Abstract
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide. In this review, we present the clinical spectrum and pathogenesis of syndromes caused by Aspergillus in COPD namely invasive aspergillosis (IA), community-acquired Aspergillus pneumonia, chronic pulmonary Aspergillosis and Aspergillus sensitisation. Some of these entities are clearly linked to COPD, while others may coexist, but are less clearly liked directly to COPD. We discuss current uncertainties as these pertain to IA in COPD cohorts and explore areas for future research in this field.
Keywords: Aspergillosis, Chronic pulmonary disease, Bronchiectasis
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide [1]. It affects more than 500 million people [2] (more than 7% of the global population), posing significant morbidity, mortality, health and economic burden [3–5]. COPD is associated with chronic debilitating respiratory symptoms, including breathlessness, cough and sputum production, as well as systemic symptoms, including fatigue, muscle wasting and deconditioning [6]. COPD exacerbations, punctuating the natural history of the disease, are associated with burdensome symptoms, quality of life decline and a high mortality rate, which in the case of severe exacerbations (those requiring hospital admission) exceeds 15% within 3 months from an event [7]. These events are frequent, since each year, 22–40% of all patients with COPD experience at least one exacerbation, while 9–16% experience more than one exacerbation. Although COPD is both preventable and treatable, the clinical course is usually progressive and irreversible.
The aetiology of exacerbations is very heterogeneous, and includes bacterial [8], viral [9] or possibly fungal infections [10], enhanced eosinophilic inflammation, mechanical and environmental causes. However, in clinical practice COPD exacerbations are still managed as a single disease entity and treated with antibiotics, bronchodilators and systemic corticosteroids, that are not always required, and pose a significant treatment burden. Frequent courses of antibiotics promote antimicrobial resistance and microbial dysbiosis, while systemic corticosteroids are associated with significant side effects, that include a predisposition to infections, such as pneumonia, but also fungal infections. Better characterisation of the aetiology of COPD exacerbations in clinical practice is therefore crucial.
Considering that isolation of Aspergillus fumigatus from respiratory secretions is a common occurrence in COPD patients, surprisingly little is known about its significance and implications. Aspergillus spp. are ubiquitous fungal pathogens causing a wide range of airway disorders with A. fumigatus the most frequently isolated species [11]. Given their small spore size (2–3 µm) and abundance (inhaled air can contain up to 100 conidia/m3) [12] Aspergillus conidia are able to reach the lung alveoli. However, these conidia are normally promptly removed by the innate immune system of the immunocompetent host and are unable to cause disease. Immunocompromised hosts like haematological malignancy or transplant patients are at the highest risk of developing invasive aspergillosis. The airway defences of patients with COPD are less effective at killing Aspergillus spores [13, 14], and they can escape killing by both professional phagocytes and epithelial cells and germinate. Recently however, there has been increased interest in the role of Aspergillus in groups previously considered to be at low risk of aspergillosis, including COPD patients.
Various clinical syndromes related to Aspergillus have been described in COPD [15]. Invasive aspergillosis (IA) is well recognised in COPD patients in intensive care units (ICU) and is associated with high mortality [16, 17], partly due to delays in diagnosis. Aspergillus may also contribute to more indolent pathology in COPD as a subset of patients with COPD will develop chronic pulmonary aspergillosis (CPA) [18]. This entity remains underdiagnosed due to its indolent nature, but may progress to severe end-stage disease, poor quality of life and life-threatening haemoptysis. In addition, A. fumigatus is isolated from the sputum of patients with COPD at steady state and during exacerbations in up to a third of cases, though its contribution to the symptoms and COPD progression is unclear [15]. Aspergillus sensitisation or presence of A. fumigatus in the airways has been linked with bronchiectasis and worse forced expiratory volume in the first second (FEV1) in COPD [19]. Finally, allergic bronchopulmonary aspergillosis (ABPA) has been diagnosed in COPD patients with or without asthma, but this entity is poorly characterised, and may not be a discrete complication of COPD. [20]
In this review, we present the clinical spectrum and pathogenesis of syndromes caused by Aspergillus in COPD namely IA, community-acquired Aspergillus pneumonia, CPA and Aspergillus sensitisation. Some of these entities are clearly linked to COPD, while others may coexist, but are less clearly linked directly to COPD (Table 1). We discuss current uncertainties as these pertain to IA in COPD cohorts and explore areas for future research in this field.
Table 1.
Aspergillus entities and linkages to COPD
| Aspergillosis entity | Comments |
|---|---|
| Well documented and clearly linked | |
| Invasive pulmonary aspergillosis | Often a challenging diagnosis |
| Invasive Aspergillus tracheobronchitis | Usually only diagnosed in ICU or autopsy |
| Chronic cavitary pulmonary aspergillosis | Infrequent and often with other pulmonary disease |
| Aspergillus sensitisation | Common but of uncertain clinical significance |
| Community-acquired Aspergillus pneumonia | Probably rare, difficult to diagnose. Similar, if not identical, to sub-acute invasive pulmonary aspergillosis |
| Aspergillus nodule(s) | Leads to suspicion for carcinoma, uncertain frequency |
| May coexist, but less clearly linked directly to COPD | |
| Allergic bronchopulmonary aspergillosis | Possibly co-incident but not causally associated |
| Aspergillus bronchitis | Probably linked to coexistent bronchiectasis |
Invasive aspergillosis
IA most commonly affects people with a wide spectrum of immunosuppressive disorders [21]. In these settings, the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) criteria define IA as proven, probable, or possible based on level of proof [22]. This ranges from the presence of decisive histopathological evidence of fungal invasion (proven) to a combination of clinical data and host risk factors either associated (probable) or not (possible) with the positivity of mycological criteria [at least 1 of the following: (1) cytology, direct microscopy and/or culture indicating presence of Aspergillus spp. in a lower respiratory tract specimen; (2) galactomannan antigen index > 0.5 in plasma/serum and/or galactomannan antigen > 0.8 in bronchoalveolar lavage (BAL) fluid. [22]
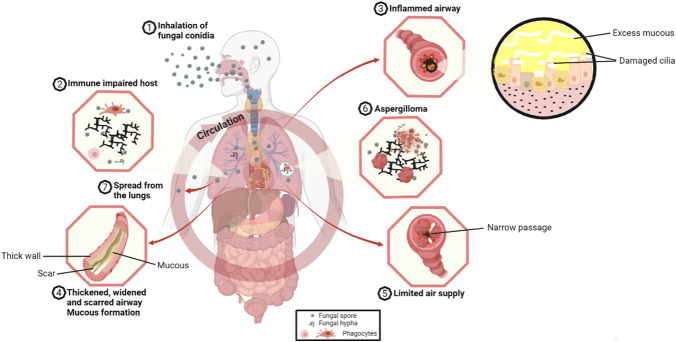
Pulmonary aspergillosis is the most frequent clinical manifestation of IA, but haematogenous spread could occur resulting in the involvement of extra-pulmonary sites such as the brain and skin [23]. Aspergillus becomes invasive in the lungs when epithelial cells and alveolar macrophages fail to clear the conidia which germinate into branching filaments called hyphae that invade the lung tissue [24] (Fig. 1). This gives rise to several invasive entities such as tracheobronchitis, acute and sub-acute disease (Fig. 2). Acute IA occurs rapidly with clinical presentation ranging from days to 3–4 weeks. Sub-acute invasive aspergillosis (SAIA) typically occurs in patients who are not profoundly immunocompromised but may be very debilitated and usually runs a slowly progressive course over 4–12 weeks [25]. IA may be angio-invasive and non-angio-invasive with angio-invasion being especially common in neutropenic patients with invasive pulmonary aspergillosis (IPA). This angio-invasion occurs as either invasion of major proximal pulmonary arteries with resultant thrombosis and distal tissue infarction or infiltration on a vessel in the centre of a well circumscribed nodule by hyphal elements [26]. The frequency of angio-invasion in IPA in COPD is uncommon [27]. Non-angio-invasive IPA is relatively more common in corticosteroid-treated patients, including those with COPD, with micro-abscesses, nodules and acute and chronic inflammation seen more frequently than angio-invasion. In a review by Franquet et al. involving nine patients with COPD and semiinvasive aspergillosis proven at autopsy or by thoracoscopically guided lung biopsy in Spain, the radiologic findings consisted of parenchymal consolidation (n = 6), nodules larger than 1 cm in diameter (n = 3), cavitation (n = 2) and multiple cavitated nodules with a variable degree of central necrosis (n = 3) [28].
Fig. 1.
Clinical presentations of aspergillosis and associated airway obstruction
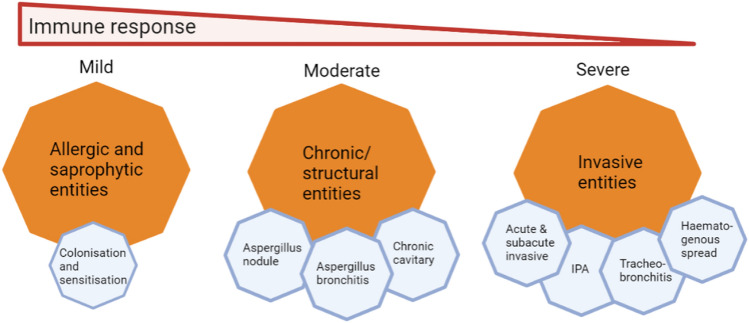
Fig. 2.
The various entities associated with Aspergillus pulmonary disease
Epidemiology of IA
IA affects primarily profoundly immunocompromised hosts, typically patients with chronic granulomatous disease, haematopoietic stem cell transplant (HSCT), prolonged neutropenia and solid organ transplant [29, 30]. However, IA in lower-risk populations is becoming increasingly recognised. These include patients with COPD [31], diabetes mellitus, renal failure, liver failure [32], high-dose corticosteroid treatment [33, 34], lung cancer [35] and those admitted to the ICU (including those with influenza and COVID-19) [31, 32, 36]. A large number of publications over the last two decades have implicated COPD as a risk factor for IA [32, 37–41].
Previous epidemiological studies may have underestimated COPD as a risk factor for IA. In a systematic review of 50 studies involving 1941 patients with IA, COPD was the underlying condition only in 26 (1.3%) [42]. This could reflect under-diagnosis of COPD. A retrospective study from Spain involving 239 patients admitted with COPD reported that IA was diagnosed in 22.1% of those with Aspergillus in respiratory secretions [37]. However, the prevalence of IA depends on the population studied; more critically ill patients are at greater risk of developing IA. Vandewoude et al. reported that, over a seven-year retrospective analysis, 83/172 (48%) critically ill patients with Aspergillus colonisation had IA [43]. In a recent systematic review, it was estimated that up to 3.9% of COPD patients admitted to hospital may have IA [2]. Aspergillus-colonised patients with GOLD stage 3 or 4 were more likely to have IA than those with earlier stages of COPD; among 150 patients mostly in early stages of COPD who had bronchoscopy, 17 (11%) had a positive Aspergillus test (culture ± galactomannan ± polymerase chain reaction [PCR]), and only 5 had probable IA [44]. Barberan et al. examined Aspergillus-colonised patients for eventual development of aspergillosis: COPD was a risk factor for developing aspergillosis and 26% of Aspergillus-colonised COPD patients developed aspergillosis [45].
