Abstract
According to the WHO, on October 16, 2020, the spreading of the SARS-CoV-2, responsible for the COVID-19 pandemic, reached 235 countries and territories, and resulting in more than 39 million confirmed cases and 1.09 million deaths globally. Monitoring of the virus outbreak is one of the main activities pursued to limiting the number of infected people and decreasing the number of deaths that have caused high pressure on the health care, social, and economic systems of different countries. Wastewater based epidemiology (WBE), already adopted for the surveillance of life style and health conditions of communities, shows interesting features for the monitoring of the COVID-19 diffusion. Together with wastewater, the analysis of airborne particles has been recently suggested as another useful tool for detecting the presence of SARS-CoV-2 in given areas. The present review reports the status of research currently performed concerning the monitoring of SARS-CoV-2 spreading by WBE and airborne particles. The former have been more investigated, whereas the latter is still at a very early stage, with a limited number of very recent studies. Nevertheless, the main results highlights in both cases necessitate more research activity for better understating and defining the biomarkers and the related sampling and analysis procedures to be used for this important aim.
Keywords: Airborne particles, Biomarkers, COVID-19, SARS-CoV-2, Wastewater, Wastewater-based epidemiology (WBE)
1. Introduction
The first detection of severe acute respiratory syndrome (SARS) disease caused by SARS-CoV-2 (officially designated by the Coronavirus Study Group of the International Committee on Taxonomy of Viruses) occurred in the city of Wuhan (China) in late December 2019 (CDC, 2020). Since that date, the Coronavirus Disease 2019 (COVID-19) has grown rapidly, and exists in 235 countries and territories and have resulted in more than 39 million confirmed cases and 1.09 million deaths globally as of October 16 (WHO., 2020a). Currently, the most credited even if not fully demonstrated transmission pathway of SARS-CoV-2 virus is the one-based on respiratory droplets, with an average diameter ≥5 μm, that could be generated by a sneeze, cough, breath or during normal speaking of infected subjects (Lewis, 2020; National Research Council, 2020; Yu et al., 2018; WHO, 2020b). However, the presence of the virus within airborne droplet nuclei with a diameter ≤5 μm and hence transmissible to distances > 1m is still a matter of debate (Anderson et al., 2020; Lewis, 2020; Morawska and Cao 2020; National Research Council, 2020; Yu et al., 2018; WHO, 2020b). During the pandemic peaks, the presence of SARS-CoV-2 was detected in the aerosol samples collected in the internal air of two hospitals of Wuhan (Liu et al., 2020). The presence of the virus was also reported by Santarpia et al. (2020) in analysing air samples of 13 isolation rooms for patients infected by SARS-CoV-2 at Nebraska University Hospital. Both authors agreed on staying in open spaces and effective room ventilation as two effective methods for decreasing the spreading of the virus. Additionally, a high number of recent studies supported the aerosols and droplets diffusion/transmission route capacity of SARS-CoV-2 (Becchetti et al., 2020; Buonanno et al., 2020; Mizukoshi et al., 2020; Moreno et al., 2020; Noorimotlagh et al., 2020; Stadnytskyi et al., 2020; Tang et al., 2020). Differently, no presence of SARS-CoV-2 was detected by Faridi et al. (2020) in air samples collected from 2 m to 5 m of distance from the beds of patients affected by COVID-19. Despite all air samples were negative, the authors suggested in vivo experiments to be conducted using actual patient cough, sneeze, and breath aerosols for obtaining airborne size carrier aerosols. In some recent research, the idea that outdoor particulate matter (PM) could be another carrier for the spreading of the COVID-19 pandemic was proposed (Coccia 2020; Bontempi 2020a; Setti et al., 2020a). This was based on the evidence of the correlation between the number of cases and the PM concentration in the air in the Italian regions where the larger number of cases were registered during the first pandemic peak of March 2020. In this context, PMs may act as physical carriers of the virus or as a possible infection-boosting factor (Comunian et al., 2020). However, some scientific debate is still going on since the absence of a demonstrated causal nexus (Altman and Krzywinski, 2015).
In analysing the Italian context, Bontempi (2020a,b) detected a significant correlation between commercial exchanges, that are strictly connected also with social interactions, and COVID-19 diffusion/transmission. This correlation resulted also higher than the one detected for the PM.
Similarly, SARS-CoV-2 was also detected in wastewater and sewage (Medema et al., 2020; Núñez-Delgado, 2020; Ahmed et al., 2020a), representing another concern for the spreading of the virus in particular by aerosols generated in wastewater treatment plants (WWTPs). In any case, also for this last aspect, there is a lack of definitive scientific evidence.
Wastewaters generated by hospitals, but in particular, those generated by domestic users, have been largely investigated in the last months (Núñez-Delgado, 2020; Medema et al., 2020; Randazzo et al., 2020; Ahmed et al., 2020a). The persistence of SARS-CoV-1 in hospital and domestic wastewater up to 2 days at 20 °C and >14 days at 4 °C was already detected by Wang et al. (2005) when no chlorine was added. More recently, Randazzo et al. (2020) found SARS-CoV-2 RNA in six Spanish wastewater treatment plants. Other possible routes for the diffusion of the virus were also considered by Nghiem et al. (2020) and Di Maria et al. (2020) concerning the municipal solid waste streams. All this suggested wastewater-based epidemiology (WBE) (Ahmed et al., 2020a, Ahmed et al., 2020b) for the early detection of virus presence and for the surveillance and monitoring of COVID-19 (Farkas et al., 2020a).
WBE was already successfully adopted for the surveillance of life style and health conditions of communities concerning the use and/or abuse of both licit and illicit drug (Choi et al., 2018; Daughton, 2001). A similar role in COVID-19 detection and management seems to be played also by the monitoring of the airborne particles generated due to both symptomatic and asymptomatic individuals (Mizumoto et al., 2020; Oran and Topol, 2020; Wang et al., 2020a,b,c,d). Furthermore, the noninvasive nature of both WBE and airborne particle sampling can also help to prevent any ethical issue of confidentiality and potential stigmatization of infected patients (Choi et al., 2018; Bagcchi, 2020).
However, the efficient use of this apporach for SARS-CoV-2 monitoring in both water environment and airborne particles requires specific standardization of viral sampling procedures and methodologies along with the appropriate identification of specific biomarkers. The biomarkers are pivotal to providing the required epidemiological information for the early detection of the virus in the environmental compartments of water and air media to forestall further transmission. The present review aims to report about the scientific evidence of SARS-CoV-2 spreading via aerosols and wastewater carriers and on the criticisms concerning the WBE application for an effective monitoring and prevention of the virus outbreak. Particular attention was also focused on the virus transmission and interaction with the host along with the indication of future research needs.
2. Coronaviruses description
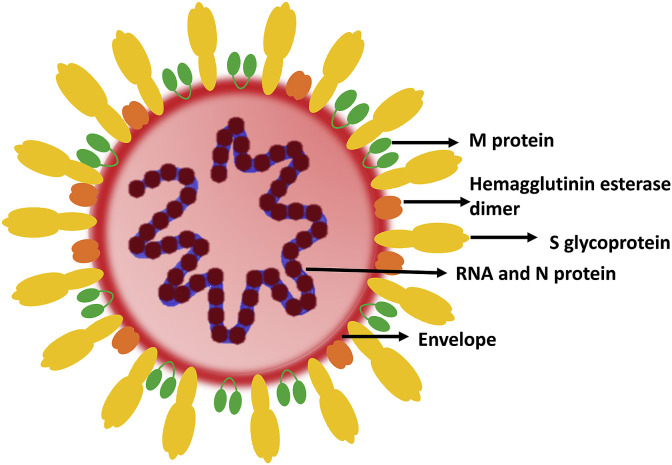
Coronaviruses (CoVs) belong to the subfamily Coronavirinae, subdivided into four genera Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus based on their phylogenetic properties (Cui et al., 2019). The members of Alphacoronavirus and Betacoronavirus possess the ability to infect mammals. CoVs possess spike-like projections on its surface like a crown, thereby the name. Attributes of the viruses belonging to the family Coronaviridae include the presence of an envelope with a positive-sense single-stranded RNA genome of 26–32kilo bases (Fig. 1 ) (Ahmed et al., 2020a, Ahmed et al., 2020b). Electron microscopic analysis demonstrated the spherical shape of the virus with an estimated diameter of 60–140 nm. In addition, the open reading frame (ORF)1a/b and the remaining ORFs of all coronaviruses encode for 16 non-structural proteins (NSPs) and envelope (E), membrane (M), nucleocapsid (N) and spike (S) proteins which constitute the structural proteins respectively (Fig. 1) (Su et al., 2016). The structural proteins form an integral part of the virus membrane and aid in functions like fusion with the host cell, assembly, and release of virions; moreover, the NSPs function in virus replication and transcription. The first 21st century outbreak associated with CoVs was caused by SARS-CoV in 2002–2003 followed by a second pandemic caused by MERS-CoV in 2012 (Jakhmola et al., 2020) https://doi.org/10.1016/j.heliyon.2020.e05706. The SARS-CoV genetically resembled the bat-CoV and was predicted to be transmitted to humans through intermediate hosts like palm civets, raccoon dogs, and Chinese ferret (Weiss, 2005). Moreover, the MERS-CoV transmitted to humans viadromedary camels, primary reservoirs of MERS-CoV (Conzade et al., 2018) https://doi.org/10.3390/v10080425. Investigations have revealed that SARS-CoV-2 is genetically closer to SARS-CoV. Moreover, a phylogenetic study sorted the various genomes of SARS-CoV-2 into three lineages (i.e., A, B, and C). America and Europe predominantly consisted of the A and C types, respectively (Forster et al., 2020). The B type was mostly confined to East Asia (Forster et al., 2020). The complete 29,903-bp–long RNA genome sequence of the SARS-CoV-2 (GenBank: MN908947.3) contained 5′-capped and 3′-polyadenylated ends and many ORFs (Wang et al., 2020a,b,c,d). Notably, the Hemagglutinin-esterase gene, prevalent in lineage A Betacoronaviruses was absent in SARS-CoV-2 (Chan et al., 2020). Like its distinct relative SARS-CoV, the SARS-CoV-2 also uses S protein to interact with the host ACE-2 receptor for cellular entry. However, another cell surface protein Neuropilin-1 has also been shown to act as entry factor for SARS-CoV-2. A study based on cryoelectron microscopy elaborated that the SARS-CoV-2 S protein has 20-fold higher affinity towards ACE-2 than SARS-CoV and this is due to 30% difference in the S protein of these two viruses. Unlike SARS-CoV or SARS-CoV-2, the MERS-CoV S protein engages with host dipeptidyl peptidase 4 (DPP4, also known as CD26) for the purpose of entry. The viral protein and host receptor interaction induces conformational changes in the S protein and facilitates entry. After interaction with the host receptor the virus is either endocytosed inside the cell or releases the genome in cytosol through membrane fusion. Once in the cytoplasm, RNA is translated into a polypeptide which is further cleaved by the proteases. Briefly, two viral encoded cysteine proteases; papain- and 3C-like proteases, cleave the polypeptides encoded by ORF1a/b into 15 NSPs (Chan et al., 2020). The virus encoded RNA dependent RNA polymerase (RdRp) performs discontinuous transcription and synthesizes sub genomic RNAs. These RNAs further get translated into various viral proteins like S, M, E, N etc. Among these the SARS-CoV-2 E protein, the smallest among all structural proteins, is localized to the endoplasmic reticulum and Golgi complex. The M protein is the most abundant transmembrane glycoprotein (Nieto-Torres et al., 2011; Siu et al., 2008) and prominently functions in the viral assembly with E and N proteins (Siu et al., 2008). Taking advantage of the host cytoplasmic membrane system, the viral RNA and proteins are assembled into virions. Subsequently, these virions are exocytosed through vesicles infecting the nearby cells. These different steps of viral life cycles can be used as drug targets for the treating the infection (Indari et al., 2021). However, adding up to the difficulties of therapeutic interventions are the newly developed alterations in the SARS-CoV-2 genome.
Fig. 1.
Coronaviruses schematic.
