Summary Paragraph:
Humans have infected a wide range of animals with SARS-CoV-2 viruses1-5, but the establishment of a new natural animal reservoir has not been observed. Here, we document that free-ranging white-tailed deer (Odocoileus virginianus) are highly susceptible to infection with SARS-CoV-2 virus, are exposed to a range of viral diversity from humans, and are capable of sustaining transmission in nature. SARS-CoV-2 virus was detected by rRT-PCR in more than one-third (129/360, 35.8%) of nasal swabs obtained from Odocoileus virginianus in northeast Ohio (USA) during January-March 2021. Deer in 6 locations were infected with 3 SARS-CoV-2 lineages (B.1.2, B.1.582, B.1.596). The B.1.2 viruses, dominant in humans in Ohio at the time, infected deer in four locations. Probable deer-to-deer transmission of B.1.2, B.1.582, and B.1.596 viruses was observed, allowing the virus to acquire amino acid substitutions in the spike protein (including the receptor-binding domain) and ORF1 that are infrequently seen in humans. No spillback to humans was observed, but these findings demonstrate that SARS-CoV-2 viruses have the capacity to transmit in US wildlife, potentially opening new pathways for evolution. There is an urgent need to establish comprehensive “One Health” programs to monitor deer, the environment, and other wildlife hosts globally.
As of November 9, 2021, SARS-CoV-2, the virus responsible for coronavirus disease 2019 (COVID-19), has caused over 5 million deaths globally6. The zoonotic origins of SARS-CoV-2 are not fully resolved7, exposing large gaps in our knowledge of susceptible host species and potential new reservoirs. Natural infections of SARS-CoV-2 linked to human exposure have been reported in domestic animals (e.g. cats, dogs, ferrets) and wildlife under human care, including several species of big cats, Asian small-clawed otters, western lowland gorillas, and mink1. Detection of SARS-CoV-2 by PCR in free-ranging wildlife has been limited to small numbers of mink in Spain and Utah (USA), which purportedly escaped from a nearby farm8, 9. An in silico study modeling SARS-CoV-2 binding sites on the angiotensin-converting enzyme 2 (ACE2) receptor across host species predicted that cetaceans, rodents, primates, and several species of deer are at higher risk for infection10. Experimental infections have identified additional animal species susceptible to SARS-CoV-2 including hamsters, North American raccoons, striped skunks, white-tailed deer, raccoon dogs, fruit bats, deer mice, domestic European rabbits, bushy-tailed woodrats, tree shrews, and multiple non-human primate species11-20. Moreover, several species were capable of intra-species SARS-CoV-2 transmission (cats, ferrets, fruit bats, hamsters, raccoon dogs, deer mice, white-tailed deer)13-15,17,21-23. Vertical transmission has also been documented in experimentally infected white-tailed deer23. Alarmingly, in July 2021 antibodies for SARS-CoV-2 were reported in 152 free-ranging white-tailed deer (seroprevalence 40%) sampled across Michigan, Pennsylvania, Illinois, and New York (USA)24, raising the possibility that SARS-CoV-2 infected deer in the Midwest and Northeast regions.
In this study, we report the detection of SARS-CoV-2 in 129 out of 360 (35.8%) free-ranging white tailed deer (Odocoileus virginianus) from northeast Ohio tested via rRT-PCR between January-March 2021. SARS-CoV-2 is a reportable disease in animals and per international health regulations these results were immediately reported to the World Organisation for Animal Health (OIE) on August 31, 2021, the first PCR-confirmed report of natural infection of SARS-CoV-2 in a cervid globally (Report ID: FUR_151387, Outbreak ID: 89973)25. Fourteen SARS-CoV-2 viruses were whole-genome sequenced and deposited in GISAID on October 5, 2021 Extended Data Table 4), representing the first deposit of SARS-CoV-2 sequences from deer in a public repository. Additionally, we recovered two viable SARS-CoV-2 isolates from our samples, providing evidence that naturally infected deer shed infectious SARS-CoV-2 virus, Genetic sequence data were used to estimate the number of human-to-deer transmission events, characterize the genetic diversity of the virus in deer, and identify phylogenetic clades of deer-only viruses arising from deer-to-deer transmission.
High infection rate of SARS-CoV-2
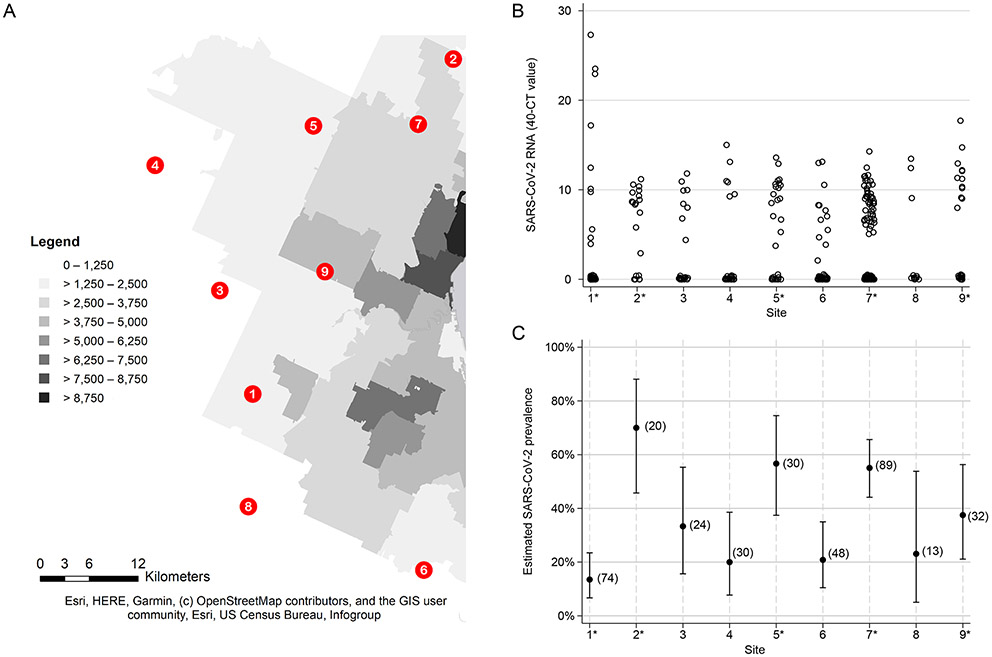
We sampled 360 free-ranging white-tailed deer across from nine locations (Figure 1A) in northeast Ohio (USA) between January-March 2021. Across all sites, SARS-CoV-2 was detected by rRT-PCR in 35.8% of nasal swabs from white-tailed deer (129/360, 95% CI 30.9% - 41.0%, Supplementary Table 1). Each site was sampled 1-3 times during the study period, for a total of 18 collection dates (Extended Data Table 1). At least 1 rRT-PCR-positive sample was identified from 17/18 collection dates with the majority of positive sample CT values less than 30 (Figure 1B). Prevalence estimates varied from 13.5% to 70% across the nine sites (Figure 1C). The highest prevalence estimates of SARS-CoV-2 were observed in four sites (2, 5, 7, and 9) situated in the northern section of the sampled area, adjacent to urban areas with higher human population densities (Figures 1A and 1C). Male deer (Chi2 = 25.45, p-value < 0.0005) and heavier deer (Wilcoxon-Mann-Whitney p-value = 0.0056) were significantly more likely to test positive for SARS-CoV-2 (Extended Data Table 2).
