Key Points
Question
What are neurologic manifestations of patients with coronavirus disease 2019?
Findings
In a case series of 214 patients with coronavirus disease 2019, neurologic symptoms were seen in 36.4% of patients and were more common in patients with severe infection (45.5%) according to their respiratory status, which included acute cerebrovascular events, impaired consciousness, and muscle injury.
Meaning
Neurologic symptoms manifest in a notable proportion of patients with coronavirus disease 2019.
Abstract
Importance
The outbreak of coronavirus disease 2019 (COVID-19) in Wuhan, China, is serious and has the potential to become an epidemic worldwide. Several studies have described typical clinical manifestations including fever, cough, diarrhea, and fatigue. However, to our knowledge, it has not been reported that patients with COVID-19 had any neurologic manifestations.
Objective
To study the neurologic manifestations of patients with COVID-19.
Design, Setting, and Participants
This is a retrospective, observational case series. Data were collected from January 16, 2020, to February 19, 2020, at 3 designated special care centers for COVID-19 (Main District, West Branch, and Tumor Center) of the Union Hospital of Huazhong University of Science and Technology in Wuhan, China. The study included 214 consecutive hospitalized patients with laboratory-confirmed diagnosis of severe acute respiratory syndrome coronavirus 2 infection.
Main Outcomes and Measures
Clinical data were extracted from electronic medical records, and data of all neurologic symptoms were checked by 2 trained neurologists. Neurologic manifestations fell into 3 categories: central nervous system manifestations (dizziness, headache, impaired consciousness, acute cerebrovascular disease, ataxia, and seizure), peripheral nervous system manifestations (taste impairment, smell impairment, vision impairment, and nerve pain), and skeletal muscular injury manifestations.
Results
Of 214 patients (mean [SD] age, 52.7 [15.5] years; 87 men [40.7%]) with COVID-19, 126 patients (58.9%) had nonsevere infection and 88 patients (41.1%) had severe infection according to their respiratory status. Overall, 78 patients (36.4%) had neurologic manifestations. Compared with patients with nonsevere infection, patients with severe infection were older, had more underlying disorders, especially hypertension, and showed fewer typical symptoms of COVID-19, such as fever and cough. Patients with more severe infection had neurologic manifestations, such as acute cerebrovascular diseases (5 [5.7%] vs 1 [0.8%]), impaired consciousness (13 [14.8%] vs 3 [2.4%]), and skeletal muscle injury (17 [19.3%] vs 6 [4.8%]).
Conclusions and Relevance
Patients with COVID-19 commonly have neurologic manifestations. During the epidemic period of COVID-19, when seeing patients with neurologic manifestations, clinicians should suspect severe acute respiratory syndrome coronavirus 2 infection as a differential diagnosis to avoid delayed diagnosis or misdiagnosis and lose the chance to treat and prevent further transmission.
This study investigates the neurologic manifestations of patients with coronavirus disease 2019.
Introduction
In December 2019, many unexplained pneumonia cases occurred in Wuhan, China, and rapidly spread to other parts of China, then to Europe, North America, and Asia. This outbreak was confirmed to be caused by a novel coronavirus (CoV).1 The novel CoV was reported to have symptoms resembling that of severe acute respiratory syndrome CoV (SARS-CoV) in 2003.2 Both shared the same receptor, angiotensin-converting enzyme 2 (ACE2).3 Therefore, this virus was named SARS-CoV-2, and in February 2020, the World Health Organization (WHO) named the disease coronavirus disease 2019 (COVID-19). As of March 5, 2020, there were 95 333 confirmed cases of COVID-19 and 3282 deaths globally.4
Coronaviruses can cause multiple systemic infections or injuries in various animals.5 The CoVs can adapt quickly and cross the species barrier, such as with SARS-CoV and Middle East respiratory syndrome CoV (MERS-CoV), causing epidemics or pandemics. Infection in humans often leads to severe clinical symptoms and high mortality.6 As for COVID-19, several studies have described typical clinical manifestations including fever, cough, diarrhea, and fatigue. Coronavirus disease 2019 also has characteristic laboratory findings and lung computed tomography (CT) abnormalities.7 However, to our knowledge, it has not been reported that patients with COVID-19 had any neurologic manifestations. Here, we report the characteristic neurologic manifestations of SARS-CoV-2 infection in 78 of 214 patients with laboratory-confirmed diagnosis of COVID-19 and treated at our hospitals, which are located in the epicenter of Wuhan.