The extremely poor prognosis of patients with COPD and IA was highlighted by Bulpa and colleagues [39]: 53 of 56 (95%) of such patients died despite optimal ICU care and antifungal treatment in 43 (77%). In an observational study involving 563 patients with IA, mortality was 38% among colonised patients, 67% in those with putative IA and 79% in those with proven IA (p < 0.001). In this cohort, COPD was the most common co-morbid condition (n = 174, 31%) [46]. Putative IPA was a strong independent risk factor for mortality in a cohort study of 50 patients admitted to ICU in France; 62.5% died in the ICU [47]. There was a statistically significant difference in the mean survival duration of patients with IA (29 days) when compared to patients without IA (86 days) in the Spanish retrospective study [37]. A retrospective cohort study of ICU patients in Belgium revealed that 127 (6.9%) out of 1850 admissions had evidence of Aspergillus in respiratory secretions; the majority (70%) did not have haematological malignancy; COPD was the most common diagnosis; and the observed mortality was 80% [32].
Several studies have identified risk factors for IA among patients with COPD. Rello et al. found corticosteroid treatment (> 20 mg of oral prednisone) and previous antibiotic use to be common among IA patients [31]. Suggested risk factors from case reports include late-stage COPD, prolonged steroid courses, viral infection and inhaled steroids [48–50]. Admission to the ICU, previous antibiotic treatment and cumulative steroid dose (> 700 mg of prednisone) in the three months prior to admission or from admission to diagnosis were independent risk factors for IA [37]. In the large series from southern China, corticosteroid exposure was only found in 13% [40], so COPD alone is important in its own right. Corticosteroid use both contributes to risk of IA and also to a worse prognosis, if not curtailed. Among 94 patients with multiple underlying diseases with IA, use of corticosteroid therapy increased the risk of dying by 10.6-fold [51].
Clinical features and diagnosis of IA
Early clinical suspicion for IA is vital as the timely commencement of antifungals can alter the prognosis of this serious disease. In COPD patients with IA, the common clinical features include fever, dyspnoea, sputum increase, wheezing, haemoptysis and chest pain [37]. Suspecting IA from imaging in patients with COPD is challenging given the non-specific nature of the findings, but bilateral findings are seen in at least 50% of patients. In COPD patients with IA, the abnormalities on chest X-ray may include infiltrates, cavitation, nodule or consolidation. The thorax CT findings include infiltrates, nodule, consolidation, cavitation the halo sign and air crescent sign [40]. The halo sign comprises ground glass opacity due to haemorrhage that surrounds a pulmonary nodule or mass. The halo sign is less common in non-angio-invasive infection. It may be seen with or without IPA in COVID-19 Associated Pulmonary Aspergillosis (CAPA) due to the in situ infarction and endotheliopathy [52]. The air crescent sign is a late sign of IA which results from retraction of the necrotic lung from the adjacent parenchyma [53]. However, these classic clinical and radiologic features may not occur as commonly in COPD-associated IPA as in neutropenic patients [54].
The gold standard for the diagnosis of IA involves detection of fungi in histopathological specimens [55]. In practice, this is rarely achieved in critically ill patients, and isolation of Aspergillus from respiratory samples is relied upon instead. This is hampered by the relatively low positive predictive value of culture of respiratory specimens [56] and the 2–7 day turnaround time [57]. Isolation of Aspergillus in a patient with COPD creates diagnostic uncertainty, particularly in unwell patients and if an alternative diagnosis is not entertained. Due to these difficulties, there has been an increasing reliance on serologic and molecular diagnostic technologies. Examples of these tests include galactomannan (GM) and PCR in BAL (if the patient is intubated) and in serum, as well as beta-D-glucan (BDG) in serum. It may be that tracheal aspiration is adequate as a mycologic specimen—Aspergillus signals on culture and PCR are higher in sputum and bronchial samples than in BAL samples [58].
Diagnosis with Aspergillus antigen detection
In immunocompromised patients, the serum GM has been established as a prognostic marker for IA. In a study involving 11 patients with grade 3 or 4 COPD, the serum GM value was found to be positive in 9 (81.8%) cases, and 5 of them (55.5%) died. Serum GM may have a role as a prognostic index in COPD cases with IPA. In a prospective study to evaluate the prognostic value of GM in the setting of severe COPD, a GM > 0.5 ng/mL, a cumulative dosage of corticosteroids > 216 mg before the ICU admission and a low creatinine clearance were predictors of poor outcome [17]. However, using a cut-off of ≥ 0.5 ng/mL for GM (Platelia, Biorad) 14 (42.4%) of the 33 patients with probable IA were positive on serum testing [37]. Further work is required to determine the utility of serum GM in diagnosing IA in COPD.
Some work has been done to compare the sensitivity of BAL fluid in the diagnosis of IPA compared to serum GM. In a prospective single-centre study involving 50 critically ill COPD patients, BAL fluid performed better than serum GM and lower respiratory tract Aspergillus isolation in the diagnosis of IPA. In this study, a possible cut-off value of 0.8 for GM from BAL lavage fluid was suggested in critically ill COPD patients [17].
Due to poor sensitivity of serum GM, there has been a greater reliance on GM testing of BAL fluid [59, 60]. The role of GM in serum or BAL fluid for the diagnosis of IA in patients with COPD was evaluated in a multicentre study conducted in Spain involving a total of 188 patients over a four-year period [61]. In this study, the sensitivity of BAL GM (optical density index [ODI] ≥ 1.0) was higher in immunocompromised patients compared to patients with COPD (81.8% vs 66.7%; p: 0.38). Among the patients with COPD, a BAL fluid ODI ≥ 0.5 provided the best performance with a sensitivity (88.9%) that equalled that of BAL fluid fungal culture. In another study involving 11 patients with IA, the optimal BAL ODI cut-off value of 1.25 was identified following analysis of receiver operating characteristics with a sensitivity of 90.9% and a specificity of 96.3% for diagnosing IA. [62]
Diagnosis with PCR
The Aspergillus PCR assays holds great promise for the rapid identification of A. fumigatus from blood and respiratory samples such as BAL and sputum [63, 64]. Few comparisons in COPD to compare diagnostic performance of PCR and culture have been done. The data are discordant, possibly reflecting sample type and stage of patient illness. In a multicentre study that prospectively evaluated 47 mechanically ventilated patients with COPD for IA, a commercial PCR assay was positive for 10 patients (21.3%) including the two (4.2%) patients with positive culture [65]. In addition, in a study evaluating IA in 175 patients of which 91 (52.6%) had COPD, the same Aspergillus PCR assay on 322 lower respiratory tract samples found 15 of the 81 patients with COPD to have probable IA [66]. The sensitivity (%), specificity (%) and diagnostic odds ratio for detection of Aspergillus of this assay in these two studies (first sample/any sample) were 86.7/93, 87.6/82.4 and 48/68.75. Compared with fungal culture (median time from sample culture to visualisation of fungal growth of 3 days), PCR significantly reduced the time to diagnosis to ∼ 4 h using Aspergillus PCR. Therefore, PCR detection of A. fumigatus has the added advantage of facilitating rapid detection of azole resistance and prompt initiation of appropriate antifungal treatment.
Numerous commercial PCR assays are now available, but few have been studied explicitly in COPD patients. One of them, AsperGenius®, (PathoNostics, Maastricht, Netherlands), is a multiplex qPCR assay that detects azole resistance mutations in A. fumigatus arising from the environment [63]. The MycoGENIE® (Ademtech, Pessac, France) assay is similar multiplex qPCR assay for Aspergillus DNA which does not detect azole resistance [63]. Another commercial PCR assay (OLM Diagnostics, Newcastle upon Tyne, UK), detects Aspergillus terreus as well as all Aspergillus species relevant as A. terreus is amphotericin B resistant [67].
Diagnosis with point-of-care antigen tests
Since the early diagnosis and appropriate treatment of IA is associated with significant improvement in survival [68, 69], there has been a push to develop point-of-care (POC) tests for IA. One POC lateral flow device (LFD) (OLM Diagnostics) test utilises a mouse monoclonal antibody, JF5, which binds to a specific glycoprotein antigen produced by Aspergillus spp [70]. A comparison of GM, 1,3-β-D-glucan and the Aspergillus LFD with conventional culture was performed on 268 BAL samples from 221 patients with underlying respiratory diseases, including COPD, in Austria [71]. Probable or proven IPA was diagnosed in 14% of this study population. The LFD showed a sensitivity of 77% and specificity of 92%. The GM sensitivity and specificity was 97 and 81% for a cut-off of 0.5, and 97% and 93% for a cut-off of 1.0.
A recent comparison between a new Aspergillus GM lateral flow assay (LFA) (IMMY, Norman, OK, USA) and the LFD (OLM Diagnostics, Newcastle upon Tyne, UK) for the diagnosis of IA showed comparable performance as sensitivities ranged between 58 and 69%, with specificities between 68 and 75% [72]. The sensitivity increased to 81% when both tests were used in combination. It is important to note that this comparison was carried out on patients at risk for IA but without underlying haematological malignancy or neutropenia. Both the LFA and LFD tests showed promise with respect to the diagnosis of IA though targeted testing of suspected cases of IA was advocated rather than general screening of all patients [72].
Recent research on developing a POC monoclonal Ab476-based LFD for detection of urinary excreted fungal GM-like antigens is showing promise [73–75]. One advantage of this test is that it is easy to collect urine samples for this and large volumes can be tested. No data are published in patients with COPD.
There are no specific studies using POC tests for diagnosis of IA exclusively in COPD patients. It is still not clear whether the POC tests are useful for CPA or Aspergillus bronchitis, and they have not been trialled on sputum samples.
Diagnosis in serum by detecting beta-D-glucan
The BDG is a key constituent of the cell wall of many pathogenic fungi and can be detected in blood of patients with invasive candidiasis, Pneumocystis pneumonia and IA [76, 77]. BDG detection appears to be an emerging tool for the diagnosis of IPA in COPD patients. A retrospective evaluation of laboratory results of four COPD patients in Turkey with IPA who had a BDG test (Fungitell; Associates of Cape Cod) showed that BDG was positive in 3 out 4 patients [78]. Tutar and colleagues who studied 11 COPD patients with IPA examined BDG in five patients, and this was positive in three (60%) of them [41]. However, due to a dearth of large-scale studies that evaluate BDG testing in the setting of IA, current guidelines [79, 80] do not recommend using BDG for the diagnosis of IA [81].
Diagnosis with Aspergillus antibody
The clinical significance of Aspergillus antibody assays for the diagnosis of IA is unclear though these may have utility in the confirmation of the diagnosis of IA and for the monitoring of the treatment of IA [82]. A recent study by Yu and colleagues [83] involving 58 cases of pulmonary aspergillosis (37 IPA and 21 CPA cases) showed potential value of serum Aspergillus IgG antibody detection in the diagnosis of IA and CPA among a cohort of non-neutropenic patients, many with chronic pulmonary disease [83]. From the receiver operating characteristic (ROC) curves, Aspergillus IgG antibody detection had a higher specificity in the IPA group than in the CPA group (0.952) with Aspergillus IgG antibody distinguishing IPA from community-acquired bacterial pneumonia and healthy controls (sensitivity = 0.923, specificity = 0.459, cut-off value = 134.46, AUC = 0.727) and distinguishing CPA from community-acquired bacterial pneumonia and healthy controls (sensitivity = 0.952, specificity = 0.692, cut-off value = 75.46, AUC = 0.873) [83].