Figure 1. SARS-CoV-2 viral RNA in white-tailed deer across the study locations.
(A) The nine study sites were spread across a 1000 km2 landscape of varying population density in Northeast Ohio. Darker shading corresponds to higher human population density (people per square mile). Sampling sites one, two, five, seven, and nine are in close proximity to human populations and are indicated as urban sites with an asterisk in panels B and C. (B) Nasal swabs from white-tailed deer were tested for the presence of SARS-CoV-2 viral RNA using real-time reverse transcriptase PCR (rRT-PCR). Estimates of SARS-CoV-2 viral RNA are represented by the Ct value of the N1 rRT-PCR target subtracted from 40. Negative samples are represented with a value of zero. (C) The prevalence of SARS-CoV-2 in the white-tailed deer at each study site was estimated using rRT-PCR. Proportion of positive samples is shown with Clopper-Pearson exact 95% confidence interval bars. Number of samples collected for each site is indicated in parentheses.
Three SARS-CoV-2 lineages identified
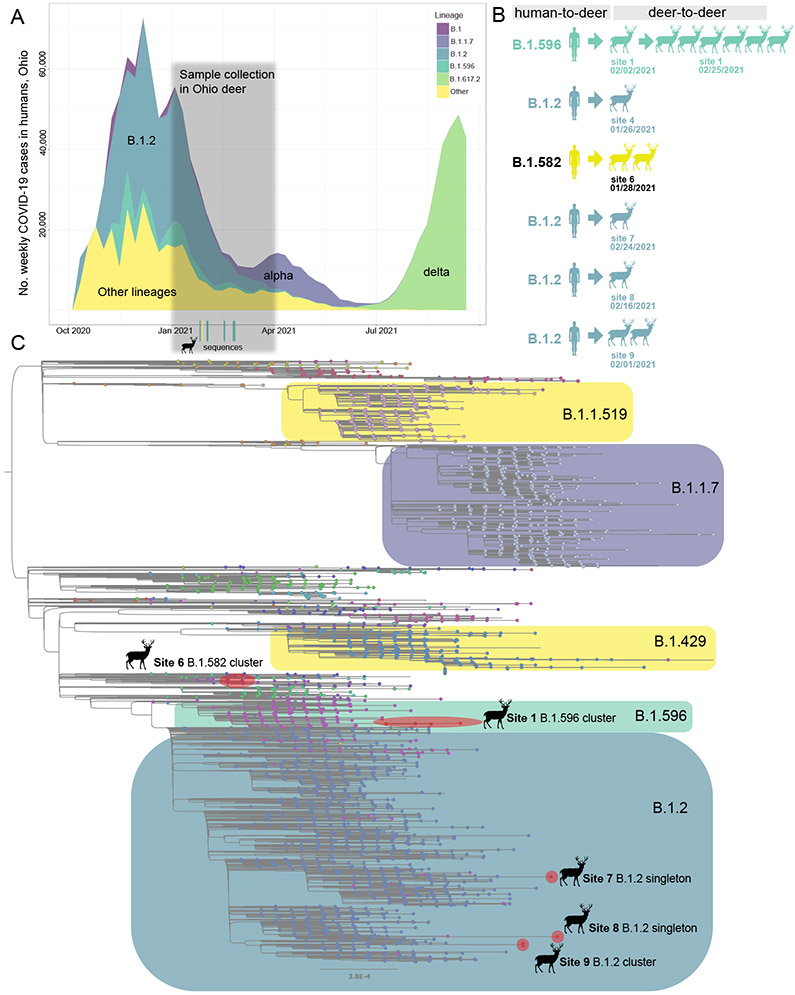
We sequenced the complete genome of 14 viruses collected from six of the nine sites, collected at seven time points spanning from 1/26/2021 to 2/25/2021 (Supplementary Table 1). The deer samples were collected approximately six weeks after the peak of Ohio’s 2020-2021 winter epidemic of SARS-CoV-2 in humans, which was dominated by B.1.2 viruses (>50% of human viruses, Figure 2A, Extended Data Table 3). B.1.2 viruses genetically similar to human viruses were detected in deer at four sites: 4, 7, 8, and 9 (Figure 2B). B.1.596, a minor lineage (~11% of human viruses), was identified in seven deer samples at site 1, spanning two collection times (2/2/2021 and 2/25/2021). A rarer lineage, B.1.582 (~1% of human samples), was identified in two deer samples at site 6. No sequences belonging to the alpha (B.1.1.7) or delta (B.1.617.2) lineages were identified in the deer samples, as these variants became widespread in the human population only after February 2021.
Figure 2. Three SARS-CoV-2 lineages identified in white-tailed deer.
(A) The number of weekly COVID-19 cases in humans in Ohio is presented from October 2020 – September 2021, shaded by the proportion of viruses sequenced each week in Ohio that belong to one of five Pango lineages (or “Other”). (B) Summary of six human-to-deer transmission events observed in Ohio, with putative deer-to-deer transmission. (C) Maximum likelihood tree inferred for SARS-CoV-2 viruses in humans and white-tailed deer in Ohio during January – March 2021. Tips are shaded by Pango lineage and major lineages are boxed, labeled, and shaded similar to Figure 2B. Viruses found in white-tailed deer (clusters or singletons) are shaded red and labeled by location (the B.1.2 virus identified at site 4 not shown due to lower sequence coverage). All branch lengths drawn to scale.
