Abstract
Background
Proton pump inhibitors (PPIs) are a class of medications that reduce acid secretion and are used for treating many conditions such as gastroesophageal reflux disease (GERD), dyspepsia, reflux esophagitis, peptic ulcer disease, and hypersecretory conditions (e.g. Zollinger‐Ellison syndrome), and as part of the eradication therapy for Helicobacter pylori bacteria. However, approximately 25% to 70% of people are prescribed a PPI inappropriately. Chronic PPI use without reassessment contributes to polypharmacy and puts people at risk of experiencing drug interactions and adverse events (e.g. Clostridium difficile infection, pneumonia, hypomagnesaemia, and fractures).
Objectives
To determine the effects (benefits and harms) associated with deprescribing long‐term PPI therapy in adults, compared to chronic daily use (28 days or greater).
Search methods
We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10), MEDLINE, Embase, clinicaltrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP). The last date of search was November 2016. We handsearched the reference lists of relevant studies. We screened 2357 articles (2317 identified through search strategy, 40 through other resources). Of these articles, we assessed 89 for eligibility.
Selection criteria
We included randomized controlled trials (RCTs) and quasi‐randomized trials comparing at least one deprescribing modality (e.g. stopping PPI or reducing PPI) with a control consisting of no change in continuous daily PPI use in adult chronic users. Outcomes of interest were: change in gastrointestinal (GI) symptoms, drug burden/PPI use, cost/resource use, negative and positive drug withdrawal events, and participant satisfaction.
Data collection and analysis
Two review authors independently reviewed and extracted data and completed the risk of bias assessment. A third review author independently confirmed risk of bias assessment. We used Review Manager 5 software for data analysis. We contacted study authors if there was missing information.
Main results
The review included six trials (n = 1758). Trial participants were aged 48 to 57 years, except for one trial that had a mean age of 73 years. All participants were from the outpatient setting and had either nonerosive reflux disease or milder grades of esophagitis (LA grade A or B). Five trials investigated on‐demand deprescribing and one trial examined abrupt discontinuation. There was low quality evidence that on‐demand use of PPI may increase risk of 'lack of symptom control' compared with continuous PPI use (risk ratio (RR) 1.71, 95% confidence interval (CI) 1.31 to 2.21), thereby favoring continuous PPI use (five trials, n = 1653). There was a clinically significant reduction in 'drug burden', measured as PPI pill use per week with on‐demand therapy (mean difference (MD) ‐3.79, 95% CI ‐4.73 to ‐2.84), favoring deprescribing based on moderate quality evidence (four trials, n = 1152). There was also low quality evidence that on‐demand PPI use may be associated with reduced participant satisfaction compared with continuous PPI use. None of the included studies reported cost/resource use or positive drug withdrawal effects.
Authors' conclusions
In people with mild GERD, on‐demand deprescribing may lead to an increase in GI symptoms (e.g. dyspepsia, regurgitation) and probably a reduction in pill burden. There was a decline in participant satisfaction, although heterogeneity was high. There were insufficient data to make a conclusion regarding long‐term benefits and harms of PPI discontinuation, although two trials (one on‐demand trial and one abrupt discontinuation trial) reported endoscopic findings in their intervention groups at study end.
Plain language summary
Stopping or reducing versus continuing long‐term proton pump inhibitor use in adults
Review question
This review aimed to build on prior work and evaluate the effects of stopping or lowering the dose of proton pump inhibitors (PPIs; acid‐reducing medicines) in adults, compared to what is commonly done in practice (i.e. continuing long‐term (more than four weeks) daily PPI use. Effects include both benefits and harms (e.g. pill use, symptom control, and cost).
Background
PPIs are used for many different conditions (e.g. heartburn, acid reflux, stomach ulceration). Studies for most of these conditions only support short‐term use (two to 12 weeks), yet these medicines are commonly continued for prolonged periods or even indefinitely. Long‐term PPI use contributes to medicine misuse and puts people at risk of experiencing unwanted drug interactions and side effects (e.g. diarrhea, headache, bone fractures). It also leads to a high cost burden on the healthcare system. 'Deprescribing' involves slowly withdrawing and stopping medicines. The most common approach is 'on‐demand' therapy, which allows people to use medicines only when they have symptoms (i.e. when heartburn begins). The overall goal of deprescribing is to minimize the number of medicines a person takes, thereby reducing inappropriate medicine use and avoiding side effects.
Study characteristics
We found six trials with 1758 participants. Of these, five studies looked at on‐demand deprescribing and one trial looked at abruptly stopping PPIs. Participants were aged 48 to 57 years, except for one trial (average age of 73 years). The majority of participants had moderate heart burn and acid reflux with milder forms of esophagitis (inflammation of the food pipe that may lead to damage).
Key results
We found that deprescribing methods led to worse symptoms control while considerably reducing pill use. Deprescribing PPIs may lead to side effects such as inflammation of the esophagus. Very few data were available to make a conclusion regarding long‐term benefits and harms of PPI reduction or discontinuation.
Quality of the evidence
Overall, the quality of evidence for this review ranged from very low to moderate. Trials were inconsistent with how they reported symptom control. There were also limitations in how the studies were conducted (e.g. participants and investigators may have known which medicine they received), which lowered the quality of evidence. Other contributing factors included small sample sizes for most trials and inconsistent results between studies.
Summary of findings
Summary of findings for the main comparison. On‐demand deprescribing compared to continued use for people with moderate GERD and mild esophagitis.
| On‐demand deprescribing compared to continued use for people with moderate GERD and mild esophagitis | ||||||
| Patient or population: people with moderate GERD and mild esophagitis, mean age 48 to 57 years Settings: outpatient Intervention: on‐demand deprescribing Comparison: continued use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Continued use | On‐demand deprescribing | |||||
| Lack of symptom control | Study population | RR 1.71 (1.31 to 2.21) | 1653 (5 studies) | ⊕⊕⊝⊝ Low1,2 | ‐ | |
| 92 per 1000 | 157 per 1000 (120 to 203) | |||||
| Moderate | ||||||
| 123 per 1000 | 210 per 1000 (161 to 272) | |||||
| Pill use (pills/week) | ‐ | The mean pill use (pills/week) in the intervention groups was 3.79 lower (4.73 to 2.84 lower) | ‐ | 1152 (3 studies) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| Cost/resource use | We found no data on cost or resource use. | Not estimable | ‐ | ‐ | ‐ | |
| Adverse drug withdrawal events ‐ esophagitis (endoscopic findings) | ‐ | ‐ |
RR 30.59 (1.84 to 508.91) |
598 (1 study) | ⊕⊕⊝⊝ Low1,2 |
Risk not estimable as no events in continued use arm. |
| Participant satisfaction | Study population | RR 1.82 (1.26 to 2.65) | 1653 (5 studies) | ⊕⊝⊝⊝ Very low2,4,5 | ‐ | |
| 88 per 1000 | 160 per 1000 (111 to 234) | |||||
| Moderate | ||||||
| 81 per 1000 | 147 per 1000 (102 to 215) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GERD: gastroesophageal reflux disease; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to a high risk of detection bias, and attrition bias. 2 Downgraded one level due to wide confidence intervals and summary statistic close to the line of no effect. 3 Downgraded one level due to high risk of detection and attrition bias. 4 Downgraded one level due to high risk of attrition and reporting bias. 5 Downgraded one level due to indirectness as poor methods of satisfaction used (willingness to continue or "inadequate relief").
Summary of findings 2. Abrupt discontinuation deprescribing compared to continued use for people with mild‐to‐moderate esophagitis.
| Abrupt discontinuation deprescribing compared to continued use for people with mild to moderate esophagitis | ||||||
| Patient or population: people with mild to moderate esophagitis, symptomatic, aged > 18 years (mean age 73 years) Settings: outpatient Intervention: abrupt discontinuation deprescribing Comparison: continued use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Continued use | Abrupt discontinuation deprescribing | |||||
| Lack of symptom control | Study population | RR 3.02 (1.74 to 5.24) | 105 (1 study) | ⊕⊝⊝⊝ Very low1,2 | ‐ | |
| 224 per 1000 | 678 per 1000 (391 to 1000) | |||||
| Moderate | ||||||
| 225 per 1000 | 679 per 1000 (392 to 1000) | |||||
| Adverse drug withdrawal events ‐ relapse (endoscopic findings) | Study population | RR 3.41 (1.91 to 6.09) | 105 (1 study) | ⊕⊝⊝⊝ Very low1,2 | ‐ | |
| 204 per 1000 | 696 per 1000 (390 to 1000) | |||||
| Moderate | ||||||
| 204 per 1000 | 696 per 1000 (390 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to concerns surrounding attrition bias and blinding. 2 Downgraded two levels due to wide confidence intervals, and small number of participants and events.
Background
See Appendix 1 for a glossary of terms and Appendix 2 for abbreviations used in this review.
Proton pump inhibitors (PPIs) are a class of medications that reduce acid secretion by inhibiting gastric H+, K+‐adenosine triphosphatase (ATPase). They are used for treating many conditions such as gastroesophageal reflux disease (GERD), dyspepsia (i.e. indigestion), reflux esophagitis (inflammation of the esophagus), peptic ulcer disease (PUD), hypersecretory conditions (e.g. Zollinger‐Ellison syndrome), and as part of the eradication therapy for Helicobacter pylori (H. pylori) bacteria (Wallace 2011). They are also used to prevent and reduce the risk of ulcers in people with a history of PUD, in critically ill people as stress ulcer prophylaxis (SUP), and people who use chronic nonsteroidal anti‐inflammatory drugs (NSAIDs) (Armstrong 1999; Armstrong 2005).
Description of the condition
Gastroesophageal reflux disease
GERD affects millions of adults globally. Approximately five million Canadians experience heartburn or regurgitation (or both) at least once weekly, where heartburn is the most common symptom reported (17%) followed by regurgitation (11%) (CDHF 2015; Tougas 1999). Similar figures also extend globally. One systematic review evaluating the epidemiology of GERD found that 10% to 20% of adults in Western countries are also affected by GERD (Dent 2005).
Many GERD publications and guidelines exist though a uniform definition of GERD among them is lacking. The Canadian Consensus on the Management of Gastroesophageal Reflux Disease in Adults (Canadian Consensus) defines GERD as the "reflux of gastric contents into the esophagus causing (a) symptoms sufficient to reduce quality of life, and/or (b) esophageal injury". The hallmark symptoms are heartburn and acid regurgitation. The Canadian Consensus also classifies GERD as either 'mild' or 'moderate and severe' disease (Armstrong 2005). The American College of Gastroenterology's (ACG) Guidelines for the Diagnosis and Management of Gastroesophageal Reflux Disease recognizes that GERD is "defined by consensus and as such is a disease comprising symptoms, end‐organ effects and complications related to the reflux of gastric contents into the esophagus, oral cavity and/or the lung" (Katz 2013). The ACG categorizes GERD based on endoscopic findings: nonerosive GERD (NERD) is symptomatic GERD with negative endoscopy, while GERD with erosive findings on endoscopy is referred to as erosive reflux disease or erosive esophagitis (EE) (Katz 2013). In 2006, the Global Consensus Group published the Montreal Definition and Classification of GERD to determine a globally acceptable definition that would be useful for patients, practitioners, and health agencies. This group defined GERD as, "a condition which develops when the reflux of stomach contents cause troublesome symptoms and/or complications", such as heartburn, regurgitation, and esophageal injury (i.e. reflux esophagitis) (Vakil 2006).
