Abstract
In March 2020, following the annual International Committee on Taxonomy of Viruses (ICTV) ratification vote on newly proposed taxa, the phylum Negarnaviricota was amended and emended. At the genus rank, 20 new genera were added, two were deleted, one was moved, and three were renamed. At the species rank, 160 species were added, four were deleted, ten were moved and renamed, and 30 species were renamed. This article presents the updated taxonomy of Negarnaviricota as now accepted by the ICTV.
Keywords: Arenaviridae, Articulavirales, Artoviridae, Aspiviridae, Bornaviridae, Bunyavirales, bunyavirus, Filoviridae, Fimoviridae, Goujianvirales, Hantaviridae, ICTV, International Committee on Taxonomy of Viruses, Jingchuvirales, megaclassification, megataxonomy, Mononegavirales, Muvirales, Mymonaviridae, Nairoviridae, Negarnaviricota, Nyamiviridae, Orthomyxoviridae, Orthornavirae, Paramyxoviridae, Peribunyaviridae, Phasmaviridae, Phenuiviridae, Pneumoviridae, Rhabdoviridae, Riboviria, Serpentovirales, Sunviridae, Tenuivirus, Tospoviridae, virus classification, virus nomenclature, virus taxonomy
INTRODUCTION
Phylum Negarnaviricota was established in 2019 by the International Committee on Taxonomy of Viruses (ICTV) for negative-sense RNA viruses that can be connected evolutionarily through their encoded RNA-directed RNA polymerase (RdRp) core domains. The phylum includes two subphyla, Haploviricotina and Polyploviricotina, for negative-sense RNA viruses that encode large (L) proteins with or without mRNA capping activity, respectively. The two subphyla include four classes (Chunqiuviricetes, Milneviricetes, Monjiviricetes, and Yunchangviricetes) and two classes (Ellioviricetes and Insthoviricetes), respectively [56, 109, 136]. The vast majority of viruses that have been assigned to phylum Negarnaviricota belong to two orders: Mononegavirales (established in 1991 [92] and amended/emended in 1995 [16], 1997 [93], 2000 [94], 2005 [95], 2011 [32], 2016 [2], 2017 [7], March 2018 [8], October 2018 [66], and 2019 [9]) and Bunyavirales (established in 2017 and amended/emended in 2018 [64, 65] and 2019 [1]). Here we present the changes that were proposed to the entire phylum Negarnaviricota via official ICTV taxonomic proposals (TaxoProps) in 2019 and that were accepted by the ICTV in March 2020 [128]. These changes are now part of the official ICTV taxonomy.
TAXONOMIC CHANGES ABOVE THE PHYLUM RANK
Until recently, Negarnaviricota, included in realm Riboviria (established in 2019 [127]), was the only established phylum in the ICTV framework. In 2020, virus taxonomy was amended to include Negarnaviricota in the new riboviriad kingdom Orthornavirae as one of five sister phyla [55] (TaxoProp 2019.006G.A.v1.Riboviria).
TAXONOMIC CHANGES AT THE SUBPHYLUM RANK
No new subphyla were created.
TAXONOMIC CHANGES AT THE CLASS RANK
No new classes were created.
TAXONOMIC CHANGES AT THE ORDER RANK
No new orders were created.
Taxonomic changes within order Goujianvirales (Haploviricotina: Yunchangviricetes)
No changes were made.
Taxonomic changes within order Jingchuvirales (Haploviricotina: Monjiviricetes)
Family Chuviridae
One new species, Taiyuan mivirus, was added to genus Mivirus for Tàiyuán leafhopper virus (TYLeV) first discovered by high-throughput sequencing (HTS) in a leafhopper (Psammotettix alienus (Dahlbom, 1850)) sampled in Tàiyuán (太原), Shānxī Province (山西省), China [129] (TaxoProp 2019.018M.A.v2.1newsp_Taiyuan_mivirus).
Taxonomic changes within order Mononegavirales (Haploviricotina: Monjiviricetes)
Family Artoviridae
The family was expanded by one new genus, Hexartovirus, including one new species, Caligid hexartovirus, for Lepeophtheirus salmonis negative-stranded RNA virus 1 (LsNSRV-1) first discovered by HTS in salmon lice (Lepeophtheirus salmonis (Krøyer, 1837)) sampled on the west coast of Norway [81]. Species Barnacle peropuvirus was moved from genus Peropuvirus into genus Hexartovirus and renamed Barnacle hexartovirus (TaxoProp 2019.021M.A.v1.1newgenus_Hexartovirus).
Family Bornaviridae
No changes were made.
Family Filoviridae
The family was expanded by one genus, Dianlovirus, including a single new species, Mengla dianlovirus, for Měnglà virus (MLAV) discovered by HTS in a Rousettus sp. bat sampled in Měnglà County (勐腊县), Yúnnán Province (云南省), China [145, 146] (TaxoProp 2019.011M.A.v1.Mengla_dianlovirus).
One new species, Bombali ebolavirus, was created in genus Ebolavirus, for Bombali virus (BOMV) first discovered by consensus PCR and confirmed by HTS in little free-tailed bats (Chaerephon pumilus (Cretzschmar, 1830–1831)) and Angolan free-tailed bats (Mops condylurus (A. Smith, 1833)) sampled in Bombali District, Northern Province, Sierra Leone [37] (TaxoProp 2019.007M.A.v2.Bombali_ebolavirus).
Family Lispiviridae
No changes were made.
Family Mymonaviridae
A new genus, Hubramonavirus, was established for two new species: Hubei hubramonavirus for Húběi rhabdo-like virus 4 (HbRLV-4) discovered by HTS in an arthropod mix collected in Húběi
Province (湖北省), China [106] and Lentinula hubramonavirus for Lentinula edodes negative-strand RNA virus 1 (LeNSRV-1) first detected by HTS in commercial shiitakes (Lentinula edodes (Berk.) Pegler (1976)) sampled in Japan [60] (TaxoProp 2019.001F.A.v1.Hubramonavirus_1gen).
Family Nyamiviridae
No changes were made.
Family Paramyxoviridae
The overlooked deletion of species Bat mumps orthorubulavirus and the overlooked renaming of species Synodus paramyxovirus to Synodus synodonvirus were corrected (TaxoProp 2019.016M.A.v1.Corrections).
The family was expanded by three new genera: genus Cynoglossusvirus for the already established species Cynoglossus paramyxovirus (now renamed Cynoglossus cynoglossusvirus); genus Hoplichthysvirus for the already established species Hoplichthys paramyxovirus (now renamed Hoplichthys hoplichthysvirus); and genus Scoliodonvirus for the already established species Scoliodon paramyxovirus (now renamed Scoliodon scoliodonvirus) (TaxoProp 2019.025M.A.v2.Paramyxoviridae_3gen5sp4rensp).
Genus Aquaparamyxovirus was expanded by one species, Oncorhynchus aquaparamyxovirus, for Pacific salmon paramyxovirus (PSPV) first isolated from Chinook salmon (Oncorhynchus tshawytscha (Walbaum, 1792)) in Oregon, USA [135]. Species Salmon aquaparamyxovirus was renamed Salmo aquaparamyxovirus (TaxoProp 2019.025M.A.v2.Paramyxoviridae_3gen5sp4rensp).
Genus Jeilongvirus was expanded by one species, Miniopteran jeilongvirus, for “bat paramyxovirus isolate Bat-ParaV/B16–40” (here renamed Shaan virus [ShaV]) first isolated from a Schreibers’s long-fingered bat (Miniopterus schreibersii (Kuhl, 1817)) feces sampled in Danyang County (단양군), North Chungcheong Province (충청북도), South Korea [79] (TaxoProp 2019.025M.A.v2.Paramyxoviridae_3gen5sp4rensp).
Genus Orthoavulavirus was expanded erroneously by two species, Avian orthoavulavirus 21 and Avian orthovulavirus 21 [sic], for the same virus, “avian paramyxovirus 17” (here renamed avian paramyxovirus 21 [APMV-21]) first isolated from bird feces collected in Seosan (서산시), South Chungcheong Province (충청남도), South Korea [48] (TaxoProps 2019.014M.A.v1.Avulavirus_1newsp and 2019.025M.A.v2.Paramyxoviridae_3gen5sp4rensp).
Genus Orthorubulavirus was expanded by one species, Mammalian orthorubulavirus 6, for Alston virus (AlsV) first isolated from pteropodid bat urine sampled in Alstonville, New South Wales, Australia [49] (TaxoProp 2019.025M.A.v2.Paramyxoviridae_3gen5sp4rensp).
Genus Pararubulavirus was expanded by one species, Hervey pararubulavirus, for Hervey virus (HerV) first isolated from pteropodid bat urine sampled in Hervey Bay, Queensland, Australia [11, 53]. (TaxoProp 2019.025M.A.v2.Paramyxoviridae_3gen5sp4rensp).
Genus Respirovirus was expanded by one species, Squirrel respirovirus, for giant squirrel virus (GSqV) first isolated from a Sri Lankan giant squirrel (Ratufa macroura (Pennant, 1769)) sampled in a German zoo [35] (TaxoProp 2019.019M.A.v2.1newsp_Squirrel_respirovirus).
Family Rhabdoviridae
Genus Almendravirus was expanded by one species, Menghai almendravirus, for Menghai rhabdovirus (MRV) first isolated from Asian tiger mosquitoes (Aedes albopictus (Skuse, 1894)) collected in Měnghǎi County (勐海县) in Yúnnán Province (云南省), China [113] (TaxoProp 2019.033M.N.v1.Menghai_almendravirus_1sp).
Genus Nucleorhabdovirus was split into three genera, Alphanucleorhabdovirus, Betanucleorhabdovirus, and Gammanucleorhabdovirus (TaxoProp 2019.031M.Ac.v1.Nucleorhabdovirus_splitgen). Established species Eggplant mottled dwarf nucleorhabdovirus, Maize Iranian mosaic nucleorhabdovirus, Maize mosaic nucleorhabdovirus, Potato yellow dwarf nucleorhabdovirus, Rice yellow stunt nucleorhabdovirus, and Taro vein chlorosis nucleorhabdovirus were assigned to genus Alphanucleorhabdovirus and renamed Eggplant mottled dwarf alphanucleorhabdovirus, Maize Iranian mosaic alphanucleorhabdovirus, Maize mosaic alphanucleorhabdovirus, Potato yellow dwarf alphanucleorhabdovirus, Rice yellow stunt alphanucleorhabdovirus, and Taro vein chlorosis alphanucleorhabdovirus, respectively. Established species Datura yellow vein nucleorhabdovirus, Sonchus yellow net nucleorhabdovirus, and Sowthistle yellow vein nucleorhabdovirus were assigned to genus Betanucleorhabdovirus and renamed Datura yellow vein betanucleorhabdovirus, Sonchus yellow net betanucleorhabdovirus, and Sowthistle yellow vein betanucleorhabdovirus, respectively. Established species Maize fine streak nucleorhabdovirus was assigned to genus Gammanucleorhabdovirus and renamed Maize fine streak gammanucleorhabdovirus (TaxoProp 2019.031M.Ac.v1.Nucleorhabdovirus_splitgen).
Three new species were established in genus Alphanucleorhabdovirus:
Morogoro maize-associated alphanucleorhabdovirus for Morogoro maize-associated virus (MMaV) first detected by HTS in maize (Zea mays L.) sampled in Morogoro, Morogoro Region, Tanzania [97];
Physostegia chlorotic mottle alphanucleorhabdovirus for Physostegia chlorotic mottle virus (PhCMoV) first isolated from lionhearts (Physostegia sp.) in Austria [76]; and
Wheat yellow striate alphanucleorhabdovirus for wheat yellow striate virus (WYSV) first isolated from common wheat (Triticum aestivum L.) sampled in Hánchéng (韩城), Shǎnxī/Shaanxi Province (陕西省), China [61] (TaxoProp 2019.031M.Ac.v1.Nucleorhabdovirus_splitgen).
Three new species were established in genus Betanucleorhabdovirus:
Alfalfa betanucleorhabdovirus for alfalfa-associated nucleorhabdovirus (AaNV) first discovered by HTS in alfalfa (Medicago sativa L.) sampled in Stadl-Paura, Upper Austria (Oberösterreich), Austria [36];
Blackcurrant betanucleorhabdovirus for blackcurrant-associated rhabdovirus (BCaRV) first discovered by HTS in blackcurrant (Ribes nigrum L.) sampled in Russia [138]; and
Trefoil betanucleorhabdovirus for birds-foot trefoil-associated virus (BFTV) first discovered by HTS in Bird’s-foot trefoil (Lotus corniculatus L.) sampled in the Qínlǐng Mountains (秦岭山), Shǎnxī/Shaanxi Province (陕西省), China [26, 131] (TaxoProp 2019.031M.Ac.v1.Nucleorhabdovirus_splitgen).
One new genus, Arurhavirus, was established to include four species:
Aruac arurhavirus for Aruac virus (ARUV) first isolated from mosquitoes (Trichoprosopon theobaldi Lane and Cerqueira, 1942) collected in Melaju Forest, Trinidad, Trinidad and Tobago [110, 126];
Inhangapi arurhavirus for Inhangapi virus (INHV) first isolated from sandflies (Lutzomyia flaviscutellata (Mangabeira, 1942)) collected in Catu Forest, Belém, Pará State, Brazil [3, 126];
Santabarbara arurhavirus for Santa Barbara virus (SBAV) first in mice sampled in Santa Bárbara do Pará, Pará State, Brazil [unpublished]; and
Xiburema arurhavirus for Xiburema virus (XIBV) first isolated from mosquitoes (Sabethes intermedius (Lutz, 1904)) sampled in Sena Madureira, Acre State, Brazil [51, 132] (2019.006M.A.v1.Arurhavirus_1gen4sp).
One new genus, Barhavirus, was established to include two new species:
Bahia barhavirus for Bahia Grande virus (BGV) first isolated from mosquitoes (Aedes, Culex, Anopheles, Psorophora spp.) collected in Texas, Louisiana, New Mexico, and North Dakota, USA [52, 126]; and also for Harlingen virus (HARV) first isolates from a isolated from salt marsh mosquitoes (Culex salinarius Coquillett, 1904) sampled in Harlingen, Texas, USA [126].
Muir barhavirus for Muir Springs virus (MSV) first isolated from mosquitoes (Aedes sp.) collected in Fort Morgan, Colorado, USA [52, 126] (TaxoProp 2019.012M.A.v1.Rhabdoviridae_5gen8sp1reasp).
One new genus, Lostrhavirus, was established to include new species Lonestar zarhavirus [sic] for lone star tick rhabdovirus (LSTRV) first detected by HTS in lone star ticks (Amblyomma americanum (Linnaeus, 1758)) collected in the USA [unpublished] (TaxoProp 2019.012M.A.v1.Rhabdoviridae_5gen8sp1reasp).
One new genus, Mousrhavirus, was established to include the previously established species Moussa virus (now renamed Moussa mousrhavirus) (TaxoProp 2019.012M.A.v1.Rhabdoviridae_5gen8sp1reasp).
One new genus, Ohlsrhavirus, was established to include five new species:
Culex ohlsrhavirus for Culex rhabdo-like virus (CRLV) first discovered by HTS in southern house mosquitoes (Culex quinquefasciatus Say, 1823) collected near Perth, Western Australia, Australia [107];
Northcreek ohlsrhavirus for North Creek virus (NORCV) first discovered by HTS in mosquitoes (Culex sitiens Wiedemann, 1828) collected in Ballina, New South Wales, Australia [23];
Ohlsdorf ohlsrhavirus for Ohlsdorf virus (OHLDV) first discovered by HTS in mosquitoes (Ochlerotatus cantans (Meigen, 1818)) collected in Hamburg, Germany [105];
Riverside ohlsrhavirus for riverside virus (RISV) first discovered by HTS in mosquitoes (Ochlerotatus sp.) collected in Gemenc, Gyékényes, and Drávaszabolcs, Hungary [98]; and
Tongilchon ohlsrhavirus for Tongilchon virus 1 (TCHV-1) first detected in mosquitoes (Culex bitaeniorhynchus Giles, 1901) collected in Tongil-chon (통일촌), Gyeonggi Province (경기도), South Korea [39] (TaxoProp 2019.032M.N.v1.Ohlsrhavirus_1gen5sp).
One new genus, Sawgrhavirus, was established to include four new species:
Connecticut sawgrhavirus for Connecticut virus (CNTV) first isolated from ticks (Ixodes dentatus Marx, 1899) taken from an eastern cottontail (Sylvilagus floridanus (J. A. Allen, 1890)) captured in Lyme, Connecticut, USA [67, 126];
Island sawgrhavirus for Long Island tick rhabdovirus (LITRV) first detected by HTS in lone star ticks (Amblyomma americanum (Linnaeus, 1758)) collected on Long Island, New York, USA [119];
Minto sawgrhavirus for New Minto virus (NMV) first isolated from rabbit ticks (Haemaphysalis leporispalustris Packard, 1869) sampled in New Minto, Alaska, USA [99, 126]; and
Sawgrass sawgrhavirus for Sawgrass virus (SAWV) isolated from American dog ticks (Dermacentor variabilis (Say, 1821)) sampled at Sawgrass Lake, Tampa Bay, Florida, USA [102, 126] (TaxoProp 2019.012M.A.v1.Rhabdoviridae_5gen8sp1reasp).
One new genus, Sunrhavirus, was established to accommodate six novel species:
Garba sunrhavirus for Garba virus (GARV) first isolated from a malachite kingfisher (Corythornis cristatus (Pallas, 1764)) trapped in Bangui, Central African Republic [51, 126];
Harrison sunrhavirus for Harrison Dam virus (HARDV) first isolated from common banded mosquitoes (Culex annulirostris Skuse, 1889) collected at Beatrice Hill, Northern Territory, Australia [71];
Kwatta sunrhavirus for Kwatta virus (KWAV) first isolated from mosquitoes (Culex sp.) collected near Paramaribo, Suriname [25, 126];
Oakvale sunrhavirus for Oak Vale virus (OVV) first isolated from mosquitoes (Culex edwardsi Barraud, 1923) sampled in Peachester, Queensland, Australia [77, 96];
Sunguru sunrhavirus for Sunguru virus (SUNV) first isolated from a domestic chicken (Gallus gallus domesticus (Linnaeus, 1758)) in Arua District, Northern Region, Uganda [58]; and
Walkabout sunrhavirus for Walkabout Creek virus (WACV) first isolated from biting midges (Culicoides austropalpalis Lee and Reye, 1955) collected near Samford, Queensland, Australia [71] (2019.004M.A.v2.Sunrhavirus).
One new genus, Zarhavirus, was created for one new species, Zahedan zarhavirus, for Zahedan rhabdovirus (ZARV) first isolated from ticks (Hyalomma anatolicum anatolicum (Koch, 1844)) collected in Zâhedân  , Sistan and Baluchestan Province
, Sistan and Baluchestan Province  , Iran [28] (TaxoProp 2019.012M.A.v1.Rhabdoviridae_5gen8sp1reasp).
, Iran [28] (TaxoProp 2019.012M.A.v1.Rhabdoviridae_5gen8sp1reasp).
One new species, Taiwan bat lyssavirus, was added to genus Lyssavirus for Taiwan bat lyssavirus (TWBLV) first isolated from a Japanese pipistrelle (Pipistrellus abramus (Temminck, 1838)) sampled in Taiwan [45] (TaxoProp 2019.001M.A.v1.Lyssavirus).
Genus Cytorhabdovirus was expanded by 12 species:
Cabbage cytorhabdovirus for cabbage cytorhabdovirus 1 (CCyV-1) first discovered by HTS in cabbage (Brassica oleracea L.) sampled in the UK [89];
Maize-associated cytorhabdovirus for maize-associated cytorhabdovirus (MaCV) first discovered by HTS in maize (Zea mays L.) collected in Lima, Peru [133];
Maize yellow striate cytorhabdovirus for maize yellow striate virus (MYSV) first discovered by HTS in maize (Zea mays L.) and common wheat (Triticum aestivum L.) collected in Sinsacate, Córdoba Province, Argentina [70];
Papaya cytorhabdovirus for papaya virus E (PpVE) first discovered by HTS in papaya (Carica papaya L.) sampled in Los Ríos Province, Ecuador [73];
Persimmon cytorhabdovirus for persimmon virus A (PeVA) first discovered by HTS in Japanese persimmon (Diospyros kaki L.f.) sampled in Japan [47];
Raspberry vein chlorosis cytorhabdovirus for raspberry vein chlorosis virus (RVCV) first discovered by HTS in red raspberries (Rubus idaeus L.) sampled in Dundee, Scotland, UK [50];
Rice stripe mosaic cytorhabdovirus for rice stripe mosaic virus (RSMV) first discovered by HTS in rice (Oryza sativa L.) sampled in Luódìng (罗定), Guǎngdōng Province (广东省), China [147];
Tomato yellow mottle-associated cytorhabdovirus for tomato yellow mottle-associated virus (TYMaV) first discovered by HTS in tomato (Solanum lycopersicum L.) sampled in Chóngqìng (重庆), China [141];
Wuhan 4 insect cytorhabdovirus for Wuhan insect virus 4 (WuIV-4) first discovered by HTS in mealy plum aphids (Hyalopterus pruni (Geoffroy, 1762)) sampled in Wǔhàn (武 汉), Húběi Province (湖北省), China [59];
Wuhan 5 insect cytorhabdovirus for Wuhan insect virus 5 (WuIV-5) first discovered by HTS in mealy plum aphids (Hyalopterus pruni (Geoffroy, 1762)) sampled in Wǔhàn (武 汉), Húběi Province (湖北省), China [59];
Wuhan 6 insect cytorhabdovirus for Wuhan insect virus 6 (WuIV-6) first discovered by HTS in mealy plum aphids (Hyalopterus pruni (Geoffroy, 1762)) sampled in Wǔhàn (武 汉), Húběi Province (湖北省), China [59]; and
Yerba mate chlorosis-associated cytorhabdovirus for yerba mate chlorosis-associated virus (YmCaV) [12] first discovered by HTS in yerba mate (Ilex paraguariensis A. St.-Hil.) sampled in Cerro Azul, Misiones Province, Argentina (TaxoProps 2019.002M.A.v3.Cytorhabdovirus and 2019.030M.A.v1.Cytorhabovirus_12newsp).
One new species, Holmes hapavirus, was added to genus Hapavirus for Holmes Jungle virus (HOJV) first isolated from common banded mosquitoes (Culex annulirostris Skuse, 1889) collected near Darwin, Northern Territory, Australia [38] (2019.003M.A.v3.Hapavirus).
Three new species were added to genus Sripuvirus:
Charleville sripuvirus for Charleville virus (CHVV) first isolated from sandflies (Phlebotomus sp.) collected in Charleville, Queensland, Australia [29, 123];
Cuiaba sripuvirus for Cuiaba virus (CUIV) isolated from a cane toad (Rhinella marina (Linnaeus, 1758)) captured in Pará State, Brazil [51, 123]; and
Hainan sripuvirus for Hainan black-spectacled toad rhabdovirus (HnBSTRV) first detected by HTS in an Asian common toad (Duttaphrynus melanostictus (Schneider, 1799)) sampled in Hǎinán Province (海南省), China [108] (TaxoProp 2019.013M.A.v1.Sripuvirus_3newsp).
Taxonomic changes within order Muvirales (Haploviricotina: Chunqiuviricetes)
No changes were made.
Taxonomic changes within order Serpentovirales (Haploviricotina: Milneviricetes)
No changes were made.
Taxonomic changes within order Articulavirales (Polyploviricotina: Insthoviricetes)
No changes were made.
Taxonomic changes within order Bunyavirales (Polyploviricotina: Ellioviricetes)
Family Arenaviridae
Genus Hartmanivirus was expanded by three species: Muikkunen hartmanivirus for Dante Muikkunen virus 1 (DaMV-1), Schoolhouse hartmanivirus for old schoolhouse viruses 1 and 2 (OScV-1/2), and Zurich hartmanivirus for veterinary pathology Zurich viruses 1 and 2 (VPZV-1/2), all first detected by HTS in captive boid snakes [44] (TaxoProp 2019.008M.A.v2.Hartmanivirus_3new sp).
Genus Mammarenavirus was expanded by four species:
Alxa mammarenavirus for RtDs-AreV/IM2014 virus (here renamed Alxa virus [ALXV]) (TaxoProp 2019.020M.A.v2.1newsp_Alxa_mammarenavirus) first discovered by HTS in a Northern three-toed jerboa (Dipus sagitta (Pallas, 1773)) sampled in Alxa Left Banner (阿拉善左旗), Inner Mongolia Autonomous Region (内蒙古自治区), China [139, 140];
Chevrier mammarenavirus for Lìjiāng virus (LIJV) first discovered by HTS in a Chevrier’s field mouse (Apodemus chevrieri (Milne-Edwards, 1868)) sampled around Lìjiāng (丽江), Yúnnán Province (云南省), China [unpublished] (TaxoProp 2019.009M.A.v2.Mammarenavirus_sp_LIJV);
Planalto mammarenavirus for Aporé virus (APOV) first discovered by HTS in a Mato Grosso colilargo (Oligoryzomys mattogrossae (J. A. Allen, 1916)) sampled in Cassilândia, Mato Grosso do Sul State, Brazil (TaxoProp 2019.010M.A.v1.Mammarenavirus_sp_APOV) [34]; and
Xapuri mammarenavirus for Xapuri virus (XAPV) first discovered by HTS in a Musser’s neacomys (Neacomys musseri Patton, da Silva, and Malcolm, 2000) sampled in Xapuri, Acre State, Brazil [33] (TaxoProp 2019.005M.A.v1.Mammarenavirus_sp_XAPV).
Family Fimoviridae
Genus Emaravirus was expanded by two species: Blackberry leaf mottle associated emaravirus for blackberry leaf mottle-associated virus (BLMaV) first discovered in blackberries (Rubus spp.) collected in various US states (TaxoProp 2019.010P.A.v1.Emaravirus_1sp) [41] and Pistacia emaravirus B for pistacia virus B (PiVB) discovered by HTS in pistachios (Pistacia vera L.) sampled in Turkey [18] (TaxoProp 2019.011P.A.v1.Emaravirus_1sp).
Family Hantaviridae
Genus Loanvirus was expanded by one species, Brno loanvirus, for Brno virus (BRNV) first discovered by HTS in a noctule (Nyctalus noctula (Schreber, 1774)) sampled in Brno, South Moravia Region (Jihomoravský kraj), Czech Republic [112] (TaxoProp 2019.017M.A.v3.1newsp_Brno_virus).
Family Peribunyaviridae
Genus Pacuvirus was expanded by two species: new species Caimito pacuvirus for Caimito virus (CAIV) first isolated from sandflies (Nyssomyia ylephiletor (Fairchild and Hertig, 1952)) sampled in El Aguacate, Panamá Province, Panama [46, 116] (TaxoProp 2019.022M.A.v2.2sp_Pacuvirus) and Chilibre pacuvirus (the former Chilibre phlebovirus, renamed and moved from genus Phlebovirus) (TaxoProps 2019.022M.A.v2.2sp_Pacuvirus and 2019.026M.A.v1.Phenuiviridae_4gen79sp).