Methods
Study Design and Participants
This retrospective, observational study was done at 3 centers (Main District, West Branch, and Tumor Center) of Union Hospital of Huazhong University of Science and Technology (Wuhan, China). These 3 centers are designated hospitals assigned by the government to treat patients with COVID-19. We retrospectively analyzed consecutive patients from January 16, 2020, to February 19, 2020, who had been diagnosed as having COVID-19, according to WHO interim guidance.8 A confirmed case of COVID-19 was defined as a positive result on high-throughput sequencing or real-time reverse-transcription polymerase chain reaction analysis of throat swab specimens. Throat swab samples were collected and placed into a collection tube containing preservation solution for the virus.9 A SARS-CoV-2 infection was confirmed by real-time reverse-transcription polymerase chain reaction assay using a SARS-CoV-2 nucleic acid detection kit according to the manufacturer’s protocol (Shanghai bio-germ Medical Technology Co). Radiologic assessments included chest and head CT, and all laboratory testing (a complete blood cell count, blood chemical analysis, coagulation testing, assessment of liver and renal function testing, C-reactive protein, creatine kinase, and lactate dehydrogenase) was performed according to the clinical care needs of the patient. Two hundred fourteen hospitalized patients with laboratory confirmation of SARS-CoV-2 were included in the analysis.9 Before enrollment, verbal consent was obtained from patients or an accompanying relative for patients who could not give consent. The study was performed in accordance with the principles of the Declaration of Helsinki. This study was approved and written informed consent was waived by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, on February 20, 2020, owing to the rapid emergence of the disease and the urgent need to collect data.
Data Collection
We reviewed electronic medical records, nursing records, laboratory findings, and radiologic examinations for all patients with laboratory-confirmed SARS-CoV-2 infection and collected data on age, sex, comorbidities (hypertension, diabetes, cardiac or cerebrovascular disease, malignancy, and chronic kidney disease), typical symptoms from onset to hospital admission (fever, cough, anorexia, diarrhea, throat pain, abdominal pain), nervous system symptoms, laboratory findings, and CT scan (chest and head if available). Subjective symptoms were provided by patients who were conscious, cognitively and mentally normal, and linguistically competent to respond to interview. Any missing or uncertain records were collected and clarified through direct communication with involved patients, health care clinicians, and their families. We defined the degree of severity of COVID-19 (severe vs nonsevere) at the time of admission using the American Thoracic Society guidelines for community-acquired pneumonia.10
All neurologic manifestations were reviewed and confirmed by 2 trained neurologists. Major disagreement between 2 reviewers was resolved by consultation with a third reviewer. Neurologic manifestations were categorized into 3 categories: central nervous system (CNS) manifestations (dizziness, headache, impaired consciousness, acute cerebrovascular disease, ataxia, and seizure), peripheral nervous system (PNS) manifestations (taste impairment, smell impairment, vision impairment, and nerve pain), and skeletal muscular injury manifestations. Impaired consciousness includes the change of consciousness level (somnolence, stupor, and coma) and consciousness content (confusion and delirium). To avoid cross-infection during the outbreak, we had to minimize patients going out for examination. Therefore, the diagnosis of nervous system manifestations mainly depended on the subjective symptoms of patients and the examinations available. Acute cerebrovascular disease includes ischemic stroke and cerebral hemorrhage diagnosed by clinical symptoms and head CT. Seizure is based on the clinical symptoms at the time of presentation. Skeletal muscle injury was defined as when a patient had skeletal muscle pain and elevated serum creatine kinase level greater than 200 U/L (to convert to microkatals per liter, multiply by 0.0167).7
Statistical Analysis
For baseline data, mean and standard deviations (SD) were used for normally distributed data and median and range for data that were not normally distributed. Categorical variables were expressed as counts and percentages. Continuous variables were compared by using the Wilcoxon rank sum test. Proportions for categorical variables were compared using the χ2 test. All statistical analyses were performed using R, version 3.3.0, software (the R Foundation). The significance threshold was set at a 2-sided P value less than .05.
Results
Demographic and Clinical Characteristics
A total of 214 hospitalized patients with confirmed SARS-CoV-2 infection were included in the analysis. Their demographic and clinical characteristics were shown in Table 1. Their mean (SD) age was 52.7 (15.5) years, and 87 were men (40.7%). Of these patients, 83 (38.8%) had at least 1 of the following underlying disorders: hypertension (51 [23.8%]), diabetes (30 [14.0%]), cardiac or cerebrovascular disease (15 [7.0%]), and malignancy (13 [6.1%]). The most common symptoms at onset of illness were fever (132 [61.7%]), cough (107 [50.0%]), and anorexia (68 [31.8%]). Seventy-eight patients (36.4%) had nervous system manifestations: CNS (53 [24.8%]), PNS (19 [8.9%]), and skeletal muscle injury (23 [10.7%]). In patients with CNS manifestations, the most common reported symptoms were dizziness (36 [16.8%]) and headache (28 [13.1%]). In patients with PNS symptoms, the most common reported symptoms were taste impairment (12 [5.6%]) and smell impairment (11 [5.1%]).
