Abstract
Background
Inhaled corticosteroids are well established for the long‐term treatment of inflammatory respiratory diseases such as asthma or chronic obstructive pulmonary disease. They have been investigated for the treatment of coronavirus disease 2019 (COVID‐19). The anti‐inflammatory action of inhaled corticosteroids might have the potential to reduce the risk of severe illness resulting from hyperinflammation in COVID‐19.
Objectives
To assess whether inhaled corticosteroids are effective and safe in the treatment of COVID‐19; and to maintain the currency of the evidence, using a living systematic review approach.
Search methods
We searched the Cochrane COVID‐19 Study Register (which includes CENTRAL, PubMed, Embase, ClinicalTrials.gov, WHO ICTRP, and medRxiv), Web of Science (Science Citation Index, Emerging Citation Index), and the WHO COVID‐19 Global literature on coronavirus disease to identify completed and ongoing studies to 7 October 2021.
Selection criteria
We included randomised controlled trials (RCTs) evaluating inhaled corticosteroids for COVID‐19, irrespective of disease severity, age, sex, or ethnicity.
We included the following interventions: any type or dose of inhaled corticosteroids. We included the following comparison: inhaled corticosteroids plus standard care versus standard care (with or without placebo).
We excluded studies examining nasal or topical steroids.
Data collection and analysis
We followed standard Cochrane methodology. For risk of bias assessment, we used the Cochrane RoB 2 tool. We rated the certainty of evidence using the GRADE approach for the outcomes of mortality, admission to hospital or death, symptom resolution, time to symptom resolution, serious adverse events, adverse events, and infections.
Main results
Inhaled corticosteroids plus standard care versus standard care (with/without placebo)
People with a confirmed diagnosis of moderate‐to‐severe COVID‐19
We found no studies that included people with a confirmed diagnosis of moderate‐to‐severe COVID‐19.
People with a confirmed diagnosis of asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
We included three RCTs allocating 3607 participants, of whom 2490 had confirmed mild COVID‐19. We analysed a subset of the total number of participants recruited to the studies (2171, 52% female) as some trials had a platform design where not all participants were allocated to treatment groups simultaneously. The included studies were community‐based, recruiting people who were able to use inhaler devices to deliver steroids and relied on remote assessment and self‐reporting of outcomes. Most people were older than 50 years and had co‐morbidities such as hypertension, lung disease, or diabetes. The studies were conducted in high‐income countries prior to wide‐scale vaccination programmes. A total of 1057 participants were analysed in the inhaled corticosteroid arm (budesonide: 860 participants; ciclesonide: 197 participants), and 1075 participants in the control arm. No studies included people with asymptomatic SARS‐CoV‐2 infection.
With respect to the following outcomes, inhaled corticosteroids compared to standard care:
– may result in little to no difference in all‐cause mortality (at up to day 30) (risk ratio (RR) 0.61, 95% confidence interval (CI) 0.22 to 1.67; 2132 participants; low‐certainty evidence). In absolute terms, this means that for every nine deaths per 1000 people not receiving inhaled corticosteroids, there were six deaths per 1000 people who did receive the intervention (95% CI 2 to 16 per 1000 people);
– probably reduces admission to hospital or death (at up to 30 days) (RR 0.72, 95% CI 0.51 to 0.99; 2025 participants; moderate‐certainty evidence);
– probably increases resolution of all initial symptoms at day 14 (RR 1.19, 95% CI 1.09 to 1.30; 1986 participants; moderate‐certainty evidence);
– may reduce the duration to symptom resolution (at up to day 30) (by −4.00 days, 95% CI −6.22 to −1.78 less than control group rate of 12 days; 139 participants; low‐certainty evidence);
– the evidence is very uncertain about the effect on serious adverse events (during study period) (RR 0.51, 95% CI 0.09 to 2.76; 1586 participants; very low‐certainty evidence);
– may result in little to no difference in adverse events (at up to day 30) (RR 0.78, 95% CI 0.47 to 1.31; 400 participants; low‐certainty evidence);
– may result in little to no difference in infections (during study period) (RR 0.88, 95% CI 0.30 to 2.58; 400 participants; low‐certainty evidence).
As studies did not report outcomes for subgroups (e.g. age, ethnicity, sex), we did not perform subgroup analyses.
Authors' conclusions
In people with confirmed COVID‐19 and mild symptoms who are able to use inhaler devices, we found moderate‐certainty evidence that inhaled corticosteroids probably reduce the combined endpoint of admission to hospital or death and increase the resolution of all initial symptoms at day 14. Low‐certainty evidence suggests that corticosteroids make little to no difference in all‐cause mortality up to day 30 and may decrease the duration to symptom resolution. We do not know whether inhaled corticosteroids increase or decrease serious adverse events due to heterogeneity in the way they were reported across the studies. There is low‐certainty evidence that inhaled corticosteroids may decrease infections.
The evidence we identified came from studies in high‐income settings using budesonide and ciclesonide prior to vaccination roll‐outs.
We identified a lack of evidence concerning quality of life assessments, serious adverse events, and people with asymptomatic infection or with moderate‐to‐severe COVID‐19. The 10 ongoing and four completed, unpublished RCTs that we identified in trial registries address similar settings and research questions as in the current body of evidence. We expect to incorporate the findings of these studies in future versions of this review.
We monitor newly published results of RCTs on inhaled corticosteroids on a weekly basis and will update the review when the evidence or our certainty in the evidence changes.
Keywords: Female; Humans; Male; Adrenal Cortex Hormones; Cause of Death; COVID-19 Drug Treatment; Respiration, Artificial; SARS-CoV-2
Plain language summary
Are inhaled corticosteroids an effective treatment for people with mild COVID‐19?
Key messages
Inhaled corticosteroids (anti‐inflammatory medicines) given via the oral inhaled route are evaluated for treatment of coronavirus disease 2019 (COVID‐19).
We identified three published studies for people with mild disease. Inhaled corticosteroids probably reduce the risk of people going to hospital or death (admission to hospital or death before hospital admission). Inhaled corticosteroids may lower the number of days people have symptoms of mild COVID‐19 and probably increase resolution of COVID‐19 symptoms at day 14. They may make little to no difference in death from any cause, and we do not have enough evidence to know whether they cause serious harms.
There are no data for people with COVID‐19 with no symptoms (asymptomatic) or people with moderate‐to‐severe COVID‐19.
We found 10 ongoing and four completed unpublished studies. We will update this review when their results become available.
What are inhaled corticosteroids?
Inhaled corticosteroids are medicines that are breathed into the lower airways through an inhaler where they reduce inflammation in the lungs. They are commonly used to treat respiratory diseases like asthma and chronic obstructive pulmonary disease. Long‐term use and incorrect inhaler technique may lead to side effects that include a mouth infection called thrush, a change in voice, and an increased risk of lung infections. Good inhaler technique means the medicine does not stay in the mouth and throat.
Why are inhaled corticosteroids possible treatments for COVID‐19?
COVID‐19 mainly affects the lungs and airways. When the immune system fights the virus, the lungs and airways become inflamed. This inflammation causes breathing difficulties, and the lungs cannot easily move oxygen into the blood and remove carbon dioxide from the blood.
What did we want to find out?
People need more and better treatment options for asymptomatic SARS‐CoV‐2 infection (the virus that causes COVID‐19) or mild, moderate, or severe COVID‐19. We wanted to know if inhaled corticosteroids are an effective and helpful treatment option for COVID‐19 in any setting (for example, home or hospital) and whether they cause unwanted effects.
We were interested in:
– death from any cause up to day 30, day 60, or longer if reported;
– admission to hospital or death within 30 days;
– whether symptoms resolved and how fast;
– quality of life;
– unwanted effects.
What did we do?
We looked for studies where the investigators compared inhaled corticosteroids and usual care to usual care only, sometimes in addition to a dummy medicine that did not contain any active ingredients (placebo) but was given in the same way. To make the comparison least skewed and more fair, patients in the studies must all have had the same random chance (like the flip of a coin) to receive the inhaled corticosteroids or the other treatment. The studies could include people of any age, sex, or ethnicity.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
Three studies compared inhaled corticosteroids plus usual care compared to usual care with or without placebo in people with a confirmed diagnosis of mild COVID‐19. These studies analysed 2171 participants mostly older than 50 years and with other medical problems, 52% of them were female, of whom 1057 received inhaled corticosteroids in our analyses. We found no studies that included people with asymptomatic infection or confirmed diagnosis of moderate‐to‐severe COVID‐19.
We also found 10 ongoing studies, and four completed studies without published results.
Main results
All studies compared inhaled corticosteroids with usual care or placebo. The studies included only people with a confirmed diagnosis of SARS‐CoV‐2 infection and mild disease. No studies looked at hospitalised people or people with asymptomatic SARS‐CoV‐2 infection. Inhaled corticosteroids
– may make little to no difference in death from any cause up to day 30;
– probably reduce the risk of admission to hospital or occurrence of death up to day 30;
– probably increase resolution of COVID‐19 symptoms at day 14 and may reduce time to symptom resolution.
We are very uncertain about a possible difference in serious unwanted effects. Moreover, inhaled corticosteroids may result in little to no difference in the number of any unwanted effects or additional infections.
What are the limitations of the evidence?
The studies were conducted in populations from wealthy countries, prior to the roll‐out of COVID‐19 vaccination programmes. We have moderate confidence in the evidence for the outcomes of symptom resolution at day 14 and hospital admission. We have low confidence in the evidence for the effects on deaths from any cause for people with mild COVID‐19 and time to symptom resolution. The confidence in the unwanted or serious unwanted effects and infections is low or very low, because of the differences in the way investigators recorded and reported results. There was no evidence for people with asymptomatic infection or moderate‐to‐severe COVID‐19 who were hospitalised.
How up to date is this evidence?
Our evidence is up‐to‐date to 7 October 2021.
Summary of findings
Summary of findings 1. Inhaled corticosteroids plus standard care compared to standard care (with or without placebo) for adults with a confirmed diagnosis of mild COVID‐19.
| Inhaled corticosteroids plus standard care compared to standard care (with or without placebo) for adults with a confirmed diagnosis of asymptomatic SARS‐CoV‐2 infection or mild COVID‐19 | ||||||
|
Patient or population: adults with a confirmed diagnosis of mild COVID‐19, of whom only 10% (219/2132) participants had received ≥ 1 vaccination Setting: outpatient Intervention: inhaled corticosteroids plus standard care Comparison: standard care (with or without placebo) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
No of participants (studies) |
Certainty of the evidence (GRADE) |
Comment | |
| Risk with standard care (with or without placebo)a | Risk with inhaled corticosteroids (plus standard care) | |||||
|
All‐cause mortality Follow‐up: at up to 30 days |
9 per 1000 | 6 per 1000 (2 to 16) |
RR 0.61 (0.22 to 1.67) |
2132 (3 studies) |
⊕⊕⊖⊖ Lowb |
Inhaled corticosteroids may result in little to no difference in all‐cause mortality up to day 30. |
|
Admission to hospital or death Follow‐up: at up to 30 days |
79 per 1000 | 57 per 1000 (40 to 78) |
RR 0.72 (0.51 to 0.99) |
2025 (2 studies) |
⊕⊕⊕⊖ Moderatec |
Inhaled corticosteroids probably reduce the risk of admission to hospital or death up to day 30. |
|
Symptom resolution: all initial symptoms resolved at day 14 |
465 per 1000 | 553 per 1000 (507 to 605) |
RR 1.19 (1.09 to 1.30) |
1986 (2 studies) |
⊕⊕⊕⊖ Moderated |
Inhaled corticosteroids probably increase the resolution of all initial symptoms at day 14. |
|
Symptom resolution: duration to symptoms resolved Follow‐up: at up to day 30 |
The mean duration to symptoms resolved was 12.00 days. | The mean duration of symptoms resolved was 8.00 days (5.78 to 10.22 days). |
MD −4.00 days (−6.22 to −1.78) |
139 (1 study) |
⊕⊕⊖⊖ Lowd,e |
Inhaled corticosteroids may decrease the duration to symptom resolution. |
|
Serious adverse events Follow‐up: during study period |
5 per 1000 | 3 per 1000 (0 to 14) |
RR 0.51 (0.09 to 2.76) |
1586 (1 study) |
⊕⊖⊖⊖ Very Lowb,d |
The evidence is very uncertain about the effect of inhaled corticosteroids on serious adverse events. |
|
Adverse events Follow‐up: at up to day 30 |
143 per 1000 | 111 per 1000 (67 to 187) |
RR 0.78 (0.47 to 1.31) |
400 (1 study) |
⊕⊕⊖⊖ Lowd,e |
Inhaled corticosteroids may result in little to no difference in adverse events. |
|
Infections Follow‐up: during study period |
34 per 1000 | 30 per 1000 (10 to 89) |
RR 0.88 (0.30 to 2.58) |
400 (1 study) |
⊕⊕⊖⊖ Lowb |
Inhaled corticosteroids may result in little to no difference in infections. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention group (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio. | ||||||
|
GRADE working group grades evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aControl group risk estimated from included studies (with 10% of participants being vaccinated at least once). bDowngraded two levels for very serious imprecision (very low number of events, wide CI). cDowngraded one level for serious imprecision (low number of participants/events and optimal information size would be 3764 participants). dDowngraded one level for serious risk of bias (partly measurement of the outcome affected by unblinded design, selection of the reported result, missing outcome data as the safety‐relevant outcome was not reported) and reporting bias (the safety‐relevant outcome was not reported). eDowngraded one level for serious imprecision (low number of participants/events and wide CI).
Background
This work is part of a series of Cochrane Reviews investigating treatments and therapies for coronavirus disease 2019 (COVID‐19). Reviews in this series share information in the background section and methodology with the first published reviews about monoclonal antibodies (Kreuzberger 2021) and convalescent plasma (Piechotta 2021) from the German research project 'CEOsys' (COVID‐19 Evidence Ecosystem).
Description of the condition
COVID‐19 is a rapidly spreading infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2; WHO 2020a). On 11 March 2020, the World Health Organization (WHO) declared the current COVID‐19 outbreak a pandemic. The severity of COVID‐19 is unprecedented in comparison to that of previous coronavirus outbreaks such as severe acute respiratory syndrome (SARS), which caused 813 deaths, and Middle East respiratory syndrome (MERS), which caused 858 deaths (WHO 2007; WHO 2019). Despite intensive international efforts to contain its spread, SARS‐CoV‐2 has resulted in a continuously rising number of cases and deaths with a clearly accelerating increase in the first months of 2021 (WHO 2021a; WHO 2021b). In the meantime, the appearance of SARS‐CoV‐2 variants with higher transmissibility is further increasing infection rates (WHO 2021c).
The risk for a severe course of disease, hospitalisation, and mortality is higher among people aged 65 years or older; smokers; and those with certain underlying medical conditions such as cancer, chronic kidney disease, chronic obstructive pulmonary disease (COPD), heart conditions, immunocompromised state, obesity, sickle cell disease, or type 2 diabetes mellitus (Huang 2020; Liang 2020; WHO 2020a; Williamson 2020). COVID‐19 case fatality ratios vary widely between countries and reporting periods, from 0.0% to more than 25% (Johns Hopkins University 2021). However, these numbers may be misleading as they tend to overestimate the infection:fatality ratio due to varying testing frequency, a lack of reporting dates, and variations in case definitions, especially in the beginning of the pandemic when the main focus was on severe cases (WHO 2020b).
The median incubation time is estimated to be five to six days, and 97.5% of symptomatic cases develop symptoms within 11.5 days of exposure (Lauer 2020). Sore throat, cough, fever, headache, fatigue, and myalgia or arthralgia are the most commonly reported symptoms (Struyf 2020). Other symptoms include dyspnoea, chills, nausea or vomiting, diarrhoea, and nasal congestion (WHO 2020a). Most infected people (approximately 80%) have mild symptoms (Wu 2020), or remain completely asymptomatic (Buitrago‐Garcia 2020). A smaller proportion (approximately 14%) are affected by severe or critical disease which requires treatment at an intensive care unit (ICU) due to respiratory failure, septic shock, or multiple organ dysfunction (Wu 2020). In light of the extent of the COVID‐19 pandemic and the scarcity of effective treatments, there is an urgent need for effective therapies to save lives and to reduce the high burden on healthcare systems, especially in the face of evolving variants of the virus with the potential for increased transmissibility and the limited global availability of vaccines.
Description of the intervention
Corticosteroids are a group of stress hormones produced from the adrenal cortex. In addition to their stress‐mediated mechanisms for generating energy substrates, corticosteroids have anti‐inflammatory and immunosuppressive properties in higher doses and are applied widely in almost all medical fields (Barnes 2006; Rhen 2005). The effects are based on the binding to intracellular receptors, which influences protein expression. In addition, glucocorticoids also develop extragenomic effects. All active ingredients are lipophilic and thus easily reach the cells via the cell membrane. Long‐term systemic corticosteroid therapy, unless in a very low dose, can be associated with many adverse effects (e.g. hypertension, osteoporosis, and diabetes). Inhaled corticosteroids have a lower risk of undesirable effects compared with systemic administration. They can be used as dose aerosols, inhalable powder, or inhalation capsules in different dosages (low, medium, and high dosages) depending on indication (Daley‐Yates 2015). Inhaled corticosteroids reach the lower respiratory tract as a finely divided aerosol via the airflow and develop their effect directly on the bronchial mucosa. This form of administration is particularly important for the treatment of inflammatory respiratory diseases such as asthma or COPD (Geddes 1992). However, many adverse effects are dose‐related and can include local effects such as oropharyngeal candidiasis, hoarseness, and increased risk of pneumonia, in addition to the systemic effects mentioned above (Daley‐Yates 2015; Price 2012).
How the intervention might work
In COVID‐19, an imbalanced inflammation is thought to play a key role in the pathophysiology of hypoxaemic respiratory failure (Schulte‐Schrepping 2020). A systemic inflammatory response with an excessive release of cytokines and inflammatory mediators can lead to lung injury with the development of acute respiratory distress syndrome (ARDS).
It has been proposed that corticosteroids could be clinically effective against severe and critical COVID‐19, due to their anti‐inflammatory and immunomodulatory properties (Villar 2020). Furthermore, in vitro studies have shown that inhaled glucocorticoids have antiviral effectiveness due to two mechanisms: downregulation of the expression of ACE2 and TMPRSS2 genes, which are critical for viral cell entry (Finney 2021; Matsuyama 2020; Peters 2020), and reduction of the replication of SARS‐CoV‐2 in airway epithelial cells (Yamaya 2020). In addition, corticosteroids reduce the exacerbation rate in COPD and asthma, which is very often caused by viral infections (Viniol 2018). Observation from the early pandemic showed that people with bronchial asthma and COPD were less likely to be hospitalised for COVID‐19, which is postulated to be due to the routine medication with inhaled corticosteroids in those people (Halpin 2020).
Why it is important to do this review
Globally, the number of new COVID‐19 cases and deaths continue to increase with a substantial impact on healthcare systems. Vaccination remains a key component of options for response to address the ongoing circulation and reduce the impact of the dominant variants of concern. Despite the efforts to increase full vaccination uptake in people who are currently insufficiently vaccinated, some pharmaceutical interventions remain a mainstay in the management of COVID‐19. Treatment decisions should be informed by high‐quality, relevant, and up‐to‐date synthesised research evidence provided by international networks. There are several systematic reviews on the use of systemic corticosteroids for the treatment of COVID‐19 based on randomised controlled trials (RCTs) and non‐randomised studies (e.g. Sterne 2020; van Paassen 2020; Wagner 2021a).