Family Phasmaviridae
The previously established genus Inshuvirus and its included species Insect inshuvirus were both abolished due to insufficient member virus information (TaxoProp 2019.028M.A.v2.Phasmaviridae_1newsp_abol1gen3sp).
New species Anopheles orthophasmavirus was included in genus Orthophasmavirus for Anopheles triannulatus orthophasmavirus (AtOPV) first discovered by HTS in mosquitoes (Anopheles triannulatus (Neiva and Pinto, 1922)) sampled in Santa Bárbara Farm, Amapá State, Brazil [103]. Two species, Nome phantom orthophasmavirus and Seattle orthophasmavirus, were abolished (TaxoProp 2019.028M.A.v2.Phasmaviridae_1newsp_abol1gen3sp).
Family Phenuiviridae
The previously unassigned genus Coguvirus was included in family Phenuiviridae (TaxoProp 2019.026M.A.v1.Phenuiviridae_4gen79sp). One new species, Coguvirus eburi, was created in the genus for citrus virus A (CiVA) first discovered by HTS in a sweet orange tree in Italy [78] (2019.004P.A.v1.Coguvirus_1sp).
Genus Banyangvirus and included species Huaiyangshan banyangvirus, Guertu banyangvirus, and Heartland banyangvirus were renamed Bandavirus, Dabie bandavirus, Guertu bandavirus, and Heartland bandavirus, respectively (TaxoProps 2019.015M.A.v1.Bandavirus and 2019.026M.A.v1.Phenuiviridae_4gen79sp). Four new bandavirus species were added to the genus:
Bhanja bandavirus for Bhanja virus (BHAV) first isolated from flat-inner-spurred haemaphysalids (Haemaphysalis intermedia Warburton and Nuttall, 1909) sampled in Orissa State, India [27, 104];
Hunter Island bandavirus for Hunter Island virus (HUIV) first isolated from ticks (Ixodes eudyptidis Maskell, 1885) sampled on Albatross Island, Tasmania, Australia [130];
Kismaayo bandavirus for Kismaayo virus (KISV; name corrected from the previously circulating “Kismayo virus” and “Kisemayo virus”) first isolated from yellow back ticks (Rhipicephalus pulchellus (Gerstäcker, 1873)) sampled in Kismaayo, Lower Juba (Jubbada Hoose) Region, Somalia [149]; and
Lone Star bandavirus [sic] for lone star virus (LSV) first isolated from lone star ticks (Amblyomma americanum (Linnaeus, 1758)) sampled in Kentucky, USA [54, 114] (TaxoProp 2019.026M.A.v1.Phenuiviridae_4gen79sp).
Genus Entovirus was created for one new species, Entoleuca entovirus, for Entoleuca phenui-like virus 1 (EnPLV-1) first discovered by HTS in Entoleuca sp. fungi sampled in Málaga Province, Spain [124] (TaxoProp 2019.026M.A.v1.Phenuiviridae_4gen79sp).
Genus Kabutovirus and included species Kabuto mountain kabutovirus and Huangpi kabutovirus were renamed Uukuvirus, Kabuto mountain uukuvirus, and Huangpi uukuvirus, respectively. The established species Uukuniemi phlebovirus was moved into genus Uukuvirus and renamed Uukuniemi uukuvirus. 14 new species were established in genus Uukuvirus:
American dog uukuvirus for American dog tick virus (ADAV) first detected by HTS in American dog ticks (Dermacentor variabilis (Say, 1821)) sampled in Heckscher State Park, New York, USA [120];
Dabieshan uukuvirus for Dàbiéshān tick virus (DbsTV) first discovered by HTS in Asian longhorned ticks (Haemaphysalis longicornis Neumann, 1901) collected in the Dàbié Mountains (大別山), China [59];
Grand Arbaud uukuvirus for Grand Arbaud virus (GAV) first isolated from ticks (Argas reflexus (Fabricius, 1794)) sampled in Bouches-du-Rhône Department, France [40, 85];
Kaisodi uukuvirus for Kaisodi virus (KASDV) first isolated from hard-bodied ticks (Haemaphysalis spinigera Neumann, 1897) sampled in Mysore State, India [14, 88, 144];
Lihan uukuvirus for Lǐhán tick virus (LITV) first discovered by HTS in Asian blue ticks (Rhipicephalus microplus (Canestrini, 1888)) sampled in Lǐhán (李韩), Húběi Province (湖北省), China [59];
Murre uukuvirus for murre virus (MURV) first isolated from common murres (Uria aalge (Pontoppidan, 1763)) sampled in Alaska, USA [85];
Pacific coast uukuvirus for Pacific coast tick virus (PACTV) first discovered by HTS in Pacific coast ticks (Dermacentor occidentalis Marx, 1892) sampled in Mendocino County, California, USA [17];
Precarious Point uukuvirus for Precarious Point virus (PPV) first isolated from seabird ticks (Ixodes uriae White, 1852) sampled on Macquarie Island, Tasmania, Australia [85, 111];
Rukutama uukuvirus for Rukutama virus (RUKV) first isolated from seabird ticks (Ixodes uriae White, 1852) sampled on Tûlenij/Tyuleny Island (Остров Тюлений), Sakhalin Oblast (Сахалинская область), Russia [63, 150];
Schmidt uukuvirus for EgAn 1825–61 virus (here renamed Nile warbler virus [NIWV]) first isolated from a willow warbler (Phylloscopus trochilus (Linnaeus, 1758)) sampled in Nile Delta, Egypt [85];
Silverwater uukuvirus for Silverwater virus (SILV) first isolated from rabbit ticks (Haemaphysalis leporispalustris Packard, 1869) sampled near Powassan, Ontario, Canada [69, 72];
Tacheng uukuvirus for Tǎchéng tick virus 2 (TcTV-2) first discovered by HTS in ticks (Dermacentor marginatus Sulzer, 1776) sampled in China [59];
Yongjia uukuvirus for Yǒngjiā tick virus 1 (YjTV-1) first discovered by HTS in East Asian mountain haemaphysalids (Haemaphysalis hystricis Supino, 1897) in China [59]; and
Zaliv Terpeniya uukuvirus for Zaliv Terpeniya virus (ZTV) first isolated from seabird ticks (Ixodes uriae White, 1852) sampled on Tyuleny Island (Тюлений oстров) in the Gulf of Patience (Залив Терпения), Sakhalin Oblast (Сахалинская область) and Commander Islands (Командорские острова), Kamchatka Krai (Камчатский край), RSFSR, USSR [62, 151] (TaxoProp 2019.026M.A.v1.Phenuiviridae_4gen79sp).
Genus Ixovirus was established for the three new species:
Blackleg ixovirus for blacklegged tick phlebovirus 1, here renamed blacklegged tick virus 1 (BLTV-1), first discovered by HTS in deer ticks (Ixodes scapularis Say, 1821) sampled in Heckscher State Park, New York, USA [120];
Norway ixovirus for Norway phlebovirus 1, here renamed Fairhair virus (FHAV), first discovered by HTS in castor bean ticks (Ixodes ricinus (Linnaeus, 1758)) sampled in Norway [91]; and
Scapularis ixovirus for blacklegged tick phlebovirus 3, here renamed blacklegged tick virus 3 (BLTV-3), first discovered by HTS in deer ticks (Ixodes scapularis Say, 1821) sampled in Heckscher State Park, New York, USA [120] (TaxoProp 2019.026M.A.v1.Phenuiviridae_4gen79sp).
Genus Lentinuvirus was created for one new species, Lentinula lentinuvirus, for Lentinula edodes negative-strand RNA virus 2 (LeNSRV-2) first discovered by HTS in shiitake (Lentinula edodes (Berk.) Pegler (1976)) sampled in Japan [60] (TaxoProp 2019.026M.A.v1.Phenuiviridae_4gen79sp).
In genus Phlebovirus, established species Sandfly fever Naples phlebovirus was renamed Naples phlebovirus. The genus was expanded by 53 new species (TaxoProp 2019.026M.A.v1.Phenuiviridae_4gen79sp):
Adana phlebovirus for Adana virus (ADAV) first isolated from Phlebotomus spp. sandflies sampled in Adana, Adana Province (Adana ili), Turkey [4];
Aguacate phlebovirus for Aguacate virus (AGUV) first isolated from Lutzomyia spp. sandflies sampled in El Aguacate, Panamá Province, Panama [82, 116];
Alcube phlebovirus for Alcube virus (ACBV) first isolated from sandflies (Phlebotomus perniciosus Newstead, 1911) sampled around Arrábida, Portugal [10];
Alenquer phlebovirus for Alenquer virus (ALEV) first isolated from a human in Ramal das Pias, Alenquer, Pará State, Brazil [83, 122];
Ambe phlebovirus for Ambe virus (ABEV) first isolated from psychodid sandflies sampled near Altamira, Pará State, Brazil [80, 118];
Anhanga phlebovirus for Anhangá virus (ANHV) first isolated from a Linnaeus’s two-toed sloth (Choloepus didactylus (Linnaeus, 1758)) sampled in Castanhal Forest, Pará State, Brazil [80];
Arumowot phlebovirus for Arumowot virus (AMTV) first isolated from mosquitoes (Culex antennatus (Becker, 1903)) sampled in Sudan [13, 84];
Buenaventura phlebovirus for Buenaventura virus (BUEV) first isolated in 1984 from Lutzomyia sp. sandflies sampled in Rio Raposo, Valle del Cauca Department, Colombia [87, 116];
Cacao phlebovirus for Cacao virus (CACV) first isolated from sandflies (Nyssomyia trapidoi (Fairchild and Hertig, 1952)) sampled in El Aguacate, Panamá Province, Panama [87, 116];
Campana phlebovirus for Campana virus (CMAV) first isolated from phlebotomine sandflies sampled in El Aguacate, Panamá Province, Panama [87];
Chagres phlebovirus for Chagres virus (CHGV) first isolated from a human sampled at Fort Sherman, Canal Zone/Cólon Province, Panama [90];
Cocle phlebovirus for Coclé virus (CCLV) first isolated from a human sampled in Penonomé, Coclé Province, Panama [87];
Dashli phlebovirus for Dāshlī virus (DASV) first isolated from Sergentomyia sp. sandflies sampled in Dāshlīborun
 , Golestān Province
, Golestān Province  , Iran [6];
, Iran [6];Durania phlebovirus for Durania virus (DRNV) first isolated from sandflies sampled in 1986 near Durania, North Santander Department, Colombia [82, 118];
Echarate phlebovirus for Echarate virus (ECHV) first isolated from a human sampled in Cusco, Peru [83];
Gabek phlebovirus for Gabek Forest virus (GFV) first isolated from a northeast African spiny mouse (Acomys cahirinus (É. Geoffroy, 1803)) sampled in Gabek Forest, near Paloich, Sudan [86];
Gordil phlebovirus for Gordil virus (GORV) first isolated from a typical lemniscomys (Lemniscomys striatus (Linnaeus, 1758)) sampled in Gordil, Vakaga Prefecture, Central African Republic [86];
Icoaraci phlebovirus for Icoaraci virus (ICOV) first isolated from from an unidentified forest rat sampled in Belém, Pará State, Brazil [19, 142];
Itaituba phlebovirus for Itaituba virus (ITAV) first isolated from a common opossum (Didelphis marsupialis Linnaeus, 1758) trapped at the Tapacurazinho stream, Itaituba, Pará State, Brazil [83, 122];
Itaporanga phlebovirus for Itaporanga virus (ITPV) first isolated from a sentinel Swiss mouse collected in Itaporanga, São Paulo State, Brazil [46, 121];
Ixcanal phlebovirus for Ixcanal virus (IXCV) first isolated from Lutzomyia sp. sandflies sampled in Aldea Ixcanal and Aldea Puerta, El Progreso Department, Guatemala [82, 118];
Karimabad phlebovirus for Karimabad virus (KARV) first isolated from Phlebotomus sp. sandflies in Karīmābād
 , Khūzestān Province
, Khūzestān Province 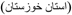 , Iran [86];
, Iran [86];La Gloria phlebovirus for La Gloria virus (LAGV) first discovered by HTS in phlebotomine sandflies sampled near La Gloria village, Panama Canal area, central Panama [68];
Lara phlebovirus for GGP-2011a virus (here renamed Rio Claro virus [RICV]) first isolated from a sentinel hamster sampled in Venezuela [unpublished];
Leticia phlebovirus for Leticia virus (LETV) first isolated from sandflies sampled in Leticia, Amazonas Department, Colombia [87];
Maldonado phlebovirus for Maldonado virus (MLOV) first isolated from a human sampled in Puerto Maldonado, Madre de Dios Region, Peru [83];
Massilia phlebovirus for Massilia virus (MASV) first isolated from sandflies (Phlebotomus perniciosus Newstead, 1911) sampled in Marseille and Nice, Provence-Alpes-Côte d’Azur, France [20, 86];
Medjerda phlebovirus for Medjerda Valley virus (MVV) first isolated from phlebotomine sandflies sampled at an archaeological site in Bizerte Governorate, Tunisia [15];
Mona Grita phlebovirus for Mona Grita virus (MOGV) first discovered by HTS in sandflies (Nyssomyia trapidoi (Fairchild and Hertig, 1952)) sampled on Isla Mona Grita, Panama Canal, central Panama [68];
Munguba phlebovirus for Munguba virus (MUNV) first isolated from sandflies (Nyssomyia umbratilis (Ward and Fraiha, 1977)) sampled in Monte Dourado, Pará State, Brazil [80, 122];
Nique phlebovirus for Nique virus (NIQV) first isolated from sandflies (Lutzomyia panamensis (Shannon, 1926)) sampled in Cerro Nique, Darién Province, Panama [83, 117];
Ntepes phlebovirus for Ntepes virus (NTPV) first isolated from Sergentomyia sp. sandflies sampled near Ntepes village, Marigat District, Baringo County, Kenya [115];
Odrenisrou phlebovirus for Odrénisrou virus (ODRV) first isolated from mosquitoes (Culex albiventris Edwards, 1922) collected in the forest of Taï National Park, Côte d’Ivoire [84];
Oriximina phlebovirus for Oriximiná virus (ORXV) first isolated from Lutzomyia sp. sandflies sampled in Saracazinho, Pará State, Brazil [83, 122];
Pena Blanca phlebovirus for Peña Blanca virus (PEBV) first discovered by HTS in sandflies sampled on Peña Blanca peninsula, Panama Canal, central Panama [68];
Punique phlebovirus for Punique virus (PUNV) first isolated from sandflies (Phlebotomus perniciosus Newstead, 1911 and Phlebotomus longicuspis Nitzulescu, 1930) sampled in Tunis, Tunisia [86];
Rio Grande phlebovirus for Rio Grande virus (RGV) first isolated from a Southern Plains woodrat (Neotoma micropus Baird, 1855) sampled in Texas, USA [46];
Saint Floris phlebovirus for Saint-Floris virus (SAFV) first isolated from a gerbil sampled in Gordil, Vakaga Prefecture, Central African Republic [86];
Salanga phlebovirus for Salanga virus (SLGV) first isolated from a Hinde’s aethomys (Aethomys hindei (Thomas, 1902)) collected in Salanga, Ombella-M’Poko Prefecture, Central African Republic [51, 148];
Salobo phlabovirus [sic] for Salobo virus (SLBOV) first isolated from a Guyenne spiny-rat (Proechimys guyannensis (E. Geoffroy, 1803)) in Pará State, Brazil [142];
Sicilian phlebovirus for sandfly fever Sicilian virus (SFSV) first isolated from a human sampled in Palermo Province, Sicily Region, Italy [101, 137];
Tapara phlebovirus for Tapará virus (TPRV) first isolated from phlebotomine sandflies in Altamira, Pará State, Brazil [80];
Tehran phlebovirus for Tehran virus (THEV) first isolated from sandflies (Phlebotomus papatasi (Scopoli, 1786)) sampled in Tehran, Iran [86];
Tico phebovirus [sic] for Tico virus (TICV) discovered by HTS in sandflies sampled in Panama Canal area, central Panama [68];
Toros phlebovirus for Toros virus (TORV) first discovered by HTS in sandflies sampled in Damyeri, Adana Province (Adana ili), Turkey [5];
Toscana phlebovirus for Toscana virus (TOSV) first isolated from sandflies (Phlebotomus perniciosus Newstead, 1911) in Toscany, Italy [86, 125];
Tres Almendras virus for Tres Almendra virus (TRAV) first discovered by HTS in sandflies (Psychodopygus panamensis (Shannon, 1926)) sampled on Tres Almendras Islands, Panama Canal area, central Panama [68];
Turuna phlebovirus for Turuna virus (TUAV) first isolated from Lutzomyia sp. sandflies sampled in Cachoeira Porteira, Pará State, Brazil [83, 122];
Uriurana phlebovirus for Uriurana virus (URIV) first isolated from phlebotomine sandflies in Tucuruí, Pará State, Brazil [80];
Urucuri phlebovirus for Urucuri virus (URUV) first isolated from a Guyenne spiny-rat (Proechimys guyannensis (E. Geoffroy, 1803)) in Utinga Forest, Belém, Pará State, Brazil [80, 122];
Viola phlebovirus for viola virus (VIOV) first discovered by HTS in sandflies (Lutzomyia longipalpis (Lutz and Neiva, 1912)) sampled in Pirizal, Mato Grosso State, Brazil [24]; and
Zerdali phlebovirus for Zerdali virus (ZERV) first discovered by HTS in sandflies sampled in Zerdali, Adana Province (Adana ili), Turkey [5].
Genus Rubodvirus was created for the two new species Apple rubodvirus 1 and 2 to accommodate apple rubbery wood viruses 1 and 2 (ARWV-1/2), respectively, first discovered using HTS in apple trees (Malus sp.) sampled in Germany and USA [100] (TaxoProp 2019.026M.A.v1.Phenuiviridae_4gen79sp).
One new species, Melon tenuivirus, was added to genus Tenuivirus for melon chlorotic spot virus (MeCSV) first isolated from muskmelon (Cucumis melo L.) sampled in Provence-Alpes-Côte d’Azur Region, France [57].
Genus Wubeivirus was abolished and its two species, Fly wubeivirus and Dipteran wubeivirus, were moved into genus Phasivirus and renamed Fly phasivirus and Dipteran phasivirus, respectively (TaxoProp 2019.026M.A.v1.Phenuiviridae_4gen79sp).
Family Tospoviridae
The overlooked adjustment of 12 tospovirid species names to correct non-Latinized binomials was implemented (TaxoProp 2019.016M.A.v1.Corrections).
Eight new species were created in genus Orthotospovirus:
Alstroemeria necrotic streak orthotospovirus for Alstroemeria necrotic streak virus (ANSV) first isolated from ornamental crops (Alstroemeria sp.) sampled in Colombia [42];
Alstroemeria yellow spot orthotospovirus for Alstroemeria yellow spot virus (AYSV) first isolated from ornamental crops (Alstroemeria sp.) imported to and sampled in the Netherlands [43];
Groundnut chlorotic fan spot orthotospovirus for groundnut chlorotic fan-spot virus (GCFSV) first isolated from peanut (Arachis hypogaea L.) sampled in Taiwan [21];
Hippeastrum chlorotic ringspot orthotospovirus for Hippeastrum chlorotic spot virus (HCRV) first isolated from amaryllis (Hippeastrum sp.) and spider lily (Hymenocallis littoralis (Jacq.) Salisb.) sampled in southwestern China [31, 143];
Mulberry vein banding associated orthotospovirus for mulberry vein banding-associated virus (MVBaV) discovered first by HTS in mulberry (Morus alba L.) sampled in Guǎngxī Zhuàng Autonomous Region (广西壮族自治区), China in 2011 [74, 75];
Pepper chlorotic spot orthotospovirus for pepper chlorotic spot virus (PCSV) first isolated from sweet pepper (Capsicum annuum L.) in Taiwan [22];
Tomato yellow ring orthotospovirus for tomato yellow ring virus (TYRV) first isolated from tomato (Solanum lycopersicum L.) in Iran [134]; and
Tomato zonate spot orthotospovirus for tomato zonate spot virus (TZSV) first isolated from tomato (Solanum lycopersicum L.) and chili pepper (Capsicum annuum L.) sampled in Yúnnán Province (云南省), China [30] (TaxoProp 2019.006P.A.v1.Orthotospovirus_8sp).
SUMMARY
A summary of the current, ICTV-accepted taxonomy of the phylum Negarnaviricota is presented in Table 1 (Goujianvirales), Table 2 (Jingchuvirales), Table 3 (Mononegavirales), Table 4 (Muvirales), Table 5 (Serpentovirales), Table 6 (Articulavirales), and Table 7 (Bunyavirales).
Table 1.
ICTV-accepted taxonomy of the order Goujianvirales (Negarnaviricota: Haploviricotina: Yunchangviricetes) as of March 2020.
| Genus | Species¶ | Virus (Abbreviation)& |
|---|---|---|
| Family Yueviridae | ||
| Yuyuevirus | Beihai yuyuevirus* | Běihǎi sesarmid crab virus 3 (BhSCV-3) |
| Shahe yuyuevirus | Shāhé yuèvirus-like virus 1 (ShYLV-1) | |
Note that viruses are real objects that are assigned to concepts that are called taxa. Species, genera, families, and orders are taxa.
type species
Taxon names are always italicized and always begin with a capital letter.
Virus names are not italicized and are not capitalized, except if the name or a name component is a proper noun. This column lists the virus names with their correct (lack of) capitalization. Lists of viruses within a given species are provisional at this point and will likely be amended in the near future.
Table 2.
ICTV-accepted taxonomy of the order Jingchuvirales (Negarnaviricota: Haploviricotina: Monjiviricetes) as of March 2020.
| Genus | Species¶ | Virus (Abbreviation)& |
|---|---|---|
| Family Chuviridae | ||
| Mivirus | Argas mivirus | Tǎchéng tick virus 4 (TcTV-4) |
| Barnacle mivirus* | Běihǎi barnacle virus 9 (BhBV-9) | |
| Beetle mivirus | Húběi coleoptera virus 3 (HbCV-3) | |
| Bole mivirus | Bólè tick virus 3 (BTV-3) | |
| Brunnich mivirus | Wēnlǐng crustacean virus 14 (WlCV-14) | |
| Changping mivirus | Chāngpíng tick virus 2 (CpTV-2) | |
| Charybdis mivirus | Wēnzhōu crab virus 3 (WzCV-3) | |
| Cockroach mivirus | Wǔchāng cockroach virus 3 (WcLFV-3) | |
| Crab mivirus | Wēnzhōu crab virus 2 (WzCV-2) | |
| Crustacean mivirus | Wēnlǐng crustacean virus 13 (WlCV-13) | |
| Dermacentor mivirus | Chāngpíng tick virus 3 (CpTV-3) | |
| Tǎchéng tick virus 5 (TcTV-5) | ||
| Hermit mivirus | Běihǎi hermit crab virus 3 (BhHCV-3) | |
| Hippoboscid mivirus | Wǔhàn louse fly virus 7 (WhLFV-7) | |
| Hubei mivirus | Húběi chuvirus-like virus 1 (HbCLV-1) | |
| Hubei odonate mivirus | Húběi chuvirus-like virus 3 (HbCLV-3) | |
| Imjin mivirus | Imjin River virus 1 (IjRV-1) | |
| Lacewing mivirus | Shuāngào insect virus 5 (SgIV-5) | |
| Lishi mivirus | Líshí spider virus 1 (LsSV-1) | |
| Lonestar mivirus | lone star tick chuvirus 1 (LSTCV-1) | |
| Louse fly mivirus | Wǔhàn louse fly virus 6 (WhLFV-6) | |
| Mosquito mivirus | Wǔhàn mosquito virus 8 (WhMV-8) | |
| Myriapod mivirus | Húběi myriapoda virus 8 (HbMV-8) | |
| Odonate mivirus | Húběi odonate virus 11 (HbOV-11) | |
| Sanxia mivirus | Sānxiá atyid shrimp virus 4 (SxASC-4) | |
| Shayang mivirus | Shāyáng fly virus 1 (SyFV-1) | |
| Suffolk mivirus | Suffolk virus (SFKV) | |
| Taiyuan mivirus | Tàiyuán leafhopper virus (TYLeV) | |
| Wenling mivirus | Wēnlǐng crustacean virus 15 (WlCV-15) | |
| Wuhan mivirus | Wǔhàn tick virus 2 (WhTV-2) | |
| Xinzhou mivirus | Xīnzhōu nematode virus 5 (XzNV-5) | |
Note that viruses are real objects that are assigned to concepts that are called taxa. Species, genera, families, and orders are taxa.
type species
Taxon names are always italicized and always begin with a capital letter.
Virus names are not italicized and are not capitalized, except if the name or a name component is a proper noun. This column lists the virus names with their correct (lack of) capitalization. Lists of viruses within a given species are provisional at this point and will likely be amended in the near future.
Table 3.