Table 1. Clinical Characteristics of Patients With COVID-19.
| Characteristic | No. (%) | P valuea | ||
|---|---|---|---|---|
| Total (N = 214) | Severe (n = 88) | Nonsevere (n = 126) | ||
| Age, mean (SD), y | 52.7 (15.5) | 58.2 (15.0) | 48.9 (14.7) | |
| Age, y | ||||
| <50 | 90 (42.1) | 24 (27.3) | 66 (52.4) | <.001 |
| ≥50 | 124 (57.9) | 64 (72.7) | 60 (47.6) | |
| Sex | ||||
| Female | 127 (59.3) | 44 (50.0) | 83 (65.9) | .02 |
| Male | 87 (40.7) | 44 (50.0) | 43 (34.1) | |
| Comorbidities | ||||
| Any | 83 (38.8) | 42 (47.7) | 41 (32.5) | .03 |
| Hypertension | 51 (23.8) | 32 (36.4) | 19 (15.1) | <.001 |
| Diabetes | 30 (14.0) | 15 (17.0) | 15 (11.9) | .29 |
| Cardiac or cerebrovascular disease | 15 (7.0) | 7 (8.0) | 8 (6.3) | .65 |
| Malignancy | 13 (6.1) | 5 (5.7) | 8 (6.3) | .84 |
| Chronic kidney disease | 6 (2.8) | 2 (2.3) | 4 (3.2) | .69 |
| Typical symptoms | ||||
| Fever | 132 (61.7) | 40 (45.5) | 92 (73.0) | <.001 |
| Cough | 107 (50.0) | 30 (34.1) | 77 (61.1) | <.001 |
| Anorexia | 68 (31.8) | 21 (23.9) | 47 (37.3) | .04 |
| Diarrhea | 41 (19.2) | 13 (14.8) | 28 (22.2) | .17 |
| Throat pain | 31 (14.5) | 10 (11.4) | 21 (16.7) | .28 |
| Abdominal pain | 10 (4.7) | 6 (6.8) | 4 (3.2) | .21 |
| Nervous system symptoms | ||||
| Any | 78 (36.4) | 40 (45.5) | 38 (30.2) | .02 |
| CNS | 53 (24.8) | 27 (30.7) | 26 (20.6) | .09 |
| Dizziness | 36 (16.8) | 17 (19.3) | 19 (15.1) | .42 |
| Headache | 28 (13.1) | 15 (17.0) | 13 (10.3) | .15 |
| Impaired consciousness | 16 (7.5) | 13 (14.8) | 3 (2.4) | <.001 |
| Acute cerebrovascular disease | 6 (2.8) | 5 (5.7) | 1 (0.8) | .03 |
| Ataxia | 1 (0.5) | 1 (1.1) | 0 | NA |
| Seizure | 1 (0.5) | 1 (1.1) | 0 | NA |
| PNS | 19 (8.9) | 7 (8.0) | 12 (9.5) | .69 |
| Impairment | ||||
| Taste | 12 (5.6) | 3 (3.4) | 9 (7.1) | .24 |
| Smell | 11 (5.1) | 3 (3.4) | 8 (6.3) | .34 |
| Vision | 3 (1.4) | 2 (2.3) | 1 (0.8) | .37 |
| Nerve pain | 5 (2.3) | 4 (4.5) | 1 (0.8) | .07 |
| Skeletal muscle injury | 23 (10.7) | 17 (19.3) | 6 (4.8) | <.001 |
| Onset of symptoms to hospital admission, median (range), d | ||||
| CNS | ||||
| Dizziness | 1 (1-30) | 1 (1-30) | 1 (1-14) | NA |
| Headache | 1 (1-14) | 1 (1-3) | 3 (1-14) | NA |
| Impaired consciousness | 8 (1-25) | 10 (1-25) | 1 (1-3) | NA |
| Acute cerebrovascular disease | 9 (1-18) | 10 (1-18) | 1 (1) | NA |
| Ataxia | 2 (2) | 2 (2) | NA | NA |
| Seizure | 2 (2) | 2 (2) | NA | NA |
| PNS | ||||
| Impairment | ||||
| Taste | 2 (1-5) | 3 (1-3) | 2 (1-5) | NA |
| Smell | 2 (1-5) | 1 (1-4) | 2 (1-5) | NA |
| Vision | 2 (1-3) | 3 (2-3) | 1 (1) | NA |
| Nerve pain | 1 (1-1) | 1 (1-1) | 1 (1) | NA |
| Skeletal muscle injury | 1 (1-11) | 1 (1-11) | 1 (1-6) | NA |
Abbreviations: CNS, central nervous system; COVID-19, coronavirus disease 2019; NA, not applicable; PNS, peripheral nervous system.
P values indicate differences between patients with severe and nonsevere infection, and P less than .05 was considered statistically significant.
According to the American Thoracic Society guidelines for community-acquired pneumonia,10 88 patients (41.1%) had severe infection and 126 patients (58.9%) had nonsevere infection. The patients with severe infection were significantly older (mean [SD] age, 58.2 [15.0] years vs 48.9 [14.7] years; P < .001) and more likely to have other underlying disorders (42 [47.7%] vs 41 [32.5%]; P = .03), especially hypertension (32 [36.4%] vs 19 [15.1%]; P < .001), and had fewer typical symptoms of COVID-19 such as fever (40 [45.5%] vs 92 [73%]; P < .001) and dry cough (30 [34.1%] vs 77 [61.1%]; P < .001). Moreover, nervous system manifestations were significantly more common in severe infections compared with nonsevere infections (40 [45.5%] vs 38 [30.2%], P = .02). They included acute cerebrovascular disease (5 [5.7%]; 4 patients with ischemic stroke and 1 with cerebral hemorrhage who died later of respiratory failure; vs 1 [0.8%]; 1 patient with ischemic stroke; P = .03, Figure), impaired consciousness (13 [14.8%] vs 3 [2.4%]; P < .001), and skeletal muscle injury (17 [19.3%] vs 6 [4.8%]; P < .001). In the severe group, 1 patient had a seizure characterized by a sudden onset of limb twitching, foaming in the mouth, and loss of consciousness, which lasted for 3 minutes.
