Abstract
Background
The increased expression of B cell‐activating factor (BAFF) has been linked to autoantibody production in autoimmune diseases (ADs). The aim of this study was to investigate the association among TNFSF13B gene (OMIM: 603969) single nucleotide polymorphisms (SNPs), TNFSF13B mRNA, and soluble BAFF (sBAFF) expression in patients with rheumatoid arthritis (RA) and primary Sjögren's syndrome (pSS). The diagnostic value of sBAFF also was evaluated by the area under the curve (AUC) of receiver operating characteristic or receptor (ROC) curves.
Methods
Genotypes of the TNFSF13B rs9514827 (−2841 T > C), rs1041569 (−2701 A > T) and rs9514828 (−871 C > T) SNPs were determined by PCR‐RFLP assay. TNFSF13B mRNA and sBAFF expression were performed by RT‐qPCR and ELISA, respectively. The study included 320 RA patients, 101 pSS patients, and 309 healthy subjects (HS).
Results
The rs9514828 T allele and the TAT haplotype were associated with an increased risk to develop RA. In both ADs, the TNFSF13B mRNA levels were increased in comparison with HS. The rs9514828 (−871 C > T) polymorphism was associated with increased gene expression in RA patients. Also, sBAFF levels were higher in both ADs, however pSS patients showed the highest sBAFF levels. sBAFF showed higher diagnostic performance for pSS with an AUC of 0.968, with a similar accuracy of anti‐SSA/Ro antibody diagnosis (AUC = 0.974).
Conclusions
Our findings demonstrate that the TNFSF13B rs9514828 (−871 C > T) polymorphism is a risk factor for RA in the western Mexican population. sBAFF levels may be a potential diagnosis biomarker in pSS.
Keywords: primary Sjogren's syndrome, rheumatoid arthritis, sBAFF levels, TNFSF13B polymorphisms
TNFSF13B rs9514828 polymorphism is a risk factor for RA in western Mexican population. sBAFF levels are increased in RA and pSS patients. sBAFF showed higher diagnostic performance for pSS with AUC of 0.968, with similar accuracy of anti‐SSA/Ro antibody diagnosis.

1. INTRODUCTION
B cell‐activating factor (BAFF), a member of TNF family ligands, has been shown to modulate B cell biology as it is involved in processes such as survival, proliferation (Schneider et al., 1999), maturation (Schneider et al., 2001), and immunoglobulin secretion (Moore et al., 1999). BAFF can be recognized by three receptors: BAFF‐R also known as BR3, B cell maturation antigen (BCMA), and transmembrane activator and calcium modulating cyclophilin ligand interactor (TACI) (Mackay & Schneider, 2009). The BAFF receptors are differentially expressed regarding the B cell maturation stages (Zhou et al., 2020). The increased expression of BAFF has been linked to autoimmune diseases (ADs); in systemic lupus erythematosus (SLE) patients' high levels of BAFF have been associated with high disease activity and increased autoantibody production (Carter et al., 2013; Petri et al., 2008; Salazar‐Camarena et al., 2015) Therefore, therapeutic BAFF inhibition is one approved treatment indicated for active, autoantibody‐positive SLE patients who are receiving standard therapy (Stohl, 2012), and recently approved for active lupus nephritis treatment (Furie et al., 2020). Furthermore, some studies have found elevated serum levels of BAFF in response to therapy with IFN‐β (Smets et al., 2021) and rituximab (Cornec et al., 2016), which has been associated with an increase in B transitional subsets and clinical response, respectively.
Despite the importance of BAFF in the B cell population, the association of BAFF with other rheumatic autoantibody‐positive diseases has been poorly explored. Increased BAFF levels were observed in serum (Bosello et al., 2008; Cheema et al., 2001; Geng & Zhang, 2012; Moura et al., 2010; Pers et al., 2005; Tan et al., 2003) and synovial fluid (Moura et al., 2010; Tan et al., 2003) of rheumatoid arthritis (RA) patients and were associated with autoantibody production and the severity of the disease. RA is a heterogeneous disease, it can be presented with high clinical variability, and various pathogenic mechanisms are implicated. The disease can be subdivided into seropositive and seronegative RA, based on the presence of rheumatoid factor (RF) and anti‐citrullinated protein antibodies (ACPA) (Derksen et al., 2017), and therefore, the underlying autoreactive B and T lymphocytes responses play a crucial role in the pathogenesis of the disease. In primary Sjögren's syndrome (pSS) the hallmark of the immunopathology is characterized by T and B lymphocytes infiltration in the exocrine glands, identified as lymphocytic sialadenitis in the minor salivary gland (MSG) biopsy, which is an important tool for disease diagnostic in addition to anti‐Ro/SSA and anti‐La/SSB antibodies presence (Mariette et al., 2003). Also, increased BAFF levels have been reported associated with the ectopic germinal center’s formation and maintenance in pSS (Carrillo‐Ballesteros et al., 2020).
The TNFSF13B gene encodes for BAFF protein and has been mapped to chromosome 13q32‐34 (Schneider et al., 1999). Three single nucleotide polymorphisms (SNPs) in the 5′ untranslated region have been associated with disease susceptibility in autoimmune diseases: rs9514827 (−2841 T > C) located 2841 bp upstream from the start of the transcription site on cDNA, rs1041569 (−2701 A > T) located 2701 bp upstream from the start of the transcription site on cDNA (Andreou et al., 2021;Theodorou et al., 2018; Zayed et al., 2013) and rs9514828 (−871 C > T) located 871 bp upstream from the start of the transcription site on cDNA (Abdel‐Hamid & Al‐Lithy, 2011; Kawasaki et al., 2002; Nezos et al., 2014; Zayed et al., 2013).
In addition, the TNFSF13B mRNA BAFF expression has been associated with the rs9514828 (−871 C > T) in healthy subjects (Almeida & Petzl‐Erler, 2013; Kawasaki et al., 2002) and SLE patients (Marín‐Rosales et al., 2019). The TNFSF13B polymorphisms have been associated with disease susceptibility in different autoimmune disorders such as immune thrombocytopenic purpura (Abdel‐Hamid & Al‐Lithy, 2011), Sjögren's syndrome (Nezos et al., 2014; Nossent et al., 2008), SLE (Kawasaki et al., 2002; Marín‐Rosales et al., 2019; Zayed et al., 2013), and RA (Kawasaki et al., 2002; Ruyssen‐Witrand et al., 2012). Also, a recent report has associated the rs1041569 (−2701 A > T) with an increased risk of inflammatory bowel disease in the Greek population (Andreou et al., 2021). However, the TNFSF13B SNPs have not been analyzed in Mexican patients with RA and pSS, and the BAFF relationship with clinical variables and autoantibodies in both diseases remains unclear.
The aim of this study was to analyze the TNFSF13B rs9514827 (−2841 T > C), rs1041569 (−2701 A > T), and rs9514828 (−871 C > T) SNPs, TNFSF13B mRNA, and soluble BAFF levels expression in two groups with different rheumatic ADs: rheumatoid arthritis and primary Sjögren's syndrome. Also, the clinical utility of sBAFF as a biomarker was evaluated.
2. MATERIALS AND METHODS
2.1. Subjects
For the genotyping analysis of the study, we included a total sample of 730 Mexican mestizo subjects from western Mexico. Three hundred and nine healthy subjects (HS) with no family history of autoimmune diseases, 320 subjects that fulfilled the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) 2010 classification criteria for Rheumatoid Arthritis (Aletaha et al., 2010) and 101 subjects that fulfilled the classification criteria of the ACR/EULAR 2016 for primary Sjogren's syndrome (Shiboski et al., 2017). All included subjects were from western Mexico with at least three generations of ancestors. At the enrollment of the study, for RA patients the disease activity score in 28 joints with erythrocyte sedimentation rate (DAS28‐ESR) (England et al., 2019) and the Health Assessment Questionnaire (HAQ) (Bruce & Fries, 2003) were assessed. For the pSS patients, the Sjögren's Syndrome Disease Activity Index (SSDAI) and Sjögren's Syndrome Disease Damage Index (SSDDI) were also obtained (Hernández‐Molina & Sánchez‐Hernández, 2013). According to the DAS28‐ESR equation, RA patients were classified into four groups: remission (<2.6), low activity (≥2.6 to ≤3.2), moderate (>3.2 to ≤5.1), and severe (>5.1). The human ethics committee of the Hospital General de Occidente approved the protocol under the number 560/18. All the participants signed an informed consent adapted by us and developed by the Research Ethics Review Committee of the World Health Organization (WHO, 2020). All procedures were performed following the ethical standards of the institutional and national research committee and according to the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2.2. DNA extraction and genotyping
Genomic DNA was obtained from peripheral blood leukocytes using a modified Miller's technique (Miller et al., 1988). DNA purity and concentration were determined by UV spectrophotometry (A260/A280 ratio of 1.8–2.0 and 260/230 ratio of 2.0–2.2) (NanoDrop lite, Thermo Scientific, Massachusetts, USA) and stored at −20°C until used for polymerase chain reaction (PCR) amplification. To identify the genotypes of the single nucleotide polymorphisms rs9514827 (−2841 T > C), rs1041569 (−2701 A > T), and rs9514828 (−871 C > T), we conducted PCR coupled to the restriction fragment length polymorphisms (RFLP) technique as previously described (Marín‐Rosales et al., 2019). The 468 bp PCR product rs9514827 (−2841 T > C) was digested with three units of AciI enzyme (New England BioLabs, Massachusetts, USA) at 37°C for 2 hours. The digestion fragments were TT: 468 bp, TC: 468, 308, 160 bp, and CC: 308 and 160 bp. The 468‐bp PCR product rs1041569 (−2701 A > T) was digested with three units of DpnII enzyme (New England BioLabs, Massachusetts, USA) at 37°C for 1 hour. The digestion fragments were AA: 205, 169, and 94 bp, TA: 263, 205, 169, and 94 bp and TT: 263 and 205 bp. Finally, the 398‐bp PCR product rs9514828 (−871 C > T) was digested with three units of AciI enzyme (New England BioLabs, Massachusetts, USA) at 37°C for 6 hours. The digestion fragments were CC: 261 and 137 bp, CT: 398, 261 and 137 bp, and TT: 398 bp.