A cohort of 19 patients with sub-acute IA treated with voriconazole all had detectable Aspergillus precipitins which reduced after 6 months of therapy [84]. In a study which assessed the relationship between domestic mould exposure, Aspergillus biomarkers and COPD severity during acute exacerbation and at stable state, anti-Aspergillus antibodies (IgG and precipitins) were associated with chronic lung function alteration and/or domestic mould exposure [85]. This finding supports the consideration of indoor mould contamination and anti-Aspergillus antibodies kinetics in COPD management.
In a retrospective study to evaluate the utility of Aspergillus fumigatus-specific serum IgG and IgA (IgAG) tests for serological IPA diagnosis in 87 non-neutropenic patients in Czech Republic, GM, (1,3)-β-d-glucan and IgAG assays were found to have sensitivity/specificity/positive predictive value (PPV)/negative predictive value (NPV) of 48.8%/91.3%/83.3%/66.7%, 82.9%/73.9%/73.9%/82.9% and 75.6%/95.7%/93.9%/81.5%, respectively [86]. Of the three tests, the IgAG assay was found to have the highest specificity and PPV. Improvement was achieved by combining the GM, BG and IgAG assays.
Further work is required to explore the role of Aspergillus antibody assays for the diagnosis of IA in the setting of COPD.
Treatment of IA in COPD patients
Recommendations for the treatment of IA are largely drawn from studies in immunocompromised patients, with minimal data available in COPD specifically. If voriconazole is given with prednisolone, the dose of voriconazole should be reduced by at least 30% because of a drug-drug interaction [87] and also because corticosteroids worsen outcome in IA.
Community-acquired Aspergillus pneumonia and/or pneumonitis
Invasive aspergillosis in immunocompromised patients may be acquired in the community [79, 88, 89]. Recent reports of invasive aspergillosis in newly hospitalised COPD patients, many of whom, but not all, were treated with corticosteroids, emphasise the high frequency of Aspergillus acquisition in the community [37, 39, 40]. Virtually all cases of ABPA, Aspergillus bronchitis and CPA are, or presumed to be, acquired out of hospital [90]. This is hardly surprising given the ubiquitous nature of A. fumigatus and other species [91, 92]. It would therefore not be surprising that susceptible patients may develop community-acquired Aspergillus pneumonia.
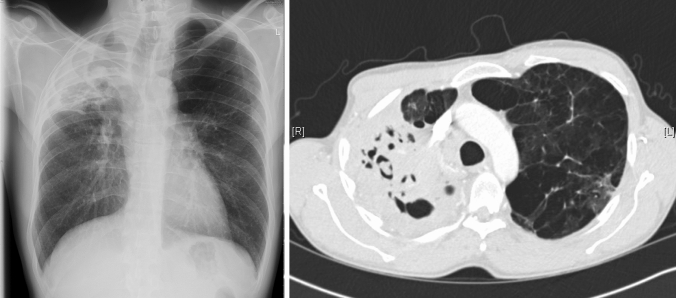
Reports of community-acquired Aspergillus pneumonia and/or pneumonitis are uncommon. The terminology is also variable. There appear to be 3 common features to these reports: A remarkable exposure to airborne spores, prior influenza or other viral infection and/or underlying pulmonary disease, usually emphysema or chronic obstructive pulmonary disease (COPD). Only a few cases are described (Fig. 3).
Fig. 3.
CT thorax of a female patient with asthma and significant COPD. A right upper lobe mass was found consistent with acute pneumonia. Evolution over the next few weeks and further imaging demonstrated the interval development of a fungal ball with an area of consolidation. This was consistent with a primary community-acquired Aspergillus pneumonia and the secondary development of chronic cavitary pulmonary aspergillosis with a fungal ball
Clancy and Nguyen presented one case and summarised the prior literature in 1998 [93]. All 12 cases they identified were infected with A. fumigatus and ranged in age from 14 months to 67 years. Microscopy for hyphae was positive in one patient, and sputum cultures were positive for Aspergillus in 7 of 10 patients (criteria for inclusion in the series). All 12 patients died, despite amphotericin B therapy in six.
Two other series require mention—both reporting a slowly progressive ‘necrotising pneumonia’ [94, 95]. Kennedy et al. report 4 cases of a necrotising pneumonia in 4 patients in Edinburgh, none immunocompromised but 2 with overt prior pulmonary disease and one a heavy smoker [94]. Binder et al. present 4 additional cases of a chronic necrotising pneumonia (chronic necrotising pulmonary aspergillosis) attributable to A. fumigatus or in one case A. flavus [95].
Since that time additional cases attributable to exposure related to damp and decomposing bark have been described [96, 97] and other cases attributable to other ‘vegetal’ [98] or decomposing plant materials [99] and gardening [100–102]. The radiological patterns described are primarily those of a miliary, bilateral diffuse pattern, with high level exposure, but unilateral, usually upper lobe cavitary disease is also well described.
Late diagnosis and lack of antifungal therapy are associated with early death and/or progressive disease. Amphotericin B therapy is probably ineffective for severe cases [93, 98, 103]. Voriconazole is recommended for treatment in the acute phase, with the addition of corticosteroids for those with overwhelming infection and/or pneumonitis, in the current state of our relative ignorance of this condition. More slowly progressive infection may respond to itraconazole.
Chronic pulmonary aspergillosis
Epidemiology of CPA
Chronic, allergic and saprophytic entities could also occur within the lungs following exposure to Aspergillus (Fig. 2). CPA is an indolent infection that affects patients with pre-existing lung disease including COPD, tuberculosis (TB), sarcoidosis, or previously treated lung cancer. CPA presents with slowly enlarging lung cavities and may remain undiagnosed resulting in poor quality of life, and secondary infections. Around 3 million people globally have CPA according to estimates, with higher prevalence in developing countries [103]. A. fumigatus is the commonest species causing CPA though A. flavus, A. niger and other rarer species have also been implicated [104].
A retrospective review of patients with CPA in Manchester, UK, over an 8-year period revealed that COPD was the commonest underlying condition occurring in one third of patients (42; 33.3%) with 9% having COPD as the primary underlying pulmonary disorder [18]. In a case series of 18 patients with CPA which heralded the nomenclature change for this disease, all patients had prior pulmonary disease, with COPD occurring in 10 (55.6%) [105]. In a retrospective study involving 70 CPA patients in Korea, 35 (50%) had COPD [106]. In Spain, a retrospective cohort study comprising 123 patients with COPD and respiratory isolation of Aspergillus spp. over a 12-year period had 7 patients with CPA in it [107].
COPD appears to be a strong risk factor for mortality in CPA patients. In a study that involved a cohort of 387 patients referred to the UK’s National Aspergillus Centre with CPA, survival was 86, 62 and 47% at 1, 5 and 10 years, respectively [108]. Following analysis, COPD was an indicator of mortality (hazard ratio 1.57, 1.05–2.36; p = 0.029). In a large study to describe the epidemiological and prognostic data of CPA patients hospitalised in France between 2008 and 2017, COPD was observed for 5 years in 46% of the 2931 CPA pts hospitalised in 2017. Emphysema was observed in 21% of patients. Among the 2605 CPA patients hospitalised in 2012, the cumulative five-year mortality rate was 41% [109].
Clinical features and diagnosis of CPA
The clinical features of CPA include chronic cough, dyspnoea, chest pain and haemoptysis which may be life-threatening. Constitutional symptoms such as sweats, anorexia, weight loss and malaise are often prominent [110]. Investigations commonly reveal thick-walled lung cavities, pleural thickening, aspergillomas and nodules (Fig. 4). Aspergillomas are made of active and dead mycelia, mucus, fibrin, epithelia cells and inflammatory cells (see Fig. 1). In COPD patients, new lung nodules are usually presumed to be malignant due to smoking history and may lead to surgery. A combination of clinical features, progressive radiological findings, and microbiological and/or serological evidence of Aspergillus infection is required to make the diagnosis of CPA after alternative diagnoses such as cancer or non-tuberculous mycobacterial lung disease have been excluded [111]. Guidelines on CPA endorsed by the ERS and ESCMID have been published [111].
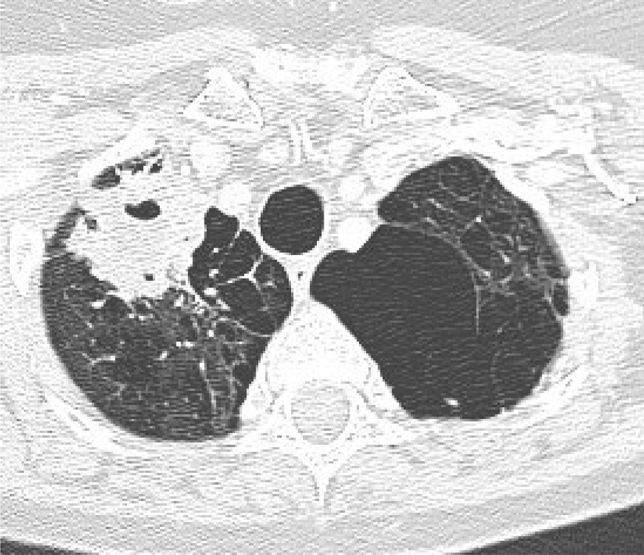
Fig. 4.
A chest radiograph and CT scan of a patient with COPD showing contraction of the right upper lobe with several large cavities and pleural thickening visible. No fungal balls are seen within the cavities. The left upper lobe is almost completely replaced by emphysematous bullae
The progression of previous anatomic alterations within the airways such as cavitations appear to predispose to the development of CPA (Fig. 5). This may partly account for the increased risk of CPA among patients with COPD, pulmonary (TB), sarcoidosis and non-tuberculous mycobacterial infection [18]. Globally, previous pulmonary TB is the leading predisposing factor for CPA, while COPD is the most common cause in areas of low TB prevalence [112]. CPA disproportionately affects low resource settings due to high prevalence of both COPD and TB. In those settings, the diagnosis of CPA may be even more elusive due to lack of access to specialised fungal diagnostics or a CT scanner. Trend analyses have shown that the majority of deaths from COPD occur in less-developed countries [113].
Fig. 5.