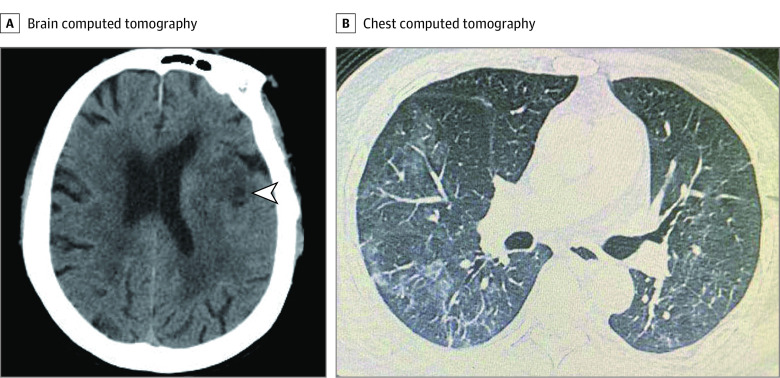
Figure. Representative Computed Tomography (CT) Images of a Patient With Coronavirus Disease 2019 With New Onset of Ischemic Stroke.
A, Brain CT image 1 day after ischemic stroke. White arrowhead indicates the ischemic lesion. B, Chest CT image 1 day after ischemic stroke.
Apart from cerebrovascular disease and impaired consciousness, most neurologic manifestations occurred early in the illness (median time, 1-2 days). Of 6 patients with acute cerebrovascular disease, 2 arrived at the emergency department owing to sudden onset of hemiplegia but without any typical symptoms (fever, cough, anorexia, and diarrhea) of COVID-19. Their lung lesions were found by an emergent lung CT and were diagnosed as having COVID-19 by a positive SARS-CoV-2 nucleic acid detection in the later stage. Some patients with fever and headache were admitted to the neurology ward after initially being ruled out of COVID-19 by routine blood test results and a screening lung CT in the clinic. However, several days later, they had typical COVID-19 symptoms such as cough, throat pain, lower lymphocyte count, and ground-glass opacity appearance on lung CT. Their diagnosis of COVID-19 was confirmed by a positive nucleic acid test and then they were transferred to the isolation ward.
Laboratory Findings in Patients and With Severe and Nonsevere Infection
Table 2 showed the laboratory findings in severe and nonsevere subgroups. Patients with severe infection had more increased inflammatory response, including higher white blood cell counts, neutrophil counts, lower lymphocyte counts, and increased C-reactive protein levels compared with those patients with nonsevere infection (white blood cell count: median, 5.4 × 109/L [range, 0.1-20.4] vs 4.5 × 109/L [range, 1.8-14.0]; P < .001; neutrophil: median, 3.8 × 109/L [range, 0.0-18.7] vs 2.6 × 109/L [range, 0.7-11.8]; P < .001; lymphocyte count: median, 0.9 × 109/L [range, 0.1-2.6] vs 1.3 × 109/L [range, 0.4-2.6]; P < .001; C-reactive protein: median, 37.1 mg/L [range, 0.1-212.0] vs 9.4 mg/L [range, 0.2-126.0]; P < .001). The patients with severe infection had higher D-dimer levels than patients with nonsevere infection (median, 0.9 mg/L [range, 0.1-20.0] vs 0.4 mg/L [range, 0.2-8.7]; P < .001), which was indicative of consumptive coagulation system. In addition, patients with severe infection had multiple organ involvement, such as serious liver (increased lactate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase levels), kidney (increased blood urea nitrogen and creatinine levels), and skeletal muscle damage (increased creatinine kinase levels).
Table 2. Laboratory Findings of Patients With COVID-19.