ICTV-accepted taxonomy of the order Mononegavirales (Negarnaviricota: Haploviricotina: Monjiviricetes) as of March 2020.
| Genus | Species¶ | Virus (Abbreviation)& |
|---|---|---|
| Family Artoviridae | ||
| Hexartovirus | Barnacle hexartovirus | Běihǎi barnacle virus 8 (BhBV-8) |
| Caligid hexartovirus* | Lepeophtheirus salmonis negative-stranded RNA virus 1 (LsNSRV-1) | |
| Peropuvirus | Beihai peropuvirus | Běihǎi rhabdo-like virus 1 (BhRLV-1) |
| Hubei peropuvirus | Húběi rhabdo-like virus 6 (HbRLV-6) | |
| Odonate peropuvirus | Húběi rhabdo-like virus 8 (HbRLV-8) | |
| Pillworm peropuvirus | Húběi rhabdo-like virus 5 (HbRLV-5) | |
| Pteromalus puparum peropuvirus* | Pteromalus puparum negative-strand RNA virus 1 (PpNSRV-1) | |
| Woodlouse peropuvirus | Běihǎi rhabdo-like virus 2 (BhRLV-2) | |
| Family Bornaviridae | ||
| Carbovirus | Queensland carbovirus* | jungle carpet python virus (JCPV) |
| Southwest carbovirus | southwest carpet python virus (SWCPV) | |
| Cultervirus | Sharpbelly cultervirus* | Wǔhàn sharpbelly bornavirus (WhSBV) |
| Orthobornavirus | Elapid 1 orthobornavirus | Loveridge’s garter snake virus 1 (LGSV-1) |
| Mammalian 1 orthobornavirus* | Borna disease virus 1 (BoDV-1) | |
| Borna disease virus 2 (BoDV-2) | ||
| Mammalian 2 orthobornavirus | variegated squirrel bornavirus 1 (VSBV-1) | |
| Passeriform 1 orthobornavirus | canary bornavirus 1 (CnBV-1) | |
| canary bornavirus 2 (CnBV-2) | ||
| canary bornavirus 3 (CnBV-3) | ||
| Passeriform 2 orthobornavirus | estrildid finch bornavirus 1 (EsBV-1) | |
| Psittaciform 1 orthobornavirus | parrot bornavirus 1 (PaBV-1) | |
| parrot bornavirus 2 (PaBV-2) | ||
| parrot bornavirus 3 (PaBV-3) | ||
| parrot bornavirus 4 (PaBV-4) | ||
| parrot bornavirus 7 (PaBV-7) | ||
| Psittaciform 2 orthobornavirus | parrot bornavirus 5 (PaBV-5) | |
| Waterbird 1 orthobornavirus | aquatic bird bornavirus 1 (ABBV-1) | |
| aquatic bird bornavirus 2 (ABBV-2) | ||
| Family Filoviridae | ||
| Cuevavirus | Lloviu cuevavirus* | Lloviu virus (LLOV) |
| Dianlovirus | Mengla dianlovirus* | Měnglà virus (MLAV) |
| Ebolavirus | Bombali ebolavirus | Bombali virus (BOMV) |
| Bundibugyo ebolavirus | Bundibugyo virus (BDBV) | |
| Reston ebolavirus | Reston virus (RESTV) | |
| Sudan ebolavirus | Sudan virus (SUDV) | |
| Tai Forest ebolavirus | Taï Forest virus (TAFV) | |
| Zaire ebolavirus* | Ebola virus (EBOV) | |
| Marburgvirus | Marburg marburgvirus* | Marburg virus (MARV) |
| Ravn virus (RAVV) | ||
| Striavirus | Xilang striavirus* | Xīlǎng virus (XILV) |
| Thamnovirus | Huangjiao thamnovirus* | Huángjiāo virus (HUJV) |
| Family Lispiviridae | ||
| Arlivirus | Gerrid arlivirus | Sānxiá water strider virus 4 (SxWSV-4) |
| Hubei arlivirus | Húběi rhabdo-like virus 3 (HbRLV-3) | |
| Lishi arlivirus* | Líshí spider virus 2 (LsSV-2) | |
| Odonate arlivirus | Húběi odonate virus 10 (HbOV-10) | |
| Tacheng arlivirus | Tǎchéng tick virus 6 (TcTV-6) | |
| Wuchang arlivirus | Wǔchāng romanomermis nematode virus 2 (WcRNV-2) | |
| Family Mymonaviridae | ||
| Hubramonavirus | Hubei hubramonavirus* | Húběi rhabdo-like virus 4 (HbRLV-4) |
| Lentinula hubramonavirus | Lentinula edodes negative-strand RNA virus 1 (LeNSRV-1) | |
| Sclerotimonavirus | Dadou sclerotimonavirus | soybean leaf-associated negative-stranded RNA virus 3 (SLaNSRV-3) |
| Drop sclerotimonavirus | Sclerotinia sclerotiorum negative-stranded RNA virus 2 (SsNSRV-2) | |
| Sclerotinia sclerotiorum negative-stranded RNA virus 4 (SsNSRV-4) | ||
| Glycine sclerotimonavirus | Fusarium graminearum negative-stranded RNA virus 1 (FgNSRV-1) | |
| soybean leaf-associated negative-stranded RNA virus 1 (SLaNSRV-1) | ||
| Hubei sclerotimonavirus [sic]1 | ||
| Illinois sclerotimonavirus | soybean leaf-associated negative-stranded RNA virus 2 (SLaNSRV-2) | |
| Phyllosphere sclerotimonavirus | soybean leaf-associated negative-stranded RNA virus 4 (SLaNSRV-4) | |
| Sclerotinia sclerotimonavirus* | Sclerotinia sclerotiorum negative-stranded RNA virus 1 (SsNSRV-1) | |
| Sclerotinia sclerotiorum negative-stranded RNA virus 3 (SsNSRV-3) | ||
| Family Nyamiviridae | ||
| Berhavirus | Beihai berhavirus | Běihǎi rhabdo-like virus 4 (BhRLV-4) |
| Echinoderm berhavirus | Běihǎi rhabdo-like virus 5 (BhRLV-5) | |
| Sipunculid berhavirus* | Běihǎi rhabdo-like virus 3 (BhRLV-3) | |
| Crustavirus | Beihai crustavirus | Běihǎi rhabdo-like virus 6 (BhRLV-6) |
| Wenling crustavirus | Wēnlǐng crustacean virus 12 (WlCV-12) | |
| Wenzhou crustavirus* | Wēnzhōu crab virus 1 (WzCV-1) | |
| Nyavirus | Midway nyavirus | Midway virus (MIDWV) |
| Nyamanini nyavirus* | Nyamanini virus (NYMV) | |
| Sierra Nevada nyavirus | Sierra Nevada virus (SNVV) | |
| Orinovirus | Orinoco orinovirus* | Orinoco virus (ONCV) |
| Socyvirus | Soybean cyst nematode socyvirus* | soybean cyst nematode virus 1 (SbCNV-1) |
| Tapwovirus | Tapeworm tapwovirus* | Wēnzhōu tapeworm virus 1 (WzTWV-1) |
| Family Paramyxoviridae | ||
| Subfamily Avulavirinae | ||
| Metaavulavirus | Avian metaavulavirus 2* | avian paramyxovirus 2 (APMV-2) |
| Avian metaavulavirus 5 | avian paramyxovirus 5 (APMV-5) | |
| Avian metaavulavirus 6 | avian paramyxovirus 6 (APMV-6) | |
| Avian metaavulavirus 7 | avian paramyxovirus 7 (APMV-7) | |
| Avian metaavulavirus 8 | avian paramyxovirus 8 (APMV-8) | |
| Avian metaavulavirus 10 | avian paramyxovirus 10 (APMV-10) | |
| Avian metaavulavirus 11 | avian paramyxovirus 11 (APMV-11) | |
| Avian metaavulavirus 14 | avian paramyxovirus 14 (APMV-14) | |
| Avian metaavulavirus 15 | avian paramyxovirus 15 (APMV-15) | |
| Avian metaavulavirus 20 | avian paramyxovirus 20 (APMV-20) | |
| Orthoavulavirus | Avian orthoavulavirus 1* | avian paramyxovirus 1 (APMV-1)2 |
| Avian orthoavulavirus 9 | avian paramyxovirus 9 (APMV-9) | |
| Avian orthoavulavirus 12 | avian paramyxovirus 12 (APMV-12) | |
| Avian orthoavulavirus 13 | avian paramyxovirus 13 (APMV-13) | |
| Avian orthoavulavirus 16 | avian paramyxovirus 16 (APMV-16) | |
| Avian orthoavulavirus 17 | Antarctic penguin virus A (APV-A) | |
| Avian orthoavulavirus 18 | Antarctic penguin virus B (APV-B) | |
| Avian orthoavulavirus 19 | Antarctic penguin virus C (APV-C) | |
| Avian orthoavulavirus 21 | avian paramyxovirus 21 (APMV-21) | |
| Avian orthovulavirus 21 [sic]3 | ||
| Paraavulavirus | Avian paraavulavirus 3* | avian paramyxovirus 3 (APMV-3) |
| Avian paraavulavirus 4 | avian paramyxovirus 4 (APMV-4) | |
| Subfamily Metaparamyxovirinae | ||
| Synodonvirus | Synodus synodonvirus* | Wēnlǐng triplecross lizardfish paramyxovirus (WTLPV) |
| Subfamily Orthoparamyxovirinae | ||
| A quaparamyxovirus | Oncorhynchus aquaparamyxovirus | Pacific salmon paramyxovirus (PSPV) |
| Salmo aquaparamyxovirus* | Atlantic salmon paramyxovirus (AsaPV) | |
| Ferlavirus | Reptilian ferlavirus* | fer-de-lance virus (FDLV) |
| Henipavirus | Cedar henipavirus | Cedar virus (CedV) |
| Ghanaian bat henipavirus | Ghana virus (GhV) | |
| Hendra henipavirus* | Hendra virus (HeV) | |
| Mojiang henipavirus | Mòjiāng virus (MojV) | |
| Nipah henipavirus | Nipah virus (NiV) | |
| Jeilongvirus | Beilong jeilongvirus* | Beilong virus (BeiV) |
| Jun jeilongvirus | J virus (JV) | |
| Lophuromys jeilongvirus 1 | Mount Mabu Lophuromys virus 1 (MMLV-1) | |
| Lophuromys jeilongvirus 2 | Mount Mabu Lophuromys virus 2 (MMLV-2) | |
| Miniopteran jeilongvirus | Shaan virus (ShaV) | |
| Myodes jeilongvirus | Pohorje Myodes paramyxovirus 1 (PMPV-1) | |
| Tailam jeilongvirus | Tailam virus (TaiV) | |
| Morbillivirus | Canine morbillivirus | canine distemper virus (CDV) |
| Cetacean morbillivirus | cetacean morbillivirus (CeMV) | |
| Feline morbillivirus | feline morbillivirus (FeMV) | |
| Measles morbillivirus* | measles virus (MeV) | |
| Phocine morbillivirus | phocine distemper virus (PDV) | |
| Rinderpest morbillivirus | rinderpest virus (RPV) | |
| Small ruminant morbillivirus | peste-des-petits-ruminants virus (PPRV) | |
| Narmovirus | Mossman narmovirus | Mossman virus (MossV) |
| Myodes narmovirus | bank vole virus 1 (BaV-1) | |
| Nariva narmovirus* | Nariva virus (NarV) | |
| Tupaia narmovirus | Tupaia paramyxovirus (TupV) | |
| Respirovirus | Bovine respirovirus 3 | bovine parainfluenza virus 3 (BPIV-3) |
| Caprine respirovirus 3 | caprine parainfluenzavirus 3 (CPIV-3) | |
| Human respirovirus 1 | human parainfluenza virus 1 (HPIV-1) | |
| Human respirovirus 3 | human parainfluenza virus 3 (HPIV-3) | |
| Murine respirovirus* | Sendai virus (SeV) | |
| Porcine respirovirus 1 | porcine parainfluenza virus 1 (PPIV-1) | |
| Squirrel respirovirus | giant squirrel virus (GSqV) | |
| Salemvirus | Salem salemvirus* | Salem virus (SalV) |
| Subfamily Rubulavirinae | ||
| Orthorubulavirus | Human orthorubulavirus 2 | human parainfluenza virus 2 (HPIV-2) |
| Human orthorubulavirus 4 | human parainfluenza virus 4a (HPIV-4a) | |
| human parainfluenza virus 4b (HPIV-4b) | ||
| Mammalian orthorubulavirus 5 | parainfluenza virus 5 (PIV-5) | |
| Mammalian orthorubulavirus 6 | Alston virus (AlsV) | |
| Mapuera orthorubulavirus | Mapuera virus (MapV) | |
| Mumps orthorubulavirus* | mumps virus (MuV) | |
| Porcine orthorubulavirus | La Piedad Michoacan Mexico virus (LPMV) | |
| Simian orthorubulavirus | simian virus 41 (SV-41) | |
| Pararubulavirus | Achimota pararubulavirus 1 | Achimota virus 1 (AchPV-1) |
| Achimotapararubulavirus 2 | Achimota virus 2 (AchPV-2) | |
| Hervey pararubulavirus | Hervey virus (HerV) | |
| Menangle pararubulavirus* | Menangle virus (MenPV) | |
| Sosuga pararubulavirus | Sosuga virus (SOSV) | |
| Teviot pararubulavirus | Teviot virus (TevPV) | |
| Tioman pararubulavirus | Tioman virus (TioPV) | |
| Tuhoko pararubulavirus 1 | Tuhoko virus 1 (ThkPV-1) | |
| Tuhoko pararubulavirus 2 | Tuhoko virus 2 (ThkPV-2) | |
| Tuhoko pararubulavirus 3 | Tuhoko virus 3 (ThkPV-3) | |
| Unassigned (to subfamilies) | ||
| Cynoglossusvirus | Cynoglossus cynoglossusvirus* | Wēnlǐng tonguesole paramyxovirus (WTSPV) |
| Hoplichthysvirus | Hoplichthys hoplichthysvirus* | Wēnlǐng hoplichthys paramyxovirus (WHPV) |
| Scoliodonvirus | Scoliodon scoliodonvirus* | Wēnzhōu pacific spadenose shark paramyxovirus (WPSSPV) |
| Family Pneumoviridae | ||
| Metapneumovirus | Avian metapneumovirus* | avian metapneumovirus (AMPV) |
| Human metapneumovirus | human metapneumovirus (HMPV) | |
| Orthopneumovirus | Bovine orthopneumovirus | bovine respiratory syncytial virus (BRSV) |
| Human orthopneumovirus* | human respiratory syncytial virus (HRSV) | |
| Murine orthopneumovirus | murine pneumonia virus (MPV) | |
| Family Rhabdoviridae | ||
| Almendravirus | Arboretum almendravirus | Arboretum virus (ABTV) |
| Balsa almendravirus | Balsa virus (BALV) | |
| Coot Bay almendravirus | Coot Bay virus (CBV) | |
| Menghai almendravirus | Menghai virus (MRV) | |
| Puerto Almendras almendravirus* | Puerto Almendras virus (PTAMV) | |
| Rio Chico almendravirus | Rio Chico virus (RCHV) | |
| Alphanemrhavirus | Xingshan alphanemrhavirus* | Xingshan nematode virus 4 (XsNV-4) |
| Xinzhou alphanemrhavirus | Xinzhou nematode virus 4 (XzNV-4) | |
| Alphanucleorhabdovirus | Eggplant mottled dwarf alphanucleorhabdovirus | eggplant mottled dwarf virus (EMDV) |
| Maize Iranian mosaic alphanucleorhabdovirus | maize Iranian mosaic virus (MIMV) | |
| Maize mosaic alphanucleorhabdovirus | maize mosaic virus (MMV) | |
| Morogoro maize-associated alphanucleorhabdovirus | Morogoro maize-associated virus (MMaV) | |
| Physostegia chlorotic mottle alphanucleorhabdovirus | Physostegia chlorotic mottle virus (PhCMoV) | |
| Potato yellow dwarf alphanucleorhabdovirus* | potato yellow dwarf virus (PYDV) | |
| Rice yellow stunt alphanucleorhabdovirus | rice yellow stunt virus (RYSV) | |
| rice transitory yellowing virus (RTYV) | ||
| Taro vein chlorosis alphanucleorhabdovirus | taro vein chlorosis virus (TaVCV) | |
| Wheat yellow striate alphanucleorhabdovirus | wheat yellow striate virus (WYSV) | |
| Arurhavirus | Aruac arurhavirus* | Aruac virus (ARUV) |
| Inhangapi arurhavirus | Inhangapi virus (INHV) | |
| Santabarbara arurhavirus | Santa Barbara virus (SBAV) | |
| Xiburema arurhavirus | Xiburema virus (XIBV) | |
| Barhavirus | Bahia barhavirus* | Bahia Grande virus (BGV) |
| Harlingen virus (HARV) | ||
| Muir barhavirus | Muir Springs virus (MSV) | |
| Betanucleorhabdovirus | Alfalfa betanucleorhabdovirus | alfalfa-associated nucleorhabdovirus (AaNV) |
| Blackcurrant betanucleorhabdovirus | blackcurrant-associated rhabdovirus (BCaRV) | |
| Datura yellow vein betanucleorhabdovirus | datura yellow vein virus (DYVV) | |
| Sonchus yellow net betanucleorhabdovirus* | Sonchus yellow net virus (SYNV) | |
| Sowthistle yellow vein betanucleorhabdovirus | sowthistle yellow vein virus (SYVV) | |
| Trefoil betanucleorhabdovirus | birds-foot trefoil-associated virus (BFTV) | |
| Caligrhavirus | Caligus caligrhavirus | Caligus rogercresseyi rhabdovirus (CRogRV) |
| Lepeophtheirus caligrhavirus* | Lepeophtheirus salmonis rhabdovirus 127 (LSalRV-127) | |
| Salmonlouse caligrhavirus | Lepeophtheirus salmonis rhabdovirus 9 (LSalRV-9) | |
| Curiovirus | Curionopolis curiovirus* | Curionopolis virus (CURV) |
| Iriri curiovirus | Iriri virus (IRIRV) | |
| Itacaiunas curiovirus | Itacaiunas virus (ITAV) | |
| Rochambeau curiovirus | Rochambeau virus (RBUV) | |
| Cytorhabdovirus | Alfalfa dwarf cytorhabdovirus | alfalfa dwarf virus (ADV) |
| Barley yellow striate mosaic cytorhabdovirus | barley yellow striate mosaic virus (BYSMV) | |
| Broccoli necrotic yellows cytorhabdovirus | broccoli necrotic yellows virus (BNYV) | |
| Cabbage cytorhabdovirus | cabbage cytorhabdovirus 1 (CCyV-1) | |
| Colocasia bobone disease-associated cytorhabdovirus | Colocasia bobone disease-associated virus (CBDaV) | |
| Festuca leaf streak cytorhabdovirus | Festuca leaf streak virus (FLSV) | |
| Lettuce necrotic yellows cytorhabdovirus* | lettuce necrotic yellows virus (LNYV) | |
| Lettuce yellow mottle cytorhabdovirus | lettuce yellow mottle virus (LYMoV) | |
| Maize-associated cytorhabdovirus | maize-associated cytorhabdovirus (MaCV) | |
| Maize yellow striate cytorhabdovirus | maize yellow striate virus (MYSV) | |
| Northern cereal mosaic cytorhabdovirus | northern cereal mosaic virus (NCMV) | |
| Papaya cytorhabdovirus | papaya virus E (PpVE) | |
| Persimmon cytorhabdovirus | persimmon virus A (PeVA) | |
| Raspberry vein chlorosis cytorhabdovirus | raspberry vein chlorosis virus (RVCV) | |
| Rice stripe mosaic cytorhabdovirus | rice stripe mosaic virus (RSMV) | |
| Sonchus cytorhabdovirus 1 | Sonchus virus (SonV) | |
| Strawberry crinkle cytorhabdovirus | strawberry crinkle virus (SCV) | |
| Tomato yellow mottle-associated cytorhabdovirus | tomato yellow mottle-associated virus (TYMaV) | |
| Wheat American striate mosaic cytorhabdovirus | wheat American striate mosaic virus (WASMV) | |
| Wuhan 4 insect cytorhabdovirus | Wuhan insect virus 4 (WuIV-4) | |
| Wuhan 5 insect cytorhabdovirus | Wuhan insect virus 5 (WuIV-5) | |
| Wuhan 6 insect cytorhabdovirus | Wuhan insect virus 6 (WuIV-6) | |
| Yerba mate chlorosis-associated cytorhabdovirus | yerba mate chlorosis-associated virus (YmCaV) | |
| Dichorhavirus | Citrus chlorotic spot dichorhavirus | citrus chlorotic spot virus (CiCSV) |
| Citrus leprosis N dichorhavirus | citrus leprosis virus N (CiLV-N) | |
| Clerodendrum chlorotic spot dichorhavirus | clerodendrum chlorotic spot virus (ClCSV) | |
| Coffee ringspot dichorhavirus | coffee ringspot virus (CoRS V) | |
| Orchidfleck dichorhavirus* | orchid fleck virus (OFV) | |
| Ephemerovirus | Adelaide River ephemerovirus | Adelaide River virus (ARV) |
| Berrimah ephemerovirus | Berrimah virus (BRMV) | |
| Bovine fever ephemerovirus* | bovine ephemeral fever virus (BEFV) | |
| Kimberley ephemerovirus | Kimberley virus (KIMV) | |
| Malakal virus (MALV) | ||
| Koolpinyah ephemerovirus | Koolpinyah virus (KOOLV) | |
| Kotonkan ephemerovirus | kotonkan virus (KOTV) | |
| Obodhiang ephemerovirus | Obodhiang virus (OBOV) | |
| Yata ephemerovirus | Yata virus (YATV) | |
| Gammanucleorhabdovirus | Maize fine streak gammanucleorhabdovirus | maize fine streak virus (MFSV) |
| Hapavirus | Flanders hapavirus* | Flanders virus (FLAV) |
| Gray Lodge hapavirus | Gray Lodge virus (GLOV) | |
| Hart Park hapavirus | Hart Park virus (HPV) | |
| Holmes hapavirus | Holmes Jungle virus (HOJV) | |
| Joinjakaka hapavirus | Joinjakaka virus (JOIV) | |
| Kamese hapavirus | Kamese virus (KAMV) | |
| La Joya hapavirus | La Joya virus (LJV) | |
| Landjia hapavirus | Landjia virus (LANV = LJAV) | |
| Manitoba hapavirus | Manitoba virus (MANV = MNTBV) | |
| Marco hapavirus | Marco virus (MCOV) | |
| Mosqueiro hapavirus | Mosqueiro virus (MQOV) | |
| Mossuril hapavirus | Mossuril virus (MOSV) | |
| Ngaingan hapavirus | Ngaingan virus (NGAV) | |
| Ord River hapavirus | Ord River virus (ORV) | |
| Parry Creek hapavirus | Parry Creek virus (PCV) | |
| Wongabel hapavirus | Wongabel virus (WONV) | |
| Ledantevirus | Barur ledantevirus | Barur virus (BARV) |
| Fikirini ledantevirus | Fikirini virus (FKRV) | |
| Fukuoka ledantevirus | Fukuoka virus (FUKV) | |
| Kanyawara ledantevirus | Kanyawara virus (KYAV) | |
| Kern Canyon ledantevirus | Kern Canyon virus (KCV) | |
| Keuraliba ledantevirus | Keuraliba virus (KEUV) | |
| Kolente ledantevirus | Kolente virus (KOLEV) | |
| Kumasi ledantevirus | Kumasi rhabdovirus (KRV) | |
| Le Dantec ledantevirus* | Le Dantec virus (LDV) | |
| Mount Elgon bat ledantevirus | Mount Elgon bat virus (MEBV) | |
| Nishimuro ledantevirus | Nishimuro virus (NISV) | |
| Nkolbisson ledantevirus | Nkolbisson virus (NKOV) | |
| Oita ledantevirus | Oita virus (OITAV) | |
| Vaprio ledantevirus | Vaprio virus (VAPV) | |
| Wuhan ledantevirus | Wuhan louse fly virus 5 (WLFV-5) | |
| Yongjia ledantevirus | Yongjia tick virus 2 (YTV-2) | |
| Lostrhavirus | Lonestar zarhavirus*4 | lone star tick rhabdovirus (LITRV) |
| Lyssavirus | Aravan lyssavirus | Aravan virus (ARAV) |
| Australian bat lyssavirus | Australian bat lyssavirus (ABLV) | |
| Bokeloh bat lyssavirus | Bokeloh bat lyssavirus (BBLV) | |
| Duvenhage lyssavirus | Duvenhage virus (DUVV) | |
| European bat 1 lyssavirus | European bat lyssavirus 1 (EBLV-1) | |
| European bat 2 lyssavirus | European bat lyssavirus 2 (EBLV-2) | |
| Gannoruwa bat lyssavirus | Gannoruwa bat lyssavirus (GBLV) | |
| Ikoma lyssavirus | Ikoma lyssavirus (IKOV) | |
| Irkut lyssavirus | Irkut virus (IRKV) | |
| Khujand lyssavirus | Khujand virus (KHUV) | |
| Lagos bat lyssavirus | Lagos bat virus (LBV) | |
| Lleida bat lyssavirus | Lleida bat lyssavirus (LLEBV) | |
| Mokola lyssavirus | Mokola virus (MOKV) | |
| Rabies lyssavirus* | rabies virus (RABV) | |
| Shimoni bat lyssavirus | Shimoni bat virus (SHIB V) | |
| Taiwan bat lyssavirus | Taiwan bat lyssavirus (TWBLV) | |
| West Caucasian bat lyssavirus | West Caucasian bat virus (WCBV) | |
| Mousrhavirus | Moussa mousrhavirus* | Moussa virus (MOUV) |
| Novirhabdovirus | Hirame novirhabdovirus | hirame rhabdovirus (HIRRV = HIRV) |
| Piscine novirhabdovirus | viral hemorrhagic septicemia virus (VHSV) | |
| Salmonid novirhabdovirus* | infectious hematopoietic necrosis virus (IHNV) | |
| Snakehead novirhabdovirus | snakehead rhabdovirus (SHRV) | |
| Ohlsrhavirus | Ohlsdorf ohlsrhavirus | Ohlsdorf virus (OHLDV) |
| Tongilchon ohlsrhavirus | Tongilchon virus 1 (TCHV-1) | |
| Riverside ohlsrhavirus | Riverside virus (RISV) | |
| Culex ohlsrharhavirus | Culex rhabdo-like virus (CRLV) | |
| Northcreek ohlsrhavirus | North Creek virus (NORCV) | |
| Perhabdovirus | Anguillid perhabdovirus | eel virus European X (EVEX) |
| Perch perhabdovirus* | perch rhabdovirus (PRV) | |
| Sea trout perhabdovirus | lake trout rhabdovirus (LTRV) | |
| Sawgrhavirus | Connecticut sawgrhavirus | Connecticut virus (CNTV) |
| Island sawgrhavirus | Long Island tick rhabdovirus (LITRV) | |
| Minto sawgrhavirus | New Minto virus (NMV) | |
| Sawgrass sawgrhavirus* | Sawgrass virus (SAWV) | |
| Sigmavirus | Drosophila affinis sigmavirus | Drosophila affinis sigmavirus (DAffSV) |
| Drosophila ananassae sigmavirus | Drosophila ananassae sigmavirus (DAnaSV) | |
| Drosophila immigrans sigmavirus | Drosophila immigrans sigmavirus (DImmSV) | |
| Drosophila melanogaster sigmavirus* | Drosophila melanogaster sigmavirus (DMelSV) | |
| Drosophila obscura sigmavirus | Drosophila obscura sigmavirus (DObsSV) | |
| Drosophila tristis sigmavirus | Drosophila tristis sigmavirus (DTriSV) | |
| Muscina stabulans sigmavirus | Muscina stabulans sigmavirus (MStaSV) | |
| Sprivivirus | Carp sprivivirus* | spring viremia of carp virus (SVCV) |
| Pike fry sprivivirus | grass carp rhabdovirus (GrCRV) | |
| pike fry rhabdovirus (PFRV) | ||
| tench rhabdovirus (TenRV) | ||
| Sripuvirus | Almpiwar sripuvirus | Almpiwar virus (ALMV) |
| Chaco sripuvirus | Chaco virus (CHOV) | |
| Charleville sripuvirus | Charleville virus (CHVV) | |
| Cuiaba sripuvirus | Cuiaba virus (CUIV) | |
| Hainan sripuvirus | Hainan black-spectacled toad rhabdovirus (HnBSTRV) | |
| Niakha sripuvirus* | Niakha virus (NIAV) | |
| Sena Madureira sripuvirus | Sena Madureira virus (SMV) | |
| Sripur sripuvirus | Sripur virus (SRIV) | |
| Sunrhavirus | Garba sunrhavirus | Garba virus (GARV) |
| Harrison sunrhavirus | Harrison Dam virus (HARDV) | |
| Kwatta sunrhavirus | Kwatta virus (KWAV) | |
| Oakvale sunrhavirus | Oak Vale virus (OVV) | |
| Sunguru sunrhavirus* | Sunguru virus (SUNV) | |
| Walkabout sunrhavirus | Walkabout Creek virus (WAKV) | |
| Tibrovirus | Bas Congo tibrovirus | Bas-Congo virus (BASV) |
| Beatrice Hill tibrovirus | Beatrice Hill virus (BHV) | |
| Coastal Plains tibrovirus | Coastal Plains virus (CPV) | |
| Ekpoma 1 tibrovirus | Ekpoma virus 1 (EKV-1) | |
| Ekpoma 2 tibrovirus | Ekpoma virus 2 (EKV-2) | |
| Sweetwater Branch tibrovirus | Sweetwater Branch virus (SWBV) | |
| Tibrogargan tibrovirus* | Bivens Arm virus (BAV) | |
| Tibrogargan virus (TIBV) | ||
| Tupavirus | Durham tupavirus* | Durham virus (DURV) |
| Klamath tupavirus | Klamath virus (KLAV) | |
| Tupaia tupavirus | tupaia rhabdovirus (TUPV) | |
| Varicosavirus | Lettuce big-vein associated varicosavirus* | lettuce big-vein associated virus (LBVaV) |
| Vesiculovirus | Alagoas vesiculovirus | vesicular stomatitis Alagoas virus (VSAV) |
| American bat vesiculovirus | American bat vesiculovirus (ABVV) | |
| Carajas vesiculovirus | Carajas virus (CJSV) | |
| Chandipura vesiculovirus | Chandipura virus (CHPV) | |
| Cocal vesiculovirus | Cocal virus (COCV) | |
| Indiana vesiculovirus* | vesicular stomatitis Indiana virus (VSIV) | |
| Isfahan vesiculovirus | Isfahan virus (ISFV) | |
| Jurona vesiculovirus | Jurona virus (JURV) | |
| Malpais Spring vesiculovirus | Malpais Spring virus (MSPV) | |
| Maraba vesiculovirus | Maraba virus (MARAV) | |
| Morreton vesiculovirus | Morreton virus (MORV) | |
| New Jersey vesiculovirus | vesicular stomatitis New Jersey virus (VSNJV) | |
| Perinet vesiculovirus | Perinet virus (PERV) | |
| Piry vesiculovirus | Piry virus (PIRYV) | |
| Radi vesiculovirus | Radi virus (RADV) | |
| Yug Bogdanovac vesiculovirus | Yug Bogdanovac virus (YBV) | |
| Zarhavirus | Zahedan zarhavirus* | Zahedan rhabdovirus (ZARV) |
| Family Sunviridae | ||
| Sunshinevirus | Reptile sunshinevirus 1* | Sunshine Coast virus (SunCV) |
| Family Xinmoviridae | ||
| Anphevirus | Bolahun anphevirus | Bolahun virus (BLHV) |
| Gambie virus (GAMV) | ||
| Dipteran anphevirus | Húběi diptera virus 11 (HbDV-11) | |
| Drosophilid anphevirus | Drosophila unispina virus 1 (DuniV-1) | |
| Odonate anphevirus | Húběi rhabdo-like virus 7 (HbRLV-7) | |
| Orthopteran anphevirus | Húběi orthoptera virus 5 (HbOV-5) | |
| Shuangao anphevirus | Shuāngào fly virus 2 (SgFV-2) | |
| Xincheng anphevirus* | Xīnchéng mosquito virus (XcMV) | |
Note that viruses are real objects that are assigned to concepts that are called taxa. Species, genera, subfamilies, families, and orders are taxa.
type species
Due to a formal classification mistake this species was not deleted. A proposal to delete this species name will be submitted prior to the next taxonomic proposal submission deadline.