Reflux esophagitis
PPIs are also commonly used on a long‐term basis for esophagitis due to chronic GERD. The severity of EE detected on endoscopy is classified by the Los Angeles (LA) Grading criteria as grades A to D, defined as follows: grade A ‐ at least one mucosal break of 5 mm or less, not extending between the tops of two mucosal folds; grade B ‐ at least one mucosal break with a length greater than 5 mm not extending between the tops of two mucosal folds; grade C ‐ at least one mucosal break involving less than 75% of the esophageal circumference, but extending between the tops of at least two mucosal folds; grade D ‐ at least one mucosal break involving 75% or more of the esophageal circumference (Lundell 1999). LA Grade C and D esophagitis are considered severe stages of esophagitis, have lower rates of healing with PPIs, and are more prone to relapse in the absence of maintenance therapy (Katz 2013). The Canadian Dyspepsia Working Group suggest that healing and symptom relief of EE is superior with PPIs as compared to histamine H2 receptor antagonists (H2RAs), and that standard doses of PPIs are superior to half or low doses of PPIs for this indication (Veldhuyzen van Zanten 2005). Barrett's esophagus is another long‐term sequelae of GERD and esophagitis that is believed to be developed from the change of normal esophageal epithelial cells to specialized intestinal glandular epithelial cells. This metaplasia, detectable via endoscopy, can progress to dysplasia, and finally adenocarcinoma (Armstrong 2005). The ACG guidelines recommend ongoing maintenance PPI therapy for people with EE and Barrett's esophagus (Katz 2013). Since Barrett's esophagus is believed to result from chronic acid reflux, the Canadian guidelines suggest that regardless of symptoms, it should be treated with lifelong PPIs of at least standard dose, and managed by specialists (Veldhuyzen van Zanten 2005).
Peptic ulcer disease
Gastric and duodenal ulcers (bleeding or nonbleeding) are often referred to under the rubric of PUD (ACPG 2000; Katz 2013; Talley 2005). There are many risk factors and possible causes for PUD, the most common being infection of the gastric mucosa with H. pylori; however, other causes include NSAID‐ or drug‐induced PUD, and hypersecretory states. The standard for H. pylori‐positive PUD is treatment with triple therapy (two antibiotics and one PPI) to eradicate the bacteria. This is typically followed by four to eight weeks of PPI therapy for gastric ulcers, but does not require subsequent PPI therapy in the setting of uncomplicated duodenal ulcers. If eradication is achieved, the rate of rebleeding is very low (1.3% over 11 to 53 months) (Gisbert 2004; Laine 2012; Malfertheiner 2009).
NSAIDs, and to a lesser extent newer selective cyclooxygenase‐2 (COX2) inhibitors, are known to cause dyspeptic symptoms and increase the risk of PUD through the inhibition of cyclooxygenase‐1 (COX1) enzyme in the gastric mucosa. There is support for the concomitant use of gastroprotective agents (PPIs, misoprostol, or H2RAs) as primary or secondary prophylaxis of peptic ulcers, especially in the presence of risk factors. These include age 65 years or over; history of bleeding PUD; and use of corticosteroids, anticoagulants, or antiplatelet agents (Veldhuyzen van Zanten 2005). If people are to be maintained on long‐term NSAID therapy, it is advised to test and treat for H. pylori due to the additive risk (Veldhuyzen van Zanten 2005).
Description of the intervention
'Deprescribing' involves slowly withdrawing and stopping medications, with the aim of reducing polypharmacy and inappropriate medication use thereby improving people's outcomes (Thompson 2013). A number of approaches to deprescribing have been outlined by the Canadian Consensus: intermittent PPI use is defined as a "daily intake of a medication for a predetermined, finite period (usually two to eight weeks) to produce resolution of reflux‐related symptoms or healing of esophageal lesions following relapse of the individual's condition"; on‐demand PPI use is defined as "the daily intake of a medication for a period sufficient to achieve resolution of the individual's reflux‐related symptoms; following symptom resolution, the medication is discontinued until the individual's symptoms recur, at which point, medication is again taken daily until the symptoms resolve" (Armstrong 2005). Other deprescribing methods may include stopping therapy abruptly or via a tapering regimen, switching to an alternate therapy (e.g. stepping down from PPI to H2RA), lowering the total daily dose, or, in the case of active H. pylori infection, treating the infection and then discontinuing PPI therapy.
Small studies have demonstrated successful deprescribing approaches, yet currently no systematically developed guidelines exist that describe known benefits and harms of deprescribing (Gnjidic 2012; Iyer 2008). Furthermore, there are no evidence‐based methods of stopping (i.e. tapering and monitoring) specific drugs to help guide prescribers and other clinicians.
The concept of deprescribing is straightforward, yet many barriers exist for clinicians and patients (Raghunath 2005; Reeve 2012; Reeve 2013). Barriers include: clinician internal pressures (i.e. believing drug therapy is effective, safe, and recommended, and desire to prescribe it), clinician workload pressures (i.e. desire to avoid repeat consultations), patient pressures (i.e. people requesting therapy or unwilling to stop an effective treatment or switch to an alternative), and marketing and commercial influences. Additionally, clinicians and patients may disagree with the appropriateness of deprescribing, possibly related to belief of lack of suitable alternatives, fear of return or worsening of a person's symptoms, and influences of negative past experiences or experiences of others. Clinicians also believe that patients may be unwilling to discontinue PPIs as it allows for continued adverse lifestyle habits that contribute to GERD symptoms (e.g. smoking or poor diet), although this has not been confirmed by existing literature (Grime 2001). Clinicians may also be reluctant to discontinue medications initiated by other health professionals. The lack of guidelines and fear/concern of withdrawal or rebound symptoms are key concerns limiting the implementation of deprescribing initiatives. In addition, polypharmacy often is not addressed, with adverse drug effects being mistaken for new conditions requiring additional treatment, thus driving the cycle of inappropriate prescribing (Anthierens 2010; Hilmer 2009).
How the intervention might work
Various deprescribing methods exist. While the underlying goal of deprescribing is the same (i.e. to stop or reduce in an attempt to discontinue or achieve the lowest effective dose), the methods and outcomes may differ. Different methods for reducing drug exposure have been reported (e.g. pharmacist‐led medication reviews, prescriber feedback, and multidisciplinary interventions), though their resulting clinical outcomes are not always reported (Gnjidic 2012). Such outcomes include positive drug withdrawal events (PDWE) and adverse drug withdrawal events (ADWE). Graves and colleagues defined ADWE as a "clinically significant set of signs or symptoms caused by the removal of a drug" (Graves 1997). Indeed, ADWEs are major contributors to unsuccessful deprescribing. In contrast, desirable outcomes (i.e. reduction/elimination of potential or actual drug‐related adverse events) may result from drug withdrawals, which will be referred to in this review as PDWEs. Successful deprescribing may also result in reduced drug interactions, increased quality of life and patient satisfaction, as well as reduced drug burden and costs.
Stopping PPIs by abrupt withdrawal or by slowly withdrawing or reducing the dose has been theorized to have beneficial effects, as well as potential harms. As suggested by Linsky and colleagues, PPIs are often viewed as having few adverse effects; however, their long‐term use has been associated with an increased risk of pneumonia, Clostridium difficile (C. difficile) colitis, and osteoporotic fractures (Linsky 2013). Limiting PPI doses or stopping PPIs via one of the three interventions that will be assessed in this review may reduce the risk of such adverse events.
One Cochrane review by Donnellan and colleagues investigated comparative efficacies of various therapies (including PPIs) in preventing relapse in people with esophagitis and endoscopy‐negative reflux disease (Donnellan 2004). The current review adds to the knowledge established by Donnellan 2004, by including additional indications for PPI use, and focusing on trials that allow for comparison of different maintenance therapy strategies with a control of no change in continuous PPI use.
One systematic review by Lee and colleagues evaluated whether people with GERD were receiving unnecessary maintenance PPI therapy or high‐dose PPI therapy (Lee 2004). The review included deprescribing trials, including randomized controlled trials (RCTs) and nonrandomized trials, of switching to H2RA, intermittent or on‐demand PPI use, and dose reduction of high‐dose PPIs. The review reported that 80% of people receiving high‐dose PPIs were able to reduce their doses successfully to standard‐dose PPIs. Based on results of participant symptom assessments, deprescribing was possible in 26% to 71% of people with GERD (Lee 2004). Despite these results, it remains unclear how the deprescribing interventions evaluated by Lee and colleagues compare to people who would continue on long‐term PPI therapy (e.g. without a change in dose), and which benefits or risks exist with deprescribing.
Jiang 2013 undertook another systematic review and meta‐analysis of on‐demand PPI. This group measured the proportion of people who were unwilling to continue on‐demand therapy compared to people unwilling to continue on daily PPI therapy or placebo. They found that on‐demand therapy reduced the risk of being unwilling to continue compared to daily PPI therapy and placebo. This meta‐analysis did not report on other outcomes such as pill burden, ADWEs, or symptom relapse and may not have included all studies relevant for our review. Further, most people in practice will continue on the same dose of PPI long‐term. Studies in the Jiang 2013 meta‐analysis did not compare people with on‐demand PPI use to people who continued on the same dose of PPI long‐term with no change in regimen. As such, the effect of on‐demand use compared to continued PPI use is not well‐established.
The present systematic review builds on prior work and evaluates evidence of deprescribing compared to what is commonly done in practice, that is, continuing chronic PPI use with no change in regimen.
Why it is important to do this review
The evidence for PPI use in many indications (e.g. GERD, PUD, SUP) only supports short‐term use (e.g. two to 12 weeks), yet these drugs are commonly continued for prolonged periods or even indefinitely, without appropriate reassessment (Donnellan 2004; Reimer 2009a).
Continuous use of PPIs for durations exceeding accepted standards appears to be commonplace, and is likely because they are very well tolerated and considered benign compared to other prescribed medications. This relatively desirable safety profile often overshadows lesser known drug interactions (e.g. hepatic enzyme inhibition, effects on drug absorption or activation) and poorly understood adverse effects and associated risks (vitamin B12 deficiency, hypomagnesemia, community‐acquired pneumonia (CAP), C. difficile infection, diarrhea, headache, and fractures) (Ament 2012; Chubineh 2012; Fohl 2011; Linsky 2013). Although these adverse effects may not be very common and true association with risk is debatable, the extensive use of PPIs in a wide range of populations without monitoring and reassessment is concerning.
The Canadian Health Network reported that six PPIs were in the top 100 prescribed drugs in 2012, with pantoprazole ranking fifth at 11,051,000 prescriptions dispensed in Canadian retail pharmacies over a 12‐month period (CHN 2013). Due to this widespread and chronic use, even rare adverse events will present over time and put people at risk.
Inappropriate PPI use is not only commonplace in Canada, it is also a major global issue. It is estimated that between 20% and 82% of people worldwide are using a PPI without an indication (Claudene 2008; Dangler 2013; Heidelbaugh 2010; Leri 2013; Molloy 2010; Reeve 2015; Wahab 2012). For example, in one cross‐sectional study of people in primary care in Denmark receiving long‐term PPI therapy, 39% had never undergone a trial withdrawal, and only 27% had a verified diagnosis indicating the need for PPI therapy (Reimer 2009a). These findings also parallel those of one Australian study (Gadzhanova 2012). The Gastroenterological Society of Australia (GESA) make specific recommendations to consider 'stepdown' therapy, cessation, or dose reduction, in targeted populations (e.g. milder grades of esophagitis or mild reflux symptoms) (GESA 2011), yet, Gadzhanova 2012 found that only one‐third of people initiated on high‐dose PPIs were stepped down, as per GESA recommendations.
Another example of inappropriate PPI therapy includes continuation of SUP after discharge from various intensive care unit (ICU) settings in the US (Heidelbaugh 2010). Guidelines suggest use of SUP (PPI or H2RA) in critically ill people with coagulopathy, mechanical ventilation for more than 48 hours, or a history of gastrointestinal (GI) ulceration or bleed within the last year (Armstrong 1999; Spirt 2006). However, many people continue SUP therapy after discharge when it is no longer indicated. Murphy and colleagues published a study demonstrating this in people in surgical ICUs (Murphy 2008). The authors concluded that PPIs and H2RAs were continued in 24.2% of people upon discharge from hospital.