| Laboratory finding | Median (range) | P valuea | ||
|---|---|---|---|---|
| Total (N = 214) | Severe (n = 88) | Nonsevere (n = 126) | ||
| Count, ×109/L | ||||
| White blood cell | 4.9 (0.1-20.4) | 5.4 (0.1-20.4) | 4.5 (1.8-14.0) | .008 |
| Neutrophil | 3.0 (0.0-18.7) | 3.8 (0.0-18.7) | 2.6 (0.7-11.8) | <.001 |
| Lymphocyte | 1.1 (0.1-2.6) | 0.9 (0.1-2.6) | 1.3 (0.4-2.6) | <.001 |
| Platelet | 209.0 (18.0-583.0) | 204.5 (18.0-576.0) | 219.0 (42.0-583.0) | .25 |
| C-reactive protein, mg/L | 12.2 (0.1-212.0) | 37.1 (0.1-212.0) | 9.4 (0.4-126.0) | <.001 |
| D-dimer, mg/L | 0.5 (0.1-20.0) | 0.9 (0.1-20.0) | 0.4 (0.2-8.7) | <.001 |
| Creatine kinase, U/L | 64.0 (8.8-12216.0) | 83.0 (8.8-12216.0) | 59.0 (19.0-1260.0) | .004 |
| Lactate dehydrogenase, U/L | 241.5 (2.2-908.0) | 302.0 (2.2-880.0) | 215.0 (2.5-908.0) | <.001 |
| Aminotransferase, U/L | ||||
| Alanine | 26.0 (5.0-1933.0) | 32.5 (5.0-1933.0) | 23.0 (6.0-261.0) | .04 |
| Aspartate | 26.0 (8.0-8191.0) | 34.0 (8.0-8191.0) | 23.0 (9.0-244.0) | <.001 |
| Blood urea nitrogen, mmol/L | 4.1 (1.5-48.1) | 4.6 (1.5-48.1) | 3.8 (1.6-13.7) | <.001 |
| Creatinine, μmol/L | 68.2 (35.9-9435.0) | 71.6 (35.9-9435.0) | 65.6 (39.4-229.1) | .03 |
Abbreviation: COVID-19, coronavirus disease 2019.
SI conversion factor: To convert aminotransferase levels to microkatals per liter, multiply by 0.0167; creatine kinase to microkatals per liter, multiply by 0.0167; lactate dehydrogenase to microkatals per liter, multiply by 0.0167.
P values indicate differences between patients with severe and nonsevere infection, and P less than .05 was considered statistically significant.
Laboratory Findings in Patients With and Without CNS Symptoms
Table 3 showed the laboratory findings of patients with and without CNS symptoms. We found that patients with CNS symptoms had lower lymphocyte levels, platelet counts, and higher blood urea nitrogen levels compared with those without CNS symptoms (lymphocyte count: median, 1.0 × 109/L [range, 0.1-2.3] vs 1.2 × 109/L [range, 0.2-2.6], P = .049; platelet count: median, 180.0 × 109/L [range, 18.0-564.0] vs 227.0 × 109/L [range, 42.0-583.0], P = .005; blood urea nitrogen: median, 4.5 mmol/L [range, 1.6-48.1] vs 4.1 mmol/L [range, 1.5-19.1], P = .04). For the severe subgroup, patients with CNS symptoms also had lower lymphocyte levels and platelet counts and higher blood urea nitrogen levels compared with those without CNS symptoms (lymphocyte count: median, 0.7 × 109/L [range, 0.1-1.6] vs 0.9 × 109/L [range, 0.2-2.6], P = .007; platelet count: median, 169.0 × 109/L [range, 18.0-564.0] vs 220.0 × 109/L [range, 109.0-576.0], P = .04; blood urea nitrogen: median, 5.0 mmol/L [range, 2.3-48.1] vs 4.4 mmol/L [range, 1.5-19.1], P = .04). For the nonsevere subgroup, there were no significant differences in laboratory findings of patients with and without CNS symptoms.
Table 3. Laboratory Findings of Patients With COVID-19 With CNS Symptomsa.
| Laboratory finding | Median (range) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Severe | Nonsevere | |||||||
| With CNS symptoms (n = 53) | Without CNS symptoms (n = 161) | P value | With CNS symptoms (n = 27) | Without CNS symptoms (n = 61) | P value | With CNS symptoms (n = 26) | Without CNS symptoms (n = 100) | P value | |
| Count, ×109/L | |||||||||
| White blood cell | 4.6 (0.1-12.5) | 4.9 (1.8-20.4) | .58 | 5.3 (0.1-12.5) | 5.5 (1.9-20.4) | .77 | 4.1 (2.4-11.0) | 4.6 (1.8-14.0) | .40 |
| Neutrophil | 2.6 (0.0-10.9) | 3.1 (0.7-18.7) | .41 | 3.8 (0.0-10.9) | 3.6 (0.7-18.7) | >.99 | 2.2 (0.9-7.4) | 2.8 (0.7-11.8) | .11 |
| Lymphocyte | 1.0 (0.1-2.3) | 1.2 (0.2-2.6) | .049 | 0.7 (0.1-1.6) | 0.9 (0.2-2.6) | .007 | 1.3 (0.7-2.3) | 1.3 (0.4-2.6) | .49 |
| Platelet | 180.0 (18.0-564.0) | 227.0 (42.0-583.0) | .005 | 169.0 (18.0-564.0) | 220.0 (109.0-576.0) | .04 | 188.5 (110.0-548.0) | 232.0 (42.0-583.0) | .09 |
| C-reactive protein, mg/L | 14.1 (0.1-212.0) | 11.4 (0.1-204.5) | .31 | 48.6 (0.1-212.0) | 26.1 (0.1-204.5) | .68 | 7.4 (3.1-111.0) | 9.8 (0.4-126.0) | .82 |
| D-dimer, mg/L | 0.5 (0.2-9.7) | 0.5 (0.1-20.0) | .75 | 1.2 (0.2-9.7) | 0.9 (0.1-20.0) | .42 | 0.4 (0.2-6.4) | 0.4 (0.2-8.7) | .46 |
| Creatine kinase, U/L | 79.0 (8.8-12216.0) | 60.5 (19.0-1260.0) | .17 | 104.0 (8.8-12 216.0) | 64.0 (19.0-1214.0) | .08 | 52.5 (28.0-206.0) | 59.0 (19.0-1260.0) | .56 |