Includes: Newcastle disease virus (NDV).
Due to a formal classification mistake, this species was incorrectly named Avian orthovulavirus 21 in TaxoProp 2019.014M.A.v1.Avulavirus_1newsp but correctly named Avian orthoavulavirus 21 in TaxoProp 2019.025M.A.v2.Paramyxoviridae_3gen5sp4rensp. Hence as of now, both species names are official. A proposal to correct this mistake will be submitted prior to the next taxonomic proposal submission deadline.
Due to a formal classification mistake this species was named Lonestar zarhavirus instead of Lonestar lostrhavirus. A proposal to correct this mistake will be submitted prior to the next taxonomic proposal submission deadline.
Taxon names are always italicized and always begin with a capital letter.
Virus names are not italicized and are not capitalized, except if the name or a name component is a proper noun. This column lists the virus names with their correct (lack of) capitalization. Lists of viruses within a given species are provisional at this point and will likely be amended in the near future.
Table 4.
ICTV-accepted taxonomy of the order Muvirales (Negarnaviricota: Haploviricotina: Chunqiuviricetes) as of March 2020.
| Genus | Species¶ | Virus (Abbreviation)& |
|---|---|---|
| Family Qinviridae | ||
| Yingvirus | Beihai yingvirus | Běihǎi sesarmid crab virus 4 (BhSCV-4) |
| Charybdis yingvirus | Wēnzhōu qínvirus-like virus 2 (WzQLV-2) | |
| Hubei yingvirus | Húběi qínvirus-like virus 1 (HbQLV-1) | |
| Sanxia yingvirus | Sānxiá qínvirus-like virus 1 (SxQLV-1) | |
| Shahe yingvirus | Shāhé qínvirus-like virus 1 (ShQLV-1) | |
| Wenzhou yingvirus | Wēnzhōu qínvirus-like virus 1 (WzQLV-1) | |
| Wuhan yingvirus * | Wǔhàn insect virus 15 (WhIV-15) | |
| Xinzhou yingvirus | Xīnzhōu nematode virus 3 (XzNV-3) | |
Note that viruses are real objects that are assigned to concepts that are called taxa. Species, genera, families, and orders are taxa.
type species
Taxon names are always italicized and always begin with a capital letter.
Virus names are not italicized and are not capitalized, except if the name or a name component is a proper noun. This column lists the virus names with their correct (lack of) capitalization. Lists of viruses within a given species are provisional at this point and will likely be amended in the near future.
Table 5.
ICTV-accepted taxonomy of the order Serpentovirales (Negarnaviricota: Haploviricotina: Milneviricetes) as of March 2020.
| Genus | Species¶ | Virus (Abbreviation)& |
|---|---|---|
| Family Aspiviridae | ||
| Ophiovirus | Blueberry mosaic associated ophiovirus | blueberry mosaic associated virus (BlMaV) |
| Citruspsorosis ophiovirus* | citrus psorosis virus (CPsV) | |
| Freesia sneak ophiovirus | freesia sneak virus (FreSV) | |
| Lettuce ring necrosis ophiovirus | lettuce ring necrosis virus (LRNV) | |
| Mirafiori lettuce big-vein ophiovirus | Mirafiori lettuce big-vein virus (MLBVV) | |
| Ranunculus white mottle ophiovirus | ranunculus white mottle virus (RWMV) | |
| Tulip mild mottle mosaic ophiovirus | tulip mild mottle mosaic virus (TMMMV) | |
Note that viruses are real objects that are assigned to concepts that are called taxa. Species, genera, families, and orders are taxa.
type species
Taxon names are always italicized and always begin with a capital letter.
Virus names are not italicized and are not capitalized, except if the name or a name component is a proper noun. This column lists the virus names with their correct (lack of) capitalization. Lists of viruses within a given species are provisional at this point and will likely be amended in the near future.
Table 6.
ICTV-accepted taxonomy of the order Articulavirales (Negarnaviricota: Polyploviricotina: Insthoviricetes) as of March 2020.
| Genus | Species¶ | Virus (Abbreviation)& |
|---|---|---|
| Family Amnoonviridae | ||
| Tilapinevirus | Tilapia tilapinevirus* | tilapia lake virus (TiLV) |
| Family Orthomyxoviridae | ||
| Alphainfluenzavirus | Influenza A virus* | influenza A virus (FLUAV) |
| Betainfluenzavirus | Influenza B virus* | influenza B virus (FLUBV) |
| Deltainfluenzavirus | Influenza D virus* | influenza D virus (FLUDV) |
| Gammainfluenzavirus | Influenza C virus* | influenza C virus (FLUCV) |
| Isavirus | Salmon isavirus* | infectious salmon anemia virus (ISAV) |
| Quaranjavirus | Johnston Atoll quaranjavirus | Johnston Atoll virus (JAV) |
| Quaranfil quaranjavirus* | Quaranfil virus (QRFV) | |
| Thogotovirus | Dhori thogotovirus | Dhori virus (DHOV) |
| Thogoto thogotovirus* | Thogoto virus (THOV) | |
type species
Taxon names are always italicized and always begin with a capital letter.
Table 7.
ICTV-accepted taxonomy of the order Bunyavirales (Negarnaviricota: Polyploviricotina: Ellioviricetes) as of March 2020.
| Genus | Species¶ | Virus (Abbreviation)& |
|---|---|---|
| Family Arenaviridae | ||
| Antennavirus | Hairy antennavirus | Wēnlǐng frogfish arenavirus 2 (WlFAV-2) |
| Striated antennavirus* | Wēnlǐng frogfish arenavirus 1 (WlFAV-1) | |
| Hartmanivirus | Haartman hartmanivirus* | Haartman Institute snake virus 1 (HISV-1) |
| Muikkunen hartmanivirus | Dante Muikkunen virus 1 (DaMV-1) | |
| Schoolhouse hartmanivirus | old schoolhouse virus 1 (OScV-1) | |
| old schoolhouse virus 2 (OScV-2) | ||
| Zurich hartmanivirus | veterinary pathology Zurich virus 1 (VPZV-1) | |
| veterinary pathology Zurich virus 2 (VPZV-2) | ||
| Mammarenavirus | Allpahuayo mammarenavirus | Allpahuayo virus (ALLV) |
| Alxa mammarenavirus | Alxa virus (ALXV) | |
| Argentinian mammarenavirus | Junín virus (JUNV) | |
| Bear Canyon mammarenavirus | Bear Canyon virus (BCNV) | |
| Brazilian mammarenavirus | Sabiá virus (SBAV) | |
| Cali mammarenavirus | Pichindé virus (PICHV) | |
| Chapare mammarenavirus | Chapare virus (CHAPV) | |
| Chevrier mammarenavirus | Lìjiāng virus (LIJV) | |
| Cupixi mammarenavirus | Cupixi virus (CUPXV) | |
| Flexal mammarenavirus | Flexal virus (FLEV) | |
| Gairo mammarenavirus | Gairo virus (GAIV) | |
| Guanarito mammarenavirus | Guanarito virus (GTOV) | |
| Ippy mammarenavirus | Ippy virus (IPPYV) | |
| Lassa mammarenavirus | Lassa virus (LASV) | |
| Latino mammarenavirus | Latino virus (LATV) | |
| Loei River mammarenavirus | Loei River virus (LORV) | |
| Lujo mammarenavirus | Lujo virus (LUJV) | |
| Luna mammarenavirus | Luli virus (LULV) | |
| Luna virus (LUAV) | ||
| Lunk mammarenavirus | Lunk virus (LNKV) | |
| Lymphocytic choriomeningitis mammarenavirus* | Dandenong virus (DANV) | |
| lymphocytic choriomeningitis virus (LCMV) | ||
| Machupo mammarenavirus | Machupo virus (MACV) | |
| Mariental mammarenavirus | Mariental virus (MRLV) | |
| Merino Walk mammarenavirus | Merino Walk virus (MRWV) | |
| Mobala mammarenavirus | mobala virus (MOBV) | |
| Mopeia mammarenavirus | Mopeia virus (MPOV) | |
| Morogoro virus (MORV) | ||
| Okahandja mammarenavirus | Okahandja virus (OKAV) | |
| Oliveros mammarenavirus | Oliveros virus (OLVV) | |
| Paraguayan mammarenavirus | Paraná virus (PRAV) | |
| Planalto mammarenavirus | Aporé virus (APOV) | |
| Pirital mammarenavirus | Pirital virus (PIRV) | |
| Ryukyu mammarenavirus | Ryukyu virus (RYKV) | |
| Serra do Navio mammarenavirus | Amapari virus (AMAV) | |
| Solwezi mammarenavirus | Solwezi virus (SOLV) | |
| Souris mammarenavirus | souris virus (SOUV) | |
| Tacaribe mammarenavirus | Tacaribe virus (TCRV) | |
| Tamiami mammarenavirus | Tamiami virus (TMMV) | |
| Wenzhou mammarenavirus | Wēnzhōu virus (WENV) | |
| Whitewater Arroyo mammarenavirus | Big Brushy Tank virus (BBRTV) | |
| Catarina virus (CTNV) | ||
| Skinner Tank virus (SKTV) | ||
| Tonto Creek virus (TTCV) | ||
| Whitewater Arroyo virus (WWAV) | ||
| Xapuri mammarenavirus | Xapuri virus (XAPV) | |
| Reptarenavirus | California reptarenavirus | CAS virus (CASV) |
| Giessen reptarenavirus | University of Giessen virus 1 (UGV-1) | |
| University of Giessen virus 2 (UGV-2) | ||
| University of Giessen virus 3 (UGV-3) | ||
| Golden reptarenavirus* | Golden Gate virus (GOGV) | |
| Ordinary reptarenavirus | tavallinen suomalainen mies virus 2 (TSMV-2) | |
| Rotterdam reptarenavirus | ROUT virus (ROUTV) | |
| University of Helsinki virus 1 (UHV-1) | ||
| Family Cruliviridae | ||
| Lincruvirus | Crustacean lincruvirus* | Wēnlǐng crustacean virus 9 (WlCV-9) |
| Family Fimoviridae | ||
| Emaravirus | Actinidia chlorotic ringspot-associated emaravirus | Actinidia chlorotic ringspot-associated virus (AcCRaV) |
| Blackberry leaf mottle associated emaravirus | blackberry leaf mottle-associated virus (BLMaV) | |
| European mountain ash ringspot-associated emaravirus* | European mountain ash ringspot-associated virus (EMARaV) | |
| Fig mosaic emaravirus | fig mosaic virus (FMV) | |
| High Plains wheat mosaic emaravirus | High Plains wheat mosaic virus (HPWMoV) | |
| Pigeonpea sterility mosaic emaravirus 1 | pigeonpea sterility mosaic virus 1 (PPSMV-1) | |
| Pigeonpea sterility mosaic emaravirus 2 | pigeonpea sterility mosaic virus 2 (PPSMV-2) | |
| Pistacia emaravirus B | pistacia virus B (PiVB) | |
| Raspberry leaf blotch emaravirus | raspberry leaf blotch virus (RLBV) | |
| Redbud yellow ringspot-associated emaravirus | redbud yellow ringspot-associated virus (RYRaV) | |
| Rose rosette emaravirus | rose rosette virus (RRV) | |
| Family Hantaviridae | ||
| Subfamily Actantavirinae | ||
| Actinovirus | Batfish actinovirus* | Wēnlǐng minipizza batfish virus (WEMBV) |
| Goosefish actinovirus | Wēnlǐng yellow goosefish virus (WEYGV) | |
| Spikefish actinovirus | Wēnlǐng red spikefish virus (WERSV) | |
| Subfamily Agantavirinae | ||
| Agnathovirus | Hagfish agnathovirus* | Wēnlǐng hagfish virus (WEHV) |
| Subfamily Mammantavirinae | ||
| Loanvirus | Brno loanvirus | Brno virus (BRNV) |
| Longquan loanvirus* | Lóngquán virus (LQUV) | |
| Mobatvirus | Laibin mobatvirus | Láibīn virus (LAIV) |
| Nova mobatvirus* | Nova virus (NVAV) | |
| Quezon mobatvirus | Quezon virus (QZNV) | |
| Orthohantavirus | Andes orthohantavirus | Andes virus (ANDV) |
| Castelo dos Sonhos virus (CASV) | ||
| Lechiguanas virus (LECV = LECHV) | ||
| Orán virus (ORNV) | ||
| Asama orthohantavirus | Asama virus (ASAV) | |
| Asikkala orthohantavirus | Asikkala virus (ASIV) | |
| Bayou orthohantavirus | bayou virus (BAYV) | |
| Catacamas virus (CATV) | ||
| Black Creek Canal orthohantavirus | Black Creek Canal virus (BCCV) | |
| Bowe orthohantavirus | Bowé virus (BOWV) | |
| Bruges orthohantavirus | Bruges virus (BRGV) | |
| Cano Delgadito orthohantavirus | Caño Delgadito virus (CADV) | |
| Cao Bang orthohantavirus | Cao Bằng virus (CBNV) | |
| Liánghé virus (LHEV) | ||
| Choclo orthohantavirus | Choclo virus (CHOV) | |
| Dabieshan orthohantavirus | Dàbiéshān virus (DBSV) | |
| Dobrava-Belgrade orthohantavirus | Dobrava virus (DOBV) | |
| Kurkino virus (KURV) | ||
| Saaremaa virus (SAAV) | ||
| Sochi virus (SOCV) | ||
| El Moro Canyon orthohantavirus | Carrizal virus (CARV) | |
| El Moro Canyon virus (ELMCV) | ||
| Huitzilac virus (HUIV) | ||
| Fugong orthohantavirus | Fúgóng virus (FUGV) | |
| Fusong orthohantavirus | Fǔsōng virus (FUSV) | |
| Hantaan orthohantavirus* | Amur virus (AMRV) | |
| Hantaan virus (HTNV) | ||
| Soochong virus (SOOV) | ||
| Jeju orthohantavirus | Jeju virus (JJUV) | |
| Kenkeme orthohantavirus | Kenkeme virus (KKMV) | |
| Khabarovsk orthohantavirus | Khabarovsk virus (KHAV) | |
| Topografov virus (TOPV) | ||
| Laguna Negra orthohantavirus | Laguna Negra virus (LANV) | |
| Maripa virus (MARV) | ||
| Rio Mamoré virus (RIOMV) | ||
| Luxi orthohantavirus | Lúxī virus (LUXV) | |
| Maporal orthohantavirus | Maporal virus (MAPV) | |
| Montano orthohantavirus | Montaño virus (MTNV) | |
| Necocli orthohantavirus | Necoclí virus (NECV) | |
| Oxbow orthohantavirus | Oxbow virus (OXBV) | |
| Prospect Hill orthohantavirus | Prospect Hill virus (PHV) | |
| Puumala orthohantavirus | Hokkaido virus (HOKV) | |
| Muju virus (MUJV) | ||
| Puumala virus (PUUV) | ||
| Rockport orthohantavirus | Rockport virus (RKPV) | |
| Sangassou orthohantavirus | Sangassou virus (SANGV) | |
| Seewis orthohantavirus | Seewis virus (SWSV) | |
| Seoul orthohantavirus | gōu virus (GOUV) | |
| Seoul virus (SEOV) | ||
| Sin Nombre orthohantavirus | New York virus (NYV) | |
| Sin Nombre virus (SNV) | ||
| Thailand orthohantavirus | Anjozorobe virus (ANJZV) | |
| Serang virus (SERV) | ||
| Thailand virus (THAIV) | ||
| Tigray orthohantavirus | Tigray virus (TIGV) | |
| Tula orthohantavirus | Adler virus (ADLV) | |
| Tula virus (TULV) | ||
| Yakeshi orthohantavirus | Yákéshí virus (YKSV) | |
| Thottimvirus | Imjin thottimvirus | Imjin virus (MJNV) |
| Thottapalayam thottimvirus* | Thottapalayam virus (TPMV) | |
| Subfamily Repantavirinae | ||
| Reptillovirus | Gecko reptillovirus* | Hainan oriental leaf-toed gecko virus (HOLGV) |
| Family Leishbuviridae | ||
| Shilevirus | Leptomonas shilevirus* | Leptomonas moramango virus (LEPMV) |
| Family Mypoviridae | ||
| Hubavirus | Myriapod hubavirus* | Húběi myriapoda virus 5 (HbMV-5) |
| Family Nairoviridae | ||
| Orthonairovirus | Artashat orthonairovirus | Artashat virus (ARTSV) |
| Chim orthonairovirus | Chim virus (CHIMV) | |
| Crimean-Congo hemorrhagic fever orthonairovirus | Crimean-Congo hemorrhagic fever virus (CCHFV) | |
| Dera Ghazi Khan orthonairovirus | Abu Hammad virus (AHV) | |
| Abu Mina virus (AMV) | ||
| Dera Ghazi Khan virus (DGKV) | ||
| Sapphire II virus (SAPV) | ||
| Dugbe orthonairovirus* | Dugbe virus (DUGV) | |
| kupe virus (KUPEV) | ||
| Estero Real orthonairovirus | Estero Real virus (ERV) | |
| Hazara orthonairovirus | Hazara virus (HAZV) | |
| Tofla virus (TFLV) | ||
| Hughes orthonairovirus | Caspiy virus (CASV) | |
| Farallon virus (FARV) | ||
| Great Saltee virus (GRS V) | ||
| Hughes virus (HUGV) | ||
| Punta Salinas virus (PSV) | ||
| Raza virus (RAZAV) | ||
| Soldado virus (SOLV) | ||
| Zirqa virus (ZIRV) | ||
| Kasokero orthonairovirus | Kasokero virus (KASV = KASOV) | |
| Leopards Hill virus (LPHV) | ||
| Yogue virus (YOGV) | ||
| Keterah orthonairovirus | Gossas virus (GOSV) | |
| Issyk-kul virus (ISKV) | ||
| Keterah virus (KTRV) | ||
| Uzun-Agach virus (UZAV) | ||
| Nairobi sheep disease orthonairovirus | Nairobi sheep disease virus (NSDV) | |
| Qalyub orthonairovirus | Bandia virus (BDAV) | |
| Geran virus (GERV) | ||
| Qalyub virus (QYBV) | ||
| Sakhalin orthonairovirus | Avalon virus (AVAV) | |
| Clo Mor virus (CMV = CLMV) | ||
| Sakhalin virus (SAKV) | ||
| Taggert virus (TAGV) | ||
| Tillamook virus (TILLV) | ||
| Tamdy orthonairovirus | Burana virus (BURV) | |
| Huángpí tick virus 1 (HpTV-1) | ||
| Tǎchéng tick virus 1 (TcTV-1) | ||
| Tamdy virus (TAMV) | ||
| Wēnzhōu tick virus (WzTV) | ||
| Thiafora orthonairovirus | Erve virus (ERVEV) | |
| Thiafora virus (TFAV) | ||
| Shaspivirus | Spider shaspivirus* | Shāyáng spider virus 1 (SySV-1) |
| Striwavirus | Strider striwavirus* | Sānxiá water strider virus 1 (SxWSV-1) |
| Family Peribunyaviridae | ||
| Herbevirus | Herbert herbevirus* | Herbert virus (HEBV) |
| Kibale herbevirus | Kibale virus (KIBV) | |
| Tai herbevirus | Taï virus (TAIV) | |
| Orthobunyavirus | Acara orthobunyavirus | Acará virus (ACAV) |
| Moriche virus (MORV) | ||
| Aino orthobunyavirus | Aino virus (AINOV) | |
| Akabane orthobunyavirus | Akabane virus (AKAV) | |
| Tinaroo virus (TINV) | ||
| Yaba-7 virus (Y7V) | ||
| Alajuela orthobunyavirus | Alajuela virus (ALJV) | |
| San Juan virus (SJV) | ||
| Anadyr orthobunyavirus | Anadyr virus (ANADV) | |
| Anhembi orthobunyavirus | Anhembi virus (AMBV) | |
| Anopheles A orthobunyavirus | Anopheles A virus (ANAV) | |
| Arumateua virus (ARTV = ARMTV) | ||
| Caraipé virus (CPEV = CRPV) | ||
| Las Maloyas virus (LMV) | ||
| Lukuni virus (LUKV) | ||
| Trombetas virus (TRMV) | ||
| Tucuruí virus (TUCV = TUCRV) | ||
| Anopheles B orthobunyavirus | Anopheles B virus (ANBV) | |
| Boracéia virus (BORV) | ||
| Bakau orthobunyavirus | Bakau virus (BAKV) | |
| Ketapang virus (KETV) | ||
| Nola virus (NOLAV) | ||
| Tanjong Rabok virus (TRV) | ||
| Telok Forest virus (TFV) | ||
| Batai orthobunyavirus | Batai virus (BATV) | |
| Batama orthobunyavirus | Batama virus (BMAV) | |
| Bellavista orthobunyavirus | Bellavista virus (BELLV) | |
| Benevides orthobunyavirus | Benevides virus (BVSV = BENV) | |
| Bertioga orthobunyavirus | Bertioga virus (BERV) | |
| Cananéia virus (CNAV) | ||
| Guaratuba virus (GTBV) | ||
| Itimirim virus (ITIV) | ||
| Mirim virus (MIRV) | ||
| Bimiti orthobunyavirus | bimiti virus (BIMV) | |
| Birao orthobunyavirus | Birao virus (BIRV) | |
| Botambi orthobunyavirus | Botambi virus (BOTV) | |
| Bozo orthobunyavirus | Bozo virus (BOZOV) | |
| Bunyamwera orthobunyavirus* | Bunyamwera virus (BUNV) | |
| Germiston virus (GERV) | ||
| Lokern virus (LOKV) | ||
| Mboké virus (MBOV) | ||
| Ngari virus (NRIV) | ||
| Northway virus (NORV) | ||
| Santa Rosa virus (SARV) | ||
| Shokwe virus (SHOV) | ||
| Stanfield virus (STAV) | ||
| Xingu virus (XINV) | ||
| Bushbush orthobunyavirus | Benfica virus (BENV = BNFV) | |
| Bushbush virus (BSBV) | ||
| Juan Díaz virus (JDV) | ||
| Buttonwillow orthobunyavirus | Buttonwillow virus (BUTV) | |
| Bwamba orthobunyavirus | Bwamba virus (BWAV) | |
| Pongola virus (PGAV) | ||
| Cache Valley orthobunyavirus | Cache Valley virus (CVV) | |
| Cholul virus (CHLV) | ||
| Tlacotalpan virus (TLAV) | ||
| Cachoeira Porteira orthobunyavirus | Cachoeira Porteira virus (CPOV) | |
| California encephalitis orthobunyavirus | California encephalitis virus (CEV) | |
| Morro Bay virus (MBV) | ||
| Capim orthobunyavirus | Capim virus (CAPV) | |
| Caraparu orthobunyavirus | Apeú virus (APEUV) | |
| Bruconha virus (BRUV) | ||
| Caraparú virus (CARV) | ||
| El Huayo virus (EHUV) | ||
| Itaya virus (ITYV) | ||
| Ossa virus (OSSAV) | ||
| Vinces virus (VINV) | ||
| Cat Que orthobunyavirus | Cát Quê virus (CQV) | |
| Oya virus (OYAV) | ||
| Catu orthobunyavirus | Catú virus (CATUV) | |
| Enseada orthobunyavirus | Enseada virus (ENSV) | |
| Faceys paddock orthobunyavirus | Facey’s paddock virus (FPV) | |
| Fort Sherman orthobunyavirus | Fort Sherman virus (FSV) | |
| Gamboa orthobunyavirus | Brus Laguna virus (BLAV) | |
| Calchaquí virus (CQIV) | ||
| Gamboa virus (GAMV) | ||
| Pueblo Viejo virus (PVV) | ||
| Soberanía virus (SOBV) | ||
| Guajara orthobunyavirus | Guajará virus (GJAV) | |
| Guama orthobunyavirus | Ananindeua virus (ANUV) | |
| Guamá virus (GMAV) | ||
| Mahogany Hammock virus (MHV) | ||
| Moju virus (MOJUV) | ||
| Guaroa orthobunyavirus | Guaroa virus (GROV) | |
| Iaco orthobunyavirus | Iaco virus (IACOV) | |
| Ilesha orthobunyavirus | Ilesha virus (ILEV) | |
| Ingwavuma orthobunyavirus | Ingwavuma virus (INGV) | |
| Jamestown Canyon orthobunyavirus | Inkoo virus (INKV) | |
| Jamestown Canyon virus (JCV) | ||
| Jerry Slough virus (JSV) | ||
| South River virus (SORV) | ||
| Jatobal orthobunyavirus | Jatobal virus (JATV) | |
| Kaeng Khoi orthobunyavirus | Kaeng Khoi virus (KKV) | |
| Kairi orthobunyavirus | Kairi virus (KRIV) | |
| Keystone orthobunyavirus | Keystone virus (KEYV) | |
| Koongol orthobunyavirus | koongol virus (KOOV) | |
| wongal virus (WONV) | ||
| La Crosse orthobunyavirus | La Crosse virus (LACV) | |
| Leanyer orthobunyavirus | Leanyer virus (LEAV) | |
| Lumbo orthobunyavirus | Lumbo virus (LUMV) | |
| Macaua orthobunyavirus | Macauã virus (MCAV) | |
| Madrid orthobunyavirus | Madrid virus (MADV) | |
| Maguari orthobunyavirus | Maguari virus (MAGV) | |
| Playas virus (PLAV) | ||
| Main Drain orthobunyavirus | Main Drain virus (MDV) | |
| Manzanilla orthobunyavirus | Manzanilla virus (MANV) | |
| Inini virus (INIV) | ||
| Marituba orthobunyavirus | Gumbo Limbo virus (GLV) | |
| Marituba virus (MTBV) | ||
| Murutucú virus (MURV) | ||
| Nepuyo virus (NEPV) | ||
| Restan virus (RESV) | ||
| Zungarococha virus (ZUNV) | ||
| Melao orthobunyavirus | Melao virus (MELV) | |
| Mermet orthobunyavirus | Mermet virus (MERV) | |
| Minatitlan orthobunyavirus | Minatitlán virus (MNTV) | |
| Palestina virus (PLSV) | ||
| MPoko orthobunyavirus | M’Poko virus (MPOV) | |
| Yaba-1 virus (Y1V) | ||
| Nyando orthobunyavirus | Eretmapodites virus (ERETV) | |
| Mojuí dos Campos virus (MDCV) | ||
| Nyando virus (NDV) | ||
| Olifantsvlei orthobunyavirus | Bobia virus (BIAV) | |
| Dabakala virus (DABV) | ||
| Olifantsvlei virus (OLIV) | ||
| Oubi virus (OUBIV) | ||
| Oriboca orthobunyavirus | Itaquí virus (ITQV) | |
| Oriboca virus (ORIV) | ||
| Oropouche orthobunyavirus | Iquitos virus (IQTV)d | |
| Madre de Dios virus (MDDV) | ||
| Oropouche virus (OROV) | ||
| Perdões virus (PDEV) | ||
| Pintupo virus (PINTV) | ||