Six human-to-deer transmission events
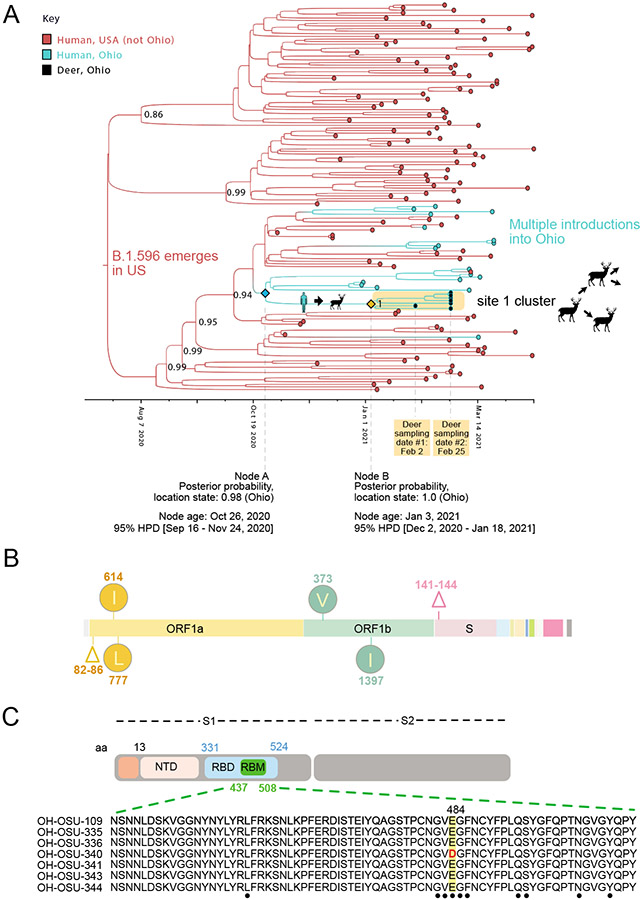
Although B.1.2 was identified in deer at four sites, our phylogenetic analysis found no evidence of B.1.2 viruses transmitting in deer across sites. Rather, each site experienced a separate human-to-deer transmission event of a slightly genetically different B.1.2 virus positioned in a different section of the B.1.2 clade on the phylogenetic tree (Figure 2C). In total, six human-to-deer transmission events were observed: B.1.582 (site 6), B.1.596 (site 1), and B.1.2 (sites 4, 7, 8, and 9). There is a degree of uncertainty about the timing of each viral entry into the deer population, owing to long branch lengths that separate the deer viruses from the ancestral human viruses on the phylogenetic tree. To estimate the timing and location of human-to-deer transmission for the larger cluster of B.1.596 deer viruses, a time-scale Bayesian MCC tree was inferred using a phylogeographic approach (Figure 3A). The MCC tree is consistent with human-to-deer transmission occurring geographically in Ohio (posterior probability = 0.98) and temporally during the winter epidemic when viral loads in humans (and the environment) would be peaking. The MCC tree indicates that 1.596 viruses were introduced into humans in Ohio multiple times from other US states during the fall of 2020 and winter of 2020-2021, forming three co-circulating Ohio clades in humans. The largest Ohio clade then seeded the deer outbreak. Deer viruses in this cluster were collected on February 2 and February 25, 2021, and the MCC tree estimates that human-to-deer transmission occurred several weeks, or possibly months, earlier (Figure 3A). Gaps in sampling in both humans and deer make it difficult to narrow this time estimate further.
Figure 3. Evolution of B.1.596 viruses in white-tailed deer.
(A) Bayesian time-scale MCC tree inferred for the cluster of 7 B.1.596 viruses identified in white-tailed deer at site 1, the 46 most closely related human B.1.596 viruses, and a random sampling of other B.1.596 viruses observed in the United States during November 2020 – March 2021. Tips are shaded by location state (host species + geography). Branches are shaded by the location state inferred from an ancestral reconstruction. Posterior probabilities are provided for key nodes. Cartoons indicate the host-switch branch where human-to-deer transmission may have occurred, followed by putative deer-to-deer transmission within site 1. The estimated timing and location state probability is provided for key nodes defining the host-switch branch. (B) Clade-defining amino acid changes observed in all 7 B.1.596 deer viruses are listed. (C) The E484D substitution in the spike protein’s receptor binding motif (RBM) is shown in one of the B.1.596 deer viruses (OH-OSU-340).
Deer-to-deer transmission and evolution
Viable SARS-CoV-2 virus was recovered from two of the deer samples (Extended Data Table 4). Deer-to-deer transmission may have occurred within the three study sites where more than one deer sample was sequenced: site 1 (B.1.596), site 6 (B.1.582), and site 9 (B.1.2). Sites 6 and 9 only had two viruses collected, both from the same sampling date (Figure 2B), limiting what can be inferred about transmission. Instead, our analysis of deer-to-deer transmission and evolution focused on the larger deer cluster of 7 B.1.596 viruses observed in site 1 that spans two collection dates (Figure 3A). A number of uncommon amino acid substitutions were observed in all 7 deer viruses in this clade (i.e. all Site 1 sequences) that were not observed in the most closely related human viruses. Five clade-defining mutations were observed in ORF1ab: a five-residue deletion in nsp1 (Δ82-86), nsp2_T434I, nsp2_P597L, nsp12_A382V, and nsp13_M474I (numbering in Figure 3B refers to ORF1a and ORF1b). A clade-defining deletion (Δ141-144) also was observed in the S1 domain of the spike protein in the 7 deer viruses. All six clade-defining mutations observed in these deer are uncommon among human viruses (< 0.05% frequency globally)26.
Uncommon amino acid changes in the spike protein S1 domain also were observed in singleton deer viruses. A B.1.2 virus from site 7 (hCoV-19/deer/USA/OH-OSU-0212/2021) has a substitution in the spike protein’s N-terminal domain (H245Y). A single B.1.596 virus from site 1 (hCoV-19/deer/USA/OH-OSU-0340/2021) has a substitution in the spike protein receptor binding motif (E484D, Figure 3C). Both mutations are relatively rare in humans, found in < 0.5% of all SARS-CoV-2 viruses sequenced globally. In experimental studies, viruses with the E484D substitution are less sensitive to neutralization by convalescent sera27. The E484D substitution has only been detected in 201 SARS-CoV-2 sequences from humans globally, 71 of which were in the United States, but none of the B.1.596 viruses in humans that were most closely related to the deer virus have this mutation. It is therefore impossible to differentiate if the E484D mutation arose in an unsampled human virus and was transmitted to deer or arose de novo in deer. Additionally, due to low availability of sequence data from deer it is not possible to determine if these spike mutations have transmitted to other deer.