Leri and colleagues also described PPI misuse. The authors conducted a retrospective review to identify PPI continuation at discharge in people who had a PPI initiated in hospital. The authors reported 78% to 82% of people were discharged on a PPI inappropriately between 2005 and 2008 (Leri 2013).
The overuse of PPIs has also led to tremendous costs. The Canadian Health Network report listed eight brands of PPIs in the top 100 products based on prescription cost (CHN 2013). Esomeprazole (Nexium) ranked at seven with a cost of CAD 257,173,000 for the cited 12‐month period (CHN 2013). In one 2007 Canadian Agency for Drugs and Technologies in Health (CADTH) report on economics surrounding treatment of dyspepsia and GERD compared five treatment regimens: on‐demand H2RA, on‐demand PPI, maintenance H2RA, maintenance PPI, and PPI with stepdown to H2RA (CADTH 2007). In the setting of EE and investigated GERD, on‐demand PPI therapy had the lowest expected one‐year costs (CAD 537/year for EE and CAD 635/year for GERD) compared with maintenance PPI treatment, which had the highest estimated cost (CAD 776/year for EE and CAD 816/year for GERD; estimates based on 2006 Ontario costs). In the NERD population, the most cost‐effective strategy was on‐demand H2RA (CAD 641/year) and the most costly strategy remained maintenance PPI therapy (CAD 827/year). In terms of efficacy, the report documented "expected weeks with heartburn" for each therapy. In all three populations, maintenance PPI therapy resulted in the lowest number of weeks with heartburn, followed second in all instances by PPI with stepdown to H2RA. The least effective treatment in all populations was on‐demand H2RA.
Several western countries have also reported high expenditure on PPIs. In 2006, GBP 425 million was spent on PPIs in England, and the same issue extends globally to GBP 7 billion (Forgacs 2008).
Inappropriate use of PPIs can contribute to polypharmacy, which is the use of multiple medications, specifically those that are not indicated. Polypharmacy may result in reduced medication adherence, falls, cognitive impairment, functional impairment, delirium, and hospitalization, all of which increase morbidity and mortality (Gnjidic 2012; Hilmer 2009; Iyer 2008). The notion of deprescribing is becoming more relevant as clinicians begin to acknowledge the risks associated with polypharmacy (Scott 2013).
Objectives
To determine the effects (benefits and harms) associated with deprescribing long‐term PPI therapy in adults, compared to chronic daily use (28 days or greater).
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐randomized trials. We applied no limitation on language or abstracts.
Types of participants
Participants were adults (aged 18 years or greater) using a PPI chronically for one month (28 days) or greater. Participants also required an indication for PPI use: GERD (nonerosive reflux disease or GERD LA Grade A to D), functional dyspepsia, or undiagnosed dyspepsia. Participants were also considered eligible if they had a history PUD (bleeding or nonbleeding, or both), H. pylori‐positive or ‐negative diagnoses, recurrent peptic esophageal stricture or Barrett's esophagitis. Participants using a PPI as SUP or for gastroprotection in the setting of low‐dose acetylsalicylic acid (ASA) (less than 325 mg/day), anti‐inflammatory doses of NSAIDs, and steroids were eligible. We excluded trials if participants were taking high‐dose NSAIDs who require chronic PPI use for gastroprotection; however, those with occasional NSAID use were not excluded.
Types of interventions
Included studies compared at least one of the deprescribing modalities with a control consisting of no change in continuous daily PPI use. Deprescribing was defined as one or more of the following interventions:
Stopping PPI therapy: either via abrupt discontinuation or a tapering regimen.
Step down: following abrupt discontinuation or tapering of PPI, an H2RA was prescribed (any H2RA at any approved dose and dosing interval per drug monograph).
-
Reduction in PPI, which included the following subcategories:
intermittent PPI use as previously defined by the Canadian Consensus (Armstrong 2005);
on‐demand PPI use as previously defined by the Canadian Consensus (Armstrong 2005);
lower dose: continuous daily PPI therapy at a lower dose compared to a control arm within the trial.
Treatment of H. pylori (using any approved triple or quadruple therapy) followed by four to eight weeks of PPI maintenance therapy for bleeding or nonbleeding PUD and then PPI discontinuation.
We excluded studies if treatment arms consisted of concomitant interventions such as rescue therapy (e.g. with antacids), lifestyle, diet modifications, or a combination of these. This did not affect inclusion if these additional interventions were handled similarly in all treatment arms.
Types of outcome measures
Primary outcomes
Lack of symptom control: reported as harms due to no change, recurring, worsening, new onset of at least one upper GI symptom (heartburn, regurgitation, dyspepsia, epigastric pain, nausea, bloating, belching).
Drug burden/PPI use.
Cost/resource use.
Secondary outcomes
PDWE: included any positive outcome (e.g. improvement in cognition, improvement in vitamin B12 levels, resolution of diarrhea) experienced as a result of the deprescribing intervention.
ADWE: included all negative outcomes or adverse effects (e.g. GI bleeding, worsening endoscopic findings) occurring as a result of deprescribing with the exception of GI symptoms, which were measured separately.
Participant satisfaction: positive or negative.
Search methods for identification of studies
We placed no restrictions on the language of publication.
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 10) (Appendix 3);
MEDLINE (1946 to 15 November 2016) (Appendix 4);
Embase (1980 to 15 November 2016) (Appendix 5);
ClinicalTrials.gov;
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP).
As deprescribing is a new concept in the literature, we used a broad range of key words. Search terms for PPIs included both the generic and common brand names (Donnellan 2004; Van Herwaarden 2014). A librarian from Cochrane reviewed the search strategy and a research librarian completed the search strategy (see Appendix 3; Appendix 4; Appendix 5). Review authors will conduct future literature searches.
Searching other resources
We handsearched reference lists of all primary studies and review articles.
Data collection and analysis
We used predefined data extraction fields and data collection forms. We used Review Manager 5 for data analysis (RevMan 2014). For RCTs with dichotomous outcomes, we collected the number of outcome events and the total number of participants in treatment and control groups.
Selection of studies
Three review authors (FJR, TB, and WT) independently reviewed search results, assessed study eligibility and trial quality, and extracted data. We resolved disagreements by discussion with another review author (CRF). The review authors retrieved and independently screened full‐text study reports and publications and identified studies for inclusion and identified/recorded reasons for exclusion of the ineligible studies. We made every attempt to exclude duplicates. We constructed a PRISMA flow diagram (Figure 1) and a Characteristics of excluded studies table based on the information collected during this process. We used a standardized eligibility checklist.
1.
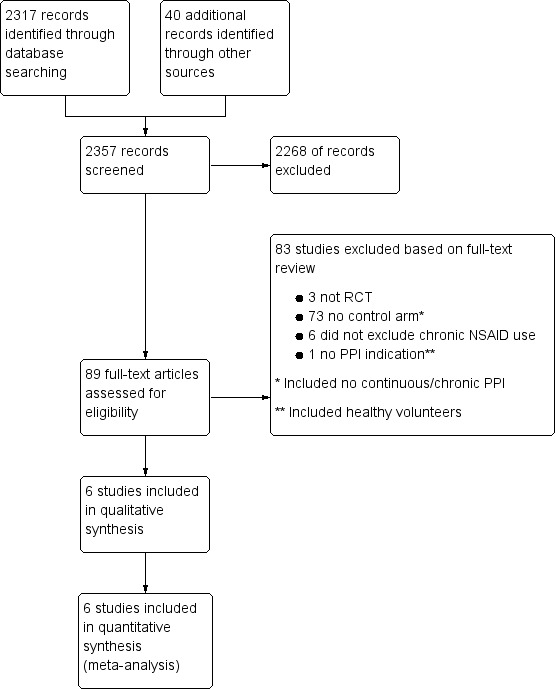
Study flow diagram. NSAID: nonsteroidal anti‐inflammatory drug; PPI: proton pump inhibitor; RCT: randomized controlled trial.
Data extraction and management
We used a standard data collection form for study characteristics and outcome data. Prior to data collection, two review authors independently completed a pilot eligibility and data extraction exercise to ensure criteria were consistently applied (Higgins 2011). Three review authors (FJR, TB, and WT) extracted the following study characteristics from included studies:
methods: study design, total duration study and run in, number of study centers and location, study setting, withdrawals, date of study;
participants: number, mean age, age range, gender, severity of condition, types of condition, inclusion criteria, and exclusion criteria;
interventions: intervention, comparison, concomitant medications;
outcomes: primary and secondary outcomes, time points reported;
other: author contact information, funding for trial, notable conflicts of interest of trial authors.
We resolved disagreements by discussion with a third review author (CRF). One review author (FJR) transferred the data into Review Manager 5 (RevMan 2014). A second author (TB) reviewed data entry for accuracy.
Assessment of risk of bias in included studies
We assessed the quality and strength of the evidence using GRADE and study bias using Cochrane's tool for assessing risk of bias (Higgins 2011).
Three review authors (FJR, TB, and WT) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion with a third review author (CRF). We ranked each potential source of bias as high, low, or unclear and provided a quote from the study to justify the bias grade (refer to Characteristics of included studies table for risk of bias assessments) using the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
Assessment of bias in conducting the systematic review
The review was conducted according to the published protocol and any deviation from the protocol is reported in the Differences between protocol and review section.
Measures of treatment effect
We reported results as risk ratios (RR) where possible with 95% confidence intervals (CI). We pooled dichotomous data using a random‐effects model (Dersimonian 1986), and continuous outcomes using mean differences (MD) when outcomes were measured on the same scale.
Unit of analysis issues
The unit of analysis was the individual participant undergoing PPI treatment. We conducted a scoping exercise prior to commencing the review and did not identify any cluster‐randomized trials for this comparison. As expected, we found no cluster‐randomized trials for this comparison after conducting comprehensive literature searching during the review.
Dealing with missing data
During the data extraction process, we attempted to contact the primary author for clarification regarding missing or unclear data. No data were imputed.
Assessment of heterogeneity
We used the Chi2 statistic and I2 statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity (Chi2 statistic with a P value less than 0.15 or I2 statistic greater than 25%) via either statistic, we planned to explore this through prespecified subgroup analysis. Due to the paucity of included trials, we did not perform predefined subgroup analyses to investigate causes of heterogeneity.
Assessment of reporting biases
We used the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions for assessment of bias (Higgins 2011). We attempted to contact study authors to request missing outcome data. Refer to Characteristics of included studies table for risk of bias assessment.
Data synthesis
We used Review Manager 5 for analysis (RevMan 2014). We calculated 95% CIs for the treatment effect and used the Mantel‐Haenszel method for dichotomous data and the inverse variance method for continuous data. We used a random‐effects models for the analysis (DerSimonian 1986).
'Summary of findings' table
We created 'Summary of findings' tables based on the type of intervention (Table 1; Table 2). We used the five GRADE considerations to assess the quality of evidence using methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and GRADEpro software (GRADEpro). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments to aid the reader's understanding of the review. We considered whether any additional outcome information was provided that we were unable to incorporate into meta‐analyses, and we planned to note this in the comments and state whether it supported or contradicted information derived from the meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Deprescribing interventions (stopping PPI, lower‐dose PPI compared to control arm, on‐demand PPI, intermittent PPI, stopping PPI and changing to H2RA, treatment of H. pylori with four to eight weeks of PPI therapy).
Diagnosis (GERD, esophagitis, functional dyspepsia, PUD, H. pylori infection).
Trial setting (primary versus secondary care).
Age (young adults versus older adults).
We planned to assess the robustness of the conclusions based on the risk of bias assessments for blinding and allocation. We were unable to conduct subgroup analysis given the limited data.
Sensitivity analysis
We planned to perform sensitivity analysis defined a priori to assess the robustness of our conclusions by: outcome reporting and participant populations, and to assess any effect of risk of bias on our estimates. Data were too limited to perform sensitivity analysis.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies tables for details.