This systematic review fills current gaps by identifying, describing, evaluating, and meta‐analysing RCTs for inhaled corticosteroids on clinical outcomes as an additional treatment option in COVID‐19. We will search for RCTs on inhaled corticosteroids for COVID‐19 on a weekly basis and update this living systematic review once new relevant evidence becomes available to ensure the review remains current. For the most up‐to‐date information about the review, the results of the searches, and any new evidence being incorporated, readers are encouraged to check the update status information. The update status information will be updated whenever the searches are rerun.
Objectives
To assess whether inhaled corticosteroids are effective and safe in the treatment of COVID‐19; and to maintain the currency of the evidence, using a living systematic review approach.
Methods
Criteria for considering studies for this review
Types of studies
The main description of methods is based on the standard template of the Cochrane Haematology review group and is in line with a series of Cochrane Reviews investigating treatments and therapies against COVID‐19. Specific adaptions related to the research question were made if necessary (see Differences between protocol and review). The protocol for this review was registered with PROSPERO on 9 July 2021 (Wagner 2021b).
To assess the efficacy and safety of inhaled corticosteroids against COVID‐19, we included RCTs, as this study design, if performed appropriately, provides the best evidence for experimental therapies in highly controlled therapeutic settings. We used the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We would also have accepted cluster‐randomised trials for inclusion, if we had found any. We found no cross‐over studies, but would have excluded them because of the short duration of the disease and potential carry‐over effects of corticosteroids.
We included the following formats, if sufficient information was available on study design, characteristics of participants, interventions, and outcomes:
full‐text publications;
preprint articles.
We included preprints to have a complete overview of the ongoing research activity, especially for tracking newly emerging studies about systemic corticosteroids against COVID‐19. We did not apply any limitation with respect to the length of follow‐up.
Types of participants
We included people with a confirmed diagnosis of COVID‐19 and moderate‐to‐severe disease and people with a confirmed diagnosis of asymptomatic SARS‐CoV‐2 infection or mild COVID‐19 (as described in the study). We did not exclude any studies based on sex, ethnicity, disease severity, or setting.
Types of interventions
We included the following intervention:
any type or dose of inhaled corticosteroids.
We included the following comparisons:
inhaled corticosteroid plus standard care versus standard care (with or without placebo).
Standard care in both arms had to be similar.
We excluded the following interventions:
topical corticosteroids.
Types of outcome measures
We evaluated core outcomes in accordance with the Core Outcome Measures in Effectiveness Trials (COMET) Initiative for people with COVID‐19 (COMET 2020; Marshall 2020), and additional outcomes that have been prioritised by consumer representatives and the panel of the German "national treatment guidance for hospitalized COVID‐19 patients" (Kluge 2022).
We defined this outcome set for hospitalised people with a confirmed diagnosis of COVID‐19 and moderate‐to‐severe disease, according to WHO clinical progression scale stage 4 to 9 (Marshall 2020) (i.e. all patients who were hospitalised because of symptomatic COVID‐19 treated with all different levels of respiratory support, such as no additional oxygen, low‐flow oxygen prongs or mask ('low‐flow oxygen'), high‐flow oxygen or non‐invasive ventilation, invasive mechanical ventilation inclusively extracorporeal membrane oxygenation) and people with a confirmed diagnosis of SARS‐CoV‐2 infection and asymptomatic or mild disease, according to the WHO clinical progression scale (Marshall 2020). Of note, the reader will encounter respiratory support both as baseline characteristic and as outcome measure – in the latter case changes in the level of support will be utilised.
In review updates, we will also evaluate patient‐reported experience measures (PREMS), as suggested by the consumer editor. However, up to October 2021, the included studies did not report PREMS.
People with a confirmed diagnosis of COVID‐19: moderate or severe disease
Efficacy of inhaled corticosteroids
Prioritised outcomes (included in the summary of findings table)
All‐cause mortality at day 30, day 60, time‐to‐event, and up to longest follow‐up.
-
Clinical status at day 30, day 60, and up to longest follow‐up, including:
-
worsening of clinical status:
participants with clinical deterioration (new need for invasive mechanical ventilation) or death;
-
improvement of clinical status:
participants discharged alive. Participants should be discharged without clinical deterioration or death.
-
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to seven days; up to 30 days, and longest follow‐up available.
Safety of inhaled corticosteroids
Serious adverse events during the study period, defined as number of participants with any serious adverse event (serious as defined according to CTCAE (Common Terminology Criteria for Adverse Events)).
Adverse events (any grade) during the study period, defined as number of participants with any adverse event.
Hospital‐acquired infections during the study period.
People with a confirmed diagnosis of asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
Efficacy of inhaled corticosteroids
Prioritised outcomes (included in the summary of findings table)
All‐cause mortality at day 30, day 60, time‐to‐event, and up to longest follow‐up.
Admission to hospital or death within 30 days.
-
Symptom resolution:
all initial symptoms resolved (asymptomatic) at day 14;
duration to symptom resolution.
Prioritised outcomes (not included in the summary of findings table)
-
Symptom resolution:
all initial symptoms resolved (asymptomatic) at day 30.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to seven days, up to 30 days, and longest follow‐up available.
Safety of inhaled corticosteroids
Serious adverse events during the study period, defined as number of participants with any serious adverse event (serious as defined according to CTCAE (Common Terminology Criteria for Adverse Events)).
Adverse events (any grade) during the study period, defined as number of participants with any adverse event.
Infections during the study period.
PREMs (e.g. through questionnaires, including information on satisfaction with the treatment to investigate possible reasons for deviations from intended interventions).
Timing of outcome measurement
In case of time‐to‐event analysis (e.g. for time to clinical improvement), we included the outcome measure based on the longest follow‐up time. We also collected information on outcomes from all other time points reported in the publications.
Search methods for identification of studies
Electronic searches
Our information specialist (MIM) conducted systematic searches in the following sources from the inception of each database to 7 October 2021 (search date for all databases) and placed no restrictions on the language of publication.
-
Cochrane COVID‐19 Study Register (CCSR) (www.covid-19.cochrane.org), comprising:
Cochrane Central Register of Controlled Trials (CENTRAL), monthly updates;
MEDLINE (PubMed), daily updates;
Embase.com, weekly updates;
ClinicalTrials.gov (www.clinicaltrials.gov), daily updates;
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch), weekly updates;
medRxiv (www.medrxiv.org), weekly updates.
-
Web of Science Core Collection (Clarivate), from 1 January 2020 onwards:
Science Citation Index Expanded (from 1945);
Emerging Sources Citation Index (from 2015).
WHO COVID‐19 Global literature on coronavirus disease (search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/).
Database search results for Web of Science were restricted to publications from 2020 to October 2021, as no treatment trials on COVID‐19 were registered prior to January 2020. For detailed search strategies, see Appendix 1.
We did not conduct separate searches of the databases required by the MECIR standards (Higgins 2021a), since these databases are already regularly searched for the production of the CCSR.
Living systematic review considerations
We will use the CCSR to monitor newly published results of RCTs on inhaled corticosteroids on a weekly basis.
Searching other resources
We identified other potentially eligible studies or ancillary publications by searching the reference lists of included studies and systematic reviews.
Living systematic review considerations
The signal for updating this review will stem from the weekly monitoring of the published relevant RCTs via the CCSR, as described under Electronic searches. Once the decision to update the review has been made, the methods mentioned in this section will be incorporated in the review update.
Data collection and analysis
Selection of studies
Two review authors (CW, AF) independently screened the results of the search for eligibility by reading the titles and abstracts using EndNote Software (EndNote X9). We coded the abstracts as either 'include' or 'exclude'. In the case of disagreement or if it was unclear whether we should retrieve the abstract, we obtained the full‐text publication for further discussion. Two review authors assessed the full‐text articles of selected studies. If the two review authors were unable to reach a consensus, they consulted the third review author to reach a final decision.
We documented the study selection process in a PRISMA flow chart (Moher 2009), and showed the total numbers of retrieved references and the numbers of included and excluded studies. We listed all studies that we excluded after full‐text assessment and the reasons for their exclusion in the Characteristics of excluded studies section.
Data extraction and management
We conducted data extraction according to Cochrane guidelines (Li 2020). Two of four review authors (MG, CW, AF, AM) extracted data independently and in duplicate, using a customised data extraction form developed in Microsoft Excel (Microsoft Excel). We solved disagreements by discussion. If no agreement was obtained, a third review author was involved to solve the disagreement.
Two of three review authors (MG, CW, AF) independently assessed eligible studies obtained in the process of study selection (as described above) for methodological quality and risk of bias. If the review authors were unable to reach a consensus, a third review author was consulted.
We extracted the following information if reported.
General information: author, title, source, publication date, country, language, duplicate publications.
Study characteristics: trial design, setting and dates, source of participants, inclusion/exclusion criteria, comparability of groups, treatment cross‐overs, compliance with assigned treatment, length of follow‐up.
Participant characteristics: age, sex, ethnicity, number of participants recruited/allocated/evaluated, number of participants with positive, negative, or unknown polymerase chain reaction (PCR) test result, additional diagnoses, severity of disease, previous treatments, concurrent treatments, comorbidities (e.g. diabetes, immunosuppression).
Interventions: type of corticosteroid, dose, frequency, timing, duration and route of administration, setting (e.g. hospitalised, non‐hospitalised), duration of follow‐up.
Control interventions: placebo, no treatment, or other intervention; dose, frequency, timing, duration, and route of administration; setting; duration of follow‐up.
Outcomes: as specified under Types of outcome measures.
Risk of bias assessment: randomisation process, deviations from the intended interventions, missing outcome data, measurement of the outcome, selection of the reported result.
Assessment of risk of bias in included studies
We used the Risk of Bias 2 (RoB 2) tool (version of 22 August 2019) to analyse the risk of bias of study results (Sterne 2019). Of interest for this review was the effect of the assignment to the intervention (the intention‐to‐treat (ITT) effect), thus, we performed all assessments with RoB 2 on this effect. The outcomes that we assessed were those specified for inclusion in the summary of findings table.
Two of five review authors (MG, CW, AF, JD, AN) independently assessed the risk of bias for each outcome. In case of discrepancies among their judgements and inability to reach consensus, we consulted another review author to reach a final decision. We assessed the following types of bias for RCTs as outlined in Chapter 8 (Higgins 2021b) and for cluster‐RCTs as outlined in Chapter 23 (Table 23.1.a; Higgins 2021c) of the Cochrane Handbook for Systematic Reviews of Interventions.
For RCTs:
bias arising from the randomisation process;
bias due to deviations from the intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome;
bias in selection of the reported result.
For cluster‐RCTs:
bias arising from the randomisation process;
bias arising from the timing of identification and recruitment of participants;
bias due to deviations from intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome;
bias in selection of the reported result.
To address these types of bias we used the signalling questions recommended in RoB 2 and made a judgement using the following options.
'Yes': if there was firm evidence that the question was fulfilled in the study (i.e. the study was at low or high risk of bias for the given the direction of the question).
'Probably yes': a judgement was made that the question was fulfilled in the study (i.e. the study was at low or high risk of bias given the direction of the question).
'No': if there was firm evidence that the question was unfilled in the study (i.e. the study was at low or high risk of bias for the given the direction of the question).
'Probably no': a judgement was made that the question was unfilled in the study (i.e. the study was at low or high risk of bias given the direction of the question).
'No information': if the study report did not provide sufficient information to allow any judgement.
We used the algorithms proposed by RoB 2 to assign each domain one of the following levels of bias.
Low risk of bias.
Some concerns.
High risk of bias.
Subsequently, we derived an overall risk of bias rating for each prespecified outcome in each study in accordance with the following suggestions.
'Low risk of bias': we judged the trial at low risk of bias for all domains for this result.
'Some concerns': we judged the trial to raise some concerns in at least one domain for this result, but not at high risk of bias for any domain.
'High risk of bias': we judged the trial at high risk of bias in at least one domain for the result, or we judged the trial to have some concerns for multiple domains in a way that substantially lowered confidence in the results.
We used the RoB 2 Excel tool to implement RoB 2 (available on the riskofbias.info website), stored, and presented our detailed RoB 2 assessments in the analyses section and as supplementary online material.
Measures of treatment effect
For continuous outcomes, we recorded the mean, standard deviation (SD), and total number of participants in both treatment and control groups. Where continuous outcomes used the same scale, we performed analyses using the mean difference (MD) with 95% confidence intervals (CI). For continuous outcomes measured with different scales, we planned to perform analyses using the standardised mean difference (SMD), but this was not needed in this review. For interpreting SMDs, we planned to re‐express SMDs in the original units of a particular scale with the most clinical relevance and impact (e.g. clinical symptoms with the WHO Clinical Progression Scale (WHO 2020c)).
For dichotomous outcomes, we recorded the number of events and total number of participants in both treatment and control groups. We reported the pooled risk ratio (RR) with a 95% CI (Deeks 2021).
We planned to extract and report hazard ratios (HRs) for time‐to‐event outcomes (e.g. time to recovery), but there were no data available. There was also no need to estimate the HR as accurately as possible from available data using the methods proposed by Parmar and Tierney (Parmar 1998; Tierney 2007).
We received the advice from a statistician to not use Peto odds ratio instead of RR when event rates were low, since we only have a few studies in our main analysis.
Unit of analysis issues
The aim of this review was to summarise trials that analysed data at the level of the participant. We collated multiple reports of one study so that the study, and not the report, was the unit of analysis.
Studies with multiple treatment groups
As recommended in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021d), for studies with multiple treatment groups of the same intervention (i.e. dose, route of administration), we planned to evaluate whether study arms were sufficiently homogeneous to be combined. If arms could not be pooled, we planned to compare each arm with the common comparator separately. For pair‐wise meta‐analysis, we planned to split the 'shared' group into two or more groups with smaller sample size, and include two or more (reasonably independent) comparisons. For this purpose, for dichotomous outcomes, we planned to divide both the number of events and the total number of participants, and for continuous outcomes, we planned to divide the total number of participants with unchanged means and SDs.
Dealing with missing data
Missing data can occur on different levels (Deeks 2021).
Missing studies: our comprehensive search for RCTs including registry entries aimed at providing an overview on all published, ongoing, and planned studies. We included too few studies to assess publication bias by a funnel plot.
Missing outcomes: if an outcome of interest to us was prespecified but not reported we considered that in the GRADE process and comment in the Discussion section.
Missing summary data: if missing summary data of an outcome of interest necessary for meta‐analysis were missing, we contacted the authors.
Missing participants: whenever possible, we contacted the original investigators to request missing data. We assumed data to be missing at random when the rate of missingness across arms was comparable and the characteristics of participants with missing data were comparable to the characteristics of participants without missing data. Otherwise, we assumed data not to be missing at random. For the primary analysis, we conducted a complete‐case analysis by excluding participants with missing outcome data from the meta‐analysis.
We performed sensitivity analyses to assess how robust results were to a worst‐case scenario assumption.
We addressed the potential impact of missing data under Potential biases in the review process.
Missing study‐level characteristics: outcomes were not available stratified by potentially important characteristics such as sex, age, or ethnicity.
We requested data for all outcomes for one study because the trial reported it partly for participants who received inhaled corticosteroids plus hydroxychloroquine (Song 2021). Furthermore, we asked the authors to specify the setting of their study, as it was unclear from the publication whether the participants were hospitalised and hence what their disease severity was at baseline.
Assessment of heterogeneity
We used the I² statistic (Higgins 2003), and visual examination, to assess possible heterogeneity (I² > 30% to signify moderate heterogeneity, I² > 75% to signify considerable heterogeneity; Deeks 2021). If heterogeneity was above 80%, we planned to explore potential causes through sensitivity and subgroup analyses. If we could not find a reason for heterogeneity, we did not perform a meta‐analysis but planned to comment on results from all studies and present these in tables.
As the heterogeneity was never above 80%, we did not explore potential causes through sensitivity and subgroup analyses. However, we will do this for future updates.
Assessment of reporting biases
We searched trials registries to identify completed trials that had not been published elsewhere, to minimise or determine publication bias. We intended to explore potential publication bias by generating a funnel plot and statistically testing this by conducting a linear regression test for meta‐analyses involving at least 10 trials (Sterne 2019). We would have considered P < 0.1 as significant for this test.
We planned to generate a funnel plot, but had fewer than 10 studies. We will produce a funnel plot for future updates.
Data synthesis
If the clinical and methodological characteristics of individual studies were sufficiently homogeneous, we pooled the data in a meta‐analysis. We performed analyses according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). We analysed trials including different severities of disease separately grouping them with respect to disease severity according to need for respiratory support at randomisation (see Types of outcome measures). We treated placebo and standard care as the same intervention, as well as standard care at different institutions and time points. For the primary analyses, data were pooled regardless of risk of bias.
We used Review Manager Web software for analyses (RevMan Web 2019). One review author entered the data into the software, and a second review author checked the data for accuracy. We used the random‐effects model for all analyses as we anticipated that true effects were related, but were not the same for included studies. If we deemed meta‐analysis inappropriate for a certain outcome because of heterogeneity of included studies both statistically or conceptually or for too high risk of bias, we presented descriptive statistics only.
If meta‐analysis was possible, we assessed the effects of potential biases in sensitivity analyses (see Sensitivity analysis). For binary outcomes, we based the estimation of the between‐study variance using the Mantel‐Haenszel method. We planned to explore heterogeneity above 80% with subgroup analyses. If we could not find a cause for the heterogeneity, we did not perform a meta‐analysis, but commented on the results as a narrative with the results from all studies presented in tables.
Subgroup analysis and investigation of heterogeneity
Because of clinical relevance, we planned subgroup analyses of mortality for the following characteristics.
Sociodemographic characteristics (e.g. sex, age, ethnicity).
Comorbidities.
Different dosage.
Different timing.
Vaccination status.
Due to insufficient data, we were unable to perform these subgroup analyses.
Sensitivity analysis
We planned sensitivity analysis of mortality for the following potential confounders.
Risk of bias assessment components (studies with a low risk of bias or some concerns versus studies with a high risk of bias).
High rate of missing data.
Because there were no studies with a high risk of bias or high rate of missing data that reported mortality, we could not perform a sensitivity analysis.
We performed sensitivity analysis for mortality for the following potential confounders.
Fixed‐effect versus random‐effects model.
Preprint versus journal publication.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence for the following outcomes, and prepared one summary of findings table per population.
Summary of findings
We used GRADEpro GDT software to create summary of findings tables. For time‐to‐event outcomes, we planned to calculate absolute effects at specific time points, as recommended in the GRADE guidance (Skoetz 2020).
According to Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions, the "most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes" should be included in the summary of findings tables (Schünemann 2020). We included outcomes prioritised according to the core outcome sets for studies for the treatment of people with confirmed COVID‐19 (COMET 2020), and patient relevance. We included the following outcomes.
People with a confirmed diagnosis of COVID‐19: moderate or severe disease
All‐cause mortality at day 30, day 60, time‐to‐event, and at hospital discharge.
-
Clinical status at day 30, day 60, and up to longest follow‐up, including:
-
worsening of clinical status:
participants with clinical deterioration (new need for invasive mechanical ventilation) or death;
-
improvement of clinical status:
participants discharged alive. Participants should be discharged without clinical deterioration or death.
-
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to 7 days; up to 30 days, and longest follow‐up available.
Serious adverse events during the study period, defined as number of participants with any serious adverse event (serious as defined according to CTCAE (Common Terminology Criteria for Adverse Events)).
Adverse events (any grade) during the study period, defined as number of participants with any adverse event.
Hospital‐acquired infections during the study period.
People with a confirmed diagnosis of asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
All‐cause mortality at day 30, day 60, time‐to‐event, and up to longest follow‐up.
Admission to hospital or death within 30 days.
-
Symptom resolution:
all initial symptoms resolved (asymptomatic) at day 14;
duration to symptom resolution.