| Patois orthobunyavirus | Abras virus (ABRV) | |
| Babahoya virus (BABV) | ||
| Pahayokee virus (PAHV) | ||
| Patois virus (PATV) | ||
| Shark River virus (SRV) | ||
| Peaton orthobunyavirus | Peaton virus (PEAV) | |
| Potosi orthobunyavirus | Potosi virus (POTV) | |
| Sabo orthobunyavirus | Sabo virus (SABOV) | |
| San Angelo orthobunyavirus | San Angelo virus (SAV) | |
| Sango orthobunyavirus | Sango virus (SANV) | |
| Schmallenberg orthobunyavirus | Douglas virus (DOUV) | |
| Sathuperi virus (SATV) | ||
| Schmallenberg virus (SBV) | ||
| Shamonda virus (SHAV) | ||
| Serra do Navio orthobunyavirus | Serra do Navio virus (SDNV) | |
| Shuni orthobunyavirus | Kaikalur virus (KAIV) | |
| Shuni virus (SHUV) | ||
| Simbu orthobunyavirus | Para virus (PARAV) | |
| Simbu virus (SIMV) | ||
| Snowshoe hare orthobunyavirus | Khatanga virus (KHATV)9 | |
| snowshoe hare virus (SSHV) | ||
| Sororoca orthobunyavirus | Sororoca virus (SORV) | |
| Tacaiuma orthobunyavirus | CoAr 1071 virus (CA1071V) | |
| CoAr 3627 virus (CA3626V) | ||
| Tacaiuma virus (TCMV) | ||
| Virgin River virus (VRV) | ||
| Tahyna orthobunyavirus | Ťahyňa virus (TAHV) | |
| Tataguine orthobunyavirus | Tataguine virus (TATV) | |
| Tensaw orthobunyavirus | Tensaw virus (TENV) | |
| Tete orthobunyavirus | Bahig virus (BAHV) | |
| Matruh virus (MTRV) | ||
| Tete virus (TETEV) | ||
| Tsuruse virus (TSUV) | ||
| Weldona virus (WELV) | ||
| Thimiri orthobunyavirus | Thimiri virus (THIV) | |
| Timboteua orthobunyavirus | Timboteua virus (TBTV) | |
| Trivittatus orthobunyavirus | Achiote virus (ACHOV) | |
| Trivittatus virus (TVTV) | ||
| Turlock orthobunyavirus | Lednice virus (LEDV) | |
| Turlock virus (TURV) | ||
| Umbre virus (UMBV) | ||
| Utinga orthobunyavirus | Utinga virus (UTIV) | |
| Witwatersrand orthobunyavirus | Witwatersrand virus (WITV) | |
| Wolkberg orthobunyavirus | Wolkberg virus (WBV) | |
| Wyeomyia orthobunyavirus | Rio Pracupi virus | |
| Taiassui virus (TAIAV) | ||
| Tucunduba virus (TUCV) | ||
| Wyeomyia virus (WYOV) | ||
| Zegla orthobunyavirus | Zegla virus (ZEGV) | |
| Pacuvirus | Caimito pacuvirus | Caimito virus (CAIV) |
| Chilibre pacuvirus | Chilibre virus (CHIV) | |
| Pacui pacuvirus* | Pacui virus (PACV) | |
| Rio Preto da Eva pacuvirus | Rio Preto da Eva virus (RPEV) | |
| Tapirape pacuvirus | Tapirapé virus (TAPV) | |
| Shangavirus | Insect shangavirus* | Shuāngào insect virus 1 (SgIV-1) |
| Family Phasmaviridae | ||
| Feravirus | Ferak feravirus* | Ferak virus (FRKV) |
| Jonvirus | Jonchet jonvirus* | jonchet virus (JONV) |
| Orthophasmavirus | Anopheles orthophasmavirus | Anopheles triannulatus orthophasmavirus (AtOPV) |
| Culex orthophasmavirus | Culex orthophasmavirus (CPLV) | |
| Ganda orthophasmavirus | Ganda orthophasmavirus (GBEEV) | |
| Kigluaikphantom orthophasmavirus* | Kigluaik phantom virus (KIGV) | |
| Odonate orthophasmavirus | Odonate orthophasmavirus (HbOV-8) | |
| Qingling orthophasmavirus | Qingling orthophasmavirus (HbOV-9) | |
| Wuchang cockroach orthophasmavirus 1 | Wǔchāng cockroach virus 1 (WcCV-1) | |
| Wuhan mosquito orthophasmavirus 1 | Wǔhàn mosquito virus 1 (WhMV-1) | |
| Wuhan mosquito orthophasmavirus 2 | Wǔhàn mosquito virus 2 (WhMV-2) | |
| Sawastrivirus | Sanxia sawastrivirus* | Sānxiá water strider virus 2 (SxWSV-2) |
| Wuhivirus | Insect wuhivirus* | Wǔhàn insect virus 2 (WhIV-2) |
| Family Phenuiviridae | ||
| Bandavirus | Bhanja bandavirus | Bhanja virus (BHAV) |
| Dabie bandavirus* | severe fever with thrombocytopenia syndrome virus (SFTSV) | |
| Guertu bandavirus | Guertu virus (GTV) | |
| Heartland bandavirus | Heartland virus (HRTV) | |
| Hunter Island bandavirus | Hunter Island virus (HUIV) | |
| Kismaayo bandavirus | Kismaayo virus (KISV) | |
| Lone Star bandavirus1 | lone star virus (LSV) | |
| Beidivirus | Dipteran beidivirus* | Húběi diptera virus 3 (HbDV-3) |
| Coguvirus | Citrus coguvirus* | citrus concave gum-associated virus (CCGaV) |
| Coguvirus eburi | citrus virus A (CiV-A) | |
| Entovirus | Entoleuca entovirus* | Entoleuca phenui-like virus 1 (EnPLV-1) |
| Goukovirus | Cumuto goukovirus | Cumuto virus (CUMV) |
| Gouleako goukovirus* | Gouléako virus (GOLV) | |
| Yichang insect goukovirus | Yíchāng insect virus (YcIV) | |
| Horwuvirus | Horsefly horwuvirus* | Wǔhàn horsefly virus (WhHV) |
| Hudivirus | Dipteran hudivirus* | Húběi diptera virus 4 (HbDV-4) |
| Hudovirus | Lepidopteran hudovirus* | Húběi lepidoptera virus 1 (HbLV-1) |
| Ixovirus | Blackleg ixovirus* | blacklegged tick virus 1 (BLTV-1) |
| Norway ixovirus | Fairhair virus (FHAV) | |
| Scapularis ixovirus | blacklegged tick virus 3 (BLTV-3) | |
| Laulavirus | Laurel Lake laulavirus* | Laurel Lake virus (LLV) |
| Lentinuvirus | Lentinula lentinuvirus* | Lentinula edodes negative-strand RNA virus 2 (LeNSRV-2) |
| Mobuvirus | Mothra mobuvirus* | Mothra virus (MTHV) |
| Phasivirus | Badu phasivirus* | Badu virus (BADUV) |
| Dipteran phasivirus | Húběi diptera virus 5 (HbDV-5) | |
| Fly phasivirus | Wǔhàn fly virus 1 (WhFV-1) | |
| Phasi Charoen-like phasivirus | Phasi Chaeron-like virus (PCLV) | |
| Wutai mosquito phasivirus | Wǔtái mosquito virus (WtMV) | |
| Phlebovirus | Adana phlebovirus | Adana virus (ADAV) |
| Aguacate phlebovirus | Aguacate virus (AGUV) | |
| Alcube phlebovirus | Alcube virus (ACBV) | |
| Alenquer phlebovirus | Alenquer virus (ALEV) | |
| Ambe phlebovirus | Ambe virus (ABEV) | |
| Anhanga phlebovirus | Anhangá virus (ANHV) | |
| Arumowot phlebovirus | Arumowot virus (AMTV) | |
| Buenaventura phlebovirus | Buenaventura virus (BUEV) | |
| Bujaru phlebovirus | Bujaru virus (BUJV) | |
| Cacao phlebovirus | Cacao virus (CACV) | |
| Campana phlebovirus | Campana virus (CMAV) | |
| Candiru phlebovirus | Ariquemes virus (ARQV) | |
| Candirú virus (CDUV) | ||
| Jacundá virus (JCNV) | ||
| Morumbi virus (MR(M)BV) | ||
| Mucura virus (MCRV/MRAV) | ||
| Serra Norte virus (SRNV) | ||
| Chagres phlebovirus | Chagres virus (CHGV) | |
| Cocle phlebovirus | Coclé virus (CCLV) | |
| Dashli phlebovirus | Dāshlī virus (DASV) | |
| Durania phlebovirus | Durania virus (DRNV) | |
| Echarate phlebovirus | Echarate virus (ECHV) | |
| Gabek phlebovirus | Gabek forest virus (GFV) | |
| Gordil phlebovirus | Gordil virus (GORV) | |
| Icoaraci phlebovirus | Icoaraci virus (ICOV) | |
| Itaituba phlebovirus | Itaituba virus (ITAV) | |
| Itaporanga phlebovirus | Itaporanga virus (ITPV) | |
| Ixcanal phlebovirus | Ixcanal virus (IXCV) | |
| Karimabad phlebovirus | Karimabad virus (KARV) | |
| La Gloria phlebovirus | La Gloria virus (LAGV) | |
| Lara phlebovirus | Rio Claro virus (RICV) | |
| Leticia phlebovirus | Leticia virus (LTCV) | |
| Maldonado phlebovirus | Maldonado virus (MLOV) | |
| Mariquita phlebovirus | Mariquita virus (MRQV) | |
| Massilia phlebovirus | Massilia virus (MASV) | |
| Medjerda phlebovirus | Medjerda Valley virus (MVV) | |
| Mona Grita phlebovirus | Mona Grita virus (MOGV) | |
| Mukawa phlebovirus | Mukawa virus (MKWV) | |
| Munguba phlebovirus | Munguba virus (MUNV) | |
| Naples phlebovirus | Arrábida virus (ARRV) | |
| Balkan virus (BALKV) | ||
| Fermo virus (FERV) | ||
| Granada virus (GRV = GRAV) | ||
| Saddaguia virus (SADV) | ||
| sandfly fever Naples virus (SFNV) | ||
| Nique phlebovirus | Nique virus (NIQV) | |
| Ntepes phlebovirus | Ntepes virus (NTPV) | |
| Odrenisrou phlebovirus | Odrénisrou virus (ODRV) | |
| Oriximina phlebovirus | Oriximiná virus (ORXV) | |
| Pena Blanca phlebovirus | Peña Blanca virus (PEBV) | |
| Punique phlebovirus | Punique virus (PUNV) | |
| Punta Toro phlebovirus | Buenaventura virus (BUEV) | |
| Capira virus (CAPIV) | ||
| Punta Toro virus (PTV) | ||
| Rift Valley fever phlebovirus* | Rift Valley fever virus (RVFV) | |
| Rio Grande phlebovirus | Rio Grande virus (RGV) | |
| Saint Floris phlebovirus | Saint-Floris virus (SAFV) | |
| Salanga phlebovirus | Salanga virus (SLGV) | |
| Salehabad phlebovirus | Adria virus (ADRV) | |
| Arbia virus (ARBV) | ||
| Bregalaka virus (BREV) | ||
| Olbia virus (OLBV) | ||
| Salehabad virus (SALV) | ||
| Zaba virus (ZABAV) | ||
| Salobo phlabovirus [sic]2 | Salobo virus (SLBOV) | |
| Sicilian phlebovirus | sandfly fever Sicilian virus (SFSV) | |
| Tapara phlebovirus | Tapará virus (TPRV) | |
| Tehran phlebovirus | Tehran virus (THEV) | |
| Tico phebovirus [sic]3 | Tico virus (TICV) | |
| Toros phlebovirus | Toros virus (TORV) | |
| Toscana phlebovirus | Toscana virus (TOSV) | |
| Tres Almendras phlebovirus | Tres Almendras virus (TRAV) | |
| Turuna phlebovirus | Turuna virus (TUAV) | |
| Uriurana phlebovirus | Uriurana virus (URIV) | |
| Urucuri phlebovirus | Urucuri virus (URUV) | |
| Viola phlebovirus | viola virus (VIOV) | |
| Zerdali phlebovirus | Zerdali virus (ZERV) | |
| Pidchovirus | Pidgey pidchovirus* | Pidgey virus (PGYV) |
| Rubodvirus | Apple rubodvirus 1* | apple rubbery wood virus 1 (ARWV-1) |
| Apple rubodvirus 2 | apple rubbery wood virus 2 (ARWV-2) | |
| Tenuivirus | Echinochloa hoja blanca tenuivirus | Echinochloa hoja blanca virus (EHBV) |
| Iranian wheat stripe tenuivirus | Iranian wheat stripe virus (IWSV) | |
| Maize stripe tenuivirus | maize stripe virus (MStV = MSpV) | |
| Melon tenuivirus | melon chlorotic spot virus (MeCSV) | |
| Rice grassy stunt tenuivirus | rice grassy stunt virus (RGSV) | |
| Rice hoja blanca tenuivirus | rice hoja blanca virus (RHBV) | |
| Rice stripe tenuivirus* | rice stripe virus (RSV = RStV) | |
| Urochloa hoja blanca tenuivirus | Urochloa hoja blanca virus (UHBV) | |
| Uukuvirus | American dog uukuvirus | American dog tick virus (ADAV) |
| Dabieshan uukuvirus | Dàbiéshān tick virus (DBSH) | |
| Grand Arbaud uukuvirus | Grand Arbaud virus (GAV) | |
| Huangpi uukuvirus | Huángpí tick virus 2 (HpTV-2) | |
| Kabuto mountain uukuvirus | Kabuto mountain virus (KAMV) | |
| Kaisodi uukuvirus | Kaisodi virus (KASDV) | |
| Lihan uukuvirus | Lǐhán tick virus (LITV) | |
| Manawa uukuvirus | Manawa virus (MWAV) | |
| Murre uukuvirus | murre virus (MURV) | |
| Pacific coast uukuvirus | Pacific coast tick virus (PACV) | |
| Precarious Point uukuvirus | Precarious Point virus (PPV) | |
| Rukutama uukuvirus | Rukutama virus (RUKV) | |
| Schmid uukuvirus | Nile Warbler virus (NIWV) | |
| Silverwater uukuvirus | Silverwater virus (SILV) | |
| Tacheng uukuvirus | Tǎchéng tick virus 2 (TCGV) | |
| Uukuniemi uukuvirus* | Chizé virus (CHZV) | |
| Fin V 707 virus (FINV) | ||
| Oceanside virus (OCV = OCEV) | ||
| Pontevès virus (PTVV) | ||
| St. Abbs Head virus (SAHV) | ||
| Uukuniemi virus (UUKV) | ||
| Yongjia uukuvirus | Yǒngjiā tick virus (YONV) | |
| Zaliv Terpeniya uukuvirus | Zaliv Terpeniya virus (ZTV) | |
| Wenrivirus | Shrimp wenrivirus* | Wēnzhōu shrimp virus 1 (WzSV-1) |
| Family Tospoviridae | ||
| Orthotospovirus | Alstroemeria necrotic streak orthotospovirus | Alstroemeria necrotic streak virus (ANSV) |
| Alstroemeria yellow spot orthotospovirus | Alstroemeria yellow spot virus (AYSV) | |
| Bean necrotic mosaic orthotospovirus | bean necrotic mosaic virus (BeNMV) | |
| Calla lily chlorotic spot orthotospovirus | calla lily chlorotic spot virus (CCSV) | |
| Capsicum chlorosis orthotospovirus | Capsicum chlorosis virus (CaCV) | |
| Chrysanthemum stem necrosis orthotospovirus | Chrysanthemum stem necrosis virus (CSNV) | |
| Groundnut bud necrosis orthotospovirus | groundnut bud necrosis virus (GBNV) | |
| Groundnut chlorotic fan spot orthotospovirus | groundnut chlorotic fan-spot virus (GCFSV) | |
| Groundnut ringspot orthotospovirus | groundnut ringspot virus (GRSV) | |
| Groundnut yellow spot orthotospovirus | groundnut yellow spot virus (GYSV) | |
| Hippeastrum chlorotic ringspot orthotospovirus | Hippeastrum chlorotic spot virus (HCRV) | |
| Impatiens necrotic spot orthotospovirus | impatiens necrotic spot virus (INSV) | |
| Iris yellow spot orthotospovirus | iris yellow spot virus (IYSV) | |
| Melon severe mosaic orthotospovirus | melon severe mosaic virus (MSMV) | |
| Melon yellow spot orthotospovirus | melon yellow spot virus (MYSV) | |
| Mulberry vein banding associated orthotospovirus | mulberry vein banding-associated virus (MVBaV) | |
| Pepper chlorotic spot orthotospovirus | pepper chlorotic spot virus (PCSV) | |
| Polygonum ringspot orthotospovirus | Polygonum ringspot virus (PolRSV) | |
| Soybean vein necrosis orthotospovirus | soybean vein necrosis virus (SVNV) | |
| Tomato chlorotic spot orthotospovirus | tomato chlorotic spot virus (TCSV) | |
| Tomato spotted wilt orthotospovirus* | tomato spotted wilt virus (TSWV) | |
| Tomato yellow ring orthotospovirus | tomato yellow ring virus (TYRV) | |
| Tomato zonate spot orthotospovirus | tomato zonate spot virus (TZSV) | |
| Watermelon bud necrosis orhtotospovirus | watermelon bud necrosis virus (WBNV) | |
| Watermelon silver mottle orthotospovirus | watermelon silver mottle virus (WSMoV) | |
| Zucchini lethal chlorosis orthotospovirus | zucchini lethal chlorosis virus (ZLCV) | |
| Family Wupedeviridae | ||
| Wumivirus | Millipede wumivirus* | Wǔhàn millipede virus 2 (WhMV-2) |
Note that viruses are real objects that are assigned to concepts that are called taxa. Species, genera, subfamilies, families, and orders are taxa.
type species
Due to a formal classification mistake this species was named Lone Star bandavirus instead of Lone star bandavirus. A proposal to correct this mistake will be submitted prior to the next taxonomic proposal submission deadline.
Due to a formal classification mistake this species was named Salobo phlabovirus instead of Salobo phlebovirus. A proposal to correct this mistake will be submitted prior to the next taxonomic proposal submission deadline.
Due to a formal classification mistake this species was named Tico phebovirus instead of Tico phlebovirus. A proposal to correct this mistake will be submitted prior to the next taxonomic proposal submission deadline
Taxon names are always italicized and always begin with a capital letter.
Virus names are not italicized and are not capitalized, except if the name or a name component is a proper noun. This column lists the virus names with their correct (lack of) capitalization. Lists of viruses within a given species are provisional at this point and will likely be amended in the near future.
ACKNOWLEDGEMENTS
We thank W. Ian Lipkin and Rafal Tokarz (Columbia University Irving Medical Center, New York, New York, USA) for providing/approving new names for “blacklegged tick phleboviruses 1 and 3” and Edward Holmes (University of Sydney, Australia) for providing/approving a new name for “Norway phlebovirus”. We would like to thank Anya Crane (NIH/NIAID/DCR/IRF-Frederick).
Funding
This work was supported in part through Laulima Government Solutions, LLC prime contract with the US National Institute of Allergy and Infectious Diseases (NIAID) under Contract No. HHSN272201800013C. J.H.K. performed this work as an employee of Tunnell Government Services (TGS), a subcontractor of Laulima Government Solutions, LLC under Contract No. HHSN272201800013C. This project has been funded in whole or in part with federal funds from the National Cancer Institute (NCI), National Institutes of Health (NIH), under Contract No. 75N91019D00024, Task Order No. 75N91019F00130. This work was also funded in part by Contract No. HSHQDC-15-C-00064 awarded by the US Department of Homeland Security (DHS) Science and Technology Directorate (S&T) for the management and operation of The National Biodefense Analysis and Countermeasures Center (NBACC), a federally funded research and development center operated by the Battelle National Biodefense Institute (V.W.); and NIH contract HHSN272201000040I/HHSN27200004/D04 and grant R24AI120942 (N.V., R.B.T.). S.S. acknowledges partial support from the Special Research Initiative of Mississippi Agricultural and Forestry Experiment Station (MAFES), Mississippi State University, and the National Institute of Food and Agriculture, US Department of Agriculture, Hatch Project 1021494.
Footnotes
The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the US Department of the Army, the US Department of Defense, the US Department of Health and Human Services, the US Department of Homeland Security (DHS) Science and Technology Directorate (S&T), or of the institutions and companies affiliated with the authors. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. In no event shall any of these entities have any responsibility or liability for any use, misuse, inability to use, or reliance upon the information contained herein. The US departments do not endorse any products or commercial services mentioned in this publication. The US Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes.
Conflict of Interest
The authors declare no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Jens H. Kuhn, Integrated Research Facility at Fort Detrick, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Frederick, Maryland, USA.
Scott Adkins, United States Department of Agriculture, Agricultural Research Service, US Horticultural Research Laboratory, Fort Pierce, Florida, USA.
Daniela Alioto, Dipartimento di Agraria, Università degli Studi di Napoli Federico II, Portici, Italy.
Sergey V. Alkhovsky, D.I. Ivanovsky Institute of Virology of N.F. Gamaleya National Center on Epidemiology and Microbiology of Ministry of Health of Russian Federation, Russia.
Gaya Amarasinghe, Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, Missouri, USA.
Simon J. Anthony, Mailman School of Public Health, Columbia University, New York, New York, USA EcoHealth Alliance, New York, New York, USA.
Tatjana Avšič-Županc, University of Ljubljana, Ljubljana Faculty of Medicine, Slovenia.
María A. Ayllón, Centro de Biotecnología y Genómica de Plantas, Universidad Politécnica de Madrid—Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria, Campus de Montegancedo, Pozuelo de Alarcón, Madrid, Spain; Departamento de Biotecnología-Biología Vegetal, Escuela Técnica Superior de Ingeniería Agronómica, Alimentaria y de Biosistemas, Universidad Politécnica de Madrid, Madrid, Spain.
Justin Bahl, Center for Ecology of Infectious Diseases, Department of Infectious Diseases, Department of Epidemiology and Biostatistics, Insitute of Bioinformatics, University of Georgia, Athens GA, USA.
Anne Balkema-Buschmann, Friedrich-Loeffler-Institut, Federal Research Institute for Animal Health, Institute of Novel and Emerging Infectious Diseases, Greifswald-Insel Riems, Germany.
Matthew J. Ballinger, Department of Biological Sciences, Mississippi State University, Mississippi State, Mississippi, USA.
Tomáš Bartonička, Department of Botany and Zoology, Masaryk University, Czech Republic.
Christopher Basler, Center for Microbial Pathogenesis, Institute for Biomedical Sciences, Georgia State University, Atlanta, Georgia, USA.
Sina Bavari, Edge BioInnovation Consulting and Mgt, Frederick, Maryland, USA.
Martin Beer, Institute of Diagnostic Virology, Friedrich-Loeffler-Institut, Greifswald-Insel Riems, Germany.
Dennis A. Bente, University of Texas Medical Branch, Galveston, Texas, USA.
Éric Bergeron, Viral Special Pathogens Branch, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Brian H. Bird, School of Veterinary Medicine, One Health Institute, University of California, Davis California USA.
Carol D. Blair, Dept. of Microbiology, Immunology & Pathology, Colorado State University, Fort Collins, CO USA.
Kim R. Blasdell, Commonwealth Scientific and Industrial Research Organisation (CSIRO), Australian Centre for Disease Preparedness, Geelong, Victoria, Australia.
Steven Bradfute, University of New Mexico Health Sciences Center, Albuquerque, New Mexico, USA.
Rachel Breyta, US Geological Survey, Western Fisheries Research Center, Seattle, Washington, USA.
Thomas Briese, Center for Infection and Immunity, and Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York, USA.
Paul A. Brown, French Agency for Food, Environmental and Occupational Heath Safety ANSES, Laboratory of Ploufragan-Plouzané-Niort, Ploufragan, France.
Ursula J. Buchholz, RNA Viruses Section, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Michael J. Buchmeier, Department of Molecular Biology and Biochemistry, University of California, Irvine, California, USA.
Alexander Bukreyev, The University of Texas Medical Branch at Galveston, Galveston Natonal Laboratory, Galveston, Texas, USA; Galveston National Laboratory, The University of Texas, Medical Branch at Galveston, Galveston, TX, USA.
Felicity Burt, Division of Virology, National Health Laboratory Service and Division of Virology, University of the Free State, Bloemfontein, Republic of South Africa.