Results of the search
We identified 2357 references; 2317 through electronic searches of CENTRAL (Wiley), MEDLINE (OvidSP), and Embase (OvidSP), and 40 through other resources. We excluded 2268 clearly irrelevant references by reading the abstracts and retrieved 89 references for further detailed assessment. We excluded 83 references for the reasons listed in the Characteristics of excluded studies table. Six references fulfilled the inclusion criteria (Characteristics of included studies table). The results of the search are presented in Figure 1.
Included studies
We included six RCTs with 1753 participants (Bayerdörffer 2016; Bour 2005; Janssen 2005; Morgan 2007; Pilotto 2003; Van der Velden 2010). The Characteristics of included studies table outlines details (e.g. sample size, participant characteristics, interventions, etc.) of all studies that met inclusion criteria.
Design
All six trials were RCTs. Four studies had an open‐label design while two were double‐blinded. To satisfy the inclusion criteria of chronic PPI usage (28 days or greater) with a control arm of no change in continuous daily PPI use, trials were required to have a run‐in phase of at least four weeks prior to randomization. For all trials, participants required symptom resolution at the end of the run‐in phase, prior to randomization. One trial went further and evaluated participants endoscopically at the beginning and end of the trial to assess the development of esophagitis (Bayerdörffer 2016). Five trials explored on‐demand therapy and one trial investigated abrupt discontinuation (Pilotto 2003). Five studies had a duration of six months, while Van der Velden 2010 conducted a trial of 13 weeks.
Sample size
The included trials were relatively small (fewer than 500). A significant number of participants were excluded after the run‐in phases, as they did not achieve symptom resolution. Pilotto 2003 included the fewest participants after the run‐in phases (n = 105) and Bayerdörffer 2016 had the largest trial (n = 598).
Setting
Morgan 2007 included participants from 23 Canadian sites. Pilotto 2003 had participants from 16 Italian centers. Van der Velden 2010 included participants from 23 general practices from central and eastern regions of the Netherlands. The trial by Bour 2005 included 41 French hospital sites (exact locations not disclosed). Janssen 2005 included participants from 58 centers (29 in Germany, 12 in France, 11 in Switzerland, and six in Hungary). Bayerdörffer 2016 included people from 61 sites (Austria, France, Germany, South Africa, and Spain).
Participants
All participants were from the outpatient setting and had either NERD or milder grades of esophagitis (LA grade A or B). Participants also varied in age. one trial only included participants aged 65 years or greater (mean age 73 years) (Pilotto 2003), while the remaining trials included relatively younger participants (mean age 48 to 57 years). Four trials reported risk factors of alcohol and tobacco use (Bour 2005; Morgan 2007; Pilotto 2003; Van der Velden 2010), and three trials indicated that heartburn was the major symptom experienced (Bayerdörffer 2016; Bour 2005; Morgan 2007).
Interventions
Pilotto 2003 examined abrupt discontinuation and the remaining trials investigated on‐demand deprescribing. Three trials used pantoprazole 20 mg pills, two used rabeprazole 10 mg and 20 mg pills, and one used esomeprazole 20 mg pills. Duration of treatment was similar for five trials (six months or up to six months), while the remaining trial was conducted over 13 weeks (Van der Velden 2010).
Outcomes
Five studies defined primary outcomes; Bayerdörffer 2016; Bour 2005; Janssen 2005; and Morgan 2007 pertained to symptom control and relief and Van der Velden 2010 reported volume of rescue medications as their primary outcome and symptom control, quality of life and final dose adjustment as secondary outcomes. Pilotto 2003 did not define primary outcomes.
Excluded studies
We excluded 83 studies. Three studies had the wrong study design (i.e. not RCTs) (Bautista 2006; Inadomi 2001; Ponce 2004). Six studies included chronic NSAID users (Annibale 1998; Bate 1997; Dent 1994; Gough 1996; Hatlebakk 1997a; Hatlebakk 1997b). In one study, participants did not have an indication for PPI use (i.e. healthy volunteers) (Niklasson 2010). Seventy‐three studies did not have a control arm (i.e. no continuous/no change in chronic PPI use) (Archambault 1988; Bardhan 1990; Bardhan 1995; Bate 1989; Bate 1990; Bate 1995; Bayerdörffer 1993; Bigard 2005; Björnsson 2006; Bytzer 2004; Carlsson 1998; Chen 1993; Dabholkar 2011; Deboever 2003; Dent 1989; DeVault 2006; Earnest 1998; Eggleston 2009; Escourrou 1999; Farley 2000; Fass 2011; Fass 2012; Fennerty 2009; Goh 1995; Goh 2007; Havelund 1988; Hoshino 1995; Howden 2001; Howden 2009; Hsu 2015; Inadomi 2003; James 1994; Johnson 2001; Johnson 2010; Jones 1997; Juul‐Hansen 2008; Kaplan‐Machlis 2000; Kaspari 2005; Klinkenberg‐Knol 1987; Kovacs 2010; Labenz 2005; Lauristen 2003; Lind 1999; Lundell 1991; Metz 2003; Nagahara 2014; Norman Hansen 2005; Peura 2009; Peura 2016; Reimer 2010; Richter 2000; Richter 2004; Robinson 1995; Sandmark 1988; Scholten 2005; Sjöstedt 2005; Sontag 1997; Sun 2015; Talley 2001; Talley 2002a; Talley 2002b; Tan 2011; Tsai 2004; Vakil 2001; Valenzuela 1991; Van Rensburg 1996; Van Rensburg 2008; Vantrappen 1988; Van Zyl 2000; Venables 1997; Vigneri 1995; Wulff 1989; Zwisler 2015). Refer to Characteristics of excluded studies table for deprescribing trials that did not meet all inclusion criteria.
Risk of bias in included studies
See Characteristics of included studies table for study details including all aspects of risk of bias. Figure 2 and Figure 3 provide a visual summary of the risk of bias in included studies presented as percentages across all included studies and for individual studies.
2.
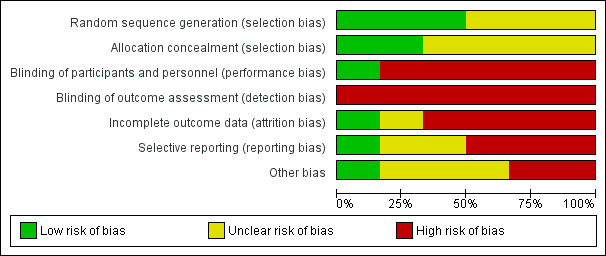
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
3.
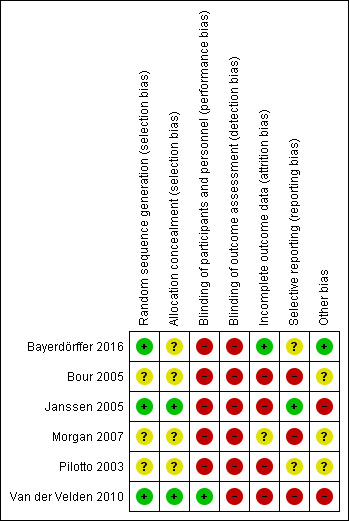
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Contribution of allocation bias was difficult to determine, as four trials did not describe how they performed allocation (Bayerdörffer 2016; Bour 2005; Morgan 2007; Pilotto 2003). The remaining two trials used sound methods: Janssen 2005 used sealed boxes containing study medication, and participants were allocated according to the numbering in ascending order, while Van der Velden 2010 used a computer‐generated list, enlisted an external party to generate sealed identical containers which were sent to the general practitioners, and participants were allocated the next available number of the set on entry into the trial.
Blinding
Of the six trials included, two trials were double blinded (Pilotto 2003; Van der Velden 2010), although the authors of van der Velden and colleagues noted that the data were analyzed unblinded. Van der Velden 2010 blinded participants by providing identical containers and by providing the control arm with daily dose of PPI plus placebo rescue medication to use as needed and providing the inverse to the intervention group (placebo daily plus PPI when needed). Pilotto 2003 did not comment how blinding was maintained and whether pills looked identical in both arms. The remaining four trials had an open‐label design (Bayerdörffer 2016; Bour 2005; Morgan 2007; Pilotto 2003). Many of the outcomes related to symptom relief, despite use of scales, relied on subjective reporting, and thus were subject to bias. For example, Bour 2005 evaluated participant‐rated symptom relief (primary outcome) using a Likert scale and it was unclear if investigators saw participant satisfaction measures before they assessed satisfaction. Janssen 2005 also measured subjective outcomes (symptom control and satisfaction/unwillingness to continue), creating high risk of bias. However, the objective measure of pill count was unbiased (measurement was not likely to be influenced by lack of blinding). Participant‐rated effect of treatment and satisfaction in the trial by Morgan 2007 were also inherently subject to bias as neither participants nor assessors were blinded.
Incomplete outcome data
Reporting of outcome data revealed a relatively high risk of bias as the majority of trials had participants lost to follow‐up or did not report on all predefined outcomes, or both.
Although the study completion rate was high for Bour 2005 (greater than 80%), the reason for dropout was unclear in all instances and there was a statistically significant higher number of participants who withdrew from the trial for reasons unknown, defined as 'other' in the on‐demand treatment group. Only 67/81 participants were analyzed for pill usage in the continuous treatment group, and only 55/71 for the on‐demand treatment group, with no explanation provided.
Janssen 2005 had a relatively higher dropout rate (22%) but maintained sufficient participation to achieve their power calculation. Although they did not report if characteristics differed between those who stayed in study, versus those who were excluded, the unwillingness to continue as cause of dropout was similar between groups (6% versus 8%) for the long‐term phase.
Morgan 2007 and Pilotto 2003 reported overall dropout rates making it difficult to assess if reasons for withdrawal from the study were similar between treatment and control groups. Endpoints in the Pilotto 2003 trial were not clearly defined, also making it difficult to assess completion of outcome data reporting. Van der Velden 2010 clearly reported dropouts and reasons, however did not comment on whether differences were significant between groups (33% dropout in the on‐demand arm, which was greater than the 20% anticipated). The authors of this study stated that 40 participants were required in each arm (Van der Velden 2010 page 44), though a power calculation was not described. Bour 2005 arbitrarily set a fixed sample size of 200, with no discussion of power or adequate sample size. In total, three of the five trials did not report a power calculation, therefore it is unclear how significant these dropout rates were (Bour 2005; Pilotto 2003; Van der Velden 2010). Bayerdörffer 2016 presented a comprehensive review in their figure two, regarding dropout rates including reasons within in each study arm.
Selective reporting
Van der Velden 2010 appeared to have significant reporting bias for several outcomes because of incomplete reporting of results. Number of rescue pills used weekly was not reported as absolute numbers, but rather participants were divided into groups based on usage that were not defined a‐priori (less than two pills, two to five pills, and six or seven pills weekly). The authors did not predefine how they would assess participant satisfaction. Predefined secondary outcomes of symptom control and quality of life were not assessed separately and rather inferred from results of two surveys (Quality of Life in Reflux and Dyspepsia (QolRad), and SF‐36 Health Survey). The use of a modified per protocol population lead to results that were more difficult to interpret, and additionally, standard deviations (SD) were not reported for all their predefined outcomes.
There was significant bias in the Pilotto 2003 trial, as outcomes were not predefined in their methods and event rates were not reported. Despite methods suggesting that investigators classified symptom severity as mild, moderate, or severe, symptom assessment was only reported as percentage of participants with symptoms. Baseline participant demographics/characteristics were presented as overall numbers rather than for each group, so it is unknown if groups were balanced at randomization and if risk factors in one arm outweighed the other.
Three trials reported all predefined outcomes (Bour 2005; Janssen 2005; Morgan 2007). However, Morgan 2007 combined satisfaction ratings in their results, which was not predefined (e.g. reported results as a combination of 'good' or 'very good' ratings and 'satisfied' or 'very satisfied' ratings). This trial did not report SDs for pill usage. We were unable to use participant satisfaction scale in the meta‐analysis as SDs were not reported for this scale.
