Abstract
Background
Interleukin‐1 (IL‐1) blocking agents have been used for treating severe coronavirus disease 2019 (COVID‐19), on the premise that their immunomodulatory effect might be beneficial in people with COVID‐19.
Objectives
To assess the effects of IL‐1 blocking agents compared with standard care alone or with placebo on effectiveness and safety outcomes in people with COVID‐19.
We will update this assessment regularly.
Search methods
We searched the Cochrane COVID‐19 Study Register and the COVID‐19 L‐OVE Platform (search date 5 November 2021). These sources are maintained through regular searches of MEDLINE, Embase, CENTRAL, trial registers and other sources. We also checked the World Health Organization International Clinical Trials Registry Platform, regulatory agency websites, Retraction Watch (search date 3 November 2021).
Selection criteria
We included randomised controlled trials (RCTs) evaluating IL‐1 blocking agents compared with standard care alone or with placebo for people with COVID‐19, regardless of disease severity.
Data collection and analysis
We followed Cochrane methodology. The protocol was amended to reduce the number of outcomes considered. Two researchers independently screened and extracted data and assessed the risk of bias with the Cochrane Risk of Bias 2 tool. We rated the certainty of evidence using the GRADE approach for the critical outcomes of clinical improvement (Day 28; ≥ D60); WHO Clinical Progression Score of level 7 or above (i.e. the proportion of participants with mechanical ventilation +/‐ additional organ support OR death) (D28; ≥ D60); all‐cause mortality (D28; ≥ D60); incidence of any adverse events; and incidence of serious adverse events.
Main results
We identified four RCTs of anakinra (three published in peer‐reviewed journals, one reported as a preprint) and two RCTs of canakinumab (published in peer‐reviewed journals). All trials were multicentre (2 to 133 centres). Two trials stopped early (one due to futility and one as the trigger for inferiority was met). The median/mean age range varied from 58 to 68 years; the proportion of men varied from 58% to 77%. All participants were hospitalised; 67% to 100% were on oxygen at baseline but not intubated; between 0% and 33% were intubated at baseline. We identified a further 16 registered trials with no results available, of which 15 assessed anakinra (four completed, four terminated, five ongoing, three not recruiting) and one (completed) trial assessed canakinumab.
Effectiveness of anakinra for people with COVID‐19
Anakinra probably results in little or no increase in clinical improvement at D28 (risk ratio (RR) 1.08, 95% confidence interval (CI) 0.97 to 1.20; 3 RCTs, 837 participants; absolute effect: 59 more per 1000 (from 22 fewer to 147 more); moderate‐certainty evidence.
The evidence is uncertain about an effect of anakinra on 1) the proportion of participants with a WHO Clinical Progression Score of level 7 or above at D28 (RR 0.67, 95% CI 0.36 to 1.22; 2 RCTs, 722 participants; absolute effect: 55 fewer per 1000 (from 107 fewer to 37 more); low‐certainty evidence) and ≥ D60 (RR 0.54, 95% CI 0.30 to 0.96; 1 RCT, 606 participants; absolute effect: 47 fewer per 1000 (from 72 fewer to 4 fewer) low‐certainty evidence); and 2) all‐cause mortality at D28 (RR 0.69, 95% CI 0.34 to 1.39; 2 RCTs, 722 participants; absolute effect: 32 fewer per 1000 (from 68 fewer to 40 more); low‐certainty evidence).
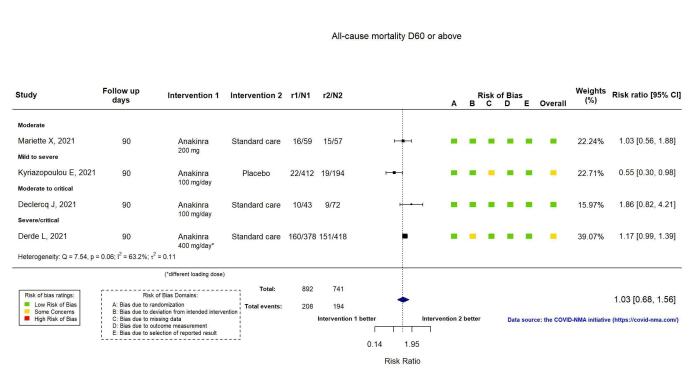
The evidence is very uncertain about an effect of anakinra on 1) the proportion of participants with clinical improvement at ≥ D60 (RR 0.93, 95% CI 0.78 to 1.12; 1 RCT, 115 participants; absolute effect: 59 fewer per 1000 (from 186 fewer to 102 more); very low‐certainty evidence); and 2) all‐cause mortality at ≥ D60 (RR 1.03, 95% CI 0.68 to 1.56; 4 RCTs, 1633 participants; absolute effect: 8 more per 1000 (from 84 fewer to 147 more); very low‐certainty evidence).
Safety of anakinra for people with COVID‐19
Anakinra probably results in little or no increase in adverse events (RR 1.02, 95% CI 0.94 to 1.11; 2 RCTs, 722 participants; absolute effect: 14 more per 1000 (from 43 fewer to 78 more); moderate‐certainty evidence).
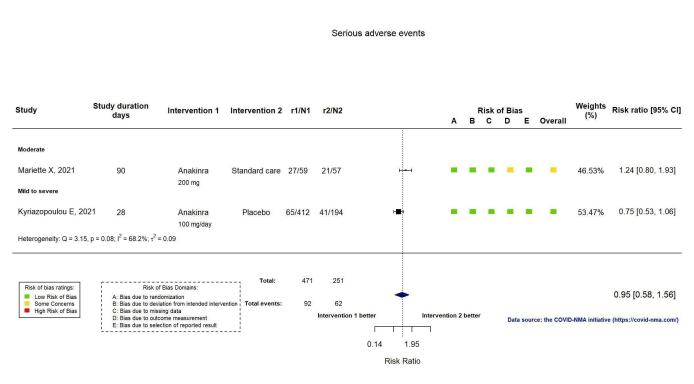
The evidence is uncertain regarding an effect of anakinra on serious adverse events (RR 0.95, 95% CI 0.58 to 1.56; 2 RCTs, 722 participants; absolute effect: 12 fewer per 1000 (from 104 fewer to 138 more); low‐certainty evidence).
Effectiveness of canakinumab for people with COVID‐19
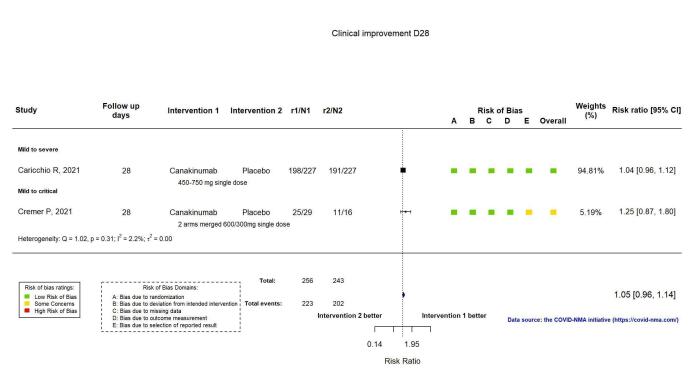
Canakinumab probably results in little or no increase in clinical improvement at D28 (RR 1.05, 95% CI 0.96 to 1.14; 2 RCTs, 499 participants; absolute effect: 42 more per 1000 (from 33 fewer to 116 more); moderate‐certainty evidence).
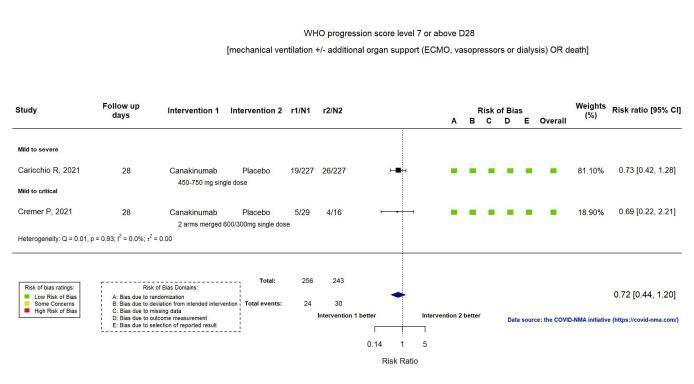
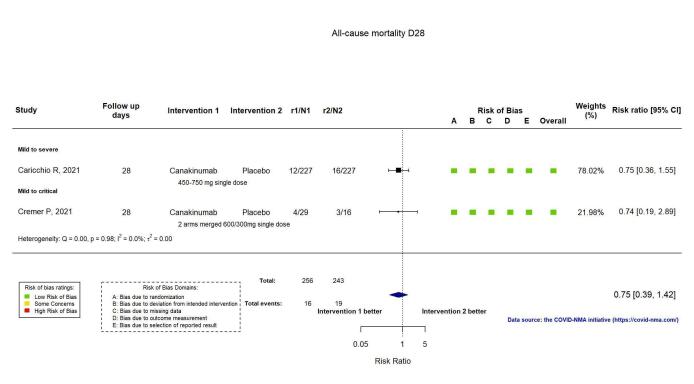
The evidence of an effect of canakinumab is uncertain on 1) the proportion of participants with a WHO Clinical Progression Score of level 7 or above at D28 (RR 0.72, 95% CI 0.44 to 1.20; 2 RCTs, 499 participants; absolute effect: 35 fewer per 1000 (from 69 fewer to 25 more); low‐certainty evidence); and 2) all‐cause mortality at D28 (RR:0.75; 95% CI 0.39 to 1.42); 2 RCTs, 499 participants; absolute effect: 20 fewer per 1000 (from 48 fewer to 33 more); low‐certainty evidence).
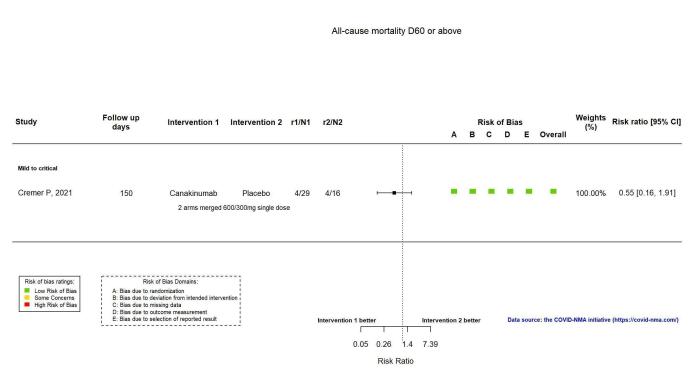
The evidence is very uncertain about an effect of canakinumab on all‐cause mortality at ≥ D60 (RR 0.55, 95% CI 0.16 to 1.91; 1 RCT, 45 participants; absolute effect: 112 fewer per 1000 (from 210 fewer to 227 more); very low‐certainty evidence).
Safety of canakinumab for people with COVID‐19
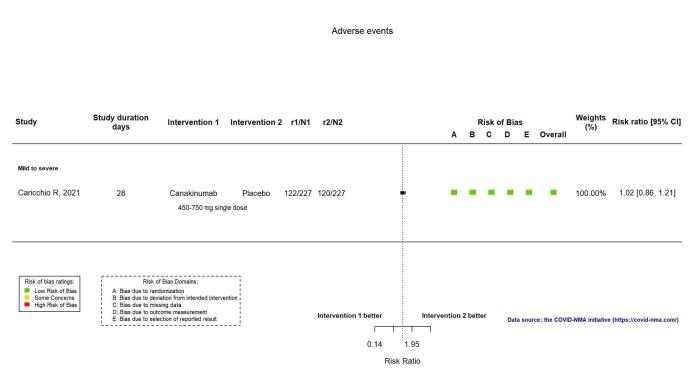
Canakinumab probably results in little or no increase in adverse events (RR 1.02; 95% CI 0.86 to 1.21; 1 RCT, 454 participants; absolute effect: 11 more per 1000 (from 74 fewer to 111 more); moderate‐certainty evidence).
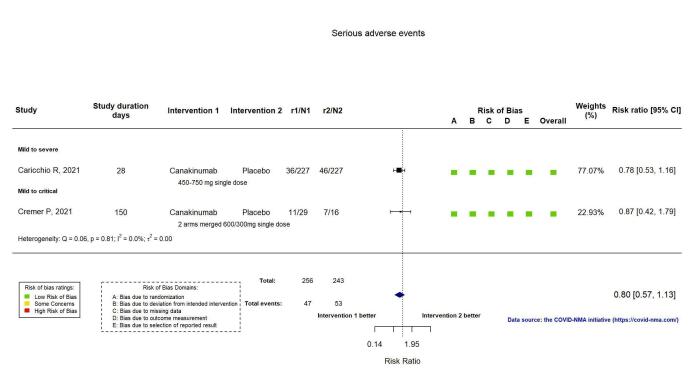
The evidence of an effect of canakinumab on serious adverse events is uncertain (RR 0.80, 95% CI 0.57 to 1.13; 2 RCTs, 499 participants; absolute effect: 44 fewer per 1000 (from 94 fewer to 28 more); low‐certainty evidence).
Authors' conclusions
Overall, we did not find evidence for an important beneficial effect of IL‐1 blocking agents. The evidence is uncertain or very uncertain for several outcomes. Sixteen trials of anakinra and canakinumab with no results are currently registered, of which four are completed, and four terminated. The findings of this review are updated on the COVID‐NMA platform (covid-nma.com).
Keywords: Aged; Female; Humans; Male; Middle Aged; COVID-19; COVID-19/drug therapy; Interleukin-1; Interleukin-1/antagonists & inhibitors; Randomized Controlled Trials as Topic; Respiration, Artificial
Plain language summary
Are medicines that block interleukin‐1 (a protein involved in immune responses) effective treatments for COVID‐19 and do they cause unwanted effects?
Key messages
• Overall, we did not find sufficient evidence to show that medicines that block interleukin‐1 (a protein involved in immune responses) are effective treatments for people with COVID‐19, or whether they cause unwanted effects.
• We found 16 studies with unpublished results. We will update this review when new data are available.
• In future, we need high‐quality studies to evaluate medicines that block interleukin‐1 to treat COVID‐19.
What is interleukin‐1 and what is its role in COVID‐19?
Interleukin‐1 (IL‐1) is a type of protein called a cytokine, which helps to regulate the body’s immune system. In particular, IL‐1 triggers inflammation to help fight infection. In COVID‐19, as the immune system fights the virus, the lungs and airways become inflamed, causing breathing difficulties. However, in some people, the immune system can over‐react (called a ‘cytokine storm’) and produce dangerously high levels of inflammation and tissue damage. This can lead to severe breathing difficulties, organ failure and death.
What are interleukin‐1 ‘blockers’?
IL‐1 blockers are medicines that stop IL‐1 from working by blocking signals from IL‐1 to other parts of the immune system. This reduces inflammation and may help the immune system to fight COVID‐19. In turn, this may reduce the need for breathing support with a ventilator (a machine that breathes for a patient) and reduce the number of deaths from COVID‐19. Three IL‐1 blockers are available: anakinra, canakinumab and rilonacept.
What did we want to find out?
We wanted to know if IL‐1 blockers are effective treatments for people with COVID‐19, compared with standard care alone or with placebo (a dummy treatment that appears identical to the medicine being tested but without any active medicine). We were particularly interested in the effects of IL‐1 blockers on:
• whether people’s symptoms got better or worse;
• how many people died; and
• any unwanted effects and serious unwanted effects.
What did we do?
We searched for studies that assessed the effects of IL‐1 blockers to treat people with COVID‐19 compared with standard care alone or with placebo. People in the studies could have suspected or confirmed COVID‐19 of any severity (mild, moderate or severe), and be any age or sex.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found six studies with 2132 people. Four studies assessed anakinra (1633 people) and two assessed canakinumab (499 people). People in the studies were aged between 58 and 68 years old on average, and the majority were men. All the people in the studies were in hospital, mainly with moderate to critical COVID‐19. The studies varied in size, from 45 to 2253 people. At the start of the studies, 67% to 100% of people were receiving oxygen, and 0% to 33% were on a ventilator.
We also found 16 studies that have not yet published their results.
Anakinra compared to usual care and placebo to treat people with COVID‐19
• Anakinra probably results in little or no improvement in COVID‐19 symptoms (defined as improvement on a clinical scale or discharge from hospital) at 28 days after treatment (three studies, 837 people) but we do not know if it makes a difference at 60 days (one study, 115 people).
• We do not know if anakinra makes a difference to the number of deaths at 28 days after treatment (two studies, 722 people) or at 60 days (four studies, 1633 people).
• Anakinra probably results in little or no increase in any unwanted effects at 28 days after treatment, but we are not sure about its effect on serious unwanted effects (two studies, 722 people).
Canakinumab compared to usual care and placebo to treat people with COVID‐19
• Canakinumab probably results in little or no improvement in COVID‐19 symptoms (defined as improvement on a clinical scale or discharge from hospital) at 28 days after treatment (two studies, 499 people).
• We do not know if canakinumab makes a difference to the number of deaths at 28 days after treatment (two studies, 499 people) or at 60 days (one study, 45 people).
• Canakinumab probably results in little or no increase in any unwanted effects (one study, 454 people), but we are not sure about its effect on serious unwanted effects (two studies, 499 people) at 28 days.
What are the limitations of the evidence?
Our confidence in the evidence is limited for several reasons. All the people in the studies were hospitalised, but some were more seriously ill than others ‐ some studies only included people on a ventilator. Usual care also differed between studies, and studies measured and reported their results using different methods.
How up to date is this evidence?
The evidence is up to date to 5 November 2021.
Summary of findings
Summary of findings 1. Anakinra compared to standard care/placebo for mild/moderate/severe/critical COVID‐19.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard care/placebo | Risk with anakinra | |||||
| Clinical improvement D28 | 737 per 1000 | 796 per 1000 (715 to 884) | RR 1.08 (0.97 to 1.20) | 837 (3 RCTs)a | ⊕⊕⊕⊝ moderateb | |
| Clinical improvement D60 or above | 847 per 1000 | 788 per 1000 (661 to 949) | RR 0.93 (0.78 to 1.12) | 115 (1 RCT)c | ⊕⊝⊝⊝ very lowd,e | |
| WHO Clinical Progression Score of level 7 or above D28 | 167 per 1000 | 112 per 1000 (60 to 204) | RR 0.67 (0.36 to 1.22) | 722 (2 RCTs)f | ⊕⊕⊝⊝ lowg,h | |
| WHO Clinical Progression Score of level 7 or above D60 or above | 103 per 1000 | 56 per 1000 (31 to 99) | RR 0.54 (0.30 to 0.96) | 606 (1 RCT)i | ⊕⊕⊝⊝ lowe,j | |
| All‐cause mortality D28 | 104 per 1000 | 71 per 1000 (35 to 144) | RR 0.69 (0.34 to 1.39) | 722 (2 RCTs)f | ⊕⊕⊝⊝ lowk | |
| All‐cause mortality D60 or above | 262 per 1000 | 270 per 1000 (178 to 408) | RR 1.03 (0.68 to 1.56) | 1633 (4 RCTs)l | ⊕⊝⊝⊝ very lowh,m,n | |
| Adverse events | 713 per 1000 | 727 per 1000 (670 to 792) | RR 1.02 (0.94 to 1.11) | 722 (2 RCTs)f | ⊕⊕⊕⊝ moderateb,o | |
| Serious adverse events | 247 per 1000 | 235 per 1000 (143 to 385) | RR 0.95 (0.58 to 1.56) | 722 (2 RCTs)f | ⊕⊕⊝⊝ lowh,o,p | |
| Time to clinical improvement Follow‐up: 28 to 90 days | 762 per 1000q | 784 per 1000 (729 to 836) | HR 1.07 (0.91 to 1.26) | 1633 (4 RCTs)l | ⊕⊕⊝⊝ lowr,s | |
| Time to WHO Clinical Progression Score of level 7 or above Follow‐up: 28 to 90 days | 187 per 1000t | 133 per 1000 (95 to 186) | HR 0.69 (0.48 to 0.99) | 722 (2 RCTs)f | ⊕⊕⊝⊝ lowe | |
| Time to death Follow‐up: 28 to 90 days | 267 per 1000u | 220 per 1000 (167 to 285) | HR 0.80 (0.59 to 1.08) | 1518 (3 RCTs)v | ⊕⊕⊝⊝ loww | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard Ratio; RR: risk ratio; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate;: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
Explanations
aDeclercq COV‐AID 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021 bImprecision downgraded by one level due to low number of participants. cDeclercq COV‐AID 2021 dIndirectness downgraded by one level: despite a multicentre design this is a single study from a single country, therefore results in this population might not be generalisable to other settings. eImprecision downgraded by two levels due to low number of participants and events. fKyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021 gInconsistency downgraded by one level: I2 = 60.0%. hImprecision downgraded by one level due to wide confidence interval consistent with the possibility for benefit and the possibility for harm, and low number of participants and events. This outcome was not downgraded an additional level for imprecision because it was downgraded one level for inconsistency, which is related to and would have contributed to the severity of the imprecision. iKyriazopoulou SAVE‐MORE 2021 jMulticentre study conducted across several countries, therefore not downgraded for indirectness. kImprecision downgraded by two levels due to wide confidence interval consistent with the possibility for benefit and the possibility for harm, and low number of participants and events. lDeclercq COV‐AID 2021; Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021 mRisk of bias downgraded by one level: some concerns regarding deviation from intended interventions and missing data. nInconsistency downgraded by one level: I2 = 63.2%. oOne additional study was identified that measured this outcome, but no results were reported. pInconsistency downgraded by one level: I2 = 68.2%. qControl group risk calculated from Declercq COV‐AID 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021. rRisk of bias downgraded by one level: some concerns regarding deviation from intended interventions, missing data and outcome measurement. sImprecision downgraded by one level due to a wide confidence interval consistent with the possibility for benefit and the possibility for no effect. tControl group risk calculated from Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021. uControl risk calculated from Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021. vDerde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021 wImprecision downgraded by two levels due to wide confidence interval consistent with the possibility for benefit and the possibility for harm, and low number of participants and events.
Summary of findings 2. Canakinumab compared to standard care/placebo for mild/moderate/severe/critical COVID‐19.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard care/placebo | Risk with canakinumab | |||||
| Clinical improvement D28 | 831 per 1000 | 873 per 1000 (798 to 948) | RR 1.05 (0.96 to 1.14) | 499 (2 RCTs)a | ⊕⊕⊕⊝ moderateb | |
| Clinical improvement D60 or above | ‐ | ‐ | ‐ | ‐ | ‐ | outcome not yet measured or reported |
| WHO Clinical Progression Score of level 7 or above D28 | 123 per 1000 | 89 per 1000 (54 to 148) | RR 0.72 (0.44 to 1.20) | 499 (2 RCTs)a | ⊕⊕⊝⊝ lowc | |
| WHO Clinical Progression Score of level 7 or above D60 or above | ‐ | ‐ | ‐ | ‐ | ‐ | outcome not yet measured or reported |
| All‐cause mortality D28 | 78 per 1000 | 59 per 1000 (30 to 111) | RR 0.75 (0.39 to 1.42) | 499 (2 RCTs)a | ⊕⊕⊝⊝ lowc | |
| All‐cause mortality D60 or above | 250 per 1000 | 138 per 1000 (40 to 478) | RR 0.55 (0.16 to 1.91) | 45 (1 RCT)d | ⊕⊝⊝⊝ very lowc,e | |
| Adverse events | 529 per 1000 | 539 per 1000 (455 to 640) | RR 1.02 (0.86 to 1.21) | 454 (1 RCT)f | ⊕⊕⊕⊝ moderateb,g | |
| Serious adverse events | 218 per 1000 | 174 per 1000 (124 to 246) | RR 0.80 (0.57 to 1.13) | 499 (2 RCTs)a | ⊕⊕⊝⊝ lowc | |
| Time to clinical improvement Follow‐up: 28 days | 889 per 1000 | 903 per 1000 (852 to 941) | HR 1.06 (0.87 to 1.29) | 454 (1 RCT)f | ⊕⊕⊕⊝ moderateb,h | |
| Time to WHO Clinical Progression Score of level 7 or above Follow‐up: 28 days | 123 per 1000 | 100 per 1000 (60 to 163) | HR 0.80 (0.47 to 1.36) | 454 (1 RCT)f | ⊕⊕⊝⊝ lowc | |
| Time to death Follow‐up: 28 to 150 days | 37 per 1000 | 26 per 1000 (13 to 52) | HR 0.71 (0.36 to 1.43) | 499 (2 RCTs)a | ⊕⊕⊝⊝ lowc | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard Ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
Explanations
aCaricchio CAN‐COVID 2021; Cremer Three C Study 2021 bImprecision downgraded by one level due to low number of participants. cImprecision downgraded by two levels due to wide confidence interval consistent with the possibility for benefit and the possibility for harm, and low number of participants. dCremer Three C Study 2021 eIndirectness downgraded by one level: despite a multicentre design this is a single study from a single country, therefore results in this population might not be generalisable to other settings. fCaricchio CAN‐COVID 2021 gWe presume that the adverse event rates, and the corresponding relative risks, are similar across diverse settings; therefore not downgraded for indirectness. hMulticentre study conducted across several countries, therefore not downgraded for indirectness.
Background
Description of the condition
The COVID‐19 pandemic, triggered by the spread of severe acute respiratory syndrome coronavirus 2, (SARS‐CoV2), has led to 5,099,852 deaths worldwide as of 12 November 2021 (Worldometer 2020).
Complex immune dysregulation is a hallmark feature of COVID‐19 disease (Peter 2021). Its pathogenesis is characterised by two distinct immune responses: a well‐co‐ordinated host immune response, which elicits mild clinical symptoms and self‐resolution in most infected individuals, or a maladaptive hyperinflammation resulting in an excessive release of cytokines, which may lead to acute respiratory insufficiency and high mortality (Cavalli 2021). Between 15% and 30% of people hospitalised with COVID‐19 will develop COVID‐19‐associated acute respiratory distress syndrome (Attaway 2021). The global burden of severe pneumonia and acute respiratory distress syndrome and the ensuing need for invasive mechanical ventilation has prompted unprecedented concerted efforts geared towards COVID‐19 drug repurposing research, including immune modulatory treatments.
Thus, as a treatment paradigm, cytokine inhibition is underpinned by a clear rationale in people with severe disease with hyperinflammation, thereby setting the stage for scaling up the use of either non‐selective cytokine production inhibition via corticosteroids or more targeted cytokine inhibitors.