3. Airborne and wastewater carriers: focus on the virome
3.1. Coronaviruses in wastewater: Sources and occurrence and potential risk of infection
The role that water can play as a vehicle for pathogen spread as a consequence of faecal contamination was historically recognized for several microorganisms and diseases. Among these, the most important viruses such as Norovirus, Enterovirus, Hepatitis A virus, and Adenovirus belong to the Calicivirade, Picornaviridae, and Adenoviridae families (WHO, 2017). These viruses were detected in several types of water (wastewater, seawater, freshwater, and drink water) and hence associated with disease outbreaks (Bonadonna and La Rosa, 2019; Gall et al., 2015; La Rosa and Fratini, 2012; Moreira and Bondelind, 2017; Girones, 2017). SARS-CoV-2 is an enveloped virus belonging to the Betacoronavirus genus in the Coronaviride family characterized by positive-sense and single-strained RNA. A significant portion of a patient infected by SARS-CoV-2 showed gastrointestinal symptoms with consequent virus shedding into faeces above those from the respiratory tract (Xiao et al., 2020a; Ahmed et al., 2020a, Ahmed et al., 2020b). In addition, Xiao et al. (2020a) reported that the virus can infect glandular epithelial cells, and the virions can be secreted from intestinal cells albeit there is limited evidence of the potential infectivity of the virus detected in faecal samples (Xiao et al., 2020b). Faeces was charged to be the main carrier of the virus in wastewater and hence a further potential route of COVID-19 pandemic spreading. As reported in Table 1 the percentage of patients affected by SARS-CoV-2 representing gastrointestinal symptoms resulted quite high, in general, > 10% up to 62.5%, as the number of patients presenting SARS-CoV-2 RNA fragments in the stools. Furthermore, for those cases investigated, the SARS-CoV-2 load in the samples of stools analyzed resulted in general > 2 E3 (copies/ml).
Table 1.
Patients monitored, number of gastrointestinal symptoms, SARS-CoV-2 RNA fragments detected in stools, and related SARS-CoV-2 loads.
| Reference | Number of patients | Gastrointestinal symptoms | Stools with RNA | SARS-CoV-2 load (copie/ml) |
|---|---|---|---|---|
| Wang et al., (2020a,b,c,d) | 153 | – | 44 | <2.6E4 |
| Lin et al. (2020) | 160 | 100 | 31 | – |
| Xiao et al. (2020a) | 73 | – | 39 | – |
| Xu et al. (2020b) | 10 | 3 | 8 | 2E3-2E7 (range) |
| Wu et al., 2020a, Wu et al., 2020b | 74 | 23 | 41 | – |
| Zhang et al. (2020) | 23 | – | 10 | 5.6E3 (mean) |
| Wu et al., 2020a, Wu et al., 2020b | 4243 | 747 | 530 | 10E3.8-10E7.6 (range) |
| Wu et al., 2020a, Wu et al., 2020b | 9 | 1 | – | 10E3-10E75 (range) |
| Chen et al. (2020) | 42 | 8 | 28 | – |
| Lo et al. (2020) | 10 | – | – | – |
| Wu et al., 2020a, Wu et al., 2020b | 1099 | 55 | – | – |
| Pan et al. (2020) | 204 | 103 | – | – |
Differently from other microorganisms (e.g., bacteria), viruses showed a very limited survival period without hosting cellules. Furthermore, due to the damage susceptible by the labile lipid envelope, SARS-COV-2 resulted inactivated in the aqueous environment even if this process could result not so rapid. The occurrence of SAR-CoV-2 in municipal wastewater was investigated in the Netherlands by Medema et al. (2020). Presence of RNA fragments was detected in 6 out of 8 wastewater treatment plants monitored. Similar results were also reported by Wu et al. (2020b) in monitoring one of the major wastewater treatment plants in Massachusetts. Ahmed et al. (2020a) monitored one pumping station and two wastewater treatment plants in Southeast Queensland (Australia), reporting 2 out of 9 samples with the presence of fragments of SARS-CoV-2 RNA. Similar studies were also performed by Wurtzer et al. (2020) and La Rosa et al. (2020) for France and Italy, respectively. All these findings suggested that wastewater could be used for COVID-19 surveillance, but, in any case, no evidence of SARS-CoV-2 infection spreading via water and wastewater was currently detected. Wang et al. (2005) reported a persistence of SARS-CoV in hospital wastewater, domestic sewage, and tap water for 2 days at 20 °C and 14 days at 4 °C. The same authors also reported that at 20 °C, the persistence of the virus in faeces and urine was of 3 days and 17 days, respectively, whereas a concentration of 10 mg/L of chlorine with a contact time ≥10 min resulted able to completely inactivate SARS-CoV. For a chlorine concentration of 20 mg/L the minimum contact time decreased to 1 min. Concerning the survival of coronavirus in tap and wastewater, Ahmed et al., 2020a, Ahmed et al., 2020b compared HCoV-229E and animal FIPV coronavirus with Poliovirus-1 (PV-1). In wastewater at 23 °C, HCoV resulted inactivated in less than 4 days, whereas PV-1 lasted for about 11 days in primary wastewater and about 6 days in secondary effluents. In tap water, both HCoV and FIPV lasted for about 12 days, whereas at 4 °C the persistence was estimated to achieve up to 100 days.
The specific RNA sequences for SARS-CoV-2 reported in the study were detected by qPCR (Foladori et al., 2020). For improving the efficiency and effectiveness of RNA detection, liquid samples were generally pre-treated for both increasing its purity degree and/or for increasing the virus concentration. By the way, there is a lack of consensus on the protocols and standard procedures to be adopted for these analyses. Among the available methods for the SARS-CoV-2 concentration, Wu et al., 2020b, Wu et al., 2020a proposed a filtration using 0.22 μm membranes followed by PEG precipitation, centrifugation at 12,000 rpm for 2h or until the pellet was visible. Ahmed et al. (2020a) proposed two methods: a first method based on direct RNA extraction from the electronegative 0.45 -μm filter 90 mm diameter; a second method based on centrifugation at 4750 rpm for 30 min followed by centrifugation of the supernatant at 3500 rpm for 15 min through a centrifugal filter with a cut of 10 kDa. Wurtzer et al. (2020) proposed ultracentrifugation at 200,000 rpm for 1h at 4 °C. For RNA detection, La Rosa et al. (2020) used three different PRC assays: a broad range of PCR for Coronavirus detection targeting ORF1ab; a nested RT-PCR targeting the spike regions for SARS-CoV-2 proposed by Nao et al. (2020). For the same purpose, Randazzo et al. (2020) adopted an RT-qPCR diagnostic panel assay proposed by CDC (2020). All these differences in the procedures adopted leaded to some difficulties in performing direct comparison between the results reported in the studies as the consequent correct and univoque interpretation of the data.
3.2. SARS-CoV-2 in airborne particulate matter
Recently, 239 scientists from 32 countries have written an open letter to the World Health Organization (WHO) arguing the importance of airborne as a transmission route for COVID-19 (Morawska and Milton, 2020). However, at the moment the airborne mechanisms of transport of the virus, including the role of particulate matter, are not clear. In this context, the number of studies concerning the role of PM10/PM2.5 as carriers of virus spreading is rapidly growing. Airborne particles containing the virus can be emitted by infected individuals during sneeze, cough, shouting, speaking and normally breathing as already reported for other respiratory viruses belonging or not to the Coronavirinae subfamily (Milton et al., 2013; Wu et al., 2020b, Wu et al., 2020a; Leung et al., 2020). Settling rapidity of larger size respiratory droplets (i.e. > 5 μm) resulted higher than their evaporation rapidity. Oppositely, the evaporation rapidity of lower size respiratory droplets resulted higher than the settling one leaving residuals called droplet nuclei. These latter may be rich of viruses aggregate and other compounds as mineral salts and proteins (Asadi et al., 2020; Borouiba, 2020). Due to their dimensions, droplet nuclei can float in the air for long periods potentially contributing to the airborne transmission mechanism (Allen and Marr, 2020; Morawska and Cao, 2020; Martano, 2020). Air quality also in terms of humidity and temperature was already indicated as another relevant aspect in the COVID-19 spreading (Wu et al., 2020b, Wu et al., 2020a). Ma et al. (2020) reported during January and February 2020 a decrease of deaths due to SARS-CoV-2 in the city of Wuhan as both air temperature and humidity increased. Opposite findings were reported by Zhou and Xie (2020) for 122 Chinese cities during the same period indicating the absence of evidence between increase in ambient temperature and decrease of infection spreading. By the way, is worthy to be noted that smaller size droplet nuclei can remain suspended in the outdoor air for several days (Brito et al., 2018) before being eventually inhaled by other individuals. This facilitates its dilution below the infective inhalation dose (about 105-106) but also the inactivation and/or complete degradation of the SARS-CoV-2 and higher temperature can facilitate this process. In any case, low air quality can affect the respiratory apparatus of exposed individuals making them more vulnerable to the virial aggression (Barceló, 2020b). With specific reference to the role of PM10/PM2.5 as potentially carriers of infection spreading, the methodological approach adopted in such studies can be classified into two main categories: (a) statistical inference between the concentration of PM and the number of infections and (b) lab analysis of the presence of RNA in the particulate matter. Bibliography research in Scopus database was performed using the keywords as “COVID-19” or “SARS-COV” or “Coronavirus” and “aerosols” or “droplets” or “PM” or “air pollution” or “air transmission” or “airborne. The research was limited to articles with open access published in 2020 and the English language. Among more than 700 papers, only 6 can be referred to the role of PM as a carrier of COVID-19 and all of them can be referred to the first approach category. Besides, the investigation of the references listed in the most relevant papers led to detect further 3 papers based on the statistical approach and 1 based on lab analysis.
Concerning the “statistical” approach, several studies (Wu et al., 2020b, Wu et al., 2020a; Borro et al., 2020; Chennakesavulu and Reddy, 2020; Coccia, 2020; Delnevo et al., 2020; Fronza et al., 2020; Li et al., 2020; Setti et al., 2020a) found a correlation/association between the PM concentrations (in some cases in terms of number of exceeding of regulated concentration of air quality standards) in several cities in Italy and China. Spearman's correlation coefficient was the most used tool implemented for statistical analysis. However, it remains controversial the cause-effect relationship between infection and PM concentration since as Altman and Krzywinski (2015) explained correctly in their work “Correlation is not causation”. Besides, confounding variables are not sufficiently investigated. In the specific case where a mere association or correlation between particulate concentration (variable - X) and the number of infected people (variable - Y) occured, the relationship cannot be interpreted a priori as a cause-effect relationship (X cause Y), because they would overlook other phenomena. For example, the number of infected people could be caused by a third factor (variable - Z) and air pollution would play a role as not the cause but as an amplifier to (“boost”) of the virus effects on the respiratory system as some studies have identified (Bontempi et al., 2020; Comunian et al., 2020; Setti et al., 2020a). Cities with ≤100 and > 100 days with the concentration of PM10 higher than the limits indicated by EU air quality standards, showed an increase in the number of infections of about 0.25% and 85% respectively (Coccia, 2020). Additionally, Wu et al., 2020b, Wu et al., 2020a highlighted how the concentration of PM2.5 can affect the distance in which the virus can be transmitted because SARS-CoV-2 droplets absorb PM2.5 and form a solid/liquid (PM2.5/SARS-CoV-2) interface. Zhao et al. (2019) found a similar result through a modelling analysis applied to the virus of Pathogenic Avian Influenza (HPAI). They stated that PM2.5 might transport the virus for longer distances than PM10, due to the lower deposition velocity. However, higher distances contribute to a lower probability of infection because of the decrease of the concentration of virus/particles. Concerning the RNA analysis approach, only the study of Setti et al. (2020b) was identified. Here, 34 PM10 samples were collected from an industrial site in the province of Bergamo in Northern Italy, over three weeks (from February 21st to March 13th, 2020) and in 7 samples have identified the presence of SARS-COV-2 RNA. About this approach, some general considerations should be reported:
-
•
The extraction method has not yet been well standardized;
-
•
The number of samples is statistically low to deduct general considerations and further studies are necessary;
-
•
The evidence of viral RNA is not indicative of the presence of active viruses that could be effectively transmitted.
In conclusion, based on what emerged from the analysis of the scientific literature, there is no scientific evidence of a possible spread of COVID-19 infection through atmospheric particulate matter. Furthermore, these findings resulted characterized by a high level of uncertainty preventing any definitive scientifically valid conclusions.