Discussion
Our finding that white-tailed deer are frequently infected with SARS-CoV-2 viruses raises profound questions about the future trajectory of SARS-CoV-2. The potential establishment of a new reservoir of SARS-CoV-2 viruses in white-tailed deer could open new pathways for evolution, transmission to other wildlife species, and potential spillback of novel variants to humans that our immune system has not seen before. SARS-CoV-2 viruses have a high capacity for adaptive evolution when infection rates are high in a community or population. It is therefore alarming that over one-third of deer in our study were positive by PCR, suggesting an active or recent infection during last winter’s major wave. A number of mutations were observed in white-tailed deer that are very low frequency in humans, including a mutation in the receptor binding motif. Such mutations could potentially be amplified in a new reservoir host with high infection rates and different constraints on evolution. There is an urgent need to expand monitoring of SARS-CoV-2 viruses in potential wildlife hosts to document the breadth of the problem in white-tailed deer nationally, understand the ecology of transmission, and track evolutionary trajectories going forward, including in other potential host species.
The impact of urban sprawl on disease ecology is well documented for Lyme disease and other multihost zoonotic systems that include white-tailed deer, rodents, and other species that have become ubiquitous and well adapted in expanding US urban and semi-urban environments, creating opportunities for pathogen exchange. Approximately 30 million free-ranging white-tailed deer are distributed broadly across urban, suburban, and rural environments in the United States, and can live at densities of greater than 45 deer per square mile in some areas28. Ohio is home to >700,000 free-ranging white-tailed deer29 and another 440 commercial deer farms30. Estimates of deer density in and around our sites range from approximately 8/km2 to upwards of 30/km2. There are no deer farms in the study area and public feeding of deer is prohibited. There is ample forage available around urban and suburban residences in gardens and plantings, drawing deer into close proximity with humans and their companion animals. Therefore, is unsurprising that deer in urban sites were at higher risk for infection in our study. Urban settings provide ample opportunities for deer to have direct and indirect contact with human-contaminated sources (e.g. trash, backyard feeders, bait stations, wildlife hospitals) that could serve as a pathway for viral spillover into wildlife. Additionally, urban and suburban environments include waterways that could be contaminated by multiple sources18,31. Viable SARS-CoV-2 is shed in human stool. SARS-CoV-2 RNA and has also been detected in wastewater32,33 and urban runoff 34; although, infectivity of SARS-CoV-2 from these sources is undetermined. The recent detection of genetically distinct SARS-CoV-2 virus fragments in New York City wastewater introduces an intriguing hypothesis that SARS-CoV-2 could be transmitting cryptically in rodents35. However, although sensitive techniques for detecting viral RNA in wastewater have vastly improved, providing a potentially useful tool for early detection of outbreaks, isolating or whole-genome sequencing viruses to characterize their genetic diversity remains challenging.
A major outstanding question is how the virus transmits between deer. Deer are social animals that live in small herds and frequently touch noses. It is unclear if baiting the deer prior to harvest contributed the increased frequency of SARS-CoV-2 in this study but concentrating deer with bait could have potentially facilitated pathogen transmission through a population. However, baiting is regularly used in deer management programs and the practice is commonly employed by deer hunters, which makes understanding the effect of baiting on SARS-CoV-2 transmission in free-ranging deer paramount for future studies. The increased rate of infection in males in this study could reflect sex-linked differences in behavior that increase disease transmission. The higher prevalence of chronic wasting disease and tuberculosis in male white-tailed deer is attributed to larger male home ranges, increased movement and contact with other deer during breeding season (fall/winter), and dynamic male social group composition and size36. Deer may experience high levels of viremia and shedding that may be conducive to environmental or aerosol transmission. Another question is whether deer experience clinical disease and whether clinical signs such as sneezing or nasal discharge increase the risk of transmission. Two previous experimental studies reported only subclinical infections in white-tailed deer challenged with SARS-CoV-2, but these are very small sample sizes14,23.
While extensive measures were taken to prevent cross-contamination during sample collection and testing, the nature field work makes it impossible to completely exclude the possibility. Cross-contamination during sample collection would not invalidate the detection of SARS-CoV-2 in white-tailed deer but would artificially inflate prevalence estimates. However, the extent of genomic diversity among the sequences recovered on a single sampling day (e.g. site 1, sampling 2) indicates cross-contamination during sample collection was likely minimal.
Although our study was limited to northeastern Ohio, these findings have implications for other US states, including Michigan, Pennsylvania, New York, and Illinois where high rates of exposure to SARS-CoV-2 in white-tailed deer was reported earlier based on serology24. Serological assays are notoriously difficult to interpret and many animal health experts hoped the results were an artifact. Moreover, the detection of antibodies does not prove active infection. Unfortunately, our study suggests that the antibodies observed in deer in other states may have arisen from active infection, and this is just the tip of the iceberg.
Moreover, it is worth noting that white-tailed deer are a relatively convenient surveillance target because of their abundance and accessibility. The detection of SARS-CoV-2 in free-ranging white-tailed deer naturally raises the question whether less accessible species are also being infected through viral spillover from humans, which calls for broader surveillance efforts.
Methods:
Sample collection.
Between January-March 2021, 360 free-ranging white-tailed deer originating from 9 study sites in northeast Ohio (USA) were euthanized as part of a deer population management program. Harvest occurred at locations that were baited with whole kernel corn for up to two weeks prior to each culling session, and additional deer were harvested opportunistically when they were observed away from the bait on a culling session day. In the field, once a deer was harvested, the head was wrapped in a plastic bag and an identification tag was attached to a leg. Each day of the program, harvested deer carcasses were transported to a central processing point where samples were collected. All samples were collected by one experienced veterinarian who wore a facemask and gloves that were changed or washed between each sample. A nasal swab was collected from each deer and placed into a tube with brain heart infusion broth (BHIB). After collection, samples were immediately chilled on ice packs then transferred into a − 80°C freezer within 12 h where they remained until testing was initiated. Samples were collected post-mortem, which was exempt from oversight by The Ohio State University Institutional Animal Care and Use Committee.
Diagnostic testing.
Samples were initially tested using the Charité/Berlin (WHO) assay37. Viral RNA was extracted from 200μl of BHIB using Omega Bio-tek Mag-Bind Viral DNA/RNA kit (cat #M6246-03). Xeno Internal Control (Life Technologies cat # A29763) was included in the extraction to ensure the accuracy of negative results. Five microliters of extracted RNA was added to Path-ID MPX One-Step Kit master mix (Life Technologies cat# 4442135) containing 12.5μl 2x Multiplex RT-PCR buffer, 2.5μl enzyme mix, 1.5μl nuclease free water, 4.5μl E assay primer/probe panel (Integrated DNA Technologies, Inc. cat #1006804), and 1μl XENO VIC Internal Control Assay (Life Technologies cat# A29765) for each sample. The cycling parameters for the real-time, reverse-transcriptase PCR (rRT-PCR) were 48°C for 10 min, 95°C 10 min, 45 cycles of 95°C 15 sec and 58°C 45 sec. Samples with a cycle threshold (Ct) of ≤40 were considered positive. If the E assay was positive, the RdRp confirmatory and discriminatory assays were completed using the above master mix formulation and thermocycler parameters, replacing the E assay primer/ probe panel with the confirmatory and discriminatory primer/probe panel (Integrated DNA Technologies, Inc. cat #s 10006805 and 10006806). The RNA from all samples that tested positive with the E assay, was retested with the CDC rRT-PCR protocol38. Samples that were 2019-nCoV N1 and N2 positive were classified as presumptive positive. A subset of presumptive positive samples were selected for retesting, in which RNA was re-extracted from original samples to verify the rRT-PCR result.