Among targeted cytokine inhibitors, IL‐1 inhibitors have garnered a great deal of clinical attention (Cavalli 2021). This can be attributed to the pivotal role that IL‐1 family members play in orchestrating the innate immune system response to tissue damage and viral infections (Sims 2010). IL‐1 blood levels, which reflect the host inflammatory response, were shown to be highly upregulated in people with severe COVID‐19 (van de Veerdonk 2020).
Description of the intervention
Inhibitors of interleukin‐1 (IL‐1) are proteins whose mode of action can be classified as targeting either the IL‐1 peptide or the IL‐1 receptor (Pile 2015). Three IL‐1 blocking agents are currently available, i.e. anakinra, canakinumab, and rilonacept, each of which can be administered subcutaneously or, in the case of canakinumab, also intravenously. There are two general mechanisms of IL‐1 inhibitors, i.e. binding to the IL‐1 receptor (anakinra) or binding directly to IL‐1 (rilonacept and canakinumab). Whilst anakinra, a bio‐engineered form of the naturally occurring interleukin‐1 receptor antagonist (IL‐1ra), blocks the action of interleukin‐1 (van de Veerdonk 2020), canakinumab is a human anti‐IL‐1ß monoclonal antibody.
How the intervention might work
Two distinct IL‐1 genes, IL1A and IL1B, encoding IL‐1α and IL‐1β, respectively, bind to IL‐1 receptor type, which is present on nearly all cells (Dinarello 2012). Once bound to its receptor, IL‐1 triggers a cascade of inflammatory mediators, chemokines and other cytokines (Dinarello 2012), including IL‐16 and IL‐8. Early prevention of IL‐1 receptor activation with IL‐1 blocking agents is hypothesised to reduce the downstream secretion of IL‐6 and IL‐8, thereby curtailing the risk of an escalation of the cytokine storm.
IL‐1 blockers have become a cornerstone treatment for a broad spectrum of aberrant hyperinflammatory immune response syndrome, ranging from Still’s disease to the treatment of cytokine storm syndromes, including macrophage activation syndrome and cytokine release syndrome (La Rosée 2019). Furthermore, IL‐1 receptor blockers have been shown to improve survival rates in people who have sepsis with macrophage activation syndrome features (Shakoory 2016).
Why it is important to do this review
In the quest for an effective treatment for COVID‐19 that focuses on taming excess cytokine production to reduce morbidity and mortality, attention has been centred on repurposing common immune‐modulating compounds used for inflammation‐associated pathologies, such as IL‐1 inhibitors. However, many drugs have been used while little evidence to support them was available. This has led to supply problems for people who are taking these drugs for the conditions for which they are licensed (e.g. rheumatoid arthritis). For this reason, there is a need for evidence‐based treatment guidelines. To our knowledge, no high‐quality living systematic review is addressing this research question.
This review will be updated as soon as new evidence substantially changes the conclusions or certainty of the evidence of the review, or at least twice a year (i.e. every six months) if no substantial changes occur.
Objectives
To assess the effects of IL‐1 blocking agents compared with standard care alone or with placebo on effectiveness and safety outcomes in people with COVID‐19.
This review is part of a larger project: the COVID network meta‐analysis (COVID‐NMA) initiative (Boutron 2020a). The COVID‐NMA initiative provides decision‐makers with a complete, high‐quality and up‐to‐date mapping and synthesis of evidence on interventions for preventing and treating COVID‐19. We developed a master protocol on the effect of all interventions for preventing and treating COVID‐19 (Boutron 2020b) and a specific protocol for IL‐1 blocking agents detailed in the methods section. Our results are made available and updated weekly on the COVID‐NMA platform at covid-nma.com.
This living review focuses on SARS‐CoV‐2 and does not consider studies evaluating treatment with IL‐1 blocking agents for other coronavirus infections affecting humans.
Methods
The peer‐reviewed protocol (October 2020 version) accepted by the Cochrane editorial team is available on Zenodo (Boutron 2020c) and is registered on PROSPERO (CRD42020214329). The protocol and registration were amended in March 2021 (Boutron 2021). The changes and justifications are described in Differences between protocol and review. The methods for the living process of the review are available in Appendix 1.
Types of studies
We included randomised controlled trials (RCTs) of any design (e.g. parallel‐group, cluster and factorial) with no restrictions on language. We excluded early‐phase clinical trials, single‐arm trials, non‐randomised studies and modelling studies of interventions for COVID‐19, as well as prognosis studies, systematic reviews and meta‐analyses, and diagnostic test accuracy studies.
Types of participants
We included trials evaluating children or adults with suspected, probable, or confirmed COVID‐19 (see classification in Appendix 2; (WHO 2020a)).
Interventions
We included the following IL‐1 blocking agents, with no restriction on dose, frequency, or mode of administration:
anakinra (interleukin‐1 receptor antagonist);
canakinumab (human anti‐IL‐1β monoclonal antibody);
rilonacept (interleukin‐1 blocker).
Comparator(s)
We considered the following types of comparators in this review:
standard care alone or with placebo;
standard of care as defined by trialists.
Outcome measures
Our outcome selection was based on the CORE outcome sets developed by the WHO (WHO Working Group 2020), and advice from content experts. We predefined the following critical and important outcome measures.
Critical outcomes
Effectiveness outcomes
We considered the following outcomes with related time points reported as days (D) of follow‐up.
Clinical improvement (D28/ ≥ D60) defined as a hospital discharge or improvement on the scale used by trialists to evaluate clinical progression and recovery. We recorded the scale and the threshold used by authors to define improvement as appropriate.
WHO Clinical Progression Score of level 7 or above (i.e. mechanical ventilation +/‐ additional organ support (extracorporeal membrane oxygenation (ECMO), vasopressors or dialysis) or death (D28/ ≥ D60).
All‐cause mortality (D28/ ≥ D60).
We reported all assessments performed at D60 and later under ≥ D60.
Safety outcomes
Incidence of any adverse events (AEs)
Incidence of serious AEs (SAEs)
For each time point, we considered the time of randomisation as D0. However, if not reported, we considered D0 as reported by the authors. When outcomes were assessed at time points other than those selected by the review, we chose the closest (e.g. D15 for D28).
We presented all critical outcomes in the summary of findings tables.
Important outcomes
Time to clinical improvement
Time to WHO Clinical Progression Score of level 7 or above
Time to death
Search methods for identification of studies
The search relied on the search for the COVID‐NMA initiative (Boutron 2020a; Boutron 2020b).
The initial search strategy was developed with an Information Specialist from the Cochrane Editorial & Methods Department (Robin Featherstone). The current search strategies are listed in full in Appendix 3.
To improve our process and optimise our resources, we evaluated two secondary sources: the L‐OVE platform and the Cochrane COVID‐19 Study Register. We found that searching both secondary sources allowed us to identify 100% of the reports of RCTs (preprint or peer‐reviewed publication) assessing treatment or preventive interventions for COVID‐19 (Pierre 2021). We consequently modified our search strategy on 7 September 2020, and now only search the L‐OVE platform, the Cochrane COVID‐19 Study Register and the Retraction Watch Database.
The Cochrane COVID‐19 Study Register is a specialised register built within the Cochrane Register of Studies (CRS), and is maintained by Cochrane Information Specialists. The register contains study reports from several sources, including:
daily searches of ClinicalTrials.gov;
weekly searches of PubMed;
weekly searches of Embase.com;
weekly searches of the WHO International Clinical Trials Registry Platform (ICTRP);
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL).
Complete data sources and search methods for the register are available at: community.cochrane.org/about-covid-19-study-register.
The COVID‐19 L‐OVE platform is a digital repository built by systematic searches in multiple databases, trial registries and preprint servers. Complete data sources and search methods are available at: app.iloveevidence.com/covid19/methods
Electronic searches
We searched the following databases on 5 November 2021.
The L‐OVE platform (app.iloveevidence.com/covid19), every working day since 7 September 2020.
The Cochrane COVID‐19 Study Register (covid-19.cochrane.org/), weekly since 7 September 2020.
Additional sources for the COVID‐NMA searched prior to September 2020 are reported elsewhere (Boutron 2020b).
If no peer‐reviewed publication was available, we included and extracted the preprint. We recognise that preprints are not peer‐reviewed and are living documents that can be updated or published. We developed a preprint tracker in collaboration with a research team from the French National Centre for Scientific Research, which systematically informs us when a preprint is updated or published (Cabanac 2021). As soon as an update was identified, we recorded the data not available in the initial report. We checked the data for discrepancies against that already extracted and extracted data from the most recent report in case of discrepancies. We updated the analysis if needed.
Searching other resources
We searched the following trial registries for unpublished and ongoing trials on 3 November 2021.
The WHO ICTRP (www.who.int/ictrp/en/) to identify ongoing and completed clinical trials on COVID‐19. We used the List By Health Topic: 2019‐nCoV / COVID‐19 filter and retrieved all studies identified.
The European Medicines Agency (EMA) clinical data website (clinicaldata.ema.europa.eu/web/cdp/home) to identify trials submitted to the EMA, and searched for the Clinical Study Report of eligible trials.
The US Food and Drug Administration (FDA) website to identify FDA approval trials (www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19).
The Retraction Watch Database for retracted trials (retractionwatch.com/retracted-coronavirus-covid-19-papers/).
Of note, we did not check the references of reports included as the living search process identifies COVID‐19 trial records prospectively from the point of trial registration.
Data collection and analysis
As part of the COVID‐NMA living systematic review (Boutron 2020b), we search, screen, and extract data daily. An updated synthesis is reported online at least bi‐weekly.
Selection of studies
We used an Excel spreadsheet to document search dates and numbers of citations identified. Two review authors independently screened the records and abstracts in duplicate using Rayyan (Ouzzani 2016). We resolved discrepancies on exclusion and screening of full texts by consensus between both reviewers or involving a third reviewer. We recorded reasons for exclusion for all studies excluded after full text review.
Data extraction and management
Two review authors independently read each preprint, peer‐reviewed publication, protocol, or other study reports, evaluated the completeness of the data availability, and assessed the risk of bias. We used a specific structured online data extraction form. The online tool automatically identified any discrepancies, and both review authors involved in the data extraction discussed these to reach a consensus.
The information we extracted included study characteristics (such as first author, publication year and journal, funding source), number of participants randomised, participant characteristics (e.g. severity of clinical presentation), comorbidities, co‐interventions, intervention details (e.g. dose, schedule), outcome measures, and risk of bias assessment.
We systematically contacted the trial authors to ask them for supplementary information unavailable from the trial reports (Appendix 4). The WHO team with whom we are working requested these data by sending a personalised email. We classified disease severity as described below, according to clinical status or clinical management. This classification relies on existing classification and clinical expertise (WHO 2020a; WHO 2020b). We considered the description of eligibility criteria as well as the baseline characteristics of participants, and classified the severity as follows.
Mild disease ambulatory: 'outpatients' whose clinical symptoms are mild with no sign of pneumonia on imaging.
Mild disease: clinical symptoms requiring hospitalisation but no need for supplemental oxygen.
Moderate disease: fever and respiratory symptoms with radiological findings of pneumonia and requiring standard oxygen therapy O2 (3 to 5 L/min).
-
Severe disease: meeting any of the following criteria:
respiratory distress (≥ 30 breaths/min);
oxygen saturation ≤ 93% at rest in ambient air or oxygen saturation ≤ 97% with O2 > 5 L/min;
PaO2/FiO2 ≤ 300 mmHg (1 mmHg = 0.133 kPa). PaO2/FiO2 in high‐altitude areas (> 1000 metres above sea level) is corrected by the following formula: PaO2/FiO2 x (atmospheric pressure (mmHg)/760);
people hospitalised on non‐invasive ventilation (NIV)/high flow nasal oxygen (HFNO).
-
Critical disease: cases meeting the following criteria:
respiratory failure requiring invasive mechanical ventilation;
shock;
other organ failure requiring intensive care unit care.
When no data related to these classifications were available, we requested the information from the authors.
For dichotomous outcomes, we calculated the relative risk (RR) with 95% confidence intervals (CIs) as a measure of effect. We extracted the number of events and the number of total participants in each trial arm. For time‐to‐event outcomes, we extracted the hazard ratio (HR) with 95% CIs. When these were not provided, we attempted to obtain them using the tools provided in Tierney 2007. For time‐to‐event outcomes, when several analyses were reported, we extracted results obtained from the intention‐to‐treat (ITT) analysis whenever these were available. If ITT results were not available, we extracted results from any modified ITT analyses.
Assessment of risk of bias in included studies
We assessed the trials using the Cochrane Risk of Bias 2 (RoB 2) tool for RCTs (Sterne 2019).
The Cochrane RoB 2 tool is structured into five domains:
risk of bias arising from the randomisation process;
risk of bias due to deviations from intended interventions;
risk of bias due to missing outcome data;
risk of bias in the measurement of the outcome;
risk of bias in the selection of the reported result.
A series of 'signalling questions' elicit information relevant to risk of bias assessment within each domain. The response options to the signalling questions are: 'yes'; 'probably yes'; 'probably no'; 'no'; and 'no information'. A risk of bias judgement for each domain is generated by an algorithm, based on answers to the signalling questions. Judgement can be 'low', 'some concerns' or 'high' risk of bias. Overall risk of bias is considered 'low' if all domains are at 'low risk'; 'some concerns' if at least one domain has 'some concerns' and no domain at 'high' risk of bias; and 'high' if at least one domain is at 'high risk'.
We assessed the risk of bias for all critical and important outcomes.
In the context of this review, we are interested in quantifying the effect of assignment to the interventions at baseline, regardless of whether the interventions were received as intended (the ITT effect).
The Cochrane Bias Methods Group developed training materials on the risk of bias assessment tool RoB 2, which is used by the systematic reviewers participating in data extraction and risk of bias assessment for the COVID‐NMA platform (available upon request).
We recorded judgements for each domain and time point by using an online data extraction tool.
Two review authors independently assessed the risk of bias of each study at the outcome level, with consensus in case of disagreement. Review authors had epidemiological training or were members of the Cochrane Response team. They were trained using the material developed by the Cochrane Bias Methods Group. Each review author independently assessed the included manuscripts and used signalling questions for each bias domain, which was fed into the related algorithm to obtain a judgement. Both review authors recorded their judgement and support for judgement, but not their answers to signalling questions. For the consensus, all disagreements in judgement were identified and discussed until consensus was achieved. If needed, a third review author was involved.
To ensure standardisation of judgement and justification, the review authors, as well as the COVID‐NMA core team, revised the assessments/support for judgement.
Standardised assessments
In the context of the COVID‐19 pandemic, we also standardised our assessment of some domains.
Domain 2. Risk of bias due to deviations from intended interventions
In trials where participants and carers were not blinded, we specified some deviations that could arise because of the trial context and could affect the trial outcomes.
-
Cross‐over from the control group to the intervention group:
when the number of participants in the control receiving the intervention was important, we rated this domain as ‘some concerns’;
when the cross‐over was planned in the protocol for participants with clinical worsening, we decided to rate this domain as ‘some concerns’ because the trial context could have influenced the decision to provide the treatment.
-
Co‐interventions:
-
the following co‐interventions could affect the trial outcomes:
remdesivir and other antivirals;
corticosteroids;
biologics.
when these co‐interventions were reported and balanced, we assessed this domain as ‘low’ risk of bias;
when these co‐interventions were reported but imbalanced, we rated this domain as ‘some concerns’ and not ‘high risk’ of bias as it is impossible to distinguish between deviation because of trial context and deviation because of intervention effect.
-
Domain 2. Analysis to estimate the effect of assignment
For critical outcomes (i.e. binary outcomes), the analysis evaluated was not always based on the analysis reported by authors, but on our analysis where we considered all participants randomised as the denominator.
For time‐to‐event outcomes, ITT analyses were considered appropriate.
-
When the analysis was not an ITT analysis, we rated this domain on a case‐by‐case basis according to:
the number of participants who crossed over and were not analysed in the group allocated;
the number of participants excluded from the analysis for reasons other than missing data, and imbalance between arms in terms of number and reasons for exclusion.
Domain 4. Risk of bias in measurement of the outcome
We prespecified the following rules.
Clinical Improvement (D28/ ≥ D60/time‐to‐event): assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but this is not likely in the context of the pandemic.
WHO Clinical Progression Score of level 7 or above (D28/ ≥ D60/time‐to‐event): assessment of this outcome is probably not influenced by knowledge of the intervention assignment.
All‐cause mortality (D28/ ≥ D60/time‐to‐event): assessment of this outcome is not influenced by knowledge of the intervention assignment.
-
Adverse events and serious adverse events:
when detection of events relies only on measures that cannot be influenced by judgement (e.g. laboratory detected events), assessment of this outcome is probably not influenced by knowledge of the intervention assignment;
when detection events rely only on measures that can be influenced by judgement (e.g. clinically and laboratory detected events), assessment of this outcome can be influenced by knowledge of the intervention assignment but this is not likely in the context of a pandemic.
Unit of analysis issues
We treated comparisons from multi‐arm or platform trials as independent two‐arm trials since we did not pool comparisons of different drugs in the same meta‐analysis. We did not identify any cross‐over or cluster‐randomised trials. If we do identify eligible cluster‐randomised trials in future updates of the review, we will extract results that properly account for the cluster design (such as based on a multilevel model or on generalised estimating equations). If such an analysis is not reported, we will try to obtain an estimate of the intraclass correlation coefficient and calculate data required for the meta‐analyses, taking the design effect into consideration.
Dealing with missing data
For missing outcome data, we extracted the number of participants who dropped out before completing the trial and how trial authors handled missing outcome data. In our primary analysis for the critical outcomes, we followed a conservative approach assuming that participants with missing outcome data did not experience the event of interest. Hence, we calculated all RRs with the number of participants randomised in each group in the denominator. We also conducted sensitivity analyses to assess the potential impact of missing outcome data on the results by using an available case analysis with the number of participants analysed (e.g. only participants without missing outcome data or only participants who received treatment) in the denominator (see below: Sensitivity analyses).
Assessment of heterogeneity
We generated descriptive statistics for both the trial and population characteristics. We examined the distribution of important clinical and methodological variables (e.g. age, disease severity, pre‐existing conditions and comorbidities, location). We used visual inspection of forest plots, the I2 statistic and the magnitude of between‐study variance (Tau2) to estimate the level of heterogeneity. In this review, we did not use prediction intervals (the interval within which the effect of a future trial is expected to lie (Riley 2011)), or comparison with appropriate empirical distributions (Turner 2012), because of the small number of trials; however, these are planned for future updates if appropriate.
Assessment of reporting bias
We assessed the risk of bias due to missing results in the synthesis according to the framework proposed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Searches in trial registries were used to identify any initiated, ongoing, or completed but not published trials meeting this review's eligibility criteria. We contacted all responsible parties to obtain an updated report of the results included in the trial registry. For published trials, we contacted the corresponding authors to get the missing data.
We checked whether the results of all our critical and important outcomes were reported as prespecified in the trial register. When registration was not prospective, we also checked the protocol or statistical analysis plan if available.
When any trial results were not available, we used a matrix indicating the availability of trial results, as recommended in the Cochrane Handbook (Higgins 2021; Kirkham 2018).
We checked whether the results were unavailable because of the P value, magnitude, or direction of the result. We considered the risk of bias due to missing results if one specified outcome of the registry was missing from the main report because of these reasons.
Due to the small number of trials, we could not assess the potential for reporting bias across studies either graphically or statistically.
Data synthesis
We have combined trials evaluating the same drug with standard care alone or with placebo comparators together under the same comparison. We included all eligible RCTs in the primary analysis, regardless of the risk of bias assessment.
We did not combine trials assessing different drugs because these drugs have a different mode of action; anakinra is an interleukin‐1 receptor antagonist, while canakinumab is a human anti‐IL‐1β monoclonal antibody.
We calculated the log RRs and their standard error for binary outcomes using the number of events and total participants in each arm, then we pooled the trial‐specific effect sizes. For time‐to‐event outcomes, we directly extracted the HRs and the respective 95% CIs from the trial reports and subsequently pooled them in the meta‐analysis.
For each direct comparison with at least two trials providing data, we presented effect estimates with 95% CIs. We used the random‐effects model as we anticipated clinical and methodological heterogeneity across trials.
We conducted all analyses using our 'R‐shiny' application (available from covid-nma.com/pairwise_meta_analysis/), based on the 'metafor' package in R.
Subgroup analyses
Because of the low number of trials, we did not carry out the prespecified subgroup analyses. Had there been sufficient data, we would have performed subgroup analyses based on participant characteristics, timing of the treatment and disease severity (mild disease only, mixed disease (i.e. participants heterogeneous in terms of severity, participants with moderate disease and participants with severe disease) and critical disease only).
Sensitivity analyses
We performed sensitivity analyses by excluding trials with a high overall risk of bias and RCTs reported as preprint only. We also ran the analyses using the number of participants analysed instead of those randomised (Chaimani 2018; Mavridis 2015; Mavridis 2018; White 2008).
Summary of findings and assessment of the certainty of the evidence
To evaluate the confidence in the results of the pairwise comparisons for critical and important outcomes, we used the GRADE approach (Schünemann 2019). We prepared summary of findings tables using the GRADEpro GDT tool to present estimated relative and absolute risks for critical and important outcomes. One review author assessed the overall certainty of the evidence for each outcome, and another review author cross‐checked it using the GRADE classification (Schünemann 2019).
Results
Description of studies
For a complete description of studies, please see the Characteristics of included studies, Characteristics of excluded studies (Appendix 5), and Characteristics of unpublished registered studies tables (Appendix 6 and Appendix 7). The full dataset used in the analyses is publicly available (Davidson 2021).
Results of the search
The results of searches are detailed in Figure 1. We retrieved a total of 48,043 references by searching electronic bibliographic databases, after excluding duplicates; 699 were eligible for full‐text screening. We included seven reports of six RCTs (five published in peer‐reviewed journals and one reported as a preprint) evaluating IL‐1 blocking agents. Four RCTs evaluated anakinra (Declercq COV‐AID 2021; Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021), and two evaluated canakinumab (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021).
1.
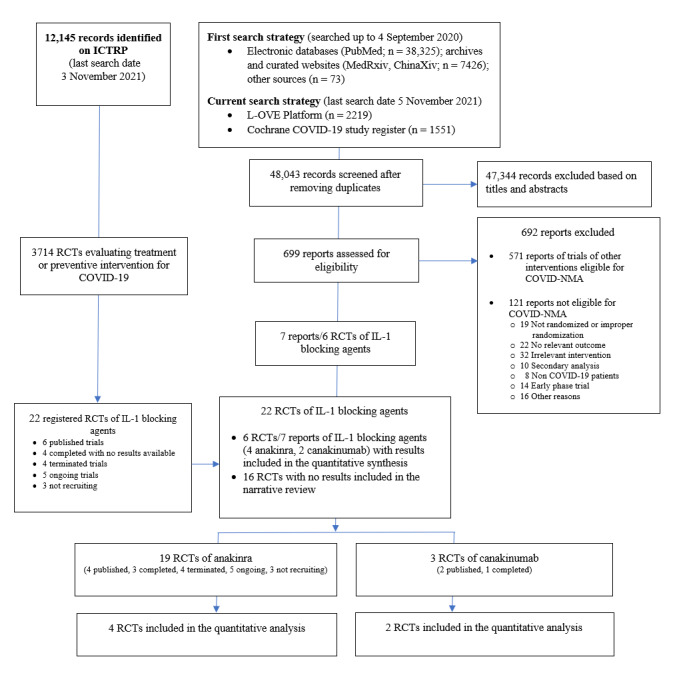
Flowchart of included randomised controlled trials (RCTs) of interleukin 1 (IL‐1) blocking agents (last search date 5 November 2021)
COVID‐NMA is a living systematic review of all trials assessing treatment and preventive interventions for COVID‐19 (Boutron 2020b). This review is a subreview of COVID‐NMA.
ICTRP: World Health Organization (WHO) International Clinical Trials Registry Platform
We did not identify any retracted articles. The search of the US Food and Drug Administration website did not retrieve any reports. The search in registries identified 22 registered trials, of which 16 had no results available.
We also contacted the named contacts for trials registered with no associated publication of results. The responses are detailed in Appendix 8.
Overall, we identified 19 RCTs of anakinra (three published in peer‐reviewed journals, one reported as a preprint, three completed with no results available, four terminated, five ongoing, three not recruiting); three RCTs of canakinumab (two published in peer‐reviewed journals, one completed); and no RCTs of rilonacept either published or registered.
Included studies
Source of the data
Reports of five trials were published in peer‐reviewed journals (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021; Declercq COV‐AID 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021) and one was available as a preprint (Derde REMAP‐CAP 2021).
Results of two published trials were posted on clinical trial registries (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021). All data correspond to those reported in the preprint and peer‐reviewed journal articles. We also contacted corresponding authors of the six trials to request additional data; three authors provided information (Caricchio CAN‐COVID 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). We are awaiting a response from the other trial authors.
Study design
Four trials used a two‐arm parallel‐group randomised design (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). In another trial, a multifactorial four‐arm adaptive platform trial assessed anakinra compared to other active treatment (tocilizumab, sarilumab) and standard care (Derde REMAP‐CAP 2021). One trial used a 2 x 2 factorial design assessing anakinra and IL‐6 blocking agents (tocilizumab and siltuximab) (Declercq COV‐AID 2021), one was a two‐arm RCT with imbalanced randomisation (1:2) (Kyriazopoulou SAVE‐MORE 2021), and one was a proof‐of‐concept study (Cremer Three C Study 2021).
Three were placebo‐controlled trials (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021; Kyriazopoulou SAVE‐MORE 2021). The sample size ranged from 45 to 2253. One trial was stopped early by the data and safety monitoring board after an interim analysis due to futility (Mariette CORIMUNO‐19 Collaborative 2021); one was stopped as the statistical trigger for inferiority of anakinra compared to other active interventions (tocilizumab, sarilumab) was met (Derde REMAP‐CAP 2021).
Study registration
All trial registration records were available, and all six trials were prospectively registered (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021; Declercq COV‐AID 2021; Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021).
Settings
All trials were multicentre (2 to 133 centres); they were conducted in France (Mariette CORIMUNO‐19 Collaborative 2021), Belgium (Declercq COV‐AID 2021), the USA (Cremer Three C Study 2021), or were multicountry trials (Caricchio CAN‐COVID 2021; Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021).
Overall, two trials were conducted during the time and setting for high prevalence of a SARS‐CoV‐2 variant of concern, Alpha variant (B.1.1.7) (Kyriazopoulou SAVE‐MORE 2021; Derde REMAP‐CAP 2021). The information, obtained from Mullen 2020, was derived from data on the prevalence of the variant in the population during the study period. One multicountry trial showed high prevalence of the Alpha variant in two of the participating countries (United Kingdom and Italy) towards the end of the trial (Derde REMAP‐CAP 2021). The other trial conducted in two countries (Italy and Greece) showed prevalence of this variant during the whole study period (Kyriazopoulou SAVE‐MORE 2021).