4. Wastewater-based epidemiology (WBE): SARS-CoV-2 monitoring and its detection techniques
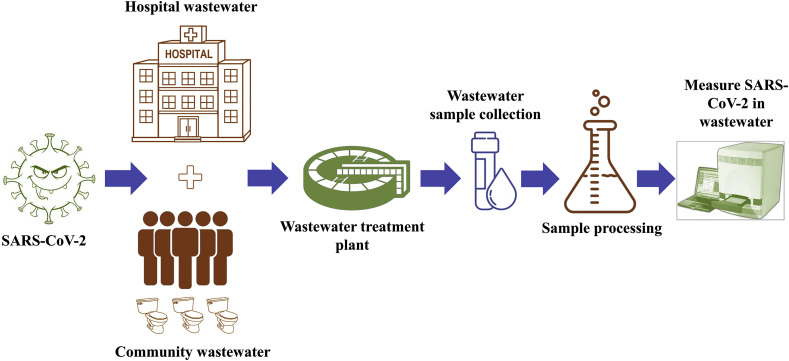
Water-borne diseases such as cholera, diarrhoea, dysentery, typhoid, and polio resulting death rate increase day-by-day globally. It is due to more than 2 billion people using drinking water sources contaminated with faeces in the worldwide (WHO, 2017). Most of these infections are from the water environment through the improper treatment of viruses in the sewage and wastewaters. In the last two decades, SARS-CoV-2 is the third coronavirus to spread worldwide (Guarner, 2020). As already reported in Table 1, several studies detected the presence of SARS-CoV-2 in stools of infected patients using as conformity test the rectal swab method (Lamers et al., 2020; Xu et al., 2020b; Zang et al., 2020). Moreover, a duration and survival of this virus in such media from 15 day up to 42 days was also reported (Jiang et al., 2020; He et al., 2020; Wölfel et al., 2020). These evidences suggested the use of wastewater monitoring for the surveillance of the presence of the virus and of the health conditions of given communities adopting the WBE approach (Medema et al., 2020; Randazzo et al., 2020) (Fig. 2 ). Several studies reported about the detection of SARS-CoV-2 RNA in wastewater samples in Nethelands (Medema et al., 2020), in Spain (Randazzo et al., 2020), in USA (Sherchan et al., 2020; Wu et al., 2020b, Wu et al., 2020a), in Australia (Ahmed et al., 2020a), in India (Kumar et al., 2020) and in Germany (Wu et al., 2020b, Wu et al., 2020a). Of particular interest was the study or Ahmed et al. (2020b) reporting the use of WBE for SARS-CoV-2 in wastewater in commercial passenger aircraft and cruise ships. One of the clinical suggests WBE as a good tool for the detection of variations in viral strains through phylogenetic analysis recognising virus trees over time and among various regions. Only a few studies are available for SARS-CoV-2 through phylogenetic analysis of wastewater samples. Nemudryi et al. (2020), found strains of SARS-CoV-2 in the wastewater of Bozeman, Montana. Rimoldi et al. (2020) revealed similarities among the isolated viral strains with those found in Europe and the Lombardy region in northern Italy through genome sequencing and phylogenetic analysis. WBE was already successfully used to monitor both life style and health of communities e.g. by the detection of the use and/or abuse of both licit and illicit drugs (Choi et al., 2018; Daughton, 2001). Some contrasting results were reported about the detection of SARS-CoV-2 in treated wastewater and surfaces water. If from one hand Naddeo and Liu (2020) observed SARS-CoV-2 survival and diffusion in both wastewater and received surface waters, from the other hand no presence in treated wastewater was reported by other authors (Singer and Wray, 2020; Wang et al., (2020a,b,c,d; Wurtzer et al., 2020). However, these results can be influenced by some factors as low concentration levels and RNA extraction spread rates requiring more research efforts for the definition of large scale WBE protocols (Hart and Halden, 2020a, Hart and Halden, 2020b). O'Brien and Xagoraraki (2019, 2020) model-based spatial and temporal evolution of viral diseases through wastewater data collection. Mao et al. (2020) proposed a small, portable paper-based device for the detection of SARS-CoV-2. In Italian based sewage wastewater and river samples, Rimoldi et al. (2020) demonstrated SARS-CoV-2 RNA detection through real-time RT-PCR.
Fig. 2.
Wastewater based epidemiology (WBE) concept.
The continuously human excreta received from the different sewer systems offer near-real-time outbreak data and these data were containing viruses from infected humans. This can be also related to their symptomatology status like no symptoms, paucisymptomatic or subclinical, and presymptomatic. By the way, it is worthy to mention that Chen et al. (2020) reported about patients with positive faecal specimen but negative pharyngeal and sputum test.
WBE identified viral concentrations in wastewater during different seasonal fluctuations. However, these concentrations were analyzed in comparison with other virus protocols. For example, the highest concentration of noroviruses was found during the winter and summer seasons (Nordgren et al., 2009). Similarly, Li et al. (2011) and Katayama et al. (2008) , reported the concentration levels of rotavirus and norovirus were higher from November to April. This variation in COVID-19 spread also occurs in sewage at locations falling inside of the climatologically favoured zones (4–12 °C) (Poole, 2020). Due to SARS-CoV-2 is a potentially lethal virus to humans, biosafety guidelines and procedures for concentrating such virus through specific filtration are given by the Center for Disease Control and Prevention (CDC, 2020). Several virus surrogates are used which depends on the experiment type, murine hepatitis virus is used as a SARS-CoV-2 surrogate (Ahmed et al., 2020a) in wastewater samples. Transmissible gastroenteritis (TMV) virus, enveloped bacteriophage, non-enveloped Escherichia, and feline infectious peritonitis viruses were used to test the persistence and disinfection of human coronaviruses in water and wastewater (Boehm et al., 2019; Silverman and Boehm, 2020; De Carvalho et al., 2017; Zielińska and Galik, 2017). Shirasaki et al. (2017) concluded that plant virus (Pepper mild mottle virus) is a suitable surrogate for the human virus. Lesimple et al. (2020) reviewed recent techniques for virus removal from wastewater like algal ponds, nanomaterials, membranes, and other treatment techniques. They have reviewed these techniques to develop WBE for both sampled (localised infection clusters) and quantified.
4.1. Protocols for WBE
The practical exploitation of WBE requires the definition of clear and agreed methodologies including all different phases from wastewater sampling to the final correlation of biomarker concentration and health status. Based on the current aim of this analysis (i.e., lifestyle or disease surveillance) and on the biomarkers used for this goal, the methodology proposed in the literature showed some differences. In any case, a common preliminary step of all the methodologies consists in the study of the area and of the population served by the sewage system for the exact identification of the size of the population interested and the presence of other wastewaters different from the urban ones entering in the sewage system (McCall et al., 2020).
4.1.1. Lifestyle surveillance
A relevant aspect concerns the selected biomarkers. These have to be a major and exclusive excretion metabolite, chemical or biological product of the specific disease under investigation, characterized by adequate stability in wastewater and the successive sampling and storage process (Hart and Halden, 2020a, Hart and Halden, 2020b; Zuccato et al., 2008). In investigating community drug abuse, Zuccato et al. (2008) excluded from the target biomarkers adopted in their study the most abundant excretion products (i.e., Glucuronic acid-conjugated metabolites) since their high instability in wastewater. Once these two main aspects have been defined, these authors suggested that the wastewater specimen has to be realized by the collection of wastewater samples at the wastewater plant inlet every 20 min for 24 h. This composite sample has to be pooled and performed by an automatic computer-controlling device, in a time-proportional mode for considering the rate fluctuations during different hours of the day. It is also recommended to withdraw more samples on consecutive days for ensuring the correct reproducibility of the tests. Before being analyzed, the samples have to be properly stored for avoiding the degradation of the biomarkers. Maida et al. (2017) suggested filtration the same day of collection and storage at 4 °C for a maximum of 2 days. The analytical procedure differs based on the origin of the biomarkers. In the case of drugs, the targeted biomarkers are urine metabolites and/or the unchanged parents of specific drugs (Table 2 ). These can be measured by a fully validated procedure (Castiglioni et al., 2006; Zuccato et al., 2008; Maida et al., 2017) consisting in the following main steps: spiking of the water samples with internal standards, acidification and extraction of the solid phase using reverse-phase/cation-exchange cartridges. Cartridges were preventively conditioned with methanol, water, and 0.01 N HCl solution. Successively, the liquid eluted obtained by methanol and 2% ammonia in methanol was analyzed by liquid chromatography-tandem mass spectrometry. Quantitative analyses were performed in the selected reaction-monitoring mode by measuring the fragmentation products of the protonated or deprotonated pseudo-molecular ions of each compound and deuterated analog. Instrumental limits, repeatability, recoveries, and limits of detection have to be also calculated (Castiglioni et al., 2006). Another validated procedure, with some minor differences to the one proposed by Castiglioni et al. (2006) was proposed by Lai et al. (2011; 2015). Once the concentration of the targeted biomarkers was determined, the whole amounts of biomarkers excreted by the population served by the wastewater treatment plant can be calculated by simply multiplying the concentration by the whole amount of wastewater collected from the plant. Since the population served by the plant was preventively investigated, it is possible to assess the amounts of biomarkers excreted per inhabitant per day (mg/inhabitant/day). The evaluation of the daily consumption of drugs per inhabitant is hence possible by multiplying the amount of mg/inhabitant/day by a corrective factor that represents the percentage of drug dose excreted multiplied by the molar mass ratio of the parent drug/targeted biomarker.
Table 2.
Biomarkers exploitable for the monitoring of life style and health at the community level through wastewater.
| Detection goal | Biomarker | Disease | Amount/Concentration detected | Reference |
|---|---|---|---|---|
| Illicit drugs | ||||
| Cocaine | BE Cocaine |
– | 267-390 (mg/day/1000 people) 109-157 (mg/day/1000 people) |
Zuccato et al. (2008) |
| Heroin | Morhpine 6-Acetylmorphine |
– | 32-173 (mg/day/1000 people) | Zuccato et al. (2008) |
| Amphetamine | Amphetamine | – | 2.5–24 (mg/day/1000 people) | Zuccato et al. (2008) |
| Methamphetamine | Methamphetamine | – | 2.4–4.5 (mg/day/1000 people) | Zuccato et al. (2008) |
| Ecstasy | Ecstasy | – | 3.4–7.3 (mg/day/1000 people) | Zuccato et al. (2008) |
| Cannabis | THC-COOH | – | 20-50 (mg/day/1000 people) | Zuccato et al. (2008) |
| Antibiotics | ||||
| Azithromycin n-Demethyl azithronicin |
Pneumonia, middle ear infection, strep throat, intestinal infection | 269-22,730 mg/L 30–74 mg/L |
Senta et al. (2019) | |
| Clarithromycin n-Demethyil clarithromycin |
Pneumonia, skin infection, H, Pylori infection, Lyme disease | 111-10,491 mg/L 13-1559 mg/L |
Senta et al. (2019) | |
| Ciprofloxacin | Respiratory tract infection, skin infection, gastroenteritis | 17-2500 mg/l | Guerra et al. (2014) | |
| Trimethoprim | Urinary tract infection | 464-6796 mg/L | (Kasprzyk-Hordern et al., 2009; Roberts and Thomas, 2006) | |
| Antivirals | ||||
| Oseltamivir phosphate Oseltamivir carboxylate |
Flu virus (influenza) | 5–529 ng/L 28–1213 ng/L |
(Leknes et al., 2012; Takanami et al., 2012) | |
| Acyclovir Carboxy-acyclovir |
Herpes simplex virus infections, chicken pox, shingles | 1780 ng/L 490–3420 ng/L |
(Funke et al., 2016; Prasse et al., 2010) | |
| Lamivudine, Carboxy lamivudine |
HIV/AIDs, hepatitis B | 52–720 ng/L 25–84 ng/L |
(Funke et al., 2016; Prasse et al., 2010) | |
| Zidovudine | HIV/AIDs | 310–380 ng/L | Prasse et al. (2010) | |
| Painkillers | ||||
| Acetaminophen | Painkiller | 5529–500,000 ng/L | (Guerra et al., 2014; Roberts and Thomas, 2006) | |
| Ibuprofen | Painkiller | 968–45,000 ng/L | (Guerra et al., 2014; Kasprzyk-Hordern et al., 2009; Roberts and Thomas, 2006) | |
| Pathogenic organisms | ||||
| Bacterial DNA Klebsiella pneumoniae |
Pneumonia, UTI, bacteremia and endophthalmitis | 6.31–6.56 log gene copies/100 mL | Shannon et al. (2007) | |
| Bacterial DNA Pseudomonas aeruginosa |
Pneumonia, UTI, gastrointestinal infections | 4.31–4.38 log gene copies/100 mL | Shannon et al. (2007) | |
| Bacterial DNA Enterococcus faecalis |
UTIs, bacteremia, septicemia | 4.66–4.85 log gene copies/100 mL | Shannon et al. (2007) | |
| Viral DNA/RNA Norovirus (GI) |
Gastroenteritis | <10–3500 viral genomes/L | Hellmer et al. (2014) | |
| Viral DNA/RNA Influenza A |
Respiratory infection | 2.6 × 105 genome copies/L | Heijnen and Medema (2011) | |
| Viral DNA/RNA Hepatitis A |
Liver infection | <10–1500 viral genomes/L | Hellmer et al. (2014) | |
| Viral DNA/RNA Severe acute respiratory syndrome (SARS CoV) |
Respiratory infection | – | Poon et al. (2004) | |
| Fungal DNA Candida species |
Candidiasis | – | Assress et al. (2019) | |
| Fungal DNA Aspergillus (Aspergillus fumigatus, Aspergillus niger and Aspergillus flavus) |
Chronic pulmonary aspergillosis, pulmonary and nasal allergies, asthma, pneumonitis | – | Assress et al. (2019) | |
4.1.2. Disease surveillance
As already discussed in the previous section 4.1.1, also in this case the stability of the biomarkers and the adequate concentrations are important issues able to affect the results of the whole analysis. If the targeted biomarkers are represented by viral DNA/RNA, the water samples were filtered by nano-ceramic filters eluted for 2 min with 1 L 1.5% w/w beef extract as indicated in EPA's protocol (U.S. EPA, 2001) within 24 h. The pH of the obtained solution was adjusted to 3.5 ± 0.1. After being flocculated for 30min. and centrifuged at 2500g for 15 min at 4 °C, the solid phase was resuspended by 30 mL of 0.15M sodium phosphate, pH 9.0–9.5, and centrifugated at 7000g for 10 min at 4 °C. After centrifugation, the supernatant was firstly neutralized to a pH of about 7.25 and the filtrated using 0.45 -μm and 0.22 -μm syringe filters. Extraction of nucleic acid was performed with 140 mL of purified virus concentrate using a commercial kit. The nucleic acid was stored at −80 °C until being further processed. The quantification of the targeted viral DNA/RNA is usually performed by quantitative PCR (qPCR) or reverse transcription (RT) qPCR (McCalla et al., 2020).