Bour 2005 reported additional analysis, but acknowledged the change to their protocol, to include statistical analysis rather than just descriptive data (e.g. subanalysis of relapse rates in different levels of on‐demand use). Janssen 2005 presented comprehensive tables and figures and included means, SDs, event numbers, absolute differences calculated, P values, and CIs. Janssen 2005 reported additional data in the results section although they were not predefined in their study design (e.g. tolerability, number of pills used). Bayerdörffer 2016 addressed all outcomes and reported data for both primary and secondary outcomes.
Other potential sources of bias
The use of perceived mean daily symptom load (MDSL) score by Janssen 2005 could contribute to bias, as this scale was defined by the authors, was not validated, and psychometric properties were unknown. Evaluating bias for the trial published by Pilotto 2003 was challenging as insufficient data were provided to make an accurate assessment.
Five of the six trials listed drug manufacturers as external funding sources: Bour 2005 by Janssen‐Cilag; Morgan 2007 by Janssen‐Ortho; Pilotto 2003 by Pharmacia, Milano, Italy; Van der Velden 2010 by Nycomed BV; and Bayerdörffer 2016 by AstraZeneca. Their role was unclear/unreported in four trials (Bayerdörffer 2016; Bour 2005; Morgan 2007; Pilotto 2003), while Van der Velden 2010 explicitly stated drug manufacturer was not involved in any aspect of participant selection, data collection, analysis, interpretation, writing and submission of manuscript. Janssen 2005 did not list sources of funding.
Effects of interventions
Primary outcomes
Lack of symptom control
All six studies measured lack of symptom control and they analyzed data separately based on the deprescribing interventions (i.e. (on‐demand therapy) and abrupt discontinuation). Analysis 1.1 summarizes the pooled outcomes (n = 1653) for on‐demand deprescribing versus continuous PPI therapy under the heading of 'Lack of symptom control'. Lack of symptom control was defined as either treatment failure (i.e. return of symptoms) or inadequate symptom relief. Two trials assessed treatment failure (Bour 2005; Janssen 2005). In Janssen 2005, treatment failure was the inverse of rate of symptom relief. In Bour 2005, symptom recurrence was interpreted as treatment failure. Three trials assessed inadequate relief (Bayerdörffer 2016; Morgan 2007; Van der Velden 2010). Van der Velden 2010 and Bayerdörffer 2016 reported dropout rates secondary to inadequate relief. Morgan 2007 reported effect of the study medication on heartburn control as 'good' or 'very good'. The remaining participants were interpreted as having inadequate symptom relief. Intention‐to‐treat (ITT) was used to report event rates, Overall, 16.3% of participants in the on‐demand deprescribing group experienced lack of symptom control versus 9.2% in continuous treatment (RR 1.71, 95% CI 1.31 to 2.21).
1.1. Analysis.
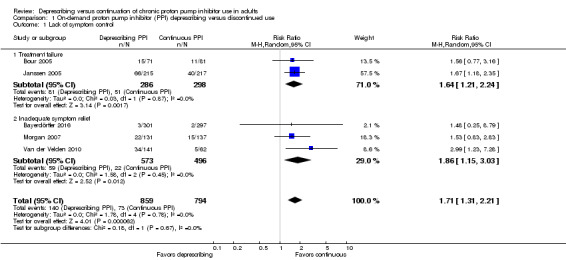
Comparison 1 On‐demand proton pump inhibitor (PPI) deprescribing versus discontinued use, Outcome 1 Lack of symptom control.
Pilotto 2003 reported the percentage of participants with return of GI reflux symptoms (i.e. lack of symptom control). The data from this trial were evaluated separately as the deprescribing intervention was stopping PPI (i.e. abrupt discontinuation). From this value, the event rate was extrapolated using ITT (Analysis 2.1). Similarly to on‐demand therapy, abrupt discontinuation was associated with an increased risk in return of GI symptoms. Given that only a single abrupt discontinuation trial reported data for this outcome, firm conclusions cannot be made.
2.1. Analysis.

Comparison 2 Abrupt discontinuation proton pump inhibitor (PPI) deprescribing versus continued use, Outcome 1 Lack of symptom control.
Drug burden/proton pump inhibitor use
Three studies (n = 1152) evaluated the number of pills used throughout the duration of each trial (Analysis 1.2) (Bayerdörffer 2016; Bour 2005; Janssen 2005). A fourth trial, Van der Velden 2010, reported weekly "rescue" pill use rather than total weekly PPI use. We did not include PPI use data from this trial as there was insufficient reporting of the outcome to allow for inclusion in meta‐analysis.
1.2. Analysis.
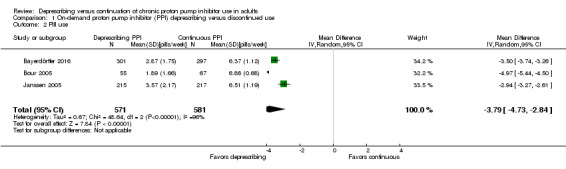
Comparison 1 On‐demand proton pump inhibitor (PPI) deprescribing versus discontinued use, Outcome 2 Pill use.
All three trials included in the analysis reported mean number of pills used per day (Bayerdörffer 2016; Bour 2005; Janssen 2005). For the purpose of this review, the mean values along with their SD were converted to weekly PPI consumption. Reported means and SDs were multiplied by a factor of seven to convert to total PPI use per week. The overall effect was a mean reduction of PPI use by 3.79 pills per week 95% CI ‐4.73 to ‐2.84 (P < 0.00001). There was significant heterogeneity (I2 greater than 96%).
Cost/resource use
We were unable to perform analyses of cost or resource use because the studies did not report this information.
Secondary outcomes
Positive drug withdrawal events
We were unable to perform analyses of PDWEs because the studies did not report this information.
Adverse drug withdrawal events
Two trials reported ADWEs; one trial had on‐demand deprescribing (Bayerdörffer 2016) and the other abrupt discontinuation (Pilotto 2003). Bayerdörffer 2016 reported 15 participants developing esophagitis in the on‐demand arm while those who continued with daily PPI use did not develop esophagitis (Analysis 1.3). Pilotto 2003 documented healing rates for participants on continuous daily PPI versus placebo (i.e. abrupt discontinuation). Based on healing rates reported, we calculated relapse rates (the inverse) and interpreted them as a marker for ADWE (Analysis 2.2). Overall, 69.6% of participants with a history of esophagitis had a relapse with abrupt discontinuation compared to 20.4% with continuous treatment.
1.3. Analysis.
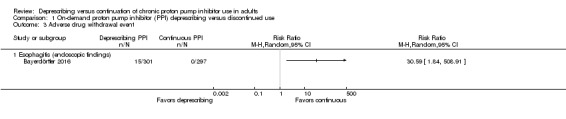
Comparison 1 On‐demand proton pump inhibitor (PPI) deprescribing versus discontinued use, Outcome 3 Adverse drug withdrawal event.
2.2. Analysis.

Comparison 2 Abrupt discontinuation proton pump inhibitor (PPI) deprescribing versus continued use, Outcome 2 Adverse drug withdrawal events.
Participant satisfaction
Five studies (n = 1653) evaluated participant satisfaction with on‐demand deprescribing as compared to continuous daily PPI use (Bayerdörffer 2016; Bour 2005; Janssen 2005; Morgan 2007; Van der Velden 2010). Participant satisfaction was interpreted using unwillingness to continue (based on premature discontinuation rates) and inadequate symptom relief (Analysis 1.4). In these studies, participants using PPIs on‐demand had a greater likelihood of being dissatisfied with therapy compared to participants using PPIs continuously (15.8% with on‐demand versus 8.8% with continuous; RR 1.82, 95% CI 1.26 to 2.65).
1.4. Analysis.
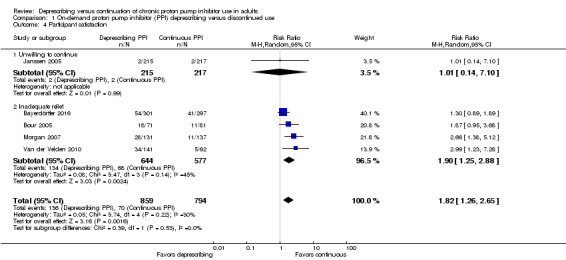
Comparison 1 On‐demand proton pump inhibitor (PPI) deprescribing versus discontinued use, Outcome 4 Participant satisfaction.
Discussion
Summary of main results
This systematic review summarized the data from six RCTs looking at deprescribing of PPIs versus continuation of chronic daily PPI therapy (Table 1; Table 2). Five trials assessed on‐demand therapy and one trial evaluated the effects of abrupt PPI discontinuation. The majority of the participants had mild GERD with or without low‐grade esophagitis. Our results show an increase in lack of symptom control and participant dissatisfaction with on‐demand deprescribing, though there was a significant reduction in pill use and burden (MD 3.79 pills weekly). Independent of the method of deprescribing, the risk of being dissatisfied with the control of upper GI symptoms was higher in the deprescribing arms. Despite these findings, a proportion of participants tolerated on‐demand therapy, though further studies are required to identify which subpopulation may benefit the most, without putting them at risk of ADWE as was seen with the Pilotto 2003 and Bayerdörffer 2016 trials. It also remains unclear whether deprescribing is effective and safe in people with severe esophagitis, as this population was not captured in the included trials. Unfortunately, we found no trials to satisfy our primary and secondary outcomes of cost and PDWEs.
On‐demand proton pump inhibitor deprescribing
Five trials evaluated deprescribing via an on‐demand approach (Bayerdörffer 2016; Bour 2005; Janssen 2005; Morgan 2007; Van der Velden 2010). Participants were aged 48 to 57 years and either had NERD or a milder form of esophagitis (LA grade A and B or Savary‐Miller grade 1 and 2).
For the primary outcome of change in upper GI symptoms, deprescribing demonstrated a return of symptoms (e.g. heartburn, regurgitation) for a proportion of people. These results were expected as participants in the on‐demand group were instructed to initiate on‐demand PPI use upon sensation of symptoms. In the combined cohort of 1653 participants randomized to on‐demand therapy, approximately 16.3% had either treatment failure or inadequate symptom relief (compared to around 9.2% on continuous PPI therapy, P less than 0.0001). This indicates that while the risk of recurrent symptoms with on‐demand therapy is greater than continuous daily PPI use, the majority of people will still tolerate the intervention. Further studies are required to identify which subpopulations comprise this group.
We pooled data for drug burden/PPI use from three on‐demand trials (n = 1152) (Bayerdörffer 2016; Bour 2005; Janssen 2005). The pooled data favored deprescribing (MD 3.79 pills weekly) and was statistically significant with a P value of less than 0.00001, although heterogeneity was high. The pooled results of this review were consistent with what is currently published in primary literature. Trials evaluating on‐demand PPI use have reported a mean pill consumption of 0.3 pills per day (Bigard 2005; Kaspari 2005; Pace 2008; Ponce 2004; Tsai 2004).
Participant satisfaction was determined by evaluating unwillingness to continue treatment and inadequate symptom relief. Data for this outcome favored chronic PPI use. Interestingly, when assessed individually, not all trials showed a significant difference in participant satisfaction ratings between groups. Janssen 2005, Bour 2005, and Bayerdörffer 2016 had CI value crossing one; however, the overall treatment effect showed significance in favor of deprescribing (RR 1.82, 95% CI 1.26 to 2.65, P < 0.002). One on‐demand PPI trial provided data for unwillingness to continue secondary to inadequate relief, which was interpreted for this review as a marker for participant dissatisfaction (and thus the inverse, as participant satisfaction) (Janssen 2005). The open‐label nature of Janssen 2005 likely influenced the results as participants were aware that they were in control of PPI use and likely favored this method rather than daily PPI use. In past literature, participant satisfaction has been reported to be in favor of chronic PPI use, though the difference from on‐demand therapy is small (Pace 2005). Our results parallel data that have been previously published. We believe that on‐demand therapy could be a viable option for people with NERD or mild‐to‐moderate GERD, without compromising satisfaction of symptom control. Further studies using more targeted measurements of participant satisfaction are needed to confirm or refute these results.