Characteristics of participants
We included a total of 2132 participants (six RCTs ) in the analysis of this review (four RCTs, 1633 randomised participants assessing anakinra; two RCTs, 499 randomised participants assessing canakinumab). The median/mean age range varied from 58 to 68 years; the proportion of men varied from 58% to 77%. All participants were hospitalised.
Participants had moderate disease (Mariette CORIMUNO‐19 Collaborative 2021), mild to severe disease (Caricchio CAN‐COVID 2021; Kyriazopoulou SAVE‐MORE 2021), mild to critical disease (Cremer Three C Study 2021), moderate to critical disease (Declercq COV‐AID 2021), severe to critical disease (Derde REMAP‐CAP 2021).
The percentage of participants on oxygen at baseline but not intubated was respectively 67% (Derde REMAP‐CAP 2021), 69% (Cremer Three C Study 2021), 87% (Declercq COV‐AID 2021), 94% (Caricchio CAN‐COVID 2021; Kyriazopoulou SAVE‐MORE 2021) and 100% (Mariette CORIMUNO‐19 Collaborative 2021). The percentage of participants intubated was 11% (Declercq COV‐AID 2021), 22% (Cremer Three C Study 2021), 33% (Derde REMAP‐CAP 2021), and none (Caricchio CAN‐COVID 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). The Kyriazopoulou SAVE‐MORE 2021 trial used a biomarker for inflammation, the soluble urokinase plasminogen activator receptor (suPAR), to identify people in a hyperinflammatory state who may be more likely to respond to IL‐1 blockade. Additionally, trials used both inflammatory and other biomarkers as part of inclusion criteria. These included C‐reactive protein (CRP) (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021; Declercq COV‐AID 2021; Mariette CORIMUNO‐19 Collaborative 2021), ferritin (Caricchio CAN‐COVID 2021; Declercq COV‐AID 2021), lymphopenia and D‐dimer (Declercq COV‐AID 2021), as well as troponin T and N‐terminal pro‐brain‐type natriuretic peptide (NT‐proBNP) (Cremer Three C Study 2021).
Details of the interventions
Anakinra was compared to placebo in one trial (Kyriazopoulou SAVE‐MORE 2021), and compared to standard care in three trials (Declercq COV‐AID 2021; Derde REMAP‐CAP 2021; Mariette CORIMUNO‐19 Collaborative 2021). Canakinumab was compared to a placebo in both trials (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021).
The treatment regimen varied slightly between trials. Two trials administered anakinra intravenously (Derde REMAP‐CAP 2021; Mariette CORIMUNO‐19 Collaborative 2021). The treatment was administered over 14 days (i.e. 300 mg was administered intravenously for the first 24 hours, followed by a maintenance dose of 100 mg intravenously four times a day for 14 days or until improvement) (Derde REMAP‐CAP 2021), or over five days (i.e. 200 mg intravenously twice daily on days one to three, 100 mg twice daily on day four, 100 mg once daily on day five) (Mariette CORIMUNO‐19 Collaborative 2021). In two trials, anakinra was administered subcutaneously; 100 mg/day once daily for 28 days or until hospital discharge (Declercq COV‐AID 2021), and 100 mg/day once daily for 7 to 10 days (Kyriazopoulou SAVE‐MORE 2021).
Canakinumab was administered intravenously as a single‐dose treatment. The dosage varied from 450 mg to 750 mg in the Caricchio CAN‐COVID 2021 study to 300 mg/600 mg (two study arms were merged) in the Cremer Three C Study 2021.
In all trials assessing anakinra, corticosteroids were administered at baseline in the majority of the participants for Kyriazopoulou SAVE‐MORE 2021 (84% anakinra vs 89% control group); Derde REMAP‐CAP 2021 (86% anakinra vs 67% control group); Declercq COV‐AID 2021 (67% anakinra vs 60% control group); while Mariette CORIMUNO‐19 Collaborative 2021 reported modest use of corticosteroid at baseline (12% anakinra group vs 15% control group) but an increase during the trial with half (51% anakinra vs 53% control group) of participants given corticosteroids.
Regarding the two trials of canakinumab, one reported use of corticosteroids at baseline in 41% vs 32% in the control group (Caricchio CAN‐COVID 2021), and one reported use of corticosteroid in 38% vs 63% in the control group (Cremer Three C Study 2021).
In the four trials reporting on anakinra, three trials reported on the administration of remdesivir at baseline (Declercq COV‐AID 2021; Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021). In all trials, the use of remdesivir was balanced, i.e. 73% vs 70% (Kyriazopoulou SAVE‐MORE 2021), 30% vs 26% (Derde REMAP‐CAP 2021), 7% vs 4% (Declercq COV‐AID 2021).
Both trials on canakinumab reported on the use of remdesivir at baseline (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021). Administration of remdesivir prior to day one was balanced in one trial (22% vs 20%) (Caricchio CAN‐COVID 2021), and unbalanced in the second trial (52% vs 38%) (Cremer Three C Study 2021).
Funding sources
Two trials were funded by pharmaceutical companies (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021), two were funded through public/non‐profit sources (Declercq COV‐AID 2021, Mariette CORIMUNO‐19 Collaborative 2021) and two through mixed public/private (pharmaceutical company) sources (Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021).
Excluded studies
We excluded a total of 692 reports; 571 were RCTs evaluating other interventions for COVID‐19 and consequently included in the COVID‐NMA platform (covid-nma.com); 121 full‐text reports were excluded from the COVID‐NMA platform. We provided details on the reasons for exclusions in Appendix 5.
Ongoing studies
We identified 16 trials with no published results from registries. More details are available in Appendix 6 and Appendix 7.
Risk of bias in included studies
Appendix 9 summarises the risk of bias assessments by outcome.
Risk of bias arising from the randomisation process
Randomisation was described adequately and was appropriate in all trials (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021; Declercq COV‐AID 2021; Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021).
Risk of bias due to deviations from intended interventions
We judged the risk of bias due to deviation from intended interventions to be low in five trials (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021; Declercq COV‐AID 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). We had some concerns about this domain for all outcomes in one trial (Derde REMAP‐CAP 2021), because the study was not blinded and no information was provided on the use of co‐interventions after the trial started. We did not downgrade any trial for the population of analysis.
Risk of bias due to missing outcome data
We judged the risk of bias due to incomplete outcome data to be low for five trials for all outcomes, since there was either no missing data (Cremer Three C Study 2021; Declercq COV‐AID 2021), or a low amount of missing data (Caricchio CAN‐COVID 2021; Derde REMAP‐CAP 2021; Mariette CORIMUNO‐19 Collaborative 2021). For one trial (Kyriazopoulou SAVE‐MORE 2021), we judged the risk of bias due to incomplete data to be low for some outcomes (all‐cause mortality D28, clinical improvement D28, adverse events and serious adverse events), as these had a low amount of missing data. However, for other outcomes with longer follow‐up and time‐to‐event outcomes, we had some concerns due to an important amount of missing data.
Risk of bias in the measurement of the outcome
In three open‐label trials (Declercq COV‐AID 2021; Derde REMAP‐CAP 2021; Mariette CORIMUNO‐19 Collaborative 2021), we judged the risk of bias to be low for all observer‐reported outcomes not involving clinical judgement (i.e. all‐cause mortality and WHO Clinical Progression Score of level 7 or above). In contrast, we had some concerns of risk of bias for the outcomes that could potentially be influenced by knowledge of the intervention assignment (i.e. clinical improvement, adverse events and serious adverse events).
In the three trials in which the outcome assessors were blinded (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021; Kyriazopoulou SAVE‐MORE 2021), we judged the risk of bias to be low for all outcomes.
Risk of bias in the selection of the reported results
All trials had prospective registries and protocols available (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021; Declercq COV‐AID 2021; Derde REMAP‐CAP 2021; Mariette CORIMUNO‐19 Collaborative 2021), or obtained upon request (Kyriazopoulou SAVE‐MORE 2021), and we judged these to be low risk of bias for all reported outcomes. Conversely, in one trial, we rated the risk of bias in the selection of the reported results as 'some concerns' because the outcome 'clinical improvement D28' was not prespecified (Cremer Three C Study 2021).
Bias due to missing results in the synthesis
We present a matrix indicating the availability of trial results for critical and important review outcomes in Appendix 10 and Appendix 11. There was no evidence of bias due to missing results, except for two trials that planned to assess adverse and serious adverse events but did not report the results (Declercq COV‐AID 2021; Derde REMAP‐CAP 2021).
Effects of interventions
Anakinra versus standard of care/placebo
We report the certainty of the evidence for critical outcomes and important outcomes in Table 1. We report the effect sizes of the outcomes for this comparison in Appendix 12.
Critical outcomes
Clinical improvement
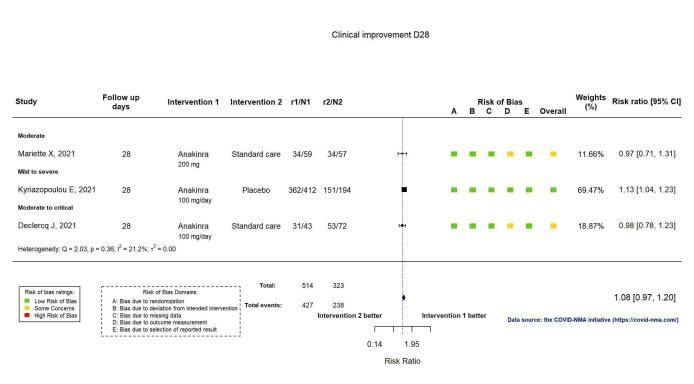
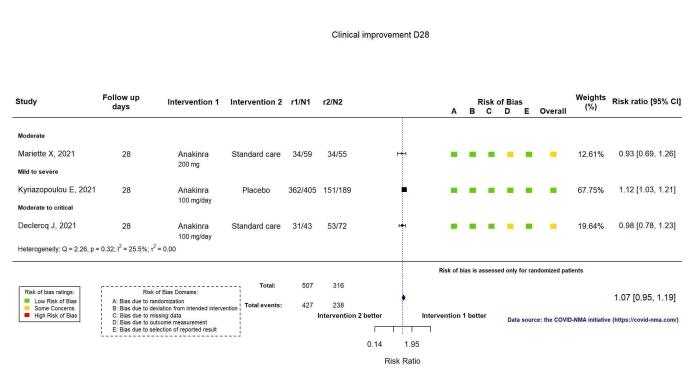
The proportion of participants achieving improvement at D28 was reported in three RCTs (Declercq COV‐AID 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). Clinical improvement was defined as an increase of at least two points on a 6‐category ordinal scale (compared with the worst status at day of randomisation) or discharge from the hospital alive (Declercq COV‐AID 2021), or as hospital discharge (Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). Anakinra probably results in little or no increase in clinical improvement at D28 (risk ratio (RR) 1.08, 95% confidence interval (CI) 0.97 to 1.20; I2 = 21.2%; 3 RCTs, 837 participants; absolute effect: 59 more per 1000 (from 22 fewer to 147 more); moderate‐certainty evidence; Figure 2).
2.
Analysis 1.1.1: Anakinra versus standard care/placebo: Clinical improvement D28
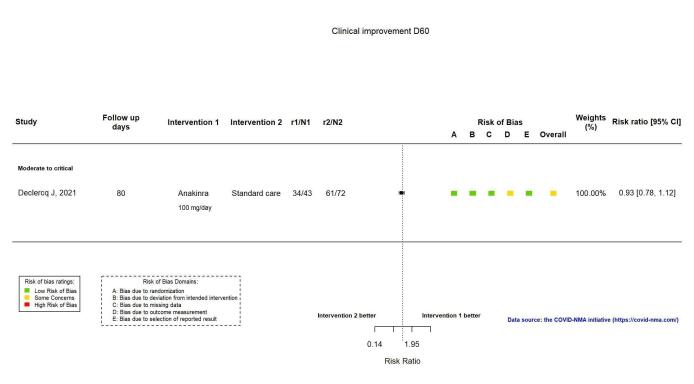
The proportion of participants achieving improvement at ≥ D60 was reported in one RCT (Declercq COV‐AID 2021). The evidence is very uncertain about an effect of anakinra on the proportion of participants with clinical improvement at ≥ D60 (RR 0.93, 95% CI 0.78 to 1.12; 1 RCT, 115 participants; absolute effect: 59 fewer per 1000 (from 186 fewer to 102 more); very low‐certainty evidence; Figure 3).
3.
Analysis 1.1.2: Anakinra versus standard care/placebo: Clinical improvement D60 or above
WHO Clinical Progression Score of level 7 or above (i.e. the proportion of participants with mechanical ventilation +/‐ additional organ support or death)
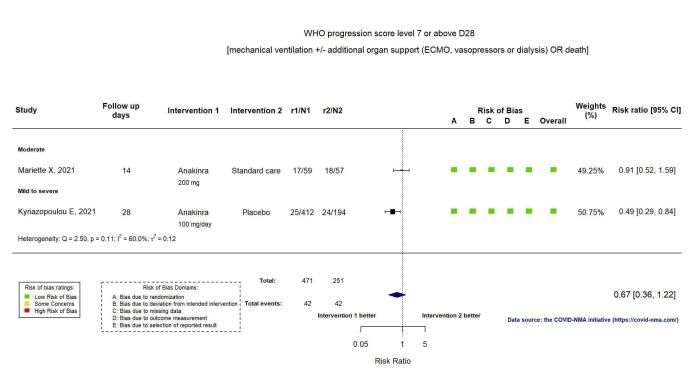
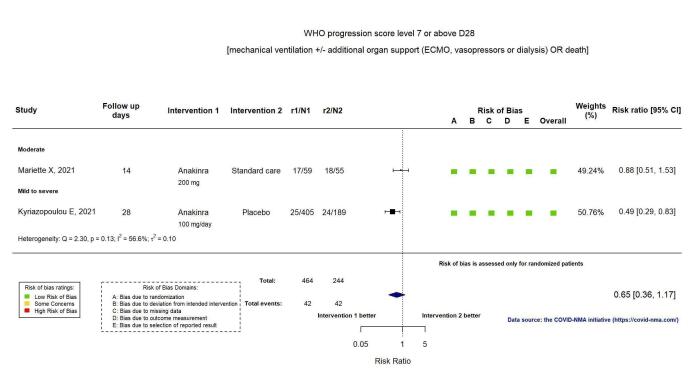
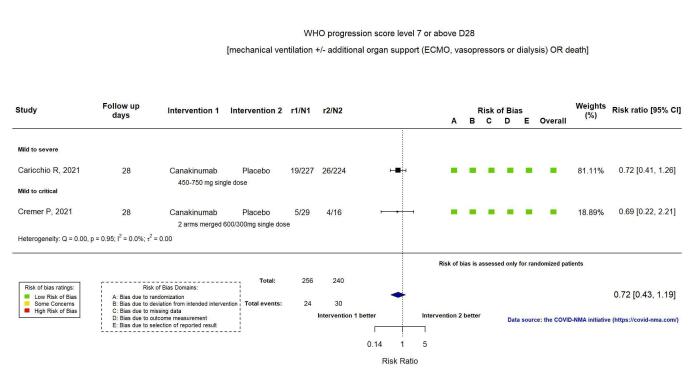
Two RCTs reported the proportion of participants with mechanical ventilation or death at D28 (Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). Overall, the evidence is uncertain for an effect of anakinra on the proportion of participants with a WHO Clinical Progression Score of level 7 or above at D28 (RR 0.67, 95% CI 0.36 to 1.22; I2 = 60.0%; 2 RCTs, 722 participants; absolute effect: 55 fewer per 1000 (from 107 fewer to 37 more); low‐certainty evidence; Figure 4).
4.
Analysis 1.1.3: Anakinra versus standard care/placebo: WHO Clinical Progression Score of level 7 or above D28
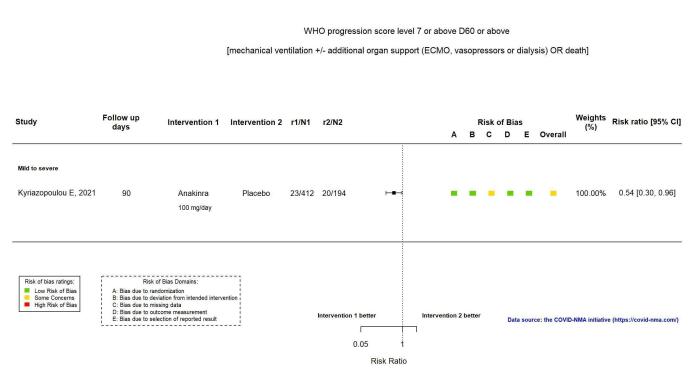
The proportion of participants with mechanical ventilation or death at ≥ D60 was reported in one RCT (Kyriazopoulou SAVE‐MORE 2021). The evidence of the effect of anakinra on mechanical ventilation or death at ≥ D60 is uncertain (RR 0.54, 95% CI 0.30 to 0.96; 1 RCT, 606 participants; absolute effect: 47 fewer per 1000 (from 72 fewer to 4 fewer); low‐certainty evidence; Figure 5).
5.
Analysis 1.1.4: Anakinra versus standard care/placebo: WHO Clinical Progression Score of level 7 or above D60 or above
All‐cause mortality
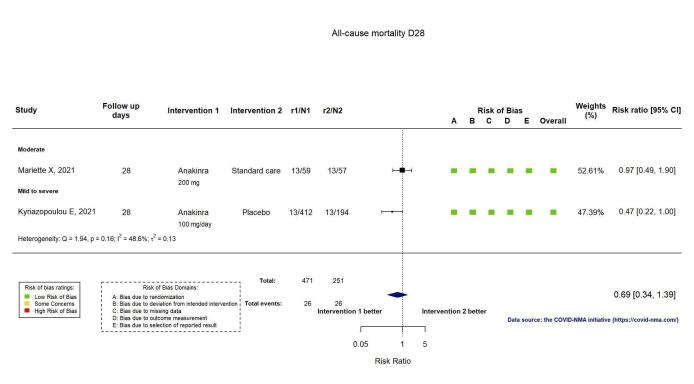
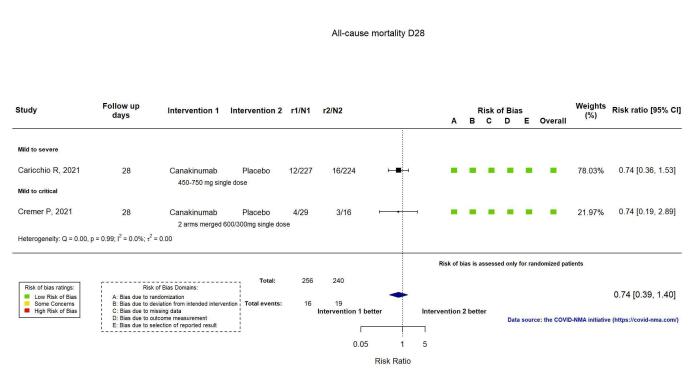
Two RCTs reported all‐cause mortality at D28 (Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021), and four at D60 or above (Declercq COV‐AID 2021; Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). The evidence of an effect of anakinra on all‐cause mortality at D28 is uncertain (RR 0.69, 95% CI 0.34 to 1.39; I2 = 48.6%; 2 RCTs, 722 participants; absolute effect: 32 fewer per 1000 (from 68 fewer to 40 more); low‐certainty evidence; Figure 6).
6.
Analysis 1.1.5: Anakinra versus standard care/placebo: All‐cause mortality D28
Four RCTs reported all‐cause mortality at ≥ D60. Overall, the evidence of an effect of anakinra on all‐cause mortality at ≥ D60 is very uncertain (RR 1.03, 95% CI 0.68 to 1.56; I2 = 63.2%; 4 RCTs, 1633 participants; absolute effect: 8 more per 1000 (from 84 fewer to 147 more); very low‐certainty evidence; Figure 7).
7.
Analysis 1.1.6: Anakinra versus placebo or standard care. Outcome: All‐cause mortality D60 or above
Adverse events (AEs)
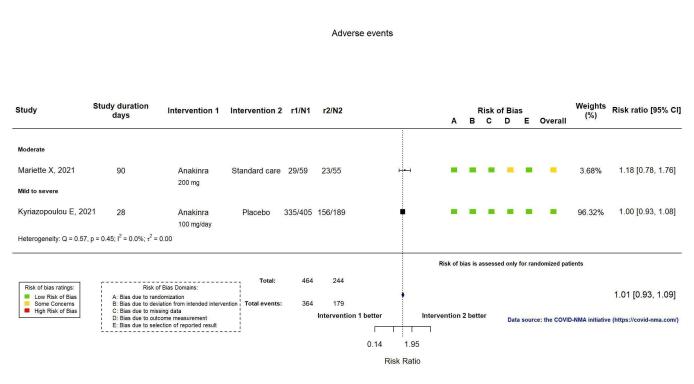
AEs were reported in two RCTs (Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). AEs were assessed by spontaneous reporting in the Mariette CORIMUNO‐19 Collaborative 2021 trial and by active monitoring in the Kyriazopoulou SAVE‐MORE 2021 trial. Anakinra probably results in little or no increase in adverse events (RR 1.02, 95% CI 0.94 to 1.11; I2 = 0%; 2 RCTs, 722 participants; absolute effect: 14 more per 1000 (from 43 fewer to 78 more); moderate‐certainty evidence; Figure 8).
8.
Analysis 1.1.7: Anakinra versus standard care/placebo: Adverse Events
Serious adverse events (SAEs)
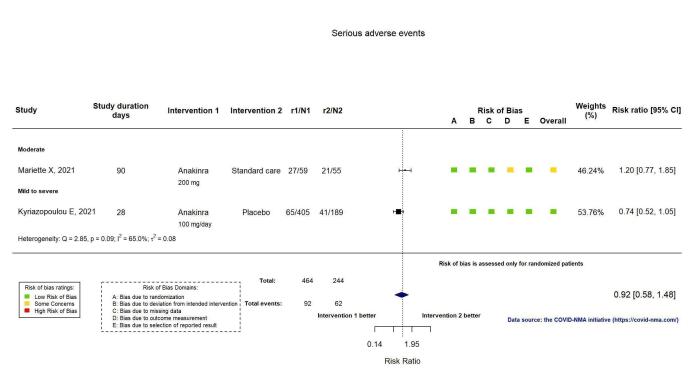
SAEs were reported in two RCTs (Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). The evidence of an effect of anakinra on serious adverse events is uncertain (RR 0.95, 95% CI: 0.58 to 1.56; I2 = 68.2%; 2 RCTs, 722 participants; absolute effect: 12 fewer per 1000 (from 104 fewer to 138 more); low‐certainty evidence; Figure 9).
9.
Analysis 1.1.8: Anakinra versus standard care/placebo: Serious adverse events
Important outcomes
Time to clinical improvement
This outcome was reported in four RCTs (Declercq COV‐AID 2021; Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). The evidence of an effect of anakinra on the time to clinical improvement is uncertain (HR 1.07, 95% CI 0.91 to 1.26; I2 = 24.5%; 4 RCTs, 1633 participants; absolute effect: 23 more per 1000 (from 33 fewer to 74 more); low‐certainty evidence; Appendix 13).
Time to WHO Clinical Progression Score of level 7 or above
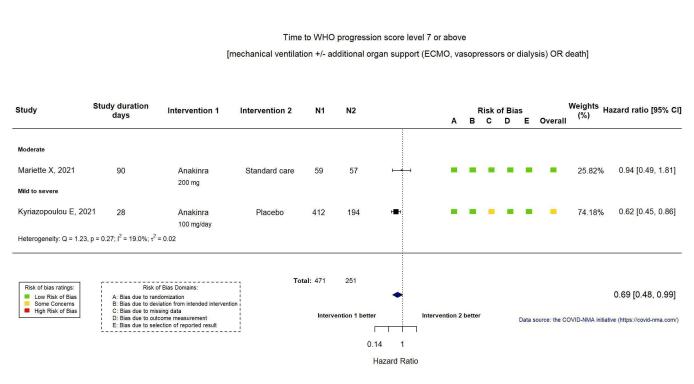
This outcome was reported in two RCTs (Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). The evidence of an effect of anakinra WHO Clinical Progression Score of level 7 or above is uncertain (HR 0.69, 95% CI 0.48 to 0.99; I2 = 19.0%; 2 RCTs, 722 participants; absolute effect: 54 fewer per 1000 (from 93 fewer to 2 fewer); low‐certainty evidence; Appendix 13).
Time to death
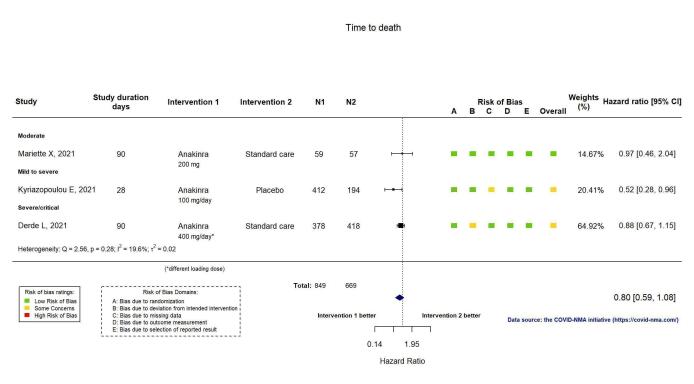
This outcome was reported in three RCTs (Derde REMAP‐CAP 2021; Kyriazopoulou SAVE‐MORE 2021; Mariette CORIMUNO‐19 Collaborative 2021). The evidence of an effect of anakinra on time to death is uncertain (HR 0.80, 95% CI 0.59 to 1.08; I2 = 19.6%; 3 RCTs, 1518 participants; absolute effect: 47 fewer per 1000 (from 100 fewer to 18 more); low‐certainty evidence; Appendix 13).
Canakinumab versus standard of care/placebo
We report the certainty evidence for critical and important outcomes in the Table 2. We report the effect sizes of the outcomes for this comparison in Appendix 14.
Critical outcomes
Clinical improvement
The proportion of participants achieving improvement at D28 was reported in two RCTs (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021). Clinical improvement was defined as an improvement of two points on a seven‐category ordinal scale or discharge from the hospital, whichever comes first (Cremer Three C Study 2021), and an improvement of clinical status by at least two points (Caricchio CAN‐COVID 2021). Canakinumab probably results in little or no increase in clinical improvement at D28 (RR 1.05, 95% CI 0.96 to 1.14; I2 = 2.2%; 2 RCTs, 499 participants; absolute effect: 42 more per 1000 (from 33 fewer to 116 more); moderate‐certainty evidence; Figure 10).