4.2. SARS-CoV-2 detection
In general, as reported by several authors (Ahmed et al., 2020a; Hart and Halden, 2020a, Hart and Halden, 2020b; Rimoldi et al., 2020; Medema et al., 2020; La Rosa et al., 2020a, b; Trottier et al., 2020; Bar-Or et al., 2020; Randazzo et al., 2020; Kocamemi et al., 2020; Green et al., 2020; Sherchan et al., 2020; Wu et al., 2020; Kumar et al., 2020; Haramoto et al., 2020), more information resulted necessary concerning the survival and persistence of the SARS-CoV-2 in water and wastewater. In any case, its detection and quantification in this media was performed by RT-qPCR method, which is based on the presence of TaqMan detection probe assay are reported by different authors (Wurtzer et al., 2020; Fongaro et al., 2019; Nemudryi et al., 2020; Medema et al., 2020; Bar-Or et al., 2020; Randazzo et al., 2020; Kocamemi et al., 2020; Green et al., 2020; Wu et al., 2020; Haramoto et al., 2020). The choice of this method is based on its reliability due to its specificity and sensitivity properties (Bustin and Nolan, 2020). Michael-Kordatou et al. (2020) reviewed different methodology protocols for the SARS-CoV-2 quantitative analysis in wastewater. Stachler and Bibby (2014) reported that several viruses were abundantly available, such as pepper mild mottle virus (PMMoV), adenovirus, human polyomavirus, torque teno virus, and norovirus, CrAssphage in the sewage of the United States and European Union countries. Many of the researchers were found CrAssphage appears to be human-associated; however, it has been detected in a limited number of seagulls, dog, duck, pig, and chicken faecal samples (Malla et al., 2019; Stachler et al., 2017). Countries like United States, United Kingdom, Australia, Japan, Thailand, and Nepal were successfully deployed CrAssphage to detect faecal pollution in environmental waters (Stachler et al., 2019; Farkas et al., 2020; Kongprajug et al., 2019; Bivins et al., 2020).
A recent study by Haramoto et al. (2020) found detection of SARS-CoV-2 RNA in wastewater through qPCR technique and none of the samples of river water was found positive for SARS-CoV-2 RNA. They found virus detection in the secondary treated wastewater was 1.4 × 102–2.5 × 103 copies/L. The survival and stability of viruses in wastewater depend on physicochemical conditions such as temperature, pH, solids, and the presence of micropollutants (Fongaro et al., 2019). Auffret et al. (2019) showed that the detrimental effect on virus persistence with high alkalinity levels of wastewater. Bivins et al. (2020) reported a 90% reduction of viable SARS-CoV-2 in wastewater and tap water through RT-qPCR detection technique at room temperature. Other researchers have reported persistence of viruses at different temperatures. For example Casanova et al. (2009) found a 99% reduction of TGEV and MHV at the temperature of 25 °C in the sewage. Ye et al. (2016) reported a 90% reduction of MHV in unpasteurized wastewater in 13 h at 25 °C. Gundy et al. (2009) found to be 99.9% reduction of feline infectious peritonitis virus and human coronavirus 229E in the primary effluent at 23 °C in 2 and 4 days, respectively. In the case of wastewater from biological treatment processes, the survival and persistence of SARS-CoV-2 RNA are unclear due to insufficient experimental data (Oliver et al., 2020). Guerrero-Latorre et al. (2020) found SARS-CoV-2 RNA in the river samples at concentrations of 2.07 × 105 to 3.19 × 106 gene copies/L. Ahmed et al. (2020) reported that among five different RT-qPCR assays (CDCN1, CDC N2, N_Sarbeco, NIID_2019- nCoV_N, and E_Sarbeco), the CDC N1 assay is the most sensitive one while the N_Sarbeco showed the least sensitivity, while positive samples among wastewater samples of a cruise ship and three aircraft were near the assay LOD (37–40 cycles), providing inconsistent results among the tested assays.
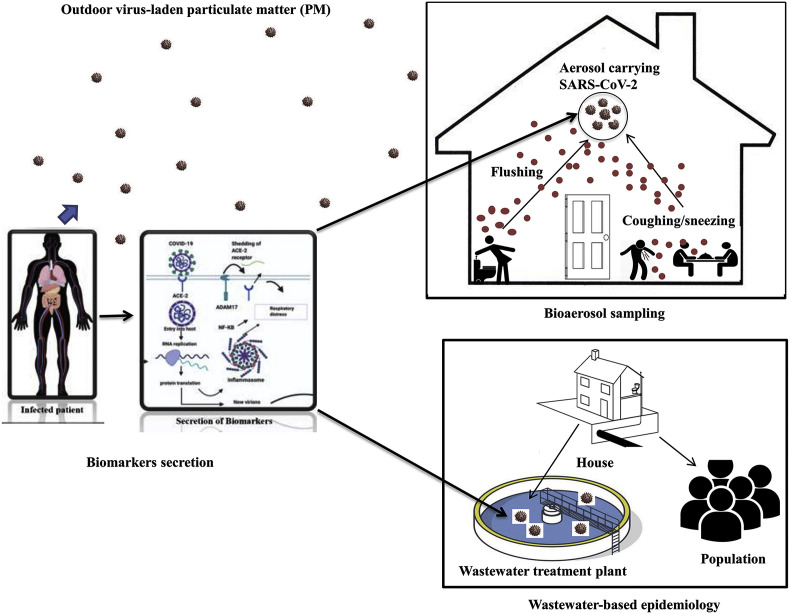
Wastewater and aerosols are ideal surveillance materials for assessing qualitative and quantitative molecular epidemiological information on population exposure to SARS-CoV-2, including biological and chemical markers associated with human activities within a community or a catchment (Carducci et al., 2020; Polo et al., 2020). Chirizzi et al. (2021) measured the concentrations of SARS-CoV-2 distribution in outdoor air samples from North and South of Italy. The authors found that the less than 0.8 copies for each size range, indicating less possible transmission through this medium, especially in a less crowded environment. Fig. 3 depict the bioaerosols sampling and WBE surveillance concept for viral particles in outdoor particulate matter, indoor aerosol, and wastewater treatment plants. One of the challenges of virus identification, unlike other microorganisms, is the inability to mutate outside the living host cells (Polo et al., 2020). The detection of human viruses in the environment can only be facilitated when excreted through faeces and urine or passed out through breath and cough or sneeze with significant concentrations and a half-life long enough to identify and characterize them.
Fig. 3.
Possible virus transmission schemes from infected to not infected individuals by outdoor and indoor aerosols and wastewater.
Some studies have also demonstrated the presence of SARS-CoV-2 RNA in aerosol samples. Chia et al. (2020) identified positive SARS-CoV-2 through the environmental sampling of hospital rooms. Santarpia et al. (2020) confirmed the presence of SARS-CoV-2 in the air samples from the hospital and isolation rooms. Liu et al. (2020) detected higher concentrations of SARS-CoV-2 RNA in the aerosol samples from the toilets used by the patients compared to the isolation room. Ong et al. (2020) detected small virus-laden droplets at the air exhaust outlet from the swab samples. Srivastava et al. (2021) also reported the prospect of rapid detection of SARS-CoV-2 in aerosol using nanomaterials enabled biosensing technique. The detection of the genetic material of SARS-CoV-2 is enabled by patient-generated airborne biomarkers of pathogens found in aerosols and other related volatile compounds, which can be exploited for making point-of-care devices (Bhalla et al., 2020; Gould et al., 2020). However, highly advanced technology is required to detect the chemical markers from environmental samples such as odor, breath, and aerosolized pathogens like SARS-CoV-2 generated by the infected patient (Fung and Mykhaylova, 2014; Bhalla et al., 2020). While the majority of the studies that were conducted to detect SARS-CoV-2 RNA in either wastewater or aerosol are based on reverse transcription-polymerase chain reaction (RT-PCR) and reverse transcription real-time polymerase chain reaction (RT-qPCR) techniques for qualitative and quantitative information, respectively, and digital PCR (Amoah et al., 2020; Polo et al., 2020; Santarpia et al., 2020; Liu et al., 2020), there have been instances where inconsistent results were obtained (Ahmed et al., 2020a; Rimoldi et al., 2020). This was due to the diverse contents of the PCR inhibitors and different concentration protocols being adapted to detect the existing molecular markers (Gibson et al., 2012; Hart and Halden, 2020a, Hart and Halden, 2020b). On this note, there is a need to identify appropriate biomarkers specific for SARS-CoV-2 detection in the environment, which have been scantly researched.
4.2.1. Biomarkers exploitable for WBE
WBE aims to assess the population's health by the detection and quantification of endogenous and exogenous urinary human biomarkers found in wastewater (i.e. wastewater-based epidemiology) (Table 2). Exogenous biomarkers have been assessed as population markers, whereas endogenous chemicals excreted by human metabolism characterized by low variance have been indicated as those suitable for estimating the population size (Choi et al., 2018). The use of WBE as a non-intrusive and non-incriminating method for obtaining real-time data truly reflecting the usage of drugs by wide communities was suggested for the first time by Daughton (2001). In this case, the authors proposed the investigation of domestic sewage for identifying the presence of illicit drugs and synthesis by-products excreted in human's faeces and urine and consequent evaluation of the amount of drug used and of the number of users.
One of the first practical applications of this approach was reported by Zuccato et al. (2005) for estimating the use of cocaine in a broad area of Northern Italy based on wastewater analysis. Based on the detection by mass spectrometry of cocaine and its main urinary metabolite (benzoylecgonine), the authors assessed an average daily use of cocaine of about 27 doses every 1000 inhabitants. Since that date, the use of this approach for the non-intrusive and wide investigation on the use of both licit and illicit drugs, pesticides, pharmaceutical and personal care products (PPCPs), alcohol consumption, and general health by oxidative stress markers, was largely exploited (Been et al., 2014; Choi et al., 2018; Kankaanpää et al., 2016; Kim et al., 2015; Lai et al., 2016; Reid et al., 2011; Rousis et al., 2017; Ryu et al., 2015). Since the success has already demonstrated in monitoring the above-described aspects, there is a large potential of using WBE for monitoring also other biomarkers related to infectious diseases (Sims and Kasprzyk-Hordern, 2020). King et al. (2009) reported the presence in the aquatic environment of Mastadenovirus A-F, responsible for gastroenteritis, ear infection, respiratory illness, and conjunctivitis, and Torque Teno Virus, responsible for hepatitis. The presence of Hepatitis A virus, Bk, MC and JC polyomavirus, responsible for gastroenteritis, hepatitis, cancer, and infection of respiratory, urinary tract and skin was reported by Moens et al. (2017). Similarly, the presence of in wastewater of Rotavirus A and Betacoronavirus (a novel coronavirus) was reported by Cook et al. (2004) and Chan et al. (2015), respectively.
