, and a hazard ratio could not be estimated.
1.4. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 4: All‐cause mortality (time‐to‐event)
Quality of life
We did not find any data for this outcome.
Adverse events, grade 3 to 4
Four studies reported this outcome for 2498 participants (see Analysis 1.10). Considering the reported event rates across studies, we estimated that remdesivir results in 39 fewer participants sustaining at least one adverse event grade 3 to 4 compared to placebo or standard care alone amongst 1000 participants. Treatment with remdesivir probably results in little or no difference in the occurrence of adverse events grade 3 to 4 within 28 days when compared to placebo or standard care alone (RR 0.92, 95% CI 0.84 to 1.01; 4 studies, 2498 participants; I² = 0%).
1.10. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 10: Adverse events, grade 3 to 4
Ventilator‐free days
One study reported this outcome for 1281 participants (see Analysis 1.12). We found that remdesivir may increase the number of ventilator‐free days compared to placebo or standard care alone (mean difference 1.90, 95% CI 0.61 to 3.19; P value = 0.004; 1 study, 1281 participants; I² = not applicable). One study provided median ventilator‐ or oxygenation‐free days at up to day 29 for 832 participants (WHO Solidarity France 2021): 29 days (IQR 20 to 29) versus 29 days (IQR 16 to 29) for remdesivir or standard care alone.
1.12. Analysis.

Comparison 1: Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19, Outcome 12: Ventilator‐free days at day 28
Individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
We have presented the summary of findings and the certainty of the evidence for adult non‐hospitalised participants with mild COVID‐19 and at least one risk factor for clinical progression, comparing a three‐day course of remdesivir to placebo or standard care alone. We did not identify any studies in individuals with asymptomatic SARS‐CoV‐2 infection, therefore we have no data on this subgroup to include in the analysis.
Primary outcomes
All‐cause mortality
We assessed all‐cause mortality at up to day 28. We did not find data for all‐cause mortality beyond day 28.
All‐cause mortality at up to day 28
One study reported this outcome for 562 participants (see Analysis 6.1). There were no events observed, thus it was not possible to determine whether remdesivir makes a difference to 28‐day mortality.
6.1. Analysis.

Comparison 6: Remdesivir and standard care versus standard care (plus/minus placebo) in mild COVID‐19, Outcome 1: All‐cause mortality at up to day 28
Improvement of clinical status (symptom alleviation up to day 14)
One study reported symptom alleviation (regression or resolution) for 126 participants at up to day 14 (see Analysis 6.2). When treated without remdesivir, an estimated 250 per 1000 participants experience improvement of clinical status within 14 days. We are uncertain whether remdesivir increases or decreases the chance of symptom alleviation by day 14 (estimated 333 per 1000, 95% CI 61 fewer to 289 more) compared to placebo (hazard ratio 1.41, 95% CI 0.73 to 2.69; 1 study, 126 participants; I² = not applicable; very low‐certainty evidence). Reasons for downgrading were serious risk of bias because of a large amount of missing data and differences between pre‐definition and reporting of the outcome, leading to selective reporting and indirectness. Additionally, the outcome measurement was performed with the FLU‐PRO plus questionnaire, initially validated for influenza and adapted to SARS‐CoV‐2 infection. Although performance seems to be good for the evaluation of COVID‐19 symptoms (Richard 2021), those seem to differ even between variants of the virus. We downgraded the certainty of the evidence another level because of imprecision due to the wide confidence interval and because the optimal information size was not reached.
6.2. Analysis.

Comparison 6: Remdesivir and standard care versus standard care (plus/minus placebo) in mild COVID‐19, Outcome 2: Clinical improvement: symptom alleviation at up to day 14
Worsening of clinical status (admission to hospital or death within 28 days)
One study reported this outcome for 562 participants (see Analysis 6.3). When treated without remdesivir, an estimated 64 per 1000 participants had to be hospitalised within 28 days. Remdesivir probably decreases clinical worsening by day 28 (estimated 18 per 1000, 95% CI 57 fewer to 16 fewer) compared to placebo (RR 0.28, 95% CI 0.11 to 0.75; 1 study, 562 participants; I² = not applicable; moderate‐certainty evidence). Our main reasons for downgrading the certainty of the evidence were serious imprecision because of the wide confidence interval and because the optimal information size was not reached.
6.3. Analysis.

Comparison 6: Remdesivir and standard care versus standard care (plus/minus placebo) in mild COVID‐19, Outcome 3: Clinical worsening: admission to hospital or death at up to day 28
Quality of life
We did not find any data for this outcome.
Serious adverse events
One study reported this outcome for 562 participants (see Analysis 6.4). In the control group, serious adverse events occurred in an estimated 67 per 1000 people. Remdesivir may decrease the rate of serious adverse events by day 28 (estimated 18 per 1000, 95% CI 60 fewer to 20 fewer) compared to placebo (RR 0.27, 95% CI 0.10 to 0.70; 1 study, 562 participants; I² = not applicable; low‐certainty evidence). Our main reasons for downgrading the certainty of the evidence were serious imprecision because of the wide confidence interval and because the optimal information size was not reached, and serious indirectness due to huge overlap with COVID‐19 symptoms, already considered in hospitalisation or death.
6.4. Analysis.

Comparison 6: Remdesivir and standard care versus standard care (plus/minus placebo) in mild COVID‐19, Outcome 4: Serious adverse events
Adverse events (any grade)
One study reported this outcome for 562 participants (see Analysis 6.5). In the control group, adverse events of any grade occurred in an estimated 463 per 1000 people. Remdesivir probably makes little or no difference to the risk of adverse events by day 29 (estimated 421 per 1000, 95% CI 111 fewer to 46 more) compared to placebo (RR 0.91, 95% CI 0.76 to 1.10; 1 study, 562 participants; I² = not applicable; moderate‐certainty evidence). Our main reasons for downgrading the certainty of the evidence were serious imprecision because of the wide confidence interval and because the optimal information size was not reached.
6.5. Analysis.

Comparison 6: Remdesivir and standard care versus standard care (plus/minus placebo) in mild COVID‐19, Outcome 5: Adverse events, any grade
Subgroup analyses
We conducted subgroup analyses for prioritised effectiveness outcomes to explore heterogeneity between predefined subgroups. In the first version of this review, we performed analyses solely for 28‐day mortality in individuals with moderate to severe disease. With the publication of in‐hospital mortality at up to day 150 in the WHO Solidarity trial, additional data for subgroups with different disease severity, based on respiratory support at baseline, became available. The only RCT in non‐hospitalised individuals with mild disease available to date did not report any deaths and therefore subgroups depending on severity at baseline could not be determined.
Age of participants
One study reported all‐cause mortality at up to day 28 divided by age groups (< 50 years, 50 to 69 years, > 69 years) for 5451 participants (see Analysis 2.1). There were no subgroup differences (Chi²= 0.10, df = 2, P = 0.95, I² not applicable).
2.1. Analysis.

Comparison 2: Subgroup analysis (age of participants): remdesivir and standard care versus standard care (plus/minus placebo), Outcome 1: All‐cause mortality at up to day 28
Pre‐existing conditions
Protocol‐specified comorbidities included diabetes, respiratory disease, hypertension, immunosuppression, obesity, and cardiac injury. One study reported all‐cause mortality at up to day 28 subdivided by pre‐existing conditions of interest (WHO Solidarity Trial Consortium 2022, interim results). They compared the effect of remdesivir in one specific subgroup (e.g. with asthma) to a control without that condition (e.g. without asthma). However, since there is a partial overlap of comorbidities between subgroups, control groups might therefore involve participants with other pre‐existing conditions of interest.
Timing of first dose administration with illness onset
One study reported all‐cause mortality at up to day 28 divided by timing of first dose administration with illness onset for 233 participants (see Analysis 3.1). There were no relevant subgroup differences (Chi²= 0.74, df = 1, P = 0.39, I² not applicable).
3.1. Analysis.

Comparison 3: Subgroup analysis (timing of first dose administration with illness onset): remdesivir and standard care versus standard care (plus/minus placebo), Outcome 1: All‐cause mortality at up to day 28
Severity of condition
Three studies reported all‐cause mortality by day 28 subdivided by respiratory support at baseline for 3194 participants (see Analysis 4.1). The evidence suggests a benefit for remdesivir compared to placebo or standard care alone only in the subgroup with low‐flow oxygen at baseline (RR 0.32, 95% CI 0.15 to 0.66; 1 study, 435 participants; I² not applicable). The test for subgroup differences suggests relevant subgroup differences and reveals high heterogeneity: Chi² = 8.32, df = 2, P = 0.02, I² = 75.7%.
4.1. Analysis.

Comparison 4: Subgroup analysis (severity of condition, based on respiratory support at baseline): remdesivir and standard care versus standard care (plus/minus placebo), Outcome 1: All‐cause mortality at up to day 28
One study reported in‐hospital mortality within 150 days subdivided by respiratory support at baseline for 8275 participants (see Analysis 4.2). The evidence does not show a relevant subgroup difference: Chi² = 4.02, df = 2, P = 0.13, I² = 50%. Compared to 28‐day mortality, there are no data reported for the subgroup with low‐flow oxygen only.
4.2. Analysis.

Comparison 4: Subgroup analysis (severity of condition, based on respiratory support at baseline): remdesivir and standard care versus standard care (plus/minus placebo), Outcome 2: In‐hospital mortality at up to day 150
Duration of remdesivir application
We compared a five‐day course of remdesivir to a 10‐day course for this subgroup. One study reported all‐cause mortality at up to day 28 subdivided by duration of remdesivir application for 584 participants (see Analysis 5.1). There were no subgroup differences (Chi²= 0.09, df = 1, P = 0.09, I² not applicable).
5.1. Analysis.

Comparison 5: Subgroup analysis (duration of remdesivir application): remdesivir and standard care versus standard care (plus/minus placebo), Outcome 1: All‐cause mortality at up to day 28
Sensitivity analysis
None of the analyses had an I2 above 80%, therefore we did not perform sensitivity analyses. Highest detected heterogeneity was 68% and 59% for adverse events of any grades and serious adverse events in the hospitalised population, respectively. For the analysis of any adverse events, heterogeneity might be caused by the divergent effects in Beigel 2020 and Spinner 2020. Whereas Beigel 2020 (placebo‐controlled, double‐blinded) reported fewer events in the control group, Spinner 2020 (open‐label) reported fewer events in the remdesivir group. For the analysis of serious adverse events, heterogeneity might be caused by the opposing effect of WHO Solidarity France 2021 (open‐label), which reported fewer events in the control group, whereas the other studies included reported fewer events in the remdesivir group.
Discussion
Summary of main results
The aim of this review was to assess the effects of remdesivir and standard care compared to standard care plus/minus placebo on clinical outcomes in patients treated for SARS‐CoV‐2 infection. This is the first update of the initial systematic review (Ansems 2021). We included nine RCTs with 11,218 participants diagnosed with SARS‐CoV‐2 infection, of whom 5982 were randomised to receive remdesivir. We classified two studies as "awaiting classification": one completed study with 60 participants and one study that was terminated early after enrolment of 249 from 1116 planed participants due to study enrolment feasibility. Furthermore, we identified five ongoing studies, one of which was suspended (recruitment was not possible due to infection incidences).
Remdesivir plus standard care versus standard care (plus/minus placebo)
Hospitalised individuals with moderate to severe COVID‐19
Remdesivir probably makes little or no difference to all‐cause mortality at up to day 28 (RR 0.93, 95% CI 0.81 to 1.06; moderate‐certainty evidence). This assertion remains unchanged since the first version of the review: no additional data could be included. However, the Canadian WHO Solidarity add‐on trial provided follow‐up data at up to day 60, which supports the assumption that remdesivir probably makes little to no difference to all‐cause mortality (RR 0.85, 95% CI 0.69 to 1.05; moderate‐certainty evidence). With the publication of the final results from the WHO Solidarity trial, data on in‐hospital mortality at up to day 150 for 8257 participants became available: RR 0.93, 95% CI 0.84 to 1.03; moderate‐certainty evidence. Overall, having evaluated five RCTs with 11,247 participants, we assume, with moderate certainty, that remdesivir has no beneficial effect on survival.
In the first version of this review we had difficulties assessing the effect of remdesivir on clinical improvement or deterioration due to differing endpoint definitions and competing risk of death. After deciding on more comprehensive surrogate parameters for the clinical course of COVID‐19, we evaluated data from four RCTs and 2514 participants. We found that remdesivir probably increases the chance of clinical improvement slightly with an estimated 685 per 1000 participants compared to 617 per 1000 treated with placebo or standard care alone (RR 1.11, 95% CI 1.06 to 1.17; moderate‐certainty evidence). Data on improvement over time were limited and less conclusive (HR 1.06, 95% CI 0.93 to 1.20). Only one study reported data for the composite of new invasive mechanical ventilation or death, with a reduced risk of clinical worsening after remdesivir application (RR 0.70, 95% CI 0.52 to 0.94; low‐certainty evidence). Time‐to‐event data in more than double the population (1734 versus 683 participants) supports the favouring direction towards remdesivir with an estimated 409 versus 544 per 1000 participants experiencing clinical worsening (HR 0.67, 95% CI 0.54 to 0.82, low‐certainty evidence). However, the certainty of the evidence was only low due to imprecision and risk of bias. Overall, remdesivir may be beneficial in the clinical course of COVID‐19, but certainty of the evidence remains low to moderate.
In the first version of this review we identified subgroup differences for all‐cause mortality at up to day 28 in the subgroup analysis for severity of condition, although with high heterogeneity (Chi² = 8.32, df = 2, P = 0.02, I² = 75.7%). The evidence suggested a benefit for remdesivir compared to placebo or standard care alone only in the subgroup with low‐flow oxygen at baseline (RR 0.32, 95% CI 0.15 to 0.66; 1 study, 435 participants). However, these findings were based on data from one study only that reported the outcome based on differentiated respiratory support at baseline (Beigel 2020). Data for this subgroup and outcome were not provided by any other matching study. However, WHO Solidarity Trial Consortium 2022 reported in‐hospital mortality at up to day 150 subdivided by respiratory support at baseline. Although each cohort was relevantly larger than the available groups for 28‐day mortality, longer‐term data could not reproduce the finding of Beigel 2020 in the subgroup with low‐flow or high‐flow oxygen at baseline (RR 0.9, 95% CI 0.79 to 1.01; 1 study, 5839 participants). Comparison however is impeded, because the subgroups are less differentiated than preferable. The tendency towards a favouring effect for remdesivir in the subgroup with less severe respiratory impairment can still not be safely interpreted due to missing evidence. We detected no differences for mortality at up to day 28 in further participant subgroups relevant for daily clinical routine, namely age, timing of first remdesivir dose, and duration of remdesivir application.
We included results from one additional RCT to assess the adverse effects profile of remdesivir compared to placebo or standard care alone (2498 participants, four studies). Remdesivir may make little or no difference to the incidence of serious adverse events (RR 0.84, 95% CI 0.65 to 1.07, low‐certainty evidence), or any adverse events (RR 1.04, 95% CI 0.92 to 1.18; low‐certainty evidence). The assumption that remdesivir does not appear to cause more adverse events than standard care alone remains the same as in the first version of this review, but the certainty of the evidence had to be adapted due to risk of bias.
Non‐hospitalised individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
Since the first version of this review, the PINETREE trial published data on the outpatient use of remdesivir in non‐hospitalised patients with mild COVID‐19 (Gottlieb 2021). Since in terms of baseline disease severity, clinical course, and duration of the treatment (3 days versus 10 days) this population differs relevantly from the hospitalised population, we analysed the data separately. To date, this is the only RCT in the outpatient setting of our knowledge. All data analysed derived from this one study with 562 participants. All the participants were symptomatic and had at least one risk factor for disease progression. None of the participants died within the study period of 28 days, but remdesivir probably decreased the rate of hospitalisation by an estimated 18 versus 64 per 1000 participants (RR 0.28, 95% CI 0.11 to 0.75, moderate‐certainty evidence). Clinical improvement in terms of symptom resolution remains uncertain due to lack of data. Remdesivir may decrease the incidence of serious adverse events by an estimated 18 versus 67 per 1000 participants (RR 0.27, 95% CI 0.10 to 0.70; low‐certainty evidence) and makes little to no difference to the risk of adverse events of any grade (RR 0.91, 95% CI 0.76 to 1.10; moderate‐certainty evidence) by day 28. Quality of life was not assessed.