Nihal Buzkan, UNIVERSITY OF KAHRAMANMARAS SÜTÇÜ IMAM FACULTY OF AGRICULTURE, DEPARTMENT OF PLANT PROTECTION AVSAR CAMPUS, 46060 KAHRAMANMARAS, TURKEY.
Charles H. Calisher, Colorado State University, Fort Collins, Colorado.
Mèngjí Cáo, National Citrus Engineering Research Center, Citrus Research Institute, Southwest University, Chongqing, 400712, China; Academy of Agricultural Sciences, Southwest University, Chongqing, 400715, China.
Inmaculada Casas, Respiratory Virus and Influenza Unit, National Microbiology Center, Instituto de Salud Carlos III, Madrid, Spain.
John Chamberlain, Virology and Pathogenesis Group, National Infection Service, Public Health England, Porton Down, United Kingdom.
Kartik Chandran, Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York, USA.
Rémi N. Charrel, Unité des Virus Emergents (Aix-Marseille Univ – IRD 190 – Inserm 1207 – IHU Méditerranée Infection), Marseille, France.
Biao Chen, Guangdong Province Key Laboratory of Microbial Signals and Disease Control, College of Agriculture, South China Agricultural University Guangdong, China.
Michela Chiumenti, Istituto per la Protezione Sostenibile delle Piante - Consiglio Nazionale delle ricerche (Institute for Sustainable Plant Protection - National Research Council), ITALY.
Il-Ryong Choi, Plant Breeding Genetics and Biotechnology Division and International Rice Research Institute, Los Baños, Philippines.
J. Christopher S. Clegg, Les Mandinaux, Le Grand Madieu, France.
Ian Crozier, Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research, Frederick, Maryland USA.
John V. da Graça, Texas A&M University-Kingsville Citrus Center, Weslaco Texas, USA.
Elena Dal Bó, CIDEFI. Facultad de Ciencias Agrarias y Forestales, Universidad de La Plata, La Plata, Argentina.
Alberto M. R. Dávila, Fundação Oswaldo Cruz-Fiocruz, Instituto Oswaldo Cruz, Laboratório de Biologia Computacional e Sistemas, Rio de Janeiro, RJ, Brasil.
Xavier de Lamballerie, Unité des Virus Emergents (Aix-Marseille Univ – IRD 190 – Inserm 1207 – IHU Méditerranée Infection), Marseille, France.
Juan Carlos de la Torre, Department of Immunology and Microbiology IMM-6, The Scripps Research Institute, La Jolla, California, USA.
Rik L. de Swart, Department Viroscience, Erasmus MC, University Medical Centre Rotterdam, The Netherlands.
Patrick L. Di Bello, Department of Botany and Plant Pathology, Oregon State University, Corvallis, OR, 97331, USA.
Nicholas Di Paola, United States Army Medical Research Institute of Infectious Diseases, Fort Detrick, Frederick, Maryland, USA.
Francesco Di Serio, Istituto per la Protezione Sostenibile delle Piante, Consiglio Nazionale delle Ricerche, Bari, Italy.
Ralf G. Dietzgen, Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, St. Lucia, Queensland, Australia.
Michele Digiaro, CIHEAM, Istituto Agronomico Mediterraneo di Bari, Valenzano, Italy.
Valerian V. Dolja, Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon, USA.
Olga Dolnik, Institute of Virology, Philipps University Marburg, Marburg, Germany.
Michael A. Drebot, Zoonotic Diseases and Special Pathogens, National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, Manitoba, Canada;.
J. Felix Drexler, Institute of Virology, Charité-Universitätsmedizin Berlin, corporate member of Free University Berlin, Humboldt-University Berlin, and Berlin Institute of Health, Berlin, Germany.
Ralf Dürrwald, Robert Koch Institut, Berlin, Germany.
Lucie Dufkova, Veterinary Research Institute, Brno, Czech Republic.
William G. Dundon, Animal Production and Health Laboratory, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Department of Nuclear Sciences and Applications, International Atomic Energy Agency, Vienna, Austria.
W. Paul Duprex, University of Pittsburgh, School of Medicine, Pittsburgh, Pennsylvania, USA.
John M. Dye, United States Army Medical Research Institute of Infectious Diseases, Fort Detrick, Frederick, Maryland, USA.
Andrew J. Easton, School of Life Sciences, University of Warwick, Coventry, UK.
Hideki Ebihara, Department of Molecular Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Toufic Elbeaino, Istituto Agronomico Mediterraneo di Bari, Valenzano, Italy.
Koray Ergünay, Virology Unit, Department of Medical Microbiology, Hacettepe University Faculty of Medicine, Ankara, Turkey.
Jorlan Fernandes, Fundação Oswaldo Cruz-Fiocruz, Instituto Oswaldo Cruz, Laboratório de Hantaviroses e Rickettsioses, Rio de Janeiro, RJ, Brasil.
Anthony R. Fooks, Animal and Plant Health Agency, Weybridge, Surrey, UK.
Pierre B. H. Formenty, World Health Organization, Geneva, Switzerland.
Leonie Forth, Institute of Diagnostic Virology, Friedrich-Loeffler-Institut, Greifswald-Insel Riems, Germany.
Ron Fouchier, Department Viroscience, Erasmus MC, University Medical Centre Rotterdam, the Netherlands.
Juliana Freitas-Astúa, Embrapa Cassava and Fruits, Cruz das Almas, Bahia, Brazil.
Selma Gago-Zachert, Institute of Biochemistry and Biotechnology, Martin Luther University Halle-Wittenberg, Halle/Saale, Germany; Department of Molecular Signal Processing, Leibniz Institute of Plant Biochemistry, Halle/Saale, Germany.
George Fú Gāo, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
María Laura García, Instituto de Biotecnología y Biología Molecular, Facultad de Ciencias Exactas, CONICET UNLP, La Plata, Argentina.
Adolfo García-Sastre, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Aura R. Garrison, United States Army Medical Research Institute of Infectious Diseases, Fort Detrick, Frederick, Maryland, USA.
Aiah Gbakima, Metabiota, Inc. Sierra Leone, Freetown, Sierra Leone.
Tracey Goldstein, One Health Institute & Karen C. Drayer Wildlife Health Center, School of Veterinary Medicine, University of California, Davis, California, USA.
Jean-Paul J. Gonzalez, Department of Microbiology & Immunology, Division of Biomedical Graduate Research Organization, School of Medicine, Georgetown University, Washington, DC 20057, USA; Centaurus Biotechnologies, CTP, Manassas, VA, USA.
Anthony Griffiths, Department of Microbiology and National Emerging Infectious Diseases Laboratories, Boston University School of Medicine, Boston, Massachusetts, USA.
Martin H. Groschup, Institute of Novel and Emerging Infectious Diseases, Friedrich-Loeffler-Institut, Greifswald-Insel Riems, Germany.
Stephan Günther, Bernhard-Nocht Institute for Tropical Medicine, WHO Collaborating Centre for Arboviruses and Hemorrhagic Fever Reference and Research, Department of Virology, Hamburg, Germany.
Alexandro Guterres, Fundação Oswaldo Cruz-Fiocruz, Instituto Oswaldo Cruz, Laboratório de Hantaviroses e Rickettsioses, Rio de Janeiro, RJ, Brasil.
Roy A. Hall, Australian Infectious Diseases Research Centre, School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, Australia.
John Hammond, United States Department of Agriculture, Agricultural Research Service, USNA, Floral and Nursery Plants Research Unit, Beltsville, Maryland, USA.
Mohamed Hassan, Department of Agricultural Botany, Faculty of Agriculture, Fayoum University, Fayoum, Egypt.
Jussi Hepojoki, University of Helsinki, Medicum, Department of Virology, Helsinki, Finland; Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland.
Satu Hepojoki, Mobidiag Ltd, Espoo, Finland; University of Helsinki, Medicum, Department of Virology, Helsinki, Finland.
Udo Hetzel, Institute of VeterinarypPathology, University of Zuerich, Switzerland.
Roger Hewson, Public Health England, Porton Down, Wiltshire, Salisbury, UK.
Bernd Hoffmann, Friedrich-Loeffler-Institut, Greifswald-Insel Riems, Germany.
Seiji Hongo, Department of Infectious Diseases, Yamagata University Faculty of Medicine, Yamagata, Japan.
Dirk Höper, Friedrich-Loeffler-Institut, Greifswald-Insel Riems, Germany.
Masayuki Horie, Hakubi Center for Advanced Research, Kyoto University, Kyoto, Japan.
Holly R. Hughes, Centers for Disease Control and Prevention, Fort Collins, Colorado, USA.
Timothy H. Hyndman, School of Veterinary Medicine, Murdoch University, Murdoch, Western Australia, Australia.
Amara Jambai, Ministry of Health and Sanitation, Freetown, Sierra Leone.
Rodrigo Jardim, Fundação Oswaldo Cruz-Fiocruz, Instituto Oswaldo Cruz, Laboratório de Biologia Computacional e Sistemas, Rio de Janeiro, RJ, Brasil.
Dàohóng Jiāng, State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, Hubei Province, China.
Qi Jin, Ministry of Health Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Hangzhou, China.
Gilda B. Jonson, Department of Agricultural Biotechnology, Center for Fungal Pathogenesis, College of Agriculture and Life Sciences, Seoul National University, Seoul, Korea.
Sandra Junglen, Institute of Virology, Charité-Universitätsmedizin Berlin, corporate member of Free University Berlin, Humboldt-University Berlin, and Berlin Institute of Health, Berlin, Germany; German Centre for Infection Research, Berlin, Germany.
Serpil Karadağ, REPUBLIC OF TURKEY MINISTRY OF AGRICULTURE AND FORESTRY,PISTACHIO RESEARCH INSTITUTE, GAZIANTEP, TURKEY.
Karen E. Keller, United States Department of Agriculture, Agricultural Research Service, Horticulture Crops Research Unit, Corvallis, Oregon, USA
Boris Klempa, Institute of Virology, Biomedical Research Center, Slovak Academy of Sciences, Bratislava, Slovakia.
Jonas Klingström, Center for Infectious Medicine, Department of Medicine Huddinge, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden.
Gary Kobinger, Department of Microbiology, Immunology & Infectious Diseases, Université Laval, Quebec City, Canada.
Hideki Kondō, Institute of Plant Science and Resources, Okayama University, Kurashiki, Japan.
Eugene V. Koonin, National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health, Bethesda, Maryland, USA.
Mart Krupovic, Archaeal Virology Unit, Institut Pasteur, Paris, France.
Gael Kurath, US Geological Survey Western Fisheries Research Center, Seattle, Washington, USA.
Ivan V. Kuzmin, US Department of Agriculture, Animal and Plant Health Inspection, National Veterinary Services Laboratories, Diagnostic Virology Laboratory, USA.
Lies Laenen, KU Leuven, Rega Institute, Zoonotic Infectious Diseases unit, Leuven, Belgium; Department of Laboratory Medicine, University Hospitals Leuven, Leuven, Belgium.
Robert A. Lamb, Department of Molecular Biosciences, Northwestern University, Evanston, Illinois, USA; Howard Hughes Medical Institute, Northwestern University, Evanston, Illinois, USA.
Amy J. Lambert, Centers for Disease Control and Prevention, Fort Collins, Colorado, USA.
Stanley L. Langevin, Department of Microbiology, University of Washington, Washington, USA.
Benhur Lee, Department of Microbiology, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Elba R. S. Lemos, Fundação Oswaldo Cruz-Fiocruz, Instituto Oswaldo Cruz, Laboratório de Hantaviroses e Rickettsioses, Rio de Janeiro, RJ, Brasil.
Eric M. Leroy, MIVEGEC (IRD-CNRS-Montpellier university) unit, French National Research Institute for Sustainable Development (IRD), Montpellier, France.
Dexin Li, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Jiànróng Lǐ, Department of Veterinary Biosciences, College of Veterinary Medicine, The Ohio State University, Columbus, Ohio, USA.
Mifang Liang, Key Laboratory for Medical Virology, NHFPC, National Institute for Viral Disease Control and Prevention, China.
Wénwén Liú, State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China.
Yàn Liú, State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China.
Igor S. Lukashevich, Department of Pharmacology and Toxicology, School of Medicine, and the Center for Predictive Medicine for Biodefense and Emerging Infectious Diseases, University of Louisville, Louisville, Kentucky, USA.
Piet Maes, KU Leuven, Rega Institute, Zoonotic Infectious Diseases unit, Leuven, Belgium.
William Marciel de Souza, Virology Research Center, University of São Paulo, Ribeirão Preto, Brazil.
Marco Marklewitz, Institute of Virology, Charité-Universitätsmedizin Berlin, corporate member of Free University Berlin, Humboldt-University Berlin, and Berlin Institute of Health, Berlin, Germany; German Center for Infection Research (DZIF), Berlin, Germany.
Sergio H. Marshall, Pontificia Universidad Católica de Valparaíso, Campus Curauma, Valparaíso, Chile.
Giovanni P. Martelli, Department of Plant, Soil and Food Sciences, University “Aldo Moro,” Bari, Italy.
Robert R. Martin, United States Department of Agriculture, Horticultural Crops Research Unit, Corvallis, Oregon, USA.
Shin-Yi L. Marzano, Department of Biology and Microbiology, Department of Plant Sciences, South Dakota State University, Brookings, South Dakota, USA.
Sébastien Massart, Liège University, Gembloux Agro-Bio Tech, TERRA, Plant Pathology Laboratory, Belgium.
John W. McCauley, Worldwide Influenza Centre, Francis Crick Institute, London, UK.
Nicole Mielke-Ehret, Biocentre Klein Flottbek, University of Hamburg, Hamburg, Germany.
Angelantonio Minafra, Istituto per la Protezione Sostenibile delle Piante - Consiglio Nazionale delle ricerche (Institute for Sustainable Plant Protection - National Research Council), ITALY.
Maria Minutolo, Dipartimento di Agraria, Università degli Studi di Napoli Federico II, Portici, Italy.
Ali Mirazimi, Folkhalsomyndigheten, Stockholm, Sweden.
Hans-Peter Mühlbach, Biocentre Klein Flottbek, University of Hamburg, Hamburg, Germany.
Elke Mühlberger, Department of Microbiology and National Emerging Infectious Diseases Laboratories, Boston University School of Medicine, Boston, Massachusetts, USA.
Rayapati Naidu, Washington State University, Department of Plant Pathology, Irrigated Agricultural Research and Extension Center, Prosser, Washington, USA.
Tomohide Natsuaki, School of Agriculture, Utsunomiya University, Utsunomiya, Tochigi, Japan.
Beatriz Navarro, Istituto per la Protezione Sostenibile delle Piante, Consiglio Nazionale delle Ricerche, Bari, Italy.
José A. Navarro, Instituto de Biología Molecular y Celular de Plantas, Universitat Politècnica de València-Consejo Superior de Investigaciones Científicas, Valencia, Spain.
Sergey V. Netesov, Novosibirsk State University, Novosibirsk, Novosibirsk Oblast, Russia.
Gabriele Neumann, Influenza Research Institute, Dept. of Pathobiological Sciences, University of Wisconsin-Madison.
Norbert Nowotny, Institute of Virology, University of Veterinary Medicine Vienna, Vienna, Austria; College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, United Arab Emirates.
Márcio R. T. Nunes, Evandro Chagas Institute, Ministry of Health, Pará, Brazil.
Are Nylund, Fish Disease Research Group, Department of Biological Sciences, University of Bergen, Bergen, Norway.
Arnfinn L. Økland, Fish Disease Research Group, Department of Biological Sciences, University of Bergen, Bergen, Norway
Renata C. Oliveira, Fundação Oswaldo Cruz-Fiocruz, Instituto Oswaldo Cruz, Laboratório de Hantaviroses e Rickettsioses, Rio de Janeiro, RJ, Brasil.
Gustavo Palacios, United States Army Medical Research Institute of Infectious Diseases, Fort Detrick, Frederick, Maryland, USA.
Vicente Pallás, Instituto de Biología Molecular y Celular de Plantas (IBMCP), Consejo Superior de Investigaciones Cientificas-Universidad Politécnica de Valencia, Valencia, Spain..
Bernadett Pályi, National Biosafety Laboratory, National Public Health Center, Budapest, Hungary.
Anna Papa, National Reference Centre for Arboviruses and Haemorrhagic Fever viruses, Department of Microbiology, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Colin R. Parrish, Baker Institute for Animal Health, College of Veterinary Medicine, Cornell University, Ithaca, New York, USA.
Alex Pauvolid-Corrêa, Department of Veterinary Integrated Biosciences and Department of Entomology, Texas A&M University, College Station, USA.
Janusz T. Pawęska, Center for Emerging Zoonotic and Parasitic Diseases, National Institute for Communicable Diseases of the National Health Laboratory Service, Sandringham-Johannesburg, Gauteng, South Africa.
Susan Payne, Department of Veterinary Pathobiology, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, Texas, USA.
Daniel R. Pérez, Department of Population Health, College of Veterinary Medicine, University of Georgia, Athens, Georgia, USA.
Florian Pfaff, Institute of Diagnostic Virology, Friedrich-Loeffler-Institut, Greifswald – Insel Riems, Germany.
Sheli R. Radoshitzky, United States Army Medical Research Institute of Infectious Diseases, Fort Detrick, Frederick, Maryland, USA.
Aziz-ul Rahman, Institute of Microbiology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Pedro L. Ramos-González, Laboratório de Biologia Molecular Aplicada, Instituto Biológico, São Paulo, SP, Brazil.
Renato O. Resende, Departamento de Biologia Celular, Universidade de Brasília, Brasília, Brazil.
Carina A. Reyes, Instituto de Biotecnología y Biología Molecular, CCT-La Plata, CONICET-UNLP, La Plata, Buenos Aires, Argentina.
Bertus K. Rima, Centre for Experimental Medicine, School of Medicine, Dentistry and Biomedical Sciences, The Queen's University of Belfast, Belfast, Northern Ireland, UK.
Gabriel Robles Luna, Instituto de Biotecnología y Biología Molecular, CCT-La Plata, CONICET-UNLP, La Plata, Buenos Aires, Argentina.
Víctor Romanowski, Instituto de Biotecnología y Biología Molecular, Centro Cientifico Technológico-La Plata, Consejo Nacional de Investigaciones Científico Tecnológico—Universidad Nacional de La Plata, La Plata, Argentina.
Paul Rota, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Dennis Rubbenstroth, Institute of Diagnostic Virology, Friedrich-Loeffler-Institut, Greifswald – Insel Riems, Germany.
Jonathan A. Runstadler, Department of Infectious Disease & Global Health, Tufts University Cummings School of Veterinary Medicine, 200 Westboro Road, North Grafton, MA 01536 USA.
Daniel Ruzek, Veterinary Research Institute, Brno, Czech Republic; Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, Branisovska 31, CZ-37005 Ceske Budejovice, Czech Republic.
Sead Sabanadzovic, Department of Biochemistry, Molecular Biology, Entomology and Plant Pathology, Mississippi State University, Mississippi State, Mississippi, USA.
Jiří Salát, Veterinary Research Institute, Brno, Czech Republic; Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, Branisovska 31, CZ-37005 Ceske Budejovice, Czech Republic.
Amadou Alpha Sall, Institut Pasteur de Dakar, Dakar, Senegal.
Maria S. Salvato, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Kamil Sarpkaya, Karabuk University (UNIKA), Faculty of Forestry , Department of Forestry Engineering, TURKEY.
Takahide Sasaya, Western Region Agricultural Research Center, National Agriculture and Food Research Organization, Fukuyama, Japan.
Martin Schwemmle, University Medical Center-University Freiburg, Faculty of Medicine, Freiburg, Germany.
Muhammad Z. Shabbir, Institute of Microbiology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Xiǎohóng Shí, MRC-University of Glasgow Centre for Virus Research, Glasgow, Scotland, UK.
Zhènglì Shí, CAS Key Laboratory of Special Pathogens, Wuhan Institute of Virology, Center for Biosafety Mega-Science, Chinese Academy of Sciences, Wuhan, Hubei, People’s Republic of China..
Yukio Shirako, Asian Center for Bioresources and Environmental Sciences, University of Tokyo, Tokyo, Japan.
Peter Simmonds, Nuffield Department of Medicine, University of Oxford, Oxford, UK.
Jana Širmarová, Veterinary Research Institute, Brno, Czech Republic.
Manuela Sironi, Bioinformatics Unit, Scientific Institute IRCCS “E. Medea,” Bosisio Parini, Italy.
Sophie Smither, CBR Division, Dstl, Porton Down, Salisbury, Wiltshire, UK.
Teemu Smura, University of Helsinki, Medicum, Department of Virology, Helsinki, Finland.
Jin-Won Song, Department of Microbiology, College of Medicine, Korea University, Seoul, Republic of Korea.
Kirsten Spann, School of Biomedical Sciences, Faculty of Health, Queensland University of Technology, Brisbane, Queensland, Australia.
Jessica R. Spengler, Viral Special Pathogens Branch, Division of High-Consequence Pathogens & Pathology, Centers for Disease Control & Prevention.
Mark D. Stenglein, Department of Microbiology, Immunology, and Pathology, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, Colorado, USA.
David M. Stone, Centre for Environment, Fisheries and Aquaculture Science, Weymouth, Dorset, UK.
Petra Straková, Veterinary Research Institute, Brno, Czech Republic.
Ayato Takada, Division of Global Epidemiology, Research Center for Zoonosis Control, Hokkaido University, Sapporo, Japan.
Robert B. Tesh, The University of Texas Medical Branch, Galveston, Texas, USA.
Natalie J. Thornburg, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Keizō Tomonaga, Institute for Frontier Life and Medical Sciences (inFront), Kyoto University, Kyoto, Japan.
Noël Tordo, Institut Pasteur, Unité des Stratégies Antivirales, WHO Collaborative Centre for Viral Haemorrhagic Fevers and Arboviruses, OIE Reference Laboratory for RVFV & CCHFV, Paris, France & Institut Pasteur de Guinée, Conakry, Guinea.
Jonathan S. Towner, Viral Special Pathogens Branch, Division of High-Consequence Pathogens Pathology, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Massimo Turina, Institute for Sustainable Plant Protection, National Research Council of Italy (CNR), Strada delle Cacce 73, 10135 Torino, Italy.
Ioannis Tzanetakis, Dept. of Entomology & Plant Pathology, Division of Agriculture, University of Arkansas System, Fayetteville, AR 72701.
Rainer G. Ulrich, Friedrich-Loeffler-Institut, Federal Research Institute for Animal Health, Institute of Novel and Emerging Infectious Diseases, Südufer 10, 17493 Greifswald-Insel Riems, Germany, and Partner site Hamburg-Lübeck-Borstel-Insel Riems, Greifswald-Insel Riems, Germany.
Anna Maria Vaira, Institute for Sustainable Plant Protection, National Research Council of Italy (IPSP-CNR), 73 Strada delle Cacce, 10135 Torino, Italy.
Bernadette van den Hoogen, Department of Viroscience, Erasmus MC, University Medical Centre Rotterdam, Rotterdam, the Netherlands.
Arvind Varsani, The Biodesign Center for Fundamental and Applied Microbiomics, Center for Evolution and Medicine School of Life Sciences, Arizona State University, Tempe, Arizona, USA; Structural Biology Research Unit, Department of Clinical Laboratory Sciences, University of Cape Town, Observatory, Cape Town, South Africa.
Nikos Vasilakis, The University of Texas Medical Branch, Galveston, Texas, USA.
Martin Verbeek, Wageningen University and Research, Biointeractions and Plant Health, Wageningen, The Netherlands.
Victoria Wahl, National Biodefense Analysis and Countermeasures Center, Fort Detrick, Frederick, MD, USA.
Peter J. Walker, School of Biological Sciences, University of Queensland, St. Lucia, Queensland, Australia.
Hui Wang, State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China.
Jianwei Wang, NHC Key Laboratory of Systems Biology of Pathogens and Christophe Mérieux Laboratory, IPB-Fondation Mérieux, Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Lin-Fa Wang, Programme in Emerging Infectious Diseases, Duke-NUS Medical School, Singapore.
Xifeng Wang, State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, China.
Tàiyún Wèi, Fujian Province Key Laboratory of Plant Virology, Institute of Plant Virology, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China.
Heather Wells, Columbia University, Mailman School of Public Health, Center for Infection and Immunity.
Anna E. Whitfield, Department of Entomology and Plant Pathology, North Carolina State University, Raleigh, North Carolina, USA.
John V. Williams, School of Medicine, University of Pittsburgh, School of Medicine, Pittsburgh, Pennsylvania, USA.
Yuri I. Wolf, National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health, Bethesda, Maryland, USA.
Zhìqiáng Wú, MOH Key Laboratory of Systems Biology of Pathogens, IPB, CAMS.
Xin Yang, Guangdong Province Key Laboratory of Microbial Signals and Disease Control, College of Agriculture, South China Agricultural University Guangdong, China.
Xīnglóu Yáng, CAS Key Laboratory of Special Pathogens, Wuhan Institute of Virology, Center for Biosafety Mega-Science, Chinese Academy of Sciences, Wuhan, Hubei, People’s Republic of China..
Xue-Jie Yu, Wuhan University School of Health Sciences, Wuhan, China.
Natalya Yutin, National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health, Bethesda, Maryland, USA.
F. Murilo Zerbini, Departamento de Fitopatologia, Instituto de Biotecnologia Aplicada à Agropecuária, Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil.
Tong Zhang, Guangdong Province Key Laboratory of Microbial Signals and Disease Control, College of Agriculture, South China Agricultural University Guangdong, China.
Yong-Zhen Zhang, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Changping, Beijing, China.
Guohui Zhou, Guangdong Province Key Laboratory of Microbial Signals and Disease Control, College of Agriculture, South China Agricultural University Guangdong, China.
Xueping Zhou, State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China.