One on‐demand trial had data for ADWE (Bayerdörffer 2016). Participants in this trial were assessed endoscopically before and after randomization. Those who did not present with esophagitis were included and randomized. Investigators performed an endoscopy at the end of the trial to assess the development of esophagitis. It found that 5% (n = 15) of participants using on‐demand PPI developed mild esophagitis (14 with LA grade A, 1 with grade B).
Abrupt discontinuation deprescribing
We identified one trial of abrupt discontinuation (Pilotto 2003). Participants in this trial experienced mild‐to‐moderate esophagitis (Grade 1 or 2), though they were much older than participants taking on‐demand therapy (mean age 73 years). This trial demonstrated a statistically significant increase in the primary outcome of lack of symptom control. Participants experienced return of symptoms, which is expected, as transient and reversible rebound effects have been previously described for both healthy people and people with GI disorders upon abrupt PPI discontinuation (Reimer 2009b). Once again, symptom relapse was not observed in the entire cohort (67.9% in abrupt discontinuation versus 22.4% in the continuous arm), suggesting almost a third of the studied population tolerated abrupt discontinuation.
The Pilotto 2003 trial also addressed the secondary outcome of ADWEs. The trial evaluated changes in endoscopic findings (i.e. healing rates), which were interpreted within this review as relapse rates by assuming the inverse. The results from this trial indicated an increased risk of ADWE with deprescribing (i.e. abrupt discontinuation) that was statistically significant in people with esophagitis grades 1 or 2. Despite these findings, it is difficult to make any conclusions as the results are based on a single study of older adults with mild‐to‐moderate disease. Furthermore, the trial had a small sample size with no reported power or predefined primary endpoints. Current recommendations also suggest avoiding abrupt discontinuation, as relapse acid hypersecretion has been demonstrated even in healthy participants (Reimer 2009b). Other potential ADWEs such as upper GI bleeding or progression of esophagitis to Barrett's esophagus, among others, were not addressed by any of the trials included.
Overall completeness and applicability of evidence
The evidence for PPI use in many indications (e.g. GERD, PUD, SUP) only supports short‐term use (e.g. two to 12 weeks), yet these drugs are commonly continued for prolonged periods or even indefinitely, without appropriate reassessment (Donnellan 2004; Reimer 2009a). Continuous use of PPIs for durations exceeding accepted standards appears to be commonplace, and is likely due to the fact that this class of medication is very well tolerated and considered benign compared to other prescribed medications. This relatively desirable safety profile often overshadows lesser known drug interactions (e.g. hepatic enzyme inhibition, effects on drug absorption or activation) and poorly understood adverse effects and associated risks (vitamin B12 deficiency, hypomagnesemia, CAP, C. difficile infection, diarrhea, headache, and fractures) (Ament 2012; Chubineh 2012; Fohl 2011; Linsky 2013). Although these adverse effects may not be very common and true association with risk is debatable, the extensive use of PPIs in a wide range of populations without monitoring and reassessment is concerning.
The six trials included in this review mimic real world practice as they assessed people with adequate control and symptom relief on chronic daily PPI therapy. The control arm of no change in current daily PPI dose reflects the all too common decision of practitioners and people not to change therapy deemed effective. The deprescribing arms of abrupt discontinuation or on‐demand PPI use represent two of the many options practitioners and people have to minimize medication use and actual or potential adverse effects of chronic PPI therapy.
Unfortunately, the search identified insufficient trials to thoroughly investigate the benefits and harms of deprescribing PPIs. As described in the Background section of this review, there are many other methods of deprescribing that were not captured here. There were also no trials that investigated our primary outcome of burden/resource use, and secondary outcome of PDWE. The systematic review identified many gaps in literature that still need to be addressed. Our literature search captured predominantly people with NERD or mild esophagitis (LA grade A and B, Savary‐Miller grade 1 and 2), which limits the generalizability of results. Outcomes such as PDWE and ADWE are also not well addressed in the literature. In addition, the optimal approach to deprescribing PPIs has not been evaluated (e.g. tapering before stopping). Studies employed different definitions of symptom relapse and participant satisfaction; consistency would be helpful to improve the quality of the body of evidence and should include participant perspective in terms of what is meaningful to them. The data presented in this systematic review were not conclusive; further studies are required to address the gaps in literature.
Quality of the evidence
The quality and strength of the evidence was assessed using the GRADE system (GRADEpro). Overall, the quality of evidence for on‐demand PPI use was very low to moderate. There was serious risk of bias for all outcomes. The quality for the outcome of lack of symptom control was low due to serious imprecision. The quality of evidence for pill use was moderate. Lastly, participant satisfaction evidence was very low in quality due to serious indirectness and imprecision. This further highlights the need for future studies to address the clinical benefits and harms of PPI deprescribing. The quality of the evidence for the abrupt discontinuation deprescribing method was very low due to high risk of bias and wide CIs.
Potential biases in the review process
While we attempted to adhere to sound methodology, this systematic review was not void of limitations. First, as deprescribing is not widely described in the scientific literature, there was potential of not capturing all relevant trials. Although we sought assistance from a specialist librarian to create a filter to capture all trials related to deprescribing, it remained, nonetheless, an unvalidated search strategy. Ideally, a grey literature search would have created a more comprehensive review, however, funding and resources did not allow for this to be investigated. Second, few trials were available to confidently support the objective of this study. This could be due to the strict criteria outlined for the control arm. To satisfy the requirement for a control arm, only RCTs with a minimum run‐in phase of 28 days were considered to fulfil the definition of "chronic PPI use". This criterion was essential to capture true chronic daily PPI users. Additionally, even though eligibility criteria were broad for participants, the trials captured in this systematic review included predominantly people with NERD and mild‐to‐moderate GERD which limits the generalizability of results. All trials were of short duration (less than one year). As such, the long‐term benefits or harms of deprescribing PPIs in chronic users are difficult to extrapolate.
Agreements and disagreements with other studies or reviews
Previous publications outline deprescribing methods for PPIs (Zarowitz 2011); however, there is very little evidence comparing deprescribing modalities to continuous daily PPI use.
With respect to on‐demand therapy, a large body of evidence supports this deprescribing alternative for people with milder forms of GERD (NERD and uninvestigated GERD) (Bytzer 2004; Lee 2004; Morgan 2007; Zacny 2005). One systematic review assessing intermittent and on‐demand acid suppression therapy (e.g. H2RA and PPI) concluded that in people with mild GERD, first treated for four to eight weeks with continuous PPI, a six‐month course of on‐demand therapy was efficacious (Zacny 2005). Of these participants, 70% to 93% were willing to continue treatment with a deprescribing regimen. A more recent Danish review by Haastrup 2014 described six clinical trials (RCT and non‐RCT), using different deprescribing methods (e.g. discontinuation, dose reduction). Indication for PPI use was unknown in two trials, and described as GERD or nonulcer dyspepsia in the remaining four trials. The authors reported PPI discontinuation without deteriorating symptom control ranging from 14% to 64%, with durations of up to one year or more, similar to results of this trial (approximately a third tolerated abrupt discontinuation and 85% tolerated stepdown to on‐demand therapy) (Haastrup 2014).
Another RCT assessed symptom relief in people with GERD treated with on‐demand omeprazole versus continuous therapy (Nagahara 2014). Their results indicated that a greater proportion of people with on‐demand treatment had symptom relief by 24 weeks (74% with on‐demand therapy versus 66.7% continuous therapy), though this was not statistically significant. They concluded that on‐demand treatment in people with NERD would be adequate maintenance therapy and that continuous therapy would be ideal for people with reflux esophagitis.
To date, we identified a single meta‐analysis by Jiang and colleagues designed to compare on‐demand PPI use versus once‐daily PPI therapy (Jiang 2013). This study included two RCTs (Janssen 2005; Tsai 2004; n = 1054). Participants were approximately 50 to 52 years of age and were diagnosed with NERD or GERD classified as Savary‐Miller grade 1. The authors concluded that on‐demand therapy was more effective (i.e. greater proportion of people willing to continue with on‐demand therapy) than daily PPI use.
Our systematic review differed in that we investigate further, and aim to assess both the efficacy and safety, and identify potential benefits and harms of deprescribing PPIs. Our results indicated the opposite of what was published by Jiang and colleagues in that a significant proportion of participants in the on‐demand group were unwilling to continue due to return of GI symptoms. This discrepancy identified gaps in literature, and further trials are required to assess the efficacy, safety, benefits, and harms of deprescribing PPIs.
Authors' conclusions
Implications for practice.
The results of this review indicate that caution is needed when implementing deprescribing methods for chronic proton pump inhibitor (PPI) users; however, few clear conclusions can be drawn regarding how to best implement PPI deprescribing. On‐demand PPI use may increase risk of symptom relapse compared to continued use, although there may be a subset of people who tolerate the intervention. If on‐demand deprescribing is tolerated, people may see a benefit of reduced pill burden by a mean of up to 3.79 pills per week. With respect the outcome of adverse drug withdrawal events (ADWE), there appears to be an increased risk of rebound esophagitis with on‐demand therapy and abrupt discontinuation, though only two trials reported data in sufficient detail. We identified no trials that allowed for conclusions regarding our primary and secondary outcomes of cost and positive drug withdrawal effects. It is important to bear in mind that the results of this systematic review only apply to people with gastroesophageal reflux disease (GERD) presenting with NERD or mild esophagitis. This excludes people with moderate‐to‐severe esophagitis, Barrett's esophagus, Zollinger‐Ellison syndrome, people with H. pylori, chronic NSAID use, or PUD, to name a few.
Implications for research.
This systematic review highlights the paucity of high‐quality data surrounding how to deprescribe PPIs appropriately. Future trials need to focus on collecting baseline participant characteristics to illicit which specific participant characteristics predict successful PPI deprescribing outcomes. Existing literature explores a variety of deprescribing methods, though as evidenced by this review, there are few trials that compare these methods to continuous PPI therapy. Future randomized controlled trials (RCT) should compare continuous PPI therapy to deprescribing methods (i.e. abrupt discontinuation, on‐demand, and intermittent therapy) in a variety of participant populations: nonerosive GERD, erosive esophagitis (EE) (low grade), and GERD that has not been investigated endoscopically (i.e. reflective of outpatient practice in resource‐limited healthcare settings). These RCTs should also focus on capturing baseline characteristics (i.e. risk factors such as smoking, alcohol use, diet, family history). New RCTs should attempt to extend mean duration of follow‐up past one year.
Furthermore, future research should focus on better defining costs, ADWE, and positive drug withdrawal events (PDWE). The following trial designs may address these gaps in the literature.
Cost‐benefit analysis designed to quantify the costs associated with the act of deprescribing as compared to the cost‐savings associated with eliminating a lifelong therapy with an agent that is not indicated.
Retrospective case‐control study to compare people who continue chronic PPI therapy as compared to people who undergo deprescribing, in order to quantify PDWE and ADWE over prolonged periods of time (i.e. greater than two years).
Retrospective case‐control study to compare deprescribing outcomes in people with high‐grade EE.
Acknowledgements
We acknowledge the following authors for kindly providing assistance: Dr Salmaan Kanji, Dr Pierre Giguere, Hannah Irving, Lisa Pizzola, Kate Walsh, Shannon Gordon, Corey Tsang, Lisa Sunstrum, and Dr Catherine Dalzell.
We acknowledge Corey Tsang and Lisa Richardson for their previous work that was the foundation of the current study.
Appendices
Appendix 1. Glossary of terms
Adenocarcinoma ‐ type of cancer originating from mucus‐secreting glands.