Genomic sequencing.
Original sample material for 76 representative presumptive positive samples were sent to the National Veterinary Services Laboratories (NVSL) for confirmatory rRT-PCR testing using the CDC protocol and whole genome sequencing. Viral RNA was amplified by PCR39 and cDNA libraries were prepared using the Nextera XT DNA Sample Preparation Kit according to manufacturer instructions. Sequencing was performed using the 500 cycle MiSeq Reagent Kit v2. Sequences were assembled using IRMA v0.6.7 and DNAStar SeqMan NGen v14.0.1. Additional sequencing was attempted at Ohio State’s Applied Microbiology Services Laboratory using a modified ARTIC V3 method (ARTIC Network, 2020). Extracted RNA was reverse transcribed and amplified by polymerase chain reaction (PCR) with the ARTIC SARS-CoV-2 FS Library Prep Kit (New England Biolabs, Ipswich MA) per manufacturer's recommended protocol. Amplified products were converted into Illumina sequencing libraries using the RNA Prep with Enrichment (L) Tagmentation Kit protocol (Illumina, San Diego, CA, USA) with unique dual indexes and 10 cycles of TagPCR. Sequencing libraries were pooled and quantified using ProNex NGS Library Quant Kit (NG1201, Promega Co. Madison, WI). 650pM libraries were loaded on P2 sequencing cartridges and analyzed with the NextSeq2000 (lllumina) with 2x101bp cycles. Data were transmitted to the BaseSpace Cloud platform (Illumina) and converted to FASTQ file format using DRAGEN FASTQ Generation v3.8.4 (Illumina). DRAGEN COVID Lineage app v3.5.3 (Illumina) was used to align sequence data and produce quality metrics and consensus genome sequences.
Data analysis.
Pangolin v3.1.11, 2021-09-17 was used to assign lineage40,41. Prevalence was estimated using the number of presumptive positive nasal swabs based upon the final CDC rRT-PCR results. Prevalence estimates, confidence intervals, and other descriptive statistics were calculated using STATA 14.2 (StataCorp LLC).
Virus isolation:
Briefly, at the NVSL, the samples were diluted between 1:2 and 1:3 in minimum essential medium with Earle’s balanced salt solution (MEM-E). Vero 76 cells (American Type Culture Collection, Manassas, VA, USA) that were mycoplasma-free were inoculated with 1.5 mL of diluted sample material and adsorbed for 1 h at 37 °C. After adsorption, a replacement medium containing 2ug/ml TPCK trypsin was added, and cells were incubated at 37 °C for up to seven days. Cell cultures with exhibiting no cytopathic effects (CPE) were frozen, thawed, and subjected to two blind passages, inoculating the fresh cultures with those lysates as described above. At the end of two blind passages or upon observation of CPE, cell culture material was tested by rRT-PCR for SARS-CoV-2 using the CDC N1 and N2 primer and probe sets.
Phylogenetic analysis.
First, a background dataset was compiled from GISAID that included all SARS-CoV-2 sequences available from humans in Ohio, USA during the study period (January 1 – March 31, 2021), downloaded on September 27, 2021 (n = 4,801 sequences). To our knowledge, these are the first SARS-CoV-2 viruses sequenced from white-tailed deer globally and no additional sequences from white-tailed deer were available in any public repository for comparison. Pangolin was used to assign a lineage to each human virus. In total, 102 lineages were identified in this data set, with the most common being B.1.2 (n = 1766), B.1.1.7 (n = 833), B.1.1.519 (n =411), B.1.429 (n = 307), and B.1.596 (n = 274). The dataset was aligned using NextClade with Wuhan-Hu-1 as a reference. The alignment was manually trimmed at the 5’ and 3’ ends. The final alignment included only coding regions and was manually edited to be in frame, with stop codons present only at the terminus of genes. A phylogenetic tree was inferred from this data set using maximum likelihood methods available in IQ-TREE version v1.6.12 with a GTR+G model of nucleotide substitution and 1,000 bootstrap replicates, using the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health (http://biowulf.nih.gov). The inferred tree was visualized in FigTree v1.4.4. Outlier sequences were removed with long branch lengths and incongruence between genetic divergence and sampling date, as assessed using TempEst v1.5.3, typically arising from poor sequence coverage. One of the 14 sequences obtained from deer in our study (hCoV-19/deer/USA/OH-OSU-0025/2021, site 4) was lower in coverage and had a very long branch length and was excluded from the final phylogenetic analysis. To examine the evolutionary origins of the cluster of 7 B.1.596 viruses obtained from deer at site 1 in more granular detail, a second phylogenetic tree was inferred that included all B.1.596 sequences available globally from NCBI’s GenBank (n = 5,586), nearly all (99.8%) from the United States, using similar methods as above. For purposes of visualization a separate phylogenetic tree was inferred that was limited to the sub-clade of B.1.596 viruses (n = 46) most closely related to the 7 deer viruses. This clade, plus 100 viruses randomly sampled from other sections of the tree as background, was used in a subsequent Bayesian phylogeographic analysis. A time-scaled Bayesian analysis using the Markov chain Monte Carlo (MCMC) method was performed the BEAST v1.10.4 package42, again using the Biowulf Linux cluster. A relaxed uncorrelated lognormal (UCLN) molecular clock was used with a flexible Bayesian skyline population, and a general-time reversible (GTR) model of nucleotide substitution with gamma-distributed rate variation among sites. Each sample was assigned to one of three categories based on host and geography: (a) viruses collected in humans in all US states except Ohio, (b) viruses collected in humans in Ohio, and (c) viruses collected in deer in Ohio. The MCMC chain was run separately three times for each of the datasets for at least 100 million iterations with subsampling every 10,000 iterations, using the BEAGLE 3 library to improve computational performance43. All parameters reached convergence, as assessed visually using Tracer v.1.7.1, with statistical uncertainty reflected in values of the 95% highest posterior density (HPD). At least 10% of the chain was removed as burn-in and runs were combined using LogCombiner v1.10.4 and a maximum clade credibility (MCC) tree was summarized using TreeAnnotator v1.10.4 and visualized in FigTree v1.4.4. The NVSL vSNP pipeline ((https://github.com/USDA-VS/vSNP) was applied for SNP based phylogenetic analysis using Wuhan-Hu-1 (NC_045512) as a reference.