Overall completeness and applicability of evidence
We identified nine RCTs, mainly from high‐ and upper‐middle‐income countries, investigating the therapeutic effects of remdesivir compared to placebo or standard care alone in a total of 11,218 hospitalised and non‐hospitalised adults with SARS‐CoV‐2 infection. The diagnosis of SARS‐CoV‐2 infection was confirmed by polymerase chain reaction (PCR) or antigen test and, in some studies, radiological signs of COVID‐19 pneumonia. The largest of the included studies stated that diagnosis of COVID‐19 was made "in the view of the responsible physician"; PCR confirmation was not required (WHO Solidarity Trial Consortium 2022). The proportion of PCR‐negative participants at baseline was reported in two studies (Gottlieb 2021; Wang 2020). The majority of participants received other experimental or standardised COVID‐19 treatment options, such as corticosteroids, antimicrobials, hydroxychloroquine, convalescent plasma, or combinations of these treatments. All trials were conducted between February 2020 and April 2021, before the emergence of the B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant. They also preceded widespread vaccination or vaccinated participants were specifically excluded (Gottlieb 2021; WHO Solidarity France 2021). There is a general underrepresentation of female participants in COVID‐related treatment studies. As suggested by the investigation of de Vries 2022 this is due to the higher proportion of men affected by severe illness in the early stage of the pandemic than structural underrepresentation.
Eight of the included studies involved hospitalised, moderately, or severely ill people with SARS‐CoV‐2 infection, or both, and compared the effect of a 10‐day course of remdesivir additional to standard care (10,876 evaluated participants) to placebo (1198 evaluated participants) or standard care alone (9678 evaluated participants). To evaluate the effects of remdesivir through meta‐analysis, we included data from seven RCTs (10,706 evaluated participants). One of the participants was adolescent between 12 and 18 years old. The analysis of safety outcomes (serious adverse events, adverse events) was affected by a relevant lack of data. Since the largest study did not report safety data (WHO Solidarity Trial Consortium 2022), we could only include data for 2498 participants from four RCTs in our analysis. One study involved non‐hospitalised symptomatic participants with SARS‐CoV‐2 infection and at least one risk factor for disease progression. They compared a three‐day course of remdesivir to placebo (both in addition to standard care) in 562 participants. Eight of the participants were adolescents between 12 and 18 years old. Different scales and definitions for disease severity and progression were used amongst studies. For hospitalised patients with moderate to severe COVID‐19, the need for respiratory support essentially determines their course within the hospital (e.g. ICU admission). We therefore analysed respiratory support at baseline and during the observational period as a surrogate for COVID‐19 disease severity. The combination of low‐ and high‐flow oxygen as well as non‐invasive and invasive mechanical ventilation in the WHO Solidarity trial unfortunately impeded pairing with data from other studies.
Since the first version of this review no additional data on 28‐day mortality became available through randomised controlled studies. Add‐on trials, published by participating sites of the multinational WHO Solidarity trial, provided further information but meta‐analysis was partially limited due to overlap in the participant cohort with the main investigation. With the publication of their final results, WHO Solidarity Trial Consortium 2022 provided the largest analysed collective (8275 evaluated participants) with a follow‐up of 150 days. It supports our former conclusion, that remdesivir may not have an effect on mortality in hospitalised patients with COVID‐19. In addition, analyses of mortality in subgroups with respect to disease severity display a tendency towards a beneficial effect of remdesivir in patients with less extensive oxygen support. However, the decreased mortality in patients with low‐flow oxygen support shown by Beigel 2020 has yet not been replicated.
For non‐hospitalised individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19, mortality appears to be insufficient to evaluate the efficacy of remdesivir treatment, because it is expected to be very low in a subgroup of less severely ill patients. We rate the composite endpoint of admission to hospital or death as quite crucial for this specific subgroup, since clinical deterioration essentially determines the person's health‐related quality of life, functional independence, and autonomy. The according data, published by Gottlieb 2021, suggest a beneficial effect of remdesivir in addition to standard care. This finding is supported indirectly by our analyses of clinical course in the hospitalised setting with moderate to severe illness. However, it is to be noted that both collectives (non‐hospitalised and hospitalised) differ significantly in the extent of additional standard care, remdesivir treatment length (three versus 10 days), and prognosis. Since we did not identify any studies on individuals with asymptomatic SARS‐CoV‐2 infection, we have no data on this subgroup. Therefore, further investigations, especially in patients with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19 in the non‐hospitalised setting, are needed.
Although we contacted all study authors, especially with regard to detailed description of the extent of respiratory support (e.g. low‐ versus high‐flow oxygen, non‐invasive versus invasive mechanical ventilation), there remained differences in reporting severity of illness and incomplete data sets, resulting in a relevant obstacle to the planned subgroup analysis. Hence, due to incompleteness of the data, uncertainty remains regarding a possible benefit of remdesivir treatment for COVID‐19 patients receiving low‐flow oxygen support only.
The applicability of our results to the current medical care for COVID‐19 patients is limited by the large proportion of unvaccinated participants exposed to early variants of SARS‐CoV‐2 in the RCTs contributing to this version of the review. The a priori risk for progression to severe disease (e.g. hospitalisation and respiratory support) has markedly decreased since the early stages of the pandemic and risk factors have changed, now essentially including insufficient immune responses to vaccination. It is difficult to establish how our findings apply to current practice due to these features of the evidence base. Hence, our conclusions account for the outlined population that has been studied in available previous RCTs and this must be carefully considered when translated into current clinical practice for treating COVID‐19 patients. Future RCTs in selected populations bearing a high risk of a severe course of COVID‐19 (e.g. immunodeficient patients with insufficient vaccination responses) infected with current variants of SARS‐CoV‐2 could provide further insight into the question of how virus variants and vaccination response status affect our conclusions.
Certainty of the evidence
We included data from seven RCTs in our meta‐analyses to assess the effects of remdesivir for hospitalised individuals with moderate to severe COVID‐19 and one RCT for non‐hospitalised individuals with mild disease. We evaluated the certainty of the evidence using the GRADE approach, with any downgrading substantiated (see Table 1; Table 2). The evidence for efficacy and safety outcomes was of moderate to very low certainty.
Individuals with moderate to severe COVID‐19
All‐cause mortality (at up to day 28 and 60)
We downgraded to moderate certainty of evidence for serious imprecision due to wide 95% confidence intervals that include both benefits and harms and for serious imprecision because data for 60‐day mortality did not reach the optimal information size, respectively.
In‐hospital mortality (at up to day 150)
We downgraded to moderate certainty of evidence for serious risk of bias because of selective reporting.
Clinical improvement: alive and ready for discharge (at up to day 28)
We downgraded to moderate certainty of evidence due to serious risk of bias because of inadequate blinding of participants, personnel, and outcome assessors.
Clinical worsening: time to new need for invasive mechanical ventilation or death (at up to day 28)
We downgraded to moderate certainty of evidence for serious risk of bias because of lack of blinding of participants, personnel, and outcome assessors.
Serious adverse events
We downgraded to low certainty of evidence for serious imprecision due to wide 95% confidence intervals that include both benefits and harms, and for risk of bias because of lack of blinding and because one study was stopped earlier than scheduled.
Adverse events (any grade)
We downgraded to low certainty of evidence because of serious imprecision due to wide 95% confidence intervals that included both benefits and harms, and risk of bias because of lack of blinding and because one study was stopped earlier than scheduled.
Individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
All‐cause mortality
There were no deaths reported within 28 days of the study period.
Clinical improvement: symptom alleviation (at up to day 14)
We downgraded to very low certainty of evidence because of serious risk of bias due to missing values and selective reporting, and serious imprecision due to the wide confidence interval, including both benefit and harm. Additionally, we downgraded for serious indirectness due to differences in pre‐defined outcome and measurement, as well as use of an adapted questionnaire.
Clinical worsening: admission to hospital or death (at up to day 28)
We downgraded to moderate certainty of evidence for serious imprecision because of the wide confidence interval and because the optimal information size was not reached.
Serious adverse events
We downgraded to low certainty of evidence for serious imprecision because of the wide confidence interval, and because the optimal information size was not met. We also downgraded for indirectness because there was a relevant overlap of COVID‐19 symptoms, which were already considered in hospitalisation or death.
Adverse events (any grade)
We downgraded to moderate certainty of evidence because of serious imprecision due to the wide confidence interval, and because the optimal information size was not met.
Potential biases in the review process
Experienced medical information specialists of the CEOsys consortium developed an all‐encompassing search strategy to identify the available evidence to answer our research question. We aimed to identify all completed, but also ongoing, studies for inclusion in this review. The sensitive search included relevant electronic databases as well as clinical trial registries. As a supplementary search, we screened reference lists of included studies. Where data were missing, we contacted study authors; for details, see Characteristics of included studies. An overview of included studies is provided in Table 5. We are confident that we have identified all relevant studies, and we will monitor ongoing studies as well as full publication of preprints closely after the publication of this review.
Differences to review protocol and first version
For a detailed description of differences see the Differences between protocol and review section. A prespecified protocol is available at an international prospective register of systematic reviews (CRD42021238065). As a major difference to the protocol, we initially planned a living approach for this review in the light of the uncertainties that came with the early phase of the pandemic. With emerging knowledge of treatment options on the one side and fast development of virus variants with altering demands on the other side, we regard a re‐evaluation of future updates based on necessity as more fitting. In contrast to our predefined inclusion criteria (adult participants), we did not exclude the studies Spinner 2020 and Gottlieb 2021, which involved adolescent participants between 12 and 18 years. Since only 0.18% and 1.42% of participants were under the age of 18, respectively, we presumed them to have a non‐relevant impact on our results.
We adapted our main outcome set in the first version and in the update according to current knowledge and patient‐oriented relevance (see Table 6). In this update this mainly concerns the modification of clinical course parameters. Since the initial review we agreed on condensed surrogates for either improvement or worsening in clinical status. This allows for a more precise conclusion but also bears the risk of underestimating other aspects of clinical course. Definition of disease severity is no longer linked to classification by the WHO but remains coupled with impairment and respiratory support. As a major difference between the first version and the update, we now include non‐hospitalised individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19. This was based on newly published data on a possible benefit for early (ambulatory) admission of remdesivir. Any change of methodology was done before analysis. We identified no other potential sources of bias in our review process.
Agreements and disagreements with other studies or reviews
The results we found do not decisively differ from those of other systematic reviews (Al‐Abdouh 2021; Angamo 2022; Tanni 2022; Vegivinti 2022) or living guidelines (Kaka 2022). Kaka 2022 published the fifth and final update of its living review in May 2022, with almost identical inclusion of RCTs and methodology (Beigel 2020; Spinner 2020; Wang 2020; WHO Solidarity France 2021; WHO Solidarity Norway 2021; WHO Solidarity Trial Consortium 2022 (interim results)). Comparable to our finding, they identified no relevant effect on mortality, but a moderate increase in improvement parameters and a small reduction in serious adverse events. Whereas they reported a small reduction in the proportion of participants receiving ventilation or ECMO from day 11 to 15, there was little to no difference in the need for new need for ventilation or ECMO from 28 days to six months. They found no benefit of a 10‐day course of remdesivir treatment, when compared to a five‐day course. Lee 2022 also solely included RCTs that evaluated the efficacy and safety of remdesivir compared to placebo or standard care alone. They used an approach of a priori probability with restricted maximum likelihood estimates and highlighted the increased probability that remdesivir reduces mortality by ≥ 1% in the subgroups without supplemental oxygen and non‐ventilated participants requiring oxygen. Analogically, the latest review with RCT meta‐analysis published in June 2022 by Beckermann 2022 emphasises the benefit of remdesivir for clinical outcomes in the subgroup of low‐flow oxygen support. It is noteworthy that the review used a targeted literature search and was funded by Gilead Science. One systematic review focused on the safety analysis and reported no or little difference in acute kidney injury and cognitive dysfunction by analysis of Beigel 2020 and Wang 2020 (Izcovich 2022).
In contrast to our review, some cited reviews did not exclusively include RCTs with a placebo or standard care control arm, but also observational cohort studies and case studies (Angamo 2022; Tanni 2022; Thiruchelvam 2022). One of them judged the current data to be insufficient for recommended usage due to high heterogeneity (Thiruchelvam 2022). None of the other reviews included the final results of the WHO Solidarity Trial Consortium 2022, due to its publication in May 2022. Our review excluded the publication Goldman 2020, which compared clinically used dosing schemes of remdesivir, but had no placebo or standard of care arm. The synthetic interpretation of the results of the aforementioned reviews and guidelines is difficult due to different methodological approaches, the type of subgroup formation, and the partial inclusion of non‐RCTs. However, we found no major differences from our conclusions in the cited reviews.
Authors' conclusions
Implications for practice.
Individuals with moderate to severe COVID‐19
The finding of the first version of this review, that remdesivir probably has little or no effect on all‐cause mortality at up to 28 days, remains unaltered due to lack of supplementary data. Additional data on longer‐term mortality up to 150 days supports this finding. Subgroup analyses by initial disease severity (characterised by the level of respiratory support at the start of treatment) led to contradictory results. Hence, the important clinical question, whether the effect of remdesivir treatment on mortality varies according to disease severity, remains unanswered. In contrast to the lack of effectiveness in terms of mortality, there is low‐ to moderate‐certainty evidence that remdesivir treatment has a beneficial effect on clinical course in terms of a reduction in the necessity for invasive ventilation, while slightly increasing the chance of the patient reaching a clinical state of being ready for discharge. In terms of safety, including the new data set, we conclude that remdesivir may not make a relevant difference to the incidence of serious adverse events or any adverse events. However, clinicians have to keep in mind that for the majority of participants included in this review, there were insufficient high‐quality data on safety available due to the specific characteristics of the platform trial included.
Individuals with mild COVID‐19
Only one study provided data for non‐hospitalised, symptomatic individuals at risk for progression at an observational period of 28 days. There were no deaths reported, but remdesivir probably decreases the rate of hospitalisation. Due to incompleteness of data on symptom alleviation we are uncertain whether remdesivir increases or decreases the chance of clinical improvement. Safety analyses show a decreased rate of serious adverse events and no relevant difference in the incidence of any adverse events.
Considering that all previous RCTs did not include vaccinated people and were conducted before the emergence of the Delta and Omicron variants of SARS‐CoV‐2, the applicability of their results to current clinical practice is limited and needs to be re‐evaluated if commensurate evidence becomes available.
Implications for research.
In this update of a systematic review on remdesivir in individuals with SARS‐CoV‐2 infection of varying degrees of severity ‐ from asymptomatic infection through mild to severe disease ‐ we included data from nine randomised controlled trials. Only one of them was performed in non‐hospitalised individuals with mild COVID‐19, hence the application of remdesivir in the earliest stage of disease. However, there are no data on the treatment of asymptomatic patients with SARS‐CoV‐2 infection and the risk of clinical deterioration. Treatment duration was three days in the outpatient setting versus five or 10 days in the hospitalised setting. Furthermore, different scales of disease severity were applied to characterise subgroups, and safety data reporting was incomplete. These aspects lower the certainty of the evidence and make it difficult to draw valid conclusions for important clinical questions during an ongoing pandemic. In particular, differences in the potential benefits or harms of remdesivir for the treatment of COVID‐19 depending on disease severity could not be analysed sufficiently.
Additional data on the efficacy and safety of remdesivir for different population subgroups (e.g. depending on age, severity of disease, vaccination or immunological status, or treatment duration), for current virus variants, for the timing of application of remdesivir in the course of the infection, and for the establishment of core outcomes for COVID‐19 research, may allow us to reduce the uncertainty around the potentially beneficial or harmful effects of remdesivir in future updates of this review.