REFERENCES
- 1.Abudurexiti A, Adkins S, Alioto D, Alkhovsky SV, Avšič-Županc T, Ballinger MJ, Bente DA, Beer M, Bergeron É, Blair CD, Briese T, Buchmeier MJ, Burt FJ, Calisher CH, Cháng C, Charrel RN, Choi IR, Clegg JCS, de la Torre JC, de Lamballerie X, Dèng F, Di Serio F, Digiaro M, Drebot MA, Duàn X, Ebihara H, Elbeaino T, Ergünay K, Fulhorst CF, Garrison AR, Gāo GF, Gonzalez J-PJ, Groschup MH, Günther S, Haenni AL, Hall RA, Hepojoki J, Hewson R, Hú Z, Hughes HR, Jonson MG, Junglen S, Klempa B, Klingström J, Kòu C, Laenen L, Lambert AJ, Langevin SA, Liu D, Lukashevich IS, Luò T, Lǚ C, Maes P, de Souza WM, Marklewitz M, Martelli GP, Matsuno K, Mielke-Ehret N, Minutolo M, Mirazimi A, Moming A, Mühlbach H-P, Naidu R, Navarro B, Nunes MRT, Palacios G, Papa A, Pauvolid-Corrêa A, Pawęska JT, Qiáo J, Radoshitzky SR, Resende RO, Romanowski V, Sall AA, Salvato MS, Sasaya T, Shěn S, Shí X, Shirako Y, Simmonds P, Sironi M, Song J-W, Spengler JR, Stenglein MD, Sū Z, Sūn S, Táng S, Turina M, Wáng B, Wáng C, Wáng H, Wáng J, Wèi T, Whitfield AE, Zerbini FM, Zhāng J, Zhāng L, Zhāng Y, Zhang Y-Z, Zhāng Y, Zhou X, Zhū L, Kuhn JH (2019) Taxonomy of the order Bunyavirales: update 2019. Arch Virol 164:1949–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso CL, Amarasinghe GK, Bányai K, Bào Y, Basler CF, Bavari S, Bejerman N, Blasdell KR, Briand F-X, Briese T, Bukreyev A, Calisher CH, Chandran K, Chéng J, Clawson AN, Collins PL, Dietzgen RG, Dolnik O, Domier LL, Dürrwald R, Dye JM, Easton AJ, Ebihara H, Farkas SL, Freitas-Astúa J, Formenty P, Fouchier RA, Fù Y, Ghedin E, Goodin MM, Hewson R, Horie M, Hyndman TH, Jiāng D, Kitajima EW, Kobinger GP, Kondo H, Kurath G, Lamb RA, Lenardon S, Leroy EM, Li C-X, Lin X-D, Liú L, Longdon B, Marton S, Maisner A, Mühlberger E, Netesov SV, Nowotny N, Patterson JL, Payne SL, Paweska JT, Randall RE, Rima BK, Rota P, Rubbenstroth D, Schwemmle M, Shi M, Smither SJ, Stenglein MD, Stone DM, Takada A, Terregino C, Tesh RB, Tian J-H, Tomonaga K, Tordo N, Towner JS, Vasilakis N, Verbeek M, Volchkov VE, Wahl-Jensen V, Walsh JA, Walker PJ, Wang D, Wang L-F, Wetzel T, Whitfield AE, Xiè JT, Yuen K-Y, Zhang Y-Z, Kuhn JH (2016) Taxonomy of the order Mononegavirales: update 2016. Arch Virol 161:2351–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aitken TH, Woodall JP, De Andrade AHP, Bensabath G, Shope RE (1975) Pacui virus, phlebotomine flies, and small mammals in Brazil: an epidemiological study. Am J Trop Med Hyg 24:358–68 [DOI] [PubMed] [Google Scholar]
- 4.Alkan C, Alwassouf S, Piorkowski G, Bichaud L, Tezcan S, Dincer E, Ergunay K, Ozbel Y, Alten B, de Lamballerie X, Charrel RN (2015) Isolation, genetic characterization, and seroprevalence of Adana virus, a novel phlebovirus belonging to the Salehabad virus complex, in Turkey. J Virol 89:4080–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkan C, Erisoz Kasap O, Alten B, de Lamballerie X, Charrel RN (2016) Sandfly-Borne Phlebovirus Isolations from Turkey: New Insight into the Sandfly fever Sicilian and Sandfly fever Naples Species. PLoS Negl Trop Dis 10:e0004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkan C, Moin Vaziri V, Ayhan N, Badakhshan M, Bichaud L, Rahbarian N, Javadian EA, Alten B, de Lamballerie X, Charrel RN (2017) Isolation and sequencing of Dashli virus, a novel Sicilian-like virus in sandflies from Iran; genetic and phylogenetic evidence for the creation of one novel species within the Phlebovirus genus in the Phenuiviridae family. PLoS Negl Trop Dis 11:e0005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amarasinghe GK, Bào Y, Basler CF, Bavari S, Beer M, Bejerman N, Blasdell KR, Bochnowski A, Briese T, Bukreyev A, Calisher CH, Chandran K, Collins PL, Dietzgen RG, Dolnik O, Dürrwald R, Dye JM, Easton AJ, Ebihara H, Fang Q, Formenty P, Fouchier RAM, Ghedin E, Harding RM, Hewson R, Higgins CM, Hong J, Horie M, James AP, Jiāng D, Kobinger GP, Kondo H, Kurath G, Lamb RA, Lee B, Leroy EM, Li M, Maisner A, Mühlberger E, Netesov SV, Nowotny N, Patterson JL, Payne SL, Paweska JT, Pearson MN, Randall RE, Revill PA, Rima BK, Rota P, Rubbenstroth D, Schwemmle M, Smither SJ, Song Q, Stone DM, Takada A, Terregino C, Tesh RB, Tomonaga K, Tordo N, Towner JS, Vasilakis N, Volchkov VE, Wahl-Jensen V, Walker PJ, Wang B, Wang D, Wang F, Wang L-F, Werren JH, Whitfield AE, Yan Z, Ye G, Kuhn JH (2017) Taxonomy of the order Mononegavirales: update 2017. Arch Virol 162:2493–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amarasinghe GK, Ceballos NGA, Banyard AC, Basler CF, Bavari S, Bennett AJ, Blasdell KR, Briese T, Bukreyev A, Caì Y, Calisher CH, Lawson CC, Chandran K, Chapman CA, Chiu CY, Choi K-S, Collins PL, Dietzgen RG, Dolja VV, Dolnik O, Domier LL, Dürrwald R, Dye JM, Easton AJ, Ebihara H, Echevarría JE, Fooks AR, Formenty PBH, Fouchier RAM, Freuling CM, Ghedin E, Goldberg TL, Hewson R, Horie M, Hyndman TH, Jiāng D, Kityo R, Kobinger GP, Kondō H, Koonin EV, Krupovic M, Kurath G, Lamb RA, Lee B, Leroy EM, Maes P, Maisner A, Marston DA, Mor SK, Müller T, Mühlberger E, Ramírez VMN, Netesov SV, Ng TFF, Nowotny N, Palacios G, Patterson JL, Pawęska JT, Payne SL, Prieto K, Rima BK, Rota P, Rubbenstroth D, Schwemmle M, Siddell S, Smither SJ, Song Q, Song T, Stenglein MD, Stone DM, Takada A, Tesh RB, Thomazelli LM, Tomonaga K, Tordo N, Towner JS, Vasilakis N, Vázquez-Morón S, Verdugo C, Volchkov VE, Wahl V, Walker PJ, Wang D, Wang L-F, Wellehan JFX, Wiley MR, Whitfield AE, Wolf YI, Yè G, Zhāng Y-Z, Kuhn JH (2018) Taxonomy of the order Mononegavirales: update 2018. Arch Virol 163:2283–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amarasinghe GK, Ayllón MA, Bào Y, Basler CF, Bavari S, Blasdell KR, Briese T, Brown PA, Bukreyev A, Balkema-Buschmann A, Buchholz UJ, Chabi-Jesus C, Chandran K, Chiapponi C, Crozier I, de Swart RL, Dietzgen RG, Dolnik O, Drexler JF, Dürrwald R, Dundon WG, Duprex WP, Dye JM, Easton AJ, Fooks AR, Formenty PBH, Fouchier RAM, Freitas-Astúa J, Griffiths A, Hewson R, Horie M, Hyndman TH, Jiāng D, Kitajima EW, Kobinger GP, Kondō H, Kurath G, Kuzmin IV, Lamb RA, Lavazza A, Lee B, Lelli D, Leroy EM, Lǐ J, Maes P, Marzano S-YL, Moreno A, Mühlberger E, Netesov SV, Nowotny N, Nylund A, Økland AL, Palacios G, Pályi B, Pawęska JT, Payne SL, Prosperi A, Ramos-González PL, Rima BK, Rota P, Rubbenstroth D, Shī M, Simmonds P, Smither SJ, Sozzi E, Spann K, Stenglein MD, Stone DM, Takada A, Tesh RB, Tomonaga K, Tordo N, Towner JS, van den Hoogen B, Vasilakis N, Wahl V, Walker PJ, Wang L-F, Whitfield AE, Williams JV, Zerbini FM, Zhāng T, Zhang Y-Z, Kuhn JH (2019) Taxonomy of the order Mononegavirales: update 2019. Arch Virol 164:1967–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaro F, Zé-Zé L, Alves MJ, Börstler J, Clos J, Lorenzen S, Becker SC, Schmidt-Chanasit J, Cadar D (2015) Co-circulation of a novel phlebovirus and Massilia virus in sandflies, Portugal. Virol J 12:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr J, Smith C, Smith I, de Jong C, Todd S, Melville D, Broos A, Crameri S, Haining J, Marsh G, Crameri G, Field H, Wang LF (2015) Isolation of multiple novel paramyxoviruses from pteropid bat urine. J Gen Virol 96:24–9 [DOI] [PubMed] [Google Scholar]
- 12.Bejerman N, de Breuil S, Debat H, Miretti M, Badaracco A, Nome C (2017) Molecular characterization of yerba mate chlorosis-associated virus, a putative cytorhabdovirus infecting yerba mate (Ilex paraguariensis). Arch Virol 162:2481–4 [DOI] [PubMed] [Google Scholar]
- 13.Berge TO (1975) International Catalogue of Arboviruses Including Certain Other Viruses of Vertebrates US Public Health Service publication no. (CDC) 75–8301, 2nd edn Department of Health, Education and Welfare, Washington, DC, USA [Google Scholar]
- 14.Bhatt PN, Kulkarni KG, Boshell MJ, Rajagopalan PK, Patil AP, Goverdhan MK, Pavri KM (1966) Kaisodi virus, a new agent isolated from Haemaphysalis spinigera in Mysore State, South India. I. Isolation of strains. Am J Trop Med Hyg 15:958–60 [DOI] [PubMed] [Google Scholar]
- 15.Bichaud L, Dachraoui K, Alwassouf S, Alkan C, Mensi M, Piorkowski G, Sakhria S, Seston M, Fares W, De Lamballerie X, Zhioua E, Charrel RN (2016) Isolation, full genomic characterization and neutralization-based human seroprevalence of Medjerda Valley virus, a novel sandfly-borne phlebovirus belonging to the Salehabad virus complex in northern Tunisia. J Gen Virol 97:602–10 [DOI] [PubMed] [Google Scholar]
- 16.Bishop DHL, Pringle CR (1995) Order Mononegavirales In: Murphy FA, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis AW, Martelli GP, Mayo MA, Summers MD (eds) Virus Taxonomy—Sixth Report of the International Committee on Taxonomy of Viruses/Archives of Virology Supplement 10. Springer-Verlag, Vienna, Austria, pp 265–7 [Google Scholar]
- 17.Bouquet J, Melgar M, Swei A, Delwart E, Lane RS, Chiu CY (2017) Metagenomic-based surveillance of Pacific Coast tick Dermacentor occidentalis identifies two novel bunyaviruses and an emerging human ricksettsial pathogen. Sci Rep 7:12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzkan N, Chiumenti M, Massart S, Sarpkaya K, Karadağ S, Minafra A (2019) A new emaravirus discovered in Pistacia from Turkey. Virus Res 263:159–63 [DOI] [PubMed] [Google Scholar]
- 19.Causey OR, Shope RE (1965) Icoaraci, a new virus related to Naples phlebotomus fever virus. Proc Soc Exp Biol Med 118:420–1 [DOI] [PubMed] [Google Scholar]
- 20.Charrel RN, Moureau G, Temmam S, Izri A, Marty P, Parola P, da Rosa AT, Tesh RB, de Lamballerie X (2009) Massilia virus, a novel Phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis 9:519–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CC, Chiu RJ (1996) A tospovirus infecting peanut in Taiwan. Acta Hortic:57–67 [Google Scholar]
- 22.Cheng Y-H, Zheng Y-X, Tai C-H, Yen J-H, Chen Y-K, Jan F-J (2014) Identification, characterisation and detection of a new tospovirus on sweet pepper. Ann Appl Biol 164:107–15 [Google Scholar]
- 23.Coffey LL, Page BL, Greninger AL, Herring BL, Russell RC, Doggett SL, Haniotis J, Wang C, Deng X, Delwart EL (2014) Enhanced arbovirus surveillance with deep sequencing: identification of novel rhabdoviruses and bunyaviruses in Australian mosquitoes. Virology 448:146–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Carvalho MS, de Lara Pinto AZ, Pinheiro A, Rodrigues JSV, Melo FL, da Silva LA, Ribeiro BM, Dezengrini-Slhessarenko R (2018) Viola phlebovirus is a novel Phlebotomus fever serogroup member identified in Lutzomyia (Lutzomyia) longipalpis from Brazilian Pantanal. Parasit Vectors 11:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Haas RA, Jonkers AH, Heinemann DW (1966) Kwatta virus, a new agent isolated from Culex mosquitoes in Surinam. Am J Trop Med Hyg 15:954–7 [DOI] [PubMed] [Google Scholar]
- 26.Debat HJ, Bejerman N (2019) Novel bird’s-foot trefoil RNA viruses provide insights into a clade of legume-associated enamoviruses and rhabdoviruses. Arch Virol 164:1419–26 [DOI] [PubMed] [Google Scholar]
- 27.Dilcher M, Alves MJ, Finkeisen D, Hufert F, Weidmann M (2012) Genetic characterization of Bhanja virus and Palma virus, two tick-borne phleboviruses. Virus Genes 45:311–5 [DOI] [PubMed] [Google Scholar]
- 28.Dilcher M, Faye O, Faye O, Weber F, Koch A, Sadegh C, Weidmann M, Sall AA (2015) Zahedan rhabdovirus, a novel virus detected in ticks from Iran. Virol J 12:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty RL, Carley JG, Standfast HA, Dyce AL, Kay BH, Snowdon WA (1973) Isolation of arboviruses from mosquitoes, biting midges, sandflies and vertebrates collected in Queensland, 1969 and 1970. Trans R Soc Trop Med Hyg 67:536–43 [DOI] [PubMed] [Google Scholar]
- 30.Dong J-H, Cheng X-F, Yin YY, Fang Q, Ding M, Li T-T, Zhang L-Z, Su X-X, McBeath J-H, Zhang Z-K (2008) Characterization of tomato zonate spot virus, a new tospovirus in China. Arch Virol 153:855–64 [DOI] [PubMed] [Google Scholar]
- 31.Dong JH, Yin YY, Fang Q, McBeath JH, Zhang ZK (2013) A new tospovirus causing chlorotic ringspot on Hippeastrum sp. in China. Virus Genes 46:567–70 [DOI] [PubMed] [Google Scholar]
- 32.Easton AJ, Pringle CR (2011) Order Mononegavirales In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (eds) Virus Taxonomy—Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press, London, United Kingdom, pp 653–57 [Google Scholar]
- 33.Fernandes J, Guterres A, de Oliveira RC, Chamberlain J, Lewandowski K, Teixeira BR, Coelho TA, Crisóstomo CF, Bonvicino CR, D’Andrea PS, Hewson R, de Lemos ERS (2018) Xapuri virus, a novel mammarenavirus: natural reassortment and increased diversity between New World viruses. Emerg Microbes Infect 7:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes J, Guterres A, de Oliveira RC, Jardim R, Dávila AMR, Hewson R, de Lemos ERS (2019) Apore virus, a novel mammarenavirus (Bunyavirales: Arenaviridae) related to highly pathogenic virus from South America. Mem Inst Oswaldo Cruz 114:e180586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forth LF, Konrath A, Klose K, Schlottau K, Hoffmann K, Ulrich RG, Höper D, Pohlmann A, Beer M (2018) A novel squirrel respirovirus with putative zoonotic potential. Viruses 10:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaafar YZA, Richert-Pöggeler KR, Maaß C, Vetten H-J, Ziebell H (2019) Characterisation of a novel nucleorhabdovirus infecting alfalfa (Medicago sativa). Virol J 16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein T, Anthony SJ, Gbakima A, Bird BH, Bangura J, Tremeau-Bravard A, Belaganahalli MN, Wells HL, Dhanota JK, Liang E, Grodus M, Jangra RK, DeJesus VA, Lasso G, Smith BR, Jambai A, Kamara BO, Kamara S, Bangura W, Monagin C, Shapira S, Johnson CK, Saylors K, Rubin EM, Chandran K, Lipkin WI, Mazet JAK (2018) The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat Microbiol 3:1084–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gubala A, Walsh S, McAllister J, Weir R, Davis S, Melville L, Mitchell I, Bulach D, Gauci P, Skvortsov A, Boyle D (2017) Identification of very small open reading frames in the genomes of Holmes Jungle virus, Ord River virus, and Wongabel virus of the genus Hapavirus, family Rhabdoviridae. Evol Bioinform Online 13:1176934317713484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hang J, Klein TA, Kim H-C, Yang Y, Jima DD, Richardson JH, Jarman RG (2016) Genome sequences of five arboviruses in field-captured mosquitoes in a unique rural environment of South Korea. Genome Announc 4:e01644–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannoun C, Corniou B, Rageau J (1970) Isolation in southern France and characterization of new tick-borne viruses related to Uukuniemi: Grand Arbaud and Ponteves. Acta Virol 14:167–70 [PubMed] [Google Scholar]
- 41.Hassan M, Di Bello PL, Keller KE, Martin RR, Sabanadzovic S, Tzanetakis IE (2017) A new, widespread emaravirus discovered in blackberry. Virus Res 235:1–5 [DOI] [PubMed] [Google Scholar]
- 42.Hassani-Mehraban A, Botermans M, Verhoeven JTJ, Meekes E, Saaijer J, Peters D, Goldbach R, Kormelink R (2010) A distinct tospovirus causing necrotic streak on Alstroemeria sp. in Colombia. Arch Virol 155:423–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassani-Mehraban A, Dullemans AM, Verhoeven JTJ, Roenhorst JW, Peters D, van der Vlugt RAA, Kormelink R (2019) Alstroemeria yellow spot virus (AYSV): a new orthotospovirus species within a growing Eurasian clade. Arch Virol 164:117–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hepojoki J, Hepojoki S, Smura T, Szirovicza L, Dervas E, Prahauser B, Nufer L, Schraner EM, Vapalahti O, Kipar A, Hetzel U (2018) Characterization of Haartman Institute snake virus-1 (HISV-1) and HISV-like viruses - the representatives of genus Hartmanivirus, family Arenaviridae. PLoS Pathog 14:e1007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu S-C, Hsu C-L, Lee M-S, Tu Y-C, Chang J-C, Wu C-H, Lee S-H, Ting L-J, Tsai K-R, Cheng M-C, Tu W-J, Hsu W-C (2018) Lyssavirus in Japanese pipistrelle, Taiwan. Emerg Infect Dis 24:782–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes HR, Russell BJ, Lambert AJ (2020) Genetic characterization of Frijoles and Chilibre species complex viruses (genus Phlebovirus; family Phenuiviridae) and three unclassified New World phleboviruses. Am J Trop Med Hyg 102:359–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ito T, Suzaki K, Nakano M (2013) Genetic characterization of novel putative rhabdovirus and dsRNA virus from Japanese persimmon. J Gen Virol 94:1917–21 [DOI] [PubMed] [Google Scholar]
- 48.Jeong J, Kim Y, An I, Wang S-J, Kim Y, Lee H-J, Choi K-S, Im S-P, Min W, Oem J-K, Jheong W (2018) Complete genome sequence of a novel avian paramyxovirus isolated from wild birds in South Korea. Arch Virol 163:223–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson RI, Tachedjian M, Rowe B, Clayton BA, Layton R, Bergfeld J, Wang L-F, Marsh GA (2018) Alston virus, a novel paramyxovirus isolated from bats causes upper respiratory tract infection in experimentally challenged ferrets. Viruses 10:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones S, McGavin W, MacFarlane S (2019) The complete sequences of two divergent variants of the rhabdovirus raspberry vein chlorosis virus and the design of improved primers for virus detection. Virus Res 265:162–5 [DOI] [PubMed] [Google Scholar]
- 51.Karabatsos N (1985) International catalogue of arboviruses including certain other viruses of vertebrates. American Society for Tropical Medicine and Hygiene, San Antonio, Texas, USA: [DOI] [PubMed] [Google Scholar]
- 52.Kerschner JH, Calisher CH, Vorndam AV, Francy DB (1986) Identification and characterization of Bahia Grande, Reed Ranch and Muir Springs viruses, related members of the family Rhabdoviridae with widespread distribution in the United States. J Gen Virol 67:1081–9 [DOI] [PubMed] [Google Scholar]
- 53.Kohl C, Tachedjian M, Todd S, Monaghan P, Boyd V, Marsh GA, Crameri G, Field H, Kurth A, Smith I, Wang L-F (2018) Hervey virus: a study on co-circulation with henipaviruses in pteropid bats within their distribution range from Australia to Africa. PLoS One 13:e0191933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kokernot RH, Calisher CH, Stannard LJ, Hayes J (1969) Arbovirus studies in the Ohio-Mississippi Basin, 1964–1967. VII. Lone star virus, a hitherto unknown agent isolated from the tick Amblyomma americanum (Linn.). Am J Trop Med Hyg 18:789–95 [PubMed] [Google Scholar]
- 55.Koonin EV, Dolja VV, Krupovic M, Arvind V, Wolf YI, Yutin N, Zerbini FM, Kuhn JH (2020) Global organization and proposed megataxonomy of the virus world. Microbiol Mol Biol Rev 84:e00061–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhn JH, Wolf YI, Krupovic M, Zhang Y-Z, Maes P, Dolja VV, Koonin EV (2019) Classify viruses—the gain is worth the pain. Nature 566:318–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lecoq H, Wipf-Scheibel C, Verdin E, Desbiez C (2019) Characterization of the first tenuivirus naturally infecting dicotyledonous plants. Arch Virol 164:297–301 [DOI] [PubMed] [Google Scholar]
- 58.Ledermann JP, Zeidner N, Borland EM, Mutebi J-P, Lanciotti RS, Miller BR, Lutwama JJ, Tendo JM, Andama V, Powers AM (2014) Sunguru virus: a novel virus in the family Rhabdoviridae isolated from a chicken in north-western Uganda. J Gen Virol 95:1436–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C-X, Shi M, Tian J-H, Lin X-D, Kang Y-J, Chen L-J, Qin X-C, Xu J, Holmes EC, Zhang Y-Z (2015) Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife 4:e05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin Y-H, Fujita M, Chiba S, Hyodo K, Andika IB, Suzuki N, Kondo H (2019) Two novel fungal negative-strand RNA viruses related to mymonaviruses and phenuiviruses in the shiitake mushroom (Lentinula edodes). Virology 533:125–36 [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Du Z, Wang H, Zhang S, Cao M, Wang X (2018) Identification and characterization of wheat yellow striate virus, a novel leafhopper-transmitted nucleorhabdovirus infecting wheat. Front Microbiol 9:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lvov DK, Timopheeva AA, Gromashevski VL, Gostinshchikova GV, Veselovskaya OV, Chervonski VI, Fomina KB, Gromov AI, Pogrebenko AG, Zhezmer VY (1973) “Zaliv Terpeniya” virus, a new Uukuniemi group arbovirus isolated from Ixodes (Ceratixodes) putus Pick.-Camb. 1878 on Tyuleniy Island (Sakhalin region) and Commodore Islands (Kamchatsk region). Arch Gesamte Virusforsch 41:165–9 [DOI] [PubMed] [Google Scholar]
- 63.Lvov SD, Gromashevsky VL, Andreev VP, Skvortsova TM, Kondrashina NG, Morozova TN, Avershin AD, Aristova VA, Dmitriev GA, Kandaurov YK, Kuznetsov AA, Galkina IV, Yamnikova SS, Shchipanova MV (1990) Natural foci of arboviruses in far northern latitudes of Eurasia In: Calisher CH (ed) Hemorrhagic fever with renal syndrome, tick-and mosquito-borne viruses. Springer, Vienna, Austria, pp 267–75 [Google Scholar]
- 64.Maes P, Alkhovsky SV, Bào Y, Beer M, Birkhead M, Briese T, Buchmeier MJ, Calisher CH, Charrel RN, Choi IR, Clegg CS, Torre JCdl, Delwart E, DeRisi JL, Bello PLD, Serio FD, Digiaro M, Dolja VV, Drosten C, Druciarek TZ, Du J, Ebihara H, Elbeaino T, Gergerich RC, Gillis AN, Gonzalez J-PJ, Haenni A-L, Hepojoki J, Hetzel U, Hồ T, Hóng N, Jain RK, Vuren PJv, Jin Q, Jonson MG, Junglen S, Keller KE, Kemp A, Kipar A, Kondov NO, Koonin EV, Kormelink R, Korzyukov Y, Krupovic M, Lambert AJ, Laney AG, LeBreton M, Lukashevic IS, Marklewitz M, Markotter W, Martelli GP, Martin RR, Mielke-Ehret N, Mühlbach H-P, Navarro B, Ng TFF, Nunes MRT, Palacios G, Pawęska JT, Peters CJ, Plyusnin A, Radoshitzky SR, Romanowski V, Salmenperä P, Salvato MS, Sanfaçon H, Sasaya T, Schmaljohn C, Schneider BS, Shirako Y, Siddell S, Sironen TA, Stenglein MD, Storm N, Sudini H, Tesh RB, Tzanetakis IE, Uppala M, Vapalahti O, Vasilakis N, Walker PJ, Wáng G, Wáng L, Wáng Y, Wèi T, Wiley MR, Wolf YI, Wolfe ND, Wú Z, Xú W, Yang L, Yāng Z, Yeh S-D, Zhāng Y-Z, Zhèng Y, Zhou X, Zhū C, Zirkel F, Kuhn JH (2018) Taxonomy of the family Arenaviridae and the order Bunyavirales: update 2018. Arch Virol 163:2295–310 [DOI] [PubMed] [Google Scholar]
- 65.Maes P, Adkins S, Alkhovsky SV, Avšič-Županc T, Ballinger MJ, Bente DA, Beer M, Bergeron É, Blair CD, Briese T, Buchmeier MJ, Burt FJ, Calisher CH, Charrel RN, Choi IR, Clegg JCS, de la Torre JC, de Lamballerie X, DeRisi JL, Digiaro M, Drebot M, Ebihara H, Elbeaino T, Ergünay K, Fulhorst CF, Garrison AR, Gāo GF, Gonzalez J-PJ, Groschup MH, Günther S, Haenni A-L, Hall RA, Hewson R, Hughes HR, Jain RK, Jonson MG, Junglen S, Klempa B, Klingström J, Kormelink R, Lambert AJ, Langevin SA, Lukashevich IS, Marklewitz M, Martelli GP, Mielke-Ehret N, Mirazimi A, Mühlbach H-P, Naidu R, Nunes MRT, Palacios G, Papa A, Pawęska JT, Peters CJ, Plyusnin A, Radoshitzky SR, Resende RO, Romanowski V, Sall AA, Salvato MS, Sasaya T, Schmaljohn C, Shí X, Shirako Y, Simmonds P, Sironi M, Song J-W, Spengler JR, Stenglein MD, Tesh RB, Turina M, Wèi T, Whitfield AE, Yeh S-D, Zerbini FM, Zhang Y-Z, Zhou X, Kuhn JH (2019) Taxonomy of the order Bunyavirales: second update 2018. Arch Virol 164:927–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maes P, Amarasinghe GK, Ayllón MA, Basler CF, Bavari S, Blasdell KR, Briese T, Brown PA, Bukreyev A, Balkema-Buschmann A, Buchholz UJ, Chandran K, Crozier I, de Swart RL, Dietzgen RG, Dolnik O, Domier LL, Drexler JF, Dürrwald R, Dundon WG, Duprex WP, Dye JM, Easton AJ, Fooks AR, Formenty PBH, Fouchier RAM, Freitas-Astúa J, Ghedin E, Griffiths A, Hewson R, Horie M, Hurwitz JL, Hyndman TH, Jiāng D, Kobinger GP, Kondō H, Kurath G, Kuzmin IV, Lamb RA, Lee B, Leroy EM, Lǐ J, Marzano S-YL, Mühlberger E, Netesov SV, Nowotny N, Palacios G, Pályi B, Pawęska JT, Payne SL, Rima BK, Rota P, Rubbenstroth D, Simmonds P, Smither SJ, Song Q, Song T, Spann K, Stenglein MD, Stone DM, Takada A, Tesh RB, Tomonaga K, Tordo N, Towner JS, van den Hoogen B, Vasilakis N, Wahl V, Walker PJ, Wang D, Wang L-F, Whitfield AE, Williams JV, Yè G, Zerbini FM, Zhang Y-Z, Kuhn JH (2019) Taxonomy of the order Mononegavirales: second update 2018. Arch Virol 164:1233–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Main AJ, Carey AB (1980) Connecticut virus: a new Sawgrass group virus from Ixodes dentatus (Acari: Ixodidae). J Med Entomol 17:473–6 [Google Scholar]