Serious adverse events during the study period, defined as number of participants with any serious adverse event (serious as defined according to CTCAE (Common Terminology Criteria for Adverse Events)).
Adverse events (any grade) during the study period, defined as number of participants with any adverse event.
Infections during the study period.
Assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty in the evidence for the above outcomes.
The GRADE approach uses five domains (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty in the body of evidence for each prioritised outcome.
We downgraded our certainty of evidence for:
serious (−1) or very serious (−2) risk of bias;
serious (−1) or very serious (−2) inconsistency;
serious (−1) or very serious (−2) uncertainty about directness;
serious (−1) or very serious (−2) imprecise or sparse data;
serious (−1) or very serious (−2) probability of reporting bias.
The GRADE system used the following criteria for assigning grade of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We followed the current GRADE guidance for these assessments in its entirety as recommended in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2020).
We used the overall risk of bias judgement, derived from the RoB 2 Excel tool, to inform our decision on downgrading for risk of bias. We phrased the findings and certainty in the evidence as suggested in the informative statement guidance (Santesso 2020).
Methods for future updates
Living systematic review considerations
Our information specialist (MIM) will provide us with new search records each month, which two review authors will screen, evaluate, extract, and integrate following the guidance for Cochrane living systematic reviews (Living Evidence Network 2019).
We will manually check platform trials that were previously identified and listed as 'studies awaiting classification' for additional treatment arms.
We will wait until the accumulating evidence changes our conclusions of the implications of research and practice before republishing the review. We will consider one or more of the following components to inform this decision.
The findings of one or more prioritised outcomes for population with virologically confirmed SARS‐CoV‐2 infection.
The credibility (e.g. GRADE rating) of one or more prioritised outcomes.
New settings, populations, interventions, comparisons, or outcomes studied.
In case of emerging policy relevance because of global controversies around the intervention, we will consider republishing an updated review even though our conclusions remain unchanged. We will review the review scope and methods approximately monthly, or more frequently if appropriate, in light of potential changes in COVID‐19 research (e.g. when additional comparisons, interventions, subgroups, or outcomes, or new review methods become available).
Results
Description of studies
Results of the search
We searched all databases and screened the resulting records up to 7 October 2021. We identified 1560 records. After removing duplicates, we screened 1329 records based on their titles and abstracts. We excluded 1305 records that did not meet the inclusion criteria. Of the remaining 24 records, we included 23 records:
three RCTs (in six records) for inclusion in this review;
five RCTs (in six records) are awaiting classification;
10 RCTs (in 11 records) are ongoing.
The study flow diagram in Figure 1 illustrates the study selection process according to PRISMA guidelines (Moher 2009).
1.
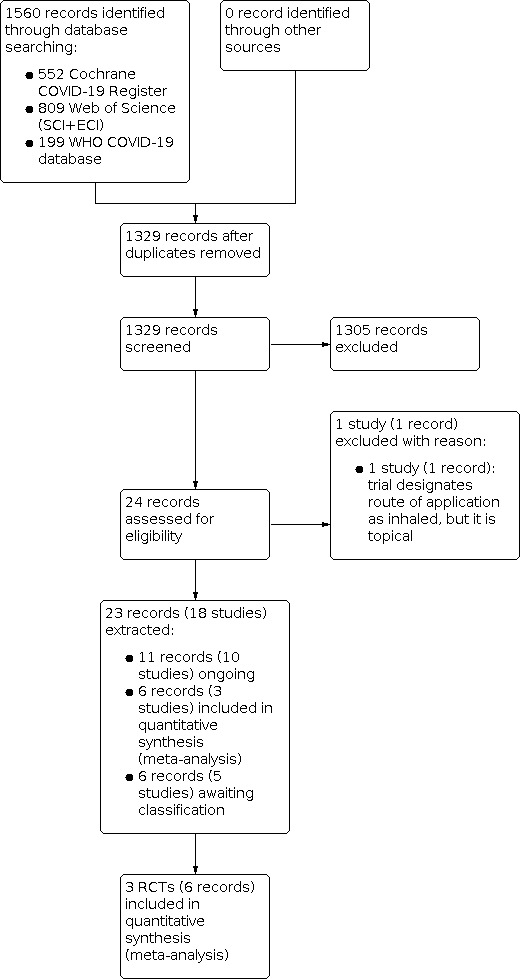
Included studies
See Characteristics of included studies table and Table 2.
1. Characteristics of included studies for the comparison: inhaled corticosteroid plus standard care versus standard care (with or without placebo).
| Study ID | Intervention and regimen | Control | Randomised to steroids and analysed in this review | Randomised to control and analysed in this review | Design | Setting | Population/disease severity at randomisation |
| Clemency 2021 | Ciclesonide 160 μg per actuation, 2 actuations twice daily (total daily dose 640 μg) + standard care | Placebo + standard care | 197 | 203 | Double‐blind RCT | Outpatient | Participants had an oxygen saturation of ≥ 93% on room air; had ≥ 1 of the following symptoms of COVID‐19: fever, cough, or dyspnoea |
| Ramakrishnan 2021 | Budesonide, 400 µg per actuation (2 puffs twice daily; total dose 1600 µg) + standard care (antipyretics and honey) | Standard care | 73 | 73 | Open‐label, RCT | Outpatient | With symptoms of COVID‐19 (new‐onset cough and fever or anosmia, or both) within 7 days |
| Yu 2021 | Budesonide 800 µg twice daily + standard care for 14 days | Standard care | 787 (analysed concurrent and SARS‐CoV‐2‐positive population) | 838 (analysed concurrent andSARS‐CoV‐2‐positive population) | Open‐label, platform trial | Outpatient | Ongoing symptoms of confirmed or suspected COVID‐19 (high temperature or new, continuous cough or change in sense of smell/taste, or a combination of these) within 14 days |
RCT: randomised controlled trial; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2.
Designs of the studies and publication status
None of the studies were cluster‐RCTs. We included three RCTs with 3607 participants, of whom 1343 were allocated to inhaled corticosteroids plus standard care and 2264 to standard care (with or without placebo). One open‐label, parallel‐group, phase 2 clinical trial allocated 146 participants of whom eight (6%) were PCR‐negative at randomisation (Ramakrishnan 2021); one open‐label, multi‐arm, adaptive platform RCT allocated 3061 participants to inhaled corticosteroids plus standard care or standard care (Yu 2021); one double‐blind placebo‐controlled trial allocated 400 participants eligible for inclusion if they had a positive SARS‐CoV‐2 molecular or antigen diagnostic sample (no information on percentage of PCR‐positive participants) (Clemency 2021).
From the allocated 3061 participants in Yu 2021, we used data from 1625 in this review. We restricted the analysis to participants who were randomised concurrently and were confirmed as SARS‐CoV‐2 positive. This was to reduce the risk of bias and indirectness arising from analysis of outcomes in participants included only on the basis of symptoms or who were randomised to usual care prior to the addition of budesonide. Comparing study participants recruited under different editions of the trial protocol, were treated according to changes in therapy or prophylaxis (e.g. vaccination) over time or were recruited in the context of other local regulations during the pandemic (e.g. contact restrictions, lockdown) and diagnostic options (e.g. access to PCR testing) would limit the applicability of evidence from this study.
The number of participants included in the analyses in this review was 2171. The number of participants analysed for the respective endpoints ranged between 139 and 2132 depending on the availability of data.
The three studies were performed as remote pragmatic outpatient trials partly relying on electronic self‐assessments and telephone calls. Ramakrishnan 2021 was performed in one centre in the community in Oxfordshire, UK, while two studies were multicentric: Yu 2021 with multisite and multimethods enrolment in the UK and Clemency 2021 in 10 centres in the US. All three included studies were performed in high‐income countries. However, the investigators of Yu 2021 undertook extensive community outreach to increase recruitment from ethnic minority and socially deprived communities.
All studies reported information on the responsible ethics committee and the financial support. Ramakrishnan 2021 and Yu 2021 were funded by departmental or governmental resources as well as non‐profit organisations: the National Institute for Health Research (NIHR) Biomedical Research Centre (Ramakrishnan 2021) and NIHR and United Kingdom Research Innovation (Yu 2021). Both studies also received support by AstraZeneca producing the intervention medication (Ramakrishnan 2021; Yu 2021, including individual authors). Clemency 2021 was funded by Covis Pharma GmbH producing the intervention medication.
Two trials were peer‐reviewed publications in indexed journals (Ramakrishnan 2021; Yu 2021); one study was available as a preprint at the time of the publication of this review (Clemency 2021). All included studies registered a study protocol prospectively.
Participants
All three studies recruited participants in the outpatient setting: in participating general medical practices (Ramakrishnan 2021; Yu 2021), via local COVID‐19 testing sites (Ramakrishnan 2021), as well online or by telephone (Yu 2021), and public and private academic and non‐academic centres (Clemency 2021). Positive PCR rates among the participants differed significantly across the studies. In Yu 2021, the virological confirmation of the SARS‐CoV‐2 infection (through PCR or rapid antigen test) was not mandatory to participate in the study and only 87% of all participants randomly assigned to budesonide, usual care alone, or other treatments were PCR tested. About 67% of those PCR‐tested participants and 82.2% of those concurrently randomised in the budesonide or usual care arms had a positive PCR‐test result. In this review, we analysed only PCR‐positive participants from the study Yu 2021 (SARS‐CoV‐2‐positive concurrent randomisation analysis population) to reduce risk of bias and indirectness. There is no information on how many participants who carried out a rapid antigen test. In Clemency 2021, the rate of positive PCR tests was not reported. However, all participants had a positive PCR or an antigen test, since it was the inclusion criterion of the study, and therefore could be defined as confirmed virologically. In Ramakrishnan 2021, 94% of participants were PCR‐positive.
In two studies all participants were adults (Ramakrishnan 2021; Yu 2021). In Clemency 2021, the participants had to be at least 12 years of age. In this study, the mean age of all participants was 43.3 (SD 16.89) years, 4% of all participants were aged less than 18 years. In Yu 2021, 36% (intervention group) and 36% (control group) of participants were between 50 and 64 years old. In Ramakrishnan 2021, the mean age of participants was 44 (range 19–71) years in the intervention group and 46 (range 19–79) years in the control group.
In Ramakrishnan 2021, the participants in both groups had, on average, one comorbidity, most frequently past or current history of asthma (16% in budesonide arm versus 14% in control arm). In Yu 2021, 80% of participants in each group (intervention group and control group) had one or more comorbidity. The most common comorbidity was high blood pressure requiring therapy in 45% participants. Ten percent of study participants had lung disease. The most common comorbidity in Clemency 2021 was arterial hypertension (23.9% of participants in intervention group and 20.7% of participants in the control group). In this, more people in the intervention group had type 2 diabetes mellitus and asthma than in the control group (diabetes mellitus: 11.2% in the intervention group versus 3.9% in the control group; P = 0.007; asthma: 9.1% in the intervention group versus 3.9% in the control group; P = 0.042).
Participants were randomised in a median of six days in Yu 2021 and three days in Ramakrishnan 2021. In Clemency 2021, the duration of symptoms prior to randomisation was not reported. However, the participants had to have a positive SARS‐CoV‐2 PCR or antigen diagnostic sample obtained in the previous 72 hours prior to randomisation. The most common symptoms in the SARS‐CoV‐2 concurrent randomisation population in Yu 2021 were feeling unwell (96.6%), cough (84.2%), myalgia (75.6%), shortness of breath (58.3%), and fever (51.9%). In Ramakrishnan 2021, it was cough (79% in the intervention group versus 70% in the control group), fever (70% in the intervention group versus 64% in the control group), and headache (57% in the intervention group versus 55% in the control group). In contrast to Yu 2021, shortness of breath had evolved only in 16% of the participants in each study arm, myalgia in 9% (intervention group) and 16% (control group). Clemency 2021 did not report the details of the symptoms at baseline.
Interventions and comparators
Two studies compared budesonide plus standard care versus standard care alone (Ramakrishnan 2021; Yu 2021). Budesonide was administered as a dry powder inhaler (Pulmicort Turbuhaler, AstraZeneca, Gothenburg, Sweden) at a dose of 400 μg per actuation (two puffs to be taken twice per day; total dose 1600 μg). In Yu 2021, the participants administered budesonide for 14 days, while in Ramakrishnan 2021, they were asked to stop taking the inhaler when they felt they had recovered (self‐reported symptom recovery) or if they achieved the primary outcome (COVID‐19‐related urgent care visits, including emergency department assessment or hospitalisation). As result, 79.9% of participants randomised to budesonide in this study reported taking budesonide for at least seven days.
Clemency 2021 compared ciclesonide metered‐dose inhaler (MDI) in addition to standard care to placebo plus standard care. Ciclesonide was administered in a dose of 160 μg per actuation, two actuations twice a day (total daily dose 640 μg). The duration of the therapy was 30 days. Placebo was not further specified in this study.
Standard care in Ramakrishnan 2021 included antipyretics for symptoms of fever (products containing paracetamol, or non‐steroidal anti‐inflammatory drugs such as aspirin and ibuprofen) and honey for symptoms of cough. In Yu 2021, standard care was specified as antipyretics and antibiotics if bacterial pneumonia was suspected. The concomitant therapy in Clemency 2021 included mostly antipyretics and only in a few cases antibiotics (5% of participants) and antivirals (1% of participants). Neutralising monoclonal antibodies were given only in one participant in the intervention group.
Outcomes
The primary outcome in Ramakrishnan 2021 was defined as COVID‐19‐related urgent care visits, including emergency department assessment or hospitalisation. Yu 2021 had two co‐primary endpoints measured within 28 days of randomisation: time to first reported recovery, defined as the first instance that a participant reported feeling recovered; and hospitalisation or death related to COVID‐19. In Ramakrishnan 2021, secondary outcomes included clinical recovery, defined by self‐reported time to symptom resolution; viral symptoms measured by the Common Cold Questionnaire (CCQ) 12 and the InFLUenza Patient‐Reported Outcome (FLUPro)13 questionnaire; blood oxygen saturations and body temperature; and SARS‐CoV‐2 viral load. In Yu 2021, secondary outcomes included the rating of how well participants felt (scale 1 to 10), time to sustained recovery, early sustained recovery, time to initial alleviation of symptoms, time to sustained alleviation of symptoms, time to initial reduction of severity of symptoms, contacts with health services, hospital assessment without admission, oxygen administration, ICU admission, mechanical ventilation, and WHO‐5 Well‐Being Index.
In Clemency 2021, the primary endpoint was time to alleviation of all COVID‐19‐related symptoms (cough, dyspnoea, chills, feeling feverish, repeated shaking with chills, muscle pain, headache, sore throat, and new loss of taste or smell) by day 30 as self‐reported in the participant's eDiary. Secondary endpoints included subsequent emergency department visits or hospital admissions for reasons attributable to COVID‐19, incidence of hospital admissions or death, all‐cause mortality, COVID‐19‐related mortality, impact on the time to hospital admission or death compared with placebo plus standard supportive care, alleviation of all COVID‐19‐related symptoms by days seven, 14, and 30 as well as additional secondary outcomes such as oxygen saturation levels, COVID‐19 viral load, and safety assessments.
Studies awaiting classification
We found five study registries with inhaled corticosteroids: two studies "completed" (Alsultan 2021; EUCTR2020‐001616‐18‐ES/NCT04355637), two studies terminated for insufficient recruitment (NCT04331054; NCT04435795), and one study with a preprint including results (Song 2021). See Table 3.
2. Characteristics of studies awaiting classification.
| Study ID | Sponsor/developer | Design | Population/disease severity | Setting | Intervention | Control | Number of participants | Status |
| Alsultan 2021 | Not stated | RCT | Excluded: expired or transmitted to ICU during the first 24 hours Included: oxygen saturation ≤ 93% |
Inpatient | Budesonide + supportive care | Supportive care | 49 | Completed |
| EUCTR2020‐001616‐18‐ES/NCT04355637 | Fundacion Clinic per a la Recerca Biomédica | RCT | Admitted for pneumonia (status #3 and #4 OMS scale) | Inpatient | Budesonide | Placebo | 300 | Completed |
| NCT04331054 | Assistance Publique – Hôpitaux de Paris | RCT | Hospitalised, but not admitted to ICU | Inpatient | SYMBICORT RAPIHALER + standard care | Standard care | 146 | Terminated (insufficient recruitment) |
| NCT04435795 | McGill University Health Centre/Research Institute of the McGill University Health Centre | RCT | Symptomatic adults positive by PCR for COVID‐19 within 5 days of enrolment with fever, cough, or shortness of breath | Outpatient | Ciclesonide intranasal and inhaled | Placebo | 215 | Terminated (could not meet target enrolment) |
| Song 2021 | National Research Foundation of Korea (NRF) grant [2020M3A9I2081699] and Korea University Guro Hospital grant (I2000171) | RCT | Low National Early Warning Score | Probably inpatient | Ciclesonide + hydroxychloroquine | Standard care | 61 | Setting unclear; no data for ciclesonide alone vs standard care |
COVID‐19: coronavirus disease 2019; ICU: intensive care unit; PCR: polymerase chain reaction; RCT: randomised controlled trial.
EUCTR2020‐001616‐18‐ES/NCT04355637 had planned to include 300 participants with moderate COVID‐19. Participants were treated with budesonide plus standard care and compared to participants treated with standard care (with/without placebo – unclear registry entry classifying the study as "open‐label" but with the use of a placebo). Song 2021 compared ciclesonide plus hydroxychloroquine to standard care and ciclesonide versus standard care in 61 participants with unclear indication for hospitalisation. For the comparison ciclesonide versus standard care, the study did not provide any data. The publication only provided data for participants in the intervention arm partly receiving a combination of ciclesonide and hydroxychloroquine, so that the observed effect cannot be attributed to the corticosteroids with certainty.
We contacted the authors of EUCTR2020‐001616‐18‐ES/NCT04355637, JRCTS031190269, and Song 2021 via email to obtain data from participants of interest but have not yet received a reply.
Ongoing studies
We identified 10 ongoing RCTs that compared inhaled corticosteroids versus standard care or placebo; see Table 4. Five studies were classified as "recruiting" or "ongoing" and the remaining five as "not yet recruiting", according to the study registration data. These 10 trials intend to recruit 4114 participants: 1426 participants with mild COVID‐19 and 2688 participants with moderate COVID‐19.