2.3. RNA extraction and reverse transcription
For the quantitative expression of the TNFSF13B gene, 36 healthy subjects, 39 RA and 32 pSS patients were selected from the whole total sample, considering the different genotypes of the rs9514828 (−871 C > T) polymorphism. Leukocytes from peripheral blood were obtained by osmotic lysis of red blood cells and preserved at −80°C with Trizol reagent (Invitrogen Life Technologies, California, USA) until use. Total RNA was extracted from these peripheral blood leukocytes by the phenol/chloroform technique described in the Trizol manufacturer protocol. RNA concentration and purity were assessed by UV spectrophotometry (A260/A280 ratio of 1.8–2.0 and 260/230 ratio of 2.0–2.2) (NanoDrop lite, Thermo Scientific, Massachusetts, USA). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using oligo‐dT and GoScript™ Reverse Transcription System (Promega Corporation, Wisconsin, USA) following the manufacturer's protocol. The cDNA samples were stored at −80°C until the real‐time PCR assays.
2.4. Quantitative PCR (qPCR)
The RT‐qPCR assays followed the guidelines of the Minimum Information for Publication of Quantitative Real‐Time PCR Experiments (MIQE) (Bustin et al., 2009). Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used as a reference gene to determine relative quantification. The primers and hydrolysis probes were designed on the Roche Universal Probe Library software (TNFSF13B: probe cat. no. 4688988001, GAPDH: probe cat. no. 05190541001), and the primers were validated by gel electrophoresis. The TNFSF13B gene expression quantification (NM_006573.4) was performed by quantitative real‐time PCR (qPCR) using the Nano Light Cycler (Roche Molecular Systems Inc, California, USA). All samples were run as duplicates. After validation of PCR efficiencies for both genes, the obtained data were analyzed.
2.5. Serum BAFF and autoantibodies levels
The sBAFF levels were quantified from sera of 101 HS, and 156 RA and 96 pSS patients with a quantitative ELISA test (R&D systems, Minneapolis, USA) performed according to the manufacturer's recommendations. The test reported an analytic sensitivity of 1.01 to 6.44 pg/ml. Furthermore, anti–citrullinated protein antibody (ACPA) levels were also quantified from sera of 314 RA patients by quantitative ELISA test (Orgentec Diagnostika GmbH, Mainz, Germany), performed according to the manufacturer's recommendations. The test reported an analytic sensitivity of 1 U/ml with a cut‐off value of 20 U/ml. The samples were read at 450/540 nm in the Multiskan™ Go Microplate Spectrophotometer (Thermo Fisher Scientific, Massachusetts, USA), absorbance measurements were obtained, and data were analyzed. The anti‐SSA/Ro and anti‐SSB/La levels were quantified in serum from pSS patients by the ORG 208 and ORG 209 ELISA kits (Orgentec Diagnostika GmbH, Mainz, Germany) respectively, according to the manufacturer's instructions. A cutoff value of 25.0 U/ml, with a range detection of 0–200 U/ml, was employed in both ELISA kits. The samples were read at 450 nm by Multiskan™ Go Microplate Spectrophotometer (Thermo Fisher Scientific, Massachusetts, USA). Antinuclear antibodies (ANA) and rheumatoid factor titers were obtained through the clinical files of patients.
2.6. Statistical analysis
The data were analyzed using GraphPad Prism 9.0 (GraphPad Software, California, USA) and MedCalc (MedCalc Software Ltd, Ostend, Belgium). Normal data distribution was tested, parametric variables were expressed as mean ± standard and non‐parametric variables were expressed as median and percentiles (25–75). The frequencies of alleles and genotypes were compared using the Chi‐square test or Fisher's exact test, when appropriate. The haplotype inference was performed using the partition, ligation, combination, subdivision, expectation maximization (PL‐CSEM) algorithm by the SHEsis software platform (Li et al., 2009; Yong & He, 2005). sBAFF serum levels were compared among groups using Kruskal‐Wallis H tests. A comparative threshold cycle (Ct) method with a cut‐off of 40 cycles was used to determine the TNFSF13B mRNA expression relative to GAPDH based on the 2−ΔCT and the 2−ΔΔCT methods (Livak & Schmittgen, 2001; Schmittgen & Livak, 2008). To analyze the relationship between quantitative variables, Spearman's correlation coefficient was used. The diagnostic performance of sBAFF was obtained through to Receiver Operating Receptor (ROC) curves and the area under the curve (AUC). The AUC of sBAFF and anti‐SSA/Ro were compared according to the DeLong et al. methodology (DeLong et al., 1988). A p‐value <0.05 was considered statistically significant. Statistically significant differences were considered when p‐values were <0.05.
3. RESULTS
3.1. Demographic and clinical features
Table 1 describes the demographic and clinical features of all subjects included in this study. HS presents the median age of 36 years old compared to RA and pSS patients at 49 and 58 years old, respectively. RA patients had a median disease evolution of 42 months. Of the whole RA patients included in the study, 88.4% of them were female. Most of the patients showed moderate disease activity with a DAS28‐ESR median of 4.33, whereas 10.37% of the RA patients were in remission. According to the HAQ, these patients had mild to moderate difficulty in their functional ability with a median of 0.760. RF and ACPA autoantibodies were positive in 70% of the RA patients. pSS patients had a median disease evolution of 36 months. Antinuclear antibodies (63%), as well as anti‐SSA/Ro (24%) and anti‐SSB/La (13%), were positive in pSS patients. The pSS disease indexes SSDAI and SSDDI were low, with median values of 2 and 1, respectively. The treatment that RA and pSS patients were taking at the time of enrollment is also presented in Table 1.
TABLE 1.
Demographic, clinical, and serological features of RA and pSS patients
| Variable | HS | RA | pSS |
|---|---|---|---|
| n = 309 | n = 320 | n = 101 | |
| Demographics | |||
| Age, years a | 36 (28–49) | 49 (40–58) | 58 (48–64) |
| Female gender (%) | 87 | 88.4 | 100 |
| Disease status | |||
| Disease evolution, months a | – | 42 (18–120) | 36 (12–96) |
| DAS28‐ESR a | – | 4.33 (3.23–5.32) | – |
| Remission (%) | – | 10.37 | – |
| LDA (%) | – | 12.71 | – |
| MDA (%) | – | 48.49 | – |
| HAD (%) | – | 28.43 | – |
| HAQ a | – | 0.76 (0.22–1.43) | – |
| SSDAI a | – | – | 2 (0–3) |
| SSDDI a | – | – | 1 (0–2) |
| Inflammation markers | |||
| ERS, mm/h a | – | 35 (23–46) | 23 (14–36) |
| CRP, mg/L a | – | 15.89 (4.23–43.11) | 3.80 (1.84–7.23) |
| Negative (%) | – | 27.24 | 54.7 |
| Positive (%) | – | 72.76 | 45.3 |
| Autoantibody status | |||
| Rheumatoid factor, UI/ml a | – | 94.20 (38.50–263.2) | 13 (7.00–32.00) |
| Positive (%) | – | 89.96 | 44.6 |
| High positive (%) | – | 66.41 | – |
| ACPA, U/ml a | – | 69.43 (18.69–272.2) | – |
| Positive (%) | – | 70.25 | – |
| High positive (%) | – | 54.14 | – |
| Anti‐SSA/Ro (%) | – | – | 24 |
| U/ml a | – | – | 107 (72.3–200) |
| Anti‐SSB/La (%) | – | – | 13 |
| U/ml a | – | – | 87 (45.7–219) |
| Antinuclear antibodies (%) | – | 48 | 63 |
| Titer | – | 1: 320 (1:160 – 1:640) | 1: 320 (1:160 – 1:640) |
| Treatment | |||
| NSAIDs (%) | – | 72.81 | – |
| Steroids (%) | – | 35.94 | 9 |
| DMARDs (%) | |||
| MTX (%) | – | 84.69 | 19 |
| Sulfasalazine (%) | – | 53.44 | 0 |
| Antimalarial (%) | – | 35.94 | 61 |
| Multitherapy | – | ||
| MTX + Sulfasalazine (%) | – | 25.94 | – |
| MTX + Antimalarial (%) | – | 10 | 11 |
| SSZ + Antimalarial (%) | – | 2.19 | – |
| Triple therapy (%) | – | 22.50 | – |
| Azathioprine (%) | – | 7.5 | 17.8 |
| sBAFF (ng/ml) a | 0.742 (0.663–0.867) | 1.093 (0.875–1.333) | 1.792 (1.381–2.483) |
Abbreviations: ACPA, anti‐citrullinated protein antibodies; CRP, C‐Reactive protein; DAS28‐ESR, disease activity score—erythrocyte sedimentation rate; DMARDs, disease‐modifying anti‐rheumatic drugs; HAQ, Health Assessment Questionary; MTX, methotrexate; NSAIDs, non‐steroidal anti‐inflammatory drug; sBAFF, soluble BAFF; SSDAI, Sjögren’s Syndrome Disease Activity Index; SSDDI, Sjögren’s syndrome disease damage index.