4.2.2. Characteristics of biomarkers for SARS-CoV-2 surveillance in wastewater
Biomarkers are measurable and reproducible biological and chemical indicators containing pathogenic deoxyribonucleic acid (DNA) and/or ribonucleic acid (RNA), proteins, inflammation resistance genes, and metabolites emanating from any biological system for assessing the potential environmental risk through its early detection (Sims and Kasprzyk-Hordern, 2020). Daughton (2012) highlighted examples of biomarker indicators to include human excretion products such as blood, faeces, urine, and other clinical samples. Since SAR-CoV-2 has shown to have similar clinical and genomic characteristics of protein sequence to SAR-CoV (Xu et al., 2020a), prompting to possible similar biomarkers in the environment (Michael-Kordatou et al., 2020; van Doremalen et al., 2020). However, recent studies have shown that, unlike the original SARS-CoV, SARS-CoV-2 has multifaceted infections, including respiratory, gastrointestinal tract, nervous system, cardiovascular, and other body organ-related infections (Zhang and Guo, 2020). This implies the production of more viral particles from infected parts, subsequently disseminating into the environment, including wastewater and air through urine, faeces, breath, and possibly odor (Gould et al., 2020; Adelodun et al., 2020a). Although more studies are still required to conclude on the frequency of SARS-CoV-2 shedding through faeces and urine and other metabolic activities in infected individuals, some studies confirmed the shedding of viral genetic particles in faeces before the onset and after the disappearance of COVID-19 symptoms (Wang et al., 2020a,b,c,d; Gupta et al., 2020; La Rosa et al., 2019), leading to the detection of SARS-CoV-2 RNA in wastewater before the actual reported COVID-19 case (Medema et al., 2020). Reid et al. (2014) highlighted the essential characteristics of compound suitability as biomarkers to include stability within the wastewater system, sufficiently present in high concentrations, sufficiently excreted in a high level of observability and must be excreted in urine without much solid partitioning. Similarly, Daughton (2012) identified some essential attributes of biomarkers to include a high level of persistence in sewage, sensitive to biological changes and or health status of the infected individual. Among the main features identified, the most relevant were represented by: the minimal variance in daily excretion among the intra-individual; minimal interference of exogenous factors; high level of detectability, and ability to identify the biological pathway or physiological system of origination. While the majority of these characteristics were used to describe the drug biomarker, the same can be attributed to SARS-CoV-2 since they are both found to exhibit through metabolism and excretion, considering the currently limited data on the pathogenesis of SARS-CoV-2. Sims and Kasprzyk-Hordern (2020) further noted some desirable characteristics of biomarkers that can be fitted by WBE techniques. The excretion of the biomarker must be through urine with concentration measured in ng/L and high enough to be detected at the downstream of the wastewater. As already mentioned before, the stability of the biomarker in the wastewater during the process of sampling and characterization is essential. Furthermore, the biomarker should be unique to the human metabolism of interest to distinguish it from exogenous biomarkers originating from other sources such as microbes or animal contamination in wastewater (Barceló, 2020a). However, it was argued that the genetic materials of SARS-CoV-2 decays naturally below the limits of detection in the environment, including wastewater and aerosol (Hart and Halden, 2020b), which could affect the quantity and quality of biomarker characteristics in these media.
4.2.3. Potential biomarkers for SARS-CoV-2 in wastewater
The selection of appropriate biomarkers can be challenging due to the complexity of the water and wastewater matrix, which requires extensive preparation of samples and pre-concentration of target analytes (Sims and Kasprzyk-Hordern, 2020; Pandopulos et al., 2020a,b). Wastewater contains complex microbial communities with diverse information on biological and chemical compounds, which are difficult to extract and characterize (Adelodun et al., 2020b). Similarly, the use of volatile compounds for the biosampling of airborne viruses has so far received less attention due to the challenges in identifying virus-specific volatile compounds (Gould et al., 2020). Nevertheless, significant progress has been recorded on biomarkers for drug/pharmaceutical monitoring (Daughton, 2020; Sims and Kasprzyk-Hordern, 2020). SARS-CoV-2 infections are found to cause serious alterations in the respiratory, pulmonary, and gastrointestinal microbiomes (Villapol, 2020; Lamote et al., 2020), thereby causing changes of metabolites which can be detected in the faeces or breath (Gould et al., 2020). The viruses contain proteins, RNA, and lipids, which are excreted or expelled via metabolic activity, making them potential marker candidates for viral infection (Gould et al., 2020). Few studies have also been conducted on the biomarkers for SARS-CoV-2. Zhang and Guo (2020) highlighted three specific biomarker targets for SARS-CoV-2 detection, and these include the viral RNA genome, spike protein, and glycans. Conticini et al. (2020) suggested chemokines and inflammatory cytokines as potential biomarkers for acute respiratory distress syndrome, an elevated condition in COVID-19 resulting from airborne particulates of SARS-CoV-2. Table 3 presents other potential biomarkers proposed in the literature that could be adopted for monitoring SARS-CoV-2 in wastewater and airborne particles using previously identified biomarkers with similar indicators to COVID-19.
Table 3.
Potential biomarkers for monitoring SARS-CoV-2 in wastewater.
| Biomarker | Type | Metabolism description | Indicator | Concentration | References |
|---|---|---|---|---|---|
| Cotinine | Exogenous | Nicotine | Urine | 5.0 ng/L−3.0 μg/L | Chen et al. (2014) |
| 5-hydxyindoleacetic acid (5-HIAA) | Endogenous | Serotonin | Urine | 30 ng/L-10 μg/L | Chen et al. (2014) |
| Homovanillic acid (HVA) | Endogenous | Catecholamine | Wastewater | 1.70 ± 0.88 mg/day | Pandopulos et al. (2020a) |
| Vanillylmandelic acid (VMA) | Endogenous | Catecholamine | Urine | 1.94 ± 1.03 mg/day | Pandopulos et al. (2020a) |
| C-reactive protein | Endogenous | Matrix metalloproteinase | Urine | 0.54–2.76 μg/mL | Stuveling et al. (2003) |
| Coprostanol | Endogenous | Cholesterol | Faeces | 30 μg/L-10 mg/L | Chen et al. (2014) |
| Cortisol | Endogenous | Steroid hormone | Faeces | 30 ng/L-10 μg/L | Chen et al. (2014) |
| Cholesterol | Endogenous | Lipid molecule, and components of cell membranes | Faeces | 30 μg/L-10 mg/L | Chen et al. (2014) |
| Androstenedione | Endogenous | Sex hormone precursor | Urine | 30 ng/L-10 μg/L | Chen et al. (2014) |
| Acesulfame | Exogenous | Artificial sweetener | Urine | 9.59–30.13 μg/L | Rico et al. (2017) |
| Lamivudine | Endogenous | Trans-sulfoxide | Urine | 2.2–579.1 ng/L | Hou et al. (2020) |
| Atenolol | Exogenous | Hypertension (drug) better blocker | Urine | 15.21–90.11 μg/L | Rico et al. (2017) |
| Isoprostanes | Endogenous | 15-F2t-isoP | Urine | 57–390 ng/g | Janicka et al. (2010) |
| 8-isoprostanes | Endogenous | 15-F2t-isoP | Exhale breath condensate | 16–110 pg m/L | (Syslová et al., 2008; Psathakis et al., 2006) |
5. Conclusions and future perspectives
The detection of specific pollutants in wastewater related to human lifestyle and health issues is an effective non-intrusive, and non-incriminating system for monitoring and returning relevant information for preserving human health and legacy for communities. This can also be an effective way of helping in the early warning of outbreaks as those caused by viral infections. Although, wastewater based epidemiology has been successfully exploited for investigating the use and abuse of both licit and illicit drugs related to lifestyle and health of communities, the criticisms remain in its use for detecting viral infections and disease. Adequate concentration and stability of the specific biomarkers in both wastewater and during the sampling and storing procedure resulted in the first critical aspects that are able to affect the liability of the successive measurements. Another criticism was observed in the absence of standardized and largely agreed methodologies and protocols, in particular for SARS-CoV-2, which which could lead to uncomparable results. Traces of SARS-CoV-2 RNA in the airborne particulate matter were also detected by some studies in the literature, suggesting this medium as another possible way for the virus spreading. However, the limited number of studies, the absence of adequate knowledge, and several criticisms concerning the analytical procedures and methodologies, indicates that much more research activity necessitate the need for better understanding of this phenomenon. Better identification of biomarkers with suitable features for viral diseases detection and definition of standardized and adequate procedures for sampling, storage, and analysis of both wastewater and airborne samples are the main aspect requiring further research activity. In view of the above, the interdisciplinary approach, and multi-dimensional research activities capable of examining the initial diffusion of the virus as well as its outbreak are strongly recommended. This requires a multiple perspectives approach, including, among others, biomedical, epidemiological and environmental expertise together with engineering, economic, political, social and demographic ones.
Credit author statement
Uttpal Anand, Pawan Kumar Jha, Vijay Tripathi, Francesco Di Maria: Conceptualization and conceiving the original idea. Uttpal Anand, Bashir Adelodun, Alberto Pivato, S. Suresh, Omkar Indari, Shweta Jakhmola: Literature survey/mining, writing - the major original draft preparation. Bashir Adelodun, Alberto Pivato, Shweta Jakhmola: Figures/Illustrations and Tables preparation. Hem Chandra Jha, Pawan Kumar Jha, Vijay Tripathi, Francesco Di Maria: Visualization, Response, Critical Review and Editing/Proofreading the manuscript. Uttpal Anand, Pawan Kumar Jha, Francesco Di Maria: Involved in the discussion on the manuscript structure. Bashir Adelodun, Pawan Kumar Jha: Retrieved and arranged the references. Hem Chandra Jha, Vijay Tripathi, Francesco Di Maria: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank the Department of Biotechnology and Ministry of Human Resource and Development, Govt. of India for the fellowship to Omkar Indari and Shweta Jakhmola, respectively, in the form of a research stipend. We gratefully acknowledge the Indian Institute of Technology Indore for the facilities and support.