10.
Analysis 2.1.1: Canakinumab versus standard care/placebo: Clinical improvement D28
WHO Clinical Progression Score of level 7 or above (i.e. the proportion of participants with mechanical ventilation +/‐ additional organ support or death)
Two RCTs reported the proportion of participants with mechanical ventilation or death at D28 (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021). The evidence of an effect of canakinumab on the proportion of participants with a WHO Clinical Progression Score of level 7 or above at D28 is uncertain (RR 0.72, 95% CI 0.44 to 1.20; I2 = 0.0%; 2 RCTs, 499 participants; absolute effect: 35 fewer per 1000 (from 69 fewer to 25 more); low‐certainty evidence; Figure 11).
11.
Analysis 2.1.2: Canakinumab versus standard care/placebo: WHO Clinical Progression Score of level 7 or above D28
All‐cause mortality
Two RCTs reported all‐cause mortality at D28 (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021), and one trial at ≥ D60 (Cremer Three C Study 2021). The evidence for an effect of canakinumab on all‐cause mortality at D28 is uncertain (RR: 0.75, 95% CI 0.39 to 1.42); I2 = 0.0%; 2 RCTs, 499 participants; absolute effect: 20 fewer per 1000 (from 48 fewer to 33 more); low‐certainty evidence; Figure 12).
12.
Analysis 2.1.3: Canakinumab versus standard care/placebo: All‐cause mortality D28
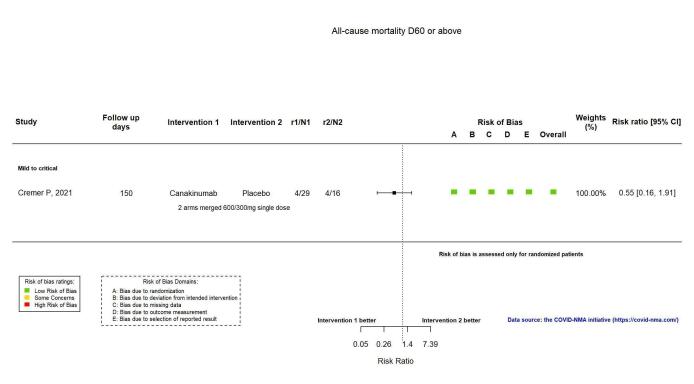
The evidence for an effect of canakinumab on all‐cause mortality at ≥ D60 is very uncertain (RR 0.55, 95% CI 0.16 to 1.91; 1 RCT, 45 participants; absolute effect: 112 fewer per 1000 (from 210 fewer to 227 more); very low‐certainty evidence; Figure 13).
13.
Analysis 2.1.4: Canakinumab versus standard care/placebo: All‐cause mortality D60 or above
Adverse events (AEs)
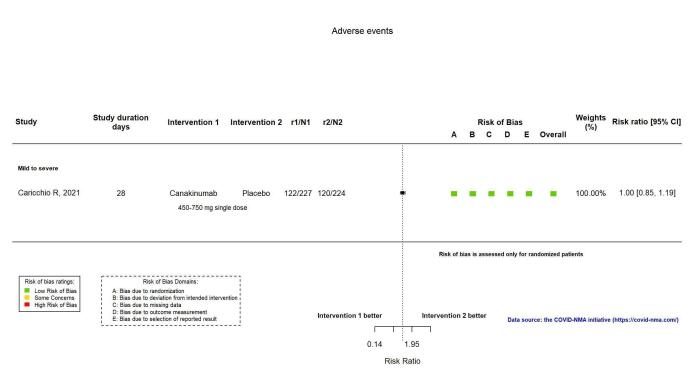
AEs were assessed by both spontaneous reporting and active monitoring in one RCT (Caricchio CAN‐COVID 2021). Canakinumab probably results in little or no increase in adverse events (RR 1.02, 95%; CI 0.86 to 1.21; 1 RCT, 454 participants; absolute effect: 11 more per 1000 (from 74 fewer to 111 more); moderate‐certainty evidence; Figure 14).
14.
Analysis 2.1.5: Canakinumab versus standard care/placebo: Adverse events
Serious adverse events (SAEs)
SAEs were reported in two RCTs (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021). The evidence comparing canakinumab with standard care alone or with placebo on serious adverse events is uncertain (RR 0.80, 95% CI 0.57 to 1.13; I2 = 0.0%; 2 RCTs, 499 participants; absolute effect: 44 fewer per 1000 (from 94 fewer to 28 more); low‐certainty evidence; Figure 15).
15.
Analysis 2.1.6: Canakinumab versus standard care/placebo: Serious adverse events
Important outcomes
Time to clinical improvement
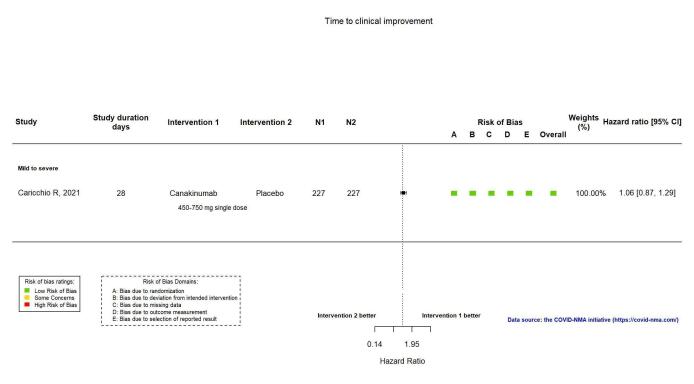
This outcome was reported in one RCT (Caricchio CAN‐COVID 2021). Canakinumab probably results in little or no increase in time to clinical improvement (HR 1.06, 95% CI 0.87 to 1.29; 1 RCT, 454 participants; absolute effect: 17 more per 1000 (from 43 fewer to 66 more); moderate‐certainty evidence; Appendix 15).
Time to WHO Clinical Progression Score of level 7 or above
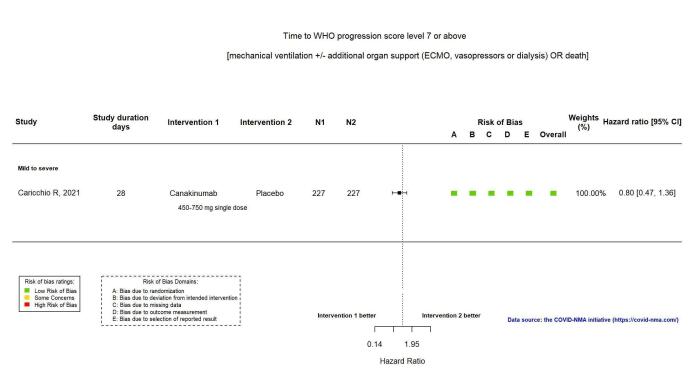
This outcome was reported in one RCT (Caricchio CAN‐COVID 2021). The evidence for an effect of canakinumab compared with standard care alone or with placebo on time to WHO Clinical Progression Score of level 7 or above is uncertain (HR 0.80, 95% CI 0.47 to 1.36; 1 RCT, 454 participants; absolute effect: 22 fewer per 1000 (from 59 fewer to 38 more); low‐certainty evidence; Appendix 15).
Time to death
This outcome was reported in two RCTs (Caricchio CAN‐COVID 2021; Cremer Three C Study 2021). The evidence for an effect of canakinumab compared with standard care alone or with placebo on time to death is uncertain (HR 0.71, 95% CI 0.36 to 1.43; I2 = 0.0%; 2 RCT, 499 participants; absolute effect: 25 fewer per 1000 (from 57 fewer to 36 more); low‐certainty evidence; Appendix 15).
Longer term follow‐up data (≥ D60) were not available for the outcomes clinical improvement and WHO Clinical Progression Score of level 7 or above for canakinumab.
Investigation of heterogeneity
The limited number of RCTs that provided results and the absence of variation across trials in some variables, such as age and gender, prevented us from performing preplanned subgroup analyses.
Sensitivity analysis
Sensitivity analyses for publication status were only possible for the comparison of anakinra versus control for one outcome (all‐cause mortality D60 or above). Results were consistent when considering only trials reported as peer‐reviewed articles (Appendix 16). No important discrepancies in the summary results were observed when we used the number analysed in the RCTs instead of the number randomised as the denominator for anakinra and canakinumab (Appendix 17).
Discussion
Summary of main findings
This review aimed to assess the effectiveness and safety of IL‐1 blocking agents for COVID‐19. We identified four RCTs that reported results for anakinra and two RCTs for canakinumab. Participants were mainly people with moderate to critical disease. One trial was reported as a preprint (Derde REMAP‐CAP 2021).
Our results suggest that anakinra and canakinumab probably result in little or no increase in clinical improvement D28. For all other critical effectiveness outcomes, evidence was of low or very low certainty. Regarding critical safety outcomes, anakinra and canakinumab probably result in little or no increase in adverse events. Evidence for serious adverse events was of low certainty.
Overall completeness and applicability of the evidence
The evidence available is not complete. We have identified 16 more registered RCTs evaluating IL‐1 blocking agents with no results available, including four completed and four terminated trials. Access to these results is expected and will allow us to update our data.
All RCTs with results were multicentre, and three involved two or more countries. The results apply only to people hospitalised with moderate to critical disease. There is some heterogeneity in the severity of the disease; some trials involved only people who were not intubated, while in others the proportion of intubated participants was 11% (Declercq COV‐AID 2021), one‐fifth (Cremer Three C Study 2021), and one‐third (Derde REMAP‐CAP 2021). Similarly, there is some heterogeneity in co‐interventions, particularly corticosteroid and remdesivir use between trials. We can also expect heterogeneity within trials in use of steroids, as results of the RECOVERY trial (Horby 2021) demonstrating the beneficial effect of corticosteroids were released while most of the trials included were ongoing.
Due to the limited number of trials, we could not use subgroup analysis or meta‐regression to explore the impact of effect modifiers such as disease severity, participant characteristics, or timing of the treatment. Owing to the rapid evolution of management of the disease over time, individual participant data would be necessary to explore heterogeneity adequately to identify if there is a subgroup of people who could benefit from IL‐1 blocking agents.
Certainty of the evidence
Overall, for anakinra, the certainty of the evidence ranged from very low for two critical outcomes (clinical improvement at ≥ D60, all‐cause mortality at ≥ D60), low for four critical outcomes (WHO Clinical Progression Score of level 7 or above at D28 and ≥ D60, all‐cause mortality at D28, serious adverse events), and moderate for two critical outcomes (clinical improvement at D28, adverse events).
Reasons for downgrading the certainty of evidence were primarily for imprecision. See Table 1.
For canakinumab, the certainty of the evidence ranged from very low for one critical outcome (all‐cause mortality ≥ D60), low for three critical outcomes (WHO Clinical Progression Score of level 7 or above at D28, all‐cause mortality at D28, serious adverse events) and moderate for two critical outcomes (clinical improvement at D28, adverse events).
Reasons for downgrading the certainty of evidence were primarily for imprecision. See Table 2.
Potential biases in the review process
We followed the guidance of the Cochrane Handbook for Systematic Reviews of Interventions to minimise bias (Higgins 2021). Our search strategy was peer‐reviewed and evaluated, we extracted data in duplicate, and contacted investigators of unpublished and published trials to obtain missing information. Weekly, we search and extract data for registered trials which are made available on our platform, and our review is updated bi‐weekly. All updates of this review are available on the COVID‐NMA platform (covid-nma.com). Nevertheless, our review has some limitations. Our protocol was updated to account for the rapid increase in knowledge on this disease. Furthermore, we included a preprint in our analysis despite the fact that this source of information has not been peer‐reviewed and may evolve over time (Oikonomidi 2020). However, only one preprint was unpublished in a peer‐reviewed journal at the time of our analysis.
Agreements and disagreements with other studies or reviews
We identified nine published systematic reviews evaluating anakinra for COVID‐19 and none for canakinumab. Of these, seven included only observational studies (Barkas 2021; Cantini 2020; Khan 2021; Kim 2020; Pasin 2021; Putman 2021; Talaie 2020). Two included and pooled results of both observational studies and randomised controlled trials (Kyriazopoulou 2021; Somagutta 2021).
Of the two other large ongoing living network meta‐analyses of COVID‐19 drug treatments (Juul 2020a; Juul 2020b; Siemieniuk 2020), the Siemieniuk 2020 review updated the search in April 2021 and identified two of the trials we included in our review (Cremer Three C Study 2021; Mariette CORIMUNO‐19 Collaborative 2021). The review by Juul and colleagues has not been updated since November 2020 (Juul 2020a; Juul 2020b).
One important issue in COVID‐19 trials and related systematic reviews is the choice of outcomes. Particularly, the outcome 'clinical improvement' relies on the WHO ordinal scale or hospital discharge. However, changes on the WHO ordinal scale may have different meanings according to the disease severity. Further, hospital discharge may vary over time according to the availability of beds. These results should consequently be interpreted with caution.
Authors' conclusions
Implications for practice
Overall, we did not find evidence for a clinically relevant beneficial effect of interleukin‐1 (IL‐1) blocking agents. The evidence is uncertain or very uncertain for several outcomes. Some important trials are ongoing. Anakinra in particular is being assessed for children in the Randomised Evaluation of COVID‐19 Therapy (RECOVERY) platform.
Implications for research
Currently, there are sixteen trials of anakinra and canakinumab with no results registered, of which four have been completed and four terminated. It is essential that trialists give access to these results through publication or posting on clinical trial registries as soon as possible.
With the data currently available, we were not able to explore heterogeneity and identify subgroups of people who might benefit from the treatment. Several hypotheses have arisen on the impact of potential effect modifiers such as participant characteristics, disease severity, the use of inflammatory biomarkers to identify people for treatment (e.g. soluble urokinase plasminogen activator receptor (suPAR)), timing of the treatment during the course of the disease, and background therapies including glucocorticoids or remdesivir. Adequate recording and reporting of these factors are warranted. The sharing of trial data and large collaborations are needed to conduct individual participant data meta‐analyses as they may yield a more in‐depth level of understanding of the effect of IL‐1 inhibitors.
Moreover, there is an important heterogenicity in outcome measures used, and interpretation of outcomes related to the management of patients is always complex in the context of a pandemic where the resources to adequately manage a patient might be missing. Consistency in the outcome assessment and reporting is needed.
The findings of this review will be updated as soon as new data are available on the COVID network meta‐analysis (COVID‐NMA) platform (covid-nma.com). Access to individual participant data will be needed to make exploration of heterogenicity possible.
Acknowledgements
We particularly thank Elise Diard for her help on the website and extraction tool development.
Members of the COVID‐NMA consortium are listed below in alphabetical order:
Solaf Alawadhi1,2,3, Camila Ávila6,13, Fulvia Baldassarre7, Rita Banzi8, Julien Barnier9, Julia Baudry10, Hanna Bergman11, Claudia Bollig12, Hillary Bonnet2,3,Isabelle Boutron1,2,3, Brian Buckley11, Guillaume Cabanac15, Anna Chaimani1,3, Sarah Charpy2, David Chavalarias17, Sarah Cohen‐Boulakia19, Elise Cogo11, Françoise Conil20, Emmanuel Coquery20, Mauricia Davidson1,2,3, Declan Devane21, Elise Diard3, Bastien Doreau14, Mishelle Engleton2, Laura Esmail2, Theodoros Evrenoglou1,3, Gilles Feron22, Gabriel 3Ferrand3, Leopold Fezeu10, Mathilde Fouet24,Joly Ghanawi25, Lina Ghosn2,3, Robin Featherstone26, Carolina Graña2,3, Giacomo Grasselli27, François Grolleau1, Candyce Hamel11, Camilla Hansen13,22, Vernon Hedge5, Nicholas Henschke11, Harald Herkner5 , Mona Hersi11 , Patrick Mallon36, Melanie Marti16, Sonia Menon3, Conor Moran38, Phlipp Kapp3 , Ameer Hohlfeld28, Tamara Kredo28, Asbjørn Hróbjartsson13,22, Chantal Julia10, Joey Kwong10, Ruben Martinez14, Pauline Martinot2, Dimitris Mavridis29, Joerg J Meerpohl12,30, Brice Meyer14, Silvia Minozzi31, Van Thu Nguyen37, Nathan Pace5, Matthew Page32, Jennifer Petkovic11, Elizabeth Pienaar28, Olivier Pierre2, Katrin Probyn11, Fiona Quirke33, Gabriel Rada6,34, Philippe Ravaud1,2,3, Pierre Ripoll20, Carolina Riveros2,3, Philippe Rivière20, Jelena Savovic35, Christine Schmucker30, Yanina Sguassero11, David Tovey3, Marialena Trivella5, Janne Vendt5, Gemma Villanueva11, Romain Vuillemot20, Emina Zoletic1,2, Stephanie Weibel5
Université de Paris, France
Centre of Research in Epidemiology and StatisticS (CRESS UMR1153), Methods team, France
Cochrane France
Cochrane Emergency and Critical Care
Epistemonikos Foundation, Chile
McMaster University, Canada
Center for Health Regulatory Policies, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Italy
Centre Max Weber, CNRS, France
Centre of Research in Epidemiology and StatisticS (CRESS UMR1153), Eren team, France
Cochrane Response
Cochrane Germany, Cochrane Germany Foundation, Freiburg, Germany
Open Patient data Explorative Network (OPEN), Odense University Hospital, Odense, Denmark
Laboratoire d'Informatique, de Modélisation et d'Optimisation des Systèmes (LIMOS), CNRS, Université Clermont Auvergne
Université Toulouse 3 – Paul Sabatier ‐ Institut de Recherche en Informatique de Toulouse – IRIT UMR 5505, France
Department of Immunization, Vaccines and Biologicals, World Health Organization Geneva, Switzerland
Institut des Systèmes Complexes de Paris IDF (ISC‐PIF), CNRS, France
WHO Collaborating Centre for Guideline Implementation and Knowledge Translation & Chinese GRADE Centre, Lanzhou University, China
Laboratoire de recherche en Informatique (LRI), CNRS, Université Paris‐Saclay, France
Laboratoire d’InfoRmatique en Image et Systèmes d’information (LIRIS), CNRS, Université Claude Bernard Lyon 1, France
Evidence Synthesis Ireland, Cochrane Ireland and HRB‐Trials Methodology Research Network, National University of Ireland, Galway, Ireland
Centre for Evidence Based Medicine Odense (CEBMO) and Cochrane Denmark, University of Southern Denmark, Denmark
French National Research Institute for Agriculture, Food and Environment (INRAE), France
Service de Neurochirurgie, Hôpital d'Instruction des Armées Percy (HIA), France
The Collaborative Approach to Meta‐Analysis and Review of Animal Data from Experimental Studies (CAMARADES), Centre for Clinical Brain Sciences, University of Edinburgh, Scotland
Cochrane Editorial and Methods Department, Cochrane Central
Department of Anesthesia, Intensive Care and Emergency, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Department of Pathophysiology and Transplantation, University of Milan, Italy
Cochrane South Africa
Department of Primary Education, University of Ioannina, Greece
Institute for Evidence in Medicine, Medical Center & Faculty of Medicine, University of Freiburg, Freiburg, Germany
Cochrane Review Group on Drugs and Alcohol; International GRADE Working Group; Department of Epidemiology, Lazio Regional Health Service, Italy
Research Methodology Division, School of Public Health and Preventive Medicine, Monash University, Australia
Health Research Board‐Trials Methodology Research Network (HRB‐TMRN), NUI Galway, Ireland
UC Evidence Center, Cochrane Chile Associated Center, Pontificia Universidad Católica de Chile, Santiago, Chile
Population Health Sciences, Bristol Medical School, University of Bristol, UK; NIHR CLAHRC West, University Hospitals Bristol and Weston NHS Foundation Trust, UK
UCD Centre for Experimental Pathogen Host Research and UCD School of Medicine, University college, Dublin, Ireland
Standford University
Infectious Diseases and General Medicine, Mater Misercordiae University Hospital, Dublin, Ireland
Editorial and peer‐reviewer contributions:
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Harald Herkner (Co‐ordinating Editor, Cochrane Emergency and Critical Care); Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Helen Wakeford (Executive Editor, Central Editorial Service, Cochrane); Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Central Editorial Service ; Copy Editor (copy editing and production): Andrea Takeda, Copy‐Edit Support, Cochrane; Peer‐reviewers (provided comments and recommended an editorial decision): Jinoos Yazdany, Professor of Medicine, UCSF, San Francisco, USA (clinical/content review); Philip Robinson, Professor of Medicine, University of Queensland, Brisbane, Australia (clinical/content review); Jeffrey Sparks, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA (clinical/content review); Francisca Sivera, Head of Department, Rheumatology, Hospital General Universitario Elda, Dept Medicina, Universidad Miguel Hernandez, Elche, Spain (clinical/content review); Liz Bickerdike, Editorial and Methods Department, Cochrane (methods review); Robin Featherstone, Information Specialist, Editorial and Methods Department, Cochrane (search review).
Appendices
Appendix 1. Living process of the review
Steering committee
We set up a steering committee of epidemiologists, methodologists, statisticians and clinicians with content expertise. This committee will meet regularly, discuss the conduct of the project, difficulties encountered and possible changes in the protocol according to new knowledge available on COVID‐19 disease. Changes in the protocol could consist for example of changes in the search strategy, eligibility criteria (e.g. study design), research questions for the pairwise meta‐analyses, outcomes.
Process and quality control
Our aim is to update the synthesis at least every week. For this purpose, we will search, screen and extract data every day. The updated synthesis will be reported online at least every week.
To standardise the process and ensure both rapidity and quality, we will proceed as follows:
We will separate the process into different tasks and set up a team for each task (i.e. a researcher/volunteer will be involved in a single task). Each team will be led by a senior researcher ensuring the quality and standardisation of the task.
For some tasks, we will develop a short training program for researchers/volunteers joining the team. This program will involve a) reading a manual detailing the task; b) performing the task on a sample as an exercise (e.g. evaluating the risk of bias of three studies) and contacting the team leader to ask about difficulties; and c) after successful training, the newcomer will perform the double data extraction with a senior well‐trained researcher.
Each team will hold a weekly meeting to discuss difficulties and ensure standardisation. All decisions and changes will be recorded.
We will set‐up an internal quality control process where a senior researcher, and former Editor‐in‐Chief of Cochrane (D Tovey), will check the data extracted and reported on the website. All points will be discussed with the data extraction team and modifications recorded for transparency.
We will develop an external quality control process for data collection involving senior researchers who will check a random sample of the data collected (e.g. member of the Cochrane Bias Methods Group for risk of bias).
We will consider the following tasks:
research mapping: screening and extracting data from registries;
screening of databases from title/abstract to full text;
data extraction;
data analyses;
assessment of evidence certainty.
The core team will perform the analysis, presentation and interpretation of the results.
Evolution of the protocol over time
The process will also evolve over time according to the new knowledge available regarding COVID‐19.
The steering committee will systematically discuss and achieve consensus on the changes of protocol proposed.
Appendix 2. Case definitions
Suspect case
A. A patient with acute respiratory illness (fever and at least one sign/symptom of respiratory disease (e.g. cough, shortness of breath)), AND with no other etiology that fully explains the clinical presentation AND a history of travel to or residence in a country/area or territory reporting local transmission of COVID‐19 disease during the 14 days prior to symptom onset.
OR
B. A patient with any acute respiratory illness AND having been in contact with a confirmed or probable COVID‐19 case (see definition of contact) in the last 14 days before onset of symptoms.
OR
C. A patient with severe acute respiratory infection (fever and at least one sign/symptom of respiratory disease (e.g. cough, shortness breath)) AND requiring hospitalisation AND no other etiology that fully explains the clinical presentation.
Probable case
A suspect case for whom testing for COVID‐19 is inconclusive (inconclusive being the result of the test reported by the laboratory).
Confirmed case
A person with laboratory confirmation of COVID‐19 infection, regardless of clinical signs and symptoms.
Of note, when the definition used to classify cases was not clearly reported, we will rely on the classification provided by authors.