Data provided in median and percentile (25–75).
3.2. Genotype, allele, and haplotype frequencies of TNFSF13B gene polymorphisms
HS were found in Hardy–Weinberg equilibrium for the three TNFSF13B gene polymorphisms (rs9514827, χ 2 = 0.2567, p = .6123; rs1041569, χ 2 = .0172, p = .8955, and rs9514828, χ 2 = .1894, p = .6633). Table 2 shows the genotype, allele, and haplotype frequencies in all groups of study as well as genetic models. Differences in allele and genotype frequencies were found only between RA and HS for the rs9514828 (−871 C > T) polymorphism. The CT genotype and T allele carriers showed increased susceptibility for RA, with an OR of 1.495 [95% CI (1.079–2.070), p c = .030] and an OR of 1.299, [95% CI (1.014–1.665), p = .038], respectively. Also, we found significant associations in the dominant and overdominant genetic models, which emphasize the role of the rs9514828 T allele. In contrast, no associations were found between TNFSF13B SNPs and pSS.
TABLE 2.
Frequencies of genotypes, alleles, and haplotypes of the TNFSF13B gene polymorphism in RA and pSS patients
| HS | RA | OR | p‐value | p c value | pSS | OR | p Value | p c value | |
|---|---|---|---|---|---|---|---|---|---|
| n = 309 (%) | n = 320 (%) | (95% CI) | n = 101 (%) | (95% CI) | |||||
| rs9514827 (−2841 T > C) | |||||||||
| Genotype | |||||||||
| TT | 205 (66.3) | 217 (67.8) | 1 | – | 70 (69.3) | 1 | – | ||
| TC | 95 (30.7) | 96 (30.0) | 0.954 [0.678–1.344] | .79 | 1.00 | 31 (30.7) | 0.956 [0.586–1.556] | .86 | 1.00 |
| CC | 9 (2.9) | 7 (2.2) | 0.734 [0.270–2.009] | .547 | 1.00 | 0 | – | .12 | .24 |
| Allele | |||||||||
| T | 505 (81.7) | 530 (82.8) | 1 | – | 171(84.7) | 1 | – | ||
| C | 113 (18.3) | 110 (17.2) | 0.927 [0.694–1.240] | .61 | – | 31 (15.3) | 0.810 [0.525–1.250] | .34 | – |
| Dominant model | |||||||||
| TT | 205 (66.3) | 217 (67.8) | 1 | – | 70 (69.3) | 1 | – | ||
| TC + CC | 104 (33.7) | 103 (32.2) | 0.936 [0.671–1.305] | .695 | – | 31 (30.7) | 0.873 [0.538–1.417] | .58 | – |
| Recessive model | |||||||||
| TT + TC | 300 (97.1) | 313 (97.8) | 1 | – | 101 (100) | 1 | – | ||
| CC | 9 (2.9) | 7 (2.2) | 0.746 [0.274–2.027] | .564 | – | 0 | – | .08 | – |
| Overdominant | |||||||||
| TT + CC | 214 (69.3) | 224 (70.0) | 1 | – | 70 (69.3) | 1 | – | ||
| TC | 95 (30.7) | 96 (30.0) | 0.965 [0.687–1.356] | .839 | – | 31 (30.7) | 0.998 [0.613–1.624] | .99 | – |
| rs1041569 (−2701 A > T) | |||||||||
| Genotype | |||||||||
| AA | 249 (80.6) | 266 (83.1) | 1 | – | 82 (81.2) | 1 | – | ||
| AT | 57 (18.4) | 53 (16.6) | 0.870 [0.577–1.314] | .509 | 1.00 | 18 (17.8) | 0.959 [0.534–1.723] | .89 | 1.00 |
| TT | 3 (1.0) | 1 (0.3) | 0.312 [0.032–3.020] | .36 | .72 | 1 (1) | 1.012 [0.104–9.866] | 1 | 1.00 |
| Allele | |||||||||
| A | 555 (89.8) | 585 (91.4) | 1 | – | 182 (90.1) | 1 | – | ||
| T | 63 (10.2) | 55 (8.6) | 0.828 [0.566–1.211] | .33 | – | 20 (9.9) | 0.968 [0.570–1.645] | .9 | – |
| Dominant model | |||||||||
| AA | 249 (80.6) | 266 (83.1) | 1 | – | 82 (81.2) | 1 | – | ||
| AT+TT | 60 (19.4) | 54 (16.9) | 0.843 [0.561–1.265] | .408 | – | 19 (18.8) | 0.962 [0.542–1.706] | .89 | – |
| Recessive model | |||||||||
| AA+AT | 306 (99.0) | 319 (99.7) | 1 | – | 100 (99) | 1 | – | ||
| TT | 3 (1.0) | 1 (0.3) | 0.320 [0.033–3.091] | .365 | – | 1 (1) | 1.020 [0.105–9.917] | 1 | – |
| Overdominant | |||||||||
| AA+TT | 252 (81.6) | 267 (83.4) | 1 | – | 83 (82.2) | 1 | – | ||
| AT | 57 (18.4) | 53 (16.6) | 0.878 [0.581–1.325] | .534 | – | 18 (17.8) | 0.959 [0.534–1.721] | .89 | – |
| rs9514828 (−871 C > T) | |||||||||
| Genotype | |||||||||
| CC | 172 (55.7) | 147 (45.9) | 1 | – | 61 (60.4) | 1 | – | ||
| CT | 119 (38.5) | 152 (47.5) | 1.495 [1.079–2.070] | .015 | .03 | 32 (31.7) | 0.758 [0.466–1.235] | .27 | .540 |
| TT | 18 (5.8) | 21 (6.6) | 1.365 [0.701–2.660] | .359 | .72 | 8 (7.9) | 1.253 [0.519–3.029] | .62 | 1.00 |
| Allele | |||||||||
| C | 463 (74.9) | 446 (69.7) | 1 | – | 154 (76.2) | 1 | – | ||
| T | 155 (25.1) | 194 (30.3) | 1.299 [1.014–1.665] | .038 | – | 48 (23.8) | 0.931 [0.642–1.350] | .71 | – |
| Dominant model | |||||||||
| CC | 172 (55.66) | 147 (45.94) | 1 | – | 61 (60.4) | 1 | – | ||
| CT + TT | 137 (44.34) | 173 (54.06) | 1.478 [1.079–2.023] | .015 | – | 40 (39.6) | 0.823 [0.521–1.301] | .4 | – |
| Recessive model | |||||||||
| CC + CT | 291 (94.17) | 299 (93.44) | 1 | – | 93 (92.1) | 1 | – | ||
| TT | 18 (5.83) | 21 (6.56) | 1.135 [0.593–2.172] | .702 | – | 8 (7.9) | 1.391 [0.586–3.303] | .45 | – |
| Overdominant | |||||||||
| CC + TT | 190 (61.5) | 168 (52.5) | 1 | – | 69 (68.3) | 1 | – | ||
| CT | 119 (38.5) | 152 (47.5) | 1.445 [1.052–1.984] | .023 | – | 32 (31.7) | 0.741 [0.459–1.194] | .22 | – |
| Haplotype | |||||||||
| TAC | 427.88 (69.20) | 419.32 (65.50) | 1 | – | 143.45 (71) | 1 | – | ||
| TAT | 14.87 (2.40) | 63.91 (10.00) | 4.358 [2.445–7.770] | <.001 | <.001 | 8.66 (4.30) | 1.783 [0.764–4.163] | .18 | 1.00 |
| TTC | 16.87 (2.87) | 2.75 (0.40) | 0.180 [0.052–0.620] | .003 | .02 | – | – | – | |
| TTT | 45.38 (7.30) | 44.02 (6.90) | 0.998 [0.650–1.550] | .996 | 1.00 | 14.08 (7.0) | 0.925 [0.493–1.734] | .81 | 1.00 |
| CAC | 18.25 (3.00) | 23.93 (3.70) | 1.362 [0.728–2.547] | .332 | 1.00 | – | – | – | |
| CAT | 94.00 (15.20) | 77.84 (12.22) | 0.847 [0.610–1.178] | .324 | 1.00 | 23.16 (11.5) | 0.727 [0.444–1.191] | .21 | 1.00 |
| CTT | 0.75 (0.10) | 8.23 (1.30) | 8.172 [1.018–62.62] | .021 | .126 | – | – | – | |
Notes: Statistical tests used for allelic and genotype frequencies: Pearson’s X2 and Fisher’s exact, when appropriate. Haplotype inference was calculated with the SHEsis platform using the PL‐CSEM method (partition, ligation, combination, subdivision, expectation maximization). Haplotype analysis included the rs9514827 (−2841 T > C), rs1041569 (−2701 A > T) and rs9514828 (−871 C > T) polymorphisms of TNFSF13B gene. All haplotypes with a frequency <0.03 were excluded from the analysis. Bonferroni correction was applied to p‐values to control for multiple comparisons and showed as a corrected p‐value (pc). The adjustment is shown only for those analyzes with more than two comparisons. Statistically significant difference, p‐value < .05. Statistically significant difference, p‐value < .05. Values in bold indicate statistically significant results.