References
- Adelodun B., Ajibade F.O., Ibrahim R.G., Bakare H.O., Choi K.-S. Snowballing transmission of COVID-19 (SARS-CoV-2) through wastewater: any sustainable preventive measures to curtail the scourge in low-income countries? Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelodun B., Ajibade F.O., Ighalo J.O., Odey G., Ibrahim R.G., Kareem K.Y., Bakare H.O., Tiamiyu A.O., Ajibade T.F., Abdulkadir S.T., Adeniran K.A., Choi K.S. Assessment of socioeconomic inequality based on virus-contaminated water usage in developing countries: a review. Environ. Res. 2020;192 doi: 10.1016/j.envre.2020.110309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., Edson J., Ehret J., Gyawali P., Hamilton K.A., Hosegood I., Hugenholtz P., Jiang G., Kitajima M., Sichani H.T., Shi J., Shimko K.M., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Zaugg J., Mueller J.F. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. J. Travel Med. 2020;27:1–11. doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.G., Marr L.C. Recognizing and controlling airborne transmission of SARSCoV-2 in indoor environments. Indoor Air. 2020;30:557–558. doi: 10.1111/ina.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman N., Krzywinski M. Points of significance: association, correlation and causation. Nat. Methods. 2015;12:899–900. doi: 10.1038/nmeth.3587. [DOI] [PubMed] [Google Scholar]
- Amoah I.D., Kumari S., Bux F. Coronaviruses in wastewater processes: source, fate and potential risks. Environ. Int. 2020;143 doi: 10.1016/j.envint.2020.105962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.L., Turnham P., Griffin J.R., Clarke C.C. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal. 2020;40:902–907. doi: 10.1111/risa.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 2020;54(6):635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assress H.A., Selvarajan R., Nyoni H., Ntushelo K., Mamba B.B., Msagati T.A.M. Diversity, co-occurrence and implications of fungal communities in wastewater treatment plants. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-50624-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffret M.D., Brassard J., Jones T.H., Gagnon N., Gagné M.J., Muehlhauser V., Masse L., Topp E., Talbot G. Impact of seasonal temperature transition, alkalinity and other abiotic factors on the persistence of viruses in swine and dairy manures. Sci. Total Environ. 2019;659:640–648. doi: 10.1016/j.scitotenv.2018.12.306. [DOI] [PubMed] [Google Scholar]
- Bagcchi S. Stigma during the COVID-19 pandemic. Lancet Infect. Dis. 2020;20(782) doi: 10.1016/S1473-3099(20)30498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceló D. Wastewater-based epidemiology to monitor COVID-19 outbreak: present and future diagnostic methods to be in your radar. Case Stud. Chem. Environ. Eng. 2020;2 doi: 10.1016/j.cscee.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceló D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. J. of Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or I., Yaniv K., Shagan M., Ozer E., Erster O., Mendelson M., Mannasse B., Shirazi R., Kramarsky-Winter E., Nir O., Abu-Ali H., Ronen Z., Rinott E., Lewis Y.E., Friedler E., Bitkover E., Paitan Y., Berchenko Y., Kushmaro A. Regressing SARS-CoV-2sewage measurements onto COVID-19 burden in the population: a proof-of-concept for quantitative environmental surveillance. MedRxiv. 2020. [DOI] [PMC free article] [PubMed]
- Becchetti L., Beccari G., Conzo G., Conzo P., De Santis D., Salustri F. AIR quality and COVID-19 adverse outcomes: divergent views and experimental findings. Environ. Res. 2020;193 doi: 10.1016/j.envres.2020.110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been F., Rossi L., Ort C., Rudaz S., Delémont O., Esseiva P. Population normalization with ammonium in wastewater-based epidemiology: application to illicit drug monitoring. Environ. Sci. Technol. 2014;48:8162–8169. doi: 10.1016/j.cscee.2020.100042. [DOI] [PubMed] [Google Scholar]
- Bhalla N., Pan Y., Yang Z., Payam A.F. Opportunities and challenges for biosensors and nanoscale Analytical tools for pandemics: COVID-19. ACS Nano. 2020;14:7783–7807. doi: 10.1021/acsnano.0c04421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., Crank K., Greaves J., North D., Wu Z., Bibby K. CrAssphage and pepper mild mottle virus as viral water quality monitoring tools–potential, research gaps, and way forward. Curr. Opin. Environ. Sci. Health. 2020;16:54–61. doi: 10.1016/j.coesh.2020.02.001. [DOI] [Google Scholar]
- Boehm A.B., Silverman A.I., Schriewer A., Goodwin K. Systematic review and meta-analysis of decay rates of waterborne mammalian viruses and coliphages in surface waters. Water Res. 2019;164 doi: 10.1016/j.watres.2019.114898. [DOI] [PubMed] [Google Scholar]
- Bonadonna L., La Rosa G. A review and update on waterborne viral diseases associated with swimming pools. Int J Environ Res Public Health 16(2) 2019;166 doi: 10.3390/ijerph16020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ. Res. 2020;186 doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. Commercial exchanges instead of air pollution as possible origin of COVID-19 initial diffusion phase in Italy: more efforts are necessary to address interdisciplinary research. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Vergalli S., Squazzoni F. Understanding COVID-19 diffusion requires an interdisciplinary, multi-dimensional approach. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borouiba L. Turbulent gas clouds and respiratory pathogen emissions, potential implications for reducing transmission of COVID-19. J. Am. Med. Assoc. 2020;323(18):1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- Borro M., Di Girolamo P., Gentile G., De Luca O., Preissner R., Marcolongo A., Ferracuti S., Simmaco M. Evidence-based considerations exploring relations between sars-cov-2 pandemic and air pollution: involvement of pm2.5-mediated up-regulation of the viral receptor ace-2. Int. J. Environ. Res. Publ. Health. 2020;17(15) doi: 10.3390/ijerph17155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito G.F.S., Sodré F.F., Almeida F.V. O impacto do material particulado na qualidade do Ar (impact of particulate matter on air quality) Rev. Virtual Quim. 2018;10:1335–1354. [Google Scholar]
- Buonanno G., Morawska L., Stabile L. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: prospective and retrospective applications. Environ. Int. 2020;145 doi: 10.1016/j.envint.2020.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S.A., Nolan T. RT-qPCR testing of SARS-CoV-2: a primer. Int. J. Mol. Sci. 2020 doi: 10.3390/ijms21083004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni S., Zuccato E., Crisci E., Chiabrando C., Fanelli R., Bagnati R. Identification and measurement of illicit drugs and their metabolites in urban wastewater by liquid chromatography–tandem mass spectrometry. Anal. Chem. 2006;78:8421–8429. doi: 10.1021/ac061095b. [DOI] [PubMed] [Google Scholar]
- CDC Coronavirus disease 2019 (COVID-2019). Available at. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases updates/summary.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fsummary.html
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., Xing F., Liu J., Yip C.C.Y., Poon R.W.S., Tsoi H.W. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Lau S.K.P., To K.K.W., Cheng V.C.C., Woo P.C.Y., Yue K.Y. Middle East Respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28:465e522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Kostakis C., Gerber J.P., Tscharke B.J., Irvine R.J., White J.M. Towards finding a population biomarker for wastewater epidemiology studies. Sci. Total Environ. 2014;487:621–628. doi: 10.1016/j.scitotenv.2013.11.075. [DOI] [PubMed] [Google Scholar]
- Chen L., Lou J., Bai Y., Wang M. COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am J Gastroenterol. 2020;115:790. doi: 10.14309/ajg.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennakesavulu K., Reddy G.R. The effect of latitude and PM 2.5 on spreading of SARS-CoV-2 in tropical and temperate zone countries. Environ. Pollut. 2020;266 doi: 10.1016/j.envpol.2020.115176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., Lim X.F., Lim A.S., Sutjipto S., Lee P.H., Son T.T. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11(2800) doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirizzi D., Conte M., Feltracco M., Dinoi A., Gregoris E., Barbaro E., La Bella G., Ciccarese G., La Salandra G., Gambaro A., Contini D. SARS-CoV-2 concentrations and virus-laden aerosol size distributions in outdoor air in north and south of Italy. Environ. Int. 2021;146 doi: 10.1016/j.envint.2020.106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi P.M., Tscharke B.J., Donner E., O'Brien J.W., Grant S.C., Kaserzon S.L., Mackie R., O'Malley E., Crosbie N.D., Thomas K.V., Mueller J.F. Wastewater-based epidemiology biomarkers: past, present and future. TrAC Trends Anal. Chem. (Reference Ed.) 2018;105:453–469. doi: 10.1016/j.trac.2018.06.004. [DOI] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunian S., Dongo D., Milani C., Palestini P. Air pollution and covid-19: the role of particulate matter in the spread and increase of covid-19's morbidity and mortality. Int J environ res public health 17(12) 2020. [DOI] [PMC free article] [PubMed]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzade Romy, Grant Rebecca, Malik Mamunur Rahman, Elkholy Amgad, Elhakim Mohamed, Samhouri Dalia, Embarek Peter K. Ben, Kerkhove Maria D. Van. virus. 2018;10(8) doi: 10.3390/v10080425. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N., Bridger J., Kendall K., Gomara M.I., El-Attar L., Gray J. The zoonotic potential of rotavirus. J. Infect. 2004;48(4):289e302. doi: 10.1016/j.jinf.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L.J.N. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Illicit drugs in municipal sewage: proposed new non-intrusive tool to heighten public awareness of societal use of illicit/abused drugs and their potential for ecological consequences. Pharmaceuticals and personal care products on the environment: scientific and regulatory issue. Symposium. 2001;791(2001):348–364. [Google Scholar]
- Daughton C.G. Using biomarkers in sewage to monitor community-wide human health: isoprostanes as conceptual prototype. Sci. Total Environ. 2012;424:16–38. doi: 10.1016/j.scitotenv.2012.02.038. [DOI] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho N.A., Stachler E.N., Cimabue N., Bibby K. Evaluation of Phi 6 persistence and suitability as an enveloped virus surrogate. Environ. Sci. Technol. 2017;51(15):8692–8700. doi: 10.1021/acs.est.7b01296. [DOI] [PubMed] [Google Scholar]
- Delnevo G., Mirri S., Roccetti M. Particulate matter and COVID-19 disease diffusion in Emilia-Romagna (Italy). Already a cold case? Computation. 2020 doi: 10.3390/COMPUTATION8020059. [DOI] [Google Scholar]
- Di Maria F., Beccaloni E., Bonadonna L., Cini C., Confalonieri E., La Rosa G., Milana M.R., Testai E., Scaini F. Minimization of spreading of SARS-CoV-2 via household waste producedby subjects affected by COVID-19 or in quarantine. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridi S., Niazi S., Sadeghi K., Naddafi K., Yavarian J., Shamsipour M., Jandaghi N.Z.S., Sadeghiniiat K., Nabizadeh R., Yunesian M., Momeniha F., Mokamel A., Hassanvand M.S., MokhtariAzad T. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas Luke K., Hillary L.S., Malham S.K., McDonald J.E., Jones D.L. Wastewater and public health: the potential of wastewatersurveillance for monitoring COVID-19. Curr. Opin. Environ. Sci. Health. 2020;17:14–20. doi: 10.1016/j.coesh.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas K., Mannion F., Hillary L.S., Malham S.K., Walker D.I. Emerging technologies for the rapid detection of enteric viruses in the aquatic environment. Curr. Opin. Environ. Sci. Health. 2020;16:1–6. doi: 10.1016/j.coesh.2020.01.007. [DOI] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manare S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fongaro G., Stoco P.H., Souza D.S.M., Grisard E.C., Magri M.E., Rogovski P., Schorner M.A., Barazzetti F.H., Christoff A.P., de Oliveira L.F.V., Bazzo M.L. 2020. SARS-CoV-2 in human sewage in Santa Catalina. 2019. [DOI] [PMC free article] [PubMed]
- Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronza R., Lusic M., Schmidt M., Lucic B. Spatial–temporal variations in atmospheric factors contribute to SARS-CoV-2 outbreak. Viruses. 2020 doi: 10.3390/v12060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung A.O., Mykhaylova N. Analysis of airborne biomarkers for point-of-care diagnostics. J. Lab. Autom. 2014;19:225–247. doi: 10.1177/2211068213517119. [DOI] [PubMed] [Google Scholar]
- Funke J., Prasse C., Ternes T.A. Identification of transformation products of antiviral drugs formed during biological wastewater treatment and their occurrence in the urban water cycle. Water Res. 2016;98:75–83. doi: 10.1016/j.watres.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Gall A.M., Marinas B.J., Lu Y., Shisler J.L. Waterborne viruses: a barrier to ~ safe drinking water. PLoS Pathog. 2015;11(6):e1004867. doi: 10.1371/journal.ppat.1004867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson K.E., Schwab K.J., Spencer S.K., Borchardt M.A. Measuring and mitigating inhibition during quantitative real time PCR analysis of viral nucleic acid extracts from large-volume environmental water samples. Water Res. 2012;46:4281–4291. doi: 10.1016/j.watres.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Gould O., Ratcliffe N., Król E., De Lacy Costello B. Breath analysis for detection of viral infection, the current position of the field. J. Breath Res. 2020;14 doi: 10.1088/1752-7163/ab9c32. [DOI] [PubMed] [Google Scholar]