3. Characteristics of ongoing studies.
| Study ID | Sponsor/developer | Design | Population/disease severity | Setting | Intervention | Control | Number of participants | Status |
| NCT04193878 | Stanford University | Triple‐blind, RCT | Severe pneumonia defined as hospitalisation for acute (< 7 days) onset of symptoms (cough, sputum production, or dyspnoea) and radiographic evidence of pneumonia by chest radiograph or CT scan and evidence of systemic inflammation (temperature < 35 °C or > 38 °C or WBC count > or < upper or lower limits for site or procalcitonin > 0.5 μg/L), or known current immunosuppression preventing inflammatory response AND hypoxaemia defined as new requirement for supplemental oxygen with oxygen saturation < 90% on room air, ≤ 96% on ≥ 2 L/minute oxygen, or > 6 L/minute or NIV (regardless of oxygen saturation) at enrolment | Inpatient | Formoterol + budesonide | Placebo | 600 | Enrolling by invitation |
| NCT04381364 | Ola Blennow, St Goran's Hospital | Open‐label, RCT | People with PCR‐positive SARS‐CoV‐2 receiving supplemental oxygen therapy with start < 48 hours | Inpatient | Ciclesonide | Standard care | 446 | Recruiting |
| NCT04937543 | UPECLIN HC FM Botucatu Unesp | Open‐label, RCT | People with PCR‐positive SARS‐CoV‐2 with arterial pulse oximetry (oxygen saturation) saturation ≥ 92% in room air | Probably inpatient | Beclomethasone + standard care; beclomethasone/formoterol/glycopyrronium + standard care | Standard care | 260 | Not yet recruiting |
| NCT04356495 | University Hospital, Bordeaux | Open‐label, RCT | SARS‐CoV‐2‐confirmed cases with absence of criteria for hospitalisation or oxygen therapy | Outpatient | Ciclesonide | Vitamin D | 820 | Recruiting |
| JRCTS031190269 | Not reported | Open‐label, RCT | SARS‐CoV‐2‐positive cases with no apparent pneumonia due to COVID‐19 | Outpatient | Ciclesonide | Not reported | 90 | Not recruiting |
| CTRI/2020/04/024948 | Lady Hardinge Medical College | RCT | Presence of moderate COVID‐19 disease as defined by presence of pneumonia (clinical and radiological signs) with respiratory rate 15–30/minute or oxygen saturation 90–94% on room air (or both). PCR throat swab‐positive patients and contacts of confirmed COVID‐19 cases will be considered as a COVID‐19 case | Inpatient | Ciclesonide | Standard care | 120 | Not yet recruiting |
| CTRI/2020/10/028581 | Dr Tushar Patel, SPARSH Chest Disease Centre, 100B Swastik Society, Opposite Samved Hospital, Navrangpura, Ahmedabad | RCT | Asymptomatic people with mild symptoms of < 5 days of duration; oxygen saturation > 94% at room air |
Outpatient | Budesonide + standard care | Standard care | 1000 | Not yet recruiting |
| EUCTR2020‐002208‐37‐DK | Respiratory Research Unit 237, Hvidovre Hospital, Denmark | Double‐blind, RCT | People admitted to a COVID‐19 emergency department < 24 hours due to COVID‐19‐like symptoms | Outpatient | Ciclesonide | Placebo | 138 | Ongoing |
| NCT05054322 | University of Medicine and Pharmacy at Ho Chi Minh City | Open‐label, RCT | Having COVID‐related symptoms within 5 days prior to randomisation | Outpatient | Fluticasone + standard care | Standard care | 500 | Recruiting |
| NCT05055414 | Korea United Pharm. Inc. | Triple‐blind, RCT | New onset of symptoms suggestive of COVID‐19 (fever, cough, sore throat, etc.) or diagnosed with COVID‐19 within 7 days of participant being seen at visit 1 | Unclear, probably outpatient | Budesonide/arformoterol | Placebo | 140 | Not yet recruiting |
COVID‐19: coronavirus disease 2019; CT: computer tomography; NIV: non‐invasive ventilation; PCR: polymerase chain reaction; RCT: randomised controlled trial; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; WBC: white blood cell.
Five RCTs are designed to study the effect of ciclesonide, two of which will compare ciclesonide plus standard care versus standard care alone, one versus vitamin D, one versus placebo (both in addition to standard care), and one study does not define a comparator.
Three studies are designed to study budesonide: one RCT will compare budesonide plus formoterol plus standard care versus placebo plus standard care, one budesonide plus standard care versus standard care alone, and one budesonide plus arformoterol plus standard care versus placebo plus standard care.
The only study that investigates inhaled fluticasone in addition to standard care compares it against the standard care alone (NCT05054322).
One study compares beclomethasone or beclomethasone‐formoterol‐glycopyrronium plus standard care with standard care alone (NCT04937543).
Excluded studies
We excluded one study that did not meet our inclusion criteria as it designated the route of application as inhaled, but it was topical (IRCT20200522047542N1).
Risk of bias in included studies
We assessed the risk of bias for three RCTs that contributed 13 study results to nine outcomes for outpatient participants (Clemency 2021; Ramakrishnan 2021; Yu 2021).
The completed RoB 2 tool with responses to all assessed signalling questions is available online at: https://zenodo.org/record/6334453#.YiX55nrMJPZ.
Overall judgements for studies that included people with a confirmed diagnosis of moderate‐to‐severe COVID‐19
We found no studies with people with a confirmed diagnosis of moderate‐to‐severe COVID‐19.
Overall judgements for studies that included people with a confirmed diagnosis of asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
Overall risk of bias by study
From 13 study results, we rated six (46%) at low risk of bias, six (46%) at high risk of bias, and we had concerns for one study. Regarding the respective studies, from Clemency 2021 four low and two high risk results were included, from Ramakrishnan 2021 one high risk and one of some concerns and from Yu 2021 two low risk and three high risk.
Overall risk of bias by outcome
Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo)
We judged admission to hospital or death, adverse events and infections at low risk across studies. For the outcome all‐cause mortality, two of three studies were at low risk of bias and one study with some concerns due to missing prespecification of the outcome (Ramakrishnan 2021). The following outcomes received high risk of bias judgements: symptom resolution: all initial symptoms resolved at day 14 (Clemency 2021; Yu 2021), symptom resolution: all initial symptoms resolved at day 30 (Clemency 2021), symptom resolution: mean time to recovery (Ramakrishnan 2021), quality of life (Yu 2021) and serious adverse events (Yu 2021) due to measurement of the outcome and selection of the reported result. In case of unblinded study design and subjective outcomes, risk of bias was high (Ramakrishnan 2021; Yu 2021). Also, the withdrawal of consent after randomisation in an open‐label study is likely to be due to the experimental context. Two outcomes in one study were added only after the conduction of the study and not part of the initial outcome set in the study registration (Clemency 2021).
Effects of interventions
See: Table 1
People with confirmed diagnosis of moderate‐to‐severe COVID‐19
We found no RCTs reporting outcomes for people with moderate‐to‐severe disease treated with inhaled corticosteroids.
People with a confirmed diagnosis of asymptomatic SARS‐CoV‐2 infection or mild COVID‐19
We found no RCTs reporting outcomes for people with asymptomatic SARS‐CoV‐2 infection.
All included participants had a confirmed diagnosis of COVID‐19 and mild symptoms according to Marshall 2020's WHO clinical progression scale.
Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo)
The evidence profile is presented in Table 1. Regarding missing participant data, we judged all study results presented below at low risk of bias in domain 3 and assumed missingness at random in line with our methods without further investigation. Missing outcome data affected quality of the evidence for serious adverse events and adverse events.
All‐cause mortality at up to day 30
Three studies with 2132 participants reported data on all‐cause mortality (Clemency 2021; Ramakrishnan 2021; Yu 2021). For two studies, the observation period was 28 days (Ramakrishnan 2021; Yu 2021), and for one study, it was 30 days (Clemency 2021). Overall, 6/1057 participants in the intervention group died compared to 10/1075 participants in the control group. Inhaled corticosteroids may result in little to no difference in all‐cause mortality up to day 30 (RR 0.61, 95% CI 0.22 to 1.67; 9 deaths per 1000 in the control group versus 6 deaths per 1000 (95% CI 2 to 16 deaths per 1000) in the intervention group; random‐effects model; I2 not applicable;low‐certainty evidence; Analysis 1.1). We downgraded the certainty of evidence from high to low due to very serious imprecision (very low number of events, wide CI).
Subgroup analyses
There were insufficient data to conduct subgroup analyses taking into account sociodemographic characteristics (e.g. sex, age, ethnicity), comorbidities, different dosages, different timings, and vaccination status. The setting of all three studies was primary care in high‐income countries, therefore, no subgroup analysis related to these factors was possible.
Sensitivity analyses
We summarised the effects of sensitivity analyses in Table 5. As in Clemency 2021 and Ramakrishnan 2021, no participant had died, there was no difference in the overall effect estimate when we compared the fixed‐effect versus random‐effects model or excluded the preprint Clemency 2021. As we found only a low risk of bias or some concerns in our included RCTs for this outcome, there was no opportunity to exclude studies at high risk of bias for sensitivity analysis (see Table 5). Across all studies, approximately 5% of data were missing, but this was balanced between study arms. Due to the low number of missing data, we did not perform a sensitivity analysis.
4. Sensitivity analyses for the comparison: inhaled corticosteroids plus standard care versus standard care with or without placebo.
| Outcome | Main analyses | Risk of bias (excluding studies at high risk of bias) | Fixed‐effect vs random‐effects model | Preprint vs journal publication |
| All‐cause mortality at up to day 30 | RR 0.61, 95% CI 0.22 to 1.67; 3 studies, 2132 participants | There were no RCTs with high risk of bias concerning this outcome. | RR 0.61, 95% CI 0.22 to 1.67; 3 studies, 2132 participants | RR 0.61, 95% CI 0.22 to 1.67; 3 studies, 2132 participants |
CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio.
Admission to hospital or death within 30 days
Two studies with 2025 participants reported data on admission to hospital or death (Clemency 2021; Yu 2021). Inhaled corticosteroids probably reduce the admission to hospital or occurrence of death up to day 30 (RR 0.72, 95% CI 0.51 to 0.99; 79 events per 1000 in the control group versus 57 events per 1000 (95% CI 40 to 78 events per 1000) in the intervention group; random‐effects model; I2 = 0%; moderate‐certainty evidence; Analysis 1.2). We downgraded the certainty of evidence from high to moderate due to serious imprecision (calculation of the optimal information size for an 80% power: 3764 participants would have been needed).
1.2. Analysis.

Comparison 1: Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo), Outcome 2: Admission to hospital or death at up to 30 days
Symptom resolution: all initial symptoms resolved (asymptomatic) at day 14
Two studies with 1986 participants reported data on all initial symptoms resolved at day 14 (Clemency 2021; Yu 2021). Inhaled corticosteroids probably increase symptom resolution at day 14 (RR 1.19, 95% CI 1.09 to 1.30; 465 events per 1000 in the control group versus 553 events per 1000 (95% CI 507 to 605 events per 1000) in the intervention group; random‐effects model; I2 = 0%; moderate‐certainty evidence; Analysis 1.3). We downgraded the certainty of evidence from high to moderate for serious risk of bias.
1.3. Analysis.

Comparison 1: Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo), Outcome 3: Symptom resolution: all initial symptoms resolved (asymptomatic) at day 14
Symptom resolution: mean time to recovery (days)
Ramakrishnan 2021 reported a mean time to recovery for 129 participants. While the mean time in the budesonide group was 8 (SD 5) days, the standard care group needed 12 (SD 8) days. Inhaled corticosteroids may decrease the duration of symptom resolution (MD −4 days, 95% CI −6.22 to −1.78 days; 139 participants; random‐effects model; I2 not applicable; low‐certainty evidence; Analysis 1.4). Our main reasons for downgrading were serious risk of bias (one level) due to the very subjective outcome measured in an open‐label study, and serious imprecision (one level) given the low number of participants.
1.4. Analysis.

Comparison 1: Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo), Outcome 4: Symptom resolution: mean time to recovery (days)
Clemency 2021 reported the median time to recovery of 19 days (interquartile range (IQR) 14 to 21) for the ciclesonide group and 19 days (IQR 16 to 23) for the placebo group. As this study did not report the mean data, we did not include the results in the meta‐analyses.
Symptom resolution: all initial symptoms resolved (asymptomatic) at day 30
One study with 400 participants reported data on all initial symptoms resolved at day 30 (Clemency 2021). Overall, 139/197 participants in the intervention group felt recovered of all initial symptoms compared to 129/203 participants in the control group (RR 1.11, 95% CI 0.97 to 1.27; random‐effects model; I2 not applicable; Analysis 1.5).
1.5. Analysis.

Comparison 1: Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo), Outcome 5: Symptom resolution: all initial symptoms resolved at up to day 30
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) up to 28 days
Yu 2021 with 1434 participants reported quality of life at 28 days on the WHO‐5 Well‐Being Questionnaire with scores ranging from 0 to 100 (MD 2.60, 95% CI 0.02 to 5.18; random‐effects model; I2 not applicable; Analysis 1.6).
1.6. Analysis.

Comparison 1: Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo), Outcome 6: Quality of life at day 28: mean in well‐being (WHO‐5 Well‐Being Questionnaire)
Serious adverse events during the study period
One study with 1586 participants reported data on serious adverse events (Yu 2021). Overall, 2/787 participants in the intervention group had a serious adverse event compared to 4/799 participants in the control group. The evidence is very uncertain about the effect of inhaled corticosteroids on serious adverse events (RR 0.51, 95% CI 0.09 to 2.76; 5 events per 1000 in the control group versus 3 events per 1000 (95% CI 0 to 14 events per 1000) in the intervention group; I2 not applicable; random‐effects model; very low‐certainty evidence; Analysis 1.7). We downgraded the certainty of evidence from high to very low for very serious imprecision due to a very low number of events and a wide CI and for serious risk of bias as the other two studies did not report serious adverse events (Clemency 2021; Ramakrishnan 2021).
1.7. Analysis.

Comparison 1: Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo), Outcome 7: Serious adverse events
Adverse events (any grade) up to day 30
One study with 400 participants reported data on adverse events (any grade) (Clemency 2021). Overall, 22/197 participants in the intervention group had an adverse event compared to 29/203 participants in the control group. Inhaled corticosteroids may result in little to no difference in adverse events (RR 0.78, 95% CI 0.47 to 1.31; 143 events per 1000 in the control group versus 111 events per 1000 (95% CI 67 to 187 events per 1000) in the intervention group; I2 not applicable; random‐effects model; low‐certainty evidence; Analysis 1.8). We downgraded the certainty of evidence from high to low due to serious imprecision with a low number of events/participants and wide CI and for serious risk of bias as the other two studies did not report adverse events or did not report them in an adequate manner (Ramakrishnan 2021; Yu 2021).
1.8. Analysis.

Comparison 1: Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo), Outcome 8: Adverse events
Ramakrishnan 2021 reported five adverse events for 146 participants; all in the intervention arm. The manner of reporting suggests that only treatment‐related adverse events or side effects were reported. Therefore, we did not include the results in a meta‐analysis.
Infections during the study period
One study with 400 participants reported data on infections (Clemency 2021). Overall, 6/197 participants in the intervention group had an infection compared to 7/203 participants in the control group. Inhaled corticosteroids may result in little to no difference in infections (RR 0.88, 95% CI 0.30 to 2.58; 34 events per 1000 in the control group versus 30 events per 1000 (95% CI 10 to 89 events per 1000) in the intervention group; I2 not applicable; random‐effects model; low‐certainty evidence; Analysis 1.9). We downgraded the certainty of evidence from high to low for very serious imprecision due to very low number of events and wide CI.
1.9. Analysis.

Comparison 1: Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo), Outcome 9: Infections
Discussion
Summary of main results
We aimed to assess the efficacy and safety of inhaled corticosteroids for the treatment of COVID‐19. We found no studies for people with asymptomatic infection or confirmed diagnosis of COVID‐19 and moderate‐to‐severe disease.
For people with a confirmed diagnosis of COVID‐19 and mild disease, we identified three RCTs (3607 participants) evaluating inhaled corticosteroids compared to standard care (with or without placebo). Two RCTs (3207 participants) compared budesonide plus standard care to standard care alone, of which we analysed all cases with PCR‐confirmed diagnosis and being concurrently randomised (1625 participants), and one RCT (400 participants) compared ciclesonide plus standard care to standard care plus placebo.
Inhaled corticosteroids may result in little to no difference in all‐cause mortality in outpatients with mild COVID‐19 (low‐certainty evidence). We could not explore differences in the effect of inhaled corticosteroids based on sex, comorbidities, different timing, and different dosage due to insufficient data.
Inhaled corticosteroids probably reduce the risk of admission to hospital or death at up to day 30 (moderate‐certainty evidence). Further, the intervention probably increases the resolution of all initial symptoms at day 14 and may decrease the duration to symptoms resolved (low‐certainty evidence).
Regarding adverse events and infections, inhaled corticosteroids may result in little to no difference, and the certainty of evidence was low for both outcomes. In terms of serious adverse events, the evidence is very uncertain about the effect of inhaled corticosteroids (very‐low certainty evidence).
Overall completeness and applicability of evidence
The included studies were conducted in high‐income countries with 2171 participants in our analyses. The numbers available for analysis ranged between 139 and 2132 owing to availability of data. All studies investigated participants with mild disease in outpatient settings. Currently, there is no evidence for people with asymptomatic SARS‐CoV‐2 infection or inpatients with moderate‐to‐severe disease.
For our main efficacy outcomes, mortality and admission to hospital or death, the overall event rate was very low (less than 1% for mortality, less than 10% for admission to hospital or death), resulting in low‐ to moderate‐certainty evidence and very small differences in effects between the intervention and control group. In two of three RCTs, there were no deaths. We do not expect the evidence to change in the near future because completed but unpublished studies awaiting classification would only yield data on about 700 participants while our review is already comprising more than 2000 participants. Furthermore, the applicability of the evidence is unlikely to be enhanced by the ongoing studies that address similar populations and medicines to the studies we already have. Due to the living character of this review, it will be updated as soon as new relevant evidence arises.
With regard to diagnostic workup and timing of the intervention, we chose to analyse only those participants who had laboratory‐confirmed SARS‐CoV‐2 infection, and who had been allocated to intervention and control arms at the same time. This meant that we excluded a large amount of data from Yu 2021. We assumed that testing is feasible in almost every setting where the intervention would be available. It is likely that changes in epidemiology, legislation, standard care, and respective media coverage have occurred over time. This could affect the outcomes of interest in this review.
The weight of Yu 2021 in the meta‐analyses of mortality, hospital admission or death, and symptom resolution ranged from 88% to 100%. Given the large weight of this study, the generalisability of the review is influenced by the characteristics of the participants: aged 50 to 64 years with comorbidities or aged 65 years and older irrespective of comorbidities. However, participants who were unable to use the inhaler appropriately were excluded. This means that direct evidence can only be derived for people within this certain age group excluding the younger people (aged less than 50 years) and any people unable to use an inhaler. No prior use of inhaled corticosteroids was allowed, meaning that participants had no long‐term experience with how to use an inhaler correctly. This might have consequences on potential benefits and harms of the inhaler and the medicine it delivers, although it should be noted that the risk of systemic adverse effects is likely to be negligible even with poor inhaler technique (swallowing) because of the favourable pharmacokinetics of budesonide with low oral bioavailability and especially ciclesonide owing to its on‐site activation (Derendorf 2006).
Ramakrishnan 2021 and Yu 2021 administered budesonide using the Pulmicort Turbuhaler device, which might not be readily available worldwide. Moreover, ciclesonide, the corticosteroid intervention in Clemency 2021, is still protected by a patent and not available as a generic drug. Hence, both availability and pricing might be a barrier to its use on a global scale (Google Patent Overview Ciclesonide).
Moreover, the timing of the recruitment periods of the included studies may be affected by rapidly emerging knowledge in epidemiology and therapeutic interventions. Most data in this review has been collected before spring 2021 (Yu 2021: 27 November 2020 to 31 March 2021; Clemency 2021: 11 June 2020 to 3 November 2020; Ramakrishnan 2021: 16 July 2020 to 9 December 2020). Consequently, most participants were not fully vaccinated (13% received one dose and 1% received the second dose in Yu 2021, and no vaccinations in Clemency 2021 and Ramakrishnan 2021). Even if the absolute number of patients with mild disease and a severe course of COVID‐19 can be reduced through vaccination, we currently assume that the effects shown in the investigated population are also valid for fully vaccinated patients showing symptoms (i.e. WHO grade 3 or 4).