Abbreviations: CI, confidence interval; HS, healthy subjects; OR, odds ratio; pSS, primary Sjögren’s syndrome; RA, rheumatoid arthritis.
The evaluated TNFSF13B SNPs were found in high to moderate linkage disequilibrium (rs9514827 vs rs1041569, D′ = 0.854; rs9514827 vs rs9514828, D′ = 0.746 and rs1041569 vs rs9514827 D′ = 0.776). The most common haplotypes in HS and RA were TAC (69.2% vs. 65.5%), CAT (15.2% vs. 12.2%), TTT (7.3% vs. 6.9%). The TAT (2.4% vs 10%) haplotype was associated with high susceptibility to RA disease with an OR of 4.358 [95% CI (2.445–7.770), p c < .001]. In contrast, the TTC haplotype was associated with less susceptibility to the disease with an OR of 0.180, [95% CI (0.052–0.620), p c = .020]. No association were found between any of the calculated haplotypes and pSS.
3.3. TNFSF13B gene expression and sBAFF serum levels
The TNFSF13B expression at the mRNA level was increased in both autoimmune diseases compared to HS, being pSS patients those with higher levels (5.04‐fold more). RA patients showed 2.43‐fold more TNFSF13B gene expression than HS (Figure 1a). According to 2−ΔCT analysis, HS has a lower TNFSF13B mRNA expression than subjects with autoimmune diseases [median of relative expression units (REU), HS = 21.49, RA = 44.16, pSS = 91.41, (HS vs RA p = .035 and HS vs pSS p = <.001)], and no difference was observed between RA and pSS patients (p = .081) (Figure 1b).
FIGURE 1.

Association of TNFSF13B gene expression according to rheumatic diseases and ‐871C > T polymorphism. The TNFSF13B gene expression was higher in RA and pSS than in HS (a and b). When comparing the gene expression between both rheumatic diseases no statistical difference was found (b). The sBAFF concentration was higher in both autoimmune disorders than in HS, mainly in pSS (c). TNFSF13B expression showed high expression in RA patients with CT and TT genotypes of the ‐871C > T polymorphism [2.77 and 6.82‐fold more, respectively (d)]. RA patients were classified according to dominant and recessive genotyping models, the carriers with the mutant alleles showed 3.51 and 4.43‐fold more expression (e and f). Also, TNFSF13B gene expression according to genetic models, showed that being a carrier of two copies of the T allele was associated with higher TNFSF13B mRNA expression (g–i). sBAFF concentration did not show a difference in RA patients, independently of genotypes (j). The TNFSF13B gene expression and sBAFF levels were similar in patients with pSS carriers of the risk allele with respect to the most frequent (k and l). Qualitative and quantitative TNFSF13B gene expression analysis was evaluated through the 2–ΔΔCt and 2–ΔCt methods. Data are shown in median and percentiles (25–75). HS: Healthy subjects, pSS: Primary Sjögren's syndrome, RA: Rheumatoid arthritis. p‐Values were obtained through the Mann–Whitney U tests or Kruskal‐Wallis H test (Dunn's post hoc test, when appropriate) according to the case. p‐Value < .05 *, p‐value < .01 **, p‐value < .001***. Statistically significant difference, p‐value < .05
To evaluate the risk of the rs9514828 (−871 C > T) polymorphism and their influence on the TNFSF13B gene expression in RA patients, we evaluated the genetic models using the 2−ΔΔCT and the 2−ΔCT analysis for each of them (Figure 1d–i). Codominant model showed that rs9514828 TT (6.82‐fold more) and rs9514828 CT (2.77‐fold more) genotype carriers had higher TNFSF13B mRNA expression compared to rs9514828 CC genotype, (TT = 6.82 and CT = 2.77) (Figure 1d). Similar differences were observed in dominant and recessive genetic models (Figure 1e,f). Also, a higher TNFSF13B mRNA expression was found according to 2−ΔCT analysis in carriers of the rs9514828 TT genotype (Figure 1g–i). Differences were not found between TNFSF13B mRNA expression and pSS (Figure 1k).
In addition, we analyzed the sBAFF levels, the pSS patients showed the highest values (median of 1.792 ng/ml) compared to RA patients (median of 1.093 ng/ml, p < .001) and HS (median 0.742 ng/ml, p = <.001). Also, RA patients had increased levels of sBAFF compared to HS (p < .001) (Figure 1c). However, codominant genetic model did not showed difference between genotypes and sBAFF levels in RA patients (rs9514828 median CC = 0.874 ng/ml, CT = 1.259 ng/ml and TT = 1.097 ng/ml, p = .2602) (Figure 1j). Also, for pSS no difference in sBAFF levels was appreciated according dominant genetic model (rs9514828 median CC = 1.471 ng/ml and CT + TT = 1.821 ng/ml, p = .1214) (Figure 1l). No correlations were found between REU of the TNFSF13B gene and sBAFF expression neither HS, RA, or pSS patients with the CC or the CT genotypes of the rs9514828 (−871 C > T) (data not shown).
3.4. sBAFF and clinical characteristics of RA and pSS
A correlation matrix between some of the variables assessed in RA patients is shown in Figure 2a. A low positive correlation was observed between levels of RF and ACPA autoantibodies, as well as between sBAFF levels and disease evolution. Despite RA patients had higher sBAFF levels than HS, we did not find an association between sBAFF levels and RA autoantibodies status. Rheumatoid arthritis patients seropositive to ACPA autoantibodies showed sBAFF median levels of 1.089 ng/ml compared to seronegative patients that had median sBAFF levels of 1.134 ng/ml (Figure 2b). Moreover, we analyzed sBAFF levels according to high or low levels of both autoantibodies (ACPA and RF). Opposite of how we expected RA patients with low levels of ACPA/RF showed higher sBAFF levels in comparison with patients with high levels of both autoantibodies (median 1.071 vs 1.225 ng/ml, p = .027) (Figure 2c).
FIGURE 2.
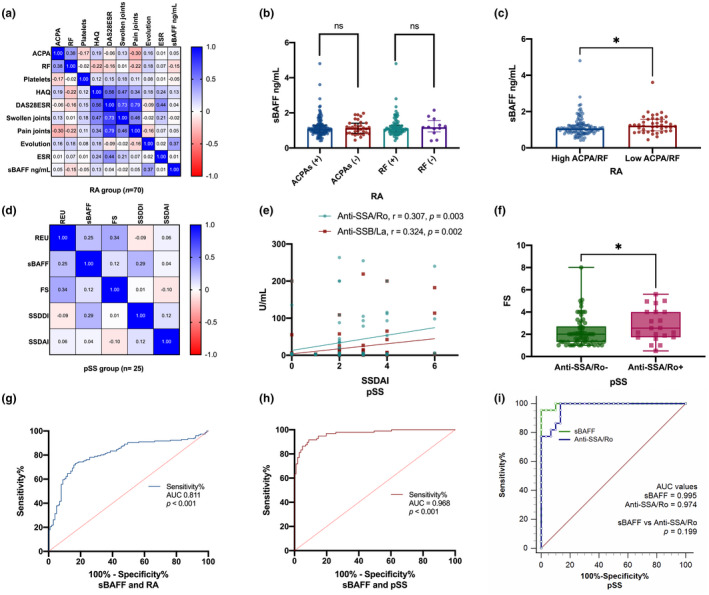
Association and correlation of sBAFF levels, autoantibodies, and clinimetric measures in RA and pSS patients. RA patients showed positive correlation between ACPAs and RF with a statistical difference (r = 0.380, p = .001). Also, the clinimetric tools had a positive correlation with variables directly related to its calculation (a). RA patients classified according to ACPAs and RF status did not show statistical difference (b). Otherwise, classified as low or high, the formers showed higher sBAFF levels (p < .05), (c). No statistical differences were found in pSS patients among TNFSF13B mRNA relative expression units (REU), sBAFF, FS, and clinimetrical tools (d). However, anti‐SSA/Ro and anti‐SSB/La showed a positive correlation with SSDAI (e), and the focus score (FS) was higher in pSS seropositive to anti‐SSA/Ro (f). The diagnostic performance of sBAFF was evaluated in RA (g) and pSS (h), with higher efficiency in pSS than in RA (AUC = 0.968 vs 0.811, respectively). Also, the diagnostic efficiency of sBAFF was compared with anti‐SSA/Ro and sBAFF showed a similar performance with no statistical difference (i). Data is shown in median and percentiles (25–75). REU was obtained through the 2–ΔCt method. ROC curves with AUC were used and compared through the Delong method. ACPA: Anti‐citrullinated protein antibodies, AUC: Area under the curve, DAS28: Disease activity score of 28 joints, ESR: Erythrosedimentation rate, FS: Focus score, HAQ: Health assessment Questionary, RA: Rheumatoid arthritis, pSS: Primary Sjögren's syndrome, REU: Relative expression units, RF: Rheumatoid factor, SSDAI: Sjögren's syndrome disease activity index, SSDDI: Sjögren's syndrome disease damage index. p‐Values were obtained through the Mann–Whitney U tests and Spearman's rank‐order correlation test. p‐value < .01 **, p‐value < .001***. Statistically significant difference, p‐value < .05
On the contrary, pSS patients did not show an association between sBAFF levels and TNFSF13B mRNA expression, SSDDI score, or the focus score (Figure 2d). However, correlation between SSDAI score and pSS autoantibodies, anti‐SSA/Ro (r = 0.307, p = .003), and anti‐SSB/La (r = 0.324, p = .002) were found (Figure 2e). Primary Sjögren's syndrome with seropositive anti‐SSA/Ro antibodies had a higher focus score than anti‐SSA/Ro seronegative (Figure 2f).
