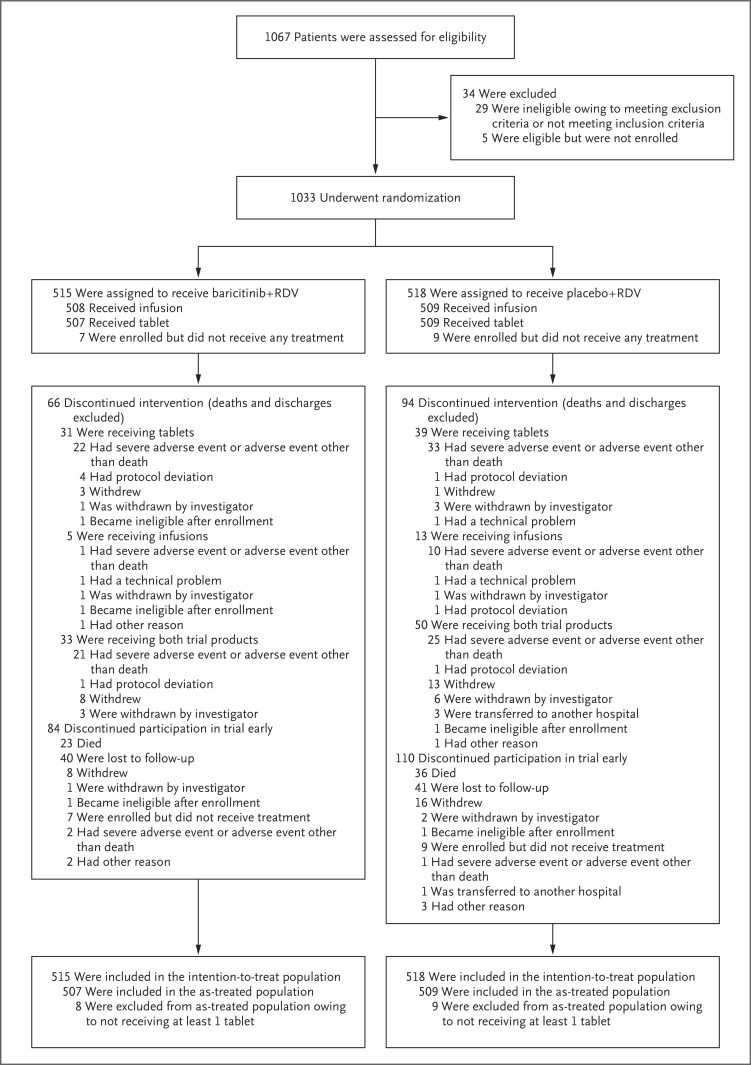
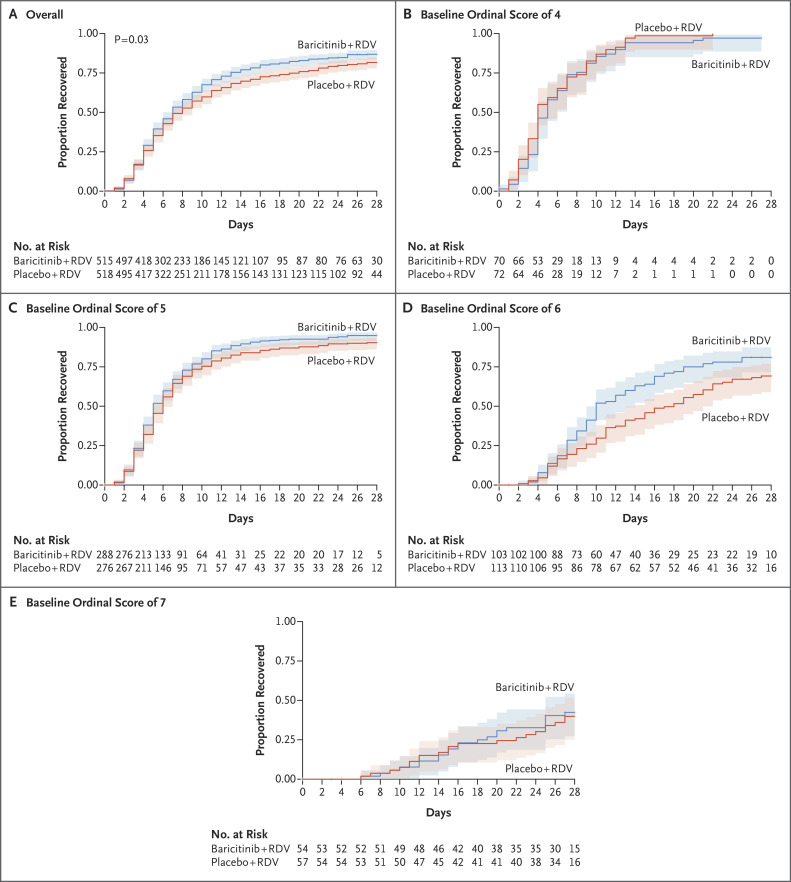
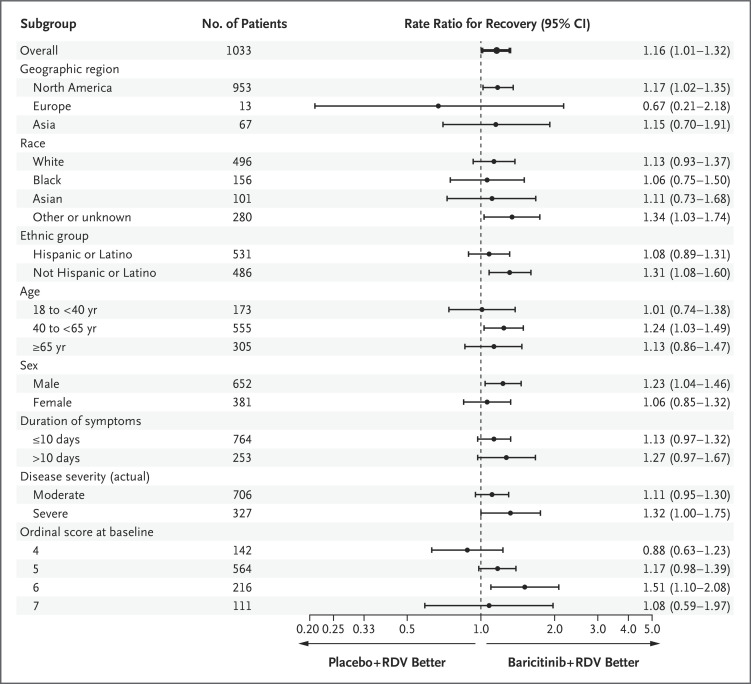
Andre C Kalil
Andre C Kalil, M.D., M.P.H.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,✉,
Thomas F Patterson
Thomas F Patterson, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Aneesh K Mehta
Aneesh K Mehta, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Kay M Tomashek
Kay M Tomashek, M.D., M.P.H.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Cameron R Wolfe
Cameron R Wolfe, M.B., B.S., M.P.H.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Varduhi Ghazaryan
Varduhi Ghazaryan, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Vincent C Marconi
Vincent C Marconi, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Guillermo M Ruiz-Palacios
Guillermo M Ruiz-Palacios, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Lanny Hsieh
Lanny Hsieh, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Susan Kline
Susan Kline, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Victor Tapson
Victor Tapson, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Nicole M Iovine
Nicole M Iovine, M.D., Ph.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Mamta K Jain
Mamta K Jain, M.D., M.P.H.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Daniel A Sweeney
Daniel A Sweeney, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Hana M El Sahly
Hana M El Sahly, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Angela R Branche
Angela R Branche, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Justino Regalado Pineda
Justino Regalado Pineda, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
David C Lye
David C Lye, M.B., B.S.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Uriel Sandkovsky
Uriel Sandkovsky, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Anne F Luetkemeyer
Anne F Luetkemeyer, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Stuart H Cohen
Stuart H Cohen, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Robert W Finberg
Robert W Finberg, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Patrick EH Jackson
Patrick EH Jackson, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Babafemi Taiwo
Babafemi Taiwo, M.B., B.S.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Catherine I Paules
Catherine I Paules, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Henry Arguinchona
Henry Arguinchona, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Paul Goepfert
Paul Goepfert, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Neera Ahuja
Neera Ahuja, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Maria Frank