Appendix 3. Search strategies
Cochrane COVID‐19 Study Register
Current Strategy (last updated 2 September 2021)
PubMed
(2019 nCoV[tiab] OR 2019nCoV[tiab] OR corona virus[tiab] OR corona viruses[tiab] OR coronavirus[tiab] OR coronaviruses[tiab] OR COVID[tiab] OR COVID19[tiab] OR nCov 2019[tiab] OR SARS‐CoV2[tiab] OR SARS CoV‐2[tiab] OR SARSCoV2[tiab] OR SARSCoV‐2[tiab] OR "COVID‐19"[Mesh] OR "COVID‐19 Testing"[Mesh] OR "COVID‐19 Vaccines"[Mesh] OR "Coronavirus"[Mesh:NoExp] OR "Receptors, Coronavirus"[Mesh] OR "SARS‐CoV‐2"[Mesh] OR "Spike Glycoprotein, Coronavirus"[Mesh]) NOT ("animals"[mh] NOT "humans"[mh]) NOT (editorial[pt] OR newspaper article[pt])
Embase
((('anti‐SARS‐CoV‐2 agent'/exp OR 'coronaviridae'/de OR 'coronavirinae'/de OR 'coronaviridae infection'/de OR 'coronavirus disease 2019'/exp OR 'coronavirus infection'/de OR 'COVID‐19 testing'/exp OR 'sars coronavirus 2 test kit'/exp OR 'sars‐related coronavirus'/de OR 'severe acute respiratory syndrome coronavirus 2'/exp OR '2019 ncov':ti,ab,kw OR 2019ncov:ti,ab,kw OR (((corona* OR corono*) NEAR/1 (virus* OR viral* OR virinae*)):ti,ab,kw) OR coronavir*:ti,ab,kw OR coronovir*:ti,ab,kw OR covid:ti,ab,kw OR covid19:ti,ab,kw OR hcov*:ti,ab,kw OR 'ncov 2019':ti,ab,kw OR 'sars cov2':ti,ab,kw OR 'sars cov 2':ti,ab,kw OR sarscov2:ti,ab,kw OR 'sarscov 2':ti,ab,kw) NOT (('animal experiment'/de OR 'animal'/exp) NOT ('human'/exp OR 'human experiment'/de))) NOT 'editorial'/it) NOT ([medline]/lim OR [pubmed‐not‐medline]/lim) AND [1‐12‐2019]/sd
CENTRAL
1 ("2019 nCoV" OR 2019nCoV OR "corona virus*" OR coronavirus* OR COVID OR COVID19 OR "nCov 2019" OR "SARS‐CoV2" OR "SARS CoV‐2" OR SARSCoV2 OR "SARSCoV‐2"):TI,AB AND CENTRAL:TARGET
2 Coronavirus:MH AND CENTRAL:TARGET
3 Coronavirus:EH AND CENTRAL:TARGET
4 #1 OR #2 OR #3
5 2019 TO 2021:YR AND CENTRAL:TARGET
6 #5 AND #4
7 INSEGMENT
8 #6 NOT #7
ClinicalTrials.gov
COVID‐19 OR 2019‐nCoV OR SARS‐CoV‐2 OR coronavirus
WHO ICTRP
COVID OR 2019‐nCoV OR SARS‐CoV‐2 OR coronavirus OR corona virus
medRxiv (via Cochrane Register of Studies)
All new medRxiv records are imported each week into the Cochrane Register of Studies. Records captured by this strategy are then evaluated:
("2019 nCoV" OR 2019nCoV OR "corona virus*" OR coronavirus* OR COVID OR COVID19 OR "nCov 2019" OR "SARS‐CoV2" OR "SARS CoV‐2" OR SARSCoV2 OR "SARSCoV‐2"):TI,AB
COVID‐19 L‐OVE platform via Episktemonikos Database
coronavir* OR coronovirus* OR betacoronavir* OR "beta‐coronavirus" OR "beta‐coronaviruses" OR "corona virus" OR "virus corona" OR "corono virus" OR "virus corono" OR hcov* OR "covid‐19" OR covid19* OR "covid 19" OR "2019‐ncov" OR cv19* OR "cv‐19" OR "cv 19" OR "n‐cov" OR ncov* OR (wuhan* and (virus OR viruses OR viral)) OR sars* OR sari OR (covid* and (virus OR viruses OR viral)) OR "severe acute respiratory syndrome" OR mers* OR "middle east respiratory syndrome" OR "middle‐east respiratory syndrome" OR "covid‐19‐related" OR "2019‐ncov‐related" OR "cv‐19‐related" OR "n‐cov‐related"
Appendix 4. Details of the requests for information sent to authors of published IL‐1 blocking agent trials
| Study ID | Author’s contact name | Treatment | Requested information | Date of contact | Reply | Data provided |
|
NCT04330638 |
Declercq J |
Anakinra | Some missing data for: outcomes, co‐interventions, participant characteristics | 3 November 2020 12 January 2021 30 June 2021 |
Awaiting response | No |
| NCT02735707 | Derde L | Anakinra | Some missing data for: outcomes, co‐interventions, participant characteristics |
19 July 2021 | Awaiting response | No |
| NCT04680949 | Kyriazopoulou E | Anakinra | Some missing data for: outcomes, co‐interventions, participant characteristics |
30 June 2021 | Expressed interest in collaborating | Requested information received on 2 July 2021 and 19 August 2021 |
|
NCT04341584 |
Mariette X | Anakinra | Some missing data for: outcomes, co‐interventions, participant characteristics | 23 March 2021 | Agreed to provide data | Requested information received on 23 July 2021 |
| NCT04362813 | Caricchio R | Canakinumab | Some missing data for: outcomes, co‐interventions, participant characteristics | 23 August 2021 | Agreed to provide data | Requested information received on 10 April 2021 |
|
NCT04365153 |
Cremer P |
Canakinumab | Some missing data for: outcomes, co‐interventions, participant characteristics | 6 November 2020 11 May 2021 |
Replied 31 May 2021 “Publication is due soon; all answers will be found there.” Study was later published on 29 July 2021. |
No |
Appendix 5. Characteristics of excluded studies table
| Study title | First author, Journal, Year | Link | Reason for exclusion |
| Nigella sativa supplementation accelerates recovery from mild COVID‐19: first randomized controlled clinical Trial (RCT) | Koshak A, OSF, 2020 | osf.io/urb6f/ | Irrelevant intervention |
| Efficacy of surgical helmet systems for protection against COVID‐19: a double‐blinded randomised control study | Schaller G, International Orthopaedics, 2020 | link.springer.com/article/10.1007/s00264‐020‐04796‐3 | No relevant outcome |
| Nature and dimensions of the cytokine storm and its attenuation by convalescent plasma in severe COVID‐19 | Bandopadhyay P, medRxiv, 2020 | www.medrxiv.org/content/10.1101/2020.09.21.20199109v1 | Other reasons |
| Human safety, tolerability, and pharmacokinetics of a novel broad‐spectrum oral antiviral compound, molnupiravir, with activity against SARS‐CoV‐2 | Painter W, medRxiv, 2020; preprint of Painter W, Antimicrob Agents Chemother, 2021 | www.medrxiv.org/content/10.1101/2020.12.10.20235747v1 | Early phase |
| Early viral clearance among covid‐19 patients when gargling with povidone‐iodine and essential oils – a clinical trial | Mohamed N, medRxiv, 2020 | www.medrxiv.org/content/10.1101/2020.09.07.20180448v1 | Irrelevant intervention |
| Efficacy of commercial mouth‐rinses on SARS‐CoV‐2 viral load in saliva: randomized control trial in Singapore |
Seneviratne CJ, medRxiv, 2020 preprint of Seneviratne CJ, Infection, 2020 | www.medrxiv.org/content/10.1101/2020.09.14.20186494v1 | Irrelevant intervention |
| Efficacy of commercial mouth‐rinses on SARS‐CoV‐2 viral load in saliva: randomized control trial in Singapore |
Seneviratne CJ, Infection, 2020 publication of Seneviratne CJ, medRxiv, 2020 | link.springer.com/article/10.1007%2Fs15010‐020‐01563‐9 | Irrelevant intervention |
| The possible beneficial role of the regular use of potent mouthwash solutions in the treatment of COVID‐19 |
Mukhtar K, medRxiv, 2020 | www.medrxiv.org/content/10.1101/2020.11.27.20234997v1 | Irrelevant intervention |
| Effect of 1% povidone iodine mouthwash/gargle, nasal and eye drop in COVID‐19 patient |
Choudhury MIM, Bioresearch Communications, 2021 | www.bioresearchcommunications.com/index.php/brc/article/view/176 | Irrelevant intervention |
| Clinical efficacy of diammonium glycyrrhizinate in the treatment of common type patients with novel coronavirus pneumonia |
Zhou WM, Chinese Journal of Virology, 2020 | www.epistemonikos.org/en/threads/5f18204d7db23a1920da4374 | Irrelevant intervention |
| The use of exhaled nitric oxide and peak expiratory flow to demonstrate improved breathability and antimicrobial properties of novel face mask made with sustainable filter paper and Folium Plectranthii amboinicii oil: additional option for mask shortage during COVID‐19 pandemic |
Duong‐Quy S, Multidiscip Respir Med, 2020 | mrmjournal.org/mrm/article/view/664 | Irrelevant intervention |
| REaCH‐Resiliency Engagement and Care in Health; A telephone befriending intervention to address the psycho‐social challenges of vulnerable population in the context of COVID‐19 pandemic: an exploratory trial in India |
Saju M, ResearchSquare, 2020 | www.researchsquare.com/article/rs‐72843/v1 | Non‐COVID‐19 patients |
| Basic psychological need‐satisfying activities during the COVID‐19 outbreak |
Behzadnia B, IAAP, 2020 | iaap‐journals.onlinelibrary.wiley.com/doi/10.1111/aphw.12228 | Non‐COVID‐19 patients |
| Web‐based relaxation intervention for stress during social isolation: randomized controlled trial |
Pizzoli S.F.M., JMIR Mental Health, 2020 | mental.jmir.org/2020/12/e22757 | Non‐COVID‐19 patients |
| Wearing of cloth or disposable surgical face masks has no effect on vigorous exercise performance in healthy individuals. |
Shaw K, Int J Environ Res Public Health, 2020 | www.mdpi.com/1660‐4601/17/21/8110 | Irrelevant intervention |
| Intraoperative aerosol box use: does an educational visual aid reduce contamination? |
Burnett GW, Korean J Anesthesiol, 2020 | ekja.org/journal/view.php?doi=10.4097/kja.20511/ | Irrelevant intervention |
| Treating COVID‐19 with chloroquine | Huang M, J Mol Cell Biol, 2020 | academic.oup.com/jmcb/article/12/4/322/5814655 | Not randomised or improper randomisation |
| Clinical efficacy of convalescent plasma for treatment of COVID‐19 |
Abolghasemia H, Transfus Apher Sci, 2020 | www.trasci.com/article/S1473‐0502(20)30180‐4/fulltext | Not randomised or improper randomisation |
| COVID‐19: comparing the applicability of shared room and single room occupancy |
Hyun M, Transbound Emerg Dis, 2020 | onlinelibrary.wiley.com/doi/10.1111/tbed.13853 | Not randomised or improper randomisation |
| COVID‐19‐associated ARDS treated with Dexamethasone (CoDEX): study design and rationale for a randomized trial. |
Tomazini B, medRxiv, 2020 | www.medrxiv.org/content/10.1101/2020.06.24.20139303v1 | Other reasons |
| Interim analysis of an open‐label randomized controlled trial evaluating nasal irrigations in non‐hospitalized patients with COVID‐19 |
Kimura K, Int Forum Allergy Rhinol, 2020 | onlinelibrary.wiley.com/doi/10.1002/alr.22703 | Other reasons |
| The impact of respiratory protective equipment on difficult airway management: a randomised, crossover, simulation study |
Schumacher J, Anaesthesia, 2020 | associationofanaesthetists‐publications.onlinelibrary.wiley.com/doi/10.1111/anae.15102 | Other reasons |
| Resuscitation of the patient with suspected/confirmed COVID‐19 when wearing personal protective equipment: a randomized multicenter crossover simulation trial |
Malysz M, Cardiology Journa, 2020 | journals.viamedica.pl/cardiology_journal/article/view/CJ.a2020.0068 | Other reasons |
| COVID‐19 aerosol box as protection from droplet and aerosol contaminations in healthcare workers performing airway intubation: a randomised cross‐over simulation study. |
Noor Azhar M, Emerg Med J. 2020 | emj.bmj.com/content/38/2/111.long | Other reasons |
| The use of personal protective equipment (PPE) by police during a public health crisis: An experimental test of public perception. |
Simpson R, J Exp Criminol, 2021 | link.springer.com/article/10.1007/s11292‐020‐09451‐w | Irrelevant intervention |
| Treatment of COVID‐19 patients with quercetin: a prospective, single ‐ centre, randomized, controlled trial |
Onal H, Authorea, 2021 | www.authorea.com/users/390404/articles/504772‐treatment‐of‐covid ‐19‐patients‐with‐quercetin‐a‐prospective‐single‐centre‐randomized‐controlled‐trial |
Irrelevant intervention |
| Chinese medicine (Q‐14) in the treatment of patients with coronavirus disease 2019 (COVID‐19): a single‐center, open label, randomised controlled trial |
Liu J, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.01.25.21249417v1 | Irrelevant intervention |
| A pilot randomized controlled trial of a group intervention via Zoom to relieve loneliness and depressive symptoms among older persons during the COVID‐19 outbreak |
Shapira S, Internet Interv, 2021 | www.sciencedirect.com/science/article/pii/S2214782921000087?via%3Dihub | Non‐COVID‐19 patients |
| Targeting TGF‐b pathway with COVID‐19 drug candidate ARTIVeda/PulmoHeal accelerates recovery from mild‐moderate COVID‐19 |
Trieu V, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.01.24.21250418v1 | Irrelevant intervention |
| An open clinical evaluation of selected Siddha regimen in expediting the management of Covid‐19 ‐ a randomized controlled study. |
Chitra SM, J Ayurveda Integr Med, 2021 | www.sciencedirect.com/science/article/pii/S0975947621000036?via%3Dihub | Irrelevant intervention |
| A randomized, comparative clinical study to evaluate the activity of CurvicTM formulation for management of SARS‐COV‐2 Infection (COVID‐19) |
Yogesh A, Journal of clinical trials, 2021 | www.longdom.org/open‐access/a‐randomized‐comparative‐clinical‐study‐to‐evaluate ‐the‐activity‐of‐cureqovitaatm‐formulation‐for‐management‐of‐sarscov2.pdf |
Irrelevant intervention |
| Phase III: randomized observer‐blind trial to evaluate lot‐to‐lot consistency of a new plant‐derived quadrivalent virus like particle influenza vaccine in adults 18–49 years of age |
Ward B, Vaccine, 2021 | www.sciencedirect.com/science/article/pii/S0264410X21000049?via%3Dihub | Irrelevant intervention |
| A stepped wedge cluster randomized control trial to evaluate the implementation and effectiveness of optimized quality‐improvement initiatives in improving quality of care for acute cardiac events in response to the COVID‐19 outbreak |
Zhou S, ResearchSquare, 2021 | www.researchsquare.com/article/rs‐239133/v1 | Non‐COVID‐19 patients |
| Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID‐19: a randomized, double‐blind clinical trial |
Farnoosh G, Authorea, 2020 | www.authorea.com/users/381612/articles/497517‐efficacy‐of‐a‐low‐dose‐of‐melatonin‐as‐an‐ adjunctive‐therapy‐in‐hospitalized‐patients‐with‐covid‐19‐a‐randomized‐double‐blind‐clinical‐trial ?commit=5be3e7266256468d59e81ff82a1b125710ba7459 |
Irrelevant intervention |
| Chyawanprash for the prevention of COVID‐19 infection among healthcare workers: a randomized controlled trial |
Gupta A, medRxiv, 2021 | www.medrxiv.org/content/10.1101/2021.02.17.21251899v1 | Irrelevant intervention |
| Impact of pulse D therapy on the inflammatory markers in patients With COVID‐19 |
Lakkireddy M, ResearchSquare, 2021 preprint of Lakkireddy M, Sci Rep, 2021 | www.researchsquare.com/article/rs‐152494/v1 | No relevant outcome |
| Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad‐spectrum oral antiviral agent with activity against SARS‐CoV‐2 |
Painter W, Antimicrob Agents Chemother, 2021; publication of Painter W, medRxiv, 2020 | aac.asm.org/content/early/2021/02/24/AAC.02428‐20 | Early phase |
| Itraconazole for COVID‐19: preclinical studies and a proof‐of‐concept randomized clinical trial |
Liesenborghs L, EBioMedicine, 2021 | www.thelancet.com/journals/ebiom/article/PIIS2352‐3964(21)00081‐5/fulltext | Early phase |
| Time to adapt in the pandemic era: a prospective randomized non –inferiority study comparing time to intubate with and without the barrier box |
Madabhushi P, BMC Anesthesiol, 2020 | bmcanesthesiol.biomedcentral.com/articles/10.1186/s12871‐020‐01149‐w | Irrelevant intervention |
| Face masks increase compliance with physical distancing recommendations during the COVID‐19 pandemic |
Seres G, OSF Preprints, 2020 | www.epistemonikos.org/documents/2ce86ff6f63b8924e7331939b7438f5f21f4c803 | Irrelevant intervention |
| Inhaled corticosteroids downregulate SARS‐CoV‐2‐related gene expression in COPD: results from a RCT |
Milne S, medRxiv, 2020 | www.medrxiv.org/content/10.1101/2020.08.19.20178368v1 | No relevant outcome |
| Efficacy of a six‐week therapist‐guided online therapy versus self‐help internet‐based therapy on COVID‐19 invoked anxiety and depression among individuals in Oman: an open‐label, pragmatic randomized controlled trial |
Al‐Alawi M, prepints JMIR, 2021; preprint of Al‐Alawi M, JMIR Mental Health, 2021 | preprints.jmir.org/preprint/26683/accepted | Non‐COVID‐19 patients |
| Safety and efficacy of anti‐il6‐receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: a comparative analysis |
Rossotti R, J Infect, 2020 | www.journalofinfection.com/article/S0163‐4453(20)30467‐9/fulltext | Not randomised or improper randomisation |
| Immune profiling reveals early disease trajectories associated with COVID‐19 mortality: a sub‐study from the ACTT‐1 trial |
Thiede JM, J Infect Dis, 2021 | academic.oup.com/jid/advance‐article/doi/10.1093/infdis/jiab035/6105271 | Secondary analysis |
| Renal involvement in patients with COVID‐19 pneumonia and outcomes after stem cell nebulization |
Torres‐Zambrano GM, medRxiv, 2021 | www.medrxiv.org/content/10.1101/2020.12.16.20236877v1 | Secondary analysis |
| Effect of clear vs standard covered masks on communication with patients during surgical clinic encounters: a randomized clinical trial |
Kratzke IM, JAMA surgery, 2021 | jamanetwork.com/journals/jamasurgery/fullarticle/2777511 | No relevant outcome |
| Efficacy of a six‐week‐long therapist‐guided online therapy versus self‐help internet‐based therapy for COVID‐19‐induced anxiety and depression: open‐label, pragmatic, randomized controlled trial |
Al‐Alawi M, JMIR Mental Health, 2021; publication of Al‐Alawi M, prepints JMIR, 2021 | mental.jmir.org/2021/2/e26683/ | Non‐COVID‐19 patients |
| Tele‐management of home isolated COVID‐19 patients via oxygen therapy with non‐invasive positive pressure ventilation and physical therapy techniques: a randomized clinical trial. |
Adly AS, J Med Internet Res, 2021 | preprints.jmir.org/preprint/23446/accepted | No relevant outcome |
| A randomized, double‐blind, placebo‐controlled phase 1 trial of inhaled and intranasal niclosamide: a broad spectrum antiviral candidate for treatment of COVID‐19 |
Backer V, Lancet, 2021 | www.thelancet.com/journals/lanepe/article/PIIS2666‐7762(21)00061‐2/fulltext | Non‐COVID‐19 patients |
| Multicentre randomised double‐blinded placebo‐controlled trial of favipiravir in adults with mild COVID‐19. |
Bosaeed M, BMJ Open, 2021 | bmjopen.bmj.com/content/11/4/e047495 | Other reasons |
| Pharmacokinetics and safety of XAV‐19, a swine glyco‐humanized polyclonal anti‐SARS‐CoV‐2 antibody, for COVID‐19‐related moderate pneumonia: a randomized, double‐blind, placebo‐controlled, phase IIa study |
Gaborit B, medRxiv, 2021 | www.medrxiv.org/content/10.1101/2021.04.15.21255549v1 | No relevant outcome |
| Randomized clinical trial to evaluate a routine full anticoagulation strategy in patients with coronavirus infection (SARS‐CoV2) admitted to hospital: rationale and design of the ACTION (AntiCoagulaTlon cOroNavirus)‐Coalition IV Trial |
Lopes RD, Am Heart J, 2021 | www.sciencedirect.com/science/article/pii/S0002870321000958 | Other reasons |
| Reusable snorkel masks adapted as particulate respirators |
Seligman H, PloS one, 2021 | journals.plos.org/plosone/article?id=10.1371/journal.pone.0249201 | No relevant outcome |
| Efficacy of m‐Health for the detection of adverse events following immunization ‐ the stimulated telephone assisted rapid safety surveillance (STARSS) randomised control trial |
Gold MS, Vaccine, 2021 | www.sciencedirect.com/science/article/pii/S0264410X20315097?via%3Dihub | No relevant outcome |
| The efficacy of computerized cognitive behavior therapy (cCBT) for depressive and anxiety symptoms in patients with COVID‐19: randomized controlled trial | Liu Z, J Med Internet Res, 2021 | preprints.jmir.org/preprint/26883/accepted | No relevant outcome |
| The effect of omega‐3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID‐19: a randomized clinical trial |
Doaei S, J Transl Med, 2021 | translational‐medicine.biomedcentral.com/articles/10.1186/s12967‐021‐02795‐5 | No relevant outcome |
| Optimal dose and safety of molnupiravir in patients with early SARS‐CoV‐2: a phase 1, dose‐escalating, randomised controlled study |
Khoo SH, medRxiv, 2021; preprint of Khoo SH, J Antim Chemo, 2021 | www.medrxiv.org/content/10.1101/2021.05.03.21256309v1 | Early phase |
| Arbidol combined with the Chinese medicine Lianhuaqingwen capsule versus arbidol alone in the treatment of COVID‐19 |
Liu L, Medicine, 2021 | journals.lww.com/md‐journal/Fulltext/2021/01290/Arbidol_combined_with_the_Chinese_medicine.111.aspx | Irrelevant intervention |
| The angiotensin II type 1 receptor blocker valsartan in the battle against COVID‐19. |
Ligt M, Obesity, 2021 | onlinelibrary.wiley.com/doi/10.1002/oby.23221 | No relevant outcome |
| In South Africa, a 2‐dose Oxford/AZ vaccine did not prevent mild to moderate COVID‐19 (cases mainly B.1.351 variant). |
Irfan N, Ann Intern Med, 2021 | www.acpjournals.org/doi/10.7326/ACPJ202105180‐050 | Other reasons |
| Tolerability, safety, pharmacokinetics, and immunogenicity of a novel SARS‐CoV‐2 neutralizing antibody, etesevimab in Chinese healthy adults: a randomized, double‐blind, placebo‐controlled, first‐in‐human phase 1 study. |
Wu X, Antimicrob Agents Chemother, 2021 | aac.asm.org/content/early/2021/05/04/AAC.00350‐21 | Early phase |
| Adjunct low‐dose ketamine infusion in critically ill patients at a Saudi Hospital (Attainment trial) |
Amer M. ,Crit. Care Med., 2021 | journals.lww.com/ccmjournal/Citation/2021/01001/701__Adjunct_Low_Dose_Ketamine_Infusion_in.669.aspx | No relevant outcome |
| Safety and efficacy of meplazumab in healthy volunteers and COVID‐19 patients: a randomized phase 1 and an exploratory phase 2 trial |
Bian H, Sig Transduct Target Ther, 2021 | www.nature.com/articles/s41392‐021‐00603‐6 | Early phase |
| Accelerated first‐in‐human clinical trial of EIDD‐2801/MK‐4482 (molnupiravir), a ribonucleoside analogue with potent antiviral activity against SARS‐CoV‐2 |
Cohen O, ResearchSquare, 2021; preprint of Holman W, Trials, 2021 | www.researchsquare.com/article/rs‐477300/v1 | Early phase |
| Effect of toclilizumab on cardiac injury and dysfunction in COVID‐19 |
Zafar A, J. Am. Coll. Cardiol., 2021 | www.sciencedirect.com/science/article/pii/S0735109721043837?via%3Dihub | Secondary analysis |
| Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease |
Lakkireddy M, Sci Rep, 2021 publication of Lakkireddy M, ResearchSquare, 2021 | www.nature.com/articles/s41598‐021‐90189‐4 | No relevant outcome |
| ChAdOx1 nCoV‐19 (AZD1222) vaccine in people living with and without HIV |
Madhi S, ResearchSquare,2021 | www.researchsquare.com/article/rs‐322470/v1 | Secondary analysis |
| T cell and antibody responses induced by a single dose of ChAdOx1 nCoV‐19 (AZD1222) vaccine in a phase 1/2 clinical trial. |
Ewer KJ, Nature medicine, 2020 | www.nature.com/articles/s41591‐020‐01194‐5 | Secondary analysis |
| Phase 1/2 trial of SARS‐CoV‐2 vaccine ChAdOx1 nCoV‐19 with a booster dose induces multifunctional antibody responses. |
Barrett JR, Nature medicine, 2020 | www.nature.com/articles/s41591‐020‐01179‐4 | Secondary analysis |
| Vitamin C may increase the recovery rate of outpatient cases of SARS‐CoV‐2 infection by 70%: reanalysis of the COVID A to Z randomized clinical trial |
Hemila H, Front. Immunol, 2021; comment of Hemila H, ResearchSquare, 2021 | www.frontiersin.org/articles/10.3389/fimmu.2021.674681/full | Other reasons |
| Virucidal effect of povidone iodine on COVID‐19 in the nasopharynx: an open‐label randomized clinical trial. |
Arefin MK, Indian J Otolaryngol Head Neck Surg, 2021 | link.springer.com/article/10.1007/s12070‐021‐02616‐7 | Irrelevant intervention |
| The efficacy of antioxidant oral supplements on the progression of COVID‐19 in non‐critically ill patients: a randomized controlled trial |
Abulmeaty MMA, Antioxidants (Basel), 2021 | www.mdpi.com/2076‐3921/10/5/804 | No relevant outcome |
| Potential use of azithromycin alone and in combination with ivermectin in fighting against the symptoms of COVID‐19 |
Rizwan F, Professional Med J, 2021 | http://theprofesional.com/index.php/tpmj/article/view/5867 | Not randomised or improper randomisation |
| The method and results of a treatment targeting SARS‐CoV‐2‐activated inflammasomes |
Lee J, ResearchSquare, 2021 | www.researchsquare.com/article/rs‐509122/v4 | Not randomised or improper randomisation |
| Safety, immunogenicity, and efficacy of a COVID‐19 vaccine (NVX‐CoV2373) co‐administered with seasonal influenza vaccines |
Toback S, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.06.09.21258556v1 | Not randomised or improper randomisation |
| Clinical efficacy of an osmotic, antiviral and anti‐inflammatory polymeric nasal film to treat covid‐19 early‐phase respiratory symptoms |
Shrivastava R, Open Access J. Clin., 2021 | www.dovepress.com/clinical‐efficacy‐of‐an‐osmotic‐antiviral‐ and‐anti‐inflammatory‐polyme‐peer‐reviewed‐fulltext‐article‐OAJCT |
Not randomised or improper randomisation |
| Tocilizumab in COVID‐19 ‐ a Bayesian reanalysis of RECOVERY |
Albuquerque A, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.06.15.21258966v1 | Secondary analysis |
| Is convalescent plasma futile in COVID‐19? A Bayesian re‐analysis of the RECOVERY randomised controlled trial |
Hamilton FW, Int J Infect Dis, 2021 | www.ijidonline.com/article/S1201‐9712(21)00523‐3/fulltext | Secondary analysis |
| Safety and immunogenicity of a recombinant stabilized prefusion SARS‐CoV‐2 spike protein vaccine (MVCCOV1901) adjuvanted with CpG 1018 and aluminum hydroxide in healthy adults: a phase 1, dose‐escalation study |
Hsieh SM, EclinicalMedicine, 2021 | www.thelancet.com/journals/eclinm/article/PIIS2589‐5370(21)00269‐8/fulltext | Not randomised or improper randomisation |
| Acute triiodothyronine treatment and red blood cell sedimentation rate (ESR) in critically ill COVID‐19 patients: a novel association? |
Pantos C, Clin Hemorheol Microcirc, 2021 | content.iospress.com/articles/clinical‐hemorheology‐and‐microcirculation/ch211215 | No relevant outcome |
| Randomised, controlled, open label, multicentre clinical trial to explore safety and efficacy of hyperbaric oxygen for preventing ICU admission, morbidity and mortality in adult patients with COVID‐19. |
Kjellberg A, BMJ Open, 2021 | bmjopen.bmj.com/content/11/7/e046738 | Other reasons |