3.5. Evaluation of sBAFF levels as potential biomarkers for RA and pSS
Because in our study we find the highest sBAFF levels in ADs, we analyzed the performance of sBAFF levels as a biomarker. Rheumatoid arthritis showed an AUC value of 0.811 (p < .001) (Figure 2g) and pSS showed an AUC of 0.968 (p < .001) (Figure 2h). As sBAFF showed high performance according to ROC analysis in pSS, we analyzed this in comparison with anti‐SSA/Ro antibody. The AUC value for anti‐SSA/Ro was 0.974 (Figure 2i), similarly to sBAFF.
4. DISCUSSION
Rheumatoid arthritis and primary Sjögren's syndrome are characterized by an exacerbated response of the immune system. RA is a chronic, inflammatory disease characterized by synovial hyperplasia that could lead to joint destruction and physical dysfunction (Tanaka, 2020). pSS is characterized by focal lymphocytic infiltration of the exocrine glands which can lead to glandular atrophy (Bowman, 2018). Even when etiology is unknown it is well documented that diverse risk factors are involved in their development. Both diseases involve the interplay of genetic (Deane et al., 2017; Imgenberg‐Kreuz et al., 2019) and environmental factors (Björk et al., 2020), which conducts to a loss of immunological tolerance with the subsequent establishment and perpetuation of the disease (Fayyaz et al., 2016; Scott et al., 2010).
BAFF has a main role among the factors that promote humoral responses and autoantibody production. This cytokine has been demonstrated to induce B cell survival (Moore et al., 1999), proliferation (Schneider et al., 1999), maturation (Schneider et al., 2001), extrafollicular responses (MacLennan & Vinuesa, 2002), and T‐independent class‐switch recombination (Castigli et al., 2005). Multiple studies had associated high levels of sBAFF with several autoimmune diseases, being SLE one of the most consistently associated (Ospina et al., 2016). BAFF is encoded TNFSF13B gene and several SNPs have been reported in association with increased risk for ADs (Gottenberg et al., 2006; Kawasaki et al., 2002; Nezos et al., 2014). In the present study, we analyzed the TNFSF13B SNPs rs9514827 (−2841 T > C), rs1041569 (−2701 A > T), and rs9514828 (−871 C > T) in HS, RA, and pSS patients from western Mexico.
The distribution of the allelic and genotypic frequencies of rs9514827 (−2841 T > C) and rs1041569 (−2701 A > T) were similar in HS and patients with ADs. In our study, the rs9514828 (−871 C > T) allele frequency was in concordance with those reported in the American population (C = 75.2 and T = 24.8) (1000 Genomes Project ‐ Rs9514828 Allele Frequency, 2021, p. 1000 genomes project), as well as, in the western Mexican population (Marín‐Rosales et al., 2019). In this study, we did not find an association between the rs9514828 (−871 C > T) polymorphism and the risk to develop pSS in our population. In contrast, an increased risk for developing RA was found in T allele carriers of the rs9514828 (−871 C > T) polymorphism, in the western Mexican population. To date, one previous study has investigated the association between the rs9514828 (−871 C > T) polymorphism and RA risk in the Japanese population, without finding a significant difference (Kawasaki et al., 2002). This difference can be explained in part by ancestry. The ancestry in the western Mexican‐Mestizos population has been reported with a composition of 64% European, 24% Amerindian and 15% African (Martínez‐Cortés et al., 2012; Rangel‐Villalobos et al., 2008).
Haplotype analysis of TNFSF13B SNPs rs9514827 (−2841 T > C), rs1041569 (−2701 A > T), and rs9514828 (−871 C > T) polymorphisms showed moderate linkage disequilibrium (rs9514827 vs rs1041569, D′ = 0.854; rs9514827 vs rs9514828, D′ = 0.746, and rs1041569 vs rs9514827 D′ = 0.776). The most prevalent haplotype was TAC in HS and patients with ADs. The TAT haplotype which includes the rs9514828T polymorphic allele was associated with an increased risk of developing RA. This is the first study that associates the TAT haplotype of the TNFSF13B gene with RA susceptibility. Furthermore, we found the TTC haplotype associated with a decreased risk of developing RA in the western Mexican population. Contrary to reported by Nossent et al., we did not find an association between TNFSF13B haplotypes and pSS risk. The CTAT haplotype was found associated with pSS positive to SSA/Ro and SSB/La antibodies, in the French population (Nossent et al., 2008). pSS patients analyzed by Nossent reported a higher frequency of Ro/La autoantibodies (77%) in contrast, our pSS patients showed a lower Ro/La seropositivity (24% for Ro and 13% for La antibodies), being an important limitation in our study.
Additionally, the rs9514827 (−2841 T > C) polymorphism has been associated with treatment response in RA patients. The rs9514827T allele carriers, non‐responders to therapy with TNF inhibitors also showed a poor response to Rituximab treatment (Ruyssen‐Witrand et al., 2012). In our study, patients with biological therapy were not included and the response to treatment was not considered.
On the contrary, we analyzed the association of the TNFSF13B rs9514828 (−871 C > T) polymorphism and TNFSF13B mRNA expression in both RA and pSS patients. An increased TNFSF13B mRNA expression was found in both ADs. In comparison with HS, RA patients showed 2.43‐fold more TNFSF13B mRNA expression whereas pSS patients had 5.04‐fold more TNFSF13B mRNA expression. The increased gene expression levels were in concordance with sBAFF protein, sBAFF levels were highest in RA and pSS patients. Also, RA carriers of the rs9514828TT genotype showed increased TNFSF13B mRNA expression, which suggests a functional effect of this variant. In contrast, in the pSS group, the TNFSF13B transcript levels did not correlate with the rs9514828 polymorphism. This discrepancy can be explained in part because pSS patients have been described as overexpression of type I interferons, which indeed was associated with increased TNFSF13B mRNA expression (Brkic et al., 2013). Also, diverse mechanisms at the epigenetic, transcriptional, and post‐transcriptional level can affect gene expression (Bao & Cao, 2016; Lahiri et al., 2012; Sjöstrand et al., 2015), however, none of this were explored in our study.
Previously, the rs9514828 (−871 C > T) polymorphism was associated with an increase in TNFSF13B mRNA expression (Kawasaki et al., 2002). According to in‐silico analysis, the presence of the rs9514828T variant can modify the binding affinity for the transcription factor myeloid zinc finger protein (MZF1) and thus influence the TNFSF13B gene expression (Kawasaki et al., 2002). However, is well known that gene expression is regulated by several post‐transcriptional factors (Alsaleh et al., 2014). Similar to the reported by Gottenberg et al., in the pSS French population, we did not find an association between rs9514828 (−871 C > T) polymorphism and TNFSF13B mRNA expression in pSS patients from western Mexico.