Antiplatelet agent ‐ prevents blood clotting.
Barrett's esophagus ‐ a change in the lower esophagus that results from GERD that can increase the risk of esophageal cancer.
Benign ‐ harmless.
CADTH ‐ Canadian Agency for Drugs and Technologies in Health.
Clostridium difficile ‐ spore‐forming bacteria that causes colitis.
Coagulopathy ‐ a condition affecting the blood's ability to clot.
Concomitant ‐ accompanying.
Duodenal ‐ relating to the first part of the small intestine which attaches to the stomach.
Dyspepsia ‐ indigestion.
Dysplasia ‐ abnormal cell development.
Efficacious ‐ effective.
Endoscopy ‐ a procedure to visualize the inside of the body (i.e. the gastrointestinal tract).
Endoscopic reflux disease ‐ visible esophageal injury seen by endoscopy.
Epidemiology ‐ the study of the incidence, distribution, and control of disease in a specific population.
Epigastric ‐ over the stomach.
Epithelial cells ‐ any of the small structures that form the layers that cover or line a body surface.
Esophagitis ‐ inflammation of the esophagus.
Gastric ‐ stomach.
Gastro ‐ related to the stomach.
H+/K+ adenosine triphosphatase (ATPase) ‐ an enzyme that contributes to the creation of stomach acid.
Helicobacter pylori(H. pylori) ‐ a form of bacteria associated with stomach and duodenal ulcers.
Hypersecretory ‐ causing excessive production.
Hypomagnesemia ‐ low blood levels of magnesium.
Morbidity ‐ rate of illness or complications.
Mortality ‐ death rate.
NSAIDS ‐ nonsteroidal anti‐inflammatory drugs.
Prophylaxis ‐ prevention.
Regurgitation ‐ the backward flow of stomach contents into the esophagus and mouth.
Rubric ‐ heading.
Selective cyclooxygenase‐2 (COX2) inhibitors ‐ a form of anti‐inflammatory drug.
Sequelae ‐ negative consequence.
Ventilation ‐ breathing.
Appendix 2. Abbreviations
| ACG | American College of Gastroenterology |
| ADWE | Adverse drug withdrawal events |
| ASA | Acetylsalicylic acid (aspirin) |
| ATPase | H+, K+‐adenosine triphosphatase |
| C. difficile | Clostridium difficile |
| CADTH | Canadian Agency for Drugs and Technologies in Health |
| COX1 | Cyclooxygenase‐1 inhibitor |
| COX2 | Cyclooxygenase‐2 inhibitor |
| EE | Erosive esophagitis |
| GERD | Gastroesophageal disease |
| GESA | Gastroenterological Society of Australia |
| GI | Gastrointestinal |
| H. pylori | Helicobater pylori |
| H2RA | Histamine‐2 receptor antagonist |
| ICU | Intensive care unit |
| IPA | International Pharmaceutical Abstracts |
| LA Grade | Los Angeles Grade |
| NERD | Nonerosive reflux disease |
| NSAID | Nonsteroidal anti‐inflammatory drug |
| ODT | On‐demand therapy |
| OPEN | Ontario Pharmacy Research Collaboration |
| PDWE | Positive drug withdrawal events |
| PPI | Proton pump inhibitor |
| PUD | Peptic ulcer disease |
| RCT | Randomized controlled trial |
| SUP | Stress ulcer prophylaxis |
Appendix 3. CENTRAL search strategy
1. exp Proton Pump Inhibitors/
2. ((proton adj2 pump adj2 inhibitor*) or PPI or PPIs).tw.
3. Esomeprazole/
4. (Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam).tw.
5. Omeprazole/
6. (omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Lomac or Omepral or Omez).tw.
7. (pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro).tw.
8. Rabeprazole/
9. (rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc).tw.
10. Lansoprazole/
11. (lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).tw.
12. dexlansoprazole/
13. (Dexlansoprazole or Kapidex or Dexilant).tw.
14. (tenatoprazole or benatoprazole or TU 199 or CAS 113712‐98‐4 or STU‐Na).tw.
15. or/1‐14
16. child/ or child, preschool/ or infant/ or infant, newborn/ or infant, low birth weight/ or infant, small for gestational age/ or infant, very low birth weight/ or infant, extremely low birth weight/ or infant, postmature/ or infant, premature/ or Pediatrics/ or Neonatology/ or Adolescent/
17. adult/ or aged/ or "aged, 80 and over"/ or frail elderly/ or middle aged/ or young adult/
18. 16 not (16 and 17)
19. 15 not 18
20. (deprescri* or de‐prescri* or unprescri* or cease* or ceasing* or cessation* or withdraw* or discontinu* or stop* or intermittent or "on demand").mp. [mp=title, original title, abstract, mesh headings, heading words, keyword]
21. 19 and 20
Appendix 4. MEDLINE search strategy
1. exp proton pump inhibitor/
2. ((proton adj2 pump adj2 inhibitor*) or PPI or PPIs).tw.
3. esomeprazole/
4. (Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam).tw.
5. omeprazole/
6. (omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Lomac or Omepral or Omez).tw.
7. pantoprazole/
8. (pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro).tw.
9. rabeprazole/
10. (rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc).tw.
11. lansoprazole/
12. (lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).tw.
13. (Dexlansoprazole or Kapidex or Dexilant).tw.
14. benatoprazole/
15. (tenatoprazole or benatoprazole or TU 199 or CAS 113712‐98‐4 or STU‐Na).tw.
16. or/1‐15
17. random:.tw. or placebo:.mp. or double‐blind:.tw.
18. 16 and 17
19. (animal$ not human$).sh,hw.
20. 18 not 19
21. child/ or newborn/ or exp infant/ or toddler/ or preschool child/ or school child/ or adolescent/
22. adult/ or exp aged/ or middle aged/
23. 21 not (21 and 22)
24. 20 not 23
25. (deprescri* or de‐prescri* or unprescri* or cease* or ceasing* or cessation* or withdraw* or discontinu* or stop* or intermittent or "on demand").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
26. 24 and 25
Appendix 5. Embase search strategy
1. exp Proton Pump Inhibitors/
2. ((proton adj2 pump adj2 inhibitor*) or PPI or PPIs).tw.
3. Esomeprazole/
4. (Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam).tw.
5. Omeprazole/
6. (omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Lomac or Omepral or Omez).tw.
7. (pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro).tw.
8. Rabeprazole/
9. (rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc).tw.
10. Lansoprazole/
11. (lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).tw.
12. dexlansoprazole/
13. (Dexlansoprazole or Kapidex or Dexilant).tw.
14. (tenatoprazole or benatoprazole or TU 199 or CAS 113712‐98‐4 or STU‐Na).tw.
15. or/1‐14
16. randomized controlled trial.pt.
17. controlled clinical trial.pt.
18. randomized.ab.
19. placebo.ab.
20. drug therapy.fs.
21. randomly.ab.
22. trial.ab.
23. groups.ab.
24. or/16‐23
25. exp animals/ not humans.sh.
26. 24 not 25
27. 15 and 26
28. child/ or child, preschool/ or infant/ or infant, newborn/ or infant, low birth weight/ or infant, small for gestational age/ or infant, very low birth weight/ or infant, extremely low birth weight/ or infant, postmature/ or infant, premature/ or Pediatrics/ or Neonatology/ or Adolescent/
29. adult/ or aged/ or "aged, 80 and over"/ or frail elderly/ or middle aged/ or young adult/
30. 28 not (28 and 29)
31. 27 not 30
32. (deprescri* or de‐prescri* or unprescri* or cease* or ceasing* or cessation* or withdraw* or discontinu* or stop* or intermittent or "on demand").mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
33. 31 and 32
Data and analyses
Comparison 1. On‐demand proton pump inhibitor (PPI) deprescribing versus discontinued use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lack of symptom control | 5 | 1653 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.31, 2.21] |
| 1.1 Treatment failure | 2 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.21, 2.24] |
| 1.2 Inadequate symptom relief | 3 | 1069 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.15, 3.03] |
| 2 Pill use | 3 | 1152 | Mean Difference (IV, Random, 95% CI) | ‐3.79 [‐4.73, ‐2.84] |
| 3 Adverse drug withdrawal event | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Esophagitis (endoscopic findings) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Participant satisfaction | 5 | 1653 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.26, 2.65] |
| 4.1 Unwilling to continue | 1 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.10] |
| 4.2 Inadequate relief | 4 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.25, 2.88] |
Comparison 2. Abrupt discontinuation proton pump inhibitor (PPI) deprescribing versus continued use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lack of symptom control | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Symptom relapse rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse drug withdrawal events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Relapse (endoscopic findings) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bayerdörffer 2016.
| Methods | Prospective, multicenter, open‐label, randomized trial | |
| Participants | n = 598 (outpatient) 86% white ethnicity Mean age 48 years All had NERD and moderate‐to‐severe GERD |
|
| Interventions |
Intervention: on‐demand esomeprazole 20 mg orally x 6 months Control: continuous esomeprazole 20 mg orally once daily x 6 months |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Only patients who were free from heartburn at visit 2 (defined as 7 symptom‐free days in the last week of the short‐term treatment phase; i.e., complete resolution of symptoms) were randomized sequentially (1:1) to one of two treatment groups for a 6‐month maintenance treatment phase."
"Randomization was performed using a computer program at AstraZeneca in balanced blocks using a blocking size of 2" (p. 3). Comment: automated computer program using block size of 2. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description on whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label: no blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label: no blinding of participants or personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Primary outcome: GI symptoms (reported in trial as proportion of participants discontinuing the study as a result of unsatisfactory treatment) Secondary outcome: satisfaction (interpreted in trial as inverse of primary outcome) Quote: "The distribution of reasons for discontinuation was similar in the two treatment groups (Table 2)" (p. 6). "ITT data were analyzed using the last‐visit‐carried‐forward method for those patients who discontinued prematurely, and all patients who discontinued were regarded as having discontinued due to unsatisfactory treatment" (p. 4). Comment: ITT and PP results reported for primary outcome. All reasons for withdrawals reported. Figure 2 describing participants' disposition through the stages of the study. Outcome: drug usage Quote: "Among patients with evaluable data (on‐demand, n = 295; continuous, n = 286), mean drug usage over the 6‐month study period was 0.41 (standard deviation [SD], 0.25) tablets (doses) per day in the on‐demand treatment" (p. 6). Comment: not all participants used in ITT analysis were evaluable for drug usage and no description explaining why: ODT: ITT n = 301 vs evaluable n = 295 CT: ITT n = 297 vs evaluable n = 286 |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes defined in methods were reported in results. |
| Other bias | Low risk |
Quote: "The intake of study medication was registered using the MEMS® device, which utilizes a microelectronic recorder recessed in the cap of a drug container (Medical Event Monitoring System, Aardex, Zug, Switzerland). At each opening and closure of the container, the date and time of day was automatically recorded. This information was analyzed at the end of the study" (p. 3). Comment: this is an objective method to record medication use, and minimizes recall bias. Comment: this study was funded by AstraZeneca R&D and many of authors including lead investigators have received financial support or (were) employees of AstraZeneca (p. 11). |
Bour 2005.