Epidemiological data.
The epidemiological curve of SARS-CoV-2 cases in Ohio from April 2020 to September 2020 was generated using the number of daily reported COVID-19 cases in the state of Ohio (all age groups), available from the US Centers for Disease Control and Prevention (https://data.cdc.gov/Case-Surveillance/COVID-19-Case-Surveillance-Public-Use-Data-with-Ge/n8mc-b4w4). All SARS-CoV-2 genetic sequences from Ohio were downloaded from GISAID on October 8, 2021 (n = 18,052) to estimate the proportion of viruses belonging to different Pango lineages during each week of the epidemic. To account for the intensity of surveillance not being even over time the number of viruses per lineage per week was normalized against the epidemiological curve derived from COVID-19 case counts and visualized using R. To further minimize biases only sequences categorized in the GISAID submission as obtained using a “baseline surveillance” sampling strategy were included in the analysis. The dataset was further trimmed to include only submissions with complete collection dates and sufficient coverage to assign a Pango lineage, resulting in a final dataset of 9,947 sequences from Ohio. For simplicity sub-lineages of B.1.617.2 (e.g., AY.3) were consolidated into the Delta category and sub-lineages of B.1.1.7 (e.g., Q.3) were consolidated into the Alpha category. Baseline surveillance data prior to December 20, 2020 was too thinly sampled to reliably estimate the proportion of viruses from different lineages from this time period, so a second figure was generated using all available sequence data. Since the proportions of Pango lineages over time proved to be very similar in the baseline data and the complete dataset, the larger dataset that dated back to October 2020 was used in the final figure.
Extended Data
Extended Data Table 1. SARS-CoV-2 prevalence stratified by site and sampling date.
Sample collection dates for all nine sites are shown indicating the number of nasal swabs collected from white-tailed deer on each date and the number of those swabs that were screened positive for SARS-CoV-2 using the WHO and Centers for Disease Control and Prevention rRT-PCR protocols in series. Overall estimated prevalence is shown with 95% confidence interval estimates (Clopper-Pearson exact). The 7 collection dates from which genetic sequences were obtained are in bold text.
| Date of collection | Samples | Positive | Estimated Prevalence |
Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|---|---|
| Site 1 | 2021-02-02 | 41 | 2 | 5% | 1% | 17% |
| 2021-02-25 | 33 | 8 | 24% | 11% | 42% | |
| Site 2 | 2021-1-27 | 4 | 3 | 75% | 19% | 99% |
| 2021-03-03 | 16 | 11 | 69% | 41% | 89% | |
| Site 3 | 2021-03-02 | 24 | 8 | 33% | 16% | 55% |
| Site 4 | 2021-01-26 | 14 | 2 | 14% | 2% | 43% |
| 2021-02-17 | 16 | 4 | 25% | 7% | 52% | |
| Site 5 | 2021-01-25 | 16 | 8 | 50% | 25% | 75% |
| 2021-02-09 | 8 | 5 | 63% | 24% | 91% | |
| 2021-03-08 | 6 | 4 | 67% | 22% | 96% | |
| Site 6 | 2021-01-28 | 33 | 3 | 9% | 2% | 24% |
| 2021-03-04 | 15 | 7 | 47% | 21% | 73% | |
| Site 7 | 2021-02-22 | 20 | 5 | 25% | 9% | 49% |
| 2021-02-23 | 19 | 8 | 42% | 20% | 67% | |
| 2021-02-24 | 50 | 36 | 72% | 58% | 84% | |
| Site 8 | 2021-02-16 | 13 | 3 | 23% | 5% | 54% |
| Site 9 | 2021-02-01 | 22 | 12 | 55% | 32% | 76% |
| 2021-03-01 | 10 | 0 | 0% | 0% | 31%* |
Extended Data Table 2. Covariate data recorded for each deer sample.
rRT-PCR positive samples are shown by group for categorical variables, p-values shown from Pearson’s Chi2. The mean of each continuous variable is show for rRT-PCR positive and negative deer respectively, p-values shows from Wilcoxon-Mann-Whitney rank-sum test (two-sided). Age was not recorded for 3 deer which tested negative for SARS-CoV-2.
| Categorical | ||||||
|---|---|---|---|---|---|---|
| Covariate | Category | Samples | Positive (%) | Odds Ratio |
Chi2 | P-Value |
| Sex | 3.10 | 25.45 | <0.0005 | |||
| Male | 149 | 76 (51) | ||||
| Female | 211 | 53 (25) | ||||
| Cull method | 0.82 | 0.72 | 0.395 | |||
| Treestand | 119 | 39 (33) | ||||
| Opportunistic | 241 | 90 (37) | ||||
| Continuous | ||||||
| Covariate | Samples | Negative mean (std) | Positive mean (std) | Difference | z (Mann-Whitney) | P-Value |
| Mass (Kg) | 360 | 52.76 (13.48) | 56.73 (13.93) | 3.972 | 2.77 | 0.0056 |
| Age (years) | 357 | 2.11 | 2.05 | −0.053 | −0.035 | 0.9717 |
Extended Data Table 3. Proportion of SARS-CoV-2 viruses identified in Ohio in humans during January 1 – February 28, 2021.
Only viruses categorized as collected for baseline surveillance are included.