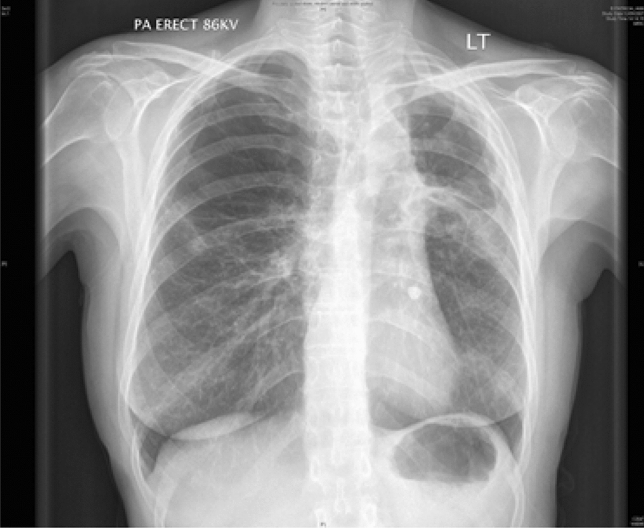
Chest X-ray of an ex-smoker (30 pack-years) patient with COPD and emphysema who developed increasing frequency of respiratory infections. Imaging shows upper lobe fibrosis with traction bronchiectasis and a positive Aspergillus IgG antibody test. A large cavity is visible occupying the whole left upper lobe
Treatment of CPA
Long-term antifungal treatment is usually required for CPA, and the triazole antifungals are the cornerstone of therapy. Treatment should be continued for at least twelve months [114] and carries response rates of 60–80%, depending on the study and antifungal agent used [106, 111, 112]. The triazoles can boost the systemic level of oral or inhaled steroids in COPD [87] thereby leading to serious complications such as Cushing’s syndrome and adrenal failure. Systemic corticosteroids should be stopped because linked to a worse clinical outcome and a threefold higher mortality [115, 116].
Surgery has a prominent role in the treatment of unilateral CPA in younger patients as it holds the promise of cure in some settings [18]. Surgical outcomes have been found to be better in patients with better lung function and localised pulmonary disease [117]. In COPD specifically, surgery may be high risk in advanced stages and is often contraindicated due to multifocal or bilateral appearance of CPA.
Aspergillus nodules
Aspergillus nodules are an unusual form of CPA mimicking carcinoma of the lung, metastases, cryptococcal nodule, coccidioidomycosis or other rare pathogens. In immunocompromised patients, IA may present as nodules. But nodules may also occur in non-immunocompromised patients and are referred to as Aspergillus nodules.
Aspergillus nodules may be single or multiple with or without cavitation, most of which are smaller than 3 cm across [118]. Aspergillus nodules have non-specific clinical and radiological manifestations, may be asymptomatic; the nodules are usually incidental discoveries on chest CT scan (Fig. 6). The Aspergillus nodules may not have distinctive features on chest CT thus making it challenging to distinguish them from malignant or other inflammatory nodules. Commoner symptoms include dyspnoea, cough, haemoptysis and weight loss [119]. This entity can only be definitively diagnosed following removal or biopsy of the nodule(s), but is suggested if some of the multiple nodules are cavitary, other infectious aetiologies are ruled out and Aspergillus IgG (or other microbiology) is positive.
Fig. 6.
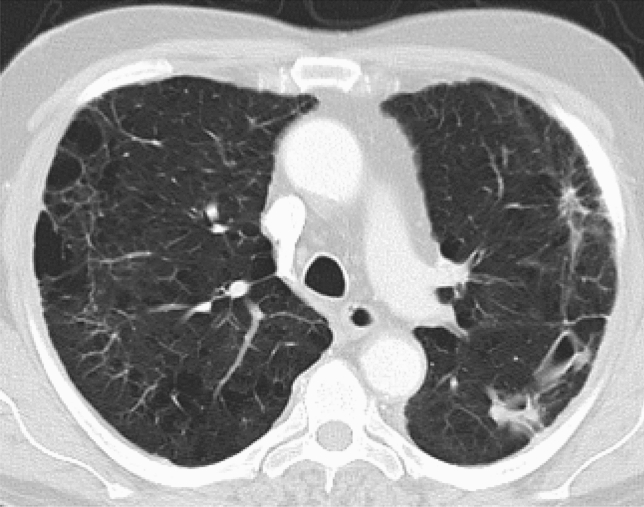
CT thorax of a long term smoker with COPD presenting with increasing shortness of breath for 20 years. CT scan shows multiple nodules on the left side, two of which had cavitated, in association with a raised Aspergillus IgG titre, all in keeping with chronic cavitary pulmonary aspergillosis
Aspergillus IgG antibody testing appears to have a low sensitivity for diagnosing Aspergillus nodules as this was positive in only a small proportion of patients in a recent study [119]. Further research is required to clearly establish the role of Aspergillus IgG antibody in the diagnosis of Aspergillus nodules and identify other means of making the diagnosis without requiring biopsy or resection. Treatment modalities include antifungal therapy and surgical procedures such as lobectomy or sublobar resection depending on the number of nodules, their location and each patient’s lung function.
Aspergillus sensitisation
Aspergillus sensitisation has been associated with increased severity of asthma. However, the relationship between COPD without asthma and fungal sensitisation remains unclear. COPD patients have been shown to have higher Aspergillus IgG (31 mg/L vs 20.7 mg/L) and more frequent Aspergillus sensitisation than controls (18% vs 4%) [120]. Research has shown that Aspergillus-sensitised individuals with COPD more often have bronchiectasis [120] as well as worse FEV1 compared to non-sensitised COPD patients (39% vs 51% predicted) [121], although this was not found in all studies [2]. The average prevalence of Aspergillus sensitisation in COPD from 5 studies comprising more than 1000 patients was found to be 13.6% [2]. Other studies have reported a prevalence of Aspergillus sensitisation in COPD of between 8.5 and 18% [122].
Allergic bronchopulmonary aspergillosis (ABPA) typically occurs in the setting of asthma or cystic fibrosis. In the recent past, there have been several articles have reported an association between COPD and ABPA though the exact pathophysiologic mechanism underpinning this association is yet to be elucidated. The first report of this association involved a 50‐year‐old smoker with COPD who presented with features of an exacerbation and was subsequently diagnosed with ABPA on the basis of positive Aspergillus skin tests (both Type I and III), elevated total serum IgE, Aspergillus specific IgE and IgG, eosinophilia and a positive sputum culture for A. fumigatus [123]. Both COPD and ABPA are characterised by the occurrence of airway inflammation, mucous hypersecretion, impaired mucociliary clearance and airflow obstruction.
A prospective case–control study involving 200 patients with COPD in India identified Aspergillus hypersensitivity in 17 (8.5%) of the patients with COPD as compared to none in the control group [124]. The serologic criteria for the diagnosis of ABPA were fulfilled in two (1.0%) of the patients. There have since been other case reports of ABPA occurring in patients with COPD who had neither asthma nor CF [125, 126]. This is a dynamic area of study, with uncertainty about the implications for clinical care.
Aspergillus bronchitis
Aspergillus bronchitis is an uncommon manifestation of Aspergillus infection. It is a chronic superficial infection of the lower airways (trachea and bronchi) with Aspergillus, without important tissue invasion, lung parenchymal destruction or overt allergic response [127]. Aspergillus bronchitis is characterised by superficial invasion of airway mucosa by Aspergillus hyphae. This gives rise to mucoid impaction, production of thick tenacious sputum with bronchial plugging. Aspergillus IgG antibody is typically detectable in serum and Aspergillus spp. repeatedly detectable in sputum or bronchoscopy fluid with culture or PCR.
Aspergillus bronchitis is most common in those with bronchiectasis, but has also been reported to occur in patients with COPD. A retrospective analysis of 38 patients presenting with tracheobronchitis among non-neutropenic/non-transplant adult patients with at least two cultures of respiratory samples yielding Aspergillus spp. identified 26 (81.3%) patients with GOLD III to IV COPD [128]. A smaller series involving 17 patients with Aspergillus bronchitis identified 6 (35%) patients with COPD [129].
The various Aspergillosis entities in COPD patients and therapies are summarised in Table 2.
Table 2.
Aspergillosis entities in COPD patients and therapies, in approximate descending order of utility and necessity (i.e. first-, second- and third-line therapies)
| Aspergillus entities | Diagnostic tests | Treatment |
|---|---|---|
| Invasive pulmonary aspergillosis | Cytology, direct microscopy and/or culture of lower respiratory tract specimen, galactomannan antigen index in plasma/serum and BAL fluid, Aspergillus PCR on plasma/serum and BAL, chest imaging | Triazoles, amphotericin B, caspofungin or micafungin, surgical resection |
| Invasive Aspergillus tracheobronchitis | Sputum and BAL fluid culture or PCR, Aspergillus IgG in serum, serum IgE, chest imaging | Triazoles |
| Chronic cavitary pulmonary aspergillosis | Aspergillus IgG or precipitins in plasma/serum, Aspergillus antigen or PCR in respiratory fluids, microscopy/histology, chest imaging | Triazoles, surgery, amphotericin B. micafungin |
| Community-acquired Aspergillus pneumonia | Aspergillus IgG or precipitins in plasma/serum | Triazoles |
| Aspergillus nodule(s) | Aspergillus IgG or precipitins in plasma/serum, Aspergillus antigen or PCR in respiratory fluids, histology, chest imaging | Excision biopsy, triazoles |
| Aspergillus sensitisation | Serum IgE to A. fumigatus serum precipitins, total IgE, skin prick test or RAST test | None, triazoles, nebulised amphotericin B |
Bronchiectasis
Bronchiectasis is a chronic airways syndrome usually defined in the presence of permanent airway dilatation (often demonstrated on high-resolution CT scanning), variable mucociliary clearance, recurrent airway symptoms of daily sputum production and episodic infective exacerbations [130–132].
The common manifestations of Aspergillus disease in bronchiectasis include CPA, APBA pulmonary aspergilloma and IA [133]. Aspergillus diseases such as ABPA may be complicated by the development of bronchiectasis whereas patients with already established bronchiectasis and lung architectural disruption can develop Aspergillus-related diseases such as aspergillomas on top of post-TB bronchiectasis [134]. Bronchiectasis and COPD are reported to coexist in 20–60% of cases, and coexisting disease is linked to greater symptomatology, reduced therapeutic options and poorer prognosis when compared to bronchiectasis or COPD alone [135–138]. Misdiagnosis of the two conditions frequently occur with computed tomography (CT) without high-resolution algorithms being identified as the main cause of under-diagnosis of bronchiectasis [134].
Bronchiectasis does share some pathogenic similarities with COPD. In both diseases, chronic bronchial infection by pathogenic microorganisms and consequent chronic inflammation with remodelling of the airways leads to impairment of local defence mechanisms. This ultimately results in the persistence of microorganisms such as Aspergillus in the bronchial tree despite treatment [139]. In bronchiectasis, Aspergillus infection of the airways is thought to directly damage the airways and impair mucociliary clearance. In normal (or asthmatic/COPD) airways in susceptible individuals, it has been theorised the Aspergillus proteases upregulate mucous production and/or drive a Th2 phenotype thus drive airway inflammation towards frank bronchiectasis [130].
Role of Aspergillus colonisation in COPD
The isolation of Aspergillus from respiratory secretions of COPD patients is common and usually dismissed as representing mere colonisation or contamination, especially outside the setting of critical illness and in the absence of imaging findings suggestive of IA or CPA. However, several studies attempted to assess its significance [27]. A prospective study conducted across four hospitals in Spain involving 240 hospitalised patients with a severe COPD exacerbation reported a 16.6% prevalence of Aspergillus spp. isolation on admission and 14.1% at 1-year follow-up. No patients were found to have IA. Those who isolated Aspergillus were more likely to have had an exacerbation in the preceding year and to also have other pathogens and especially Pseudomonas aeruginosa in sputum [140]. Aspergillus isolation was associated with an increased length of stay (11.8 vs 7.5 days). In a prospective study from the UK, 37% of patients isolated A. fumigatus at baseline at steady state. Isolation of A. fumigatus was associated with higher inhaled corticosteroid dose and higher sputum total cell and neutrophil count. However, there was no link with exacerbation frequency or FEV1 [119]. The association with a higher dose of inhaled corticosteroids was also shown in a retrospective pair-matched study from China; patients with Aspergillus isolation had more prolonged hospital stay (15 vs 12 days) [141]. Finally, patients with COPD who reported activities related to fungal exposure reported more exacerbations. This may suggest a potential association with Aspergillus exposure [142].