A consistent finding in our study was the increased sBAFF levels in RA and pSS patients. As BAFF is a cytokine involved directly in B cell response and autoantibody production, we analyzed if the sBAFF levels were associated with autoantibodies and disease activity in both ADs. In contrast, we did not find associations between sBAFF levels and SNPs or mRNA expression, we hypothesize that serum sBAFF levels could be influenced by the local BAFF production. It has been described that fibroblast‐like synoviocytes contributes to local BAFF production in RA (Yoshitomi, 2019). Also, in pSS has been determined a local BAFF expression in ectopic GC‐like structures in minor salivary glands (Carrillo‐Ballesteros et al., 2020). Regarding sBAFF levels and autoantibodies, we could not establish an association when comparing sBAFF levels according to ACPA and RF positive vs negative. However, we found higher sBAFF levels in patients with low ACPA/RF levels in comparison with high ACPA/RF antibodies. The above, contrasts with two previous reports, where a positive correlation between sBAFF levels and ACPA/RF autoantibodies was found (Bosello et al., 2008; Pers et al., 2005). These differences can be explained in part because RA patients included in those studies, were according to 1987 ACR classification criteria (Bergling et al., 2013), which mainly classify established RA. In fact, we found that RA patients with very early disease (<3 months) showed higher sBAFF levels than the established disease (data not shown). It has been suggested that RA treatment with glucocorticoids and antirheumatic drugs can modify BAFF (Reyes et al., 2008) and autoantibodies (Bugatti et al., 2018) expression. Similarly, we did not find an association between the sBAFF levels and anti‐SSA/Ro and anti‐SSB/La antibodies, in pSS patients. A previous report on Caucasian pSS patients observed a positive correlation between sBAFF levels and anti‐SSA/Ro and anti‐SSB/La antibodies (Quartuccio et al., 2013). Despite low frequencies of anti‐SSA/Ro and anti‐SSB/La antibodies found in our pSS patients, we observed a positive correlation between anti‐SSA/Ro antibody and SSDAI score, as well as anti‐SSA/Ro antibody and Focus score. In addition, the majority of pSS patients included in our study, show positive MSG biopsies, which emphasized the predominant role of the local inflammatory environment in the disease. Because we measure serum soluble BAFF levels the cellular source of this cytokine in each condition was unknown, which could be considered a limitation of this study. Finally, we explored the potential of sBAFF levels as a diagnostic biomarker in RA and pSS. The AUC obtained for RA and pSS were 0.811 and 0.968, demonstrating higher diagnostic performance for pSS. The cutoff for sBAFF of >1.08 ng/ml in pSS showed 89.6% of sensibility and 92.08% of specificity, with a likelihood ratio of 11.31, with a similar accuracy of anti‐SSA/Ro antibody diagnosis. Other authors have previously reported sBAFF as a useful tool for SLE diagnosis, their use was able to predict flares and could be associated with lupus nephritis (Petri et al., 2008). Based on the low frequency of anti‐SSA/Ro in pSS patients included in this study and the high performance of sBAFF, this cytokine could be considered a diagnostic biomarker in pSS. In conclusion, our findings demonstrate that the TNFSF13B rs9514828 (−871 C > T) polymorphism is a risk factor for RA in the western Mexican population. sBAFF levels may be a potential diagnosis biomarker in pSS.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
AUTHOR CONTRIBUTIONS
Proposed the conceptualization of the study design and administrated the project: Claudia Azucena Palafox‐Sánchez. Involved in the clinical evaluation of the patients: Miguel Marín‐Rosales and Sergio Cerpa‐Cruz. Participated in patient acquisition and sample processing: Jhonatan Antonio Alvarez‐Gómez and Enrique Santillán‐López. Performed PCR and RFLPs assay: Enrique Santillán‐López, Noemí Espinoza‐García, and Beatriz Alejandra Treviño‐Talavera. Conducted RT‐qPCR tests of the samples: Diana Celeste Salazar‐Camarena. Collaborated in the elaboration of ELISAs: Nefertari Sagrero‐Fabela. Carried out the statistical analysis of the data: Enrique Santillán‐López and Edith Oregon‐Romero. Contributed to the manuscript writing: Claudia Azucena Palafox‐Sánchez and Enrique Santillán‐López. Reviewed the manuscript: José Francisco Muñoz‐Valle and Alvaro Cruz. All authors have read and agree to the published version of the manuscript.
ETHICS APPROVAL
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the Hospital General de Occidente under the number 560/18.
ACKNOWLEDGMENTS
Funding was provided to CAPS by Universidad de Guadalajara PRO‐SNI 2018‐2019.
Santillán‐López, E. , Muñoz‐Valle, J. F. , Oregon‐Romero, E. , Espinoza‐García, N. , Treviño‐Talavera, B. A. , Salazar‐Camarena, D. C. , Marín‐Rosales, M. , Cruz, A. , Alvarez‐Gómez, J. A. , Sagrero‐Fabela, N. , Cerpa‐Cruz, S. , Palafox‐Sánchez, C. A. (2022). Analysis of TNFSF13B polymorphisms and BAFF expression in rheumatoid arthritis and primary Sjögren's syndrome patients. Molecular Genetics & Genomic Medicine, 10, e1950. 10.1002/mgg3.1950
Funding information
Grant from Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara [PRO‐SNI 2018–2019]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1000 genomes project ‐ rs9514828 allele frequency . (2021). https://www.ncbi.nlm.nih.gov/snp/rs9514828#frequency_tab
- Abdel‐Hamid, S. M. , & Al‐Lithy, H. N. (2011). B cell activating factor gene polymorphisms in patients with risk of idiopathic thrombocytopenic purpura. The American Journal of the Medical Sciences, 342(1), 9–14. 10.1097/maj.0b013e31820e7f05 [DOI] [PubMed] [Google Scholar]
- Aletaha, D. , Neogi, T. , Silman, A. J. , Funovits, J. , Felson, D. T. , Bingham, C. O. , Birnbaum, N. S. , Burmester, G. R. , Bykerk, V. P. , Cohen, M. D. , Combe, B. , Costenbader, K. H. , Dougados, M. , Emery, P. , Ferraccioli, G. , Hazes, J. M. , Hobbs, K. , Huizinga, T. W. , Kavanaugh, A. , … Hawker, G. (2010). 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European league against rheumatism collaborative initiative. Annals of the Rheumatic Diseases, 69(9), 1580–1588. 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- Almeida, E. R. A. , & Petzl‐Erler, M. L. (2013). Expression of genes involved in susceptibility to multifactorial autoimmune diseases: Estimating genotype effects. International Journal of Immunogenetics, 40(3), 178–185. 10.1111/j.1744-313x.2012.01152.x [DOI] [PubMed] [Google Scholar]
- Alsaleh, G. , François, A. , Philippe, L. , Gong, Y.‐Z. , Bahram, S. , Cetin, S. , Pfeffer, S. , Gottenberg, J.‐E. , Wachsmann, D. , Georgel, P. , & Sibilia, J. (2014). MiR‐30a‐3p negatively regulates BAFF synthesis in systemic sclerosis and rheumatoid arthritis fibroblasts. PLoS One, 9(10), e111266. 10.1371/journal.pone.0111266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou, N.‐P. , Legaki, E. , Dovrolis, N. , Boyanov, N. , Georgiou, K. , Gkouskou, K. , & Gazouli, M. (2021). B‐cell activating factor (BAFF) expression is associated with Crohn's disease and can serve as a potential prognostic indicator of disease response to infliximab treatment. Digestive and Liver Disease, 53(5), 574–580. 10.1016/j.dld.2020.11.030 [DOI] [PubMed] [Google Scholar]
- Bao, Y. , & Cao, X. (2016). Epigenetic control of B cell development and B‐cell‐related immune disorders. Clinical Reviews in Allergy & Immunology, 50(3), 301–311. 10.1007/s12016-015-8494-7 [DOI] [PubMed] [Google Scholar]
- Berglin, E. , & Dahlqvist, S. R. (2013). Comparison of the 1987 ACR and 2010 ACR/EULAR classification criteria for rheumatoid arthritis in clinical practice: A prospective cohort study. Scandinavian Journal of Rheumatology, 42(5), 362–368. 10.3109/03009742.2013.776103 [DOI] [PubMed] [Google Scholar]
- Björk, A. , Mofors, J. , & Wahren‐Herlenius, M. (2020). Environmental factors in the pathogenesis of primary Sjögren's syndrome. Journal of Internal Medicine, 287(5), 475–492. 10.1111/joim.13032 [DOI] [PubMed] [Google Scholar]
- Bosello, S. , Youinou, P. , Daridon, C. , Tolusso, B. , Bendaoud, B. , Pietrapertosa, D. , Morelli, A. , & Ferraccioli, G. (2008). Concentrations of BAFF correlate with autoantibody levels, clinical disease activity, and response to treatment in early rheumatoid arthritis. The Journal of Rheumatology, 35(7), 1256–1264. [PubMed] [Google Scholar]
- Bowman, S. (2018). Primary Sjögren's syndrome. Lupus, 27(1_suppl), 32–35. 10.1177/0961203318801673 [DOI] [PubMed] [Google Scholar]