| Lactate dehydrogenase, U/L | 243.0 (2.2-880.0) | 241.0 (3.5-908.0) | .77 | 334.0 (2.2-880.0) | 299.0 (3.5-743.0) | .32 | 198.0 (2.5-417.0) | 226.0 (121.0-908.0) | .14 |
| Aminotransferase, U/L | |||||||||
| Alanine | 27.0 (5.0-261.0) | 26.0 (6.0-1933.0) | .21 | 35.0 (5.0-259.0) | 31.0 (7.0-1933.0) | .32 | 25.5 (13.0-261.0) | 23.0 (6.0-135.0) | .68 |
| Aspartate | 29.5 (13.0-213.0) | 26.0 (8.0-8191.0) | .10 | 35.5 (14.0-213.0) | 34.0 (8.0-8191.0) | .32 | 23.0 (13.0-198.0) | 23.5 (9.0-244.0) | .56 |
| Blood urea nitrogen, mmol/L | 4.5 (1.6-48.1) | 4.1 (1.5-19.1) | .04 | 5.0 (2.3-48.1) | 4.4 (1.5-19.1) | .04 | 3.9 (1.6-9.4) | 3.8 (1.7-13.7) | .57 |
| Creatinine, μmol/L | 71.7 (37.1-1299.2) | 66.3 (35.9-9435.0) | .06 | 71.7 (37.1-1299.2) | 68.4 (35.9-9435.0) | .25 | 72.0 (40.3-133.6) | 63.4 (39.4-229.1) | .27 |
Abbreviations: CNS, central nervous system; COVID-19, coronavirus disease 2019.
SI conversion factor: To convert aminotransferase levels to microkatals per liter, multiply by 0.0167; creatine kinase to microkatals per liter, multiply by 0.0167; lactate dehydrogenase to microkatals per liter, multiply by 0.0167.
P values indicate differences between patients with and without CNS symptoms, and P less than .05 was considered statistically significant.
Laboratory Findings in Patients With and Without PNS Symptoms
Table 4 showed the laboratory findings of patients with and without PNS symptoms. We found that there were no significant differences in laboratory findings of patients with PNS and those without PNS. Similar results were also found in the severe subgroup and nonsevere subgroup, respectively.
Table 4. Laboratory Findings of Patients With COVID-19 With PNS Symptoms.
| Laboratory finding | Median (range) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Severe | Nonsevere | ||||||||
| With PNS symptoms (n = 19) | Without PNS symptoms (n = 195) | P value | With PNS symptoms (n = 7) | Without PNS symptoms (n = 81) | P value | With PNS symptoms (n = 12) | Without PNS symptoms (n = 114) | P value | ||
| Count, ×109/L | ||||||||||
| White blood cell | 4.8 (2.8-7.5) | 4.9 (0.1-20.4) | .74 | 4.5 (3.1-6.8) | 5.6 (0.1-20.4) | .11 | 4.9 (2.8-7.5) | 4.4 (1.8-14.0) | .27 | |
| Neutrophil | 2.8 (1.5-5.4) | 3.0 (0.0-18.7) | .74 | 2.6 (1.5-5.3) | 4.1 (0.0-18.7) | .10 | 2.9 (1.9-5.4) | 2.5 (0.7-11.8) | .21 | |
| Lymphocyte | 1.2 (0.6-2.6) | 1.1 (0.1-2.6) | .43 | 1.2 (0.6-1.6) | 0.9 (0.1-2.6) | .26 | 1.2 (0.7-2.6) | 1.3 (0.4-2.4) | .92 | |
| Platelet | 204.0 (111.0-305.0) | 213.0 (18.0-583.0) | .56 | 204.0 (111.0-245.0) | 205.0 (18.0-576.0) | .56 | 214.5 (155.0-305.0) | 219.0 (42.0-583.0) | .81 | |
| C-reactive protein, mg/L | 12.0 (3.1-81.0) | 12.3 (0.1-212.0) | .45 | 7.5 (3.1-76.4) | 43.7 (0.1-212.0) | .13 | 13.0 (3.1-81.0) | 8.8 (0.4-126.0) | .60 | |
| D-dimer, mg/L | 0.4 (0.2-9.5) | 0.5 (0.1-20.0) | .40 | 0.5 (0.2-9.5) | 1.3 (0.1-20.0) | .27 | 0.4 (0.2-4.5) | 0.4 (0.2-8.7) | .99 | |
| Creatine kinase, U/L | 67.0 (32.0-1214.0) | 64.0 (8.8-12216.0) | .41 | 105.0 (32.0-1214.0) | 83.0 (8.8-12216.0) | .76 | 66.0 (42.0-171.0) | 57.5 (19.0-1260.0) | .29 | |
| Lactate dehydrogenase, U/L | 205.0 (2.5-517.0) | 242.0 (2.2-908.0) | .28 | 170.0 (46.0-517.0) | 309.0 (2.2-880.0) | .05 | 254.0 (2.5-481.0) | 215.0 (2.9-908.0) | .67 | |
| Aminotransferase, U/L | ||||||||||
| Alanine | 26.0 (5.0-116.0) | 27.0 (6.0-1933.0) | .70 | 19.0 (5.0-80.0) | 35.0 (8.0-1933.0) | .23 | 26.0 (8.0-116.0) | 23.0 (6.0-261.0) | .56 | |
| Aspartate | 22.0 (8.0-115.0) | 27.0 (9.0-8191.0) | .29 | 22.0 (8.0-53.0) | 35.5 (12.0-8191.0) | .13 | 22.0 (14.0-115.0) | 23.5 (9.0-244.0) | >.99 | |
| Blood urea nitrogen, mmol/L | 4.1 (1.6-8.8) | 4.1 (1.5-48.1) | .76 | 4.2 (3.5-8.8) | 4.7 (1.5-48.1) | .96 | 3.7 (1.6-5.3) | 3.9 (1.7-13.7) | .66 | |
| Creatinine, μmol/L | 62.5 (48.1-121.4) | 68.3 (35.9-9435.0) | .46 | 71.4 (58.3-121.4) | 71.7 (35.9-9435.0) | .72 | 59.9 (48.1-77.3) | 66.6 (39.4-229.1) | .24 | |
Abbreviations: COVID-19, coronavirus disease 2019; PNS, peripheral nervous system.
SI conversion factor: To convert aminotransferase levels to microkatals per liter, multiply by 0.0167; creatine kinase to microkatals per liter, multiply by 0.0167; lactate dehydrogenase to microkatals per liter, multiply by 0.0167.
P values indicate differences between patients with and without PNS symptoms, and P less than .05 was considered statistically significant.