- 68.Marklewitz M, Dutari LC, Paraskevopoulou S, Page RA, Loaiza JR, Junglen S (2019) Diverse novel phleboviruses in sandflies from the Panama Canal area, Central Panama. J Gen Virol 100:938–49 [DOI] [PubMed] [Google Scholar]
- 69.Matsuno K, Weisend C, Kajihara M, Matysiak C, Williamson BN, Simuunza M, Mweene AS, Takada A, Tesh RB, Ebihara H (2015) Comprehensive molecular detection of tick-borne phleboviruses leads to the retrospective identification of taxonomically unassigned bunyaviruses and the discovery of a novel member of the genus Phlebovirus. J Virol 89:594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maurino F, Dumón AD, Llauger G, Alemandri V, de Haro LA, Mattio MF, Del Vas M, Laguna IG, Giménez Pecci MdlP (2018) Complete genome sequence of maize yellow striate virus, a new cytorhabdovirus infecting maize and wheat crops in Argentina. Arch Virol 163:291–95 [DOI] [PubMed] [Google Scholar]
- 71.McAllister J, Gauci PJ, Mitchell IR, Boyle DB, Bulach DM, Weir RP, Melville LF, Davis SS, Gubala AJ (2014) Genomic characterisation of Almpiwar virus, Harrison Dam virus and Walkabout Creek virus; three novel rhabdoviruses from northern Australia. Virol Rep 3–4:1–17 [Google Scholar]
- 72.McLean DM, Larke RPB (1963) Powassan and Silverwater viruses: ecology of two Ontario arboviruses. Can Med Assoc J 88:182–5 [PMC free article] [PubMed] [Google Scholar]
- 73.Medina-Salguero AX, Cornejo-Franco JF, Grinstead S, Mollov D, Mowery JD, Flores F, Quito-Avila DF (2019) Sequencing, genome analysis and prevalence of a cytorhabdovirus discovered in Carica papaya. PLoS One 14:e0215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meng J, Liu P, Zhu L, Zou C, Li J, Chen B (2015) Complete genome sequence of mulberry vein banding associated virus, a new tospovirus infecting mulberry. PLoS One 10:e0136196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meng JR, Liu PP, Zou CW, Wang ZQ, Liao YM, Cai JH, Qin BX, Chen BS (2013) First report of a Tospovirus in mulberry. Plant Dis 97:1001. [DOI] [PubMed] [Google Scholar]
- 76.Menzel W, Richert-Pöggeler KR, Winter S, Knierim D (2018) Characterization of a nucleorhabdovirus from Physostegia. Acta Hortic 1193:29–38 [Google Scholar]
- 77.Muller MJ, Standfast HA (1986) Vectors of ephemeral fever group viruses In: St George TD, Kay BH, Blok J (eds) Arbovirus research in Australia - Proceedings of the fourth symposium. CSIRO/QMIR, Brisbane, Australia, pp 295–300 [Google Scholar]
- 78.Navarro B, Zicca S, Minutolo M, Saponari M, Alioto D, Di Serio F (2018) A negative-stranded RNA virus infecting citrus trees: the second member of a new genus within the order Bunyavirales. Front Microbiol 9:2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noh JY, Jeong DG, Yoon S-W, Kim JH, Choi YG, Kang S-Y, Kim HK (2018) Isolation and characterization of novel bat paramyxovirus B16–40 potentially belonging to the proposed genus Shaanvirus. Sci Rep 8:12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nunes-Neto JP, Souza WM, Acrani GO, Romeiro MF, Fumagalli M, Vieira LC, Medeiros DBdA, Lima JA, de Lima CPS, Cardoso JF, Figueiredo LTM, da Silva SPD, Tesh R, Nunes MRT, Vasconcelos PFdC (2017) Characterization of the Bujaru, frijoles and Tapara antigenic complexes into the sandfly fever group and two unclassified phleboviruses from Brazil. J Gen Virol 98:585–94 [DOI] [PubMed] [Google Scholar]
- 81.Økland AL, Nylund A, Øvergård A-C, Skoge RH, Kongshaug H (2019) Genomic characterization, phylogenetic position and in situ localization of a novel putative mononegavirus in Lepeophtheirus salmonis. Arch Virol 164:675–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palacios G, da Rosa AT, Savji N, Sze W, Wick I, Guzman H, Hutchison S, Tesh R, Lipkin WI (2011) Aguacate virus, a new antigenic complex of the genus Phlebovirus (family Bunyaviridae). J Gen Virol 92:1445–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palacios G, Tesh R, Travassos da Rosa A, Savji N, Sze W, Jain K, Serge R, Guzman H, Guevara C, Nunes MR, Nunes-Neto JP, Kochel T, Hutchison S, Vasconcelos PFC, Lipkin WI (2011) Characterization of the Candiru antigenic complex (Bunyaviridae: Phlebovirus), a highly diverse and reassorting group of viruses affecting humans in tropical America. J Virol 85:3811–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palacios G, Savji N, Travassos da Rosa A, Desai A, Sanchez-Seco MP, Guzman H, Lipkin WI, Tesh R (2013) Characterization of the Salehabad virus species complex of the genus Phlebovirus (Bunyaviridae). J Gen Virol 94:837–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palacios G, Savji N, Travassos da Rosa A, Guzman H, Yu X, Desai A, Rosen GE, Hutchison S, Lipkin WI, Tesh R (2013) Characterization of the Uukuniemi virus group (Phlebovirus: Bunyaviridae): evidence for seven distinct species. J Virol 87:3187–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palacios G, Tesh RB, Savji N, Travassos da Rosa APA, Guzman H, Bussetti AV, Desai A, Ladner J, Sanchez-Seco M, Lipkin WI (2014) Characterization of the Sandfly fever Naples species complex and description of a new Karimabad species complex (genus Phlebovirus, family Bunyaviridae). J Gen Virol 95:292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palacios G, Wiley MR, Travassos da Rosa APA, Guzman H, Quiroz E, Savji N, Carrera J-P, Bussetti AV, Ladner JT, Lipkin WI, Tesh RB (2015) Characterization of the Punta Toro species complex (genus Phlebovirus, family Bunyaviridae). J Gen Virol 96:2079–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pavri KM, Casals J (1966) Kaisodi virus, a new agent isolated from Haemaphysalis spinigera in Mysore state, South India. Am J Trop Med Hyg 15:961–3 [DOI] [PubMed] [Google Scholar]
- 89.Pecman A, Kutnjak D, Gutiérrez-Aguirre I, Adams I, Fox A, Boonham N, Ravnikar M (2017) Next generation sequencing for detection and discovery of plant viruses and viroids: comparison of two approaches. Front Microbiol 8:1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peralta PH, Shelokov A, Brody JA (1965) Chagres virus: a new human isolate from Panama. Am J Trop Med Hyg 14:146–51 [DOI] [PubMed] [Google Scholar]
- 91.Pettersson JH-O, Shi M, Bohlin J, Eldholm V, Brynildsrud OB, Paulsen KM, Andreassen Å, Holmes EC (2017) Characterizing the virome of Ixodes ricinus ticks from northern Europe. Sci Rep 7:10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pringle CR, Alexander DJ, Billeter MA, Collins PL, Kingsbury DW, Lipkind MA, Nagai Y, Orvell C, Rima B, Rott R, ter Meulen V (1991) The order Mononegavirales. Arch Virol 117:137–402006902 [Google Scholar]
- 93.Pringle CR (1997) The order Mononegavirales—current status. Arch Virol 142:2321–6 [PubMed] [Google Scholar]
- 94.Pringle CR (2000) Order Mononegavirales In: van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR, Wickner RB (eds) Virus Taxonomy—Seventh Report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, California, USA, pp 525–30 [Google Scholar]
- 95.Pringle CR (2005) Order Mononegavirales In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) Virus Taxonomy—Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press, San Diego, California, USA, pp 609–14 [Google Scholar]
- 96.Quan P-L, Williams DT, Johansen CA, Jain K, Petrosov A, Diviney SM, Tashmukhamedova A, Hutchison SK, Tesh RB, Mackenzie JS, Briese T, Lipkin WI (2011) Genetic characterization of K13965, a strain of Oak Vale virus from Western Australia. Virus Res 160:206–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Read DA, Featherston J, Rees DJG, Thompson GD, Roberts R, Flett BC, Mashingaidze K, Pietersen G, Kiula B, Kullaya A, Mbega ER (2019) Molecular characterization of Morogoro maize-associated virus, a nucleorhabdovirus detected in maize (Zea mays) in Tanzania. Arch Virol 164:1711–5 [DOI] [PubMed] [Google Scholar]
- 98.Reuter G, Boros A, Pál J, Kapusinszky B, Delwart E, Pankovics P (2016) Detection and genome analysis of a novel (dima)rhabdovirus (Riverside virus) from Ochlerotatus sp. mosquitoes in Central Europe. Infect Genet Evol 39:336–41 [DOI] [PubMed] [Google Scholar]
- 99.Ritter DG, Calisher CH, Muth DJ, Shope RE, Murphy FA, Whitfield SG (1978) New Minto virus: a new rhabdovirus from ticks in Alaska. Can J Microbiol 24:422–6 [DOI] [PubMed] [Google Scholar]
- 100.Rott ME, Kesanakurti P, Berwarth C, Rast H, Boyes I, Phelan J, Jelkmann W (2018) Discovery of negative-sense RNA viruses in trees infected with apple rubbery wood disease by next-generation sequencing. Plant Dis 102:1254–63 [DOI] [PubMed] [Google Scholar]
- 101.Sabin AB (1951) Experimental studies on Phlebotomus (pappataci, sandfly) fever during World War II. Arch Gesamte Virusforsch 4:367–410 [DOI] [PubMed] [Google Scholar]
- 102.Sather GE, Lewis AL, Jennings W, Bond JO, Hammon WM (1970) Sawgrass virus: a newly described arbovirus in Florida. Am J Trop Med Hyg 19:319–26 [DOI] [PubMed] [Google Scholar]
- 103.Scarpassa VM, Debat HJ, Alencar RB, Saraiva JF, Calvo E, Arcà B, Ribeiro JMC (2019) An insight into the sialotranscriptome and virome of Amazonian anophelines. BMC Genomics 20:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shah KV, Work TH (1969) Bhanja virus: a new arbovirus from ticks Haemaphysalis intermedia Warburton and Nuttall, 1909, in Orissa, India. Indian J Med Res 57:793–8 [PubMed] [Google Scholar]
- 105.Shahhosseini N, Lühken R, Jöst H, Jansen S, Börstler J, Rieger T, Krüger A, Yadouleton A, de Mendonça Campos R, Cirne-Santos CC, Ferreira DF, Garms R, Becker N, Tannich E, Cadar D, Schmidt-Chanasit J (2017) Detection and characterization of a novel rhabdovirus in Aedes cantans mosquitoes and evidence for a mosquito-associated new genus in the family Rhabdoviridae. Infect Genet Evol 55:260–8 [DOI] [PubMed] [Google Scholar]
- 106.Shi M, Lin XD, Tian JH, Chen LJ, Chen X, Li CX, Qin XC, Li J, Cao JP, Eden JS, Buchmann J, Wang W, Xu J, Holmes EC, Zhang YZ (2016) Redefining the invertebrate RNA virosphere. Nature 540:539–43 [DOI] [PubMed] [Google Scholar]
- 107.Shi M, Neville P, Nicholson J, Eden J-S, Imrie A, Holmes EC (2017) High-resolution metatranscriptomics reveals the ecological dynamics of mosquito-associated RNA viruses in western Australia. J Virol 91:e00680–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi M, Lin X-D, Chen X, Tian J-H, Chen L-J, Li K, Wang W, Eden J-S, Shen J-J, Liu L, Holmes EC, Zhang Y-Z (2018) The evolutionary history of vertebrate RNA viruses. Nature 556:197–202 [DOI] [PubMed] [Google Scholar]
- 109.Siddell SG, Walker PJ, Lefkowitz EJ, Mushegian AR, Adams MJ, Dutilh BE, Gorbalenya AE, Harrach Bz, Harrison RL, Junglen S, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Nibert M, Rubino L, Sabanadzovic S, Sanfaçon H, Simmonds P, Varsani A, Zerbini FM, Davison AJ (2019) Additional changes to taxonomy ratified in a special vote by the International Committee on Taxonomy of Viruses (October 2018). Arch Virol 164:943–6 [DOI] [PubMed] [Google Scholar]
- 110.Spence L, Anderson CR, Aitken THG, Downs WG (1966) Aruac virus, a new agent isolated from Trinidadian mosquitoes. Am J Trop Med Hyg 15:231–4 [DOI] [PubMed] [Google Scholar]
- 111.St George TD, Doherty RL, Carley JG, Filippich C, Brescia A, Casals J, Kemp DH, Brothers N (1985) The isolation of arboviruses including a new flavivirus and a new bunyavirus from Ixodes (Ceratixodes) uriae (Ixodoidea: Ixodidae) collected at Macquarie Island, Australia, 1975–1979. Am J Trop Med Hyg 34:406–12 [DOI] [PubMed] [Google Scholar]
- 112.Straková P, Dufkova L, Širmarová J, Salát J, Bartonička T, Klempa B, Pfaff F, Höper D, Hoffmann B, Ulrich RG, Růžek D (2017) Novel hantavirus identified in European bat species Nyctalus noctula. Infect Genet Evol 48:127–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun Q, Zhao Q, An X, Guo X, Zuo S, Zhang X, Pei G, Liu W, Cheng S, Wang Y, Shu P, Mi Z, Huang Y, Zhang Z, Tong Y, Zhou H, Zhang J (2017) Complete genome sequence of Menghai rhabdovirus, a novel mosquito-borne rhabdovirus from China. Arch Virol 162:1103–6 [DOI] [PubMed] [Google Scholar]
- 114.Swei A, Russell BJ, Naccache SN, Kabre B, Veeraraghavan N, Pilgard MA, Johnson BJB, Chiu CY (2013) The genome sequence of lone star virus, a highly divergent bunyavirus found in the Amblyomma americanum tick. PLoS One 8:e62083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tchouassi DP, Marklewitz M, Chepkorir E, Zirkel F, Agha SB, Tigoi CC, Koskei E, Drosten C, Borgemeister C, Torto B, Junglen S, Sang R (2019) Sand fly-associated phlebovirus with evidence of neutralizing antibodies in humans, Kenya. Emerg Infect Dis 25:681–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tesh RB, Chaniotis BN, Peralta PH, Johnson KM (1974) Ecology of viruses isolated from Panamanian phlebotomine sandflies. Am J Trop Med Hyg 23:258–69 [DOI] [PubMed] [Google Scholar]
- 117.Tesh RB (1975) Multiplication of Phlebotomus fever group arboviruses in mosquitos after intrathoracic inoculation. Journal of Medical Entomology 12:1–4 [DOI] [PubMed] [Google Scholar]
- 118.Tesh RB, Boshell J, Young DG, Morales A, De Carrasquilla CF, Corredor A, Modi GB, Travassos Da Rosa APA, McLean RG, De Rodriguez C, Gaitan MO (1989) Characterization of 5 new phleboviruses recently isolated from sand flies in tropical America. Am J Trop Med Hyg 40:529–33 [PubMed] [Google Scholar]
- 119.Tokarz R, Sameroff S, Leon MS, Jain K, Lipkin WI (2014) Genome characterization of Long Island tick rhabdovirus, a new virus identified in Amblyomma americanum ticks. Virol J 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tokarz R, Williams SH, Sameroff S, Sanchez Leon M, Jain K, Lipkin WI (2014) Virome analysis of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks reveals novel highly divergent vertebrate and invertebrate viruses. J Virol 88:11480–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trapp EE, Paes de Andrade AH, Shope RE (1965) Itaporanga, a newly reecognized arbovirus from Sao Paulo State, Brazil. Proc Soc Exp Biol Med 118:421–2 [DOI] [PubMed] [Google Scholar]
- 122.Travassos da Rosa APA, Tesh RB, Pinheiro FP, Travassos da Rosa JFS, Peterson NE (1983) Characterization of eight new phlebotomus fever serogroup arboviruses (Bunyaviridae: Phlebovirus) from the Amazon region of Brazil. Am J Trop Med Hyg 32:1164–71 [DOI] [PubMed] [Google Scholar]
- 123.Vasilakis N, Tesh RB, Widen SG, Mirchandani D, Walker PJ (2019) Genomic characterisation of Cuiaba and Charleville viruses: arboviruses (family Rhabdoviridae, genus Sripuvirus) infecting reptiles and amphibians. Virus Genes 55:87–94 [DOI] [PubMed] [Google Scholar]
- 124.Velasco L, Arjona-Girona I, Cretazzo E, López-Herrera C (2019) Viromes in Xylariaceae fungi infecting avocado in Spain. Virology 532:11–21 [DOI] [PubMed] [Google Scholar]
- 125.Verani P, Ciufolini MG, Nicoletti L, Balducci M, Sabatinelli G, Coluzzi M, Paci P, Amaducci L (1982) Studi ecologici ed epidemiologici del virus Toscana, un arbovirus isolato da flebotomi. Ann Ist Super Sanita 18:397–9 [PubMed] [Google Scholar]
- 126.Walker PJ, Firth C, Widen SG, Blasdell KR, Guzman H, Wood TG, Paradkar PN, Holmes EC, Tesh RB, Vasilakis N (2015) Evolution of genome size and complexity in the Rhabdoviridae. PLoS Pathog 11:e1004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Walker PJ, Siddell SG, Lefkowitz EJ, Mushegian AR, Dempsey DM, Dutilh BE, Harrach B, Harrison RL, Hendrickson RC, Junglen S, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Nibert M, Rubino L, Sabanadzovic S, Simmonds P (2019) Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019). Arch Virol 164:2417–29 [DOI] [PubMed] [Google Scholar]
- 128.Walker PJ, Siddell SG, Lefkowitz EJ, Mushegian AR, Adriaenssens E, Dempsey DM, Dutilh BE, Harrach Bz, Harrison RL, Hendrickson RC, Junglen S, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Nibert M, Rubino L, Sabanadzovic S, Simmonds P, Varsani A, Zerbini FM, Davison AJ (2020) Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses (2020). Arch Virol [DOI] [PubMed] [Google Scholar]
- 129.Wang H, Liu Y, Liu W, Cao M, Wang X (2019) Sequence analysis and genomic organization of a novel chuvirus, Tàiyuán leafhopper virus. Arch Virol 164:617–20 [DOI] [PubMed] [Google Scholar]
- 130.Wang J, Selleck P, Yu M, Ha W, Rootes C, Gales R, Wise T, Crameri S, Chen H, Broz I, Hyatt A, Woods R, Meehan B, McCullough S, Wang L-F (2014) Novel phlebovirus with zoonotic potential isolated from ticks, Australia. Emerg Infect Dis 20:1040–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Y, Hua W, Wang J, Hannoufa A, Xu Z, Wang Z (2013) Deep sequencing of Lotus corniculatus L. reveals key enzymes and potential transcription factors related to the flavonoid biosynthesis pathway. Mol Genet Genomics 288:131–9 [DOI] [PubMed] [Google Scholar]
- 132.Wanzeller ALM, Martins LC, Diniz Júnior JAP, de Almeida Medeiros DB, Cardoso JF, da Silva DEA, de Oliveira LF, de Vasconcelos JM, Nunes MRT, da S. G Vianez JL Júnior, Vasconcelos PFC (2014) Xiburema virus, a hitherto undescribed virus within the family Rhabdoviridae isolated in the Brazilian Amazon Region. Genome Announc 2:e00454–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Willie K, Stewart LR (2017) Complete genome sequence of a new maize-associated cytorhabdovirus. Genome Announc 5:e00591–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Winter S, Koerbler M, Shahraeen N, Katul L, Lesemann D-E (2003) Characterization of a new tospovirus species infecting tomato in Iran. J Plant Dis Prot 110:74 [Google Scholar]
- 135.Winton JR, Batts WN, Powers RL, Purcell MK (2019) Complete genome sequences of the index isolates of two genotypes of Pacific salmon paramyxovirus. Microbiol Resour Announc 8:e01521–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wolf YI, Kazlauskas D, Iranzo J, Lucía-Sanz A, Kuhn JH, Krupovic M, Dolja VV, Koonin EV (2018) Origins and evolution of the global RNA virome. MBio 9:e02329–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Woyessa AB, Omballa V, Wang D, Lambert A, Waiboci L, Ayele W, Ahmed A, Abera NA, Cao S, Ochieng M, Montgomery JM, Jima D, Fields B (2014) An outbreak of acute febrile illness caused by sandfly fever Sicilian virus in the Afar Region of Ethiopia, 2011. Am J Trop Med Hyg 91:1250–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu L-P, Yang T, Liu H-W, Postman J, Li R (2018) Molecular characterization of a novel rhabdovirus infecting blackcurrant identified by high-throughput sequencing. Arch Virol 163:1363–6 [DOI] [PubMed] [Google Scholar]
- 139.Wu Z, Du J, Lu L, Yang L, Dong J, Sun L, Zhu Y, Liu Q, Jin Q (2018) Detection of Hantaviruses and Arenaviruzses [sic] in three-toed jerboas from the Inner Mongolia Autonomous Region, China. Emerg Microbes Infect 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu Z, Lu L, Du J, Yang L, Ren X, Liu B, Jiang J, Yang J, Dong J, Sun L, Zhu Y, Li Y, Zheng D, Zhang C, Su H, Zheng Y, Zhou H, Zhu G, Li H, Chmura A, Yang F, Daszak P, Wang J, Liu Q, Jin Q (2018) Comparative analysis of rodent and small mammal viromes to better understand the wildlife origin of emerging infectious diseases. Microbiome 6:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu C, Sun X, Taylor A, Jiao C, Xu Y, Cai X, Wang X, Ge C, Pan G, Wang Q, Fei Z, Wang Q (2017) Diversity, distribution, and evolution of tomato viruses in China uncovered by small RNA sequencing. J Virol 91:e00173–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu FL, Liu DY, Nunes MRT, Da Rosa A, Tesh RB, Xiao SY (2007) Antigenic and genetic relationships among Rift Valley fever virus and other selected members of the genus Phlebovirus (Bunyaviridae). Am J Trop Med Hyg 76:1194–200 [PubMed] [Google Scholar]
- 143.Xu Y, Lou S-G, Li XL, Zheng Y-X, Wang W-C, Liu Y-T (2013) The complete S RNA and M RNA nucleotide sequences of a hippeastrum chlorotic ringspot virus (HCRV) isolate from Hymenocallis littoralis (Jacq.) Salisb in China. Arch Virol 158:2597–601 [DOI] [PubMed] [Google Scholar]
- 144.Yadav PD, Nyayanit DA, Shete AM, Jain S, Majumdar TP, Chaubal GY, Shil P, Kore PM, Mourya DT (2019) Complete genome sequencing of Kaisodi virus isolated from ticks in India belonging to Phlebovirus genus, family Phenuiviridae. Ticks Tick Borne Dis 10:23–33 [DOI] [PubMed] [Google Scholar]
- 145.Yang X-L, Zhang Y-Z, Jiang R-D, Guo H, Zhang W, Li B, Wang N, Wang L, Waruhiu C, Zhou J-H, Li S-Y, Daszak P, Wang L-F, Shi Z-L (2017) Genetically diverse filoviruses in Rousettus and Eonycteris spp. bats, China, 2009 and 2015. Emerg Infect Dis 23:482–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang X-L, Tan CW, Anderson DE, Jiang R-D, Li B, Zhang W, Zhu Y, Lim XF, Zhou P, Liu X-L, Guan W, Zhang L, Li S-Y, Zhang Y-Z, Wang L-F, Shi Z-L (2019) Characterization of a filovirus (Měnglà virus) from Rousettus bats in China. Nat Microbiol 4:390–5 [DOI] [PubMed] [Google Scholar]
- 147.Yang X, Huang J, Liu C, Chen B, Zhang T, Zhou G (2016) Rice stripe mosaic virus, a novel cytorhabdovirus infecting rice via leafhopper transmission. Front Microbiol 7:2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao G, Krishnamurthy S, Cai Z, Popov VL, Travassos da Rosa AP, Guzman H, Cao S, Virgin HW, Tesh RB, Wang D (2013) Identification of novel viruses using VirusHunter - an automated data analysis pipeline. PLoS One 8:e78470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Бутенко АМ, Громашевский ВЛ, Львов ДК, Попов ВФ (1979) Вирус Кисемайо - представитель антигенной группы Бханджа. Вопр Вирусол:661–5 [PubMed] [Google Scholar]
- 150.Львов ДК, Альховский СВ, Щелканов МЮ, Щетинин АМ, Дерябин ПГ, Аристова ВА, Гительман АК, Самохвалов ЕИ, Ботиков АГ (2014) Генетическая характеристика вирусов Сахалин (SAKV - Sakhalin virus), Парамушир (PMRV - Paramushir virus) (Bunyaviridae, Nairovirus, группа Сахалин) и Рукутама (RUKV - Rukutama virus) (Bunyaviridae, Phlebovirus, группа Укуниеми), изолированных от облигатных паразитов колониальных морских птиц - клещей Ixodes (Ceratixodes) uriae, White 1852 и I. signatus Birulya, 1895 в бассейнах Охотского и Берингова морей. Вопр Вирусол 59:11–7 [PubMed] [Google Scholar]
- 151.Львов ДК, Альховский СВ, Щелканов МЮ, Щетинин АМ, Дерябин ПГ, Гительман АК, Самохвалов ЕИ, Ботиков АГ (2014) Генетическая характеристика штаммов вируса Залив Терпения (ZTV - Zaliv Terpeniya virus) (Bunyaviridae, Phlebovirus, антигенный комплекс Укуниеми), изолированного в высоких широтах Северной Евразии из облигатных эктопаразитов чистиковых птиц (Alcidae Leach, 1820) - клещей Ixodes (Ceratixodes) uriae White, 1852 и от комаров Culex modestus Ficalbi, 1889 в субтропиках Закавказья. Вопр Вирусол 59:12–8 [PubMed] [Google Scholar]