- Green H., Wilder M., Middleton F.A., Collins M., Fenty A., Gentile K., Kmush B., Zeng T., Larsen D.A. Quantification of SARS-CoV-2 and cross-assembly phage (crAssphage) from wastewater to monitor coronavirus transmission within communities. medRxiv. 2020. [DOI]
- Guarner J. Three emerging coronaviruses in two decades: the story of SARS, MERS, and now COVID-19. Am. J. Clin. Pathol. 2020;153:420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra P., Kim M., Shah A., Alaee M., Smyth S.A. Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci. Total Environ. 2014;473–474:235–243. doi: 10.1016/j.scitotenv.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ Virol1(1) 2009;10 doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- Gupta S., Parker J., Smits S., Underwood J., Dolwani S. Persistent viral shedding of SARS-CoV-2 in faeces - a rapid review. Color. 2020:611–620. doi: 10.1111/codi.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Modeling wastewater temperature and attenuation of sewage-borne biomarkers globally. Water Res. 2020. [DOI] [PubMed]
- He X., Lau E.H., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X., Mo X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Heijnen L., Medema G. Surveillance of Influenza A and the pandemic influenza A (H1N1) 2009 in sewage and surface water in The Netherlands. J. Water Health. 2011;9:434–442. doi: 10.2166/wh.2011.019. [DOI] [PubMed] [Google Scholar]
- Hellmer M., Paxeus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergstrom T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis a virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80:6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C., Hua Z., Xu P., Xu H., Wang Y., Liao J., Di B. Estimating the prevalence of hepatitis B by wastewater-based epidemiology in 19 cities in China. Sci. Total Environ. 2020. [DOI] [PubMed]
- Indari Omkar, Jakhmola Shweta, Elangovan Manivannan, Jha Hem. C. An update on antiviral therapy against SARS-CoV-2: How far have we come? Frontiers in Pharmacology. 2021 doi: 10.3389/fphar.2021.632677. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakhmola Shweta, Indari Omkar, Kashyap Dharmendra, Varshney Nidhi, Rani Annu, Sonkar Charu, Baral Budhadev, Chatterjee Sayantani, Das Ayan, Kumar Rajesh, Jha Hem Chandra. Recent updates on COVID-19: A holistic review. Heliyon. 2020;6(12) doi: 10.1016/j.heliyon.2020.e05706. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicka M., Kot-Wasik A., Kot J., Namieśnik J. Isoprostanes-biomarkers of lipid peroxidation: their utility in evaluating oxidative stress and analysis. Int. J. Mol. Sci. 2010;11:4631–4659. doi: 10.3390/ijms11114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Luo M., Zou Z., Wang X., Chen C., Qiu J. Asymptomatic SARS-CoV-2 infected case with viral detection positive in stool but negative in nasopharyngeal samples lasts for 42 days. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- Kankaanpää A., Ariniemi K., Heinonen M., Kuoppasalmi K., Gunnar T. Current trends in Finnish drug abuse: wastewater based epidemiology combined with other national indicators. Sci. Total Environ. 2016:568,864–874. doi: 10.1016/j.scitotenv.2016.06.060. [DOI] [PubMed] [Google Scholar]
- Kasprzyk-Hordern B., Dinsdale R.M., Guwy A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009;43:363–380. doi: 10.1016/j.watres.2008.10.047. [DOI] [PubMed] [Google Scholar]
- Katayama H., Haramoto E., Oguma K., Yamashita H., Tajima A., Nakajima H., Ohgaki S. One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res. 2008;42(6–7):1441–1448. doi: 10.1016/j.watres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Kim K.Y., Lai F.Y., Kim H.Y., Thai P.K., Mueller J.F., Oh J.E. The first application of wastewater-based drug epidemiology in five South Korean cities. Sci. Total Environ. 2015 doi: 10.1016/j.scitotenv.2015.04.065. [DOI] [PubMed] [Google Scholar]
- King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press; 2009. Virus taxinomy: classification and nomenclature of viruses. [Google Scholar]
- Kocamemi B.A., Kurt H., Hacioglu S., Yarali C., Saatci A.M., Pakdemirli B. First data-set on SARS-CoV-2 detection for istanbul wastewaters in Turkey. medRxiv. 2020 doi: 10.1101/2020.05.03.20089417. [DOI] [Google Scholar]
- Kongprajug A., Mongkolsuk S., Sirikanchana K. CrAssphage as a potential human sewage marker for microbial source tracking in Southeast Asia. Environ. Sci. Technol. Lett. 2019;6(3):159–164. doi: 10.1021/acs.estlett.9b00041. [DOI] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Fratini M., della Libera S., Iaconelli M., Muscillo M. Emerging and potentially emerging viruses in water environments. Ann. Ist. Super Sanita. 2012;48(4):397e406. doi: 10.4415/ANN_12_04_07. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Bonanno Ferraro G., Veneri C., Iaconelli M., Bonadonna L., Lucentini L., Suffredini E. Evidence from environmental monitoring. Sci. Total Environ. December 2019;750 doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F.Y., Anuj S., Bruno R., Carter S., Gartner C., Hall W., et al. Systematic and dayto- day effects of chemical-derived population estimated on wastewater-based drug epidemiology. Environ. Sci. Technol. 2015;49:999–1008. doi: 10.1021/es503474d. [DOI] [PubMed] [Google Scholar]
- Lai F.Y., O'Brien J., Bruno R., Hall W., Prichard J., Kirkbride P., Gartner G., Thai P., Carter S., Lloyd B., Burns L., Mueller J. Spatial variations in the consumption of illicit stimulant drugs across Australia: a nationwide application of wastewater-based epidemiology. Sci. Total Environ. 2016;568:810–818. doi: 10.1016/j.scitotenv.2016.05.207. [DOI] [PubMed] [Google Scholar]
- Lai F.Y., Ort C., Gartner C., Carter S., Prichard J., Kirkbride P., et al. Refining the estimation of illicit drug consumptions from wastewater analysis: co-analysis of prescription pharmaceuticals and uncertainty assessment. Water Res. 2011;45:4437–4448. doi: 10.1016/j.watres.2011.05.042. [DOI] [PubMed] [Google Scholar]
- Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., Ravelli R.B., van Schayck J.P., Mykytyn A.Z., Duimel H.Q., van Donselaar E. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020 doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamote K., Janssens E., Schillebeeckx E., Lapperre T.S., De Winter B.Y., Van Meerbeeck J.P. The scent of COVID-19: viral (semi-)volatiles as fast diagnostic biomarkers? J. Breath Res. 2020;14 doi: 10.1088/1752-7163/aba105. [DOI] [PubMed] [Google Scholar]
- Leknes H., Sturtzel I.E., Dye C. Environmental release of oseltamivir from a Norwegian sewage treatment plant during the 2009 influenza A (H1N1) pandemic. Sci. Total Environ. 2012;414:632–638. doi: 10.1016/j.scitotenv.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Lesimple A., Jasim S.Y., Johnson D.J., Hilal N. The role of wastewater treatment plants as tools for SARS-CoV-2 early detection and removal. J Water Process Eng. 2020;101544 doi: 10.1016/j.jwpe.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K.-H., McDevitt J.J., Hau B.J.P., et al. Respiratory virus shedding in exhaled breath and efficacy of face mask. Nat. Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. Is the coronavirus airborne? Experts can't agree. Nature. 2020 doi: 10.1038/d41586-020-00974-w. [DOI] [PubMed] [Google Scholar]
- Li D., Gu A.Z., Zeng S.Y., Yang W., He M., Shi H.C. Monitoring and evaluation of infectious rotaviruses in various wastewater effluents and receiving waters revealed correlation and seasonal pattern of occurrences. J. Appl. Microbiol. 2011;110(5):1129–1137. doi: 10.1111/j.1365-2672.2011.04954.x. [DOI] [PubMed] [Google Scholar]
- Li H., Xu X.-L., Dai D.-W., Huang Z.-Y., Ma Z., Guan Y.-J. Air pollution and temperature are associated with increased COVID-19 incidence: a time series study. Int. J. Infect. Dis. 2020;97:278–282. doi: 10.1016/j.ijid.2020.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Lu, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- Liu P., Jiang J.Z., Hua Y., Wang X., Hou F., Wan X.F., Chen J., Zou J., Chen J. Are pangolins the intermediate host of the 2019 novel coronavirus (2019-nCoV)? Biorxiv. 2020 doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, Long Iek, Lio, Fu Chon, Cheong, Hon Hou, Lei, Ion Chin, Cheong, Hong Tak, Zhong Xu Tian Yakun Sin Nin Ngan Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020;16(10):1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Liu Y., He X., Wang B., Fu S., Yan J., Niu J., Zhou J., Bin L. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China, Sci. Total Environ. 2020;724:138226. doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maida C.M., Di Gaudio F., Tramuto F., Mazzucco W., Piscionieri D., Cosenza C., Viviani G. Illicit drugs consumption evaluation by wastewater-based epidemiology in the urban area of Palermo city (Italy) Ann. Ist. Super Sanita. 2017;53(3):192–198. doi: 10.4415/ANN_17_03_03. [DOI] [PubMed] [Google Scholar]
- Malla B., Shrestha R.G., Tandukar S., Sherchand J.B., Haramoto E. Performance evaluation of human-specific viral markers and application of pepper mild mottle virus and crAssphage to environmental water samples as fecal pollution markers in the Kathmandu Valley, Nepal. Food Environ Virol. 2019;11(3):274–287. doi: 10.1007/s12560-019-09389-x. [DOI] [PubMed] [Google Scholar]
- Mao K., Hua Z., Zhugen Y. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environ. Sci. Technol. 2020;54:3733–3735. doi: 10.1021/acs.est.0c01174. [DOI] [PubMed] [Google Scholar]
- Martano P. Droplet fate in a cough puff. Atmosphere 11(8) 2020;841 doi: 10.3390/atmos11080841. [DOI] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020. [DOI] [PubMed]
- McCall C., Wu H., Miyani B., Xagoraraki I. Identification of multiple potential viral diseases in a large urban center using wastewater surveillance. Water Research. 2020;184 doi: 10.1016/j.watres.2020.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton D.K., Fabian M.P., Cowling B.J., Grantham M.L., McDevitt J.J. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9(3):e1003205. doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi A., Nakama C., Okumura J., Azuma K. Assessing the risk of COVID-19 from multiple pathways of exposure to SARS-CoV-2: modeling in health-care settings and effectiveness of nonpharmaceutical interventions. Environ. Int. 2020;147 doi: 10.1016/j.envint.2020.106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:1–5. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens U., Calvignac-Spencer S., Lauber C., Ramqvist T., Feltkamp M.C.W., Daugherty M.D., Verschoor E.J., Ehlers B., Consortium, I.R ICTV virus taxonomy profile: polyomaviridae. J. Gen. Virol. 2017;98:1159–1160. doi: 10.1099/jgv.0.000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Milton D.K. It is time to address airborne transmission of COVID-19. Clin Infect Dis. 71, ciaa939. 2020. [DOI] [PMC free article] [PubMed]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira N.A., Bondelind M. Safe drinking water and waterborne outbreaks. J. Water Health. 2017;15(1):83–96. doi: 10.2166/wh.2016.103. [DOI] [PubMed] [Google Scholar]
- Moreno T., Pintó R.M., Bosch A., Moreno N., Alastuey A., Minguillón M.C., Anfruns-Estrada E., Guix S., Fuentes C., Buonanno G., Stabile L. Tracing surface and airborne SARS-CoV-2 RNA inside public buses and subway trains. Environ. Int. 2020;147 doi: 10.1016/j.envint.2020.106326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddeo V., Liu H. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Sci.: Water Res Technol. 2020;6(5):1213–1216. doi: 10.1039/D0EW90015J. [DOI] [Google Scholar]
- Nao N., Shirato K., Katano H., Matsuyama S., Takeda M. 2020. Detection of second case of 2019-nCoV infection in Japan. National Institute of Infectious Diseases. 2020 [Google Scholar]
- National Research Council . Rapid Expert Consultation on the Possibility of Bioaerosol Spread of SARS-CoV-2 for the COVID-19 Pandemic. National Academies Press, Washington; The National Academies Press: April 1, 2020. 2020. [DOI] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Vanderwood K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Reports Med. 2020;100098 doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Stud Chem Environ Eng. 2020;1 doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Torres J.L., DeDiego M.L., Álvarez E., Jiménez-Guardeño J.M., Regla-Nava J.A., Llorente M., Kremer L., Shuo S., Enjuanes L. Subcellular location and topology of severe acute respiratory syndrome coronavirus envelope protein. Virology. 2011;415(2):69–82. doi: 10.1016/j.virol.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorimotlagh Z., Jaafarzadeh N., Martínez S.S., Mirzaee S.A. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ. Res. 2020;193 doi: 10.1016/j.envres.2020.110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren J., Matussek A., Mattsson A., Svensson L., Lindgren P.E. Prevalence of norovirus and factors influencing virus concentrations during one year in a full-scale wastewater treatment plant. Water Res. 2009;43(4):1117–1125. doi: 10.1016/j.watres.2008.11.053. [DOI] [PubMed] [Google Scholar]
- Núñez-Delgado A. What do we know about the SARS-1 CoV-2 coronavirus in the environment? Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E., Xagoraraki I. Removal of viruses in membrane bioreactors. J. Environ. Eng. 2020 doi: 10.1061/(ASCE)EE.1943-7870.0001743. [DOI] [Google Scholar]