Maria Frank, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Myoung-don Oh
Myoung-don Oh, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Eu S Kim
Eu S Kim, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Seow Y Tan
Seow Y Tan, M.B., B.S.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Richard A Mularski
Richard A Mularski, M.D., M.S.H.S.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Henrik Nielsen
Henrik Nielsen, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Philip O Ponce
Philip O Ponce, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Barbara S Taylor
Barbara S Taylor, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
LuAnn Larson
LuAnn Larson, R.N., B.S.N.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Nadine G Rouphael
Nadine G Rouphael, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Youssef Saklawi
Youssef Saklawi, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Valeria D Cantos
Valeria D Cantos, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Emily R Ko
Emily R Ko, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
John J Engemann
John J Engemann, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Alpesh N Amin
Alpesh N Amin, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Miki Watanabe
Miki Watanabe, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Joanne Billings
Joanne Billings, M.D., M.P.H.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Marie-Carmelle Elie
Marie-Carmelle Elie, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Richard T Davey
Richard T Davey, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Timothy H Burgess
Timothy H Burgess, M.D., M.P.H.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Jennifer Ferreira
Jennifer Ferreira, Sc.M.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Michelle Green
Michelle Green, M.P.H.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Mat Makowski
Mat Makowski, Ph.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Anabela Cardoso
Anabela Cardoso, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Stephanie de Bono
Stephanie de Bono, M.D., Ph.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Tyler Bonnett
Tyler Bonnett, M.S.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Michael Proschan
Michael Proschan, Ph.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Gregory A Deye
Gregory A Deye, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Walla Dempsey
Walla Dempsey, Ph.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Seema U Nayak
Seema U Nayak, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
Lori E Dodd