What's new
| Date | Event | Description |
|---|---|---|
| 23 May 2022 | New citation required and conclusions have changed | Conclusions adapted: conclusion for outpatients added |
| 23 May 2022 | New search has been performed | Review updated: three further studies for inpatients and one study for outpatients included |
History
Review first published: Issue 8, 2021
Notes
Parts of the review's Methods section are adopted from templates of Cochrane Haematology and a similar protocol published by Piechotta 2020, and the corresponding review (Piechotta 2021).
Risk of bias
Risk of bias for analysis 1.10 Adverse events, grade 3 to 4.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beigel 2020 | Low risk of bias | Randomisation was stratified by study site and disease severity in a 1:1 ratio and the allocation sequence was probably concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Some concerns | Participants and care takers were adequately blinded. The analysis was inappropriate (as‐treated population). | Some concerns | There is an uncertain amount of missing data due to competing risk of death. It is uncertain, whether the result is biased or not. | Low risk of bias | Measuring of the outcome was appropriate. The outcome was assessed using standardised methods and assessors were unaware of the treatment assignments. | Low risk of bias | The data was analysed in accordance with a pre‐specified protocol. | Some concerns | Overall judged some concerns due to inappropriate analyses and non‐plausible participant selection. Information on randomisation process is not provided, but blinding was appropriate, and outcome measurement was according to a pre‐specified protocol. There is an uncertain amount of missing data due to competing risk of death. |
| Spinner 2020 | Low risk of bias | Block‐randomisation was not stratified and web‐based. Sites did not have access to the randomisation list. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Participants and care takers were aware of the assigned intervention. There was a neglectable number of participants with deviations from the intended intervention and the analysis was appropriate (intention‐to‐treat population). | Some concerns | There is an uncertain amount of missing data due to competing risk of death. It is uncertain, whether the result is biassed or not. | Low risk of bias | Outcome assessors were aware of the treatment assignments. Adverse events grade 3‐4 are unlikely to be influenced by diagnostic bias due to their severity. | Some concerns | A study protocol provided details of time points, analyses and methods for categorisation and registration of adverse events. Adverse events grade 3 and higher were not compared between groups as stated by the statistical analysis plan. | Some concerns | Overall some concerns for risk of bias. Details about randomisation, blinding of outcome assessors and a protocol were provided. There is an uncertain amount of missing data due to competing risk of death. It was an open‐label study, but it was unlikely to affect the outcome measurement. Outcome was not reported as stated in a pre‐defined statistical analysis plan. |
| Wang 2020 | Some concerns | Randomisation was carried out 2:1 and in stratified blocks. Concealed envelopes were prepared. There were baseline differences in gender distribution, respiratory status at baseline, co‐morbidities and time from symptom onset suggesting possible randomisation problems. | Low risk of bias | The study was carried out double‐blinded and the outcome was assessed by safety set. | Some concerns | There is an uncertain amount of missing data due to competing risk of death. It is uncertain, whether the result is biassed or not. | Low risk of bias | Outcome assessors were unaware of the treatment assignments and could not have been affected by knowledge of intervention. | Low risk of bias | The trial protocol provided details of time points and analysis. The statistical analysis plan could not be found. | Some concerns | Overall judged some concerns for risk of bias. Details about randomisation, blinding of outcome assessors and a protocol were provided. There were baseline differences between intervention and control group. There is an uncertain amount of missing data due to competing risk of death. |
| WHO Solidarity France 2021 | Low risk of bias | Randomisation appears appropriate with concealed allocation. There is an underrepresentation of female participants. | Low risk of bias | The study was open‐label and deviation from intended intervention could be because of experimental context. However, the small amount of deviation could be random, and an appropriate analysis was used. | Low risk of bias | Data was available for all participants. | Some concerns | Recording and assessment of seriousness of adverse events in an unblinded trial could be influenced by the knowledge of treatment group by the assessors, but direction of bias remains unclear and high number of trial centres with many different assessors should rule out systematic bias. | Low risk of bias | Results were measured, analysed and reported according to a pre‐defined protocol. | Some concerns | Overall judged some concerns. Randomisation, measurement, analysis and reporting of results were appropriate. The open‐label design could have influenced the assessment and could have caused deviations in the intended intervention. |
Risk of bias for analysis 1.12 Ventilator‐free days at day 28.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| WHO Solidarity Canada 2022 | Low risk of bias | The allocation sequence was random and likely to be concealed. | High risk of bias | It was an open‐label study with deviations from the intended intervention which could have impacted the result due to inappropriate analysis and insufficient reporting on the population used. | High risk of bias | There is a relevant amount of missing data that could depend on its true value. | Some concerns | A pre‐defined protocol was not provided. Measurement of the outcome was probably performed adequately, but knowledge of the intervention could have influenced the assessment. | Some concerns | There was no provision of a pre‐defined protocol or statistical plan. The reported result was not listed as an outcome in the trial registry record. | High risk of bias | Overall judged high risk of bias due to deviations from the intervention and inappropriate analysis. There was a relevant amount of missing data and no pre‐definition of assessment and analysis. |
Risk of bias for analysis 4.2 In‐hospital mortality at up to day 150.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 4.2.1 No oxygen at baseline | ||||||||||||
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Some concerns | There was no statistical analysis plan and pre‐defined protocol did not state the form of analysis. Reported analyses are appropriate but could have been selected. | Some concerns | Overall judged some concerns. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were appropriate but not pre‐defined with a risk of selective reporting. |
| Subgroup 4.2.2 Low‐flow or high‐flow oxygen at baseline | ||||||||||||
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Some concerns | There was no statistical analysis plan and pre‐defined protocol did not state the form of analysis. Reported analyses are appropriate but could have been selected. | Some concerns | Overall judged some concerns. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were appropriate but not pre‐defined with a risk of selective reporting. |
| Subgroup 4.2.3 Mechanical ventilation at baseline | ||||||||||||
| WHO Solidarity Trial Consortium 2022 | Low risk of bias | Patients were randomised by a study website, which probably concealed the allocation sequence. There were no baseline imbalances that would suggest a problem with randomisation | Low risk of bias | Participants and care takers were aware of the assigned intervention, but there were no deviations from intended interventions and the analysis was appropriate (intention‐to‐treat population). | Low risk of bias | Data was available for nearly all participants (<5% missing) randomised. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected the outcome measurement. | Some concerns | There was no statistical analysis plan and pre‐defined protocol did not state the form of analysis. Reported analyses are appropriate but could have been selected. | Some concerns | Overall judged some concerns. Details about randomisation and missing data were provided. It was an open‐label study, but this could not have affected the outcome measurement. Outcome measurement and analyses were appropriate but not pre‐defined with a risk of selective reporting. |
Acknowledgements
This work is part of a series of reviews investigating treatments and therapies for COVID‐19 as part of the project CEOsys. Text passages in the Background section (e.g. Description of the intervention and Why it is important to do this review) are shared between reviews of this series. We thank the authors of the first published reviews of this series for providing and sharing this information. Moreover, we thank the Cochrane Haematology working group for use of the template for the description of methods.
We thank Rachel Richardson (Associate Editor, Evidence Production and Methods Department, Cochrane Central Executive Team) for her valuable advice and comments regarding the updating of this review.
We thank Vanessa Piechotta (Research Associate and PhD student, Cochrane Haematology, University Cologne) for her substantial contribution to the first version of the review.
Cochrane Haematology supported us in the development of this review. The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Toby Lasserson, Deputy Editor‐in‐Chief, Cochrane Evidence Production and Methods Directorate
Managing Editor (selected peer reviewers, provided comments, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Lara Kahale and Sam Hinsley, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Jenny Bellorini, Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Leticia Kawano‐Dourado, Hcor Research Institute, Hospital do Coracao, São Paulo, Brazil; Pulmonary Division, InCor, University of São Paulo, Brazil (clinical/content review), Hariklia Nguyen (consumer review), Roses Parker, Research Fellow, Cochrane (methods review), Robin Featherstone, Cochrane Central Editorial Service (search review). One additional peer reviewer provided clinical/content peer review, but chose not to be publicly acknowledged.
We thank all authors who provided additional information on their studies.
The research was part of a project supported by the German Federal Ministry of Education and Research (NaFoUniMedCovid19, funding number: 01KX2021; part of the project CEOsys). The contents of this document reflect only the authors' views, and the German Ministry is not responsible for any use that may be made of the information it contains.
FG, FF, MG, and VT are thankful for the support of their colleagues with the Department of Anaesthesia and Intensive Care at the University of Leipzig Medical Centre whilst working on the manuscript of this review during the COVID‐19 pandemic. We thank Dr Sven Laudi at the Department of Anaesthesia and Intensive Care at Leipzig University Medical Services for his biostatistical advice.
Appendices
Appendix 1. Search strategies
Cochrane COVID‐19 Study Register
Search string: remdesivir* OR GS5734 OR "GS 5734"
Study characteristics: 1) "Intervention assignment": "Randomised" OR "Unclear" 2) "Study type": "Interventional" AND "Study design": "Parallel/Crossover" OR "Unclear" OR "Other"
= 428 references
Web of Science Core Collection (Advanced search)
#1 TI=(remdesivir* OR GS5734 OR "GS 5734") OR AB=(remdesivir* OR GS5734 OR "GS 5734")
#2 TI=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2") OR AB=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")
#3 #1 AND #2
#4 TI=(random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII") OR AB=(random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII")
#5 #3 AND #4 Indexes=SCI‐EXPANDED, ESCI
= 795 references
WHO COVID‐19 Global literature on coronavirus disease
Title, abstract, subject: (remdesivir* OR GS5734 OR "GS 5734") AND (random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII")
without MEDLINE, EMBASE, Web of Science, PMC, and PubMed = 569 references
Data and analyses
Comparison 1. Remdesivir and standard care versus standard care (plus/minus placebo) in moderate to severe COVID‐19.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality at up to day 28 | 4 | 7142 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 1.2 All‐cause mortality at up to day 60 | 1 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 1.3 In‐hospital mortality at up to day 150 | 1 | 8275 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 1.4 All‐cause mortality (time‐to‐event) | 2 | 6513 | Hazard Ratio (IV, Random, 95% CI) | 0.88 [0.67, 1.16] |
| 1.5 Clinical improvement: alive and ready to discharge | 4 | 2514 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.06, 1.17] |
| 1.6 Clinical improvement: alive and ready to discharge (time‐to‐event) | 2 | 1225 | Hazard Ratio (IV, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 1.7 Clinical worsening: new need for invasive mechanical ventilation or death | 1 | 683 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.52, 0.94] |
| 1.8 Clinical worsening: new need for invasive mechanical ventilation or death (time‐to‐event) | 2 | 1734 | Hazard Ratio (IV, Random, 95% CI) | 0.67 [0.54, 0.82] |
| 1.9 Adverse events, any grade | 4 | 2498 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 1.10 Adverse events, grade 3 to 4 | 4 | 2498 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 1.11 Serious adverse events | 4 | 2498 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.07] |
| 1.12 Ventilator‐free days at day 28 | 1 | 1281 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.61, 3.19] |
Comparison 2. Subgroup analysis (age of participants): remdesivir and standard care versus standard care (plus/minus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality at up to day 28 | 1 | 5451 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.84, 1.13] |
| 2.1.1 Age < 50 years | 1 | 1913 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.72, 1.45] |
| 2.1.2 Age 50 to 69 years | 1 | 2569 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.78, 1.18] |
| 2.1.3 Age > 69 years | 1 | 969 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.74, 1.28] |
Comparison 3. Subgroup analysis (timing of first dose administration with illness onset): remdesivir and standard care versus standard care (plus/minus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause mortality at up to day 28 | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.47, 2.05] |
| 3.1.1 ≤ 10 days of symptom onset | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.29, 1.95] |
| 3.1.2 > 10 days of symptom onset | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.45, 4.88] |
Comparison 4. Subgroup analysis (severity of condition, based on respiratory support at baseline): remdesivir and standard care versus standard care (plus/minus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 All‐cause mortality at up to day 28 | 3 | 6833 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.64, 1.18] |
| 4.1.1 No oxygen at baseline | 3 | 1794 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.48, 1.89] |
| 4.1.2 Low‐flow oxygen at baseline | 1 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.15, 0.66] |
| 4.1.3 Low‐flow or high‐flow oxygen at baseline | 1 | 3639 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.04] |
| 4.1.4 Mechanical ventilation at baseline | 2 | 965 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.71, 1.58] |
| 4.2 In‐hospital mortality at up to day 150 | 1 | 8275 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.80, 1.13] |
| 4.2.1 No oxygen at baseline | 1 | 1730 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.45, 1.25] |
| 4.2.2 Low‐flow or high‐flow oxygen at baseline | 1 | 5839 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.79, 1.01] |
| 4.2.3 Mechanical ventilation at baseline | 1 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.91, 1.30] |
Comparison 5. Subgroup analysis (duration of remdesivir application): remdesivir and standard care versus standard care (plus/minus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 All‐cause mortality at up to day 28 | 1 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.18, 2.41] |
| 5.1.1 5‐day remdesivir | 1 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.07, 3.66] |
| 5.1.2 10‐day remdesivir | 1 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.13, 4.58] |
Comparison 6. Remdesivir and standard care versus standard care (plus/minus placebo) in mild COVID‐19.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 All‐cause mortality at up to day 28 | 1 | 562 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.2 Clinical improvement: symptom alleviation at up to day 14 | 1 | 126 | Hazard Ratio (IV, Random, 95% CI) | 1.41 [0.73, 2.71] |
| 6.3 Clinical worsening: admission to hospital or death at up to day 28 | 1 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.11, 0.75] |
| 6.4 Serious adverse events | 1 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.10, 0.70] |
| 6.5 Adverse events, any grade | 1 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.76, 1.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beigel 2020.
| Study characteristics | |
| Methods |
|
| Participants |
Baseline characteristics
Inclusion criteria:
Illness of any duration, and at least 1 of the following:
Exclusion criteria:
Previous treatments: lopinavir/ritonavir (Kaletra) |
| Interventions |
|
| Outcomes |
Primary study outcome: time to recovery: the day of recovery was defined as the first day on which the participant satisfies 1 of the following 3 categories from the ordinal scale:
Review outcomes Primary outcomes
Secondary outcomes
|
| Identification | |
| Notes |
|
Gottlieb 2021.
| Study characteristics | |
| Methods |
|
| Participants |
Baseline characteristics
Key Inclusion criteria:
Either:
Exclusion criteria:
Previous treatments: NR |
| Interventions |
|
| Outcomes |
Primary study outcomes:
Review outcomes: Primary outcomes
|
| Identification | |
| Notes |
|
Mahajan 2021.
| Study characteristics | |
| Methods |
|
| Participants |
Baseline characteristics
Inclusion criteria
Exclusion criteria
Previous treatments: NR |
| Interventions |
|
| Outcomes |
Primary study outcome: clinical status from day 12 to 24 on 6‐point ordinal scale Review outcomes Primary outcomes
Secondary outcomes
|
| Identification | |
| Notes |
|
Spinner 2020.
| Study characteristics | |
| Methods |
|
| Participants |
Baseline characteristics
Inclusion criteria:
Exclusion criteria
Previous treatments: not reported |
| Interventions |
|
| Outcomes |
Primary study outcome: clinical status assessed by a 7‐point ordinal scale on day 11
Review outcomes Primary outcomes
Secondary outcomes
|
| Identification | |
| Notes |
|
Wang 2020.
| Study characteristics | |
| Methods |
|
| Participants |
Baseline characteristics
Inclusion criteria
Exclusion criteria:
Previous treatments (received before and after enrolment): injection of interferon alfa‐2b; lopinavir–ritonavir; vasopressors; renal replacement therapy; antibiotics; corticosteroids |
| Interventions |
|
| Outcomes |
Primary study outcome: time to clinical improvement at up to day 28. Clinical improvement was defined as a 2‐point reduction in participant's admission status on a 6‐point ordinal scale, or live discharge from the hospital, whichever came first. The scale is as follows: 6. death; 5. hospital admission for ECMO or mechanical ventilation; 4. hospital admission for non‐invasive ventilation or high‐flow oxygen therapy; 3. hospital admission for oxygen therapy (but not requiring high‐flow or non‐invasive ventilation); 2. hospital admission but not requiring oxygen therapy; 1. discharged or having reached discharge criteria (defined as clinical recovery, i.e. normalisation of pyrexia, respiratory rate < 24 breaths per minute, saturation of peripheral oxygen > 94% on room air, and relief of cough, all maintained for at least 72 hours). Review outcomes Primary outcomes
Secondary outcomes
|
| Identification | |
| Notes |
|
WHO Solidarity Canada 2022.