In contrast to SARS‐CoV‐2‐specific monoclonal antibodies, inhaled corticosteroids do not directly interact with the spike protein of the virus, and so different variants of the virus are unlikely to influence the potential benefits and harms of the inhaled corticosteroids. The delta variant of SARS‐CoV‐2 is more virulent than its predecessors, but pathogenicity appears to be similar or even favourable to the evaluated intervention. Together with the pleiotropic immunomodulatory effect of corticosteroids, we assume that in contrast to more targeted therapies also in future variants, evidence can be seen as direct. Delta variant virus particles have been shown in vitro to have a higher ACE2 receptor affinity and increased infectivity caused by a change in antigenic properties (Motozono 2021). The variant evades cellular immunity and increases infectivity. From this perspective, a reduction of the expression of the ACE2 receptor, which is postulated for inhaled corticosteroids (Finney 2021; Peters 2020), could be a promising mechanism of action against the delta variant of SARS‐CoV‐2. However, the potential enhanced effect needs to be verified in future RCTs.
Quality of the evidence
Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo)
We included data from three RCTs in the analysis of efficacy and safety of inhaled corticosteroids. The population of interest was people with a confirmed diagnosis of mild COVID‐19.
We rated the certainty of the evidence as moderate to very low (see Table 1). We downgraded the certainty of evidence due to risk of bias arising from the unblinded study design for all patient‐reported outcomes. However, we acknowledge that within trial platforms such as PRINCIPLE (Yu 2021), blinding is difficult to implement. Low number of events/imprecision affected all endpoints except symptom resolution at day 14 and led to downgrading by one point. In addition, missing safety outcome data (adverse events and serious adverse events) led to downgrading for reporting bias. At least the latter issue could easily be improved in future publications.
Potential biases in the review process
To avoid potential biases in the review process we did all steps in duplicate (e.g. screening search results, data extraction, bias assessment, assessing certainty using the GRADE approach) and had intense discussions involving both clinicians and methodologists to agree on data analyses and interpretation. As the definition of clinical outcomes such as symptom resolution varied across included studies it is highly relevant to involve clinical experts, not to misinterpret data.
In addition to peer‐reviewed, full‐text articles, we also included one preprint. The results of the sensitivity analyses did not differ between with or without preprint. We are aware of the potentially lower quality of preprint publications, and that results could change once the peer‐reviewed journal publications are available. In cases of missing data, we contacted study authors for additional data or relevant details if we needed more information. However, we have not yet received an answer. We are confident that we identified all relevant studies and will monitor ongoing studies as well as full publications of preprints closely after the publication of this review.
Since only a few data (about 5%) were missing across all studies, we do not expect this to result in a risk of bias.
Agreements and disagreements with other studies or reviews
We could not find current published RCT‐based peer‐reviewed systematic reviews focussing on inhaled corticosteroids in people without prior use. Apart from that, one the regularly updated living guideline for people with confirmed COVID‐19 of 24 September 2021 did not include the intervention (WHO 2021d). One living systematic review published in March 2021 only included the preprint of Ramakrishnan 2021 without further mention of inhaled corticosteroids (Siemieniuk 2020).
One living network meta‐analysis includes budesonide as an intervention, using data from Ramakrishnan 2021 and Yu 2021 in the analysis of mortality and clinical progression (COVID‐NMA 2021). Notably, they seemed to have included participants without laboratory‐confirmed testing or from asynchronous allocation from Yu 2021. The different choice of the cohort for analysis comes with implications for directness and applicability of the evidence. Irrespective of this different approach, COVID‐NMA 2021 assessed the certainty of the evidence similarly to our review. The main differences were to outcomes related to the different populations from the same included studies and the exclusion of the Clemency 2021 preprint.
Authors' conclusions
Implications for practice.
In people with confirmed COVID‐19 and mild symptoms who are able to use inhaler devices, we found moderate‐certainty evidence that inhaled corticosteroids probably reduce the combined endpoint of mortality or hospitalisation and increases symptom resolution at day 14. Low‐certainty evidence suggests that inhaled corticosteroids make little to no difference in all‐cause mortality at up to day 30 and decrease the duration to symptom resolution.
Due to heterogeneity in the reporting of serious adverse events across the studies, we do not know whether inhaled corticosteroids increase or decrease the risk of this outcome. There is low‐certainty evidence that inhaled corticosteroids may decrease infections.
Most practitioners in family and internal medicine are familiar with prescription, efficacy, adherence, and favourable safety profiles of inhaled corticosteroids in hyperinflammatory pulmonary disease. However, data for use in COVID‐19 are not as clear. Moreover, the data presented in our review only apply directly to a subpopulation of people with COVID‐19 with mild disease and within a group of adults aged 50 years and above who have not been using inhaled corticosteroids. The evidence we identified came from studies in high‐income settings prior to vaccination roll‐out and current variants (delta, omicron) of SARS‐CoV‐2, yet we assume that neither has a relevant effect on the expected benefits and harms.
Implications for research.
We noted the lack of safety data for serious adverse events, adverse events, and infections reported by included studies: only one evaluated serious adverse events for 1586 participants, only one randomised controlled trial (RCT) provided adequate information on adverse events and infections for 400 participants.
As studies reported a lack of compliance with the treatment, future assessment of patient‐reported experience measures (PREMs) could help to identify further unwanted effects or targets to improve compliance.
There was no information related to treatment of participants with asymptomatic SARS‐CoV‐2 infection that could be of special interest in countries with (temporally) reduced medical resources. Also, the use of inhaled corticosteroids for people with moderate‐to‐severe disease has not yet been examined. Studies in more severely ill populations should measure ventilator‐free days, or the occurrence of new invasive mechanical ventilation or death.
We suggest there is a need for further research into the impact of inhaled corticosteroids on quality of life in people with COVID‐19 and different dosages or different inhaled corticosteroids. We deem it crucial to use blinding through placebo‐control to reduce the risk of bias.
The 10 ongoing and five completed, unpublished RCTs we identified in trial registries address broadly similar questions to those in the published evidence to date. We expect to incorporate the findings of these studies in future versions of this review.
In line with our living approach to this work, we will monitor newly published results of RCTs on inhaled corticosteroids on a monthly basis and will update the review when the evidence or our confidence in the evidence changes.
What's new
| Date | Event | Description |
|---|---|---|
| 15 March 2022 | Amended | Affiliation of information specialist corrected under Methods‐Search methods for identification of studies |
History
Review first published: Issue 3, 2022
| Date | Event | Description |
|---|---|---|
| 11 March 2022 | Amended | Correction of typographical error in Background section |
Notes
Parts of the review's methods section and of the background were adopted from Cochrane Reviews published in the COVID‐19 series (Kreuzberger 2021; Piechotta 2021).
Risk of bias
Risk of bias for analysis 1.1 All‐cause mortality at up to day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Clemency 2021 | Low risk of bias | We judged this domain low as the randomisation process was adequate because it is done remotely and moreover consider concealment adequate. Slight baseline differences were most likely due to chance. | Low risk of bias | Deviations were balanced and most likely not due to the trial context in this double‐blinded trial. | Low risk of bias | Although there were around 5% of participants lost to follow‐up, it seems not probable that the outcome has been affected relevantly by those dropouts. | Low risk of bias | We judged low risk of bias, scheduled contact at day 30. | Low risk of bias | We judged this domain low due to pre‐specification of the outcome. | Low risk of bias | Overall, we judged low risk of bias. |
| Ramakrishnan 2021 | Low risk of bias | Stratified block randomisation, sequence held in secure network location at Oxford University, characteristics incl. SARS‐CoV‐2 status are well balanced. | Low risk of bias | We judged this domain low due to only a low number of participants withdrew consent. | Low risk of bias | We judged this domain low due to a small number of missing data. In the intervention group about 1% and in the control group about 4% of the participants withdrew consent due to the allocation. | Low risk of bias | We judged this domain low because the measurements were similar between groups. | Some concerns | We judged some concerns because the outcome was not pre‐specified. | Some concerns | Overall we judged some concerns due to missing pre‐specification of the outcome. |
| Yu 2021 | Low risk of bias | Participants were randomised via web‐based Sortition and the allocation sequence was intimated to GP and patient via email/letter. There are no baseline differences that would suggest a problem with randomisation | Low risk of bias | We judged risk of bias low because there were no reported deviations from intended interventions. | Low risk of bias | We judged this domain low because it is not likely that missingness in the outcome depended on its true value. There were about 5% of the participants without a diary entry. | Low risk of bias | We judged this domain low because the measurements were similar between groups. | Low risk of bias | The data were in accordance with the protocol, so that we assessed this domain low. | Low risk of bias | Overall judged low risk of bias. |
Risk of bias for analysis 1.2 Admission to hospital or death at up to 30 days.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Clemency 2021 | Low risk of bias | We judged this domain low as the randomisation process was adequate because it is done remotely and moreover consider concealment adequate. Slight baseline differences were most likely due to chance. | Low risk of bias | Deviations were balanced and most likely not due to the trial context in this double‐blinded trial. | Low risk of bias | Although there were around 5% of participants lost to follow‐up, it seems not probable that the outcome has been affected relevantly by those dropouts. | Low risk of bias | We judged low risk of bias, scheduled contact at day 30. | Low risk of bias | We judged this domain low due to pre‐specification of the outcome. | Low risk of bias | Overall, we judged low risk of bias. |
| Yu 2021 | Low risk of bias | Participants were randomised via web‐based Sortition and the allocation sequence was intimated to GP and patient via email/letter. There are no baseline differences that would suggest a problem with randomisation | Low risk of bias | We judged risk of bias low because there were no reported deviations from intended interventions. | Low risk of bias | We judged this domain low because it is not likely that missingness in the outcome depended on its true value. There were about 5% of the participants without a diary entry. | Low risk of bias | We judged this domain low because the measurements were similar between groups. | Low risk of bias | The data were in accordance with the protocol, so that we assessed domain 5 low. | Low risk of bias | Overall judged low risk of bias. |
Risk of bias for analysis 1.3 Symptom resolution: all initial symptoms resolved (asymptomatic) at day 14.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Clemency 2021 | Low risk of bias | We judged this domain low as the randomisation process was adequate because it is done remotely and moreover consider concealment adequate. Slight baseline differences were most likely due to chance. | Low risk of bias | Deviations were balanced and most likely not due to the trial context in this double‐blinded trial. | Low risk of bias | Although there were around 5% of participants lost to follow‐up, it seems not probable that the outcome has been affected relevantly by those dropouts. | Low risk of bias | We judged low risk of bias, as the combination of structured contacts and eDiary provided a high probability that the outcome would be detected. | High risk of bias | We judged this domain high due to a subsequent addition of this outcome to the trial protocol month after having completed the trial. | High risk of bias | Overall, we judged high risk of bias due to subsequent addition of this outcome (probably during the analysis process). |
| Yu 2021 | Low risk of bias | Participants were randomised via web‐based Sortition and the allocation sequence was intimated to GP and patient via email/letter. There are no baseline differences that would suggest a problem with randomisation | Low risk of bias | We judged risk of bias low because there were no reported deviations from intended interventions. | Low risk of bias | We judged this domain low because it is not likely that missingness in the outcome depended on its true value. There were about 5% of the participants without a diary entry. | High risk of bias | We judged this domain high because it is likely that the knowledge of the intervention has influenced the subjective self‐assessment of this outcome. Missing placebo control could introduce relevant confounding to subjective self‐assessment of this endpoint. | Low risk of bias | The data were in accordance with the protocol, so that we assessed domain 5 low. | High risk of bias | Overall judged high risk of bias due to measurement of the outcome. This introduces potential high risk of bias towards the experimental. |
Risk of bias for analysis 1.4 Symptom resolution: mean time to recovery (days).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ramakrishnan 2021 | Low risk of bias | Stratified block randomisation, sequence held in secure network location at Oxford University, characteristics incl. SARS‐CoV‐2 status are well balanced. | Low risk of bias | We judged this domain low due to only a low number of participants withdrew consent | Low risk of bias | We judged this domain low due to no evidence of relevant missing data. In the intervention group about 1% and in the control group about 4% of the participants withdrew consent due to the allocation. | High risk of bias | We judged this domain high due to the unanswered question when are the participants considered recovered and the outcome is very subjective. | Some concerns | We judged this domain some concerns because the outcome was not pre‐specified. | High risk of bias | Overall judged high risk of bias due to the unanswered question when are the participants considered recovered and the subjective perspective of the outcome. |
Risk of bias for analysis 1.5 Symptom resolution: all initial symptoms resolved at up to day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Clemency 2021 | Low risk of bias | We judged this domain low as the randomisation process was adequate because it is done remotely and moreover consider concealment adequate. Slight baseline differences were most likely due to chance. | Low risk of bias | Deviations were balanced and most likely not due to the trial context in this double‐blinded trial. | Low risk of bias | Although there were around 5% of participants lost to follow‐up, it seems not probable that the outcome has been affected relevantly by those dropouts. | Low risk of bias | We judged low risk of bias, as the combination of structured contacts and eDiary provided a high probability that the outcome would be detected. | High risk of bias | We judged this domain high due to a subsequent addition of this outcome to the trial protocol month after having completed the trial. | High risk of bias | Overall, we judged high risk of bias due to subsequent addition of this outcome (probably during the analysis process). |
Risk of bias for analysis 1.6 Quality of life at day 28: mean in well‐being (WHO‐5 Well‐Being Questionnaire).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Yu 2021 | Low risk of bias | Participants were randomised via web‐based Sortition and the allocation sequence was intimated to GP and patient via email/letter. There are no baseline differences that would suggest a problem with randomisation | Low risk of bias | We judged risk of bias low because there were no reported deviations from intended interventions. | Low risk of bias | We judged this domain low because it is not likely that missingness in the outcome depended on its true value. There were about 5% of the participants without a diary entry. | High risk of bias | We judged this domain high because it is likely that the knowledge of the intervention has influenced the subjective self‐assessment of this outcome. | Low risk of bias | The data were in accordance with the protocol, so that we assessed domain 5 low. | High risk of bias | Overall judged high risk of bias due to measurement of the outcome. |
Risk of bias for analysis 1.7 Serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Yu 2021 | Low risk of bias | Participants were randomised via web‐based Sortition and the allocation sequence was intimated to GP and patient via email/letter. There are no baseline differences that would suggest a problem with randomisation | Low risk of bias | We judged risk of bias low because there were no reported deviations from intended interventions. | Low risk of bias | We judged this domain low because it is not likely that missingness in the outcome depended on its true value. There were about 5% of the participants without a diary entry. | High risk of bias | We judged this domain high because it is likely that the knowledge of the intervention has influenced the subjective self‐assessment of this outcome. | Low risk of bias | The data were in accordance with the protocol, so that we assessed domain 5 low. | High risk of bias | Overall judged high risk of bias due to measurement of the outcome. |
Risk of bias for analysis 1.8 Adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Clemency 2021 | Low risk of bias | We judged this domain low as the randomisation process was adequate because it is done remotely and moreover consider concealment adequate. Slight baseline differences were most likely due to chance. | Low risk of bias | Deviations were balanced and most likely not due to the trial context in this double‐blinded trial. | Low risk of bias | Some adverse events lead to trial discontinuation. But they had been reported, so we do not assume, that the ~5% "lost to follow up" affected the outcome. | Low risk of bias | We judged low risk of bias, as the combination of structured contacts and eDiary provided a high probability that the outcome would have been detected. | Low risk of bias | We judged this domain low due to pre‐specification of the outcome. | Low risk of bias | Overall, we judged low risk of bias. |
Risk of bias for analysis 1.9 Infections.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Clemency 2021 | Low risk of bias | We judged this domain low as the randomisation process was adequate because it is done remotely and moreover consider concealment adequate. Slight baseline differences were most likely due to chance. | Low risk of bias | Deviations were balanced and most likely not due to the trial context in this double‐blinded trial. | Low risk of bias | Although there were around 5% of participants lost to follow‐up, it seems not probable that the outcome has been affected relevantly by those dropouts. | Low risk of bias | We judged low risk of bias, as the combination of structured contacts and eDiary provided a high probability that the outcome would be detected. | Low risk of bias | We judged this domain low due to pre‐specification of the outcome. | Low risk of bias | Overall, we judged low risk of bias. |
Acknowledgements
ALF, FF, and MG are thankful for the support of their colleagues with the Department of Anesthesia and Intensive Care at the University of Leipzig Medical Center (Leipzig, Germany) while working on the manuscript of this review during the COVID‐19 pandemic.
The research was part of a project supported by the German Federal Ministry of Education and Research (NaFoUniMedCovid19, funding number: 01KX2021; part of the 'CEOSys' project). The contents of this document reflect only the review authors' views and the German Ministry is not responsible for any use that may be made of the information it contains.
Cochrane Airways group supported us in the development of this review. The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Toby Lasserson, Cochrane Evidence Production & Methods Directorate.
Managing Editor (selected peer reviewers, provided comments, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Lara Kahale, Cochrane Central Editorial Service.
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service.
Copy Editor (copy‐editing and production): Anne Lawson, Cochrane Copy Edit Support.
Peer‐reviewers (provided comments and recommended an editorial decision): Syed Shahzad Hasan, University of Huddersfield, UK and Iain Crossingham, East Lancashire Hospitals NHS Trust, UK (clinical/content review); Stella Maria O'Brien (consumer review); Nuala Livingstone, Cochrane Evidence Production & Methods Directorate (methods review); and Elizabeth Stovold (search review).