| Rank | Lineage | Percentage | Number |
|---|---|---|---|
| 1 | B.1.2 | 56.3% | 462 |
| 2 | B.1.596 | 8.3% | 68 |
| 3 | B.1.1.519 | 7.1% | 58 |
| 4 | B.1.429 | 3.4% | 28 |
| 5 | B.1.1.7 | 3.0% | 25 |
| 6 | B.1.234 | 2.7% | 22 |
| 7 | P.2 | 2.4% | 20 |
| 8 | B.1.311 | 1.6% | 13 |
| 9 | B.1.427 | 1.5% | 12 |
| 10 | B.1.400 | 1.2% | 10 |
| 11 | B.1.582 | 1.2% | 10 |
| 12 | B.1.1 | 1.1% | 9 |
| 13 | B.1.243 | 1.1% | 9 |
| 14 | B.1 | 0.7% | 6 |
| 15 | B.1.568 | 0.7% | 6 |
| 16 | B.1.595 | 0.6% | 5 |
| 17 | B.1.1.337 | 0.5% | 4 |
| 18 | B.1.240 | 0.5% | 4 |
| 19 | B.1.448 | 0.5% | 4 |
| 20 | B.1.1.207 | 0.4% | 3 |
| 21 | B.1.1.434 | 0.4% | 3 |
| 22 | B.1.265 | 0.4% | 3 |
| 23 | B.1.361 | 0.4% | 3 |
| 24 | B.1.438.4 | 0.4% | 3 |
| 25 | B.1.577 | 0.4% | 3 |
| 26 | B.1.588 | 0.4% | 3 |
| 27 | B.1.1.265 | 0.2% | 2 |
| 28 | B.1.298 | 0.2% | 2 |
| 29 | B.1.517 | 0.2% | 2 |
| 30 | B.1.565 | 0.2% | 2 |
| 31 | Q.8 | 0.2% | 2 |
| 32 | B.1.1.291 | 0.1% | 1 |
| 33 | B.1.1.316 | 0.1% | 1 |
| 34 | B.1.1.348 | 0.1% | 1 |
| 35 | B.1.110.3 | 0.1% | 1 |
| 36 | B.1.139 | 0.1% | 1 |
| 37 | B.1.324 | 0.1% | 1 |
| 38 | B.1.346 | 0.1% | 1 |
| 39 | B.1.404 | 0.1% | 1 |
| 40 | B.1.409 | 0.1% | 1 |
| 41 | B.1.493 | 0.1% | 1 |
| 42 | B.1.503 | 0.1% | 1 |
| 43 | B.1.564 | 0.1% | 1 |
| 44 | C.23 | 0.1% | 1 |
| 45 | C.31 | 0.1% | 1 |
Extended Data Table 4. Whole genome sequence data.
Sequences generated from original sample nasal swabs collected from white-tailed deer as a part of this study are shown with GISAID and NCBI Sequence Read Archive (SRA) accession numbers, collection date, site of collection, and the submitting laboratory indicating if the sequencing was completed at The Ohio State University Applied Microbiology Services Laboratory (Ohio State) or USDA National Veterinary Services Laboratories (NVSL). The two samples from which viable SARS-CoV-2 was isolated are denoted with bold text.
| Virus name | Site | Collection date | GenBank SRA Accession |
GISAID Accession |
Submitting laboratory |
|---|---|---|---|---|---|
| hCoV-19/deer/USA/OH-OSU-0025/2021 | 4 | 2021-01-26 | SRR17187543 | EPI_ISL_4878314 | Ohio State |
| hCoV-19/deer/USA/OH-OSU-0057/2021 | 6 | 2021-01-28 | SRR17187550 | EPI_ISL_4847029 | NVSL |
| hCoV-19/deer/USA/OH-OSU-0058/2021 | 6 | 2021-01-28 | SRR17187551 | EPI_ISL_4847030 | NVSL |
| hCoV-19/deer/USA/OH-OSU-0078/2021 | 9 | 2021-02-01 | SRR17187544 | EPI_ISL_4878315 | Ohio State |
| hCoV-19/deer/USA/OH-OSU-0079/2021 | 9 | 2021-02-01 | SRR17187552 | EPI_ISL_4847031 | Ohio State |
| hCoV-19/deer/USA/OH-OSU-0109/2021 | 1 | 2021-02-02 | SRR17187545 | EPI_ISL_4878316 | NVSL |
| hCoV-19/deer/USA/OH-OSU-0141/2021 | 8 | 2021-02-16 | SRR17187553 | EPI_ISL_4847032 | NVSL |
| hCoV-19/deer/USA/OH-OSU-0212/2021 | 7 | 2021-02-24 | SRR17187542 | EPI_ISL_4847033 | NVSL |
| hCoV-19/deer/USA/OH-OSU-0335/2021 | 1 | 2021-02-25 | SRR17187546 | EPI_ISL_4878317 | NVSL |
| hCoV-19/deer/USA/OH-OSU-0336/2021 | 1 | 2021-02-25 | SRR17187547 | EPI_ISL_4878318 | NVSL |
| hCoV-19/deer/USA/OH-OSU-0340/2021 | 1 | 2021-02-25 | SRR17187548 | EPI_ISL_4878319 | NVSL |
| hCoV-19/deer/USA/OH-OSU-0341/2021 | 1 | 2021-02-25 | SRR17187549 | EPI_ISL_4878320 | NVSL |
| hCoV-19/deer/USA/OH-OSU-0343/2021 | 1 | 2021-02-25 | SRR17187554 | EPI_ISL_4878321 | NVSL |
| hCoV-19/deer/USA/OH-OSU-0344/2021 | 1 | 2021-02-25 | SRR17187555 | EPI_ISL_4878322 | Ohio State |
Supplementary Material
Acknowledgments:
This work was supported by The Ohio State University Infectious Diseases Institute and The Ohio State University Center of Microbiome Science (Targeted Investment: eSCOUT – Environmental Surveillance for COVID-19 in Ohio: Understanding Transmission), Centers of Excellence for Influenza Research and Surveillance, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract HHSN272201400006C and HHSN272201400008C, and the NIAID Intramural Research Program. The authors thank Hannah Cochran, Elizabeth Ohl, Amber Cleggett, Sydney Treglia, Amanda M. Williams, Francesca Savona, Jacob W. Smith, Dakota Sizemore, and the Cleveland Metroparks Deer Management Team.
Footnotes
Disclaimer. The content does not necessarily reflect the views or policies of the U.S. Government or imply endorsement of any products.
Competing interests: Authors declare that they have no competing interests.
Data Availability:
For all 14 SARS-CoV-2 viruses from white-tailed deer sequenced in this study, sequencing data are available on NCBI Sequence Read Archive (SRA) and assembled genome sequences are on GISAID. SRA and GISAID accession numbers are provided in Extended Data Table 4.
Main References:
- 1.USDA APHIS. Confirmed Cases of SARS-CoV-2 in Animals in the United States, <https://www.aphis.usda.gov/aphis/dashboards/tableau/sars-dashboard> (2021).
- 2.McAloose D et al. From People to Panthera: Natural SARS-CoV-2 Infection in Tigers and Lions at the Bronx Zoo. mBio 11, e02220–20. doi: 10.1128/mBio.02220-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oude Munnink BB et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 371, 172–177, doi: 10.1126/science.abe5901 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz C et al. SARS-CoV-2-Specific Antibodies in Domestic Cats during First COVID-19 Wave, Europe. Emerg Infect Dis. 27, 3115–3118, doi: 10.3201/eid2712.211252 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sit THC, et al. Infection of dogs with SARS-CoV-2. Nature 586, 776–778, 10.1038/s41586-020-2334-52 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. World Health Organization Coronavirus (COVID-19) Dashboard, <https://covid19.who.int/> (2021).