| Application of nasal spray containing dimethyl sulfoxide (DSMO) and ethanol during the COVID‐19 pandemic may protect healthcare workers: A randomized controlled trials |
Hosseinzadeh A, medRxiv, 2021 | www.medrxiv.org/content/10.1101/2021.07.06.21259749v1 | Irrelevant intervention |
| RIC in COVID‐19 ‐ a clinical trial to investigate whether remote ischemic conditioning (RIC) can prevent deterioration to critical care in patients with COVID‐19. |
Davidson SM, Cardiovasc Drugs Ther, 2021 | link.springer.com/article/10.1007/s10557‐021‐07221‐y | No relevant outcome |
| Double‐blind, randomized, placebo‐controlled trial with N‐acetylcysteine for treatment of severe acute respiratory syndrome caused by Coronavirus Disease 2019 (COVID‐19) |
De Alencar JCG, Clin Infect Dis, 2021 | academic.oup.com/cid/article/72/11/e736/5910353 | Irrelevant intervention |
| Inhaled nitric oxide for the treatment of COVID‐19 and other viral pneumonias in adults |
Wolak T, Am J Respir Crit Care Med, 2021 | www.atsjournals.org/doi/10.1164/ajrccm‐conference.2021.203.1_MeetingAbstracts.A3849 | Other reasons |
| Assessing the potential correlation of polymorphisms in the IL6R with relative IL6 elevation in severely ill COVID‐19 patients'. |
Smieszek SP, Cytokine, 2021 | www.sciencedirect.com/science/article/pii/S1043466621002490?via%3Dihub | Secondary analysis |
| The systematic effect of Mesenchymal stem cell therapy in critical COVID‐19 patients: a prospective double controlled trial. |
Adas G, Cell Transplant, 2021 | journals.sagepub.com/doi/10.1177/09636897211024942 | Not randomised or improper randomisation |
| A single intramuscular injection of monoclonal antibody MAD0004J08 induces in healthy adults SARS‐CoV‐2 neutralising antibody titres exceeding those induced by infection and vaccination |
Lanin S, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.08.03.21261441v1 | Early phase |
| Accelerated first‐in‐human clinical trial of EIDD‐2801/MK‐4482 (molnupiravir), a ribonucleoside analog with potent antiviral activity against SARS‐CoV‐2 |
Holman W, Trials, 2021; publication of Cohen O, ResearchSquare, 2021 | trialsjournal.biomedcentral.com/articles/10.1186/s13063‐021‐05538‐5 | Early phase |
| Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID‐19 Pandemic |
Elgazzar A, ResearchSquare, 2020 | www.researchsquare.com/article/rs‐100956/v4 | Not randomised or improper randomisation |
| Emergence of SARS‐CoV‐2 Resistance with Monoclonal Antibody Therapy |
Choudhary M, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.09.03.21263105v1 | Not randomised or improper randomisation |
| Melatonin effects on sleep quality and outcomes of COVID‐19 patients: An open‐label, Randomized, Controlled Trial |
Mousavi SA, J Med Virol, 2021 | onlinelibrary.wiley.com/doi/10.1002/jmv.27312 | Other reasons |
| Optimal dose and safety of molnupiravir in patients with early SARS‐CoV‐2: a Phase I, open‐label, dose‐escalating, randomized controlled study |
Khoo SH, J Antim Chemo, 2021; publication of Khoo SH, medRxiv, 2021 | academic.oup.com/jac/advance‐article/doi/10.1093/jac/dkab318/6358705 | Early phase |
| Preliminary Analysis of Safety and Immunogenicity of a SARS‐CoV‐2 Variant Vaccine Booster |
Kai Wu, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.05.05.21256716v1 | Not randomised or improper randomisation |
| Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV‐19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) |
Flaxman A, Lancet, 2021 | www.thelancet.com/journals/lancet/article/PIIS0140‐6736(21)01699‐8/fulltext | Not randomised or improper randomisation |
| The effects of narrative exposure therapy on COVID‐19 patients with post‐traumatic stress symptoms: a randomized controlled trial. |
Fan Y, Shi Y, Zhang J, Sun D, Wang X, Fu G, Mo D, Wen J, Xiao X, Kong L. | www.sciencedirect.com/science/article/pii/S0165032721006054?via%3Dihub | No relevant outcome |
| Exogenous surfactant versus placebo in the treatment of moderate and severe ARDS in COVID‐19: the pilot study of a clinical trial | Ghahremani M, ResearchSquare, 2021 | www.researchsquare.com/article/rs‐136365/v1 | Other reasons |
| Induction of robust neutralizing antibodies against the COVID‐19 Delta variant with ChAdOx1 nCoV‐19 or BNT162b2 as a booster following a primary vaccination series with CoronaVac |
Patamatamkul S, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.09.25.21264099v1 | Not randomised or improper randomisation |
| Intensive treatment with ivermectin and iota‐carrageenan as pre‐exposure prophylaxis for COVID‐19 in health care workers from Tucuman, Argentina |
Chahla RE, Am J Ther, 2021 | lww.com/pages/results.aspx?txtKeywords=10.1097%2fMJT.0000000000001433 | Other reasons |
| Dutasteride reduces viral shedding, inflammatory responses and time‐to‐remission in COVID‐19: biochemical findings of a randomized double‐blind placebo controlled interventional trial (DUTA AndroCoV‐Trial ‐ Biochemical) |
Cadegiani FA, Research Square, 2020 | assets.researchsquare.com/files/rs‐135815/v1/f751da60‐614d‐4b38‐b572‐be7bb9951064.pdf | No relevant outcome |
| Impact of decreasing respiratory rate while tolerating moderate hypercapnia on lung injury markers in patients with COVID‐19 related acute respiratory distress syndrome |
Damiani F, Am J Respir Crit Care Med, 2021 | www.atsjournals.org/doi/10.1164/ajrccm‐conference.2021.203.1_MeetingAbstracts.A2497 | Irrelevant intervention |
| Salivary SARS‐CoV‐2 load reduction with mouthwash use: A randomized pilot clinical trial. |
Eduardo FP, Heliyon, 2021 | www.cell.com/heliyon/fulltext/S2405‐8440(21)01449‐3?_returnURL=https%3A%2F%2Flinkinghub. elsevier.com%2Fretrieve%2Fpii%2FS2405844021014493%3Fshowall%3Dtrue |
Irrelevant intervention |
| Investigation of the Presence of SARS‐CoV‐2 in Aerosol After Dental Treatment. |
Akin H, Int Dent J, 2021 | www.sciencedirect.com/science/article/pii/S0020653921000988?via%3Dihub | No relevant outcome |
| Factors associated with mortality among moderate and severe patients with COVID‐19 in India: a secondary analysis of a randomised controlled trial |
Mammen J, BMJ, 2021 | bmjopen.bmj.com/content/11/10/e050571 | Secondary analysis |
| Beneficial effects of novel aureobasidium pullulans strains produced beta‐1,3‐1,6 glucans on interleukin‐6 and D‐dimer levels in COVID‐19 patients; results of a randomized multiple‐arm pilot clinical study |
Raghavan k, , 2021 | www.sciencedirect.com/science/article/pii/S0753332221010271?via%3Dihub | Irrelevant intervention |
| Evaluation of SARS‐CoV‐2 spike protein antibody titers in cord blood after COVID‐19 vaccination during pregnancy in Polish healthcare workers: preliminary results. |
Zdanowski W, vaccines, 2021 | www.mdpi.com/2076‐393X/9/6/675 | Not randomised or improper randomisation |
| Safety, virologic efficacy, and pharmacokinetics of CT‐P59, a neutralizing monoclonal antibody against SARS‐CoV‐2 spike receptor‐binding protein: two randomized, placebo‐controlled, phase I studies in healthy individuals and patients with mild SARS‐CoV‐2 infection. |
Kim JY, Clin Ther, 2021 | www.clinicaltherapeutics.com/article/S0149‐2918(21)00308‐8/fulltext | Early phase |
| Covid‐19 in the phase 3 trial of mRNA‐1273 during the Delta‐variant surge |
Baden L, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.09.17.21263624v1 | Not randomised or improper randomisation |
| Tolerability, safety and immunogenicity of intradermal delivery of a fractional dose mRNA ‐1273 SARS‐CoV‐2 vaccine in healthy adults as a dose sparing strategy |
Roozen G, medrxiv, 2021; same as Roozen G, SSRN, 2021 | www.medrxiv.org/content/10.1101/2021.07.27.21261116v1 | Early phase |
| Tolerability, safety and immunogenicity of intradermal delivery of a fractional dose mRNA ‐1273 SARS‐CoV‐2 vaccine in healthy adults as a dose sparing strategy |
Roozen G, SSRN, 2021; same as Roozen G, medrxiv, 2021 | papers.ssrn.com/sol3/papers.cfm?abstract_id=3892129 | Early phase |
| The safety and immunogenicity of concomitant administration of COVID‐19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults: a phase IV, multicentre randomised controlled trial with blinding (ComFluCOV) |
Lazarus R, SSRN, 2021 | papers.ssrn.com/sol3/papers.cfm?abstract_id=3931758 | Irrelevant intervention |
| Safety, tolerability, pharmacokinetic characteristics, and immunogenicity of MW33: a phase 1 clinical study of the SARS‐CoV‐2 RBD‐targeting monoclonal antibody |
Meng X, Emerg Microbes Infect, 2021 | www.tandfonline.com/doi/full/10.1080/22221751.2021.1960900 | Early phase |
| Various combinations of favipiravir, lopinavir‐ritonavir, darunavir‐ritonavir, high‐dose oseltamivir, and hydroxychloroquine for the treatment of COVID‐19: a randomized controlled trial (FIGHT‐COVID‐19 Study) |
Atipornwanich K, SSRN, 2021 | papers.ssrn.com/sol3/papers.cfm?abstract_id=3936499 | Not randomised or improper randomisation |
| Non‐invasive vagus nerve stimulation for respiratory symptoms of COVID‐19: results from a randomized controlled trial (SAVIOR I) |
Tornero C, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.09.24.21264045v1 | No relevant outcome |
| Clinical trial of efficacy and toxicity of disoproxil tenofovir fumarate and emtricitabine for mild to moderate SARS‐CoV‐2 infections |
Arruda EA, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.09.28.21264242v1 | No relevant outcome |
| Intranasal dexamethasone: a new clinical trial for the control of inflammation and neuroinflammation in Covid‐19 patients |
Caadenas G, ResearchSquare, 2021 | www.researchsquare.com/article/rs‐693766/v1 | Other reasons |
| Efficacy of a nasal spray containing iota‐carrageenan in the postexposure prophylaxis of COVID‐19 in hospital personnel dedicated to patients care with COVID‐19 disease. |
Figueroa JM, Int J Gen Med, 2021 | www.dovepress.com/efficacy‐of‐a‐nasal‐spray‐containing‐iota‐ carrageenan‐in‐the‐postexpos‐peer‐reviewed‐fulltext‐article‐IJGM |
Irrelevant intervention |
| Safety and immunogenicity of CoronaVac and ChAdOx1 against the SARS‐CoV‐2 circulating variants of concern (Alpha, Delta, Beta) in Thai healthcare workers |
Angkasekwinai N, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.10.03.21264451v1 | Not randomised or improper randomisation |
| Immune memory response after a booster injection of mRNA‐1273 for severe acute respiratory syndrome Coronavirus‐2 (SARS‐CoV‐2) |
Chu L, medrxiv, 2021 | www.medrxiv.org/content/10.1101/2021.09.29.21264089v1 | Irrelevant intervention |
| A booster dose is immunogenic and will be needed for older adults who have completed two doses vaccination with CoronaVac: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial |
Li M, medRxiv, 2021 | www.medrxiv.org/content/10.1101/2021.08.03.21261544v1 | Irrelevant intervention |
| C‐reactive protein as a biomarker for improved efficacy of lenzilumab in patients with COVID‐19: results from the LIVE‐AIR trial | Temesgen Z, Chest, 2021 | www.sciencedirect.com/science/article/pii/S0012369221036540?via%3Dihub | No relevant outcome |
Appendix 6. Characteristics of unpublished registered studies: anakinra versus placebo
| Registration number | Status | Registration date | Design | Estimated sample size | Interventions | Control interventions |
| IRCT20120703010178N20 | Completed | June 2020 | Parallel | 30 | Anakinra | Standard care |
| NCT04443881 | Completed | June 2020 | Parallel | 180 | Anakinra | Standard care |
| EUCTR2020‐001739‐28‐BE | Completed | April 2020 | Adaptive | 210 | Anakinra | Standard care |
| NCT04364009 | Terminated | April 2020 | Parallel | 240 | Anakinra | Standard care |
| NCT04366232 | Terminated | April 2020 | Parallel | 50 | Anakinra | Standard care |
| EUCTR2020‐001734‐36‐FR | Terminated | April 2020 | Parallel | 240 | Anakinra | Standard care |
| NCT04324021 | Terminated | March 2020 | Parallel | 54 | Anakinra | Standard care |
| NCT04643678 | Ongoing | November 2020 | Parallel | 80 | Anakinra | Standard care |
| EUCTR2020‐005828‐11‐GR | Ongoing | December 2020 | Parallel | 500 | Anakinra | Placebo |
| NCT04412291 | Ongoing | June 2020 | Parallel | 120 | Anakinra | Standard care |
| NCT04381936 | Ongoing | May 2020 | Factorial | 40000 | Anakinra | Standard care |
| EUCTR2020‐001825‐29‐ES | Ongoing | May 2020 | Parallel | 180 | Anakinra | Standard care |
| NCT04362111 | Not recruiting | April 2020 | Parallel | 20 | Anakinra | Placebo |
| NCT04603742 | Not recruiting | October 2020 | Parallel | 100 | Anakinra | Placebo |
| NCT04424056 | Not recruiting | June 2020 | Parallel | 216 | Anakinra+/‐Ruxolitinib | Standard care |
Appendix 7. Characteristics of unpublished registered studies: canakinumab versus placebo
| Registration number | Status | Registration date | Design | Estimated sample size | Interventions | Control interventions |
| NCT04510493 | Completed | August 2020 | Parallel | 116 | Canakinumab | Placebo |
Appendix 8. Details of requests to authors of unpublished trials (i.e. update on the status of the study: if ongoing, communicate the expected completion date; if complete, request to share results before publication)
| Study ID | Trial status registry | Treatment | Date of contact | Reply |
| IRCT20120703010178N20 |
Completed |
Anakinra |
6 November 2020 30 June 2021 |
Answered, but no data provided yet. |
|
NCT04443881 |
Completed | Anakinra |
6 November 2020 30 June 2021 |
Answered, but no data provided yet (30 June 2021). |
| EUCTR2020‐001739‐28‐BE |
Completed | Anakinra | 30 June 2021 | Answered; the trial is at the data cleaning/data analysis phase. Willing to share data when available. |
|
NCT04364009 |
Terminated | Anakinra |
6 November 2020 30 June 2021 |
No response |
|
NCT04366232 |
Terminated |
Anakinra | 6 November 2020 30 June 2021 |
No response |
| EUCTR2020‐001734‐36‐FR |
Terminated |
Anakinra |
6 November 2020 30 June 2021 |
No response |
|
NCT04324021 |
Terminated | Anakinra | 11/03/2020 |
Answered; the study has been closed, no data provided yet. |
| NCT04643678 | Ongoing | Anakinra | 30 June 2021 | Answered; the study is now completed and at the data analysis phase. Willing to share data when available. |
| EUCTR2020‐005828‐11‐GR | Ongoing | Anakinra | 30 June 2021 | No response |
| NCT04412291 | Ongoing | Anakinra | 30 August 2021 | No response yet |
| NCT04381936 | Ongoing | Anakinra | 30 August 2021 | No response yet |
| EUCTR2020‐001825‐29‐ES | Ongoing | Anakinra | 6 November 2020 30 June 2021 |
No response |
|
NCT04362111 |
Not recruiting | Anakinra | 6 November 2020 30 June 2021 |
No response |
|
NCT04603742 |
Not recruiting | Anakinra | 30 June 2021 | No response |
| NCT04424056 | Not recruiting | Anakinra+/‐Ruxolitinib | Not yet contacted | |
| NCT04510493 | Completed | Canakinumab | 6 November 2020 30 June 2021 |
No response |
Appendix 9. Risk of bias assessments
Anakinra versus standard care/placebo
Clinical improvement D28
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Declercq COV‐AID 2021 | Low | Low | Low | Some concernsa | Low | Some concerns |
| Kyriazopoulou SAVE‐MORE 2021 | Low | Low | Low | Low | Low | Low |
| Mariette CORIMUNO‐19 Collaborative 2021 | Low | Low | Low | Some concernsb | Low | Some concerns |
aMethod of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study (outcome assessor). Clinical improvement (defined as an increase of at least two points on a 6‐category ordinal scale or discharge from the hospital alive) requires clinical judgement and could be affected by knowledge of intervention receipt, but is not considered likely to in the context of a pandemic. Risk assessed to be some concerns for this outcome. bMethod of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study. Clinical improvement (defined as discharge by day 28) requires clinical judgement and could be affected by knowledge of intervention receipt. Risk assessed to be some concerns for this outcome.
Clinical improvement D60 or above
| Study | 1.Randomization | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Declercq COV‐AID 2021 | Low | Low | Low | Some concernsc | Low | Some concerns |
cMethod of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study (outcome assessor). Clinical improvement (defined as an increase of at least two points on a 6‐category ordinal scale or discharge from the hospital alive) requires clinical judgement and could be affected by knowledge of intervention receipt, but is not considered likely to in the context of a pandemic. Risk assessed to be some concerns for this outcome.
WHO Clinical Progression Score of level 7 or above D28
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kyriazopoulou SAVE‐MORE 2021 | Low | Low | Low | Low | Low | Low |
| Mariette CORIMUNO‐19 Collaborative 2021 | Low | Low | Low | Low | Low | Low |
WHO Clinical Progression Score of level 7 or above D60 or above
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kyriazopoulou SAVE‐MORE 2021 | Low | Low | Some concernsd | Low | Low | Some concerns |
d606 participants randomised; 568 participants analysed. Data not available for all or nearly all participants randomised. No evidence that the result is not biased. Reasons: lost to follow‐up (24 vs 14). Missingness could depend on the true value of the outcome. Not likely that missingness depended on the true value of the outcome (same reasons and equal proportion of missingness between arms). Risk assessed to be some concerns for this outcome.
All‐cause mortality D28
| Study | 1.Randomization | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Kyriazopoulou SAVE‐MORE 2021 | Low | Low | Low | Low | Low | Low |
| Mariette CORIMUNO‐19 Collaborative 2021 | Low | Low | Low | Low | Low | Low |
All‐cause mortality D60 or above
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Declercq COV‐AID 2021 | Low | Low | Low | Low | Low | Low |
| Derde REMAP‐CAP 2021 | Low | Some concernse | Low | Low | Low | Some concerns |
| Kyriazopoulou SAVE‐MORE 2021 | Low | Low | Some concernsf | Low | Low | Some concerns |
| Mariette CORIMUNO‐19 Collaborative 2021 | Low | Low | Low | Low | Low | Low |
eQuote: "Open‐label design" Comment: Unblinded study (participants and personnel/carers). No participant cross‐over. No information on administration of co‐interventions of interest, antivirals, biologics and corticosteroids were reported. Hence, no information on whether deviations arose because of the trial context. Our analysis for the binary outcome is an intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention. Risk assessed to be some concerns for this outcome. f606 participants randomised; 568 participants analysed. Data not available for all or nearly all participants randomised. No evidence that the result is not biased. Reasons: lost to follow‐up (24 vs 14). Missingness could depend on the true value of the outcome. Not likely that missingness depended on the true value of the outcome (same reasons and equal proportion of missingness between arms). Risk assessed to be some concerns for this outcome.
Incidence of any adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kyriazopoulou SAVE‐MORE 2021 | Low | Low | Low | Low | Low | Low |
| Mariette CORIMUNO‐19 Collaborative 2021 | Low | Low | Low | Some concernsg | Low | Some concerns |
gMethod of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study. The authors reported on adverse events that may contain both clinically and laboratory‐detected outcomes. All these outcomes can be influenced by knowledge of the intervention assignment. Risk assessed to be some concerns for this outcome.
Incidence of serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kyriazopoulou SAVE‐MORE 2021 | Low | Low | Low | Low | Low | Low |
| Mariette CORIMUNO‐19 Collaborative 2021 | Low | Low | Low | Some concernsh | Low | Some concerns |
hMethod of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study. The authors reported on serious adverse events that may contain both clinically and laboratory‐detected outcomes. All these outcomes can be influenced by knowledge of the intervention assignment. Risk assessed to be some concerns for this outcome.
Time to clinical improvement
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Declercq COV‐AID 2021 | Low | Low | Low | Some concernsi | Low | Some concerns |
| Derde REMAP‐CAP 2021 | Low | Some concernsj | Low | Some concernsk | Low | Some concerns |
| Kyriazopoulou SAVE‐MORE 2021 | Low | Low | Some concernsl | Low | Low | Some concerns |
| Mariette CORIMUNO‐19 Collaborative 2021 | Low | Low | Low | Some concernsm | Low | Some concerns |
iMethod of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study (outcome assessor). Clinical improvement (defined as an increase of at least two points on a 6‐category ordinal scale or discharge from the hospital alive) requires clinical judgement and could be affected by knowledge of intervention receipt, but is not considered likely to in the context of a pandemic. Risk assessed to be some concerns for this outcome. jQuote: "Open‐label design" Comment: Unblinded study (participants and personnel/carers). No participant cross‐over. No information on administration of co‐interventions of interest, antivirals, biologics and corticosteroids were reported. Hence, no information on whether deviations arose because of the trial context. Participants were analysed according to their randomised groups for the outcome. Of note, 9 (tocilizumab), 2 (sarilumab), 8 (anakinra) participants were excluded from the analysis postrandomisation because outcome data were not available, which is accounted for in domain 3. This method was considered appropriate to estimate the effect of assignment to intervention for this outcome. Risk assessed to be some concerns for this outcome. kMethod of measuring the outcome probably appropriate. Measurement or ascertainment of outcome does not differ among groups. Unblinded study (outcome assessor). Clinical improvement (defined as hospital discharge) requires clinical judgement and could be affected by knowledge of intervention receipt. Risk assessed to be some concerns for this outcome. l606 participants randomised; 568 participants analysed. Data not available for all or nearly all participants randomised. No evidence that the result is not biased. Reasons: lost to follow‐up (24 vs 14). Missingness could depend on the true value of the outcome. Not likely that missingness depended on the true value of the outcome (same reasons and equal proportion of missingness between arms). Risk assessed to be some concerns for this outcome. mMethod of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study. Clinical improvement (defined as discharge by day 28) requires clinical judgement and could be affected by knowledge of intervention receipt. Risk assessed to be some concerns for this outcome.
Time to WHO Clinical Progression Score of level 7 or above
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Kyriazopoulou SAVE‐MORE 2021 | Low | Low | Some concernsn | Low | Low | Some concerns |
| Mariette CORIMUNO‐19 Collaborative 2021 | Low | Low | Low | Low | Low | Low |
n606 participants randomised; 568 participants analysed. Data not available for all or nearly all participants randomised. No evidence that the result is not biased. Reasons: lost to follow‐up (24 vs 14). Missingness could depend on the true value of the outcome. Not likely that missingness depended on the true value of the outcome (same reasons and equal proportion of missingness between arms). Risk assessed to be some concerns for this outcome.
Time to death
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Derde REMAP‐CAP 2021 | Low | Some concernso | Low | Low | Low | Some concerns |
| Kyriazopoulou SAVE‐MORE 2021 | Low | Low | Some concernsp | Low | Low | Some concerns |
| Mariette CORIMUNO‐19 Collaborative 2021 | Low | Low | Low | Low | Low | Low |
oQuote: "Open‐label design" Comment: Unblinded study (participants and personnel/carers). No participant cross‐over. No information on administration of co‐interventions of interest, antivirals, biologics and corticosteroids were reported. Hence, no information on whether deviations arose because of the trial context. Participants were analysed according to their randomised groups for the outcome. Of note, 9 (tocilizumab), 2 (sarilumab), 8 (anakinra) participants were excluded from the analysis postrandomisation because outcome data were not available, which is accounted for in domain 3. This method was considered appropriate to estimate the effect of assignment to intervention for this outcome. Risk assessed to be some concerns for this outcome. p606 participants randomised; 568 participants analysed. Data not available for all or nearly all participants randomised. No evidence that the result is not biased. Reasons: lost to follow‐up (24 vs 14) Missingness could depend on the true value of the outcome. Not likely that missingness depended on the true value of the outcome (same reasons and equal proportion of missingness between arms). Risk assessed to be some concerns for this outcome.
Canakinumab vs Placebo
Clinical improvement D28
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Caricchio CAN‐COVID 2021 | Low | Low | Low | Low | Low | Low |
| Cremer Three C Study 2021 | Low | Low | Low | Low | Some concernsq | Some concerns |
qThe protocol (dated 11 May 2020), statistical analysis plan, registry (earliest version dated 24 April 2020) were available. Outcome not prespecified. No information on whether the result was selected from multiple outcome measurements or analyses of the data. Trial probably not analysed as prespecified. Risk assessed to be some concerns for this outcome.