Pathogenesis of aspergillosis in COPD
The pathogenesis of COPD involves infections, chronic inflammation and oxidative stress [143]. A. fumigatus is the most common pathogen isolated from the sputum of patients with COPD at steady state and during exacerbations [14]. The phagocytosis of bacteria and apoptotic cells by the alveolar macrophages in COPD patients is impaired though it is uncertain if this applies to the phagocytosis of Aspergillus conidia [144]. Patients with COPD are thought to have impaired ciliary function resulting from exposure to cigarette smoke and recurrent chest infections. This may promote the binding of the Aspergillus conidia to the airways in a concentration-dependent manner. The epithelial responses to A. fumigatus result in remodelling of the respiratory epithelium with a heightened expression of Th2 cytokines [13]. Cytoskeletal collapse and apoptosis eventually occur. In COPD, it is possible that a combination of several factors limits the ability to eliminate the conidia of Aspergillus. Defects of the innate immune system (inherited or acquired), regular use of high-dose steroids in addition to frequent use of broad-spectrum antibiotics in case of exacerbations, may all play a role in promoting the colonisation of the respiratory epithelium by Aspergillus.
Pentraxin 3 (PTX3) is an acute phase protein released during inflammation or injury by several cells. PTX3 binds to the conidia of A. fumigatus and serves as an opsonising agent [145] while also triggering the classical complement pathway and mediating innate immunity to A. fumigatus [146–148]. A recent study carried out among a Chinese COPD population showed a significant association between PTX3 rs1840680 single nucleotide polymorphisms and the susceptibility to pulmonary aspergillosis [149]. Plasma PTX3 levels have been found to substantially increase in the setting of fungal infection [150] and may have utility in the diagnosis of IA in COPD patients.
There is growing evidence that severe COPD predisposes to the development of IA especially, but not exclusively, in the setting of steroid use [31, 32, 37, 39, 48]. It is still unclear why some COPD patients get colonised by Aspergillus spp., whereas others develop IA. High doses of oral steroids reportedly promote the growth of Aspergillus [151] while enhancing Th2 cytokines and dampening Th1 cytokine response [152]. Steroids also inhibit neutrophil action and decrease alveolar macrophage antifungal activity by limiting the production of reactive oxidant intermediates [153, 154]. In vitro studies suggest that steroids promote the growth of A. fumigatus as 30–40% increase in growth rate of A. fumigatus and A. flavus exposed to pharmacological doses of hydrocortisone was demonstrated [151]. The exact mechanism for this increased growth is not fully understood, but the presence of a ligand/receptor system in the fungi has been proposed as a putative mechanism for this association.
A retrospective study of 239 patients admitted with COPD with Aspergillus isolation identified the accumulated dosage of corticosteroids equivalent to > 700 mg prednisolone in the three months prior to admission to be an independent predictor of IA [37]. In this same study, the receipt of antibiotic treatment in the 3 months prior to admission was also a predictor of IA. Inhaled corticosteroids have also been identified as a risk factor for IA [155]. A study conducted among 11 patients with COPD from which Aspergillus isolates were cultured from lower respiratory tract samples revealed that 8 (72.7%) of the patients had used an inhaled steroid, while 10 (90.9%) had used a systemic steroid within the last 3 weeks [41].
A retrospective case–control study of 30 COPD patients with IA and 60 COPD control patients without IA in China identified treatment with three or more antibiotics during hospitalisation and antibiotic treatment longer than 10 days to be risk factors for IA [40]. The use of broad-spectrum antibiotics in the setting of COPD is thought to disrupt the normal flora of the respiratory epithelium thereby promoting the proliferation of fungal pathogens such as Aspergillus [156]. The ability of Aspergillus to form biofilms on bronchial epithelial cells which are difficult to eradicate with antifungals may also be a contributory factor [157].
Uncertainties, future research
Further research is required in several areas. Optimum means of diagnosing IA in COPD with sputum culture/PCR, Aspergillus antibody and antigen, BDG and identification of new biomarkers are key areas to explore further. Prospective studies are needed to establish the significance of Aspergillus isolation in COPD exacerbations, including intervention studies, and consensus reached on whether this requires treatment. The utility of Aspergillus biomarkers such as BDG and Aspergillus antibody assays in COPD monitoring of therapy for IA in COPD needs to be clearly elucidated.
Likewise, genetic risk factors for IA in need to be investigated. There are many described in leukaemia, haematopoietic stem cell transplantation and solid organ transplantation, but this is largely unexplored in COPD. In addition to PTX3 polymorphisms, other genetic defects may be implicated, such as those affecting the Th1 cytokine pathway. The role of fungal exposure in the development of CPA in patients with COPD also needs to be better defined. The efficacy of IFNγ immunotherapy in the setting of CPA/COPD overlap remains an underexplored but promising area of research.
In conclusion, COPD poses a significant global health concern and urgent measures are required to mitigate the current situation. Healthcare professionals need to be aware of the risk of IA and various forms of aspergillosis in their COPD patients to aid early diagnosis, facilitate prompt management and reduce mortality.
Author contributions
AO, CK and DWD wrote the main manuscript text with contributions from AM and CI. CI prepared Figs. 1 and 2. All authors reviewed the manuscript.
Funding
No funding was received for this article.
Availability of data and materials
Not applicable.
Declarations
Conflict of interest
Denning and family hold Founder shares in F2G Ltd, a University of Manchester spin-out antifungal discovery company. He acts, or has recently acted, as a consultant to Pulmatrix, Pulmocide, Zambon, iCo Therapeutics, Mayne Pharma, Biosergen, Bright Angel Therapeutics, Cipla and Metis. He sits on the DSMB for a SARS CoV2 vaccine trial. In the last three years, he has been paid for talks on behalf of Dynamiker, Hikma, Gilead, Merck, Mylan and Pfizer. The other co-authors have no conflict of interest to declare.
Ethical approval
Not applicable.
References
- 1.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. The Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammond EE, McDonald CS, Vestbo J, Denning DW. The global impact of Aspergillus infection on COPD. BMC Pulm Med. 2020;20:241. doi: 10.1186/s12890-020-01259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en/. Date last updated: 27 Oct 2016.
- 4.World Health Organization. Burden of COPD. http://www.who.int/respiratory/copd/burden/en/. Date last updated: 15 Mar 2017.
- 5.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53:1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 7.Mathioudakis AG, Janssens W, Sivapalan P, Singanayagam A, Dransfield MT, Jensen JS, et al. Acute exacerbations of chronic obstructive pulmonary disease: in search of diagnostic biomarkers and treatable traits. Thorax. 2020;75:520–527. doi: 10.1136/thoraxjnl-2019-214484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26:160073. doi: 10.1183/16000617.0073-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kefala AM, Fortescue R, Alimani GS, Kanavidis P, McDonnell MJ, Magiorkinis E, et al. Prevalence and clinical implications of respiratory viruses in stable chronic obstructive pulmonary disease (COPD) and exacerbations: a systematic review and meta-analysis protocol. BMJ Open. 2020;10:e035640. doi: 10.1136/bmjopen-2019-035640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiew PY, Dicker AJ, Keir HR, Poh ME, Pang SL, Mac Aogáin M, et al. A high-risk airway mycobiome is associated with frequent exacerbation and mortality in COPD. Eur Respir J. 2021;57:2002050. doi: 10.1183/13993003.02050-2020. [DOI] [PubMed] [Google Scholar]
- 11.Messer SA, Jones RN, Fritsche TR. International surveillance of Candida spp. and Aspergillus spp.: report from the SENTRY antimicrobial surveillance program (2003) J Clin Microbiol. 2006;44:1782–1787. doi: 10.1128/JCM.44.5.1782-1787.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulussen C, Hallsworth JE, Álvarez-Pérez S, Nierman WC, Hamill PG, Blain D, et al. Ecology of aspergillosis: insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb Biotechnol. 2017;10:296–322. doi: 10.1111/1751-7915.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertuzzi M, Hayes GE, Icheoku UJ, van Rhijn N, Denning DW, Nir Osherov N, et al. Anti-Aspergillus activities of the respiratory epithelium in health and disease. J Fungi. 2018;4:8. doi: 10.3390/jof4010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wrench C, Belchamber KBR, Bercusson A, Shah A, Barnes PJ, Armstrong-James D, et al. Reduced Clearance of Fungal Spores by Chronic Obstructive Pulmonary Disease GM-CSF– and M-CSF–derived Macrophages. Am J Respir Cell Mol Biol. 2018;58:271–273. doi: 10.1165/rcmb.2017-0351LE. [DOI] [PubMed] [Google Scholar]
- 15.Pashley CH, Fairs A, Morley JP, et al. Routine processing procedures for isolating filamentous fungi from respiratory sputum samples may underestimate fungal prevalence. Med Mycol. 2012;50:433–438. doi: 10.3109/13693786.2011.615762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taccone FS, Van den Abeele A, Bulpa P, Misset B, Meersseman W, Cardoso T, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7. doi: 10.1186/s13054-014-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He H, Li Q, Chang S, Ding L, Sun B, Li F, Zhan Q. Prognostic value of serum galactomannan index in critically ill patients with chronic obstructive pulmonary disease at risk of invasive pulmonary aspergillosis. Chin Medl J. 2014;127:23–28. [PubMed] [Google Scholar]
- 18.Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. 2011;37:865–872. doi: 10.1183/09031936.00054810. [DOI] [PubMed] [Google Scholar]
- 19.Everaerts S, Lagrou K, Vermeersch K, Dupont LJ, Vanaudenaerde BM, Janssens W. Aspergillus fumigatus detection and risk factors in patients with COPD–bronchiectasis overlap. Int J Mol Sci. 2018;19:523. doi: 10.3390/ijms19020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah A, Panjabi C. Allergic bronchopulmonary aspergillosis: a perplexing clinical entity. Allergy Asthma Immunol Res. 2016;8:282–297. doi: 10.4168/aair.2016.8.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segal BH, Romani LR. Invasive aspergillosis in chronic granulomatous disease. Med Mycol. 2009;47:S282–S290. doi: 10.1080/13693780902736620. [DOI] [PubMed] [Google Scholar]
- 22.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Invasive Aspergillosis. In Specialty Imaging: HRCT of the Lung (Second Edition), 2017. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/invasive-aspergillosis.