Laboratory Findings in Patients With and Without Skeletal Muscle Injury
The eTable in the Supplement shows the laboratory findings of patients with and without skeletal muscle injury. Compared with the patients without muscle injury, patients with muscle injury had significantly higher levels of creatine kinase (median, 400.0 U/L [range 203.0-12216.0] vs median, 58.5 U/L [range 8.8-212.0]; P < .001), regardless of their severity. Meanwhile, patients with muscle injury had higher neutrophil counts, lower lymphocyte counts, higher C-reactive protein levels, and higher D-dimer levels. The abnormalities were manifestations of increased inflammatory response and blood coagulation function. In addition, we found that patients with muscle injury had multiorgan damage, including more serious liver (increased lactate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase levels) and kidney (increased blood urea nitrogen and creatinine levels) abnormalities.
For the severe group, patients with skeletal muscle injury had decreased lymphocyte counts and more serious liver injury (increased lactate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase levels) and kidney injury (increased creatinine levels).
Discussion
To our knowledge, this is the first report on detailed neurologic manifestations of the hospitalized patients with COVID-19. As of February 19, 2020, of 214 patients included in this study, 88 (41.1%) had severe infection and 126 (58.9%) had nonsevere infection. Of these, 78 (36.4%) had various neurologic manifestations that involved CNS, PNS, and skeletal muscles. Compared with patients with nonsevere infection, patients with severe infection were older and had more hypertension but fewer typical symptoms such as fever and cough. Patients with severe infection were more likely to develop neurologic manifestations, especially acute cerebrovascular disease, conscious disturbance, and skeletal muscle injury. Most neurologic manifestations occurred early in the illness (the median time to hospital admission was 1-2 days). Some patients without typical symptoms (fever, cough, anorexia, and diarrhea) of COVID-19 came to the hospital with only neurologic manifestation as their presenting symptoms. Therefore, for patients with COVID-19, we need to pay close attention to their neurologic manifestations, especially for those with severe infections, which may have contributed to their death. Moreover, during the epidemic period of COVID-19, when seeing patients with these neurologic manifestations, physicians should consider SARS-CoV-2 infection as a differential diagnosis to avoid delayed diagnosis or misdiagnosis and prevention of transmission.
In January 2020,3 ACE2 was identified as the functional receptor for SARS-CoV-2, which is present in multiple human organs, including nervous system and skeletal muscles.11 The expression and distribution of ACE2 remind us that the SARS-CoV-2 may cause some neurologic manifestations through direct or indirect mechanisms. Autopsy results of patients with COVID-19 showed that the brain tissue was hyperemic and edematous and some neurons degenerated.12 Neurologic injury has been confirmed in the infection of other CoVs such as in SARS-CoV and MERS-CoV. The researchers detected SARS-CoV nucleic acid in the cerebrospinal fluid of those patients and also in their brain tissue on autopsy.13,14
Central nervous system symptoms were the main form of neurologic injury in patients with COVID-19 in this study. The pathologic mechanism may be from the CNS invasion of SARS-CoV-2, similar to SARS and MERS viruses. As with other respiratory viruses, SARS-COV-2 may enter the CNS through the hematogenous or retrograde neuronal route. The latter can be supported by the fact that some patients in this study had smell impairment. We also found that the lymphocyte counts were lower for patients with CNS symptoms than without CNS symptoms. This phenomenon may be indicative of the immunosuppression in patients with COVID-19 with CNS symptoms, especially in the severe subgroup. Moreover, we found patients with severe infection had higher D-dimer levels than that of patients with nonsevere infection. This may be the reason why patients with severe infection are more likely to develop cerebrovascular disease.