- Brkic, Z. , Maria, N. I. , van Helden‐Meeuwsen, C. G. , van de Merwe, J. P. , van Daele, P. L. , Dalm, V. A. , Wildenberg, M. E. , Beumer, W. , Drexhage, H. A. , & Versnel, M. A. (2013). Prevalence of interferon type I signature in CD14 monocytes of patients with Sjögren's syndrome and association with disease activity and BAFF gene expression. Annals of Rheumatic Diseases, 72(5), 728–735. 10.1136/annrheumdis-2012-201381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, B. , & Fries, J. F. (2003). The Stanford health assessment questionnaire: Dimensions and practical applications. Health and Quality of Life Outcomes, 1(1), 20. 10.1186/1477-7525-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugatti, S. , Manzo, A. , Montecucco, C. , & Caporali, R. (2018). The clinical value of autoantibodies in rheumatoid arthritis. Frontiers in Medicine, 5, 339. 10.3389/fmed.2018.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin, S. A. , Benes, V. , Garson, J. A. , Hellemans, J. , Huggett, J. , Kubista, M. , Mueller, R. , Nolan, T. , Pfaffl, M. W. , Shipley, G. L. , Vandesompele, J. , & Wittwer, C. T. (2009). The MIQE guidelines: Minimum information for publication of quantitative real‐time PCR experiments. Clinical Chemistry, 55(4), 611–622. 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- Carrillo‐Ballesteros, F. J. , Palafox‐Sánchez, C. A. , Franco‐Topete, R. A. , Muñoz‐Valle, J. F. , Orozco‐Barocio, G. , Martínez‐Bonilla, G. E. , Gómez‐López, C. E. , Marín‐Rosales, M. , López‐Villalobos, E. F. , Luquin, S. , Castañeda‐Chávez, A. , & Oregon‐Romero, E. (2020). Expression of BAFF and BAFF receptors in primary Sjögren's syndrome patients with ectopic germinal center‐like structures. Clinical and Experimental Medicine, 20(4), 615–626. 10.1007/s10238-020-00637-0 [DOI] [PubMed] [Google Scholar]
- Carter, L. M. , Isenberg, D. A. , & Ehrenstein, M. R. (2013). Elevated serum BAFF levels are associated with rising anti–double‐stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis and Rheumatism, 65(10), 2672–2679. 10.1002/art.38074 [DOI] [PubMed] [Google Scholar]
- Castigli, E. , Wilson, S. A. , Scott, S. , Dedeoglu, F. , Xu, S. , Lam, K.‐P. , Bram, R. J. , Jabara, H. , & Geha, R. S. (2005). TACI and BAFF‐R mediate isotype switching in B cells. The Journal of Experimental Medicine, 201(1), 35–39. 10.1084/jem.20032000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema, G. S. , Roschke, V. , Hilbert, D. M. , & Stohl, W. (2001). Elevated serum B lymphocyte stimulator levels in patients with systemic immune‐based rheumatic diseases. Arthritis and Rheumatism, 44(6), 1313–1319. [DOI] [PubMed] [Google Scholar]
- Cornec, D. , Costa, S. , Devauchelle‐Pensec, V. , Jousse‐Joulin, S. , Marcorelles, P. , Berthelot, J.‐M. , Chiche, L. , Hachulla, E. , Hatron, P.‐Y. , Goeb, V. , Vittecoq, O. , Saraux, A. , & Pers, J.‐O. (2016). Blood and salivary‐gland BAFF‐driven B‐cell hyperactivity is associated to rituximab inefficacy in primary Sjögren's syndrome. Journal of Autoimmunity, 67, 102–110. 10.1016/j.jaut.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Deane, K. D. , Demoruelle, M. K. , Kelmenson, L. B. , Kuhn, K. A. , Norris, J. M. , & Holers, V. M. (2017). Genetic and environmental risk factors for rheumatoid arthritis. Best Practice & Research Clinical Rheumatology, 31(1), 3–18. 10.1016/j.berh.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong, E. R. , DeLong, D. M. , & Clarke‐Pearson, D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics, 44(3), 837–845. [PubMed] [Google Scholar]
- Derksen, V. F. A. M. , Huizinga, T. W. J. , & van der Woude, D. (2017). The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Seminars in Immunopathology, 39(4), 437–446. 10.1007/s00281-017-0627-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- England, B. R. , Tiong, B. K. , Bergman, M. J. , Curtis, J. R. , Kazi, S. , Mikuls, T. R. , O'Dell, J. R. , Ranganath, V. K. , Limanni, A. , Suter, L. G. , & Michaud, K. (2019). 2019 update of the American College of Rheumatology recommended rheumatoid arthritis disease activity measures. Arthritis Care & Research, 71(12), 1540–1555. 10.1002/acr.24042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyaz, A. , Kurien, B. T. , & Scofield, R. H. (2016). Autoantibodies in Sjögren's Syndrome. Rheumatic Disease Clinics of North America, 42(3), 419–434. 10.1016/j.rdc.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie, R. , Rovin, B. H. , Houssiau, F. , Malvar, A. , Teng, Y. K. O. , Contreras, G. , Amoura, Z. , Yu, X. , Mok, C.‐C. , Santiago, M. B. , Saxena, A. , Green, Y. , Ji, B. , Kleoudis, C. , Burriss, S. W. , Barnett, C. , & Roth, D. A. (2020). Two‐year, randomized, controlled trial of belimumab in lupus nephritis. New England Journal of Medicine, 383(12), 1117–1128. 10.1056/nejmoa2001180 [DOI] [PubMed] [Google Scholar]
- Geng, Y. , & Zhang, Z. (2012). Comparative study on the level of B lymphocyte stimulator (BlyS) and frequency of lymphocytes between sero‐negative and sero‐positive rheumatoid arthritis patients. International Journal of Rheumatic Diseases, 15(5), 478–485. 10.1111/j.1756-185x.2012.01814.x [DOI] [PubMed] [Google Scholar]
- Gottenberg, J.‐E. , Sellam, J. , Ittah, M. , Lavie, F. , Proust, A. , Zouali, H. , Sordet, C. , Sibilia, J. , Kimberly, R. , Mariette, X. , & Miceli‐Richard, C. (2006). No evidence for an association between the −871 T/C promoter polymorphism in the B‐cell‐activating factor gene and primary Sjögren's syndrome. Arthritis Research & Therapy, 8(1), R30. 10.1186/ar1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Molina, G. , & Sánchez‐Hernández, T. (2013). Clinimetric methods in Sjögren's syndrome. Seminars in Arthritis and Rheumatism, 42(6), 627–639. 10.1016/j.semarthrit.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Imgenberg‐Kreuz, J. , Rasmussen, A. , Sivils, K. , & Nordmark, G. (2019). Genetics and epigenetics in primary Sjögren's syndrome. Rheumatology, 60(5), key330‐. 10.1093/rheumatology/key330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, A. , Tsuchiya, N. , Fukazawa, T. , Hashimoto, H. , & Tokunaga, K. (2002). Analysis on the association of human BLYS (BAFF, TNFSF13B) polymorphisms with systemic lupus erythematosus and rheumatoid arthritis. Genes & Immunity, 3(7), 424–429. 10.1038/sj.gene.6363923 [DOI] [PubMed] [Google Scholar]
- Lahiri, A. , Pochard, P. , Pottier, L. L. , Tobón, G. J. , Bendaoud, B. , Youinou, P. , & Pers, J.‐O. (2012). The complexity of the BAFF TNF‐family members: Implications of autoimmunity. Journal of Autoimmunity, 39(3), 189–198. 10.1016/j.jaut.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Zhang, Z. , He, Z. , Tang, W. , Li, T. , Zeng, Z. , He, L. , & Shi, Y. (2009). A partition‐ligation‐combination‐subdivision EM algorithm for haplotype inference with multiallelic markers: Update of the SHEsis (http://analysis.bio‐x.cn). Cell Research, 19(4), 519–523. 10.1038/cr.2009.33 [DOI] [PubMed] [Google Scholar]
- Livak, K. J. , & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods, 25(4), 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Mackay, F. , & Schneider, P. (2009). Cracking the BAFF code. Nature Reviews Immunology, 9(7), 491–502. 10.1038/nri2572 [DOI] [PubMed] [Google Scholar]
- MacLennan, I. C. M. , & Vinuesa, C. G. (2002). Dendritic cells, BAFF, and APRIL innate players in adaptive antibody responses. Immunity, 17(3), 235–238. 10.1016/s1074-7613(02)00398-9 [DOI] [PubMed] [Google Scholar]
- Mariette, X. , Roux, S. , Zhang, J. , Bengoufa, D. , Lavie, F. , Zhou, T. , & Kimberly, R. (2003). The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren's syndrome. Annals of the Rheumatic Diseases, 62(2), 168–171. 10.1136/ard.62.2.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín‐Rosales, M. , Cruz, A. , Salazar‐Camarena, D. C. , Santillán‐López, E. , Espinoza‐García, N. , Muñoz‐Valle, J. F. , Ramírez‐Dueñas, M. G. , Oregón‐Romero, E. , Orozco‐Barocio, G. , & Palafox‐Sánchez, C. A. (2019). High BAFF expression associated with active disease in systemic lupus erythematosus and relationship with rs9514828C>T polymorphism in TNFSF13B gene. Clinical and Experimental Medicine, 19(2), 183–190. 10.1007/s10238-019-00549-8 [DOI] [PubMed] [Google Scholar]
- Martínez‐Cortés, G. , Salazar‐Flores, J. , Fernández‐Rodríguez, L. G. , Rubi‐Castellanos, R. , Rodríguez‐Loya, C. , Velarde‐Félix, J. S. , Muñoz‐Valle, J. F. , Parra‐Rojas, I. , & Rangel‐Villalobos, H. (2012). Admixture and population structure in Mexican‐mestizos based on paternal lineages. Journal of Human Genetics, 57(9), 568–574. 10.1038/jhg.2012.67 [DOI] [PubMed] [Google Scholar]
- Miller, S. A. , Dykes, D. D. , & Polesky, H. F. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research, 16(3), 1215. 10.1093/nar/16.3.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, P. A. , Belvedere, O. , Orr, A. , Pieri, K. , LaFleur, D. W. , Feng, P. , Soppet, D. , Charters, M. , Gentz, R. , Parmelee, D. , Li, Y. , Galperina, O. , Giri, J. , Roschke, V. , Nardelli, B. , Carrell, J. , Sosnovtseva, S. , Greenfield, W. , Ruben, S. M. , … Hilbert, D. M. (1999). BLyS: Member of the tumor necrosis factor family and B lymphocyte stimulator. Science, 285(5425), 260–263. 10.1126/science.285.5425.260 [DOI] [PubMed] [Google Scholar]