- O'Brien E., Xagoraraki I. A water-focused one-health approach for early detection and prevention of viral outbreaks. One Health. 2019;7 doi: 10.1016/j.onehlt.2019.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver M.M.H., Hewa G.A., Pezzaniti D., Haque M.A., Haque S., Haque M.M., Moniruzzaman M., Rahman M.M., Saha K.K., Kadir M.N. COVID-19 and recycled wastewater irrigation: a review of implications. Preprint 2020. 2020. [DOI]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA - J. Am. Med. Assoc. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection. Ann. Intern. Med. 2020;32610:9–10. doi: 10.7326/m20-3012. [DOI] [PubMed] [Google Scholar]
- Pan Lei, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei. China. Am. J. Gastroenterol. 2020:115,766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandopulos A.J., Bade R., Tscharke B.J., O'Brien J.W., Simpson B.S., White J.M., Gerber C. Application of catecholamine metabolites as endogenous population biomarkers for wastewater-based epidemiology. Sci. Total Environ. 2020;142992 doi: 10.1016/j.scitotenv.2020.142992. [DOI] [PubMed] [Google Scholar]
- Pandopulos A.J., Gerber C., Tscharke B.J., O'Brien J., White J.M., Bade R. A sensitive analytical method for the measurement of neurotransmitter metabolites as potential population biomarkers in wastewater. J. Chromatogr. A. 2020;1612 doi: 10.1016/j.chroma.2019.460623. [DOI] [PubMed] [Google Scholar]
- Polo D.D., Quintela-Baluja M., Corbishley A., Jones D.L., Singer A.C., Graham D.W., Romalde P.J.L. Making waves: wastewater-based epidemiology for SARS-CoV-2 – developing robust approaches for surveillance and prediction is harder than it looks. Water Res. 2020;186 doi: 10.1016/j.watres.2020.116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole L. 2020. Seasonal influences on the spread of SARS-CoV-2 (COVID19), causality, and forecastabililty (3-15-2020). Causality, and forecastabililty (3-15-2020) March 15, 2020. [DOI]
- Poon L.L.M., Chan K.H., Wong O.K., Cheung T.K.W., Ng I., Zheng B., Seto W.H., Yuen K.Y., Guan Y., Peiris J.S.M. Detection of SARS coronavirus in patients with severe acute respiratory syndrome by conventional and real-time quantitative reverse transcription-PCR assays. Clin. Chem. 2004;50:67–72. doi: 10.1373/clinchem.2003.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasse C., Schlusener M.P., Schulz R., Ternes T.A. Antiviral drugs in wastewater and surface waters: a new pharmaceutical class of environmental relevance? Environ. Sci. Technol. 2010;44:1728–1735. doi: 10.1021/es903216p. [DOI] [PubMed] [Google Scholar]
- Psathakis K., Mermigkis D., Papatheodorou G., Loukides S., Panagou P., Polychronopoulos V., Siafakas N.M., Bouros D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur. J. Clin. Invest. 2006;36:362–367. doi: 10.1111/j.1365-2362.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M.J., Baz-Lomba J.A., Ryu Y., Thomas K.V. Using biomarkers in wastewater to monitor community drug use: a conceptual approach for dealing with new psychoactive substances. Sci. Total Environ. 2014;487:651–658. doi: 10.1016/j.scitotenv.2013.12.057. [DOI] [PubMed] [Google Scholar]
- Reid M.J., Langford K.H., Mørland J., Thomas K.V. Analysis and interpretation of specific ethanol metabolites, ethyl sulfate, and ethyl glucuronide in sewage effluent for the quantitative measurement of regional alcohol consumption. Alcohol Clin. Exp. Res. 2011;35 doi: 10.1111/j.1530-0277.2011.01505.x. [DOI] [PubMed] [Google Scholar]
- Rico M., Andrés-Costa M.J., Picó Y. Estimating population size in wastewater-based epidemiology. Valencia metropolitan area as a case study. J. Hazard Mater. 2017;323:156–165. doi: 10.1016/j.jhazmat.2016.05.079. [DOI] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P., Thomas K. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006;356:143–153. doi: 10.1016/j.scitotenv.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Rousis N.I., EGracia-Lor E., Zuccato E., Bade R., Baz-Lomba J.A. In: Summary of Excreted and Waterborne Viruses Global Water Pathogen Project. Rose J.B., Jimenez-Cisneros B., editors. Org/book/summary-Of-Excreted-And-Waterborne-Viruses Michigan State University; 2017. Castrignan, E.,_Causanilles, A., covaci, A., Rusinol, M., Girones, R.http://www.waterpathogens [Google Scholar]
- Ryu Y., Reid M.J., Thomas K.V. Liquid chromatography-high resolution mass spectrometry with immunoaffinity clean-up for the determination of the oxidative stress biomarker 8-iso-prostaglandin F2alpha in wastewater. J. Chromatogr. A. 2015;1409:146–151. doi: 10.1016/j.chroma.2015.07.060. [DOI] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., Crown K.K., Brett-Major D.M., Schnaubelt E.R., Broadhurst M.J., Lawler J.V., Reid S.P., Lowe J.J. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senta I., Kostanjevecki P., Krizman-Matasic I., Terzic S., Ahel M. Occurrence and behavior of macrolide antibiotics in municipal wastewater treatment: possible importance of metabolites, synthesis byproducts, and transformation products. Environ. Sci. Technol. 2019;53:7463–7472. doi: 10.1021/acs.est.9b01420. [DOI] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Di Gilio A., Palmisani J., Buono P., Fornari G., Perrone M., Piazzalunga A., Barbieri P., Rizzo E., Miani A. 2020. Evaluation of the Potential Relationship between Particulate Matter (PM) Pollution and COVID-19 Infection Spread in Italy. SIMA. [Google Scholar]
- Setti L., Passarini F., Gennaro G., De Barbieri P., Perrone M.G., Borelli M., Palmisani J., Gilio A., Di Torboli V., Pallavicini A., Piscitelli P., Miani A. SARS-Cov-2 RNA found on particulate matter of Bergamo in northern Italy: first preliminary evidence. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K.E., Lee D.-Y., Trevors J.T., Beaudette L.A. Application of real-time quantitative PCR for the detection of selected bacterial pathogens during municipal wastewater treatment. Sci. Total Environ. 2007;382:121–129. doi: 10.1016/j.scitotenv.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki N., Matsushita T., Matsui Y., Murai K. Assessment of the efficacy of membrane filtration processes to remove human enteric viruses and the suitability of bacteriophages and a plant virus as surrogates for those viruses. Water Res. 2017;115:29–39. doi: 10.1016/j.watres.2017.02.054. [DOI] [PubMed] [Google Scholar]
- Silverman A.I., Boehm A.B. Systematic review and meta-analysis of the persistence and disinfection of human coronaviruses and their viral surrogates in water and wastewater. Environ. Sci. Technol. Lett. 2020;7(8):544–553. doi: 10.1021/acs.estlett.0c00313. [DOI] [PubMed] [Google Scholar]
- Sims N., Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A., Wray R. Detection and survival of SARS-coronavirus in human stool, urine, wastewater and sludge. Preprint 2020. 2020. [DOI]
- Siu Y.L., Teoh K.T., Lo J., Chan C.M., Kien F., Escriou N., Tsao S.W., Nicholls J.M., Altmeyer R., Peiris J.S., Bruzzone R. The M, E and N structural proteins of the SARS coronavirus are required for efficient assembly, trafficking and release of virus-like particles. J. Virol. 2008 doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M., Srivastava N., Mishra P.K., Malhotra B.D. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.142363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachler E., Bibby K. Metagenomic evaluation of the highly abundant human gut bacteriophage CrAssphage for source tracking of human fecal pollution. Environ. Sci. Technol. Lett. 2014;1(10):405–409. doi: 10.1021/ez500266s. [DOI] [Google Scholar]
- Stachler E., Crank K., Bibby K. Co-occurrence of crAssphage with antibiotic resistance genes in an impacted urban watershed. Environ. Sci. Technol. Lett. 2019;6(4):216–221. doi: 10.1021/acs.estlett.9b00130. [DOI] [Google Scholar]
- Stachler E., Kelty C., Sivaganesan M., Li X., Bibby K., Shanks O.C. Quantitative CrAssphage PCR assays for human fecal pollution measurement. Environ. Sci. Technol. 2017;51(16):9146–9154. doi: 10.1021/acs.est.7b02703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. U.S.A. 2020;117(22):11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuveling E.M., Hillege H.L., Bakker S.J.L., Gans R.O.B., De Jong P.E., De Zeeuw D. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003;63:654–661. doi: 10.1046/j.1523-1755.2003.00762.x. [DOI] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Weifeng S., Jun L., Alexander C.K.L., Jiyong Z., Wenjun L., Bi G.F.G. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syslová K., Kačer P., Kuzma M., Klusáčková P., Fenclová Z., Lebedová J., Pelclová D. Determination of 8-iso-prostaglandin F2α in exhaled breath condensate using combination of immunoseparation and LC-ESI-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008;867:8–14. doi: 10.1016/j.jchromb.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Takanami R., Ozaki H., Giri R.R., Taniguchi S., Hayashi S. Antiviral drugs zanamivir and oseltamivir found in wastewater and surface water in osaka. Japan. J. Water Environ. Technol. 2012;10:57–68. doi: 10.2965/jwet.2012.57. [DOI] [Google Scholar]
- Tang S., Mao Y., Jones R.M., Tan Q., Ji J.S., Li N., Shen J., Lv Y., Pan L., Ding P., Wang X. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J., Darques R., Mouheb N.A., Partiot E., Bakhache W., Deffieu M.S., Gaudin R. Post-lockdown detection of SARS-CoV-2 RNA in the wastewater of Montpellier, France. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., Wit E. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1–4. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl. Res. 2020;226:57–69. doi: 10.1016/j.trsl.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B., Li Z., Song N., Jin M., Wu X.M., Xiao W.J., Zhu X.M., Gu C.Q., Yin J., Wei W., Yao W., Liu C., Li J.F., Ou G.R., Wang M.N., Fang T.Y., Wang G.J., Qiu Y.H., Wu H.H., Chao F.H., Li J.W. Excretion and detection of SARS coronavirus and its nucleic acid from digestive system. World J. Gastroenterol. 2005;11(28):4390–4395. doi: 10.3748/wjg.v11.i28.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liu Z., Chen Z., Huang X., Xu M., He T., Zhang Z. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J. Med. Virol. 2020;92(6):667–674. doi: 10.1002/jmv.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Luwen, Li Xun, Chen Hui, Yan Shaonan, Li Dong, Li Yan, Gong Zuojiong. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am. J. Nephrol. 2020;51:343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Yao H., Xu X., Zhang P., Zhang M., Shao J., Xiao Y., Wang H. Limits of detection of 6 approved RT–PCR kits for the novel SARS-Coronavirus-2 (SARS-CoV-2) Clin. Chem. 2020;66(7):977–979. doi: 10.1093/clinchem/hvaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kang H., Liu X., Tong Z. Asymptomatic cases with SARS-CoV-2 infection. J. Med. Virol. 2020;92:1401–1403. doi: 10.1002/jmv.25990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus/susan R. Weiss, sonia navas-martin. Microbiol. Mol. Biol. Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Guidelines for Drinking-Water Quality, fourth ed. incorporating the 1st addendum. 2017. https://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/ [PubMed]
- WHO. COVID-19 2020 situation summary – updated 19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/summary.html#covid19-pandemic
- WHO. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Available at. 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Moniz K., Erickson T., Chai P., Thompson J., Alm E. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv preprint. 2020. [DOI] [PMC free article] [PubMed]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. medRxiv. 2020. [DOI]
- Xiao F., Sun J., Xu Y., Li F., Zhao J., Huan J., Zhao J. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26:1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., Wang Y., Guo X. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(244) doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 50(10), 5077-5085. 2016. [DOI] [PubMed]
- Yu H., Afshar-Mohajer N., Theodore A.D., Lednicky J.A., Fan Z.H., Wu C.Y. An efficient virus aerosol sampler enabled by adiabatic expansion. J. Aerosol Sci. 2018;117:74–84. doi: 10.1016/j.jaerosci.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato E., Chiabrando C., Castiglioni S., Bagnati R., Fanelli R. Vol. 116. Environmental Health Perspectives; 2008. pp. 1027–1032. (Estimating Community Drug Abuse by Wastewater Analysis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang R., Castro M.F.G., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M., Liu Z., Brulois K.F., Wang X., Greenberg H.B., Diamond M.S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol.5(47), eabc3582. 2020. [DOI] [PMC free article] [PubMed]
- Zhang L., Guo H. Biomarkers of COVID-19 and technologies to combat SARS-CoV-2. Adv. Biomark. Sci. Technol. 2020;2:1–23. doi: 10.1016/j.abst.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Yuhuan G., Fanping M., Yuhai B., Penghui Y., Fusheng W. Virus shedding patterns in nasopharyngeal and fecal specimens of COVID-19 patients. 2020. [DOI] [PMC free article] [PubMed]
- Zhao Y., Richardson B., Takle E., Chai L., Schmitt D., Xin H. Airborne transmission may have played a role in the spread of 2015 highly pathogenic avian influenza outbreaks in the United States. Sci. Rep. 2019 doi: 10.1038/s41598-019-47788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xie J. Association between ambient temperature and COVID-19 infection in 122 cities from China, Sci. Total Environ. 2020;724:138201. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska M., Galik M. Use of ceramic membranes in a membrane filtration supported by coagulation for the treatment of dairy wastewater. Water Air Soil Pollut. 2017 doi: 10.1007/s11270-017-3365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]