| Study characteristics | |
| Methods |
|
| Participants |
Baseline characteristics (Canadian cohort of the WHO Solidarity Trial Consortium, unless otherwise noted)
Inclusion criteria:
Exclusion criteria:
Previous treatments: NR |
| Interventions |
|
| Outcomes |
Primary study outcome: in‐hospital mortality Review outcomes (new outcomes, not reported in the WHO Solidarity Trial Consortium and used for the analyses in this review, are marked) Primary outcomes
Secondary outcomes
|
| Identification | |
| Notes |
|
WHO Solidarity France 2021.
| Study characteristics | |
| Methods |
|
| Participants |
Baseline characteristics (French cohort of the WHO Solidarity Trial Consortium and additional participants, not evaluated in the WHO Solidarity Trial Consortium)
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
Primary study outcome: clinical status at day 15 as measured on the 7‐point ordinal scale of the WHO Master Protocol (version 3.0, 3 March 2020)
(1) not hospitalised, no limitation on activities; (2) not hospitalised, limitation on activities; (3) hospitalised, not requiring supplemental oxygen; (4) hospitalised, requiring supplemental oxygen; (5) hospitalised, on non‐invasive ventilation or high‐flow oxygen devices; (6) hospitalised, on invasive mechanical ventilation or ECMO; and (7) dead Review outcomes (new outcomes, not reported in the WHO Solidarity Trial Consortium and used for the analyses in this review, are marked) Primary outcomes
Secondary outcomes
|
| Identification | |
| Notes |
|
WHO Solidarity Norway 2021.
| Study characteristics | |
| Methods |
|
| Participants |
Baseline characteristics
Inclusion criteria:
Exclusion criteria:
Previous treatments: NR |
| Interventions |
|
| Outcomes |
Primary study outcome: all‐cause, in‐ hospital mortality Review outcomes (new outcomes, not reported for those patients in the WHO Solidarity Trial Consortium and used for the analyses in this review, are marked) Primary outcomes
Secondary outcomes
|
| Identification | |
| Notes |
|
WHO Solidarity Trial Consortium 2022.
| Study characteristics | |
| Methods |
|
| Participants |
Baseline characteristics
Inclusion criteria:
Exclusion criteria:
Previous treatments: NR |
| Interventions |
|
| Outcomes |
Primary study outcome: all‐cause mortality, subdivided by the severity of disease at the time of randomisation, measured using patient records throughout the study Review outcomes Primary outcomes
Secondary outcomes
|
| Identification | |
| Notes |
|
Abbreviations:
ALT = alanine transaminase
ARDS = acute respiratory distress syndrome
AST = aspartate transaminase
CAD = coronary artery disease
CKD = chronic kidney disease
CT = computed tomography
ECMO = extracorporeal membrane oxygenation
eGFR = estimated glomerular filtration rate
HR = hazard ratio
ICU = intensive care unit
IMP = investigational medicinal product
IQR = interquartile range
IWRS = interactive web response system
N = total number of participants
n = number of participants
NA = not applicable
NR = not reported
NEWS = National Early Warning Score
NIAID = National Institute of Allergy and Infectious Diseases
OR = odd ratio
PaO2/FiO2 = ratio of arterial oxygen partial pressure to fractional inspired oxygen
PCR = polymerase chain reaction
RCT = randomised controlled trial
RT‐PCR = reverse transcription polymerase chain reaction
SaO2 = arterial oxygen saturation
SD = standard deviation
SoC = standard of care
SpO2 = peripheral oxygen saturation
ULN = upper limit of normal
WHO = World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd‐Elsalam 2021 | The study was retracted |
| Ader 2020 | Duplicate |
| Ader 2021 | No data about the remdesivir intervention |
| Alpern 2020 | Not a randomised controlled trial |
| Anderson 2021 | Not a randomised controlled trial |
| Antinori 2020 | Not a randomised controlled trial |
| Banerjee 2020 | Not a randomised controlled trial |
| CTRI/2020/12/029613 | No intervention with remdesivir |
| CTRI/2020/12/029615 | Combination of remdesivir with other treatments |
| CTRI/2021/01/030830 | Intervention with remdesivir not compared to standard care or placebo |
| CTRI/2021/12/038637 | Not a randomised controlled trial |
| CTRI/2021/12/039011 | Not a randomised controlled trial |
| CTRI/2022/03/041252 | Not a randomised controlled trial |
| Deresinski 2020 | Not a randomised controlled trial |
| Elliott 2020 | Not a randomised controlled trial |
| Elliott 2021 | Combination of remdesivir with other treatments |
| EUCTR2020‐000841‐15‐ES | Intervention with remdesivir not compared to standard care or placebo |
| EUCTR2020‐000936‐23 | Duplicate |
| Euctr2020‐003510‐12‐dk | Wrong patient population |
| EUCTR2020‐004928‐42‐HU | Wrong patient population |
| Goldberg 2021 | Not a randomised controlled trial |
| Goldman 2020 | Combination of remdesivir with other treatments |
| Goldman 2020a | Intervention with remdesivir not compared to standard care or placebo |
| Grein 2020 | Not a randomised controlled trial |
| Grundmann 2022 | Full text not retrievable |
| IRCT20151227025726N28 | Intervention with remdesivir not compared to standard care or placebo |
| IRCT20210324050760N1 | Not a randomised controlled trial |
| ISRCTN15874265 | Combination of remdesivir with other treatments |
| ISRCTN85762140 | Wrong patient population |
| Jang 2021 | Combination of remdesivir with other treatments |
| Kalil 2021 | Combination of remdesivir with other treatments |
| Lapadula 2020 | Not a randomised controlled trial |
| LBCTR2020043495 | Duplicate |
| Medical Brief | Full‐text not retrievable |
| NCT04252664a | Duplicate |
| NCT04256395 | Not a randomised controlled trial |
| NCT04280705 | Duplicate |
| NCT04292899 | Combination of remdesivir with other treatments |
| NCT04302766 | Wrong patient population |
| NCT04321928 | No intervention with remdesivir |
| NCT04323761 | Intervention with remdesivir not compared to standard care or placebo |
| NCT04353596 | No intervetion with remdesivir |
| NCT04401579 | Combination of remdesivir with other treatments |
| NCT04410354 | Combination of remdesivir with other treatments |
| NCT04480333 | Wrong patient population |
| NCT04488081 | Combination of remdesivir with other treatments |
| NCT04492475 | Combination of remdesivir with other treatments |
| NCT04501978 | Intervention with remdesivir not compared to standard care or placebo |
| NCT04518410 | No intervention with remdesivir |
| NCT04539262 | Wrong patient population |
| NCT04583956 | Combination of remdesivir with other treatments |
| NCT04583969 | Combination of remdesivir with other treatments |
| NCT04610541 | Intervention with remdesivir not compared to standard care or placebo |
| NCT04640168 | Combination of remdesivir with other treatments |
| NCT04647695 | Combination of remdesivir with other treatments |
| NCT04678739 | Combination of remdesivir with other treatments |
| NCT04693026 | Combination of remdesivir with other treatments |
| NCT04713176 | Combination of remdesivir with other treatments |
| NCT04728880 | Not a randomised controlled trial |
| NCT04746183 | No intervention with remdesivir |
| NCT04832880 | Combination of remdesivir with other treatments |
| NCT04847622 | Not a randomised controlled trial |
| Olender 2020 | Intervention with remdesivir not compared to standard care or placebo |
| Olender 2021 | Not a randomised controlled trial |
| Padilla 2022 | Not a randomised controlled trial |
| Pan 2020 | Duplicate |
| Pan 2021 | Duplicate |
| PER‐101‐20 | Intervention with remdesivir not compared to standard care or placebo |
| Rosas 2021 | Combination of remdesivir with other treatments |
| Saito 2020 | Not a randomised controlled trial |
| Shakir 2022 | Not a randomised controlled trial |
| Shih 2020 | Not a randomised controlled trial |
| Soto 2020 | Not a randomised controlled trial |
| Sun 2020 | Not a randomised contolled trial |
| Tran 2020 | Not a randomised controlled trial |
| Winstead 2021 | Not a randomised controlled trial |
Characteristics of studies awaiting classification [ordered by study ID]
NCT04596839.
| Methods | Trial design: RCT
Sample size:
Setting: inpatient Language: Bengali Number of centres: multicentre Type of intervention: drug |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary study outcome:
Secondary study outcomes:
Review outcomes Inpatient setting:
|
| Notes |
|
REDPINE 2022.
| Methods | Trial design: RCT
Sample size: 249
Setting: inpatient Language: English Number of centres: 63 Type of intervention: drug |
| Participants |
Key Inclusion criteria
Key exclusion criteria
|
| Interventions |
Intervention details
Treatment details of the control group:
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
|
ALT = alanine transaminase
AST = aspartate transaminase
ECMO = extracorporeal membrane oxygenation
eGFR = estimated glomerular filtration rate
ICU = intensive care unit
IMV = invasive mechanical ventilation
NR = not reported
RCT = randomised controlled trial
RDV = remdesivir
RRT = renal replacement therapy
RT‐PCR = reverse transcription polymerase chain reaction
SoC = standard of care
Characteristics of ongoing studies [ordered by study ID]
IRCT20210709051824N1.
| Study name | 'Assessment of utility of remdesivir in patients with acute kidney injury or cronic kidney disease in admitted COVID‐19 patients' |
| Methods | Trial design: RCT
Sample size: NR
Setting: NR (probable inpatient, since inclusion criterion: lung involvement above 20% or hypoxia) Language: English Number of centres: NR Type of intervention: drug |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Details of intervention:
Treatment details of control group:
Concomitant therapy: NR |
| Outcomes |
Primary study outcomes:
Secondary study outcomes: NR |
| Starting date | Date of registration: 8 December 2021 |
| Contact information | Mahboobeh Freidoon Shahid Beheshti University of Medical Sciences, Tehran Iran (Islamic Republic of) |
| Notes |
|
NCT04252664.
| Study name | 'A trial of remdesivir in adults with mild and moderate COVID‐19' |
| Methods | Trial design: RCT
Sample size: NR
Setting: inpatient Language: Chinese Number of centres: 1 (Jin Yin‐tan Hospital Wuhan, Hubei, China, 100013) Type of intervention: drug |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Details of intervention:
Treatment details of control group:
Concomitant therapy: NR |
| Outcomes |
Primary study outcome: Time to clinical recovery (TTCR) (time frame: up to 28 days) TTCR is defined as the time (in hours) from initiation of study treatment (active or placebo) until normalisation of fever, respiratory rate, and oxygen saturation, and alleviation of cough, sustained for at least 72 hours, or live hospital discharge, whichever comes first. Normalisation and alleviation criteria:
Secondary outcome measures:
Review outcomes: Inpatient setting:
|
| Starting date | 12 February 2020 |
| Contact information | Bin Cao, China‐Japan Friendship Hospital |
| Notes |
|
NCT04351724.
| Study name | 'A multicenter, randomized, active controlled, open label, platform trial on the efficacy and safety of experimental therapeutics for patients with COVID‐19 (caused by infection with severe acute respiratory syndrome coronavirus‐2)' |
| Methods | Trial design: RCT
Sample size: NR
Setting: inpatient, except for sub‐study B, which may also include outpatients with COVID‐19 Language: English Number of centres: 9 ((Austria) |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Details of intervention: different antivirals, depending on intervention arm (lopinavir/ritonavir; chloroquine or hydroxychloroquine; remdesivir) and other substances (sub‐studies A‐C: rivaroxaban, thromboprophylaxis, candesartan, non‐RAS blocking antihypertensives, asunercept, pentaglobin) Drug: remdesivir
Treatment details of control group:
Concomitant therapy: NR |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 16 April 2020 |
| Contact information | Bernd Jilma ‐ Medical University of Vienna |
| Notes |
|
NCT04843761.
| Study name | 'ACTIV‐3b: therapeutics for severely ill inpatients with COVID‐19 (TESICO)' |
| Methods | Trial design: RCT
Sample size: NR
Setting: inpatient Language: English Number of centres: 51 Type of intervention: drug |
| Participants |
Inclusion criteria:
Exclusion criteria:
Agent‐specific exclusion criteria
|
| Interventions |
Details of intervention:
Treatment details of control group:
Concomitant therapy: corticosteroid (in line with NIH treatment guidelines, corticosteroids such as dexamethasone, prednisone, methylprednisolone or hydrocortisone may be administered as SOC) |
| Outcomes |
Primary outcome: Recovery, assessed at 90 days
Secondary outcomes:
|
| Starting date | 20 April 2021 |
| Contact information | Samuel Brown, MD Intermountain Medical Center/University of Utah |
| Notes |
|
NCT04978259.
| Study name | 'Long‐term follow‐up of a randomized multicenter trial on impact of long‐COVID in hospitalized COVID‐19 patients ‐ SOLIDARITY Finland Long COVID‐19' |
| Methods | Trial design: RCT
Sample size: NR
Setting: inpatient Language: English Number of centres: 1 (University of Helsinki) Type of intervention: drug |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention details
Treatment details of control group:
|
| Outcomes |
Primary study outcomes
Other outcomes:
|
| Starting date | 24 July 2021 |
| Contact information | Kari AO Tikkinen, University of Helsinki |
| Notes |
|
Abbreviations
ALT/ALAT = alanine transaminase
AST/ASAT = aspartate transaminase
ECMO = extracorporeal membrane oxygenation
eGFR = estimated glomerular filtration rate
ICU = intensive care unit
IGA = immunoglobulin A
IGM = immunoglobulin M
INR = international normalised ratio
NIAID = National Institute of Allergy and Infectious Diseases
NR = not reported
PaO2/FiO2 = ratio of arterial oxygen partial pressure to fractional inspired oxygen
PCR = polymerase chain reaction
RCT = randomised controlled trial
RDV = remdesivir
RT‐PCR = reverse transcription polymerase chain reaction
SaO2 = arterial oxygen saturation
SAE = serious adverse events
SoC = standard of care
WHO = World Health Organization
Differences between protocol and review
Differences between protocol and review (first version)
Types of outcome measures
We specified outcomes regarding the effectiveness and safety of remdesivir for individuals with COVID‐19 and either moderate to severe or mild to asymptomatic disease after a guideline consortium (CEOsys) that took place after protocol registration. This approach was implemented in all reviews of CEOsys. We created outcome categories and added/specified the following outcomes for hospitalised participants with moderate or severe COVID‐19, as follows.
All‐cause mortality at day 28, day 60, time‐to‐event, and at hospital discharge.
-
Clinical status, assessed by need for respiratory support with standardised scales (e.g. WHO Clinical Progression Scale (WHO 2020c), WHO Ordinal Scale for Clinical Improvement (WHO 2020c)) at day 28, day 60, and up to longest follow‐up, including:
-
Improvement of clinical status:
weaning or liberation from invasive mechanical ventilation in surviving participants;
ventilator‐free days;
duration to liberation from invasive mechanical ventilation;
liberation from supplemental oxygen in surviving participants;
duration to liberation from supplemental oxygen.
-
Worsening of clinical status:
new need for mechanical ventilation;
new need for invasive mechanical ventilation;
new need for non‐invasive mechanical ventilation or high‐flow oxygen;
new need for oxygen by mask or nasal prongs.
-
Need for dialysis at up to 28 days.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to seven days, up to 30 days, and the longest follow‐up available.
Need for admission to intensive care unit (ICU).
Duration of ICU length of stay, or time to discharge from ICU.
Duration of hospitalisation, or time to discharge from hospital.