- Moura, R. A. , Cascao, R. , Perpetuo, I. , Canhao, H. , Vieira‐Sousa, E. , Mourao, A. F. , Rodrigues, A. M. , Polido‐Pereira, J. , Queiroz, M. V. , Rosario, H. S. , Souto‐Carneiro, M. M. , Graca, L. , & Fonseca, J. E. (2010). Cytokine pattern in very early rheumatoid arthritis favours B‐cell activation and survival. Rheumatology, 50(2), 278–282. 10.1093/rheumatology/keq338 [DOI] [PubMed] [Google Scholar]
- Nezos, A. , Papageorgiou, A. , Fragoulis, G. , Ioakeimidis, D. , Koutsilieris, M. , Tzioufas, A. G. , Moutsopoulos, H. M. , Voulgarelis, M. , & Mavragani, C. P. (2014). B‐cell activating factor genetic variants in lymphomagenesis associated with primary Sjogren's syndrome. Journal of Autoimmunity, 51, 89–98. 10.1016/j.jaut.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Nossent, J. C. , Lester, S. , Zahra, D. , Mackay, C. R. , & Rischmueller, M. (2008). Polymorphism in the 5′ regulatory region of the B‐lymphocyte activating factor gene is associated with the Ro/La autoantibody response and serum BAFF levels in primary Sjogren's syndrome. Rheumatology (Oxford, England), 47(9), 1311–1316. 10.1093/rheumatology/ken246 [DOI] [PubMed] [Google Scholar]
- Ospina, F. E. , Betancur, J. F. , Suso, J. P. , Muñoz‐Buitron, E. , Cañas, C. A. , & Tobón, G. J. (2016). Role of the cytokine BAFF in autoimmune diseases: Physiopathology and therapeutic targets. Revista Colombiana de Reumatología (English Edition), 23(3), 177–194. 10.1016/j.rcreue.2016.11.003 [DOI] [Google Scholar]
- Pers, J.‐O. , Daridon, C. , Devauchelle, V. , Jousse, S. , Saraux, A. , Jamin, C. , & Youinou, P. (2005). BAFF overexpression is associated with autoantibody production in autoimmune diseases. Annals of the New York Academy of Sciences, 1050(1), 34–39. 10.1196/annals.1313.004 [DOI] [PubMed] [Google Scholar]
- Petri, M. , Stohl, W. , Chatham, W. , McCune, W. J. , Chevrier, M. , Ryel, J. , Recta, V. , Zhong, J. , & Freimuth, W. (2008). Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis and Rheumatism, 58(8), 2453–2459. 10.1002/art.23678 [DOI] [PubMed] [Google Scholar]
- Quartuccio, L. , Salvin, S. , Fabris, M. , Maset, M. , Pontarini, E. , Isola, M. , & Vita, S. D. (2013). BLyS upregulation in Sjögren's syndrome associated with lymphoproliferative disorders, higher ESSDAI score and B‐cell clonal expansion in the salivary glands. Rheumatology, 52(2), 276–281. 10.1093/rheumatology/kes180 [DOI] [PubMed] [Google Scholar]
- Rangel‐Villalobos, H. , Muñoz‐Valle, J. F. , González‐Martín, A. , Gorostiza, A. , Magaña, M. T. , & Páez‐Riberos, L. A. (2008). Genetic admixture, relatedness, and structure patterns among Mexican populations revealed by the Y‐chromosome. American Journal of Physical Anthropology, 135(4), 448–461. 10.1002/ajpa.20765 [DOI] [PubMed] [Google Scholar]
- Reyes, L. I. , León, F. , González, P. , Rozas, M. F. , Labarca, C. , Segovia, A. , Neira, O. , & Naves, R. (2008). Dexamethasone inhibits BAFF expression in fibroblast‐like synoviocytes from patients with rheumatoid arthritis. Cytokine, 42(2), 170–178. 10.1016/j.cyto.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Ruyssen‐Witrand, A. , Rouanet, S. , Combe, B. , Dougados, M. , Loët, X. L. , Sibilia, J. , Tebib, J. , Mariette, X. , & Constantin, A. (2012). Association between ‐871C>T promoter polymorphism in the B‐cell activating factor gene and the response to rituximab in rheumatoid arthritis patients. Rheumatology (Oxford, England), 52(4), 636–641. 10.1093/rheumatology/kes344 [DOI] [PubMed] [Google Scholar]
- Salazar‐Camarena, D. C. , Ortiz‐Lazareno, P. C. , Cruz, A. , Oregon‐Romero, E. , Machado‐Contreras, J. R. , Muñoz‐Valle, J. F. , Orozco‐López, M. , Marín‐Rosales, M. , & Palafox‐Sánchez, C. A. (2015). Association of BAFF, APRIL serum levels, BAFF‐R, TACI and BCMA expression on peripheral B‐cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus, 25(6), 582–592. 10.1177/0961203315608254 [DOI] [PubMed] [Google Scholar]
- Schmittgen, T. D. , & Livak, K. J. (2008). Analyzing real‐time PCR data by the comparative CT method. Nature Protocols, 3(6), 1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Schneider, P. , MacKay, F. , Steiner, V. , Hofmann, K. , Bodmer, J.‐L. , Holler, N. , Ambrose, C. , Lawton, P. , Bixler, S. , Acha‐Orbea, H. , Valmori, D. , Romero, P. , Werner‐Favre, C. , Zubler, R. H. , Browning, J. L. , & Tschopp, J. (1999). BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. The Journal of Experimental Medicine, 189(11), 1747–1756. 10.1084/jem.189.11.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, P. , Takatsuka, H. , Wilson, A. , Mackay, F. , Tardivel, A. , Lens, S. , Cachero, T. G. , Finke, D. , Beermann, F. , & Tschopp, J. (2001). Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. Journal of Experimental Medicine, 194(11), 1691–1698. 10.1084/jem.194.11.1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, D. L. , Wolfe, F. , & Huizinga, T. W. (2010). Rheumatoid arthritis. The Lancet, 376(9746), 1094–1108. 10.1016/s0140-6736(10)60826-4 [DOI] [PubMed] [Google Scholar]
- Shiboski, C. H. , Shiboski, S. C. , Seror, R. , Criswell, L. A. , Labetoulle, M. , Lietman, T. M. , Rasmussen, A. , Scofield, H. , Vitali, C. , Bowman, S. J. , Mariette, X. , & International Sjögren's Syndrome Criteria Working Group . (2017). 2016 American College of Rheumatology/European league against rheumatism classification criteria for primary Sjögren's syndrome. Annals of the Rheumatic Diseases, 76(1), 9–16. 10.1136/annrheumdis-2016-210571 [DOI] [PubMed] [Google Scholar]
- Sjöstrand, M. , Johansson, A. , Agrawi, L. , Olsson, T. , Wahrem‐Herlenius, M. , & Espinosa, A. (2015). The expression of BAFF is controlled y IRF transcription factors. Journal of Immunology (Baltimore, Md.: 1950), 196(1), 91–96. 10.4049/jimmunol.1501061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets, I. , Prezzemolo, T. , Imbrechts, M. , Mallants, K. , Mitera, T. , Humblet‐Baron, S. , Dubois, B. , Matthys, P. , Liston, A. , & Goris, A. (2021). Treatment‐induced BAFF expression and B cell biology in multiple sclerosis. Frontiers in Immunology, 12, 676619. 10.3389/fimmu.2021.676619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl, W. (2012). Biologic differences between various inhibitors of the BLyS/BAFF pathway: Should we expect differences between belimumab and other inhibitors in development? Current Rheumatology Reports, 14(4), 303–309. 10.1007/s11926-012-0254-6 [DOI] [PubMed] [Google Scholar]
- Tan, S. , Xu, D. , Roschke, V. , Perry, J. W. , Arkfeld, D. G. , Ehresmann, G. R. , Migone, T. , Hilbert, D. M. , & Stohl, W. (2003). Local production of B lymphocyte stimulator protein and APRIL in arthritic joints of patients with inflammatory arthritis. Arthritis and Rheumatism, 48(4), 982–992. 10.1002/art.10860 [DOI] [PubMed] [Google Scholar]
- Tanaka, Y. (2020). Rheumatoid arthritis. Inflammation and Regeneration, 40(1), 20. 10.1186/s41232-020-00133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou, E. , Nezos, A. , Antypa, E. , Ioakeimidis, D. , Koutsilieris, M. , Tektonidou, M. , Moutsopoulos, H. M. , & Mavragani, C. P. (2018). B‐cell activating factor and related genetic variants in lupus related atherosclerosis. Journal of Autoimmunity, 92, 87–92. 10.1016/j.jaut.2018.05.002 [DOI] [PubMed] [Google Scholar]
- WHO . (2020). Templates for informed consent forms. https://www.who.int/groups/research‐ethics‐review‐committee/guidelines‐on‐submitting‐research‐proposals‐for‐ethics‐review/templates‐for‐informed‐consent‐forms [Google Scholar]
- Yong, Y. , & He, L. (2005). SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Research, 15(2), 97–98. 10.1038/sj.cr.7290272 [DOI] [PubMed] [Google Scholar]
- Yoshitomi, H. (2019). Regulation of immune responses and chronic inflammation by fibroblast‐like synoviocytes. Frontiers in Immunology, 10, 1395. 10.3389/fimmu.2019.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed, R. A. , Sheba, H. F. , Elazaem, M. A. K. A. , Elsaadany, Z. A. , Elmessery, L. O. , Mahmoud, J. A. , Rahman, D. R. A. , & Abdou, F. R. (2013). B‐cell activating factor promoter polymorphisms in egyptian patients with systemic lupus erythematosus. Annals of Clinical and Laboratory Science, 43(3), 289–294. [PubMed] [Google Scholar]
- Zhou, Y. , Zhang, Y. , Han, J. , Yang, M. , Zhu, J. , & Jin, T. (2020). Transitional B cells involved in autoimmunity and their impact on neuroimmunological diseases. Journal of Translational Medicine, 18(1), 131. 10.1186/s12967-020-02289-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