- 24.Ben-Ami R, Lewis RE, Kontoyiannis DP. Enemy of the (immunosuppressed) state: an update on the pathogenesis of Aspergillus fumigatus infection. Br J Haematol. 2010;150:406–417. doi: 10.1111/j.1365-2141.2010.08283. [DOI] [PubMed] [Google Scholar]
- 25.Dogra V, Sinha AK, Saxena R, Talwar D. Aspergillus march: from ABPA to aspergilloma to subacute invasive aspergillosis. Allergy Asthma Clin Immunol. 2016;12:64. doi: 10.1186/s13223-016-0170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hope WW, Walsh TJ, Denning DW. The invasive and saprophytic syndromes due to Aspergillus spp. Medical Mycology. 2005;43:207–238. doi: 10.1080/13693780400025179. [DOI] [PubMed] [Google Scholar]
- 27.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270–277. doi: 10.1136/thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]
- 28.Franquet T, Müller NL, Giménez A, Domingo P, Plaza V, Bordes R. Semiinvasive pulmonary aspergillosis in chronic obstructive pulmonary disease: radiologic and pathologic findings in nine patients. AJR Am J Roentgenol. 2000;174:51–56. doi: 10.2214/ajr.174.1.1740051. [DOI] [PubMed] [Google Scholar]
- 29.Gavaldà J, Meije Y, Fortún J, Roilides E, Saliba F, Lortholary O, ESCMID Study Group for Infections in Compromised Hosts Invasive fungal infections in solid organ transplant recipients. Clin Microbiol Infect. 2014;20:27–48. doi: 10.1111/1469-0691.12660. [DOI] [PubMed] [Google Scholar]
- 30.Garbino J, Fluckiger U, Elzi L, Imhof A, Bille J, Zimmerli S. Survey of aspergillosis in non-neutropenic patients in Swiss teaching hospitals. Clin Microbiol Infect. 2011;17:1366–1371. doi: 10.1111/j.1469-0691.2010.03402.x. [DOI] [PubMed] [Google Scholar]
- 31.Rello J, Esandi ME, Mariscal D, Gallego M, Domingo C, Valles J. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: report of eight cases and review. Clin Infect Dis. 1998;26:1473–1475. doi: 10.1086/517672. [DOI] [PubMed] [Google Scholar]
- 32.Meersseman W, Vandecasteele SJ, Wilmer A, Verbeken E, Peetermans WE, Van Wijngaerden E. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621–625. doi: 10.1164/rccm.200401-093OC. [DOI] [PubMed] [Google Scholar]
- 33.Cornet M, Mallat H, Somme D, Guérot E, Kac G, et al. Fulminant invasive pulmonary aspergillosis in immunocompetent patients–a two-case report. Clin Microbiol Infect. 2003;9:1224–1227. doi: 10.1111/j.1469-0691.2003.00792.x. [DOI] [PubMed] [Google Scholar]
- 34.Kimura S-I. Invasive aspergillosis in hematological patients. Med Mycol J. 2016;57:J77–J88. doi: 10.3314/mmj.57.J77. [DOI] [PubMed] [Google Scholar]
- 35.Yan X, Li M, Jiang M, Zou L, Luo F, Jiang Y. Clinical characteristics of 45 patients with invasive pulmonary aspergillosis: retrospective analysis of 1711 lung cancer cases. Cancer. 2009;115:5018–5025. doi: 10.1002/cncr.24559. [DOI] [PubMed] [Google Scholar]
- 36.Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guinea J, Torres-Narbona M, Gijón P, Munoz P, Pozo F, Peláez T, et al. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: incidence, risk factors, and outcome. Clin Microbiol Infect. 2010;16(7):870–877. doi: 10.1111/j.1469-0691.2009.03015.x. [DOI] [PubMed] [Google Scholar]
- 38.Ribaud P, Chastang C, Latgé JP, Baffroy-Lafitte L, Parquet N, Devergie A, et al. Survival and prognostic factors of invasive aspergillosis after allogenic bone marrow transplantation. Clin Infect Dis. 1999;28:322–330. doi: 10.1086/515116. [DOI] [PubMed] [Google Scholar]
- 39.Bulpa PA, Dive AM, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J. 2007;30:782–800. doi: 10.1183/09031936.00062206. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Li L, Huang WJ, Wang LX, Li WF, Yuan WF. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: a case control study from China. Clin Microbiol Infect. 2012;18:403–408. doi: 10.1111/j.1469-0691.2011.03503.x. [DOI] [PubMed] [Google Scholar]
- 41.Tutar N, Metan G, Koç AN, Yilmaz I, Bozkurt I, Simsek ZO, et al. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Multidiscip Respir Med. 2013;8:59. doi: 10.1186/2049-6958-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32:358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 43.Vandewoude KH, Blot SI, Depuydt P, Benoit D, Temmerman W, Colardyn F, Vogelaers D. Clinical relevance of Aspergillus isolation from respiratory tract samples in critically ill patients. Crit Care. 2006;10:R31. doi: 10.1186/cc4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waqas S, Dunne K, Talento AF, Wilson G, Martin-Loeches I, Keane J, Rogers TR. Prospective observational study of respiratory Aspergillus colonization or disease in patients with various stages of chronic obstructive pulmonary disease utilizing culture versus nonculture techniques. Med Mycol. 2020;14:myaa077. doi: 10.1093/mmy/myaa077. [DOI] [PubMed] [Google Scholar]
- 45.Barberan J, Alcazar B, Malmierca E, de la Llana FG, Dorca J, del Castillo D, et al. Repeated Aspergillus isolation in respiratory samples from non-immunocompromised patients not selected based on clinical diagnoses: colonisation or infection? BMC Infect Dis. 2012;12:295. doi: 10.1186/1471-2334-12-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taccone FS, Van den Abeele A, Bulpa P, Misset B, Meersseman W, Cardoso T, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7. doi: 10.1186/s13054-014-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delsuc C, Cottereau A, Frealle E, Bienvenu A, Dessein R, Jarraud S, et al. Putative invasive pulmonary aspergillosis in critically ill patients with chronic obstructive pulmonary disease: a matched cohort study. Crit Care. 2015;19:421. doi: 10.1186/s13054-015-1140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garnacho-Montero J, Amaya-Villar R, Ortiz-Leyba C, et al. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: risk factors, clinical presentation and outcome. Crit Care. 2005;9:191–199. doi: 10.1186/cc3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bobba RK, Arsura EL. Aspergillus infection in a hospitalised veteran population. Clin Microbiol Infect. 2004;10:679. doi: 10.1111/j.1469-0691.2004.00912.x. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues J, Niederman MS, Fein AM, Pai PB. Nonresolving pneumonia in steroid-treated patients with obstructive lung disease. Am J Med. 1992;93:29–34. doi: 10.1016/0002-9343(92)90676-3. [DOI] [PubMed] [Google Scholar]
- 51.Kiertiburanakul S, Thibbadee C, Santanirand P. Invasive aspergillosis in a tertiary-care hospital in Thailand. J Med Assoc Thai. 2007;90:895–902. [PubMed] [Google Scholar]
- 52.Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuhlman JE, Fishman EK, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985;157:611–614. doi: 10.1148/radiology.157.3.3864189. [DOI] [PubMed] [Google Scholar]
- 54.Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19:253–259. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]
- 55.Arvanitis M, Mylonakis E. Diagnosis of invasive aspergillosis: recent developments and ongoing challenges. Eur J Clin Invest. 2015;45:646–652. doi: 10.1111/eci.12448. [DOI] [PubMed] [Google Scholar]
- 56.Horvath JA, Dummer S. The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am J Med. 1996;100:171–178. doi: 10.1016/s0002-9343(97)89455-7. [DOI] [PubMed] [Google Scholar]
- 57.Eigl S, Prattes J, Reinwald M, Thornton CR, Reischies F, Spiess B, et al. Influence of mould-active antifungal treatment on the performance of the Aspergillus-specific bronchoalveolar lavage fluid lateral-flow device test. Int J Antimicrob Agents. 2015;46:401–405. doi: 10.1016/j.ijantimicag.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 58.Jabeen K, Farooqi J, Irfan M, Ali SA, Denning DW. Diagnostic dilemma in COVID-19-associated pulmonary aspergillosis. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cordonnier C, Botterel F, Ben Amor R, et al. Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis. Clin Microbiol Infect. 2009;15:81–86. doi: 10.1111/j.1469-0691.2008.02122.x. [DOI] [PubMed] [Google Scholar]
- 60.Eigl S, Hoenigl M, Spiess B, et al. Galactomannan testing and Aspergillus PCR in same-day bronchoalveolar lavage and blood samples for diagnosis of invasive aspergillosis. Med Mycol. 2017;55:528–534. doi: 10.1093/mmy/myw102. [DOI] [PubMed] [Google Scholar]
- 61.Fortún J, Martín-Dávila P, de la Pedrosa EGG, Silva JT, Garcia-Rodríguez J, Benito D, et al. Galactomannan in bronchoalveolar lavage fluid for diagnosis of invasive aspergillosis in non-hematological patients. J Infect. 2016;72:738–744. doi: 10.1016/j.jinf.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X-B, Chen G-P, Lin Q-C, Lin X, Zhang H-Y, Wang J-H. Bronchoalveolar lavage fluid galactomannan detection for diagnosis of invasive pulmonary aspergillosis in chronic obstructive pulmonary disease. Med Mycol. 2013;51:688–695. doi: 10.3109/13693786.2013.777162. [DOI] [PubMed] [Google Scholar]
- 63.Guegan H, Robert-Gangneux F, Camus C, Belaz S, Marchand T, Baldeyrou M, et al. Improving the diagnosis of invasive aspergillosis by the detection of Aspergillus in broncho-alveolar lavage fluid: comparison of non-culture-based assays. J Infect. 2018;76:196–205. doi: 10.1016/j.jinf.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Imbert S, Gauthier L, Joly I, Brossas JY, Uzunov M, Touafek F, et al. Aspergillus PCR in serum for the diagnosis, follow-up and prognosis of invasive aspergillosis in neutropenic and non-neutropenic patients. Clin Microbiol Infect. 2016;22:562.e1–562.e8. doi: 10.1016/j.cmi.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aquino VR, Nagel F, Andreolla HF, de Paris F, Xavier MO, Goldani LZ, Denning DW, Pasqualotto AC. The performance of real-time PCR, galactomannan, and fungal culture in the diagnosis of invasive aspergillosis in ventilated patients with chronic obstructive pulmonary disease (COPD) Mycopathologia. 2012;174:163–9. doi: 10.1007/s11046-012-9531-1. [DOI] [PubMed] [Google Scholar]