Consistent with the previous studies,7 muscle symptoms were also common in our study. We speculate that the symptom was owing to skeletal muscle injury, as confirmed by elevated creatine kinase levels. We found that patients with muscle symptoms had higher creatine kinase and lactate dehydrogenase levels than those without muscle symptoms. Furthermore, creatine kinase and lactate dehydrogenase levels in patients with severe infection were much higher than those of patients with nonsevere infection. This injury could be associated with ACE2 in skeletal muscle.15 However, SARS-CoV, using the same receptor, was not detected in skeletal muscle by postmortem examination.16 Therefore, whether SARS-CoV-2 infects skeletal muscle cells by binding with ACE2 needs to be further studied. One other reason was the infection-mediated harmful immune response that caused the nervous system abnormalities. Significantly elevated proinflammatory cytokines in serum may cause skeletal muscle damage.
Limitations
This study has several limitations. First, only 214 patients were studied, which could cause biases in clinical observation. It would be better to include more patients from Wuhan, other cities in China, and even other countries. Second, all data were abstracted from the electronic medical records; certain patients with neurologic symptoms might not be captured if their neurologic manifestations were too mild, such as with taste impairment and smell impairment. Third, because most patients were still hospitalized and information regarding clinical outcomes was unavailable at the time of analysis, it was difficult to assess the effect of these neurologic manifestations on their outcome, and continued observations of the natural history of disease are needed. Fourth, during the outbreak period of COVID-19, because of the influx of many patients infected with SARS-CoV-2, advanced neuroimaging, such as magnetic resonance imaging and diagnostic procedures such as lumbar puncture and electromyography/nerve conduction velocity, was purposefully avoided to reduce the risk of cross infection. Therefore, in our study, most of the symptoms were a patient’s subjective descriptions. We could not distinguish whether these neurologic manifestations are caused by the virus directly or by the pulmonary disease or other organ damage indirectly.
Conclusions
In conclusion, SARS-CoV-2 may infect nervous system and skeletal muscle as well as the respiratory tract. In those with severe infection, neurologic involvement is greater, which includes acute cerebrovascular diseases, impaired consciousness, and skeletal muscle injury. Their clinical conditions may worsen, and patients may die sooner. This study may offer important new clinical information on COVID-19 that would help clinicians raise awareness of its involvement of neurologic manifestations. It is especially meaningful to learn that for those with severe COVID-19, rapid clinical deterioration or worsening could be associated with a neurologic event such as stroke, which would contribute to its high mortality rate. Moreover, during the epidemic period of COVID-19, when seeing patients with these neurologic manifestations, clinicians should consider SARS-CoV-2 infection as a differential diagnosis to avoid delayed diagnosis or misdiagnosis and prevention of transmission.
eTable. Laboratory findings of COVID-19 patients with skeletal muscle injury.
References
- 1.Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382(8):727-733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang XL, Wang XG, et al. . A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273. doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020. doi: 10.1101/2020.01.26.919985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Coronavirus disease 2019 (COVID-19) situation report-45. Accessed March 5, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200305-sitrep-45-covid-19.pdf?sfvrsn=ed2ba78b_2
- 5.Su S, Wong G, Shi W, et al. . Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490-502. doi: 10.1016/j.tim.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Middle East respiratory syndrome coronavirus (MERS-CoV). Published November 2019. Accessed January 19, 2020. https://www.who.int/emergencies/mers-cov/en/
- 7.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. January 2020. Accessed February 5, 2020. https://www.who.int/internal-publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 9.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metlay JP, Waterer GW, Long AC, et al. . Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Disease Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi: 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631-637. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Health Commission of the People′s Republic of China. Diagnosis and treatment of the novel coronavirus pneumonia (Trial version 7) [D]. Published 2020. Accessed March 3, 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf
- 13.Marc D, Dominique JF, Élodie B, et al. . Human Coronavirus: Respiratory Pathogens Revisited as Infectious Neuroinvasive, Neurotropic, and Neurovirulent Agents. CRC Press; 2013:93-122. [Google Scholar]
- 14.Arabi YM, Balkhy HH, Hayden FG, et al. . Middle East Respiratory Syndrome. N Engl J Med. 2017;376(6):584-594. doi: 10.1056/NEJMsr1408795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabello-Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F. Renin-angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev. 2015;35(3):437-463. doi: 10.1002/med.21343 [DOI] [PubMed] [Google Scholar]
- 16.Ding Y, He L, Zhang Q, et al. . Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622-630. doi: 10.1002/path.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Laboratory findings of COVID-19 patients with skeletal muscle injury.