WHO Clinical Progression Score of level 7 or above D28
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Caricchio CAN‐COVID 2021 | Low | Low | Low | Low | Low | Low |
| Cremer Three C Study 2021 | Low | Low | Low | Low | Low | Low |
All‐cause mortality D28
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Caricchio CAN‐COVID 2021 | Low | Low | Low | Low | Low | Low |
| Cremer Three C Study 2021 | Low | Low | Low | Low | Low | Low |
All‐cause mortality D60 or above
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Cremer Three C Study 2021 | Low | Low | Low | Low | Low | Low |
Incidence of any adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Caricchio CAN‐COVID 2021 | Low | Low | Low | Low | Low | Low |
Incidence of serious adverse events
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Caricchio CAN‐COVID 2021 | Low | Low | Low | Low | Low | Low |
| Cremer Three C Study 2021 | Low | Low | Low | Low | Low | Low |
Time to clinical improvement
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Caricchio CAN‐COVID 2021 | Low | Low | Low | Low | Low | Low |
Time to WHO Clinical Progression Score of level 7 or above
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Caricchio CAN‐COVID 2021 | Low | Low | Low | Low | Low | Low |
Time to death
| Study | 1. Randomization | 2. Deviations from intervention | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias |
| Caricchio CAN‐COVID 2021 | Low | Low | Low | Low | Low | Low |
| Cremer Three C Study 2021 | Low | Low | Low | Low | Low | Low |
Appendix 10. Matrix indicating availability of trial results for the critical and important outcomes of the comparison: anakinra versus standard care/placebo
Anakinra versus standard care/placebo: critical outcomes
| Critical outcomes | |||||||||||
| Trial ID | Study follow‐up (in days) | Sample size: anakinra | Sample size: standard care or placebo | Clinical improvement | WHO Clinical Progression Score of level 7 or above | All‐cause mortality | AE | SAE | |||
| Day 28 | Day ≥ 60 | Day 28 | Day ≥ 60 | Day 28 | Day ≥ 60 | ||||||
| Declercq COV‐AID 2021 | 90 | 43 | 72 | ✓ | ✓ | * | * | * | ✓ | X | X |
| Derde REMAP‐CAP 2021 | 90 | 378 | 418 | * | * | * | * | * | ✓ | X | X |
| Kyriazopoulou SAVE‐MORE 2021 | 90 | 412 | 194 | ✓ | * | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Mariette CORIMUNO‐19 Collaborative 2021 | 90 | 59 | 57 | ✓ | * | ✓ | * | ✓ | ✓ | ✓ | ✓ |
Key AE: adverse event SAE: serious adverse event ✓ A study result is available for inclusion in the synthesis. X No study result is available for inclusion, (probably) because the P value, magnitude or direction of the results generated were considered unfavourable by the study investigators. * No study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude or direction of the results. ? No study result is available for inclusion, and it is unclear if the outcome was assessed in the study.
Anakinra versus standard care/placebo: important outcomes
| Important outcomes | ||||||
| Trial ID | Study follow‐up (in days) | Sample size: anakinra | Sample size: standard care or placebo | Time to clinical improvement | Time to WHO Clinical Progression Score of level 7 or above | Time to death |
| Declercq COV‐AID 2021 | 90 | 43 | 72 | ✓ | * | * |
| Derde REMAP‐CAP 2021 | 90 | 378 | 418 | ✓ | * | ✓ |
| Kyriazopoulou SAVE‐MORE 2021 | 90 | 412 | 194 | ✓ | ✓ | ✓ |
| Mariette CORIMUNO‐19 Collaborative 2021 | 90 | 59 | 57 | ✓ | ✓ | ✓ |
Key AE: adverse event SAE: serious adverse event ✓ A study result is available for inclusion in the synthesis. X No study result is available for inclusion, (probably) because the P value, magnitude or direction of the results generated were considered unfavourable by the study investigators. * No study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude or direction of the results. ? No study result is available for inclusion, and it is unclear if the outcome was assessed in the study.
Appendix 11. Matrix indicating availability of trial results for the critical and important outcomes of the comparison: canakinumab 300/600 mg versus standard care or canakinumab 450 to 750 mg single dose versus placebo
Canakinumab 300/600 mg versus standard care or canakinumab 450 to 750 mg single dose versus placebo: critical outcomes
| Critical outcomes | |||||||||||
| Trial ID | Study follow‐up (in days) | Sample size: canakinumab 300/600 mg | Sample size: standard care or placebo | Clinical improvement | WHO Clinical Progression Score of level 7 or above | All‐cause mortality | AE | SAE | |||
| Day 28 | Day ≥ 60 | Day 28 | Day ≥ 60 | Day 28 | Day ≥ 60 | ||||||
| Caricchio CAN‐COVID 2021 | 28 | 227 | 227 | ✓ | * | ✓ | * | ✓ | * | ✓ | ✓ |
| Cremer Three C Study 2021 | 150 | 29 | 16 | ✓ | * | ✓ | * | ✓ | ✓ | * | ✓ |
Key AE: adverse event SAE: serious adverse event ✓ A study result is available for inclusion in the synthesis. X No study result is available for inclusion, (probably) because the P value, magnitude or direction of the results generated were considered unfavourable by the study investigators. * No study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude or direction of the results. ? No study result is available for inclusion, and it is unclear if the outcome was assessed in the study.
Canakinumab 300/600 mg versus standard care or canakinumab 450 to 750 mg single dose versus placebo: important outcomes
| Important outcomes | ||||||
| Trial ID | Study follow‐up (in days) |
Sample size: canakinumab |
Sample size: standard care or placebo | Time to clinical improvement | Time to WHO Clinical Progression Score score of level 7 or above | Time to death |
| Caricchio CAN‐COVID 2021 | 28 | 227 | 227 | ✓ | ✓ | ✓ |
| Cremer Three C Study 2021 | 150 | 29 | 16 | * | * | ✓ |
Key AE: adverse event SAE: serious adverse event ✓ A study result is available for inclusion in the synthesis. X No study result is available for inclusion, (probably) because the P value, magnitude or direction of the results generated were considered unfavourable by the study investigators. * No study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude or direction of the results. ? No study result is available for inclusion, and it is unclear if the outcome was assessed in the study.
Appendix 12. Data analysis table of outcomes' effect sizes: anakinra versus standard care or placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size |
| Clinical improvement D28 | 3 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 (0.97 to 1.20) |
| Clinical improvement D60 or above | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 (0.78 to 1.12) |
| WHO Clinical Progression Score of level 7 or above D28 | 2 | 722 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 (0.36 to 1.22) |
| WHO Clinical Progression Score of level 7 or above D60 or above | 1 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 (0.30 to 0.96) |
| All‐cause mortality D28 | 2 | 722 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 (0.34 to 1.39) |
| All‐cause mortality D60 or above | 4 | 1633 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 (0.68 to 1.56) |
| Adverse events | 2 | 722 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 (0.94 to 1.11) |
| Serious adverse events | 2 | 722 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 (0.58 to 1.56) |
| Time to clinical improvement | 4 | 1633 | Hazard Ratio (95% CI) | 1.07 (0.91 to 1.26) |
| Time to WHO Clinical Progression Score of level 7 and above | 2 | 722 | Hazard Ratio (95% CI) | 0.69 (0.48 to 0.99) |
| Time to death | 3 | 1518 | Hazard Ratio (95% CI) | 0.80 (0.59 to 1.08) |
Appendix 13. Analysis of important outcomes: anakinra versus standard care or placebo
Analysis 1.2.1: Time to clinical improvement (Figure 16)
16.
Analysis 1.2.1: Anakinra versus standard care/placebo: Time to clinical improvement
Analysis 1.2.2: Time to WHO Clinical Progression Score of level 7 or above (Figure 17)
17.
Analysis 1.2.2: Anakinra versus standard care/placebo: Time to WHO Clinical Progression Score of level 7 or above
Analysis 1.2.3: Time to death (Figure 18)
18.
Analysis 1.2.3: Anakinra versus standard care/placebo: Time to death
Appendix 14. Data analysis table of outcomes' effect sizes: canakinumab versus standard care or placebo
| Outcome | No. of studies | No. of participants | Statistical method | Effect size |
| Clinical improvement D28 | 2 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 (0.96 to 1.14) |
| WHO Clinical Progression Score of level 7 or above D28 | 2 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 (0.44 to 1.20) |
| All‐cause mortality D28 | 2 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 (0.39 to 1.42) |
| All‐cause mortality D60 or above | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 (0.16 to 1.91) |
| Adverse events | 1 | 454 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 (0.86 to 1.21) |
| Serious adverse events | 2 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 (0.57 to 1.13) |
| Time to clinical improvement | 1 | 454 | Hazard Ratio (95% CI) | 1.06 (0.87 to 1.29) |
| Time to WHO Clinical Progression Score of level 7 or above | 1 | 454 | Hazard Ratio (95% CI) | 0.80 (0.47 to 1.36) |
| Time to death | 2 | 499 | Hazard Ratio (95% CI) | 0.71 (0.36 to 1.43) |
Appendix 15. Analysis of important outcomes: canakinumab versus standard of care or placebo
Analysis 2.2.1: Time to clinical improvement (Figure 19)
19.
Analysis 2.2.1: Canakinumab versus standard care/placebo: Time to clinical improvement
Analysis 2.2.2: Time to WHO Clinical Progression Score of level 7 or above (Figure 20)
20.
Analysis 2.2.2: Canakinumab versus standard care/placebo: Time to WHO Clinical Progression Score of level 7 or above
Analysis 2.2.3: Time to death (Figure 21)
21.
Analysis 2.2.3: Canakinumab versus standard care/placebo: Time to death
Appendix 16. Sensitivity analyses involving studies published as peer‐reviewed full texts
Sensitivity analysis 1.3.1 (publication status): Anakinra versus standard care/placebo: All‐cause mortality D60 or above (Figure 22)
22.
Sensitivity analysis 1.3.1 (publication status): Anakinra versus standard care/placebo: All‐cause mortality D60 or above
Appendix 17. Sensitivity analyses involving number of participants analysed
Anakinra versus standard care/placebo
Sensitivity analysis 1.4.1: Clinical improvement D28 (Figure 23)
23.
Sensitivity analysis 1.4.1 (number analysed): Anakinra versus standard care/placebo: Clinical improvement D28
Sensitivity analysis 1.4.2: Clinical improvement D60 or above (Figure 24)
24.
Sensitivity analysis 1.4.2 (number analysed): Anakinra versus standard care/placebo: Clinical improvement D60 or above
Sensitivity analysis 1.4.3: WHO Clinical Progression Score of level 7 or above D28 (Figure 25)
25.
Sensitivity analysis 1.4.3 (number analysed): Anakinra versus standard care/placebo: WHO Clinical Progression Score of level 7 or above D28
Sensitivity analysis 1.4.4: WHO Clinical Progression Score of level 7 or above D60 or above (Figure 26)
26.
Sensitivity analysis 1.4.4 (number analysed): Anakinra versus standard care/placebo: WHO Clinical Progression Score of level 7 or above D60 or above
Sensitivity analysis 1.4.5: All‐cause mortality D28 (Figure 27)
27.
Sensitivity analysis 1.4.5 (number analysed): Anakinra versus standard care/placebo: All‐cause mortality D28
Sensitivity analysis 1.4.6: All‐cause mortality D60 or above (Figure 28)
28.
Sensitivity analysis 1.4.6 (number analysed): Anakinra versus standard care/placebo: All‐cause mortality D60 or above
Sensitivity analysis 1.4.7: Adverse events (Figure 29)
29.
Sensitivity analysis 1.4.7 (number analysed): Anakinra versus standard care/placebo: Adverse events
Sensitivity analysis 1.4.8: Serious adverse events (Figure 30)
30.
Sensitivity analysis 1.4.8 (number analysed): Anakinra versus standard care/placebo: Serious adverse events
Canakinumab versus standard care/placebo
Sensitivity analysis 2.3.1: Clinical improvement D28 (Figure 31)
31.
Sensitivity analysis 2.3.1 (number analysed): Canakinumab versus standard care/placebo: Clinical improvement D28
Sensitivity analysis 2.3.2: WHO Clinical Progression Score of level 7 or above D28 (Figure 32)
32.
Sensitivity analysis 2.3.2 (number analysed): Canakinumab versus standard care/placebo: WHO Clinical Progression Score of level 7 or above D28
Sensitivity analysis 2.3.3: All‐cause mortality D28 (Figure 33)
33.
Sensitivity analysis 2.3.3 (number analysed): Canakinumab versus standard care/placebo: All‐cause mortality D28
Sensitivity analysis 2.3.4: All‐cause mortality D60 or above (Figure 34)
34.
Sensitivity analysis 2.3.4 (number analysed): Canakinumab versus standard care/placebo: All‐cause mortality D60 or above
Sensitivity analysis 2.3.5: Adverse events (Figure 35)
35.
Sensitivity analysis 2.3.5 (number analysed): Canakinumab versus standard care/placebo: Adverse events
Sensitivity analysis 2.3.6: Serious adverse events (Figure 36)
36.
Sensitivity analysis 2.3.6 (number analysed): Canakinumab versus standard care/placebo: Serious adverse events
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Caricchio CAN‐COVID 2021.
| Study characteristics | ||
| Methods | Design: RCT Blinding: double‐blinding Date of study: from 30 April 2020 to 17 August 2020 Location: Multicentre: France, Italy, Russia, Spain, UK and USA Follow‐up duration (days): 28 | |
| Participants |
Population: people with suspected or confirmed COVID‐19 (mild‐severe) admitted to 39 centres in France, Italy, Russia, Spain, UK and the USA. Arms
Randomised: 454 participants (n1= 227; n2 = 227) Analysed: 451 Characteristics of participants Mean/median age: 58 267 males (59%) Admitted to ICU: n = 107 Severity: mild: n = 26; moderate: n = 321; severe: n = 104; critical= 0 Participants on oxygen without intubation: n = 425; intubated: n = 0 C‐reactive protein (median): 77 to 89 mg/L Inclusion criteria Hospitalised people with severe COVID‐19; at least 12 years old (United States) or 18 years old (Europe); hypoxaemia but did not require IMV; diagnosis of infection with SARS‐CoV‐2 within 7 days prior to randomisation; diagnosis of pneumonia with pulmonary infiltrates on chest x‐ray or computed tomographic scan within 5 days prior to randomisation; peripheral capillary oxygen saturation of 93% or less on room air or arterial oxygen partial pressure/fraction of inspired oxygen less than 300 mm Hg; blood levels of CRP of 20 mg/L or greater or ferritin of 600 μg/L or greater. Exclusion criteria Treated with therapies targeting IL‐1 or IL‐6; suspected or known untreated active infection due to another pathogen; if progression to death was imminent within 24 hours according to the investigator. |
|
| Interventions | Intervention: canakinumab (single dose infused IV over 2 hours: 450 mg for body weight 40 to < 60 kg, 600 mg for 60 to 80 kg, 750 mg for > 80 kg in 250 mL of 5% dextrose) Control: placebo Definition of standard care: standard care treatment for COVID‐19 per local practice. Use of glucocorticoids, convalescent serum or plasma, antivirals, and anticoagulants was permitted during the trial. Per protocol, immunomodulatory therapies, such as biologic agents targeting IL‐1 or IL‐6, or tumour necrosis factor were prohibited. | |
| Co‐interventions |
Steroid use at baseline or any time during the study At baseline Canakinumab: 92 (41%) Placebo: 73 (32%) |
|
| Outcomes | Primary outcome of the trial The proportion of participants who survived without ever requiring IMV from day 3 to day 29 (inclusive) Note: the definition of clinical improvement extracted is improvement of clinical status by at least 2 points. | |
| Overall comment | In addition to the published article, the trial registry, protocol, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. There were no substantive differences between the published article and the registry in population, procedures, interventions or outcomes. The report describes the participants as having severe COVID‐19, and chest x‐ray or CT scan and blood oxygen inclusion criteria indicate severe disease. However, assessments at baseline on the WHO ordinal scale range from mild (hospitalised, no oxygen therapy) to severe (non‐invasive ventilation or high‐flow oxygen); this scale has been used to categorise participants for this extraction. The study achieved its target sample size. The study reported an interim analysis including data up to day 29. This study was updated on 25 October 2021 with data from contact with authors. |
|
| Notes |
Funding: private (Novartis Pharma AG)
Conflict of interest: yes, declared.
Protocol: yes. In English Statistical plan: yes Data‐sharing stated: yes, upon publication, beginning date: 1 March 2021 |
|
Cremer Three C Study 2021.
| Study characteristics | ||
| Methods | Design: RCT Blinding: double‐blinding Date of study: from 28 April 2020 to 25 August 2020 Location: multicentre; USA Follow‐up duration (days): 150 | |
| Participants |
Population: people with confirmed COVID‐19 (mild‐critical) admitted to 2 centres in the USA Arms
Randomised: 45 participants (n1= 15; n2 = 14; n3 = 16) Analysed: 45 Characteristics of participants Mean/median age: 68 33 males (73%) Admitted to ICU: n = NR Severity: mild: n = 4; moderate: n = 20; severe: n = 11; critical n = 10 Participants on oxygen without intubation: n = 31; intubated: n = 10 C‐reactive protein: median: 153 mg/L Inclusion criteria ≥ 18 years old; hospitalised for COVID‐19 infection with a documented upper respiratory tract specimen positive for SARS‐CoV2 RNA; had a troponin T > 99th percentile upper reference range; NT‐proBNP greater than the age‐adjusted upper reference limit; C‐reactive protein (CRP) > 50 mg/L. The 5th generation Roche Troponin T assay was used (hsTnT), and a value > 12 ng/L was considered abnormal. Exclusion criteria Alternative explanation for troponin elevation; chronic systolic heart failure with EF < 35%; age < 18 years old; uncontrolled systemic bacterial or fungal infection; concomitant viral infection (e.g. influenza or other respiratory virus); pregnant; on mechanical circulatory support; on mechanical ventilation for more than 48 hours; resuscitated cardiac arrest; known hypersensitivity to canakinumab or any of its excipients; neutrophil count < 1000/mm3; history of myeloproliferative disorder or active malignancy receiving chemotherapy; known active tuberculosis or history of incompletely treated tuberculosis; current treatment with immunosuppressive agents; chronic prednisone use > 10 mg/daily (for more than 3 weeks prior to admission); has a history of solid‐organ or bone marrow transplant; severe pre‐existing liver disease with clinically significant portal hypertension; end‐stage renal disease on chronic renal replacement therapy; enrolment in another investigational study using immunosuppressive therapy; in the opinion of the investigator and clinical team, should not participate in the study; if male and sexually active, must have documented vasectomy or must practice birth control and not donate sperm during the study and for 3 months after study drug administration; women of child‐bearing potential, defined as all women physiologically capable of becoming pregnant, unless they are using highly effective methods of contraception during dosing of investigational drug |
|
| Interventions |
Intervention Canakinumab (600mg IV single dose) Canakinumab (300mg IV single dose) Control: placebo Definition of standard care: enrolled participants are eligible to receive COVID‐19 therapies as deemed clinically appropriate, irrespective of their participation |
|
| Co‐interventions |
Steroid use at baseline or any time during the study At baseline Canakinumab 600mg: 8 (53%) Canakinumab 300mg: 3 (21%) Placebo: 10 (63%) |
|
| Outcomes | Primary outcome of the trial Time to clinical improvement up to day 14, defined as the time in days from randomisation to either an improvement of two points on a seven‐category ordinal scale or discharge from the hospital, whichever occurred first | |
| Overall comment | In addition to the published article, the study registry (with outcome data posted online) and the protocol were used in data extraction and risk of bias assessment. The study achieved the target sample size specified in the trial registry. There is no change from the trial registration in the population, intervention and control treatments, or in the primary outcomes. On 10 August 2021, this study was updated based on the published report in the European Heart Journal. |
|
| Notes | Funding: private (Novartis) Conflict of interest: yes, declared. The authors declare no potential conflict of interest. Protocol: yes. In English Statistical plan: yes Data‐sharing stated: no | |
Declercq COV‐AID 2021.
| Study characteristics | ||
| Methods | Design: RCT Blinding: unblinded Date of study: from 4 April 2020 to 6 December 2020 Location: multicentre; Belgium Follow‐up duration (days): 90 | |
| Participants |
Population: people with confirmed COVID‐19 (moderate‐critical) admitted to 16 centres in Belgium. Arms
Randomised: 342 participants (n1 = 43; n2 = 32; n3 = 37; n4 = 82; n5 = 76; n6 = 72) Analysed: 342 Characteristics of participants Mean/median age: 65 265 males (77%) Admitted to ICU: n = 172 Severity: mild: n = 6; moderate: n = 169; severe: n = 128; critical: n = 39 Participants on oxygen without intubation: n = 297; intubated: n = 39 C‐reactive protein (median): 120 to 150 mg/L Inclusion criteria Older than 18 years; had a laboratory proven diagnosis of COVID‐19 with symptoms between 6 and 16 days; a ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2; P:F ratio) of less than 350 mmHg on room air or less than 280 mmHg on supplemental oxygen and bilateral pulmonary infiltrates; either a single ferritin concentration measurement of more than 2000 μg/L at inclusion when they immediately required high flow oxygen or mechanical ventilation, or a ferritin concentration of more than 1000 μg/L, which had been increasing over the previous 24 h, or lymphopenia below 800/mL with two of the following criteria: an increasing ferritin concentration of more than 700 μg/L, an increasing lactate dehydrogenase concentration of more than 300 international units (IU)/L, an increasing CRP concentration of more than 70 mg/L, or an increasing D‐dimers concentration of more than 1000 ng/mL. If the participant had three of the previous criteria at hospital admission with lymphopenia of less than 800/μL, there was no need to document an increase over 24 h. Exclusion criteria Mechanical ventilation for more than 24 h at randomisation; a clinical frailty score greater than three before SARS‐CoV‐2 infection; unlikelihood to survive beyond 48 h based on clinical assessment; an active co‐infection defined on clinical grounds (positive blood or sputum cultures); thrombocytopenia of less than 50,000/μL; neutropenia of less than 1500/μL; a history of bowel perforation or diverticulitis; high dose systemic steroid or immunosuppressive drug use for a COVID‐19‐unrelated disorder. |
|
| Interventions |
Intervention Anakinra (100 mg once daily subcutaneously for 28 days or until hospital discharge) Anakinra (100 mg once daily subcutaneously for 28 days or until hospital discharge) + tocilizumab (8 mg/kg IV single dose (not exceeding 800 mg)) Anakinra (100 mg once daily subcutaneously for 28 days or until hospital discharge) + siltuximab (11 mg/kg IV single dose) Tocilizumab (8 mg/kg IV single dose (not exceeding 800 mg)) Siltuximab (11 mg/kg IV single dose) Control: standard care Definition of standard care: most participants (42%) randomly assigned before August 2020 received hydroxychloroquine as per standard care and most participants (84%) randomly assigned from August 2020 onwards received dexamethasone as per standard care. |
|
| Co‐interventions |
Steroid use at baseline or any time during the study At baseline Anakinra: 29 (67%) Anakinra + tocilizumab: NR Anakinra + siltuximab: NR Tocilizumab: NR Siltuximab: NR Standard care: 43 (60%) |
|
| Outcomes | Primary outcome of the trial Time to clinical improvement, defined as the time in days from randomisation until either an increase of at least two points on a 6‐category ordinal scale (compared with the worst status at day of randomisation) or to discharge from the hospital alive, whichever occurred first. The 6‐category ordinal scale was defined as 1 = death; 2 = hospitalised, on invasive mechanical ventilation or extracorporeal membrane oxygenation; 3 = hospitalised, on non‐invasive ventilation or high‐flow oxygen devices; 4 = hospitalised, requiring supplemental oxygen; 5 = hospitalised, not requiring supplemental oxygen; 6 = not hospitalised. | |
| Overall comment | In addition to the published article, the study registry, supplementary material, protocol and statistical analysis plan were used in data extraction and risk of bias assessment. The WHO Rapid Evidence Appraisal for COVID‐19 Therapies (REACT) Working Group "Association Between Administration of IL‐6 Antagonists and Mortality Among Patients Hospitalized for COVID‐19: A Meta‐analysis." was also available (REACT 2021). The study achieved the target sample size specified in the trial registry. There are no important changes from the trial registration in the primary outcome, procedures, intervention and control treatments. Time to clinical improvement is reported after first randomisation, and after second randomisation but not by arm. Total adverse events were not reported (but this had been prespecified). |
|
| Notes |
Funding: public/non‐profit (Belgian Health Care Knowledge Center; VIB Grand Challenges (Flemish Institute for Biotechnology)) Conflict of interest: yes, declared. Conflicts of interest of the first and last authors are: JD, KFAVD, BM, CB, VB, LH, LN, and EDL have received personal PhD training fellowships from FWO Flanders. BNL received an European Research Council Advanced Grant and several FWO grants, as well as a University of Ghent Methusalem Grant. Protocol: yes. In English Statistical plan: yes Data‐sharing stated: yes |
|
Derde REMAP‐CAP 2021.