- 66.Guinea J, Padilla C, Escribano P, Muñoz P, Padilla B, et al. Evaluation of MycAssay™ Aspergillus for diagnosis of invasive pulmonary Aspergillosis in patients without hematological cancer. PLoS ONE. 2013;8:e61545. doi: 10.1371/journal.pone.0061545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prattes J, Hoenigl M, Zinke SE, Heldt S, Eigl S, Johnson GL, Bustin S, Stelzl E, Kessler HH. Evaluation of the new AspID polymerase chain reaction assay for detection of Aspergillus species: a pilot study. Mycoses. 2018;61:355–359. doi: 10.1111/myc.12757. [DOI] [PubMed] [Google Scholar]
- 68.Neofytos D, Treadway S, Ostrander D, Alonso CD, Dierberg KL, Nussenblatt V, et al. Epidemiology, outcomes, and mortality predictors of invasive mold infections among transplant recipients: a 10-year, single-center experience. Transpl Infect Dis. 2013;15:233–242. doi: 10.1111/tid.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramos ER, Jiang Y, Hachem R, Kassis C, Kontoyiannis DP, Raad I. Outcome analysis of invasive aspergillosis in hematologic malignancy and hematopoietic stem cell transplant patients: the role of novel antimold azoles. Oncologist. 2011;16:1049–1060. doi: 10.1634/theoncologist.2010-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thornton CR. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol. 2008;15:1095–1105. doi: 10.1128/CVI.00068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prattes J, Flick H, Iler FP, Koidl C, Raggam RB, Michael Palfner M, et al. Novel tests for diagnosis of invasive Aspergillosis in patients with underlying respiratory diseases. Am J Respir Crit Care Med. 2014;190:922–929. doi: 10.1164/rccm.201407-1275OC. [DOI] [PubMed] [Google Scholar]
- 72.Jenks JD, Mehta SR, Taplitz R, Aslam S, Reed SL, Hoenigl M. Point-of-care diagnosis of invasive aspergillosis in non-neutropenic patients: Aspergillus galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test in bronchoalveolar lavage. Mycology. 2018;62:230–6. doi: 10.1111/myc.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reischies FM, Raggam RB, Prattes J, Krause R, Eigl S, List A, et al. Urine galactomannan-to-creatinine ratio for detection of invasive aspergillosis in patients with hematological malignancies. J Clin Microbiol. 2016;54:771–774. doi: 10.1128/JCM.02969-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duettmann W, Koidl C, Krause R, Lackner G, Woelfler A, Hoenigl M. Specificity of mannan antigen and anti-mannan antibody screening in patients with haematological malignancies at risk for fungal infection. Mycoses. 2016;59:374–378. doi: 10.1111/myc.12482. [DOI] [PubMed] [Google Scholar]
- 75.Fisher BT, Zaoutis TE, Park JR, Bleakley M, Englund JA, Kane C, et al. Galactomannan antigen testing for diagnosis of invasive aspergillosis in pediatric hematology patients. J Pediatric Infect Dis Soc. 2012;1:103–111. doi: 10.1093/jpids/pis044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Obayashi T, Yoshida M, Mori T, Goto H, Yasuoka A, Iwasaki H, Teshima H, Kohno S, Horiuchi A, Ito A, et al. Plasma (1→3)-β-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet. 1995;345:17–20. doi: 10.1016/s0140-6736(95)91152-9. [DOI] [PubMed] [Google Scholar]
- 77.Gow NAR, Latge JP, Munro CA. The fungal cell wall: structure, biosynthesis, and function. In: Heitman J, Howlett BJ, Crous PW, Stukenbrock EH, James TY, Gow NAR, editors. The fungal kingdom. Washington, DC, USA: American Society of Microbiology; 2018. [Google Scholar]
- 78.Metan G, Tutar N, Koc AN (2013) 1,3-Beta-D-glucan for the diagnosis of invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Abstract Ref ID: 19384, 6th Trends in Medical Mycology. [DOI] [PMC free article] [PubMed]
- 79.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Florl C, Lewis RE, Munoz P, Verweij PE, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24:e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Patterson TF, Thompson GR, III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dichtl K, Forster J, Ormanns S, Horns H, Suerbaum S, Seybold U, Wagener J. Comparison of β-D-Glucan and galactomannan in serum for detection of invasive Aspergillosis: retrospective analysis with focus on early diagnosis. J Fungi. 2020;6:253. doi: 10.3390/jof6040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kappe R, Rimek D. Antikorper-Nachweis bei invasiver Aspergillose [Antibody detection in patients with invasive aspergillosis] Mycoses. 2004;47:55–59. doi: 10.1111/j.1439-0507.2004.01035.x. [DOI] [PubMed] [Google Scholar]
- 83.Yu Q, He J, Xing B, et al. Potential value of serum Aspergillus IgG antibody detection in the diagnosis of invasive and chronic pulmonary aspergillosis in non-agranulocytic patients. BMC Pulm Med. 2020;20:89. doi: 10.1186/s12890-020-1125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cadranel J, Philippe B, Hennequin C, et al. Voriconazole for chronic pulmonary aspergillosis: a prospective multicenter trial. Eur J Clin Microbiol Infect Dis. 2012;31:3231–3239. doi: 10.1007/s10096-012-1690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fréalle E, Reboux G, Le Rouzic O, Bautin N, Willemin MC, Pichavant M, Delourme J, Sendid B, Gosset P, Nseir S, Fry S. Impact of domestic mould exposure on Aspergillus biomarkers and lung function in patients with chronic obstructive pulmonary disease. Environ Res. 2021;195:110850. doi: 10.1016/j.envres.2021.110850. [DOI] [PubMed] [Google Scholar]
- 86.Dobias R, Jaworska P, Tomaskova H, Kanova M, Lyskova P, Vrba Z, Holub C, Svobodová L, Hamal P, Raska M. Diagnostic value of serum galactomannan, (1,3)-β-d-glucan, and Aspergillus fumigatus-specific IgA and IgG assays for invasive pulmonary aspergillosis in non-neutropenic patients. Mycoses. 2018;61:576–586. doi: 10.1111/myc.12765. [DOI] [PubMed] [Google Scholar]
- 87.Niazi-Ali S, Atherton GT, Walczak M, Denning DW. Drug-drug interaction database for safe prescribing of systemic antifungal agents. Ther Adv Infect Dis. 2021;30:20499361211010605. doi: 10.1177/20499361211010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Einsele H, Quabeck K, Müller D, et al. Prediction of invasive pulmonary aspergillosis from colonisation of lower respiratory tract before marrow transplantation. The Lancet. 1998;352:P1443. doi: 10.1016/s0140-6736(05)61265-2. [DOI] [PubMed] [Google Scholar]
- 89.Cornillet A, Camus C, Nimubona S, et al. Comparison of epidemiological, clinical, and biological features of invasive Aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis. 2006;43:577–584. doi: 10.1086/505870. [DOI] [PubMed] [Google Scholar]
- 90.Rocchi S, Reboux G, Millon L. Résistance aux antifongiques azolés d'origine environnementale : quelles alternatives pour l'avenir ? [Azole resistance with environmental origin: What alternatives for the future?] J Mycol Med. 2015;25:249–56. doi: 10.1016/j.mycmed.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 91.Warris A, Verweij PE. Clinical implications of environmental sources for Aspergillus. Med Mycol. 2005;43:S59–65. doi: 10.1080/13693780400025260. [DOI] [PubMed] [Google Scholar]
- 92.Hedayati MT, Mayahi S, Denning DW. A study on Aspergillus species in houses of asthmatic patients from Sari City, Iran and a brief review of the health effects of exposure to indoor Aspergillus. Environ Monit Assess. 2010;168:481–487. doi: 10.1007/s10661-009-1128-x. [DOI] [PubMed] [Google Scholar]
- 93.Clancy CJ, Nguyen MH. Acute community-acquired pneumonia due to Aspergillus in presumably immunocompetent hosts: clues for recognition of a rare but fatal disease. Chest. 1998;114:629–634. doi: 10.1378/chest.114.2.629. [DOI] [PubMed] [Google Scholar]
- 94.Kennedy WP, Malone DN, Blyth W. Necrotizing pulmonary aspergillosis. Thorax. 1970;25:691–701. doi: 10.1136/thx.25.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Binder RE, Faling LJ, Pugatch RD, Mahasaen C, Snider GL. Chronic necrotizing pulmonary aspergillosis: a discrete clinical entity. Medicine (Baltimore) 1982;61:109–124. doi: 10.1097/00005792-198203000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Conrad DJ, Warnock M, Blanc P, Cowan M, Golden JA. Microgranulomatous aspergillosis after shoveling wood chips: report of a fatal outcome in a patient with chronic granulomatous disease. Am J Ind Med. 1992;22:411–418. doi: 10.1002/ajim.4700220313. [DOI] [PubMed] [Google Scholar]
- 97.Butler L, Brockley T, Denning D, Richardson M, Chisholm R, Sinha S, O’Driscoll R. Acute Aspergillus pneumonia associated with mouldy tree bark-chippings, complicated by anti-glomerular basement membrane disease causing permanent renal failure. Med Mycol Case Rep. 2013;2:125–127. doi: 10.1016/j.mmcr.2013.06.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Batard E, Renaudin K, Morin O, Desjars P, Germaud P. Fatal acute granulomatous pulmonary aspergillosis in a healthy subject after inhalation of vegetal dust. Eur J Clin Microbiol Infect Dis. 2003;22:357–359. doi: 10.1007/s10096-003-0939-x. [DOI] [PubMed] [Google Scholar]
- 99.Siddiqui S, Anderson VL, Hilligoss DM, Abinun M, Kuijpers TW, Masur H, Holland SM. Fulminant mulch pneumonitis: an emergency presentation of chronic granulomatous disease. Clin Infect Dis. 2007;45:673–681. doi: 10.1086/520985. [DOI] [PubMed] [Google Scholar]
- 100.Zuk JA, King D, Zakhour HD, Delaney JC. Locally invasive pulmonary aspergillosis occurring in a gardener: an occupational hazard? Thorax. 1989;44:678–679. doi: 10.1136/thx.44.8.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meeker DP, Gephardt GN, Cordasco EM, Wiedemann HP. Hypersensitivity pneumonitis versus invasive pulmonary aspergillosis: two cases with unusual pathologic findings and review of the literature. Am Rev Respir Dis. 1991;143:431–436. doi: 10.1164/ajrccm/143.2.431. [DOI] [PubMed] [Google Scholar]
- 102.Russell K, Broadbridge C, Murray S, Waghorn D, Mahoney A. Gardening can seriously damage your health. Lancet. 2008;371:2056. doi: 10.1016/s0140-6736(08)60877-6. [DOI] [PubMed] [Google Scholar]
- 103.Global Action Fund for Fungal Infection. Fungal disease frequency. Available online: https://www.gaffi.org/why/fungal-disease-frequency/.