Viral clearance, assessed with reverse transcription polymerase chain reaction (RT‐PCR) test for SARS‐CoV‐2 at baseline and up to three, seven, and 15 days.
Serious adverse events, defined as number of participants with event.
Adverse events (any grade, grade 1 to 2, grade 3 to 4), defined as number of participants with event.
We combined three different types of advanced respiratory support (high‐flow oxygen, non‐invasive mechanical ventilation, and invasive mechanical ventilation) as one outcome measure with the term 'mechanical ventilation' for the following reasons.
Their application in clinical routine usually gives indirect evidence about a clinically relevant worsening of organ functions in an individual patient.
Their application is accompanied by a need for higher level of monitoring and care (e.g. admission to ICU).
For the individual patient, the application of each of these advanced respiratory support devices means a relevant loss of independence and quality of life, compared to application of low‐flow oxygen therapy or hospitalisation without any respiratory support.
Assessment of heterogeneity
We clarified our approach to exploring heterogeneity. We intended to conduct subgroups by type of respiratory support at baseline irrespective of the amount of statistical variation observed between the studies. We used sensitivity analysis rather than subgroup analysis to explore heterogeneity if the I2 was over 80%.
Types of subgroup analyses
We expanded the subgroup analysis, and additionally plan to conduct separate analysis if more data become available in the next updates of this review, for the following.
Age of participants (divided into applicable age groups, e.g. 18 to 65 years, 65 to 79 years, 80 years and older).
Pre‐existing conditions (e.g. diabetes, respiratory disease, hypertension, immunosuppression, obesity, cardiac injury).
Timing of first dose administration with illness onset.
-
Severity of condition:
no oxygen versus low‐flow oxygen versus mechanical ventilation (including high‐flow oxygen, non‐invasive ventilation, invasive mechanical ventilation, and extracorporeal membrane oxygenation).
-
Duration of remdesivir application:
5‐day course of remdesivir versus 10‐day course of remdesivir.
Although we contacted all study authors, especially in terms of detailed description of the extent of respiratory support (e.g. low‐ versus high‐flow oxygen, non‐invasive versus invasive mechanical ventilation), there remained differences in the reporting of severity of illness and incomplete data sets, which resulted in a relevant obstacle to the planned subgroup analysis.
Differences between reviews (first version versus first update)
Considerations for the update
The update of this living systematic review included additional data of two studies, the final results from the large WHO Solidarity trial, as well as three separately published add‐on or sub‐trials of the aforementioned multinational Solidarity trial (Abd‐Elsalam 2021; Gottlieb 2021; WHO Solidarity Canada 2022; WHO Solidarity France 2021; WHO Solidarity Norway 2021; WHO Solidarity Trial Consortium 2022), with consequential change in prioritised outcomes, credibility, and thus estimated effects. The process was supported by Cochrane specialists and in accordance with the "Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode" (Cochrane LSR). The following adaptions have been implemented:
Abstract
The text has been adapted according to changes in inclusion of outpatient participants, outcome set, results, and conclusion.
Plain language summary
The text has been adapted according to changes in inclusion of outpatient participants, outcome set, results, and conclusion.
Background
The text has been adapted according to current knowledge on SARS‐CoV‐2 and COVID‐19, as well as implementation and recommendations on the use of remdesivir.
Objectives
The text has been adapted according to inclusion of participants independent of care setting and approach for a living review removed.
Methods
Types of outcome measures
After the initial review, knowledge and experience in patient‐relevant outcome measures became more profound and adaptions to address former discrepancies were necessary. Changes to the outcome set are in line with further reviews produced within the German research project 'CEOsys' (COVID‐19 Evidence‐Ecosystem; CEOsys 2021), which have been published more recently with Cochrane (Griesel 2022; Kramer 2022). The main intention of all adaptions is to provide a clear representation of estimated effects, most crucial for affected people.
Hospitalised individuals with moderate to severe COVID‐19
Primary outcomes
All‐cause mortality at up to day 28, day 60, and up to longest follow‐up.
In‐hospital mortality at up to longest follow‐up.
Clinical improvement: proportion of participants alive and ready to be discharged at up to day 28, up to longest follow‐up, and time‐to‐event. Participants should be discharged without clinical deterioration or death.
Clinical worsening: proportion of participants with new need for invasive mechanical ventilation or deceased within 28 days, up to longest follow‐up, and time‐to‐event.
Adverse events (any grade) during the study period, defined as number of participants with any event.
Serious adverse events during the study period, defined as number of participants with any event.
Secondary outcomes
All‐cause mortality, time‐to‐event.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHO Quality of Life 100‐question patient‐reported questionnaire (WHOQOL‐100)) at up to seven days, up to 28 days, and longest follow‐up available.
Adverse events grades 3 and 4.
Ventilator‐free days (defined as days alive and free from mechanical ventilation).
Non‐hospitalised individuals with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
Primary outcomes
All‐cause mortality at day 28, up to longest follow‐up, and time‐to‐event.
Clinical improvement: proportion of participants with symptom resolution (all symptoms resolved) at up to day 14, day 28, up to longest follow‐up, and time‐to‐event.
Clinical worsening: proportion of participants admitted to the hospital or deceased within 14 days, 28 days, up to longest follow‐up, and time‐to‐event.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to seven days, up to 28 days, and longest follow‐up available.
Serious adverse events during the study period, defined as number of participants with any event.
Adverse events (any grade) during the study period, defined as number of participants with any event.
The key outcome measures (all‐cause mortality, clinical course, adverse and serious adverse events) remain as primary outcomes. However, the definition of measures for clinical deterioration or improvement were adapted to eliminate the competing risk of death. Additionally, we decided to condense the former measures of clinical status into one surrogate for each direction (deterioration or improvement) to allow a more precise statement. Outcome measures subordinate to primary outcomes are now listed as secondary outcomes. All‐cause mortality (time‐to‐event), quality of life,adverse events (grade 3 and 4), ventilator‐free days have been graded as "secondary" due to continuing lack of reporting and hence limited informative value. Need for dialysis, admission to intensive care unit (ICU), duration of ICU length of stay or time to discharge from ICU, duration of hospitalisation or time to discharge from hospital and viral clearance have been eliminated from the core outcome set because of redundancy and to provide a compact overview.
New data on the usage of remdesivir in the outpatient setting led us to add this potential population in our analyses: non‐hospitalised individuals with asymptomatic SARS‐CoV‐2 infection or mild disease.
Subgroup analyses
In the first version of this review we conducted subgroup analyses for all‐cause mortality at up to day 28 exclusively. We performed additional analyses where longer follow‐up data on mortality were available.
Sensitivity analyses
We removed the analysis "Comparison of adolescent and adult participants versus adult participants", as we considered this to be more appropriate for subgroup analysis (age of participants). Since only 1.4% in the population of non‐hospitalised participants and less than 0.1% in the population of the hospitalised participants were adolescent (12 to 18 years of age), we judged the influence on analyses to be infinitesimal.
Results
We have adapted the text according to changes in study characteristics and effects of intervention. We moved two studies from ongoing to awaiting classification. One study, formerly included, has been excluded due to retraction.
Discussion
We have adapted the text according to changes in effects of intervention, integration in current pandemic status (e.g. vaccination, variants of concerns), and current literature on the intervention.
Authors' conclusion
We have adapted the text according to changes in effects of intervention.
Tables and figures
We added an additional summary of findings table for the population of participants with asymptomatic SARS‐CoV‐2 infection or mild COVID‐19. Only primary outcomes were displayed according to changes in outcome set (Table 6). We added an additional table to display changes in the outcome set (Table 6). We have adapted Table 3, Table 4, and Table 5 according to changes. We have removed the former Table "Narrative summary of outcomes of included studies" due to accumulation of redundant information. We have adapted the PRISMA flow diagram according to changes in the search.
Living systematic review considerations
For the first version of this review, we followed a living systematic review approach and our Information Specialist (MIM) provided us with new search records weekly, which we screened, extracted, evaluated, and integrated following the guidance for Cochrane living systematic reviews (Cochrane LSR). We manually checked platform trials that were previously identified for additional treatment arms. We waited until the accumulating evidence changed our conclusions in the implications for research and practice before republishing the update of the review.
This update represents a major update, providing a comprehensive review of current available RCTs according to Cochrane methodological standards conducted in a team effort by clinicians and methodologists. We believe that relevant changes in international recommendations, pandemic course, and exposition of the population should determine the necessity of future updates rather than specific time frames. We have therefore refrained from maintaining this review as a living systematic review, as originally planned.
Contributions of authors
FG: clinical expertise, study selection, data extraction and assessment, conception and writing of the manuscript.
KA: methodological expertise, study selection, data extraction and assessment, conception and writing of the manuscript.
KD: methodological expertise, study selection, data extraction and assessment.
VT: clinical expertise, study selection, data extraction and assessment, writing of the manuscript.
MIM: Information Specialist, development of the search strategy, writing of the manuscript.
NS: methodological expertise and advice, writing and proofreading of the manuscript.
CB: methodological expertise and advice, conception, writing and proofreading of the manuscript.
AM: clinical expertise, data extraction and assessment, writing of the manuscript.
MG: clinical expertise, data extraction and assessment.
FF: clinical expertise and advice, conception, writing and proofreading of the manuscript.
MS: clinical expertise and advice, conception, writing and proofreading of the manuscript.
Sources of support
Internal sources
-
University Hospital of Cologne, Germany
Cochrane Cancer, Department of Internal Medicine
-
University Hospital RWTH Aachen, Germany
Department of Intensive Care Medicine
-
Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt‐Universität zu Berlin, Germany
Department of Infectious Diseases and Respiratory Medicine
-
University Hospital Leipzig, Germany
Department of Anesthesiology and Intensive Care Medicine
External sources
-
Federal Ministry of Education and Research, Germany
This review is part of the CEOsys project funded by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021.
Declarations of interest
FG: works as an Intensive Care Medicine Consultant and is member of the CEOsys project. The latter was funded in 2021 by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021, paid to the institution.
KA: is member of the CEOsys project, which was funded in 2021 by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021, paid to the institution.
KD: is member of the CEOsys project, which was funded in 2021 by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021, paid to the institution.
VT: works as an Intensive Care Medicine Consultant and is member of the CEOsys project (no direct funding).
MIM: is member of the CEOsys project, which was funded in 2021 by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021, paid to the institution. MIM is part of Cochrane Metabolic and Endocrine Disorders; she was not part of the editorial process.
NS: none known; she is Co‐ordinating Editor of Cochrane Haematology, but was not involved in the editorial process for this review.
CB: none known.
AM: has no relevant conflicts of interest to declare. Affiliation with not‐for‐profit organisation: co‐ordination of Section COVRIIN and work in Office of STAKOB (Competence and Treatment Centres for high consequence infectious diseases) at Robert Koch Institute Centre for Biological Threats and Special Pathogens (ZBS), Section Clinical Management and Infection Control.
MG: works as an Intensive Care Medicine Consultant and is member of the CEOsys project, which was funded in 2021 by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021, paid to the institution.
FF: works as an Intensive Care Medicine Consultant and is member of the CEOsys project (no direct funding).
MS: has no known conflicts of interest to declare.
contributed equally as first authors
contributed equally as last authors
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Beigel 2020 {published data only}2020‐001052‐18ISRCTN13035264jRCT2031190264
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19 - final report. New England Journal of Medicine 2020;383:1813-26. [DOI: 10.1056/NEJMoa2007764] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19 - preliminary report. New England Journal of Medicine 2020;383:1813-26. [CLINICALTRIALS.GOV: NCT04280705] [DOI: 10.1056/NEJMoa2007764] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of COVID-19 - preliminary report. New England Journal of Medicine 10 July 2020. [DOI: 10.1056/NEJMc2022236] [PMID: ] [DOI] [PubMed]
- Epstein M. Avoiding the termination of ACTT. European Heart Journal 2020. [DOI: 10.1093/eurheartj/ehaa546] [DOI] [PubMed]
- EUCTR2020-001052-18-DE. A multicenter, adaptive, randomised blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalised adults - version for European Union/United Kingdom sites. www.clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2020-001052-18-DE (first received 27 March 2020). [EUCTR: EUCTR2020-001052-18-DE]
- Galiuto L, Patrono C. Conflicting results on the efficacy of remdesivir in hospitalised COVID-19 patients: comment on the adaptive COVID-19 treatment trial. European Heart Journal 2020. [DOI: 10.1093/eurheartj/ehaa934] [DOI] [PMC free article] [PubMed]
- ISRCTN13035264. Adaptive COVID-19 treatment trial in the EU & UK. www.isrctn.com/ISRCTN13035264 (first received 24 July 2020). [ISRCTN: ISRCTN13035264]
- jRCT2031190264. A multicenter, adaptive, randomised blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalised adults. https://jrct.niph.go.jp/latest-detail/jRCT2031190264 (first received 24 March 2020).
- Ko F. Remdesivir reduces time to recovery in adults hospitalised with COVID-19: a meaningful step in therapeutic discovery. Journal of Clinical Outcomes Management 2020. [DOI: 10.12788/jcom.0010] [DOI]
- NCT04280705. Adaptive COVID-19 treatment trial (ACTT). clinicaltrials.gov/ct2/show/NCT04280705 (first received 21 February 2020).
- Olalla J. Remdesivir for the treatment of COVID-19 - preliminary report. New England Journal of Medicine 10 July 2020. [DOI: 10.1056/NEJMc2022236] [DOI] [PubMed]
- Paladugu S, Donato AA. Remdesivir improved time to recovery in adults hospitalised with COVID-19 and lower respiratory tract involvement. Annals of Internal Medicine 2020;173(2):JC4-5. [DOI: 10.7326/ACPJ202007210-005] [PMID: ] [DOI] [PubMed]
- Paules CI, Gallagher SK, Rapaka RR, Davey RT, Doernberg SB, Grossberg R, et al. Remdesivir for the prevention of invasive mechanical ventilation or death in coronavirus disease 2019 (COVID-19): a post hoc analysis of the adaptive COVID-19 treatment trial-1 cohort data. Clinical Infectious Diseases 2021;74:1260-4. [DOI: 10.1093/cid/ciab695] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottlieb 2021 {published data only}2020‐003510‐12
- EUCTR2020-003510-12. A study to test a drug named remdesivir to evaluate the efficacy and safety of the drug in treating patients with COVID-19 in an outpatient setting (non-hospitalised). www.clinicaltrialsregister.eu/ctr-search/trial/2020-003510-12/GB (first received 2 September 2020). [EUCTR: EUCTR2020-003510-12]
- Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. New England Journal of Medicine 2021;386(4):305-15. [CLINICALTRIALS.GOV: NCT04501952] [DOI: 10.1056/NEJMoa2116846] [EUCTR: 2020-003510-12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Paredes R, Vaca C, Mera J, Webb BJ, Perez G, et al. Remdesivir for the treatment of high-risk non-hospitalised individuals with COVID-19: a randomised, double-blind, placebo-controlled trial. Late Breaking Abstracts - Open Forum Infectious Diseases 2021;8(Suppl 1):S806-7.
- NCT04501952. Study to evaluate the efficacy and safety of remdesivir (GS-5734™) treatment of coronavirus disease 2019 (COVID-19) in an outpatient setting. clinicaltrials.gov/ct2/show/NCT04501952 (first received 6 August 2020). [CLINICALTRIALS.GOV: NCT04501952]
Mahajan 2021 {published data only}
- Mahajan L, Singh AP, Gifty. Clinical outcomes of using remdesivir in patients with moderate to severe COVID-19: a prospective randomised study. Indian Journal of Anaesthesia 2021;65:41-6. [DOI: 10.4103/ija.IJA_149_21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinner 2020 {published data only}EUCTR2020‐000842‐32ISRCTN85762140
- Castagna A, Cheong Hui DS, Mullane KM, Jain M, Galli M, Chang SC, et al. Baseline characteristics associated with clinical improvement and mortality in hospitalised patients with moderate COVID-19. Open Forum Infectious Diseases 2020;7(Suppl 1):S340. [DOI: 10.1093/ofid/ofaa439.742] [DOI]
- Criner GJ, Criner GJ, Ahn MY, Huhn G, Subramanian A, Lumbreras C, et al. 561. Safety of remdesivir vs standard care in patients with moderate Covid-19. Open Forum Infectious Diseases 2020;7(Suppl 1):S345–6.