Appendices
Appendix 1. Search strategies
The search strategy was used to inform two Cochrane Reviews (inhaled and systemic corticosteroids, therefore also including search terms related to systemic corticosteroids). Cochrane COVID‐19 Study Register
Search string:
corticosteroid* OR corticoid* OR prednison* OR dehydrocortison* OR deltason* OR decortin* OR orasone* OR deltra* OR meticorten* OR cortancyl* OR deltacorten* OR dacortin* OR adasone* OR "delta‐cortison" OR panasol* OR decorton* OR metacortandracin* OR paracort* OR predicor* OR decortisyl* OR delta‐1‐cortison* OR "delta‐dome" OR deltadehydrocortison* OR ofisolon* OR panafcort* OR predicorten* OR predni* OR econonson* OR promifen* OR servison* OR deltison* OR lisacort* OR meproson* OR rayos OR sterapred* OR "liquid pred" OR cortan* OR rectodelt* OR predeltin* OR prednisolon* OR methylprednisolon* OR medrol OR "pred forte" OR medrone OR urbason OR wyacort OR "Delta‐F" OR duralon* OR medrate OR omnipred OR adlone OR caberdelta OR depmedalon* OR "Depo Moderin" OR "Depo‐Nisolone" OR Emmetipi OR esameton* OR firmacort OR medlon* OR "Mega‐Star" OR meprolon* OR metilbetason* OR metrocort OR metypresol OR metysolon* OR orapred OR "Predni‐M‐Tablinen" OR radilem OR sieropresol OR solpredon* OR "A‐MethaPred" OR prelone OR medrone OR aprednislon OR pediapred OR hostacortin OR "Di‐Adreson‐F" OR adnisolon* OR capsoid OR cortalon* OR cortisolon* OR deltacortril OR estilsona OR panafcortelone OR sterane OR "Delta‐Cortef" OR econopred OR dacortin OR decaprednil OR "Delta‐Diona" OR "Delta‐Phoricol" OR deltahydrocortison* OR deltasolon* OR deltidrosol OR dhasolone OR fisopred OR frisolona OR gupison* OR hydeltra OR hydeltrasol OR klismacort OR kuhlprednon OR lenisolon* OR "Lepi‐Cortinolo" OR "Linola‐H" OR longiprednil OR metacortandralon* OR "Meti Derm" OR meticortelon* OR opredsone Or precortisyl OR "Pred‐Clysma" OR predeltilon* OR prenilone OR hydrocortancyl OR "Solu Moderin" OR predonin* OR metypred OR prednisol OR dexamethason* OR "BB 1101" OR decadron OR hexadrol OR fortecortin OR dexameth OR dexone OR hexadecadrol OR desamethason* OR ozurdex OR deronil OR baycuten OR aacidexam OR spersadex OR dexacortal OR gammacorten OR visumetazon* OR adexone OR "Alba‐Dex" OR cortidexason OR decacort OR decadrol OR dectancyl OR desameton OR loverine OR millicorten OR orgadrone OR alin OR auxiloson OR cortisumman OR decalix OR decameth OR decasone OR dekacort OR deltafluorene OR "Dexa‐Mamallet" OR dexafluorene OR dexalocal OR dexamecortin OR dexamonozon OR dexapos OR dexinoral OR fluorodelta OR lokalison OR methylfluorprednisolon* OR mymethason* OR "Dexa‐Rhinosan" OR "Dexa‐Scheroson" OR "Dexa‐sine" OR dexacortin OR dexafarma OR dinormon OR baycadron OR "Aeroseb‐Dex" OR Maxidex OR Dextenza OR dexasone OR dexpak OR hydrocortison* OR cortisol OR cortef OR hydrocorton* OR cetacort OR barseb OR aeroseb OR "Cort‐Dome" OR cortenema OR cortril OR cortifan OR cortispray OR dermacort OR domolene OR eldecort OR hautosone OR "Heb‐Cort" OR hytone OR Komed OR Nutracort OR Proctocort OR Rectoid OR Hydrocort OR locoid OR Solu‐Glyc OR glucocorticoid* OR alclometason* OR amcinonid* OR beclomethason* OR betamethason* OR budesonid* OR ciclesonid* OR clobetas* OR clocortolon* OR desoximetason* OR dichlorison* OR diflorason* OR diflucortolon* OR difluprednate OR drocinonid* OR flumethason* OR fluocinolon* OR fluocinonid* OR fluocortin OR fluocortolon* OR fluorometholon* OR fluperolon* OR flupredni* OR flurandrenolone* OR fluticason* OR FX006 OR halometason* OR medryson* OR melengestrol OR paramethason* OR rimexolon* OR terofenamat* OR triamcinolon* OR mometason*
Study characteristics: 1) "Intervention assignment": “Randomised” OR 2) "Study design": "Parallel/Crossover" AND "Unclear" OR 3) "Study type": "Adaptive/Platform" = 324 studies (552 references) Selections on the CCSR used to monitor RCTs and quasi RCTs on a weekly basis: Results available: "Report Results" Study type: "Interventional" Intervention assignment: "Randomised" or "Quasi Randomised"
Web of Science Core Collection (Advanced search)
#1 TI=( corticosteroid* OR corticoid* OR prednison* OR dehydrocortison* OR deltason* OR decortin* OR orasone* OR deltra* OR meticorten* OR cortancyl* OR deltacorten* OR dacortin* OR adasone* OR "delta‐cortison" OR panasol* OR decorton* OR metacortandracin* OR paracort* OR predicor* OR decortisyl* OR delta‐1‐cortison* OR "delta‐dome" OR deltadehydrocortison* OR ofisolon* OR panafcort* OR predicorten* OR predni* OR econonson* OR promifen* OR servison* OR deltison* OR lisacort* OR meproson* OR rayos OR sterapred* OR "liquid pred" OR cortan* OR rectodelt* OR predeltin* OR prednisolon* OR methylprednisolon* OR medrol OR "pred forte" OR medrone OR urbason OR wyacort OR "Delta‐F" OR duralon* OR medrate OR omnipred OR adlone OR caberdelta OR depmedalon* OR "Depo Moderin" OR "Depo‐Nisolone" OR Emmetipi OR esameton* OR firmacort OR medlon* OR "Mega‐Star" OR meprolon* OR metilbetason* OR metrocort OR metypresol OR metysolon* OR orapred OR "Predni‐M‐Tablinen" OR radilem OR sieropresol OR solpredon* OR "A‐MethaPred" OR prelone OR medrone OR aprednislon OR pediapred OR hostacortin OR "Di‐Adreson‐F" OR adnisolon* OR capsoid OR cortalon* OR cortisolon* OR deltacortril OR estilsona OR panafcortelone OR sterane OR "Delta‐Cortef" OR econopred OR dacortin OR decaprednil OR "Delta‐Diona" OR "Delta‐Phoricol" OR deltahydrocortison* OR deltasolon* OR deltidrosol OR dhasolone OR fisopred OR frisolona OR gupison* OR hydeltra OR hydeltrasol OR klismacort OR kuhlprednon OR lenisolon* OR "Lepi‐Cortinolo" OR "Linola‐H" OR longiprednil OR metacortandralon* OR "Meti Derm" OR meticortelon* OR opredsone Or precortisyl OR "Pred‐Clysma" OR predeltilon* OR prenilone OR hydrocortancyl OR "Solu Moderin" OR predonin* OR metypred OR prednisol OR dexamethason* OR "BB 1101" OR decadron OR hexadrol OR fortecortin OR dexameth OR dexone OR hexadecadrol OR desamethason* OR ozurdex OR deronil OR baycuten OR aacidexam OR spersadex OR dexacortal OR gammacorten OR visumetazon* OR adexone OR "Alba‐Dex" OR cortidexason OR decacort OR decadrol OR dectancyl OR desameton OR loverine OR millicorten OR orgadrone OR alin OR auxiloson OR cortisumman OR decalix OR decameth OR decasone OR dekacort OR deltafluorene OR "Dexa‐Mamallet" OR dexafluorene OR dexalocal OR dexamecortin OR dexamonozon OR dexapos OR dexinoral OR fluorodelta OR lokalison OR methylfluorprednisolon* OR mymethason* OR "Dexa‐Rhinosan" OR "Dexa‐Scheroson" OR "Dexa‐sine" OR dexacortin OR dexafarma OR dinormon OR baycadron OR "Aeroseb‐Dex" OR Maxidex OR Dextenza OR dexasone OR dexpak OR hydrocortison* OR cortisol OR cortef OR hydrocorton* OR cetacort OR barseb OR aeroseb OR "Cort‐Dome" OR cortenema OR cortril OR cortifan OR cortispray OR dermacort OR domolene OR eldecort OR hautosone OR "Heb‐Cort" OR hytone OR Komed OR Nutracort OR Proctocort OR Rectoid OR Hydrocort OR locoid OR Solu‐Glyc OR glucocorticoid* OR alclometason* OR amcinonid* OR beclomethason* OR betamethason* OR budesonid* OR ciclesonid* OR clobetas* OR clocortolon* OR desoximetason* OR dichlorison* OR diflorason* OR diflucortolon* OR difluprednate OR drocinonid* OR flumethason* OR fluocinolon* OR fluocinonid* OR fluocortin OR fluocortolon* OR fluorometholon* OR fluperolon* OR flupredni* OR flurandrenolone* OR fluticason* OR FX006 OR halometason* OR medryson* OR melengestrol OR paramethason* OR rimexolon* OR terofenamat* OR triamcinolon* OR mometason*) OR AB=( corticosteroid* OR corticoid* OR prednison* OR dehydrocortison* OR deltason* OR decortin* OR orasone* OR deltra* OR meticorten* OR cortancyl* OR deltacorten* OR dacortin* OR adasone* OR "delta‐cortison" OR panasol* OR decorton* OR metacortandracin* OR paracort* OR predicor* OR decortisyl* OR delta‐1‐cortison* OR "delta‐dome" OR deltadehydrocortison* OR ofisolon* OR panafcort* OR predicorten* OR predni* OR econonson* OR promifen* OR servison* OR deltison* OR lisacort* OR meproson* OR rayos OR sterapred* OR "liquid pred" OR cortan* OR rectodelt* OR predeltin* OR prednisolon* OR methylprednisolon* OR medrol OR "pred forte" OR medrone OR urbason OR wyacort OR "Delta‐F" OR duralon* OR medrate OR omnipred OR adlone OR caberdelta OR depmedalon* OR "Depo Moderin" OR "Depo‐Nisolone" OR Emmetipi OR esameton* OR firmacort OR medlon* OR "Mega‐Star" OR meprolon* OR metilbetason* OR metrocort OR metypresol OR metysolon* OR orapred OR "Predni‐M‐Tablinen" OR radilem OR sieropresol OR solpredon* OR "A‐MethaPred" OR prelone OR medrone OR aprednislon OR pediapred OR hostacortin OR "Di‐Adreson‐F" OR adnisolon* OR capsoid OR cortalon* OR cortisolon* OR deltacortril OR estilsona OR panafcortelone OR sterane OR "Delta‐Cortef" OR econopred OR dacortin OR decaprednil OR "Delta‐Diona" OR "Delta‐Phoricol" OR deltahydrocortison* OR deltasolon* OR deltidrosol OR dhasolone OR fisopred OR frisolona OR gupison* OR hydeltra OR hydeltrasol OR klismacort OR kuhlprednon OR lenisolon* OR "Lepi‐Cortinolo" OR "Linola‐H" OR longiprednil OR metacortandralon* OR "Meti Derm" OR meticortelon* OR opredsone Or precortisyl OR "Pred‐Clysma" OR predeltilon* OR prenilone OR hydrocortancyl OR "Solu Moderin" OR predonin* OR metypred OR prednisol OR dexamethason* OR "BB 1101" OR decadron OR hexadrol OR fortecortin OR dexameth OR dexone OR hexadecadrol OR desamethason* OR ozurdex OR deronil OR baycuten OR aacidexam OR spersadex OR dexacortal OR gammacorten OR visumetazon* OR adexone OR "Alba‐Dex" OR cortidexason OR decacort OR decadrol OR dectancyl OR desameton OR loverine OR millicorten OR orgadrone OR alin OR auxiloson OR cortisumman OR decalix OR decameth OR decasone OR dekacort OR deltafluorene OR "Dexa‐Mamallet" OR dexafluorene OR dexalocal OR dexamecortin OR dexamonozon OR dexapos OR dexinoral OR fluorodelta OR lokalison OR methylfluorprednisolon* OR mymethason* OR "Dexa‐Rhinosan" OR "Dexa‐Scheroson" OR "Dexa‐sine" OR dexacortin OR dexafarma OR dinormon OR baycadron OR "Aeroseb‐Dex" OR Maxidex OR Dextenza OR dexasone OR dexpak OR hydrocortison* OR cortisol OR cortef OR hydrocorton* OR cetacort OR barseb OR aeroseb OR "Cort‐Dome" OR cortenema OR cortril OR cortifan OR cortispray OR dermacort OR domolene OR eldecort OR hautosone OR "Heb‐Cort" OR hytone OR Komed OR Nutracort OR Proctocort OR Rectoid OR Hydrocort OR locoid OR Solu‐Glyc OR glucocorticoid* OR alclometason* OR amcinonid* OR beclomethason* OR betamethason* OR budesonid* OR ciclesonid* OR clobetas* OR clocortolon* OR desoximetason* OR dichlorison* OR diflorason* OR diflucortolon* OR difluprednate OR drocinonid* OR flumethason* OR fluocinolon* OR fluocinonid* OR fluocortin OR fluocortolon* OR fluorometholon* OR fluperolon* OR flupredni* OR flurandrenolone* OR fluticason* OR FX006 OR halometason* OR medryson* OR melengestrol OR paramethason* OR rimexolon* OR terofenamat* OR triamcinolon* OR mometason*)
#2 TI=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2") OR AB=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")
#3 #1 AND #2
#4 TI=(random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII") OR AB=(random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII")
#5 #3 AND #4
Indexes=SCI‐EXPANDED, ESCI Timespan=2020‐2021 = 809 references
WHO COVID‐19 Global literature on coronavirus disease
Title, abstract, subject: (corticosteroid* OR corticoid* OR prednis* OR hydrocorti* OR methylpredni* OR deltahydrocorti* OR dehydrocorti* OR dexameth* OR desameth* OR glucocorticoid* OR beclomethason* OR budesonid* OR ciclesonid* OR fluticason* OR mometason*) AND (random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII") ‐ without databases: ICTRP, MEDLINE, EMBASE, Web of Science, PMC, PubMed = 199 references
Data and analyses
Comparison 1. Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality at up to day 30 | 3 | 2132 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.22, 1.67] |
| 1.2 Admission to hospital or death at up to 30 days | 2 | 2025 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 0.99] |
| 1.3 Symptom resolution: all initial symptoms resolved (asymptomatic) at day 14 | 2 | 1986 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.09, 1.30] |
| 1.4 Symptom resolution: mean time to recovery (days) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Symptom resolution: all initial symptoms resolved at up to day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Quality of life at day 28: mean in well‐being (WHO‐5 Well‐Being Questionnaire) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.7 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.8 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.9 Infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1: Inhaled corticosteroids (plus standard care) versus standard care (with or without placebo), Outcome 1: All‐cause mortality at up to day 30
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Clemency 2021.
| Study characteristics | ||
| Methods | Trial design: multicentre, double‐blind, RCT Type of publication: preprint Setting: outpatient Recruitment dates: 11 June 2020 to 3 November 2020 Country: US Language: English Number of centres: 10 Trial registration number: NCT04377711 Date of trial registration: 5 May 2020 |
|
| Participants | Age: mean:
Sex:
Proportion of confirmed infections: positive SARS‐CoV‐2 molecular or antigen diagnostic sample was inclusion criteria Ethnicity: Asian, black or African American, Native Hawaiian or other Pacific Islander, white Number of participants:
Severity of condition according to study definition: participants had an oxygen saturation of ≥ 93% on room air Comorbidities: hypertension, drug hypersensitivity, hyperlipidaemia, type 2 diabetes mellitus, asthma Inclusion criteria:
Exclusion criteria:
Previous treatments: not reported |
|
| Interventions | Intervention group: ciclesonide 160 μg per actuation, 2 actuations twice a day (total daily dose 640 μg) + standard care Control group: placebo + standard care Concomitant therapy: paracetamol, NSAIDS, antibiotics, antivirals, monoclonal antibodies Duration of follow‐up: 30 days Treatment cross‐overs: no Compliance with assigned treatment: yes |
|
| Outcomes | Primary study outcome: time to alleviation of all COVID‐19‐related symptoms (cough, dyspnoea, chills, feeling feverish, repeated shaking with chills, muscle pain, headache, sore throat, and new loss of taste or smell) by day 30 Secondary outcomes: incidence of subsequent emergency department visits or hospital admissions for reasons attributable to COVID‐19, incidence of hospital admissions or death, all‐cause mortality, COVID‐19‐related mortality, percentage of participants with alleviation of COVID‐19‐related symptoms, time to hospital admission or death, alleviation of all COVID‐19‐related symptoms by days 7, 14, and 30, oxygen saturation levels, COVID‐19 viral load, and safety assessments |
|
| Notes | Date of publication: 12 September 2021 Sponsor/funding: Covis Pharma GmbH |
|
Ramakrishnan 2021.
| Study characteristics | ||
| Methods | Trial design: randomised, open‐label Type of publication: journal publication Setting: outpatient Recruitment dates: 16 July 2020 to 9 December 2020 Country: UK Language: English Number of centres: not reported Trial registration number: NCT04416399 Date of trial registration: 4 June 2020 |
|
| Participants | Age: mean:
Sex:
Proportion of confirmed infections: positive: 94% in the intervention group and 94% in the control group Ethnicity: white: 65 (93%) in the intervention group and 64 (93%) in the control group; non‐white: 5 (7%) in the intervention group and 5 (7%) in the control group Number of participants (recruited/allocated/evaluated): 146 recruited, of them 73 in the intervention group and 73 in the control group allocated and 70 in the intervention group and 69 in the control group evaluated Severity of condition according to study definition: with symptoms of COVID‐19 (new‐onset cough and fever or anosmia or both) within 7 days Comorbidities: cardiovascular disease, diabetes, past or current asthma Inclusion criteria:
Exclusion criteria:
Previous treatments (e.g. experimental drug therapies, oxygen therapy, ventilation): no |
|
| Interventions | Intervention group: inhaled budesonide 400 µg per actuation (2 puffs twice per day; total dose 1600 µg) + standard care Control group: standard care Concomitant therapy: antipyretics for symptoms of fever (products containing paracetamol, or NSAIDs such as aspirin and ibuprofen) and honey for symptoms of cough Duration of follow‐up: 14 days Treatment cross‐overs: no Compliance with assigned treatment: yes |
|
| Outcomes | Primary outcome: COVID‐19‐related urgent care visits, including emergency department assessment or hospitalisation Secondary outcome: clinical recovery, as defined by self‐reported time to symptom resolution; viral symptoms measured by the Common Cold Questionnaire (CCQ) 12 and the InFLUenza Patient‐Reported Outcome (FLUPro)13 questionnaire; blood oxygen saturations and body temperature; and SARS‐CoV‐2 viral load |
|
| Notes | Date of publication: 9 April 2021 Sponsor/funding: National Institute for Health Research Biomedical Research Centre and AstraZeneca |
|
Yu 2021.
| Study characteristics | ||
| Methods | Trial design: randomised platform trial Type of publication: journal publication Setting: outpatient Recruitment dates: 27 November 2020 to 31 March 2021 Country: UK Language: English Number of centres: not reported Trial registration number: ISRCTN86534580 Date of trial registration: 25 March 2021 |
|
| Participants | Age: mean
Sex:
Proportion of confirmed infections:
Missing: 7% in the intervention group and 13.6% in the control group Ethnicity:
Number of participants:
Severity of condition according to study definition: ongoing symptoms of confirmed or suspected COVID‐19 (high temperature or new, continuous cough or change in sense of smell/taste, or a combination of these) within 14 days Comorbidities: asthma, chronic obstructive pulmonary disease, lung disease, diabetes mellitus, heart problems, liver disease, stroke or neurological problem, hypertension requiring medication Inclusion criteria:
Exclusion criteria:
Previous treatments (e.g. experimental drug therapies, oxygen therapy, ventilation): no |
|
| Interventions | Intervention group: inhaled budesonide 800 µg twice daily for 14 days + standard care Control group: standard care (antipyretics, antibiotics) Concomitant therapy: no |
|
| Outcomes | Primary outcomes: time to self‐reported recovery, defined as the first instance that a participant reported feeling recovered from possible COVID‐19; hospitalisation or death or both (both within 28 days) Secondary outcomes: rating of how well participants felt (scale 1–10); time to sustained recovery (date participant first reported feeling recovered and subsequently remained well until 28 days); early sustained recovery (reported feeling recovered within the first 14 days from randomisation and remained recovered until day 28); time to initial alleviation of symptoms (date participant first reported all symptoms as minor or none); time to sustained alleviation of symptoms; time to initial reduction of severity of symptoms; contacts with health services; hospital assessment without admission; oxygen administration; intensive care unit admission; mechanical ventilation; WHO‐5 Well‐Being Index |
|
| Notes | Date of publication: 10 August 2021 Sponsor/funding: National Institute of Health Research and United Kingdom Research Innovation |
|
COVID‐19: coronavirus disease 2019; MDI: metered‐dose inhaler; NSAID: non‐steroidal anti‐inflammatory drug; RCT: randomised controlled trial; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; SD: standard deviation; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| IRCT20200522047542N1 | Designated route of application was inhaled but was topical. |
Characteristics of studies awaiting classification [ordered by study ID]
Alsultan 2021.
| Methods | Trial design: randomised trial Sample size: 49 Setting: inpatient Language: English Number of centres: 1 Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled budesonide 200 µg, twice daily for 5 days + supportive care Control group: supportive care (oxygen supplementation, vitamins, anticoagulants, dexamethasone, prone position, non‐invasive ventilation (CPAP or BIPAP), antibiotics, and fluids) Concomitant therapy: no |
| Outcomes | No distinction between primary and secondary outcomes: time of hospitalisation (mean), time on oxygen supplementation from admission to cure (mean), admission PiO2/FiO2 ratio vs discharge PiO2/FiO2 ratio (mean), admission oxygen saturation + oxygen supplementation vs discharge oxygen saturation (mean) |
| Notes | Recruitment status: completed Prospective completion date: not stated Date last update was posted: not stated Sponsor/funding: not stated |
EUCTR2020‐001616‐18‐ES/NCT04355637.