Lori E Dodd, Ph.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1,
John H Beigel
John H Beigel, M.D.
1From the University of Nebraska Medical Center, Omaha (A.C.K., L.L.); University of Texas Health San Antonio, University Health, and the South Texas Veterans Health Care System, San Antonio (T.F.P., P.O.P., B.S.T.), UT Southwestern Medical Center, Parkland Health and Hospital System (M.K.J.) and Baylor Scott and White Health (U.S.), Dallas, and Baylor College of Medicine, Houston (H.M.E.S.) — all in Texas; Emory University (A.K.M., N.G.R., Y.S., V.C.M.) and Grady Memorial Hospital (V.D.C.), Atlanta, and Atlanta Veterans Affairs Medical Center, Decatur (V.C.M.) — both in Georgia; the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K.M.T., V.G., R.T.D., M.P., G.A.D., W.D., S.U.N., L.E.D., J.H.B.), and the Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences (T.H.B.), Bethesda, and Emmes (J.F., M.G., M.M.) and Clinical Monitoring Research Program Directorate, Frederick National Laboratory (T.B.), Rockville — both in Maryland; Duke University, Durham, NC (C.R.W., E.R.K., J.J.E.); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (G.M.R.-P.) and Instituto Nacional de Enfermedades Respiratorias (J.R.P.), Mexico City; University of California Irvine, Irvine (L.H., A.N.A., M.W.), Cedars–Sinai Medical Center, Los Angeles (V.T.), University of California, San Diego, La Jolla (D.A.S.), University of California, San Francisco, San Francisco (A.F.L.), University of California, Davis, Davis (S.H.C.), and Stanford University, Stanford (N.A.) — all in California; University of Minnesota Medical School, Minneapolis (S.K., J.B.); University of Florida, Gainesville (N.M.I., M.-C.E.); University of Rochester, Rochester, NY (A.R.B.); National Center for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, and Yong Loo Lin School of Medicine (D.C.L.), and Changi General Hospital (S.Y.T.), Singapore; University of Massachusetts Medical School, Worcester (R.W.F.); University of Virginia, Charlottesville (P.E.H.J.); Northwestern University, Chicago (B.T.); Penn State Health Milton S. Hershey Medical Center, Hershey, PA (C.I.P.); Providence Sacred Heart Medical Center, Spokane, WA (H.A.); University of Alabama at Birmingham, Birmingham (P.G.); Denver Health and Hospital Authority, Denver (M.F.); Seoul National University Hospital, Seoul (M.O.), and Seoul National University Bundang Hospital, Seongnam (E.S.K.) — both in South Korea; Kaiser Permanente Northwest, Portland, OR (R.A.M.); Aalborg University Hospital, Aalborg, Denmark (H.N.); and Eli Lilly, Indianapolis (A.C., S.B.).
1