| Study characteristics | ||
| Methods |
Design: RCT Blinding: unblinded Date of study: from 25 March 2020 to 10 April 2021 Location: multicentre: UK, Netherlands, Ireland, Australia, New Zealand, Canada, Finland, Italy, Saudi‐Arabia Follow‐up duration (days): 90 |
|
| Participants |
Population: people with suspected or confirmed COVID‐19 (moderate to critical) admitted to 133 centres in 9 countries Arms
Randomised: 2253 participants (n1 = 972; n2 = 485; n3 = 378; n4 = 418) Analysed: 2197 Characteristics of participants N at baseline: 2216 Mean/median age: 60 1536 males (70%) Admitted to ICU: n = 2216 Severity: mild: n = 0; moderate: n = 4; severe: n = 1482; critical: n = 730 Participants on oxygen without intubation n = 1482; intubated n = 728 C‐reactive protein (median): 124 mg/L Inclusion criteria Participants aged > 18 years; suspected or microbiologically confirmed COVID‐19; receiving or not respiratory or cardiovascular organ support within 24 hours in an ICU. Exclusion criteria Death deemed to be imminent and inevitable during the next 24 hours; one or more of the participant, substitute decision maker or attending physician are not committed to full active treatment; more than 14 days have elapsed while admitted to hospital with symptoms of an acute illness due to suspected or proven pandemic infection or more than 24 hours elapsed since ICU admission; previous participation in this REMAP within the last 90 days; patient has already received any dose of one or more of any form of interferon, anakinra, tocilizumab, or sarilumab during this hospitalisation; long‐term therapy with any of these agents prior to this hospital admission; patient has been randomised in a trial evaluating an immune modulation agent for proven or suspected COVID‐19 infection where the protocol of that trial requires ongoing administration of study drug; known condition or treatment resulting in ongoing immune suppression including neutropenia prior to this hospitalisation; intention to prescribe systemic corticosteroids for any reason, other than participation in the corticosteroid domain of this platform, is an exclusion criterion to receive IFN‐β1a; known hypersensitivity to proteins produced by E coli will result in exclusion criterion to receive anakinra; known or suspected pregnancy is an exclusion criterion to receive the anakinra, IFN‐β1a, tocilizumab, and sarilumab interventions; a baseline alanine aminotransferase or an aspartate aminotransferase that is more than five times the upper limit of normal is an exclusion criterion to receive tocilizumab or sarilumab; a baseline platelet count < 50 x 109/L is an exclusion criterion to receive tocilizumab or sarilumab. |
|
| Interventions |
Intervention: Tocilizumab (8 mg/kg IV infusion, maximum 800 mg, a second infusion could be administered 12 to 24 hours after first) Sarilumab (400 mg IV single dose) Anakinra (initial dose: 300 mg intravenously for the first 24 hours ‐ maintenance dose: 100 mg intravenously 4 times a day for 14 days or until either free from invasive mechanical ventilation for more than 24 hours, or discharge from ICU) Control: standard care Definition of standard care: NR |
|
| Co‐interventions |
Steroid use at baseline or any time during the study At baseline Tocilizumab: 770 (82%) Sarilumab: 422 (89%) Anakinra: 317 (86%) Standard care: 269 (67%) |
|
| Outcomes |
Primary outcome of the trial An ordinal scale that is a composite of in‐hospital mortality and duration of respiratory and cardiovascular organ support, censored at 21 days, where all deaths within hospital and up to day 90 were assigned the worst outcome Note: the definition of clinical improvement extracted is hospital discharge |
|
| Overall comment | In addition to the preprint version of the article, the study registry and protocol were used in data extraction and risk of bias assessment. The report contains definite results of tocilizumab, sarilumab and anakinra from the Immune Modulation Therapy domain of the REMAP‐CAP clinical trial (an international, adaptive platform trial). There is no change from the trial registration in the intervention and control treatments. The platform initially included only participants admitted to an intensive care unit and receiving respiratory or cardiovascular organ support, a moderate state enrolling hospitalised participants not receiving respiratory or cardiovascular organ support was added subsequently. A blinded International Trial Steering Committee (ITSC) closed all arms of the domain on 10 April 2021. The primary outcome indicated in the registry reflects the primary outcome reported in the paper. Adverse events are not reported. | |
| Notes |
Funding: Mixed (PREPARE consortium by the European Union; FP7‐HEALTH‐2013‐INNOVATION‐1; RECOVER consortium by the European Union Horizon 2020 research and innovation program; Australian National Health and Medical Research Council; Health Research Council of New Zealand; Canadian Institute of Health Research Strategy for Patient‐Oriented Research Innovative Clinical Trials Program Grant; UK NIHR; NIHR Imperial Biomedical Research Centre; Health Research Board of Ireland; UPMC Learning While Doing Program; Translational Breast Cancer Research Consortium; Global Coalition for Adaptive Research; French Ministry of Health; Minderoo Foundation; Wellcome Trust Innovations Project; Netherlands Organization for Health Research and Development ZonMw; NIHR Research Professorship; NIHR Clinician Scientist Fellowship; Australian National Health and Medical Research Council Career Development Fellowship; Roche Products Ltd; Sanofi (Aventis Pharma Ltd); Swedish Orphan Biovitrum AB (Sobi); Faron Pharmaceuticals (drug provision in some countries) )
Conflict of interest: yes, declared. Dr Gordon is funded by an NIHR Research Professorship. Protocol: yes. In English Statistical plan: yes Data‐sharing stated: yes |
|
Kyriazopoulou SAVE‐MORE 2021.
| Study characteristics | ||
| Methods | Design: RCT Blinding: double‐blinding Date of study: from 23 December 2020 to 31 March 2021 Location: multicentre: Greece, Italy Follow‐up duration (days): 28 | |
| Participants |
Population: people with confirmed COVID‐19 (mild‐severe) admitted to 37 centres in Greece and Italy Arms
Randomised: 606 participants (n1 = 412; n2 = 194) Analysed: 594 Characteristics of participants Mean/median age: 62 344 males (58%) Admitted to ICU: n = 0 Severity: mild: n = 33; moderate: n = 118; severe: n = 440; critical = 0 Participants on oxygen without intubation: n = 558; intubated: n = 0 C‐reactive protein (median): 50.6 mg/L Inclusion criteria Adults of either gender; for women, unwillingness to remain pregnant during the study period; confirmed infection by SARS‐CoV‐2 virus by molecular test; findings in chest‐X‐ray or in chest computed tomography compatible with lower respiratory tract infection; need for hospitalisation; plasma suPAR ≥ 6 ng/ml Exclusion criteria Any stage IV malignancy; any do not resuscitate decision; ratio or partial oxygen pressure to fraction of inspired oxygen less than 150; need of non‐invasive ventilation (CPAP or BPAP) or mechanical ventilation; any primary immunodeficiency; less than 1500 neutrophils/mm3; oral or intravenous intake of corticosteroids at a daily dose ≥ 0.4 mg/kg prednisone for a period greater than the last 15 days; any anti‐cytokine biological treatment including JAK inhibitors the last one month; severe hepatic failure; end‐stage renal failure necessitating haemofiltration or peritoneal haemodialysis; pregnancy or lactation. |
|
| Interventions | Intervention: anakinra (100 mg subcutaneously once daily for 7 to 10 days) Control: placebo Definition of standard care: all participants were receiving predefined standard‐of‐care (SoC) which consisted of regular monitoring of physical signs, oximetry and anticoagulation. Participants with severe disease by the WHO definition were also receiving intravenous 6 mg daily dexamethasone for 10 days. Remdesivir treatment was left at the discretion of the attending physicians; other biologicals targeting cytokines and kinase inhibitors were not allowed. | |
| Co‐interventions |
Steroid use at baseline or any time during the study At baseline Anakinra: 342 (84%) Placebo: 168 (89%) |
|
| Outcomes | Primary outcome of the trial Overall comparison of the distribution of frequencies of the scores from the 11‐point WHO Clinical Progression ordinal Scale (CPS) between the two arms of treatment at Day 28 Note: the definition of clinical improvement extracted is hospital discharge | |
| Overall comment | In addition to the published/preprint articles, the protocol, statistical analysis plan, prospective study registries, supplementary appendices and contact with authors were used in data extraction and risk of bias assessment. Protocol and statistical analysis plan were not available at the time of data extraction. The study achieved the target sample size specified in the trial registries. There is no change from the trial registration in the population or intervention and control treatments. Some time points for outcomes listed in the registry were not reported in the paper (e.g. negative viral conversion on day 7 (reported on day 28, day 7 results gained from contact with authors), serious adverse events on day 60 and 90 (reported on day 28). This study was updated on 28 July 2021 with data gained from contact with authors. This study was updated on 25 August 2021 with day 90 data gained from further contact with authors. This study was updated on 25 October 2021 with data from the peer‐reviewed published report. |
|
| Notes |
Funding: mixed (Hellenic Institute for the Study of Sepsis and Swedish Orphan Biovitrum AB (Sobi))
Conflict of interest: yes, declared.
Protocol: yes. In English Statistical plan: yes Data‐sharing stated: yes, upon publication |
|
Mariette CORIMUNO‐19 Collaborative 2021.
| Study characteristics | ||
| Methods | Design: RCT Blinding: unblinded Date of study: from 8 April 2020 to 26 April 2020 Location: Multicentre; France Follow‐up duration (days): 90 | |
| Participants |
Population: people with COVID‐19 (moderate) admitted to 16 centres in France. Arms
Randomised: 116 participants (n1 = 59; n2 = 57) Analysed: 114 Characteristics of participants Mean/median age: 66 80 males (70%) Admitted to ICU: n = 0 Severity: mild: n = 0; moderate: n = 114; severe: n = 0; critical n = 0 Participants on oxygen without intubation: n = 114; intubated: n = 0 C‐reactive protein (median): 120 to 121 mg/L Inclusion criteria CORIMUNO‐19 cohort: SARS‐CoV‐2 infection (positive on real‐time RT‐PCR or chest CT scan typical of COVID‐19 pneumonia, or both) Mild‐to‐moderate, severe, or critical pneumonia (i.e. receiving oxygen at a flow of > 3 L/min via mask or nasal cannula and a score of ≥ 5 points on the WHO Clinical Progression Scale (WHO‐CPS) 10‐point ordinal scale CORIMUNO‐ANA‐1: C‐reactive protein serum concentration of more than 25 mg/L Not requiring ICU at admission Mild‐to‐moderate COVID‐19 pneumonia with a WHO‐CPS score of 5 points, receiving at least 3 L/min of oxygen but without ventilation assistance (e.g. high‐flow oxygen, non‐invasive ventilation, or mechanical ventilation) Exclusion criteria Known hypersensitivity to anakinra or any of its excipients Pregnancy Current documented bacterial infection An absolute neutrophil count of 1.0 × 109 per L or less A platelet concentration < 50 G/L Serum aspartate aminotransferase or serum alanine aminotransferase of more than five‐times the upper limit of normal Severe renal insufficiency defined by an estimated glomerular filtration rate of less than 30 mL/min. |
|
| Interventions |
Intervention: Anakinra (200 mg IV twice daily on days 1 to 3, 100 mg twice daily on day 4, 100 mg once daily on day 5) Control: standard care Definition of standard care: usual care (antibiotic drugs, antiviral drugs, corticosteroids, vasopressor support, anticoagulants) was provided at the discretion of the site clinicians. |
|
| Co‐interventions |
Steroid use at baseline or any time during the study At baseline Anakinra: 7 (12%) Standard care: 8 (15%) At any time Anakinra: 30 (51%) Standard care: 29 (53%) |
|
| Outcomes |
Primary outcome of the trial
The proportion of participant who had died or needed non‐invasive or mechanical ventilation by day 4 (i.e. a score of > 5 points on the WHO‐CPS); and survival with no need for mechanical or non‐invasive ventilation (including high‐flow oxygen) at day 14. Note: The definition of clinical improvement extracted is discharged at day 28. |
|
| Overall comment | In addition to the published paper, the trial registry, statistical analysis plan (SAP) and supplementary methods and results were used in data extraction and assessment of risk of bias. The SAP was dated 21 September 2020 and was version 2.1. This was likely after unblinded data was available for analysis, hence it was not used in the risk of bias assessment of domain 5, selection of the reported result. One coprimary outcome in the registry was not reported (decrease of at least one point in WHO progression scale score), but raw data for WHO progression scale scores were reported. This was one of a series of randomised controlled trials testing different therapeutic regimens. Quote: "On April 23, 2020, the DSMB met and recommended suspension of recruitment for futility on the basis of the interim analysis of the 102 first patients recruited, although the futility boundaries were not formally crossed. The sponsor decided to discontinue the study on April 26, 2020." As a result, the target sample size specified in the registry was not achieved. Quote: "Another trial within the CORIMUNO platform (CORIMUNO‐ANA‐2) that aims to assess the effect of anakinra in patients with more severe COVID‐19 who are in intensive care units (WHO‐CPS score 6 points) has now been completed and is being analysed." |
|
| Notes | Funding: public/non‐profit (The Ministry of Health, Programme Hospitalier de Recherche Clinique, Foundation for Medical Research, and AP‐HP Foundation) Conflict of interest: yes, declared. The writing committee declares no competing interests. Protocol: yes. In English Statistical plan: yes Data‐sharing stated: yes, within 3 months of publication, and for 10 years thereafter | |
BPAP: bilevel positive airway pressure; COVID‐19: coronavirus disease 2019; CPAP: continuous positive airway pressure; CRP: C‐reactive protein; CT: computed tomography; FWO: The Research Foundation – Flanders; ICU: intensive care unit; IL: interleukin; IV: intravenous; JAK: janus kinase; NR: not reported; NT‐proBNP: N‐terminal pro b‐type natriuretic peptide; PCR: polymerase chain reaction; RCT: randomised controlled trial; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; suPAR: soluble urokinase plasminogen activator receptor; WHO: World Health Organization
Differences between protocol and review
Below are the changes made in the review compared to the protocol.
Outcomes: to avoid multiplicity, we reduced the number of outcomes. For the selected outcome domains, we now consider only two time points (D28 and ≥ D60). We no longer evaluate the outcome domain WHO Clinical Progression Score of level 6 or above as it appears to be subject to variation due to local guidelines and resources. It is, therefore, an inconsistent indicator when assessed across studies.
Risk of bias assessment: we did not consider anticoagulants as a relevant co‐intervention for assessing risk of bias in the domain deviations from intervention after discussion with content experts.
Subgroup analyses: we did not carry out the planned subgroup analyses because of the low number of trials.
Contributions of authors
Conception and design of the review: MD, AC, LG, DD, JJM, GR, AH, GG, DT, PR, IB
Co‐ordination of the review: AC, LG, IB
Search and selection of studies for inclusion in the review: GF, CR, PK
Collection of data for the review: MD, LG, CG, NH, EG, GV, PK, HB
Assessment of the risk of bias in the included studies: MD, LG, CG, NH, EG, GV, PK, HB, IB
Analysis of data: AC, TE
Assessment of the certainty in the body of evidence: NH, GV
Interpretation of data: MD, SM, CM, DD, JJM, GR, AH, GG, DT, PR, IB
Writing of and commenting on the review: MD, SM, AC, TE, LG, CG, NH, EC, GV, GF, CR, PK, HB, CM, DD, JJM, GR, AH, GG, DT, PR, IB
Sources of support
Internal sources
Cochrane France, France
Center of Research in Epidemiology and StatisticS (CRESS), France
Centre d’Epidémiologie Clinique (GHU Cochin, Hôtel Dieu), France
Assistance Publique Hôpitaux de Paris (APHP), France
Université de Paris, France
Centre National de la Recherche Scientifique (CNRS), France
External sources
-
French Ministry of Health, Other
Funding provided to produce review
-
Agence Nationale de la Recherche (ANR), France
Funding provided to produce review
-
World Health Organization (WHO), Switzerland
Funding provided to produce review
-
Federal Ministry of Education and Research, Germany
This review is supported through funding for the project COVID‐19 evidence eco‐system (COVID‐19 Evidenzökosystem“ (CEO‐sys)) under a funding scheme issued by the National Research Network of University Medical Centers on COVID‐19 (Nationales Forschungsnetzwerk der Universitätsmedizin zu Covid‐19) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung, BMBF).
Declarations of interest
Mauricia Davidson: none known.
Sonia Menon: works as systematic reviewer for p95 consultancy company.
Anna Chaimani: none known.
Theodoros Evrenoglou: none known.
Lina Ghosn: none known.
Carolina Graña: none known.
Nicholas Henschke: is employed by Cochrane Response, an evidence consultancy initiative from Cochrane. Cochrane Response was commissioned by the WHO to perform work on the living systematic review and living network meta‐analysis for COVID‐19 studies.
Elise Cogo: is employed by Cochrane Response, an evidence consultancy initiative from Cochrane. Cochrane Response was commissioned by the WHO to perform work on the living systematic review and living network meta‐analysis for COVID‐19 studies.
Gemma Villanueva: is employed by Cochrane Response, an evidence consultancy initiative from Cochrane. Cochrane Response was commissioned by the WHO to perform work on the living systematic review and living network meta‐analysis for COVID‐19 studies.
Gabriel Ferrand: none known.
Carolina Riveros: none known.
Philipp Kapp: none known.
Hillary Bonnet: none known.
Conor Moran: none known.
Declan Devane: works for Cochrane Ireland and Evidence Synthesis Ireland which are funded within the National University of Ireland Galway (Ireland) by the Health Research Board (HRB) and the Health and Social Care, Research and Development (HSC R&D) Division of the Public Health Agency in Northern Ireland.
Joerg J Meerpohl: reports funding from the Federal Ministry of Health and the Federal Ministry of Education and Research.
Gabriel Rada: none known.
Asbjørn Hróbjartsson: none known.
Giacomo Grasselli: receives personal fees for lectures from Getinge, Fisher&Paykel, Draeger Medical, Biotest, Thermofisher and MSD; support for travel‐meeting expenses from Biotest and Getinge (all outside the present work). GG also received an unrestricted research grant from Fisher&Paykel (unrelated to the present work).
David Tovey: has a part‐time paid consultancy with the Université de Paris.
Philippe Ravaud: is a minority shareholder of INATO. PR was the methodologist of the CORIMUNO‐19 platform which generated the Mariette CORIMUNO‐19 Collaborative 2021 trial. PR did not undertake any inclusion decisions/data extraction or risk of bias assessments for the Mariette CORIMUNO‐19 Collaborative 2021 trial.
Isabelle Boutron: is director of Cochrane France and co‐convenor of the Cochrane Bias methods group.
These authors contributed equally to this work.
These authors contributed equally to this work.
New
References
References to studies included in this review
Caricchio CAN‐COVID 2021 {published and unpublished data}
- Caricchio R, Abbate A, Gordeev I, Meng J, Hsue PY, Neogi T, et al, CAN-COVID Investigators.Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA 2021;326(3):230-9. [DOI: 10.1001/jama.2021.9508] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cremer Three C Study 2021 {published data only}
- Cremer PC, Sheng CC, Sahoo D, Dugar S, Prada RA, Wang KM et al, the Three C study group.Double-blind randomized proof-of-concept trial of canakinumab in patients with COVID-19 associated cardiac injury and heightened inflammation. European Heart Journal Open 2021;43(10):1055-63. [DOI: 10.1002/clc.23451] [DOI] [PMC free article] [PubMed] [Google Scholar]
Declercq COV‐AID 2021 {published data only (unpublished sought but not used)}
- Declercq J, Van Damme KF, Leeuw ED, Maes B, Bosteels C, Tavernier SJ, et al.Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial. Lancet. Respiratory Medicine 2021;9(12):1427-38. [DOI: 10.1016/S2213-2600(21)00377-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derde REMAP‐CAP 2021 {published data only (unpublished sought but not used)}
- Derde LP, The REMAP-CAP Investigators.Effectiveness of tocilizumab, sarilumab, and anakinra for critically ill patients with COVID-19. The REMAP-CAP COVID-19 immune modulation therapy domain randomized clinical trial. medRxiv 2021. [DOI: 10.1101/2021.06.18.21259133] [DOI]
Kyriazopoulou SAVE‐MORE 2021 {published and unpublished data}
- Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, et al.Early anakinra treatment for COVID-19 guided by urokinase plasminogen receptor. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.05.16.21257283] [DOI]
- Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, et al.Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nature Medicine 2021;27(10):1752-60. [DOI: 10.1038/s41591-021-01499-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mariette CORIMUNO‐19 Collaborative 2021 {published and unpublished data}
- Mariette X, the CORIMUNO-19 Collaborative group.Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet. Respiratory Medicine 2021;9(3):295-304. [DOI: 10.1016/S2213-2600(20)30556-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Attaway 2021
- Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U.Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ 2021;372:n436. [DOI] [PubMed] [Google Scholar]
Barkas 2021
- Barkas F, Ntekouan SF, Kosmidou M, Liberopoulos E, Liontos A, Milionis H.Anakinra in hospitalized non-intubated patients with coronavirus disease 2019: a systematic review and meta-analysis. Rheumatology 2021;60(12): 5527-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2020a
- Boutron I, Chaimani A, Meerpohl JJ, Hróbjartsson A, Devane D, Rada G, et al.The COVID-NMA project: building an evidence ecosystem for the COVID-19 pandemic. Annals of Internal Medicine 2020;173(12):1015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2020b
- Boutron I, Chaimani A, Devane D, Meerpohl JJ, Rada G, Hróbjartsson A, et al.Interventions for the prevention and treatment of COVID-19: a living mapping of research and living network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013769. [DOI: 10.1002/14651858.CD013769] [DOI] [Google Scholar]
Boutron 2020c
- Boutron I, Chaimani A, Ghosn L.Interleukin (IL)-1 blocking agents for the treatment of COVID-19 A living systematic review. Zenodo 23 October 2020. [DOI: 10.5281/zenodo.5698219] [DOI]
Boutron 2021
- Boutron I, Chaimani A, Ghosn L.Interleukin (IL)-1 blocking agents for the treatment of COVID-19 A living systematic review. Zenodo 8 November 2021. [DOI: 10.5281/zenodo.5698123] [DOI]
Cabanac 2021
- Cabanac G, Oikonomidi T, Boutron I.Day-to-day discovery of preprint-publication links. Scientometrics 2021;126:5285–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantini 2020
- Cantini F, Goletti D, Petrone L, Najafi Fard S, Niccoli L, Foti R.Immune therapy, or antiviral therapy, or both for COVID-19: a systematic review. Drugs 2020;80(18):1929-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cavalli 2021
- Cavalli G, Dagna L.The right place for IL-1 inhibition in COVID-19. Lancet. Respiratory Medicine 2021;9(3):223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2018
- Chaimani A, Mavridis D, Higgins JP, Salanti G, White IR.Allowing for informative missingness in aggregate data meta-analysis with continuous or binary outcomes: extensions to metamiss. Stata Journal 2018;18(3):716-40. [PMC free article] [PubMed] [Google Scholar]
Davidson 2021
- Davidson M, Menon S, Chaimani A, Evrenoglou T, Ghosn L, Graña C, et al.Dataset: Interleukin (IL)-1 blocking agents for the treatment of COVID-19 A living systematic review. Zenodo 15 January 2022. [DOI: 10.5281/zenodo.5853927] [DOI]
Dinarello 2012
- Dinarello CA, Simon A, Meer JW.Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nature Reviews Drug Discovery 2012;11(8):633-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 21 February 2021. Available at gradepro.org.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Horby 2021
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L et al.Dexamethasone in Hospitalized Patients with Covid-19. New England Journal of Medicine 25 February 2021;384(8):693-704. [Google Scholar]
Juul 2020a
- Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al.Interventions for treatment of COVID-19: a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLOS Medicine 2020;17(9):e1003293. Erratum in: PLoS Med. 2020 Dec 29;17(12):e1003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juul 2020b
- Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E et al.Interventions for treatment of COVID-19: second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). medRxiv [Preprint] 2020.
Khan 2021
- Khan FA, Stewart I, Fabbri L, Moss S, Robinson K, Smyth AR, et al.Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 2021;76(9):907-19. [DOI] [PubMed] [Google Scholar]
Kim 2020
- Kim MS, An MH, Kim WJ, Hwang TH.Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLOS Medicine 2020;17(12):e1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2018
- Kirkham JJ, Altman DG, Chan AW, Gamble C, Dwan KM, Williamson PR.Outcome reporting bias in trials: a methodological approach for assessment and adjustment in systematic reviews. BMJ 2018;362:k3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyriazopoulou 2021
- Kyriazopoulou E, Huet T, Cavalli G, Gori A, Kyprianou M, Pickkers P, et al, International Collaborative Group for Anakinra in COVID-19.Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. Lancet Rheumatology 2021;3(10):e690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
La Rosée 2019
- La Rosée P, Horne A, Hines M, Bahr Greenwood T, Machowicz R, Berliner N, et al.Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019;133(23):2465-77. [DOI] [PubMed] [Google Scholar]
Mavridis 2015
- Mavridis D, White IR, Higgins JP, Cipriani A, Salanti G.Allowing for uncertainty due to missing continuous outcome data in pairwise and network meta-analysis. Statistics in Medicine 2015;34(5):721-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mavridis 2018
- Mavridis D, Chaimani A, EXhimiou O, Salanti G.Missing outcome data in meta-analysis. Evidence Based Mental Health 2018;21(3):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullen 2020
- Mullen JL, Tsueng G, Abdel Latif A, Alkuzweny M, Cano M, Haag E et al.Outbreak.info. Available at: https://outbreak.info Accessed 24 January 2022.
Oikonomidi 2020
- Oikonomidi T, Boutron I, Pierre O, Cabanac G, Ravaud P, COVID-19 NMA Consortium.Changes in evidence for studies assessing interventions for COVID-19 reported in preprints: meta-research study. BMC Medicine 2020;18(1):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.Rayyan — a web and mobile app for systematic reviews. Systematic Reviews 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasin 2021
- Pasin L, Cavalli G, Navalesi P, Sella N, Landoni G, Yavorovskiy AG, et al.Anakinra for patients with COVID-19: a meta-analysis of non-randomized cohort studies. European Journal of Internal Medicine 2021;86:34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peter 2021
- Peter AE, Sandeep BV, Rao BG, Kalpana VL.Calming the storm: natural immunosuppressants as adjuvants to target the cytokine storm in COVID-19. Frontiers in Pharmacology 2021;11:83777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pierre 2021
- Pierre O, Riveros C, Charpy S, Boutron I.Secondary electronic sources demonstrated very good sensitivity for identifying studies evaluating interventions for COVID-19. Journal of Clinical Epidemiology 2021;141:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pile 2015
- Pile KD, Graham GG, Mahler SM.Interleukin 1 Inhibitors. In: Parnham M, editors(s). Encyclopedia of Inflammatory Diseases. Basel, Switzerland: Springer Basel, 2015:1-5. [Google Scholar]
Putman 2021
- Putman M, Chock YP, Tam H, Kim AH, Sattui SE, Berenbaum F, et al, COVID-19 Global Rheumatology Alliance.Antirheumatic disease therapies for the treatment of COVID-19: a systematic review and meta-analysis. Arthritis & Rheumatology 2021;73(1):36-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
REACT 2021
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group.Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021;326 (6):499–518. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ.Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Shakoory 2016
- Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al.Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Critical Care Medicine 2016;44(2):275-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siemieniuk 2020
- Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al.Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ 2020;370:m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sims 2010
- Sims JE, Smith DE.The IL-1 family: regulators of immunity. Nature Reviews Immunology 2010;10(2):89-102. [DOI] [PubMed] [Google Scholar]
Somagutta 2021
- Somagutta MK, Lourdes Pormento MK, Hamid P, Hamdan A, Khan MA, Desir R, et al.The safety and efficacy of anakinra, an interleukin-1 antagonist in severe cases of COVID-19: a systematic review and meta-analysis. Infection & Chemotherapy 2021;53(2):221-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al.RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Talaie 2020
- Talaie H, Hosseini SM, Nazari M, Fakhri Y, Mousavizadeh A, Vatanpour H, et al.Is there any potential management against COVID-19? A systematic review and meta-analysis. DARU Journal of Pharmaceutical Sciences 2020;28(2):765-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR.Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8(16). [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP.Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews.. International Journal of Epidemiology 2012;41(3):818-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
van de Veerdonk 2020
- de Veerdonk FL, Netea MG.Blocking IL-1 to prevent respiratory failure in COVID-19. Critical Care 2020;24(1):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2008
- White IR, Higgins JP, Wood AM.Allowing for uncertainty due to missing data in meta-analysis--part 1: two-stage methods. Statistics in Medicine 2008;27(5):711-27. [DOI] [PubMed] [Google Scholar]
WHO 2020a
- World Health Organization.Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance; 12 January 2020. Available at: apps.who.int/iris/handle/10665/332299.
WHO 2020b
- World Health Organization.Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available at https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19).
WHO Working Group 2020
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection.A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Worldometer 2020
- COVID Live - Coronavirus Statistics - Worldometer. Available at worldometers.info/coronavirus/ Accessed 12 November 2021.