| Methods | Trial design: randomised, open‐label Sample size: 300 Setting: inpatient Language: English Number of centres: 10 centres listed "recruiting", another 4 centres listed "not recruiting" Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria
|
| Interventions | Intervention group: inhaled budesonide 800 µg Control group: placebo Concomitant therapy: no |
| Outcomes | Primary outcomes: proportion of participants with therapeutic failure (as a composite outcome: people requiring non‐invasive or invasive mechanical ventilation, high flow oxygen therapy, all‐cause mortality, or a combination) Secondary outcome: clinical evolution (discharge, ARDS, death, ICU refusal), temperature, heart rate, blood pressure, PaO2/FiO2, systemic biomarkers, duration of hospital stay, complications during admittance (infectious, cardiovascular, metabolic, others), all‐cause mortality, vital status, changes in clinical status (OMS 7‐point scale) |
| Notes | Recruitment status: completed Prospective completion date: not reported Date last update was posted: 22 September 2021 Sponsor/funding: Fundacion Clinic per a la Recerca Biomédica |
NCT04331054.
| Methods | Trial design: open‐label, RCT Sample size: 146 Setting: inpatient Language: English Number of centres: not reported Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled SYMBICORT RAPIHALER 2 puffs twice daily for 30 days + standard care Control group: standard care Concomitant therapy: no |
| Outcomes | Primary outcomes: time (in days) to clinical improvement within 30 days after randomisation, defined as the time from randomisation to an improvement of 2 points (from the status at randomisation) on a 7‐category ordinal scale or live discharge from the hospital, whichever came first within 30 days. The 7‐category ordinal scale consisted of the following categories:
These parameters will be evaluated daily during hospitalisations. Secondary outcomes: mortality rate at day 30; time (in days) from randomisation to death up to 30 days; number of days alive outside ICU within 30 days; number of days alive free of invasive or non‐invasive ventilation within 30 days; number of days alive with oxygen therapy within 30 days; maximal oxygen rate within 30 days; difference between PaO2/FiO2 ratio at randomisation and day 7 (or at the time of stopping oxygen therapy or discharge if occurs before day 7); number of days alive outside hospital within 30 days; use of antibiotics for respiratory (confirmed or suspected) infection within 30 days; difference between C‐reactive protein levels at randomisation and day 7 (or at the time of discharge if occurs before day 7) Safety outcomes included events that occurred during treatment, serious adverse events, and premature discontinuation of treatment up to 30 days after randomisation |
| Notes | Recruitment status: terminated (insufficient recruitment) Prospective completion date: 28 May 2021 Date last update was posted: 3 August 2021 Sponsor/funding: Assistance Publique – Hôpitaux de Paris |
NCT04435795.
| Methods | Trial design: randomised controlled, triple‐blind trial Sample size: 215 Setting: outpatient Language: English Number of centres: 1 Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: intranasal ciclesonide 50 μg twice daily to each nostril and inhaled ciclesonide 600 μg twice daily for 14 days Control group: intranasal normal saline twice daily and inhaled placebo 3 puffs by MDI twice daily Concomitant therapy: no |
| Outcomes | Primary outcome: proportion of participants with no symptoms of cough, fever, or dyspnoea at day 7 Secondary outcomes: proportion of participants with no symptoms of cough, fever, or dyspnoea at day 14; overall feeling – proportion who reporting they are "very much improved" or "much improved" at day 7; overall feeling – proportion who reporting they are "very much improved" or "much improved" at day 14; improvement in dyspnoea at days 7 and 14 (dyspnoea defined as reporting "shortness of breath" or "chest congestion" or "chest tightness". In those who reported dyspnoea at baseline, resolution will be defined as having no symptoms in these 3 areas); Visual Analog scale for Cough – wet or dry cough at days 7 and 14; hospitalisation for SARS‐CoV‐2 and hospitalisation for SARS‐CoV‐2‐related illness at day 14; 2‐point change in PROMIS Dyspnoea Severity Scale at days 7 and 14; 2‐point changes in PROMIS Dyspnoea Severity Scale at days 7 and 14; incidence of new oxygen use during the trial (defined as oxygen use not present at randomisation) at day 14; all‐cause mortality at days 14 and 29; PROMIS Anxiety 7a scale 1–5 (1 = never had symptoms and 5 = always had symptoms) at days 7 and 14; PROMIS Sleep Disturbance 4a scale from 1 to 5 (1 = very good and 5 = very poor) at days 7 and 14 |
| Notes | Recruitment status: terminated (could not meet target enrolment) Prospective completion date: 8 June 2021 Date last update was posted: 30 July 2021 Sponsor/funding: McGill University Health Centre/Research Institute of the McGill University Health Centre |
Song 2021.
| Methods | Trial design: open‐label, randomised Type of publication: journal publication Setting: inpatient Recruitment dates: 8 May 2020 to 31 March 2021 Country: South Korea Language: English Number of centres: 6 Trial registration number: NCT04330586 Date of trial registration: 31 March 2020 |
| Participants | Age: mean:
Sex:
Proportion of confirmed infections: 100% (confirmed by qRT‐PCR) Ethnicity: not stated Number of participants:
Severity of condition according to study definition: low NEWS Comorbidities: diabetes, hypertension, cerebrovascular diseases Inclusion criteria:
Exclusion criteria:
Previous treatments (e.g. experimental drug therapies, oxygen therapy, ventilation): no |
| Interventions | Intervention group: inhaled ciclesonide 320 µg twice daily for 14 days Control group: standard care Concomitant therapy: standard care (intravenous fluid, supplementary oxygen, antibiotics) Duration of follow‐up: 14 days Treatment cross‐overs: no Compliance with assigned treatment: yes |
| Outcomes | Primary outcome: SARS‐CoV‐2 eradication rate based on qRT‐PCR on day 14 Secondary outcomes: SARS‐CoV‐2 eradication rate based on at days 7 and 10 from study enrolment; rate of clinical improvement (resolution of all systemic and respiratory symptoms) at days 7, 10, and 14 from study enrolment; rate of clinical failure within 28 days; safety/tolerability of ciclesonide |
| Notes | Date of publication: 12 August 2021 Sponsor/funding: National Research Foundation of Korea (NRF) grant [2020M3A9I2081699] and Korea University Guro Hospital grant (I2000171) |
ARDS: acute respiratory distress syndrome; BIPAP: bilevel positive airway pressure; COVID‐19: coronavirus disease 2019; CPAP: continuous positive airway pressure; CT: computer tomography; ICU: intensive care unit; IL: interleukin; NEWS: National Early Warning Score; OMS: ordinal measurement scale; PCR: polymerase chain reaction; PiO2/FiO2: inspired oxygen tension/fraction of inspired oxygen; PaO2/FiO2: arterial oxygen partial pressure/fraction of inspired oxygen; PROMIS: Patient‐Reported Outcomes Measurement Information System; qRT‐PCR: quantitative reverse transcription polymerase chain reaction; RCT: randomised controlled trial; RT‐PCR: real‐time polymerase chain reaction.
Characteristics of ongoing studies [ordered by study ID]
CTRI/2020/04/024948.
| Study name | Efficacy of hydroxychloroquine, ciclesonide and ivermectin in treatment of moderate COVID‐19 illness: an open‐label randomised controlled study |
| Methods | Trial design: randomised, parallel‐group trial Sample size: 120 Setting: inpatient Language: English Number of centres: 1 Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled ciclesonide 200 μg twice daily for 7 days Control group: supportive management as per national guidelines Concomitant therapy: no |
| Outcomes | Primary outcome: proportion of people having virological cure on day 6 of treatment initiation Secondary outcomes: proportion of people with resolution of symptoms/signs on days 7 and 14; proportion of rescue criteria on days 7 and 14; adverse effects on days 7 and 14 |
| Starting date | 15 May 2020 |
| Contact information | Anupam Prakash Department of Medicine Lady Hardinge Medical College Shahid Bhagat Singh Marg New Delhi 110001 India prakashanupam@hotmail.com |
| Notes | Recruitment status: not yet recruiting Prospective completion date: not reported Date last update was posted: 30 April 2020 Sponsor/funding: Lady Hardinge Medical College |
CTRI/2020/10/028581.
| Study name | Clinical trial to study the effect of budesonide taken through inhalation in mild COVID cases |
| Methods | Trial design: randomised, parallel‐group, controlled trial Sample size: 1000 Setting: outpatient Language: English Number of centres: 4 Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled Rotacaps or Dry powder inhalation of budesonide 200 μg twice daily + standard care for 10–14 days depending on onset of symptoms Control group: standard care for mild COVID‐19 cases as provided by the centre Concomitant therapy: no |
| Outcomes | Primary outcome: hospitalisation with time points of 10–14 days depending on the onset of symptoms Secondary outcomes: none |
| Starting date | 23 October 2020 |
| Contact information | Dr Tushar Patel SPARSH Chest Disease Centre, 100B Swastik Society, Opposite Samved Hospital, Navrangpura, Ahmedabad Respiratory Medicine Department, GCS Hospital, Near Chamunda Bridge, Naroda road, Ahmedabad 380025 Ahmadabad GUJARAT 380009 India drtusharpatel@yahoo.com |
| Notes | Recruitment status: not yet recruiting Prospective completion date: not reported Date last update was posted: 23 October 2020 Sponsor/funding: Dr Tushar Patel, SPARSH Chest Disease Centre, 100B Swastik Society, Opposite Samved Hospital, Navrangpura, Ahmedabad |
EUCTR2020‐002208‐37‐DK.
| Study name | CIMMCov: a randomised clinical study for prevention of severe disease in mild‐to‐moderate COVID‐19 patients, using the inhaled medication ciclesonide |
| Methods | Trial design: randomised, controlled, double‐blind trial Sample size: 138 Setting: outpatient Language: English Number of centres: not reported Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled ciclesonide 320 μg, twice daily Control group: placebo: inhalation vapour, liquid Concomitant therapy: no |
| Outcomes | Primary outcome: reduction in healthcare resource utilisation (defined as renewed contact to general practitioner, emergency department, admission to hospital, or a combination) due to COVID‐19‐related symptoms at 28 days Secondary outcomes: time to clinical recovery; intensive care unit admission rate; all‐cause hospitalisation at days 14 and 28; COVID‐specific hospitalisation at days 14 and 28; symptom burden, measured as change in Asthma Control Questionnaire, the COPD Assessment Test, and St George Respiratory Questionnaire score at days 1, 14, 28 days; proportion of participants receiving mechanical ventilation during hospitalisations; mortality at days 14, 28, and 90; safety and tolerability of the study drug (number of adverse effects, proportion of early discontinuation) |
| Starting date | 29 April 2021 |
| Contact information | |
| Notes | Recruitment status: ongoing Prospective completion date: not reported Date last update was posted: not reported Sponsor/funding: Respiratory Research Unit 237, Hvidovre Hospital, Denmark |
JRCTS031190269.
| Study name | A multicenter, open‐label, randomised controlled phase II study to evaluate the efficacy and safety of inhaled ciclesonide for asymptomatic and mild patients with COVID‐19 (RACCO trial) |
| Methods | Trial design: randomised, open‐label Sample size: 90 Setting: outpatient Language: English/Japanese Number of centres: "multicentre" Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled ciclesonide 400 µg 3 times per day Control group: not reported Concomitant therapy: not reported |
| Outcomes | Primary outcome: pneumonia incidence on day 8 of ciclesonide inhalation Secondary outcomes: changes in clinical findings; changes in laboratory findings; SARS‐CoV‐2 virus genome amount; incidence rates of adverse events |
| Starting date | 3 April 2020 |
| Contact information | Sugiyama Haruhito: hasugiya@hosp.ncgm.go.jp |
| Notes | Recruitment status: "not recruiting" Prospective completion date: not reported Date last update was posted: 24 September 2020 Sponsor/funding: not reported |
NCT04193878.
| Study name | Arrest respiratory failure from pneumonia (ARREST PNEUMONIA) |
| Methods | Trial design: randomised, controlled, triple‐blind trial Sample size: 600 Setting: inpatient Language: English Number of centres: 10 academic medical centres Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled aerosolised formoterol 20 μg/2 mL and budesonide 1.0 mg/2 mL every 12 hours for 10 doses. Pulmicort Respules (budesonide) and Perforomist (formoterol) Control group: placebo. aerosolised saline 4 mL of 0.9% saline twice daily for up to 5 days Concomitant therapy: no |
| Outcomes | Primary outcome: acute respiratory failure within 7 days of randomisation; HFNC or NIV (or both) use for > 36 hours OR invasive mechanical ventilation for > 36 hours OR death in a patient placed on respiratory support (HFNC, NIV, ventilator) who dies before 36 hours Secondary outcome: hospital length of stay within 60 days of randomisation; duration of need for supplemental oxygen within 60 days of randomisation; proportion of patients intubated for respiratory failure within 7 days of randomisation |
| Starting date | 1 June 2020 |
| Contact information | Principal Investigator: Joe Levitt, MD Stanford University Principal Investigator: Emir Festic, MD Mayo Clinic |
| Notes | Recruitment status: enrolling by invitation Prospective completion date: 1 April 2024 Date last update was posted: 19 March 2021 Sponsor/funding: Stanford University |
NCT04356495.
| Study name | Trial of COVID‐19 outpatient treatment in individuals with risk factors for aggravation (COVERAGEFrance) |
| Methods | Trial design: multicentre, open‐label, RCT Sample size: 820 Setting: outpatient Language: English Number of centres: 11 centres Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled ciclesonide 160 µg (2 puffs twice a day) for 10 days Control group: vitamin supplement ("AZINC forme et vitalité"), 2 tablets daily for 10 days Concomitant therapy: no |
| Outcomes | Primary outcomes: Pilot phase: proportion of participants who had a grade 3 or 4 adverse event from inclusion (day 0) to day 14 Efficacy phase: death from inclusion (day 0) to day 14; proportion of participants with an occurrence of death Efficacy phase: oxygen therapy from inclusion (day 0) to day 14; proportion of participants who had an indication for oxygen therapy Efficacy phase: hospitalisation from inclusion (day 0) to day 14; proportion of participants who had an indication for hospitalisation Secondary outcomes: proportion of hospitalisations, overall and by cause from inclusion (day 0) to day 28; death and causes of death from inclusion (day 0) to day 28; proportion of intensive care hospitalisations, overall and by cause from inclusion (day 0) to day 28; proportion of participants with negative SARS‐CoV‐2 RT‐PCR at day 7; haematological markers (white blood count, prothrombin level, INR) evolution from inclusion (day 0) to day 7; inflammatory markers evolution (PCT, CRP) from inclusion (day 0) to day 7; number and proportion of grade 1, 2, 3, 4 adverse events from inclusion (day 0) to day 28; number and proportion of grade 1, 2, 3, 4 adverse reactions from inclusion (day 0) to day 28; acceptability of treatment assessed by interview from inclusion (day 0) to day 10; proportion of participants who received ≥ 1 day of antibiotic therapy from inclusion (day 0) to day 28; proportion of participants who experienced a worsening of oxygen saturation from inclusion (day 0) to day 28; proportion of participants who completed the prescribed protocol treatment from inclusion (day 0) to day 10 |
| Starting date | 29 July 2020 |
| Contact information | Denis MALVY, denis.malvy@chu‐bordeaux.fr Xavier ANGLARET, xavier.anglaret@u‐bordeaux.fr |
| Notes | Recruitment status: recruiting Prospective completion date: 29 January 2022 Date last update was posted: 3 August 2021 Sponsor/funding: University Hospital, Bordeaux, France |
NCT04381364.
| Study name | Inhalation of ciclesonide for patients with COVID‐19: a randomised open treatment study (HALT COVID‐19) |
| Methods | Trial design: randomised, multicentre, open‐label Sample size: 446 Setting: inpatient Language: English Number of centres: 6 centres recruiting Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled ciclesonide 320 µg twice daily for 14 days Control group: standard care Concomitant therapy: no |
| Outcomes | Primary outcome: duration of received supplemental oxygen therapy 30 days after study inclusion Secondary outcome: invasive mechanical ventilation or all‐cause death (key secondary outcome) 30 days after study inclusion; all‐cause mortality 30 days after study inclusion; invasive mechanical ventilation 30 days after study inclusion; remaining dyspnoea symptoms at 30–35 days and 5–7 months after inclusion |
| Starting date | 29 May 2020 |
| Contact information | Daniel Brodin, MD; +46736313527; daniel.brodin@capiostgoran.se Daniel P Andersson, MD, PhD; +46704490004; daniel.p.andersson@ki.se |
| Notes | Recruitment status: recruiting Prospective completion date: 1 December 2021 Date last update was posted: 10 May 2021 Sponsor/funding: Ola Blennow, St Goran's Hospital, Stockholm, Sweden |
NCT04937543.
| Study name | Efficacy of inhaled therapies in the treatment of acute symptoms associated with COVID‐19 (TRIVID) |
| Methods | Trial design: open‐label, RCT Sample size: 260 Setting: probably inpatient Language: English Number of centres: not reported Type of intervention: treatment |
| Participants | Inclusion criteria:
|
| Interventions | Intervention group: inhaled beclomethasone (dose not stated) + standard care; beclomethasone/formoterol/glycopyrronium (dose not stated) + standard care Control group: standard care Concomitant therapy: no |
| Outcomes | Primary outcome: proportion of participants using health resources 28 days after treatment Secondary outcome: airway obstruction using spirometry 28 days after treatment; small airway obstruction using CT 28 days after treatment |
| Starting date | 28 June 2021 |
| Contact information | Suzana Minamoto: suzana.tanni@unesp.br Luana Pagan: luanapagan@alunos.fmb.unesp.br |
| Notes | Recruitment status: not yet recruiting Prospective completion date: 30 October 2021 Date last update was posted: 30 June 2021 Sponsor/funding: UPECLIN HC FM Botucatu Unesp |
NCT05054322.
| Study name | FLuticasone in cOvid Treatment (FLOT) |
| Methods | Trial design: open‐label, RCT Sample size: 500 Setting: outpatient Language: English Number of centres: not reported Type of intervention: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled fluticasone 125 μg with spacer, 4 puffs, twice a day + standard care Control group: standard care Concomitant therapy: no |
| Outcomes | Primary outcome: incidence of adverse outcomes day 28 after randomisation Secondary outcomes: duration of isolation based on WHO's criteria day 28 after randomisation; incidence of patients with oxygen saturation by pulse oximetry (oxygen saturation) < 94% day 28 after randomisation; self‐reported recovery rate day 28 after randomisation |
| Starting date | 22 September 2021 |
| Contact information | Thi Tuyet Lan Le, PhD, MD; tuyetlanyds@gmail.com |
| Notes | Recruitment status: recruiting Prospective completion date: 31 January 2022 Date last update was posted: 23 September 2021 Sponsor/funding: University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam |
NCT05055414.
| Study name | Arformoterol/budesonide for COVID‐19 (ABC) |
| Methods | Trial design: randomised, controlled, triple‐blind trial Sample size: 140 Setting: unclear, probably outpatient Language: English Number of centres: not reported Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: inhaled budesonide/arformoterol dry powder inhaler 3 inhalations twice daily at 3 days and 2 inhalations twice daily at 11 days Control group: placebo for 2 weeks Concomitant therapy: no |
| Outcomes | Primary outcome: time to clinical improvement on WHO Ordinal Scale at 28 days Secondary outcomes: WHO Ordinal Scale for Clinical Improvement at 28 days; WHO Ordinal Scale change at 28 days; clinical cure rate at 28 days |
| Starting date | 1 November 2021 |
| Contact information | Korea United Pharm Inc. |
| Notes | Recruitment status: not yet recruiting Prospective completion date: 1 October 2022 Date last update was posted: 24 September 2021 Sponsor/funding: Korea United Pharm. Inc. |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; COPD: chronic obstructive pulmonary disease; COVID‐19: coronavirus disease 2019; CRP: C‐reactive protein; CT: computer tomography; GFR: glomerular filtration rate; HFNC: high flow nasal cannula; INR: international normalised ratio; NIV: non‐invasive ventilation; PCR: polymerase chain reaction; PCT: procalcitonin; RCT: randomised controlled trial; RT‐PCR: real‐time polymerase chain reaction; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; WHO: World Health Organization.