- 104.Denning DW. Chronic forms of pulmonary aspergillosis. Clin Microbiol Infect. 2001;7:25–31. doi: 10.1111/j.1469-0691.2001.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 105.Denning DW, Riniotis K, Dobrashian R, Sambatakou H. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis. 2003;37:S265–S280. doi: 10.1086/376526. [DOI] [PubMed] [Google Scholar]
- 106.Jhun BW, Jeon K, Eom JS, Lee JH, Suh GY, Kwon OJ, Koh W. Clinical characteristics and treatment outcomes of chronic pulmonary Aspergillosis. Med Mycol. 2013;51:811–817. doi: 10.3109/13693786.2013.806826. [DOI] [PubMed] [Google Scholar]
- 107.Molinos-Castro S, Pesqueira-Fontán PM, Rodríguez-Fernández S, et al. Clinical factors associated with pulmonary Aspergillosis in patients with chronic obstructive pulmonary disease. Enferm Infecc Microbiol Clin. 2020;38:4–10. doi: 10.1016/j.eimc.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 108.Lowes D, Al-Shair K, Newton PJ, Morris J, Harris C, Rautemaa-Richardson R, Denning DW. Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J. 2017;49:1601062. doi: 10.1183/13993003.01062-2016. [DOI] [PubMed] [Google Scholar]
- 109.Maitre T, Cottenet J, Godet C, Bonniaud P, Quantin C, Cadranel J. Chronic pulmonary aspergillosis in France: prevalence, associated chronic pulmonary diseases and prognosis in a 10-year retrospective study of the French nationwide administrative hospital database. Eur Respir J. 2019;54:PA4556. doi: 10.1183/13993003.congress-2019.PA4556. [DOI] [Google Scholar]
- 110.Hou X, Zhang H, Kou L, Wei L, Lu J, Li J. Clinical features and diagnosis of chronic pulmonary aspergillosis in Chinese patients. Medicine (Baltimore) 2017;96:e8315. doi: 10.1097/MD.0000000000008315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47:45–68. doi: 10.1183/13993003.00583-2015. [DOI] [PubMed] [Google Scholar]
- 112.Hayes GE, Novak-Frazer L. Chronic pulmonary Aspergillosis—where are we? And where are we going? J Fungi. 2016;2:18. doi: 10.3390/jof2020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burney PGJ, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990–2010. Eur Respir J. 2015;45:1239–1247. doi: 10.1183/09031936.00142414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sehgal IS, Dhooria S, Muthu V, Prasad KT, Aggarwal AN, Chakrabarti A, Choudhary H, Garg M, Agarwal R. Efficacy of 12-months oral itraconazole versus 6-months oral itraconazole to prevent relapses of chronic pulmonary aspergillosis: an open-label, randomised controlled trial in India. Lancet Infect Dis. 2022;22:1052–1061. doi: 10.1016/S1473-3099(22)00057-3. [DOI] [PubMed] [Google Scholar]
- 115.Gu Y, Ye X, Wang Y, Shen K, Zhong J, Chen B, et al. Clinical features and prognostic analysis of patients with Aspergillus isolation during acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med. 2021 doi: 10.1186/s12890-021-01427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Naito M, Kurahara Y, Yoshida S, Ikegami N, Kobayashi T, Minomo S, et al. Prognosis of chronic pulmonary aspergillosis in patients with pulmonary non-tuberculous mycobacterial disease. Respir Investig. 2018;56:326–331. doi: 10.1016/j.resinv.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 117.Babatasi G, Massetti M, Chapelier A, Fadel E, Macchiarini P, Khayat A, et al. Surgical treatment of pulmonary aspergilloma: current outcome. J Thorac Cardiovasc Surg. 2000;19:906–912. doi: 10.1016/S0022-5223(00)70085-7. [DOI] [PubMed] [Google Scholar]
- 118.Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47:45–68. doi: 10.1183/13993003.00583-2015. [DOI] [PubMed] [Google Scholar]
- 119.Muldoon EG, Sharman A, Page I, Bishop P, Denning DW. Aspergillus nodules; another presentation of chronic pulmonary Aspergillosis. BMC Pulm Med. 2016;16:123. doi: 10.1186/s12890-016-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Everaerts S, Lagrou K, Dubbeldam A, Lorent N, Vermeersch K, Van Hoeyveld E, et al. Sensitization to Aspergillus fumigatus as a risk factor for bronchiectasis in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2629–2638. doi: 10.2147/COPD.S141695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bafadhel M, McKenna S, Agbetile J, Fairs A, Desai D, Mistry V, et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur Respir J. 2014;43:64–71. doi: 10.1183/09031936.00162912. [DOI] [PubMed] [Google Scholar]
- 122.Connell D, Shah A. The contribution of Aspergillus fumigatus to COPD exacerbations: a sensitive topic. Eur Respir J. 2020;56:2002223. doi: 10.1183/13993003.02223-2020. [DOI] [PubMed] [Google Scholar]
- 123.Agarwal R, Srinivas R, Jindal SK. Allergic bronchopulmonary aspergillosis complicating chronic obstructive pulmonary disease. Mycoses. 2007;51:83–85. doi: 10.1111/j.1439-0507.2007.01432.x. [DOI] [PubMed] [Google Scholar]
- 124.Agarwal R, Hazarika B, Gupta D, Aggarwal AN, Chakrabarti A, Jindal SK. Aspergillus hypersensitivity in patients with chronic obstructive pulmonary disease: COPD as a risk factor for ABPA? Med Mycol. 2010;48:988–994. doi: 10.3109/13693781003743148. [DOI] [PubMed] [Google Scholar]
- 125.Mir E, Shah A. Allergic bronchopulmonary aspergillosis in a patient with chronic obstructive pulmonary disease. Prim Care Respir J. 2012;21:111–114. doi: 10.4104/pcrj.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Agarwal K, Chowdhary A, Gaur SN. A rare case of allergic bronchopulmonary aspergillosis in a patient with chronic obstructive pulmonary disease. Indian J Allergy Asthma Immunol. 2012;26:20. [Google Scholar]
- 127.Aspergillus bronchitis. Aspergillus and Aspergillosis. Online: https://www.aspergillus.org.uk/new_treatment/aspergillus-bronchitis/. Accessed 18 Aug 2022.
- 128.Barberán J, Sánchez-Haya E, del Castillo D, Sanz F, Alcázar B, Malmierca E, ASP Investigator Group Report of 38 cases of tracheobronchitis in non-immunocompromised patients with dual isolation of Aspergillus in lower respiratory tract samples. Rev Esp Quimioter. 2014;27:110–4. [PubMed] [Google Scholar]
- 129.Chrdle A, Mustakim S, Rowland J, Bright-Thomas RJ, et al. Aspergillus bronchitis without significant immunocompromised. Ann NY Acad Sci. 2012;1272:73–85. doi: 10.1111/j.1749-6632.2012.06816.x. [DOI] [PubMed] [Google Scholar]
- 130.De Soyza A, Aliberti S. Bronchiectasis and Aspergillus: How are they linked? Med Mycol. 2017;55:69–81. doi: 10.1093/mmy/myw109. [DOI] [PubMed] [Google Scholar]
- 131.O'Donnell AE. Bronchiectasis. Chest. 2008;134:815–823. doi: 10.1378/chest.08-0776. [DOI] [PubMed] [Google Scholar]
- 132.Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis non-CF Guideline Group British thoracic society guideline for non-CF bronchiectasis. Thorax. 2010;65:1–58. doi: 10.1136/thx.2010.142778. [DOI] [PubMed] [Google Scholar]
- 133.Yang B, Kim T, Ryu J, Park HY, Hwangbo B, Kong S-Y, Kwon Y-S, Lee SJ, Ra SW, Oh Y-M, et al. Increased incidence and associated risk factors of Aspergillosis in patients with bronchiectasis. J Pers Med. 2021;11:422. doi: 10.3390/jpm11050422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Polverino E, Dimakou K, Hurst J, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J. 2018;52:1800328. doi: 10.1183/13993003.00328-2018. [DOI] [PubMed] [Google Scholar]
- 135.Du Q, Jin J, Liu X, et al. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS One. 2016;11:e0150532. doi: 10.1371/journal.pone.0150532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goeminne PC, Scheers H, Decraene A, et al. Risk factors for morbidity and death in non-cystic fibrosis bronchiectasis: a retrospective cross-sectional analysis of CT diagnosed bronchiectatic patients. Respir Res. 2012;13:21. doi: 10.1186/1465-9921-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J COPD. 2017;12:1401–1411. doi: 10.2147/COPD.S132961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martínez-García MA, Máiz Carro L, De la Rosa CD. The overlap with bronchiectasis. In: Anzueto A, Heijdra Y, Hurst JR, editors. Controversies in COPD (ERS Monograph) Sheffield: European Respiratory Society; 2015. pp. 96–108. [Google Scholar]
- 139.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 140.Huerta A, Soler S, Esperatti M, Guerrero M, Menendez R, Gimeno A, Zalacaín R, Mir N, Aguado JM, Torres A. Importance of Aspergillus spp. isolation in acute exacerbations of severe COPD: prevalence, factors and follow-up: the FUNGI-COPD study. Respir Res. 2014;15:17. doi: 10.1186/1465-9921-15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tong X, Cheng A, Xu H, Jin J, Yang Y, Zhu S, Li Y. Aspergillus fumigatus during COPD exacerbation: a pair-matched retrospective study. BMC Pulm Med. 2018;18:55. doi: 10.1186/s12890-018-0611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kosmidis C, Hashad R, Mathioudakis AG, McCahery T, Richardson MD, Vestbo J. Impact of self-reported environmental mould exposure on COPD outcomes. Pulmonology. 2021 doi: 10.1016/j.pulmoe.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 143.GOLD, Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, 2015, http://www.goldcopd.org/.
- 144.Wu Y, Xu H, Li L, Yuan W, Zhang D, Huang W. Susceptibility to Aspergillus infections in rats with chronic obstructive pulmonary disease via deficiency function of alveolar macrophages and impaired activation of TLR2. Inflammation. 2016;39:1310–8. doi: 10.1007/s10753-016-0363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garlanda C, Jaillon S, Doni A, Bottazzi B, Mantovani A. PTX3, a humoral pattern recognition molecule at the interface between microbe and matrix recognition. Curr Opin Immunol. 2016;38:39–44. doi: 10.1016/j.coi.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wisniewski HG, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 2004;15:129–146. doi: 10.1016/j.cytogfr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 147.Salvatori G, Campo S. Current understanding of PTX3 protective activity on Aspergillus fumigatus infection. Med Mycol. 2012;50:225–233. doi: 10.3109/13693786.2011.648215. [DOI] [PubMed] [Google Scholar]
- 148.Garlanda C, Hirsch E, Bozza S, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 149.Qian He Q, Li H, Rui Y, Liu L, He B, Shi Y, Xin SuX. Pentraxin 3 gene polymorphisms and pulmonary Aspergillosis in chronic obstructive pulmonary disease patients. Clin Infect Dis. 2018;66:261–267. doi: 10.1093/cid/cix749. [DOI] [PubMed] [Google Scholar]
- 150.Biagi E, Col M, Migliavacca M, et al. PTX3 as a potential novel tool for the diagnosis and monitoring of pulmonary fungal infections in immuno-compromised pediatric patients. J Pediatr Hematol Oncol. 2008;30:881–885. doi: 10.1097/MPH.0b013e318180bc1d. [DOI] [PubMed] [Google Scholar]
- 151.Ng TTC, Robson GD, Denning DW. Hydrocortisone enhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology. 1994;140:2475–2479. doi: 10.1099/13500872-140-9-2475. [DOI] [PubMed] [Google Scholar]
- 152.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 153.Schaffner A. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J Clin Invest. 1985;76:1755–1764. doi: 10.1172/JCI112166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Diamond RD. Inhibition of monocyte-mediated damage to fungal hyphae by steroid hormones. J Infect Dis. 1983;147:160. doi: 10.1093/infdis/147.1.160. [DOI] [PubMed] [Google Scholar]
- 155.Leav BA, Fanburg B, Hadley S. Invasive pulmonary aspergillosis associated with high-dose inhaled fluticasone. N Engl J Med. 2000;343:586. doi: 10.1056/NEJM200008243430818. [DOI] [PubMed] [Google Scholar]
- 156.Ader F, Bienvenu A, Rammaert B, Nseir S. Management of invasive aspergillosis in patients with COPD: Rational use of voriconazole. Int J Chron Obstruct Pulmon Dis. 2009;4:279–287. doi: 10.2147/copd.s4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Seidler MJ, Salvenmoser S, Müller FM. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob Agents Chemother. 2008;52:4130–4136. doi: 10.1128/AAC.00234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.