- 7.Andersen KG, Rambaut A, Lipkin WI, Holmes EC & Garry RF The proximal origin of SARS-CoV-2. Nature Medicine 26, 450–452, doi: 10.1038/s41591-020-0820-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shriner SA et al. SARS-CoV-2 Exposure in Escaped Mink, Utah, USA. Emerg Infect Dis 27, 988–990, doi: 10.3201/eid2703.204444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguiló-Gisbert J et al. First Description of SARS-CoV-2 Infection in Two Feral American Mink (Neovison vison) Caught in the Wild. Animals 11, doi: 10.3390/ani11051422 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damas J et al. Broad Host Range of SARS-CoV-2 Predicted by Comparative and Structural Analysis of ACE2 in Vertebrates. bioRxiv, 2020.2004.2016.045302, doi: 10.1101/2020.04.16.045302 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francisco R et al. Experimental susceptibility of North American raccoons (Procyon lotor) and striped skunks (Mephitis mephitis) to SARS-CoV-2. bioRxiv, 2021.2003.2006.434226, doi: 10.1101/2021.03.06.434226 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlottau K et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. The Lancet Microbe 1, e218–e225, doi: 10.1016/S2666-5247(20)30089-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freuling C et al. Susceptibility of Raccoon Dogs for Experimental SARS-CoV-2 Infection. Emerging Infectious Disease journal 26, 2982, doi: 10.3201/eid2612.203733 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer M,V et al. Susceptibility of White-Tailed Deer (Odocoileus virginianus) to SARS-CoV-2. Journal of Virology 95, e00083–00021, doi: 10.1128/JVI.00083-21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagre A et al. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for spillback to New World rodents. PLOS Pathogens 17, e1009585, doi: 10.1371/journal.ppat.1009585 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu S et al. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduction and Targeted Therapy 5, 157, doi: 10.1038/s41392-020-00269-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sia SF et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583, 834–838, doi: 10.1038/s41586-020-2342-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosco-Lauth A et al. Peridomestic Mammal Susceptibility to Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Emerging Infectious Disease journal 27, 2073, doi: 10.3201/eid2708.210180 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mykytyn AZ et al. Susceptibility of rabbits to SARS-CoV-2. Emerg Microbes Infect 10, 1–7, doi: 10.1080/22221751.2020.1868951 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y et al. Susceptibility of tree shrew to SARS-CoV-2 infection. Scientific Reports 10, 16007, doi: 10.1038/s41598-020-72563-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y-I et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host & Microbe 27, 704–709.e702, doi: 10.1016/j.chom.2020.03.023 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi J et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science, eabb7015, doi: 10.1126/science.abb7015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cool K et al. Infection and transmission of ancestral SARS-CoV-2 and its alpha variant in pregnant white-tailed deer. bioRxiv, 2021.2008.2015.456341, doi: 10.1101/2021.08.15.456341 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandler JC et al. SARS-CoV-2 exposure in wild white-tailed deer (Odocoileus virginianus). Proc Natl Acad Sci USA 118, e2114828118, doi: 10.1073/pnas.2114828118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Organisation for Animal Health (OIE), W. A. H. I. S. Follow-up report 18, SARS-CoV-2 in animals, United States of America, FUR_151387, <https://wahis.oie.int/#/report-info?reportId=38714> (2021).
- 26.Mullen JL et al. outbreak.info, <https://outbreak.info/> (2020). [Google Scholar]
- 27.Liu Z et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe 29, 477–488 e474, doi: 10.1016/j.chom.2021.01.014 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walters BF, Woodall CW & Russell MB White-tailed deer density estimates across the eastern United States, 2008. Retrieved from the Data Repository for the University of Minnesota, (2016). [Google Scholar]
- 29.ODNR, D. o. W. Managing Ohio’s Deer Herd, <https://ohiodnr.gov/static/documents/wildlife/wildlife-management/Managing%20Ohio's%20Deer%20Herd%20pub087.pdf> (
- 30.Shepstone Management Company. (ed Inc. Whitetail Deer Farmers of Ohio; ) (2010). [Google Scholar]
- 31.Franklin AB & Bevins SN Spillover of SARS-CoV-2 into novel wild hosts in North America: A conceptual model for perpetuation of the pathogen. Science of The Total Environment 733, 139358, doi: 10.1016/j.scitotenv.2020.139358 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bivins A et al. Persistence of SARS-CoV-2 in Water and Wastewater. Environmental Science & Technology Letters 7, 937–942, doi: 10.1021/acs.estlett.0c00730 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ai Y et al. Wastewater-based epidemiology for tracking COVID-19 trend and variants of concern in Ohio, United States. medRxiv, 2021.2006.2008.21258421, doi: 10.1101/2021.06.08.21258421 (2021). [DOI] [Google Scholar]
- 34.Bernard K et al. Detection of SARS-CoV-2 in urban stormwater: An environmental reservoir and potential interface between human and animal sources. Science of the Total Environment In revision (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smyth DS et al. Tracking Cryptic SARS-CoV-2 Lineages Detected in NYC Wastewater. medRxiv, 2021.2007.2026.21261142, doi: 10.1101/2021.07.26.21261142 (2021). [DOI] [Google Scholar]
- 36.O’Brien DJ et al. Epidemiology of Mycobacterium bovis in free-ranging white-tailed deer, Michigan, USA, 1995–2000. Preventive Veterinary Medicine 54, 47–63, doi: 10.1016/S0167-5877(02)00010-7 (2002). [DOI] [PubMed] [Google Scholar]
Methods References:
- 37.Corman VM et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25, 2000045, doi: 10.2807/1560-7917.ES.2020.25.3.2000045 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CDC. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel <https://www.fda.gov/media/134922/download> (2021). [DOI] [PMC free article] [PubMed]
- 39.Paden CR et al. Rapid, Sensitive, Full-Genome Sequencing of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 26, 2401–2405. doi: 10.3201/eid2610.201800 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Toole A et al. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol 7, veab064, doi: 10.1093/ve/veab064 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rambaut A et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nature Microbiology 5, 1403–1407, doi: 10.1038/s41564-020-0770-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suchard MA et al. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol 4, vey016, doi: 10.1093/ve/vey016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayres DL et al. BEAGLE 3: Improved Performance, Scaling, and Usability for a High-Performance Computing Library for Statistical Phylogenetics. Syst Biol 68, 1052–1061, doi: 10.1093/sysbio/syz020 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For all 14 SARS-CoV-2 viruses from white-tailed deer sequenced in this study, sequencing data are available on NCBI Sequence Read Archive (SRA) and assembled genome sequences are on GISAID. SRA and GISAID accession numbers are provided in Extended Data Table 4.