- EUCTR2020-000842-32-ES. This study will test a drug named remdesivir (GS-5734 TM) to evaluate the safety and effectiveness of the drug in treating patients with moderate coronavirus disease 2019 (COVID-19) compared to standard of care treatment. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-000842-32/ES (first received 11 March 2020).
- Marty FM, Malhotra P, Gottlieb RL, Tashima KT, Galli M, Chai LYA, et al. Remdesivir vs standard care in patients with moderate Covid-19. Open Forum Infectious Diseases 2020;7(Suppl 1):S166–7.
- NCT04292730. Study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with moderate coronavirus disease (COVID-19) compared to standard of care treatment. clinicaltrials.gov/ct2/show/results/NCT04292730 (first received 3 March 2020).
- Spinner CD, Gottlieb RL, Criner GJ, Arribas Lopez JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomised clinical trial. JAMA 2020;324(11):1048-57. [DOI: 10.1001/jama.2020.16349] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang O, Brar I, Spinner C, Robinson P, Roestenberg M, Calmy A, et al. Impact of baseline alanine aminotransferase levels on the safety and efficacy of remdesivir in moderate COVID-19 patients. Hepatology 2020;72(Suppl 1):88A-89A. [DOI: 10.1002/hep.31578] [DOI]
Wang 2020 {published data only}
- Horby P, Cao B, Wang Y, Wang C. Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe pneumonia caused by COVID-19 virus infection: study protocol for a phase 3 randomised, double-blind, placebo-controlled, multicentre trial. Research Square 2020. [DOI: 10.21203/rs.2.24058/v2] [DOI] [PMC free article] [PubMed]
- NCT04257656. A trial of remdesivir in adults with severe COVID-19. clinicaltrials.gov/ct2/show/NCT04257656 (first received 6 February 2020).
- Oikonomou K, Ko F. Remdesivir in hospitalised adults with severe COVID-19: lessons learned from the first randomised trial. Journal of Clinical Outcomes Management 2020;27(3):104-6. [DOI: 10.12788/jcom.0002] [DOI]
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395(10236):1569-78. [CLINICALTRIALS.GOV: NCT04257656] [DOI: 10.1016/s0140-6736(20)31022-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang D, Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395(10238):1569, poster. [DOI] [PMC free article] [PubMed]
- Wang Y, Zhou F, Zhang D, Zhao J, Du R, Hu Y, et al. Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe COVID-19: study protocol for a phase 3 randomised, double-blind, placebo-controlled, multicentre trial. Trials 2020;21(1):422. [CLINICALTRIALS.GOV: NCT04257656] [DOI: 10.1186/s13063-020-04352-9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO Solidarity Canada 2022 {published data only}
- Ali K, Azher T, Baqi M, Binnie A, Borgia S, Carrier FM, et al. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial. Canadian Medical Association Journal 2022;194(3):E242-51. [DOI: 10.1503/cmaj.211698] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04330690. Treatments for COVID-19: Canadian arm of the solidarity trial (CATCO). https://clinicaltrials.gov/ct2/show/NCT04330690 (first received 1 April 2020). [NCT: 04330690]
WHO Solidarity France 2021 {published data only}
- Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D, et al. Remdesivir for the treatment of hospitalised patients with COVID-19: final results from the DisCoVeRy randomised, controlled, open-label trial. Medrxiv 2022. [DOI: 10.1101/2022.03.30.22273206] [DOI]
- Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet 2022;22(2):P209-21. [CLINICALTRIALS.GOV: NCT04315948] [DOI: 10.1016/S1473-3099(21)00485-0] [EUCTR: EudraCT2020-000936-23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ader F. Protocol for the discovery trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults. BMJ Open 2020;10:e041437. [CLINICALTRIALS.GOV: NCT04315948] [DOI: 10.1136/bmjopen-2020-041437] [EUCTR: EUCTR2020-000936-23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2020-000936-23-FR. Multi-centre, adaptive, randomised trial of the safety and efficacy of treatments of COVID-19 in hospitalised adults - discovery. www.clinicaltrialsregister.eu/ctr-search/trial/2020-000936-23/FR (first received 9 March 2020). [DOI] [PMC free article] [PubMed]
- EUCTR2020-000936-23-PT. Safety and efficacy of treatments of COVID-19 in hospitalised adults (discovery). www.clinicaltrialsregister.eu/ctr-search/trial/2020-000936-23/PT (first received 7 March 2020).
- Lingas G, Néant N, Gaymard A, Belhadi D, Peytavin G, Hites M, et al. Effect of remdesivir on viral dynamics in COVID-19 hospitalised patients: a modelling analysis of the randomized, controlled, open-label DisCoVeRy trial. Journal of Antimicrobial Chemotherapy 2022;77:1404-12. [DOI: 10.1093/jac/dkac048] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04315948. Trial of treatments for COVID-19 in hospitalised adults (discovery). clinicaltrials.gov/ct2/show/NCT04315948 (first received 20 March 2020).
- Vanden Eynde JJ. COVID-19: an update about the discovery clinical trial. Pharmaceuticals 2020;13(5):98-107. [DOI: 10.3390/ph13050098] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO Solidarity Norway 2021 {published data only}
- Barratt-Due A, Olsen IC, Henriksen KN, Kåsine T, Lund-Johansen F, Hoel H, et al. Evaluation of remdesivir and hydroxychloroquine on viral clearance in COVID-19 patients: results from the NOR-solidarity randomised trial (preprint). SSRN 2021. [CLINCIALTRIALS.GOV: NCT04321616] [DOI: 10.2139/ssrn.3774182] [DOI]
- Barratt-Due A, Olsen IC, Nezvalova-Henriksen K, Kåsine T, Lund-Johansen F, Hoel H, et al. Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID-19: a randomised trial. Annals of Internal Medicine 2021;174(9):1261-9. [CLINICALTRIALS.GOV: NCT04321616] [DOI: 10.7326/M21-0653] [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2020-000982-18-NO. A clinical study to evaluate the efficacy of different anti-viral drugs in SARS-CoV-2 infected patients (COVID-19). www.clinicaltrialsregister.eu/ctr-search/trial/2020-000982-18/NO (first received 24 March 2020).
- NCT04321616. The efficacy of different anti-viral drugs in (severe acute respiratory syndrome-corona virus-2) SARS-CoV-2. clinicaltrials.gov/ct2/show/NCT04321616 (first received 25 March 2020). [NCT: NCT04321616]
WHO Solidarity Trial Consortium 2022 {published data only}ISRCTN83971151
- CTRI/2020/04/024773. A clinical trial to study the effects of additional treatments for patients hospitalised and receiving treatment due to COVID -19. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=42897 (first received 21 April 2020). [CTRI: CTRI/2020/04/024773]
- Deresinski S. Treatment of COVID-19 patients with remdesivir, hydroxychloroquine, lopinavir, or interferon beta-1a. Infectious Disease Alert 2021;40(4).
- EUCTR2020-001366-11-IT. An international randomised trial of additional treatments for COVID-19 in hospitalised patients who are all receiving the local standard of care. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001366-11/IT (first received 20 April 2020).
- EUCTR2020-001366-11-LT. An international randomised trial of additional treatments for COVID-19 in hospitalised patients who are all receiving the local standard of care. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001366-11/LT (first received 7 April 2020).
- EUCTR2020-001549-38-DE. An international randomised trial of additional treatments for COVID-19 in hospitalised patients who are all receiving the local standard of care - WHO-solidarity-Germany. www.clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2020-001549-38-DE (first received 6 April 2020).
- Harrington DP, Baden LR, Hogan JW. A large, simple trial leading to complex questions. New England Journal of Medicine 2021;384:576-7. [DOI: 10.1056/NEJMe2034294] [DOI] [PMC free article] [PubMed]
- ISRCTN83971151. Public health emergency solidarity trial of treatments for COVID-19 infection in hospitalised patients. www.isrctn.com/ISRCTN83971151 (first received 25 March 2020). [DOI: ]
- Kupferschmidt K. WHO's treatment megatrial is at a standstill. Science 2021;371(6533):972-3. [DOI: 10.1126/science.371.6533.972] [DOI] [PubMed] [Google Scholar]
- LBCTR2020043495. An international randomised trial of additional treatments for COVID-19 in hospitalised patients who are all receiving the local standard of care. lbctr.moph.gov.lb/Trials/Details/4522 (first received 22 April 2020).
- NCT04575064. An international randomised trial of additional treatments for COVID-19 in hospitalised patients who are all receiving the local standard of care - WHO-solidarity-Germany. clinicaltrials.gov/ct2/show/NCT04575064 (first received 5 October 2020).
- NCT04647669. WHO COVID-19 solidarity trial for COVID-19 treatments. clinicaltrials.gov/ct2/show/NCT04647669 (first received 1 December 2020).
- NCT05024006. An international randomised trial of additional treatments for COVID-19 in hospitalised patients who are all receiving the local standard of care - Philippines. clinicaltrials.gov/ct2/show/study/NCT05024006 (first received 27 August 2021).
- Pan H, Peto R, Karim QA, Alejandria M, Henao-Restrepo AM, García CH, et al. Repurposed antiviral drugs for COVID-19 – interim WHO solidarity trial results. medRxiv 2020:2020.10.15.20209817. [CLINICALTRIALS.GOV: NCT04315948] [DOI: 10.1101/2020.10.15.20209817] [ISRCTN: ISRCTN83971151] [DOI] [PMC free article] [PubMed]
- Sharma V. The effect of antiviral drugs on COVID-19 outcomes and mortality. Critical Care Alert 2021;29(3):1-3.
- WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet 2022;399:1941-53. [DOI: 10.1016/S0140-6736(22)00519-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19 - interim WHO solidarity trial results. New England Journal of Medicine 2021;384(8):497-511. [CLINICALTRIALS.GOV: NCT04315948] [DOI: 10.1056/NEJMoa2023184] [EUCTR: EUCTR2020-001366-11] [ISRCTN: ISRCTN83971151] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abd‐Elsalam 2021 {published data only}
- Abd-Elsalam S, Salama M, Soliman S, Naguib AA, Ibrahim IS, Torky M, et al. Remdesivir efficacy in COVID-19 treatment: a randomised controlled trial. American Journal of Tropical Medicine and Hygiene 2021;106(3):886-90. [CLINCALTRIALS.GOV: NCT04345419] [DOI: 10.4269/ajtmh.21-0606] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Ader 2020 {published data only}
- Ader F, Discovery French trial management team. Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults. BMJ Open 2020;10(9):e041437. [DOI: 10.1136/bmjopen-2020-041437] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ader 2021 {published data only}
- Ader F, PeiMer-Smadja N, Poissy J, Bouscambert-Duchamp M, Belhadi D, Diallo A, et al. Antiviral drugs inhospitalised patients with COVID-19 - the DisCoVeRy trial. Medrxiv 2021. [CLINICALTRIALS.GOV: NCT04315948] [DOI: 10.1101/2021.01.08.20248149] [DOI]
Alpern 2020 {published data only}
- Alpern JD, Gertner E. Off‐label therapies for COVID‐19 - are we all in this together? Clinical Pharmacology & Therapeutics;108(2):182-4. [DOI] [PMC free article] [PubMed]
Anderson 2021 {published data only}
- Anderson MR, Bach PB, Baldwin MR. Hospital length of stay for patients with severe COVID-19: implications for remdesivir’s value. PharmacoEconomics - Open 2021;5:129-31. [DOI: 10.1007/s41669-020-00243-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Antinori 2020 {published data only}
- Antinori S, Cossu MV, Ridolfo AL, Rech R, Bonazzetti C, Pagani G, et al. Compassionate remdesivir treatment of severe COVID-19 pneumonia in intensive care unit (ICU) and non-ICU patients: clinical outcome and differences in post-treatment hospitalisation status. Pharmacological Research 2020;158:104899. [DOI: 10.1016/j.phrs.2020.104899] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banerjee 2020 {published data only}
- Banerjee I, Mohabeer P, Shukla A, Kashyap A, Robinson J. COVID-19: recent advances in epidemiology, virology, etiopathogenesis, clinical trials and vaccine development. Journal of Biomedical Sciences 2020;7(1):18-27. [Google Scholar]
CTRI/2020/12/029613 {published data only}
- CTRI/2020/12/029613. Drug to prevent lung injury secondary to COVID 19. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=50026 (first received 7 December 2020).
CTRI/2020/12/029615 {published data only}
- CTRI/2020/12/029615. Remdesivir plus tocilizumab efficacy trial in moderate to severe COVID-19 patients. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=49731 (first received 7 December 2020).
CTRI/2021/01/030830 {published data only}
- CTRI/2021/01/030830. Therapeutics for inpatients with COVID-19 (TICO). www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=52067 (first received 29 January 2021).
CTRI/2021/12/038637 {published data only}
- CTRI/2021/12/038637. A clinical trial to study the safety and efficacy of a drug, DESREM LQTM in patients with moderate to severe COVID-19. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/12/038637 (first received 24 December 2021).
CTRI/2021/12/039011 {published data only}
- CTRI/2021/12/039011. A study to assess the safety and efficacy of remdesivir in COVID-19 infected Indian patients. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/12/039011 (first received 29 December 2021).
CTRI/2022/03/041252 {published data only}
- CTRI/2022/03/041252. A clinical study to understand the effect of remdesivir intravenous injection in hospitalised moderate to severe COVID-19 patients. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=65800 (first received 22 March 2022).
Deresinski 2020 {published data only}
- Deresinski S. Remdesivir and COVID-19. Infectious Disease Alert 2020;39(10).
Elliott 2020 {published data only}
- Elliott W, Chan J. Remdesivir injection. Internal Medicine Alert 2020;42(11).
Elliott 2021 {published data only}
- Elliot W, Chan J. Remdesivir injection and baricitinib tablets. Internal Medicine Alert 2021.
EUCTR2020‐000841‐15‐ES {published data only}
- EUCTR2020-000841-15-ES. A phase 3 randomised study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with severe COVID-19 [Estudio de fase 3 aleatorizado para evaluar la seguridad y la actividad antiviral de remdesivir (GS-5734™) en participantes con infección grave por el COVID-19]. www.clinicaltrialsregister.eu/ctr-search/trial/2020-000841-15/ES (first received 11 March 2020).
EUCTR2020‐000936‐23 {published data only}
- EUCTR2020-000936-23. Multi-centre, adaptive, randomised trial of the safety and efficacy of treatments of COVID-19 in hospitalised adults - DisCoVeRy. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-000936-23 (first received 9 March 2020).
Euctr2020‐003510‐12‐dk {published data only}
- EUCTR2020-003510-12-DK. A study to test a drug named remdesivir to evaluate the efficacy and safety of the drug in treating patients with COVID-19 in an outpatient setting (non-hospitalised). www.clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2020-003510-12-DK (first received 31 August 2020).
EUCTR2020‐004928‐42‐HU {published data only}
- EUCTR2020-004928-42-HU. Clinical trial of remdesivir in COVID-19 patients. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-004928-42/HU (first received 12 October 2020). [EUCTR: 2020-004928-42]
Goldberg 2021 {published data only}
- Goldberg E, Ben Zvi H, Sheena L, Sofer S, Krause I, Sklan EH, et al. A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary centre in Israel. Clinical Microbiology and Infection 2021;27:917.e1-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldman 2020 {published data only}
- Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. New England Journal of Medicine 2020;383:1827-37. [CLINICALTRIALS.GOV: NCT04292899] [DOI: 10.1056/NEJMoa2015301] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN15874265. Study to assess the safety and effectiveness of remdesivir in people with severe COVID-19. www.isrctn.com/ISRCTN15874265 (first received 29 October 2020).
- NCT04292899. Study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with severe coronavirus disease (COVID-19). clinicaltrials.gov/ct2/show/NCT04292899 (first received 3 March 2020).