Differences between protocol and review
Title
For simplification and clarification we deleted "prevention" from the registered title because we did not intend to investigate inhaled corticosteroids as primary prevention in uninfected people. In line with other COVID‐19 reviews of this series, we refer to treatment of COVID‐19 at any stage of the disease including the asymptomatic infection.
Types of studies
We added that cross‐over trials in acute COVID‐19 would have been excluded because of the short duration of the disease and potential carry‐over effect; however, we did not identify any.
Types of outcomes
In review updates, we will add patient‐reported experience measures (PREMS) as an outcome, as suggested by the consumer editor during the review process.
Summary of findings table
We omitted symptom resolution: all initial symptoms resolved at day 30 from the summary of findings table and abstract. To further reduce the number of outcomes in the table to seven, we also omitted quality of life.
Contributions of authors
MG: data extraction, risk of bias assessment, characteristics of included studies, meta‐analysis, writing of the review, clinical expertise, taking responsibility for reading and checking the review before submission.
CW: screening, data extraction, risk of bias assessment, characteristics of included studies, characteristics of ongoing studies, characteristics of studies awaiting classification, meta‐analysis, writing of the review, taking responsibility for reading and checking the review before submission.
AM: clinical expertise, writing of the review, extraction, taking responsibility for reading and checking the review before submission.
MS: clinical expertise, checking characteristics of ongoing studies, taking responsibility for reading and checking the review before submission.
FF: clinical expertise, securing the funding, taking responsibility for reading and checking the review before submission.
MM: design and conduct of searches, drafting of search methods section, taking responsibility for reading and checking the review before submission.
AN: clinical expertise, risk of bias assessment, checking characteristics of ongoing studies, writing of the review, taking responsibility for reading and checking the review before submission.
JD: clinical expertise, risk of bias assessment, checking characteristics of ongoing studies, writing of the review, taking responsibility for reading and checking the review before submission.
AF: clinical expertise, data extraction, risk of bias assessment, writing of the review, taking responsibility for reading and checking the review before submission.
NS: methodological expertise and advice, conception and writing of the review, securing the funding, taking responsibility for reading and checking the review before submission.
Sources of support
Internal sources
-
University Hospital of Cologne, Germany
Cochrane Cancer, Department I of Internal Medicine
-
Leipzig University Hospital, Germany
Department of Anaesthesiology and Intensive Care
-
Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt‐Universität zu Berlin, Berlin, Germany, Germany
Department of Infectious Diseases and Respiratory Medicine
-
Christian Medical College, Vellore, Tamil Nadu, India
Department of Respiratory Medicine and Department of Pulmonary Medicine
External sources
-
Federal Ministry of Education and Research, Germany
NaFoUniMedCovid19 (funding number: 01KX2021) part of the 'CEO‐Sys' project
Declarations of interest
MG: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the 'CEOSys' project, which was paid to the institution); works as a resident with the Department of Anesthesiology and Intensive Care at the University of Leipzig Medical Center; is a member of the German Society for Anaesthesia and Intensive Care.
CW: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the 'CEOSys' project, which was paid to the institution); is a part of Cochrane Haematology editorial team, and was not involved in the editorial process.
AM: is a member of the 'CEOSys' project (no direct funding) and co‐ordinator of COVRIIN section and works in office of STAKOB (Competence and Treatment Centres for high consequence infectious diseases) at Robert Koch Institute Centre for Biological Threats and Special Pathogens (ZBS), Section Clinical Management and Infection Control.
MS: none.
FF: is a member of the 'CEOSys' project (no direct funding); works as an Intensive Care Medicine Consultant with the Department of Anesthesiology and Intensive Care at the University of Leipzig Medical Center; is a member of the German Society for Anaesthesia and Intensive Care and the German Interdisciplinary Association for Intensive Care and Emergency Medicine, and leading role in German guideline on respiratory failure and invasive mechanical ventilation.
MM: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the 'CEOSys' project, which was paid to the institution).
AN: none.
JD: none.
AF: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the 'CEOSys' project, which was paid to the institution), and works as a fellow with the Department of Anesthesiology and Intensive Care at the University of Leipzig Medical Center.
NS: none. Part of Cochrane Haematology editorial team, and was not involved in the editorial process.
contributed equally (first author)
contributed equally (first author)
contributed equally (last author)
contributed equally (last author)
Edited (no change to conclusions)
References
References to studies included in this review
Clemency 2021 {published data only}
- Clemency BM, Varughese R, Gonzalez-Rojas Y, Morse CG, Phipatanakul W, Koster DJ, et al.A randomised controlled trial of inhaled ciclesonide for outpatient treatment of symptomatic COVID-19 infections. medRxiv 2021:1-24. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramakrishnan 2021 {published data only}
- Ramakrishnan S, Nicolau DV Jr, Langford B, Mahdi M, Jeffers H, Mwasuku C, et al.Inhaled budesonide in the treatment of early COVID-19(STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respiratory Medicine 2021;9(7):763-72. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan S, Nicolau DV Jr, Langford B, Mahdi M, Jeffers H, Mwasuku C, et al.Inhaled budesonide in the treatment of early COVID-19 illness: a randomised controlled trial. medRxiv 2021:1-25. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOIC Patient information sheet. stoic.ndm.ox.ac.uk/patient-information-sheet (accessed prior to 22/02/2022).
Yu 2021 {published data only}
- Yu LM, Bafadhel M, Dorward J, Hayward G, Saville BR, Gbinigie O, et al.Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial. medRxiv 2021:1-43. [DOI: ] [Google Scholar]
- Yu LM, Bafadhel M, Dorward J, Hayward G, Saville BR, Gbinigie O, et al.Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet 2021;398(10301):1-13. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
IRCT20200522047542N1 {published data only}
- IRCT20200522047542N1.Effect of inhaled corticosteroids in the treatment of anosmia in patients with COVID-19. en.irct.ir/trial/48379 (first received 23 June 2021).
References to studies awaiting assessment
Alsultan 2021 {published data only}
- Alsultan M, Obeid A, Alsamarrai O, Anan MT, Bakr A, Soliman N, et al.Efficacy of colchicine and budesonide in improvement outcomes of patients with coronavirus infection 2019 in Damascus, Syria: a randomised control trial. Interdisciplinary Perspectives on Infectious Diseases 2021;2021:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR2020‐001616‐18‐ES/NCT04355637 {published data only}
- EUCTR2020-001616-18-ES.Treatment with inhaled corticoids in patients with COVID-19 admitted to hospital with pneumonia. www.clinicaltrialsregister.eu/ctr-search/search?query=2020-001616-18 (first received 23 June 2021).
- NCT04355637.Inhaled corticosteroid treatment of COVID-19 patients with pneumonia. www.clinicaltrials.gov/ct2/show/NCT04355637 (first received 23 June 2021).
NCT04331054 {published data only}
- NCT04331054.Protective role of inhaled steroids for Covid-19 infection (INHASCO). clinicaltrials.gov/ct2/show/NCT04331054 (first received 29 March 2020).
NCT04435795 {published data only}
- NCT04435795.Inhaled ciclesonide for outpatients with COVID19 (CONTAIN). clinicaltrials.gov/ct2/show/NCT04435795 (first received 16 June 2020).
Song 2021 {published data only}
- Song JY, Yoon JG, Seo YB, Lee J, Eom JS, Lee JS, et al.Ciclesonide inhaler treatment for mild-to-moderate COVID-19: a randomised, open-label, phase 2 trial. Journal of Clinical Medicine 2021;10(16):3545. [DOI: 10.3390/jcm10163545] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
CTRI/2020/04/024948 {published data only}
- CTRI/2020/04/024948.A clinical trial to study the effects of hydroxychloroquine, ciclesonide and ivermectin in treatment of moderate COVID-19 illness. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=43364&EncHid=&modid=&compid=%27,%2743364det%27 (first received 30 April 2020).
CTRI/2020/10/028581 {published data only}
- CTRI/2020/10/028581.Clinical trial to study the effect of budesonide taken through inhalation in mild COVID cases. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=47042&EncHid=&modid=&compid=%27,%2747042det%27 (first received 23 October 2020).
EUCTR2020‐002208‐37‐DK {published data only}
- EUCTR2020-002208-37-DK.CIMMCov: a randomised clinical study for prevention of severe disease in mild-to-moderate COVID-19 patients, using the inhaled medication ciclesonide. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-002208-37 (first received 8 June 2020).
JRCTS031190269 {published data only}
- JRCTs031190269.A multicenter, open-label, randomised controlled phase II study to evaluate the efficacy and safety of inhaled ciclesonide for asymptomatic and mild patients with COVID-19 (RACCO trial). jrct.niph.go.jp/en-latest-detail/jRCTs031190269 (first received 27 March 2020).
- Terada-Hirashima J, Suzuki M, Uemura Y, Hojo M, Mikami A, Sugiura W, et al.Efficacy and safety of inhaled ciclesonide in treating patients with asymptomatic or mild COVID-19 in the RACCO trial: protocol for a multicenter, open-label, randomised controlled trial. JMIR Research Protocols 2020;9(12):e23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04193878 {published data only}
- NCT04193878.Arrest respiratory failure from pneumonia (ARREST). clinicaltrials.gov/ct2/show/record/NCT04193878 (first received 10 December 2019).
NCT04356495 {published data only}
- NCT04356495.Trial of COVID-19 outpatient treatment in individuals with risk factors for aggravation (COVERAGEFrance). clinicaltrials.gov/ct2/show/record/NCT04356495 (first received 11 April 2020).
NCT04381364 {published data only}
- NCT04381364.Inhalation of ciclesonide for patients with COVID-19: a randomised open treatment study (HALT COVID-19) (HALT). clinicaltrials.gov/ct2/show/NCT04381364 (first received 23 June 2021).
NCT04937543 {published data only}
- NCT04937543.Efficacy of inhaled therapies in the treatment of acute symptoms associated with COVID-19 (TRIVID). clinicaltrials.gov/ct2/show/NCT04937543 (first received 31 August 2021).
NCT05054322 {published data only}
- NCT05054322.FLuticasone in cOvid Treatment (FLOT). clinicaltrials.gov/ct2/show/NCT05054322 (first received 20 September 2021).
NCT05055414 {published data only}
- NCT05055414.Arformoterol/budesonide for COVID-19. clinicaltrials.gov/ct2/show/NCT05055414 (first received 16 September 2021).
Additional references
Barnes 2006
- Barnes PJ.Corticosteroid effects on cell signalling. European Respiratory Journal 2006;27(2):413-26. [DOI: 10.1183/09031936.06.00125404] [DOI] [PubMed] [Google Scholar]
Buitrago‐Garcia 2020
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci Aziz M, et al.Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Medicine 2020;17(9):e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
COMET 2020
- COMET.Core outcome set developers' response to COVID-19. www.comet-initiative.org/Studies/Details/1538 (accessed 16 July 2021).
COVID‐NMA 2021
- Pharmacologic treatments for COVID-19 patients – budesonide. covid-nma.com/living_data/index.php?treatment1=budesonide&submit=Validate#comparisons_div (accessed 26 October 2021).
Daley‐Yates 2015
- Daley-Yates PT.Inhaled corticosteroids: potency, dose equivalence and therapeutic index. British Journal of Clinical Pharmacology 2015;80(3):372-80. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG.Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Derendorf 2006
- Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W.Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. European Respiratory Journal 2006;28(5):1042-50. [DOI: 10.1183/09031936.00074905] [DOI] [PubMed] [Google Scholar]
EndNote X9 [Computer program]
- EndNote X9.Clarivate, 2020.
Finney 2021
- Finney LJ, Glanville N, Farne H, Aniscenko J, Fenwick P, Kemp SV, et al.Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. Journal of Allergy and Clinical Immunology 2021;147(2):510-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geddes 1992
- Geddes DM.Inhaled corticosteroids: benefits and risks. Thorax 1992;47(6):404-7. [DOI: 10.1136/thx.47.6.404] [DOI] [PMC free article] [PubMed] [Google Scholar]
Google Patent Overview Ciclesonide
- Use of ciclesonide for the treatment of respiratory diseases. patents.google.com/patent/US8371292B2/en (accessed 28 October 2021).
GRADEpro GDT [Computer program]
- GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Halpin 2020
- Halpin DM, Faner R, Sibila O, Badia JR, Agusti A.Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respiratory Medicine 2020;8(5):436-8. [DOI: 10.1016/S2213-2600(20)30167-] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from: training.cochrane.org/handbook/archive/v6.1.
Higgins 2021a
- Higgins JP, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al.Methodological expectations of Cochrane intervention reviews. www.community.cochrane.org/mecir-manual/ (accessed 3 August 2021).
Higgins 2021b
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA.Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021c
- Higgins J, Eldridge S, Li T.Chapter 23: Including variants on randomised trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021d
- Higgins JP, Li T, Deeks JJ.Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Huang 2020
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johns Hopkins University 2021
- Johns Hopkins University.Mortality analyses. coronavirus.jhu.edu/data/mortality (accessed 16 July 2021).
Kluge 2022
- Kluge S, Janssens U, Welte T, Weber-Carstens S, Schälte G, Spinner CD et al.S3 Guideline – recommendations for inpatient therapy of patients with COVID-19 [S3-Leitlinie – Empfehlungen zur stationären Therapie von Patienten mit COVID-19]. www.awmf.org/uploads/tx_szleitlinien/113-001LGl_S3_Empfehlungen-zur-stationaeren-Therapie-von-Patienten-mit-COVID-19_2022-03.pdf (accessed 7 March 2022); ( ): . [DOI] [PubMed]
Kreuzberger 2021
- Kreuzberger N, Hirsch C, Chai KL, Piechotta V, Valk SJ, Estcourt LJ, et al.SARS‐CoV‐2‐neutralising monoclonal antibodies for treatment of COVID‐19. Cochrane Database of Systematic Reviews 2021, Issue 9. Art. No: CD013825. [DOI: 10.1002/14651858.CD013825.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauer 2020
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al.The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Annals of Internal Medicine 2020;172(9):577-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2020
- Li T, Higgins JP, Deeks JJ.Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Liang 2020
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al.Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncology 2020;21(3):335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Living Evidence Network 2019
- Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode (Version December 2019). Available at community.cochrane.org/review-production/production-resources/living-systematic-reviews (accessed 16 July 2021).
Marshall 2020
- Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al.A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuyama 2020
- Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, et al.Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proceedings of the National Academy of Sciences of the United States of America 2020;117(13):7001-3. [DOI: 10.1073/pnas.2002589117] [DOI] [PMC free article] [PubMed] [Google Scholar]
Microsoft Excel [Computer program]
- Microsoft Excel.Microsoft Corporation. Microsoft Corporation, 2018. office.microsoft.com/excel.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI] [PubMed] [Google Scholar]
Motozono 2021
- Motozono C, Toyoda M, Zahradnik J, Saito A, Nasser H, Tan TS, et al.SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe 2021;29(7):1124-36. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L.Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Peters 2020
- Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, et al.COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. American Journal of Respiratory and Critical Care Medicine 2020;202:83-90. [DOI: 10.1164/rccm.202003-0821OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piechotta 2021
- Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, et al.Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2012
- Price D, Yawn B, Brusselle G, Rossi A.Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Primary Care Respiratory Journal 2012;22:92-100. [DOI: 10.4104/pcrj.2012.00092] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2019 [Computer program]
- Review Manager Web (RevMan Web).The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Rhen 2005
- Rhen T, Cidlowski JA.Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. New England Journal of Medicine 2005;353:1711-23. [DOI: 10.1056/NEJMra050541] [DOI] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl A, Alper B, et al.GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI] [PubMed] [Google Scholar]
Schulte‐Schrepping 2020
- Schulte-Schrepping J, Reusch N, Paclik D, Baβler K, Schlickeiser S, Zhang B, et al.Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 2020;182(6):1419-40. [DOI: 10.1016/j.cell.2020.08.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Siemieniuk 2020
- Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al.Drug treatments for COVID-19: living systematic review and network meta-analysis (latest update). BMJ 2020;370(1):m2980. [DOI: 10.1136/bmj.m2980] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skoetz 2020
- Skoetz N, Goldkuhle M, Dalen EC, Akl EA, Trivella M, Mustafa RA, et al.GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and evidence profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe N, Boutron I, et al.RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Sterne 2020
- Sterne JA.Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324(13):1330-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Struyf 2020
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al.Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR.Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Paassen 2020
- Paassen J, Vos JS, Hoekstra EM, Neumann KM, Boot PC, Arbous SM.Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Critical Care 2020;24(696):1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villar 2020
- Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al.Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respiratory Medicine 2020;8(3):267-76. [DOI: 10.1016/S2213-2600(19)30417-5] [DOI] [PubMed] [Google Scholar]
Viniol 2018
- Viniol C, Vogelmeier CF.Exacerbations of COPD. European Respiratory Review 2018;27(147):1-9. [DOI: 10.1183/16000617.0103-2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2021a
- Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, et al.Systemic corticosteroids for the treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD014963. [DOI: 10.1002/14651858.CD014963] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization.Cumulative number of reported probable cases of SARS. www.who.int/csr/sars/country/2003_07_11/en (accessed 1 July 2021).
WHO 2019
- World Health Organization.Middle East respiratory syndrome coronavirus (MERS-CoV). www.who.int/emergencies/mers-cov/en (accessed 16 July 2021).
WHO 2020a
- World Health Organization.Report of the WHO‐China Joint Mission on coronavirus disease 2019 (COVID‐19). www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report (accessed 16 July 2021).
WHO 2020b
- World Health Organization.Estimating mortality from COVID-19 – scientific brief. www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 (accessed 16 July 2021).
WHO 2020c
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection.A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI] [PMC free article] [PubMed]
WHO 2021a
- World Health Organization.WHO Coronavirus Disease (COVID-19) Dashboard. covid19.who.int (accessed 16 July 2021).
WHO 2021b
- World Health Organization.Weekly epidemiological update – 23 February 2021. www.who.int/publications/m/item/weekly-epidemiological-update---23-february-2021 (accessed 16 July 2021).
WHO 2021c
- World Health Organization.SARS-CoV-2 variants. www.who.int/csr/don/31-december-2020-sars-cov2-variants/en (accessed 16 July 2021).
WHO 2021d
- Therapeutics and COVID-19: living guideline. www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.1 (accessed 22 February 2022). [https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.3]
Williamson 2020
- Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al.Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2020
- Wu Z, McGoogan JM.Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. [DOI] [PubMed] [Google Scholar]
Yamaya 2020
- Yamaya M, Nishimura H, Deng X, Sugawara M, Watanabe O, Nomura K, et al.Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respiratory Investigation 2020;58(3):155-68. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wagner 2021b
- Wagner C, Griesel M, Fichtner F, Skoetz N, Mikolajewska A, Stegemann M, et al.Efficacy and safety of inhaled corticosteroids for the treatment and prevention of SARS-CoV-2 infection (part of German Ecosystem Ceo-Sys). www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=266913 (accessed 22 February 2022). [PROSPERO: CRD42021266913]