Goldman 2020a {published data only}
- Goldman JD, Lye DCB, Hui DS‐C, Marks K, Bruno R, Montejano R, et al. Impact of baseline alanine aminotransferase levels on the safety and efficacy of remdesivir in severe COVID-19 patients. Hepatology 2020;72:279A. [Google Scholar]
Grein 2020 {published data only}
- Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe COVID-19. New England Journal of Medicine 2020;382(24):2327-36. [DOI: 10.1056/NEJMoa2007016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grundmann 2022 {published data only}
- Grundmann A, Wu C, Hardwick M, Baillie JK, Openshaw P, Semple MG, et al. Impact of dexamethasone and remdesivir on neurological complications during COVID-19 (preprint). SSRN 2022. [DOI] [PMC free article] [PubMed]
IRCT20151227025726N28 {published data only}
- IRCT20151227025726N28. Evaluating the effects of remdesivir in severe COVID-19 in hospitalised patients. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20151227025726N28 (first received 21 October 2021).
IRCT20210324050760N1 {published data only}
- IRCT20210324050760N1. Effect of remdesivir in treatment of COVID-19. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20210324050760N1 (first received 21 April 2021).
ISRCTN15874265 {published data only}
- ISRCTN15874265. Study to assess the safety and effectiveness of remdesivir in people with severe COVID-19. www.isrctn.com/ISRCTN15874265 (first received 29 October 2020).
ISRCTN85762140 {published data only}
- ISRCTN85762140. Study to assess the safety and effectiveness of remdesivir in people with moderate COVID-19. www.isrctn.com/ISRCTN85762140 (first received 2 November 2020).
Jang 2021 {published data only}
- Jang Y, Shin JS, Lee MK, Jung E, An T, Kim U, et al. Comparison of antiviral activity of gemcitabine with 2′-gluoro-2′-deoxycytidine and combination therapy with remdesivir against SARS-CoV-2. International Journal of Molecular Sciences 2021;22(4):1581-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalil 2021 {published data only}
- Elliott W, Chan J. Remdesivir injection and baricitinib tablets. Internal Medicine Alert 2021;43(1).
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, ACTT-2 study group members. Baricitinib plus remdesivir for hospitalized adults with COVID-19. New England Journal of Medicine 2020;384(9):795-807. [CLINICALTRIALS.GOV: NCT04401579] [DOI: 10.1056/NEJMoa2031994] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04401579. Adaptive COVID-19 treatment trial 2 (ACTT-2). clinicaltrials.gov/ct2/show/NCT04401579 (first received 26 May 2020).
Lapadula 2020 {published data only}
- Lapadula G, Bernasconi DP, Bellani G, Soria A, Rona R, Bombino M, et al. Remdesivir use in patients requiring mechanical ventilation due to COVID-19. Open Forum Infectious Diseases 2020;7(11):ofaa481. [DOI: 10.1093/ofid/ofaa481] [DOI] [PMC free article] [PubMed] [Google Scholar]
LBCTR2020043495 {published data only}
- LBCTR2020043495. An international randomised trial of additional treatments for COVID-19 in hospitalised patients who are all receiving the local standard of care. https://lbctr.moph.gov.lb/Trials/Details/4522 (first received 22 April 2020). [LBCTR: 2020043495]
Medical Brief {published data only}
- Medical Brief. Remdesivir for COVID-19 improves time to recovery – peer-reviewed adaptive COVID-19 teatment trial. www.medicalbrief.co.za/archives/remdesivir-for-covid-19-improves-time-to-recovery-peer-reviewed-adaptive-covid-19-treatment-trial/ 2020;304.
NCT04252664a {published data only}
- NCT04252664. A trial of remdesivir in adults with mild and moderate COVID-19. clinicaltrials.gov/ct2/show/NCT04252664 (first received 5 February 2020).
NCT04256395 {published data only}
- NCT04256395. Efficacy of a self-test and self-alert mobile applet in detecting susceptible infection of COVID-19 (COVID-19). clinicaltrials.gov/ct2/show/NCT04256395 (first received 5 February 2020).
NCT04280705 {published data only}
- NCT04280705. Adaptive COVID-19 treatment trial (ACTT). clinicaltrials.gov/ct2/show/NCT04280705 (first received 21 February 2020).
NCT04292899 {published data only}
- NCT04292899. Study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with severe coronavirus disease (COVID-19). clinicaltrials.gov/ct2/show/NCT04292899 (first received 3 March 2020).
NCT04302766 {published data only}
- NCT04302766. Expanded access remdesivir (RDV; GS-5734™). clinicaltrials.gov/ct2/show/NCT04302766 (first received 10 March 2020).
NCT04321928 {published data only}
- NCT04321928. Personalised health education against the health damage of novel coronavirus (COVID-19) outbreak in Hungary (PROACTIVE-19). clinicaltrials.gov/ct2/show/NCT04321928 (first received 25 March 2020).
NCT04323761 {published data only}
- NCT04323761. Expanded access treatment protocol: temdesivir (RDV; GS-5734) for the treatment of SARS-CoV2 (CoV) infection (COVID-19). clinicaltrials.gov/ct2/show/NCT04323761 (first received 27 March 2020).
NCT04353596 {published data only}
- NCT04353596. Stopping ACE-inhibitors in COVID-19 (ACEI-COVID). clinicaltrials.gov/ct2/show/record/NCT04353596?view=record (first received 20 April 2020).
NCT04401579 {published data only}
- NCT04401579. Adaptive COVID-19 treatment trial 2 (ACTT-2). clinicaltrials.gov/ct2/show/NCT04401579 (first received 26 May 2020).
NCT04410354 {published data only}
- NCT04410354. Study of merimepodib in combination with remdesivir in adult patients with advanced COVID-19. clinicaltrials.gov/ct2/show/NCT04410354 (first received 1 June 2020).
NCT04480333 {published data only}
- NCT04480333. Safety, tolerability and pharmacokinetics of inhaled nanoparticle formulation of remdesivir (GS-5734) and NA-831. clinicaltrials.gov/ct2/show/NCT04480333 (first received 21 July 2020).
NCT04488081 {published data only}
- NCT04488081. I-SPY COVID-19 trial: an adaptive platform trial for critically ill patients (I-SPY_COVID). clinicaltrials.gov/ct2/show/NCT04488081 (first received 27 July 2020).
NCT04492475 {published data only}
- NCT04492475. Adaptive COVID-19 treatment trial 3 (ACTT-3). clinicaltrials.gov/ct2/show/NCT04492475 (first received 30 July 2020).
NCT04501978 {published data only}
- NCT04501978. ACTIV-3: therapeutics for inpatients with COVID-19 (TICO). clinicaltrials.gov/ct2/show/NCT04501978 (first received 6 August 2020).
NCT04518410 {published data only}
- NCT04518410. ACTIV-2: a study for outpatients with COVID-19. clinicaltrials.gov/ct2/show/NCT04518410 (first received 19 August 2020).
NCT04539262 {published data only}
- NCT04539262. Study in participants with early stage coronavirus disease 2019 (COVID-19) to evaluate the safety, efficacy, and pharmacokinetics of remdesivir administered by inhalation. clinicaltrials.gov/ct2/show/NCT04539262 (first received 4 September 2020).
NCT04583956 {published data only}
- NCT04583956. ACTIV-5 / big effect trial (BET-A) for the treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04583956 (first received 12 October 2020).
NCT04583969 {published data only}
- NCT04583969. ACTIV-5 / big effect trial (BET-B) for the treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04583969 (first received 12 October 2020).
NCT04610541 {published data only}
- NCT04610541. REMdesivir-HU clinical study and severe COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04610541 (first received 30 October 2020).
NCT04640168 {published data only}
- NCT04640168. Adaptive COVID-19 treatment trial 4 (ACTT-4). clinicaltrials.gov/ct2/show/NCT04640168 (first received 23 November 2020).
NCT04647695 {published data only}
- NCT04647695. IFN-beta 1b and remdesivir for COVID19. clinicaltrials.gov/ct2/show/NCT04647695 (first received 1 December 2020).
NCT04678739 {published data only}
- NCT04678739. Efficacy and safety of remdesivir and tociluzumab for the management of severe COVID-19: a randomised controlled trial. clinicaltrials.gov/ct2/show/NCT04678739 (first received 22 December 2020).
NCT04693026 {published data only}
- NCT04693026. Efficacy of remdisivir and baricitinib for the treatment of severe COVID 19 patients. clinicaltrials.gov/ct2/show/NCT04693026 (first received 5 January 2021).
NCT04713176 {published data only}
- NCT04713176. Efficacy and safety of DWJ1248 with remdesivir in severe COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04713176 (first received 19 January 2021).
NCT04728880 {published data only}
- NCT04728880. Remdesivir in adults with COVID-19: Mansoura university hospital experience. clinicaltrials.gov/ct2/show/NCT04728880 (first received 28 January 2021).
NCT04746183 {published data only}
- NCT04746183. AGILE (early phase platform trial for COVID-19). clinicaltrials.gov/ct2/show/NCT04746183 (first received 9 February 2021).
NCT04832880 {published data only}
- NCT04832880. Factorial randomised trial of rendesivir and baricitinib plus dexamethasone for COVID-19 (the AMMURAVID Trial) (AMMURAVID). clinicaltrials.gov/ct2/show/NCT04832880 (first received 6 April 2021).
NCT04847622 {published data only}
- NCT04847622. Study to evaluate the clinical outcomes in adults with COVID-19 who have been treated with remdesivir. clinicaltrials.gov/ct2/show/NCT04847622 (first received 19 April 2021).
Olender 2020 {published data only}
- NCT04292899. Study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with severe coronavirus disease (COVID-19). clinicaltrials.gov/ct2/show/NCT04292899 (first received 3 March 2020).
- Olender SA, Perez KK, Go AS, Balani B, Price-Haywood EG, Shah NS, et al. Remdesivir for severe coronavirus disease 2019 (COVID- 19) versus a cohort receiving standard of care. Clinical Infectious Diseases 2020;73(11):e4166-74. [CLINICALTRIALS.GOV: NCT04292899] [DOI: 10.1093/cid/ciaa1041] [EUPAS: EUPAS34303] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olender 2021 {published data only}
- Olender SA, Waluna TL, Martinez E, Perez KK, Castana A, Wang S, et al. Remdesivir versus standard-of-care for severe coronavirus disease 2019 infection: an analysis of 28-day mortality. Open Forum Infectious Diseases 2021;8(7):ofab278. [DOI: 10.1093/ofid/ofab278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Padilla 2022 {published data only}
- Padilla S, Polotskaya K, Fernández M, Gonzalo-Jiménez N, la Rica A, García JA, et al. Survival benefit of remdesivir in hospitalised COVID-19 patients with high SARS-CoV-2 viral loads and low-grade systemic inflammation. Journal of Antimicrobial Chemotherapy 2022;77:2257-64. [DOI: 10.1093/jac/dkac144] [DOI] [PubMed] [Google Scholar]
Pan 2020 {published data only}
- Pan H, Peto R, Karim QA, Alejandria M, Henao-Restrepo AM, García CH, WHO Solidarity trial consortium. Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results. medRxiv 2020;384:497-511. [DOI: 10.1101/2020.10.15.20209817] [ISRCTN83971151] [NCT04315948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 2021 {published data only}
- Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Karim QA, WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19 - Interim WHO solidarity trial results. New England Journal of Medicine 2021;384:497-511. [DOI: 10.1056/NEJMoa2023184] [EUCTR2020-001366-11] [ISRCTN83971151] [NCT04315948] [DOI] [PMC free article] [PubMed] [Google Scholar]
PER‐101‐20 {published data only}
- PER-101-20. Randomised master protocol for immune modulators for treating COVID-19. www.ins.gob.pe/ensayosclinicos/rpec/recuperarECPBNuevoEN.asp?numec=101-20 (first received 30 December 2020).
Rosas 2021 {published data only}
- NCT04409262. A study to evaluate the efficacy and safety of remdesivir plus tocilizumab compared with remdesivir plus placebo in hospitalised participants with severe COVID-19 pneumonia (REMDACTA). clinicaltrials.gov/ct2/show/NCT04409262 (first received 1 June 2020).
- Rosas IO, Diaz G, Gottlieb RL, Lobo SM, Robinson P, Hunter BD, et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Medicine 2021;47(11):1258-70. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saito 2020 {published data only}
- Saito S, Hayakawa K, Mikami A, Izumi S, Funazaki H, Ashida S, et al. Investigator initiated clinical trial of remdesivir for the treatment of COVID-19 in Japan. Global Health & Medicine 2021;3:62-6. [DOI: 10.35772/ghm.2020.01106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shakir 2022 {published data only}
- Shakir A, Bhasin N, Swami R, Mehta K, Sinha S, Ali SS, et al. Renal and hepatic outcomes after remdesivir therapy in coronavirus disease-2019-positive patients with renal dysfunction at baseline or after starting therapy. Saudi Journal of Kidney Diseases and Transplantation 2021;32(4):1034-42. [DOI] [PubMed] [Google Scholar]
Shih 2020 {published data only}
- Shih WCJ, Yao C, Xie T. Data monitoring for the Chinese clinical trials of remdesivir in treating patients with COVID-19 during the pandemic crisis. Therapeutic Innovation & Regulatory Science 2020;54(5):1236-55. [DOI: 10.1007/s43441-020-00159-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soto 2020 {published data only}
- Soto A, Quiñones-Laveriano DM, Garcia PJ, Gotuzzo E, Henao-Restrepo AM. Rapid responses to the COVID-19 pandemic through science and global collaboration: the solidarity clinical trial [Respuestas rápidas a la pandemia de COVID-19 a través de la ciencia y la colaboración global: el ensayo clínico solidaridad]. Revista Peruana de Medicina Experimental y Salud Pública 2020;37:356-60. [DOI: 10.17843/rpmesp.2020.372.5546] [DOI] [PubMed] [Google Scholar]
Sun 2020 {published data only}
- Sun D. Remdesivir for treatment of COVID-19: combination of pulmonary and IV administration may offer additional benefit. AAPS Journal 2020;22:1-6. [DOI: 10.1208/s12248-020-00459-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tran 2020 {published data only}
- Tran B. Early data on remdesivir for severe COVID-19: a promising start? Internal Medicine Alert 2020;42(13).
Winstead 2021 {published data only}
- Winstead RJ, Christensen J, Sterling S, Morales M, Kumar D, Bryson A, et al. Effect of remdesivir on COVID-19 PCR positivity and cycle threshold in kidney transplant recipients. Transplantology 2021;2(3):291-3. [DOI: 10.3390/transplantology2030028] [DOI] [Google Scholar]
References to studies awaiting assessment
NCT04596839 {published data only}
- NCT04596839. Antiviral activity and safety of remdesivir in Bangladeshi patients with severe coronavirus disease (COVID-19). https://clinicaltrials.gov/ct2/show/NCT04596839 (first received 22 October 2020).
REDPINE 2022 {published data only}EUCTR2020‐005416‐22
- EUCTR2020-005416-22. A phase 3 study of remdesivir in participants with severe renal impairment who are in hospital for COVID-19. www.clinicaltrialsregister.eu/ctr-search/trial/2020-005416-22/PT#P (first received 24 February 2021).
- NCT04745351. Study to evaluate the efficacy and safety of remdesivir in participants with severely reduced kidney function who are hospitalised for coronavirus disease 2019 (COVID-19) (REDPINE). clinicaltrials.gov/ct2/show/NCT04745351 (first received 9 February 2021).
References to ongoing studies
IRCT20210709051824N1 {published data only}IRCT20210709051824N1
- IRCT20210709051824N1. Assessment of utility of remdesivir in patients with acute kidney injury or cronic kidney disease in admitted COVID-19 patients. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20210709051824N1 (first received 22 December 2021).
NCT04252664 {published data only}
- NCT04252664. A trial of remdesivir in adults with mild and moderate COVID-19. clinicaltrials.gov/ct2/show/NCT04252664 (first received 5 February 2020).
NCT04351724 {published data only}
- NCT04351724. Austrian coronavirus adaptive clinical trial (COVID-19) (ACOVACT). clinicaltrials.gov/ct2/show/NCT04351724 (first received 17 April 2020).
NCT04843761 {published data only}
- NCT04843761. ACTIV-3b: therapeutics for severely ill inpatients with COVID-19 (TESICO). clinicaltrials.gov/ct2/show/NCT04843761 (first received 14 April 2021).
NCT04978259 {published data only}
- NCT04978259. Long-term follow-up of a randomised multicenter trial on impact of long-COVID in hospitalised COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04978259 (first received 27 July 2021).
Additional references
Al‐Abdouh 2021
- Al-Abdouh A, Bizanti A, Barbarawi M, Jabri A, Kumar A, Fashanu OE, et al. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis of randomised controlled trials. Contemporary Clinical Trials 2021;101:106272. [DOI: 10.1016/j.cct.2021.106272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Angamo 2022
- Anagamo MT, Mohammed MA, Peterson GM. Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis. Infection 2022;50(1):27-41. [DOI: 10.1007/s15010-021-01671-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beckermann 2022
- Beckermann R, Gori A, Jeyakumar S, Malin JJ, Paredes R, Póvoa P, et al. Remdesivir for the treatment of patients hospitalized with COVID-19 receiving supplemental oxygen: a targeted literature review and meta-analysis. Scientific Reports 2022;12(1):9622. [DOI: 10.1038/s41598-022-13680-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandal 2021
- Brandal LT, MacDonald E, Veneti L, Ravlo T, Lange H, Naseer U, et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Eurosurveillance 2021;26(50):2101147. [DOI: 10.2807/1560-7917.ES.2021.26.50.2101147] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buitrago‐Garcia 2022
- Buitrago-Garcia D, Ipekci AM, Heron L, Imeri H, Araujo-Chaveron L, Arevalo-Rodriguez I, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: Update of a living systematic review and meta-analysis. PLOS Medicine 2022;19(5):e1003987. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2022
- Centers for Disease Control and Prevention. Symptoms of COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed 6 June 2022).
CEOsys 2021
- German COVID-19 evidence-ecosystem. www.covid-evidenz.de (accessed prior to 22 July 2021).
Choy 2020
- Choy KT, Wong AYL, Kaewpreedee P, Sia SF, Chen D, Hui KPY, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Research 2020;178:104786. [DOI: 10.1016/j.antiviral.2020.104786] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane LSR
- Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. Available from community.cochrane.org/review-production/production-resources/living-systematic-reviews (accessed 16 March 2022 ).
COMET 2020
- Core outcome set developers’ response to COVID-19. www.comet-initiative.org/Studies/Details/1538 (accessed 2 November 2020).
Covidence 2021 [Computer program]
- Covidence. Version accessed 15 January 2021. Melbourne, Australia: Veritas Health Innovation, 2021. Available at covidence.org.
CRD42021238065
- CRD42021238065. Remdesivir for the treatment of hospitalized adults COVID-19 patients (part of German Ecosystem CEO-sys). www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021238065 (first received 26 February 2021).
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Available from training.cochrane.org/handbook.
de Vries 2022
- Vries ST, Starokozhko V, Schellens IMM, Wijnans L, Enzmann H, Cavaleri M, et al. Attention for sex in COVID-19 trials: a review of regulatory dossiers. BMJ Global Health 2022;7:e008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Wit 2020
- Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, et al. Prophylactic and therapeutic remdesivir (GS5734) treatment in the rhesus macaque model of MERS-CoV infection. Proceedings of the National Academy of Sciences 2020;117(12):6771-6. [DOI: 10.1073/pnas.1922083117] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eldridge 2016
- Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Higgins JT, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0). Additional considerations for cluster-randomized trials. www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-cluster-randomized-trials (accessed 1 March 2021).
EMA 2020
- European Medicines Agency. Summary on compassionate use remdesivir Gilead. www.ema.europa.eu/en/documents/other/summary-compassionate-use-remdesivir-gilead_en.pdf (accessed 9 May 2022).
EMA 2022
- European Medicines Agency. COVID-19 treatments. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-treatments (accessed 6 June 2022).
EUA 2020
- European Commission authorises first treatment against COVID-19. eeas.europa.eu/headquarters/headquarters-homepage/82135 (accessed 10 March 2021).
EUA 2021
- Fact sheet for health care providers Emergency Use Authorisazation (EUA) of remdesivir (GS-5734™). www.askgileadmedical.com/downloads/pdfs/EUA%20Fact%20Sheet%20for%20HCPs.pdf (accsessed 10 March 2021).
EUA 2022
- Emergency Use Authorization (EUA) for remdesivir, an unapproved product Center for Drug Evaluation and Research (CDER) Memorandum. https://www.fda.gov/media/155772/download (accessed 6 May 2022).
FDA 2022a
- US Food and Drug Administration. Coronavirus (COVID-19) - Drugs. https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (accessed 6 June 2022).
FDA 2022b
- US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Approves First COVID-19 Treatment for Young Children. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-approves-first-covid-19-treatment-young-children 25 April 2022.
Funk 2021
- Funk T, Pharris A, Spiteri G, Bundle N, Melidou A, Carr M, et al. Characteristics of SARS-CoV-2 variants of concern B.1.1.7, B.1.351 or P.1: data from seven EU/EEA countries, weeks 38/2020 to 10/2021. Eurosurveillance 2021;26(16):2100348. [DOI: 10.2807/1560-7917.ES.2021.26.16.2100348] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gautret 2020
- Gautret P, Million M, Jarrot PA, Jau LC, Colson P, Fenollar F, et al. Natural history of COVID-19 and therapeutic options. Expert Review of Clinical Immunology 2020;16(12):1159-84. [DOI: 10.1080/1744666X.2021.1847640] [DOI] [PubMed] [Google Scholar]
Griesel 2022
- Griesel M, Wagner C, Mikolajewska A, Stegemann M, Fichtner F, Metzendorf M-I, et al. Inhaled corticosteroids for the treatment of COVID‐19. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: CD015125. [DOI: 10.1002/14651858.CD015125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in metaanalyses. BMJ 2003;327:557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022a
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Higgins 2022b
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Higgins 2022c
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Higgins 2022d
- Higgins JP, Tianging L, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Huang 2020
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497-506. [DOI: 10.1016/S0140-6736(20)30183-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Izcovich 2022
- Izcovich A, Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Kum E, et al. Adverse effects of remdesivir, hydroxychloroquine and liponavir/ritonavir when used for COVID-19: systematic review and meta-analysis of randomised trials. BMJ Open 2022;12(3):e048502.2. [DOI: 10.1136/bmjopen-2020-048502] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johns Hopkins 2022
- Johns Hopkins Coronavirus Resource Center. Mortality Analyses. https://coronavirus.jhu.edu/data/mortality (accessed 6 June 2022).
Kaka 2022
- Kaka AS, MacDonald R, Linskens EJ, Langsetmo L, Vela K, Duan-Porter W, et al. Major Update 2: Remdesivir for adults with COVID-19: a living systematic review and meta-analysis for the American College of Physicians Practice Points. Annals of Internal Medicine 2022;175(5):701-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karagiannidis 2020
- Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respiratory Medicine 2020;8:853-62. [DOI: 10.1016/ S2213-2600(20)30316-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2022
- Kramer A, Prinz C, Fichtner F, Fischer A-L, Thieme V, Grundeis F. Janus kinase inhibitors for the treatment of COVID-19. Cochrane Database of Systematic Reviews 2022, Issue 6. Art. No: CD015209. [DOI: 10.1002/14651858.CD015209] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kreuzberger 2021
- Kreuzberger N, Hirsch C, Chai KL, Piechotta V, Valk SJ, Estcourt LJ, et al. SARS‐CoV‐2‐neutralising monoclonal antibodies for treatment of COVID‐19. Cochrane Database of Systematic Reviews 2021, Issue 9. Art. No: CD013825. [DOI: 10.1002/14651858.CD013825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauer 2020
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Annals of Internal Medicine 2020;172(9):577–82. [DOI: 10.7326/M20-0504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2022
- Lee TC, Murthy S, DelCorpo O, Senécal J, Butler-Laporte G, Sohani ZN, et al. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. Clinical Microbiology and Infection 2022;19:S1198-743X(22)00230-0. [DOI: 10.1016/j.cmi.2022.04.018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewnard 2022
- Lewnard JA, Hong VX, Patel MM, Kahn R, Lipsitch M, Tartof SY. Clinical outcomes associated with Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in southern California. medRxiv 2022. [DOI: ] [DOI] [PMC free article] [PubMed]
Li 2020
- Li T, Higgins JP, Deeks JJ (editors). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Available from training.cochrane.org/handbook. [EPUB: training.cochrane.org/handbook]
Liang 2020
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncology 2020;21(3):335-7. [DOI: 10.1016/S1470-2045(20)30096-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lo 2017
- Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, et al. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Scientific Reports 2017;7(1):1-7. [DOI: 10.1038/srep43395.] [DOI] [PMC free article] [PubMed] [Google Scholar]
MAGICapp [Computer program]
- MAGICapp. Brønnøysund (NOR): Norwegian MAGIC Evidence Ecosystem Foundation (powered by UserVoice Inc.), (accessed 25 February 2021). Available at magicapp.org.
McGuigan 2006
- McGuigan C, Hassan-Abdallah A, Srinivasan S, Wang Y, Siddiqui A, Daluge SM, et al. Application of phosphoramidate ProTide technology significantly improves antiviral potency of carbocyclic adenosine derivatives. Journal of Medicinal Chemistry 2006;49(24):7215-26. [DOI: 10.1021/jm060776w] [DOI] [PubMed] [Google Scholar]
Microsoft 2018 [Computer program]
- Mircosoft Excel. Microsoft Corporation, 2018. Available at office.microsoft.com/excel.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04431453
- Gilead Science. Study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of remdesivir (GS-5734™) in participants from birth to < 18 years of age with coronavirus disease 2019 (COVID-19) (CARAVAN). https://www.clinicaltrials.gov/ct2/show/study/NCT04431453 (first received 16 June 2020).
NICE guideline 2021
- National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing COVID-19. https://www.nice.org.uk/guidance/NG191 2021 (last updated 19 May 2022).
NIH guideline 2022
- National Institutes of Health, COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/ (accessed 6 June 2022). [PubMed]
Nyberg 2022
- Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andres N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022;399(10332):1303-12. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform metaanalyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI: ] [DOI] [PubMed] [Google Scholar]
Piechotta 2020
- Piechotta V, Valk SJ, Chai KL, Wood EM, Lamikanra A, Kimber C, et al. Safety and effectiveness of convalescent plasma or hyperimmune globulin for people with COVID-19: a rapid review. OSF Registries 2020. [DOI: 10.17605/OSF.IO/DWF53] [DOI] [PMC free article] [PubMed]
Piechotta 2021
- Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Potere 2020
- Potere N, Valeriani E, Candeloro M, Tana M, Porreca E, Abbate A, et al. A higher mortality rate and long ventilation times differentiate COVID-19 from severe respiratory infections in flu waves [Eine höhere Letalität und lange Beatmungsdauer unterscheiden COVID-19 von schwer verlaufenden Atemwegsinfektionen in Grippewellen]. Critical Care 2020;24(1):389. [DOI: 10.25646/7111] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2021 [Computer program]
- Review Manager Web (RevMan Web). Version 3.4.0. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Richard 2021
- Richard SA, Epsi NJ, Pollett S, Lindholm DA, Malloy AMW, Maves R, et al. Performance of the inFLUenza Patient-Reported Outcome Plus (FLU-PRO Plus) instrument in patients with coronavirus disease 2019. Open Forum Infectious Diseases 2021;8(12):ofab517. [DOI: 10.1093/ofid/ofab517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Richardson 2020
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323(20):2052-9. [DOI: 10.1001/jama.2020.6775] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl A, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014] [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Sheahan 2017
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Science Translational Medicine 2017;9(396):eaal3653. [DOI: 10.1126/scitranslmed.aal3653] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheahan 2020
- Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir and interferon beta against MERS-CoV. Nature Communications 2020;11(1):222. [DOI: 10.1038/s41467-019-13940-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegel 2017
- Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. Journal of Medicinal Chemistry 2017;60(5):1648-61. [DOI: 10.1021/acs.jmedchem.6b01594] [DOI] [PubMed] [Google Scholar]
Skoetz 2020
- Skoetz N, Goldkuhle M, Van Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and evidence profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI: 10.1016/j.jclinepi.2019.10.015] [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
Stevens 2022
- Stevens LJ, Pruijssers AJ, Lee HW, Gordon CJ, Tchesnokov EP, Gribble J, et al. Mutations in the SARS-CoV-2 RNA dependent RNA polymerase confer resistance to remdesivir by distinct mechanisms. Science Translational Medicine 2022 Aug 3 [Epub ahead of print]. [DOI: 10.1126/scitranslmed.abo0718] [DOI] [PMC free article] [PubMed]
Struyf 2021
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanni 2022
- Tanni SE, Silvinato A, Floriano I, Bacha HA, Barbosa AN, Bernardo WM. Use of remdesivir in patients with COVID-19: a systematic review and meta-analysis. Jornal Brasileiro de Pneumologia 2022;48(1):e20210393. [DOI: 10.36416/1806-3756/e20210393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thiruchelvam 2022
- Thiruchelvam K, Kow CS, Hadi MA, Hasan SS. The use of remdesivir for the management of patients with moderate-to-severe COVID-19: a systematic review. Expert Review of Anti-infective Therapy 2022;20(2):211-29. [DOI: 10.1080/14787210.2021.1949984] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vangeel 2022
- Vangeel L, Chiu W, De Jonghe S, Maes P, Slechten B, Raymenants J, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Research 2022;198:105252. [DOI: 10.1016/j.antiviral.2022.105252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vegivinti 2022
- Vegivinti CT, Evanson KW, Lyons H, Akosman I, Barrett A, Hardy N, et al. Efficacy of antiviral therapies for COVID-19: a systematic review of randomised controlled trials. BMC Infectious Diseases 2022;22:107. [DOI: 10.1186/s12879-022-07068-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2021
- Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, et al. Systemic corticosteroids for the treatment of COVID‐19. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD014963. [DOI: 10.1002/14651858.CD014963] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research 2020;30(3):269-71. [DOI: 10.1038/s41422-020-0282-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Cumulative number of reported probable cases of SARS. https://www.who.int/publications/m/item/summary-of-probable-sars-cases-with-onset-of-illness-from-1-november-2002-to-31-july-2003 (accessed 3 June 2022).
WHO 2019
- World Health Organization. Middle East respiratory syndrome coronavirus (MERSCoV). https://www.who.int/health-topics/middle-east-respiratory-syndrome-coronavirus-mers#tab=tab_1 (accessed 3 June 2022).
WHO 2020a
- World Health Organization. Timeline: WHO's COVID-19 response. www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline (accessed 3 June 2022).
WHO 2020b
- World Health Organization. Estimating mortality from COVID-19 - scientific brief. www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 (accessed 3 June 2022).
WHO 2020c
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI: 10.1016/S1473-3099(20)30483-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2022a
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. covid19.who.int (accessed 3 June 2022).
WHO 2022b
- Tracking SARS-CoV-2 variants. https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed 6 June 2022).
WHO 2022c
- World Health Organization. Therapeutics and COVID-19: living guideline. www.who.int/publications/i/item/therapeutics-and-covid-19-living-guideline (accessed 6 May 2022). [PubMed]
WHO living guideline 2022
- Geneva: World Health Organization. Therapeutics and COVID-19: Living guideline. WHO/2019-nCoV/therapeutics/2022.3 22 April 2022.
Williamson 2020a
- Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430-6. [DOI: 10.1038/s41586-020-2521-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 2020b
- Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 2020;585:7824. [DOI: 10.1038/s41586-020-2423-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolter 2022
- Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa: a data linkage study. Lancet 2022;399(10323):437-46. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2020
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. [DOI: 10.1001/jama.2020.2648] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ansems 2021
- Ansems K, Grundeis F, Dahms K, Mikolajewska A, Thieme V, Piechotta V, et al. Remdesivir for the treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD014962. [DOI: 10.1002/14651858.CD014962] [DOI] [PMC free article] [PubMed] [Google Scholar]