| Methods | Prospective, multicenter, open‐label, randomized trial | |
| Participants | n = 152 (outpatient) Mean age 49 years Moderate GERD
History GERD 6.1 years |
|
| Interventions |
Intervention: on‐demand rabeprazole 10 mg orally x 6 months Control: continuous rabeprazole 10 mg orally once daily x 6 months |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | *Savary‐Miller classification of esophagitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "responders were randomized into two groups..." (p. 806). Comment: as per Section 8.9.2.3 of the Cochrane Handbook for Systematic Reviews of Interventions, insufficient information to assess sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: as per Table 8.5d of the Cochrane Handbook for Systematic Reviews of Interventions, no description of allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: "multicentre, randomized, open‐label study" (p. 806). Comment: as per Table 8.5d of the Cochrane Handbook for Systematic Reviews of Interventions, open‐label trial, participants and personnel aware of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quotes: "patients asked to report on a diary card;" "QOL was assessed using the Reflux‐Qual questionnaire;" "patient satisfaction was also assessed by an analogue visual scale;" "overall patient satisfaction as measured by both the investigator and the participant at randomization day and 6 months" (p. 807). Comment: subjective outcomes, participants and assessors not blinded, symptom relief (primary outcome) was participant‐rated using Likert scale; outcome assessment was not blinded, uncertain if investigators saw participant satisfaction measure before they assessed satisfaction. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quotes: "the completion rate was 88.8% (72 of 81) for the CT group and 84.5% (60 of 71) for the ODT group" (note: 1 participant in CT group withdrew at last visit, 2 in ODT group, all were included in final analyses ‐ so actual completion rate 88% in CT (71/81) and 82% in ODT (13/71)). "patients who withdrew before trial completion were regarded as not achieving symptom relief" (p. 807). Comment: table outlining reasons for withdrawal (p. 810): authors stated no difference between groups, but statically significant difference in withdrawal for "other" reasons (which was not explained), 6 in ODT vs 1 in CT; also 2 withdrawals for recurrence and 1 for lack of efficacy in ODT vs 0 in CT (these missing data related to true outcome as per Table 8.5d of the Cochrane Handbook for Systematic Reviews of Interventions; as per Section 8.13.2.2 reasons for missing outcomes were different even though overall numbers were similar). ITT implied in above quote for symptom relief, but not explicitly stated and unsure if used for other outcomes. Only 67 participants analyzed for pill usage in CT group, only 55 for ODT group (no reasons provided). |
| Selective reporting (reporting bias) | High risk |
Quotes: "overall patient satisfaction assessed by the investigator at baseline overlapped patients' evaluations, with no difference between the two groups (P=0.859)" (p. 809); "it was decided to arbitrarily fix the number of patients to 200;" "when finalizing the analysis plan...it was decided that some statistical tests were necessary to better compare the groups" (p. 807). Comment: p. 809‐810: as above, unsure if PP or ITT for all outcomes in comparative phase; most outcomes described in methods were reported in results in prespecified way; methods described measurement of mean daily rabeprazole use while authors carried out subanalysis of relapse rates in different levels of ODT use; no comparison of adverse events between groups. For investigator rating of satisfaction at baseline, actual ratings not provided (p. 809). *not bias but important that sample size was arbitrarily fixed with no discussion of power or adequate sample size. |
| Other bias | Unclear risk |
Quote: "this study was supported by a grant from Janssen‐Cilag." Comment: manufacturer sponsored (unclear role, if any), baseline imbalance in main symptom (p. 808) but results based on main symptom at baseline. |
Janssen 2005.
| Methods | Prospective, multicenter, open‐label, randomized trial | |
| Participants | n = 432 Mean age 51 years ˜ 25% grade 0 GERD (normal mucosa) ˜ 75% grade I GERD (patchy red lesions without white coating or with central white coating) |
|
| Interventions |
Intervention: on‐demand pantoprazole 20 mg orally as needed (maximum 1 pill daily) x 6 months Control: continuous pantoprazole 20 mg orally daily x 6 months |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Outcomes used for review
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomization was performed using a computer‐generated list" (p. 348). Comment: good technique. |
| Allocation concealment (selection bias) | Low risk |
Quote: "The study medication was packed in a sealed box and allocated according to the numbering in ascending order" (p. 348). Comment: good technique as sequential/sealed medications. However, did not comment that boxes were identical in appearance. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: "The patient and the investigator first learned to which treatment group the patient was assigned when the patient opened the box" (p. 348). Comment: high risk of bias as this was an open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quotes: For symptom control: "Patients were instructed to document the symptom intensity..." (assessment by participant diary, p. 349). For symptom control outcome: "At each visit, the investigator questioned the patient about the intensity of the symptoms..." (investigator's assessment, p. 349). For pill use outcome: "All unused medication was counted and documented by the investigator..." (paragraph 3, p. 348). For unwillingness to continue outcome: no quote, and not a predefined outcome. Comment: high risk of bias for symptom control (outcome was subjective). Low risk of bias for pill count (measurement was not likely to be influenced by lack of blinding). High risk of bias for satisfaction/unwillingness to continue (outcome was subjective and highly influenced by participant). |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "Ninety‐five patients were excluded from the PP population because they were classified as protocol violators. The most common violations were use of a prohibited concomitant medication (30 patients), loss to follow‐up (29 patients), and having >10% of diary cards unavailable for evaluation (18 patients)" (p. 343). Comment: from 558 enrolled participants, 432 entered the long‐term phase (ITT). From 432 participants, 22% (n = 95) violated the protocol. There was high number of dropouts in the randomized phase. This represented a significant amount of missing data. Despite this, the number of participants required (n = 300, or 150 participants per arm) for an 80% power was achieved. Data for each outcome was provided in full for the primary and secondary outcomes. For primary outcomes: table 2 (p. 355) and figure 2 (p. 356). For secondary outcomes: table 3 (p. 356). |
| Selective reporting (reporting bias) | Low risk |
Quote: "Statistical analysis was performed in both the intent‐to‐treat (ITT) and PP populations. Both populations were defined separately in the acute and long‐ term phases, as shown in Figure 1. The ITT population (acute phase) comprised all patients for whom it could not be ruled out that study medication was received. In the long‐term phase, the ITT population was defined as eligible patients for whom it could not be ruled out that study medication was received and who attended at least one follow‐up visit after starting the long‐term phase. The PP analysis excluded protocol violators" (data sets analyzed, p. 351). Comment: all data were reported in ITT or PP. The study protocol was not available but the published report included all expected outcomes, including those that were prespecified. In addition, tables and figures were comprehensive with means, standard deviations, event numbers, absolute differences calculated, P values and confidence intervals. Note: other data were reported in the results section, although not predefined as outcomes (e.g. tolerability, number of pills used). |
| Other bias | High risk | MDSL scale was defined and possibly created by authors and was not validated ‐ could infer some bias especially since participants, investigators, and outcome assessment not blinded and outcomes were subjective. People with healed esophagitis after a 4‐week run‐in phase were selected for randomization. Excluded people with unhealed esophagitis, and who would require longer duration of PPI therapy. No sources of funding/conflict of interest stated. |
Morgan 2007.
| Methods | Prospective, multicenter, open‐label, randomized trial | |
| Participants | n = 268 Mean age 48 years ˜ 58% no heartburn ˜ 22% mild heartburn ˜ 19% moderate heartburn |
|
| Interventions |
Intervention: on‐demand rabeprazole 20 mg orally once daily up to 6 months Control: continuous rabeprazole 20 mg orally once daily up to 6 months |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Refer to data collection sheet for more details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "those eligible to continue were subsequently randomly assigned to either 20 mg rabeprazole ODT or 20 mg once daily COT [continuous therapy] (in a 1:1 ratio)" (p. 821). Comment: as per Section 8.9.2.3 of the Cochrane Handbook for Systematic Reviews of Interventions, insufficient information to assess sequence generation. |
| Allocation concealment (selection bias) | Unclear risk |
Quote: none. Comment: as per Table 8.5d of the Cochrane Handbook for Systematic Reviews of Interventions, no description of allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: "...in this open‐label, randomized, controlled multicentre study" (p. 821). Comment: as per Table 8.5d of the Cochrane Handbook for Systematic Reviews of Interventions, open‐label trial, participants and personnel aware of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "heartburn occurrence, and worst overall severity on days with heartburn, medication compliance...were recorded in a daily diary" (p. 822). "the diary also included weekly assessments in which patients rated heartburn control...and satisfaction" (p. 822). "the overall effect of the medication regimen on heartburn control...was assessed by the physician and patient at study termination; patients were also asked to rate their overall satisfaction with their heartburn control" (p. 822). Comment: subjective outcomes (participant rating effect of treatment, reporting on satisfaction) were used and neither the participants nor assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Quote: "87% completed six months of maintenance treatment." "of the 34 participants (13%) who withdrew before completing the study, nine (3%) withdrew due to poor heartburn control during the maintenance treatment phase, eight (3%) were lost to follow‐up, six (2%) withdrew due to an adverse event, five (2%) withdrew consent, two (1%) were withdrawn due to noncompliance, two (1%) were withdrawn due to protocol violations, one (0.4%) withdrew due to pregnancy and one (0.4%) withdrew for other reasons" (p. 823). "the proportion of patients who discontinued treatment due to insufficient heartburn control was not significantly different between COT (2.2%) and ODT groups (4.6%)" (p. 823). Comment: reasons for missing data not separated into intervention/control groups except for withdrawal due to insufficient heartburn control. |
| Selective reporting (reporting bias) | High risk |
Quote: "the difference in the percentage of heartburn‐free days...was estimated together with the 95% CI [confidence interval] on an intention‐to‐treat basis." "for all secondary variables, between‐group comparisons were made on an intention‐to‐treat basis" (p. 822). "the overall effect...was assessed by the physician and patient at study termination" (p. 822); → "the majority of patients rated the effect of the study medication on heartburn control as 'good' or 'very good', with no significant difference noted between treatment groups (COT [continuous therapy] 89% versus ODT 83% P = 0.2803)" (p. 824). "patients were also asked to rate their overall satisfaction with their heartburn control" (p. 822); → "significantly more subjects on COT rated themselves 'satisfied' or 'very satisfied'... (92% and 79%, P = 0.0070)" (p. 825). Comment: all outcomes described in methods were reported in results; however, analyzed combination of ratings, which were not outlined in methods (e.g. measured combination of 'good' or 'very good' ratings, 'satisfied' or 'very satisfied' which were not prespecified in methods) as per Table 8.5d of the Cochrane Handbook for Systematic Reviews of Interventions ≥ 1 primary outcomes were reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified; did not report standard deviation values for pill usage, participant satisfaction scale could not be used in meta‐analysis and standard deviations not reported for this scale. |
| Other bias | Unclear risk |
Quote: "this work was supported by Janssen‐Ortho Inc." Comment: manufacturer sponsored (unclear role, if any). |
Pilotto 2003.
| Methods | Prospective, multicenter, double‐blind, randomized trial | |
| Participants | n = 105 Mean age 73 years (range 65 to 93 years) Symptomatic (heartburn, regurgitation, pain) 43% grade I* esophagitis 52% grade II* esophagitis 5% grade III* esophagitis 67% hiatus hernia 62% Helicobacter pylori‐negative |
|
| Interventions |
Intervention: abrupt discontinuation placebo daily x 6 months Control: continuous pantoprazole 20 mg orally, daily x 6 months |
|
| Outcomes |
Outcomes used for review
Note: no predefined outcomes reported |
|
| Notes | *Savary‐Miller classification of esophagitis Refer to data collection sheet for more details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients with healed oesophagitis were randomized to receive pantoprazole, 20 mg daily, or placebo during the following 6 months (double‐blind phase)" (p. 1400). Comment: method of randomization not outlined. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors did not outline how allocation of interventions were carried out and if they were concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Outcome: symptoms Quote: "symptoms were assessed during a structured interview. The patient was questioned about the principal symptoms...The severity of the symptoms was classified by the investigator." Comment: no comment on how blinding was maintained; no comment about whether placebo and PPI looked and tasted the same. Symptom questionnaire was assessed by investigator. Not sure if this affected bias (p. 1402). Outcome: healing of esophagitis Quote: "Patients who perceived symptoms of reflux oesophagitis for at least 3 consecutive days between the scheduled study visits were asked to contact the investigator and an additional endoscopy was performed. Patients with an endoscopically verified relapse of oesophagitis were excluded from further participation in the study." Comment: no comment on how blinding was maintained; no comment about whether placebo and PPI looked and tasted the same. However, participants were told to report symptoms for 3 days and then they were assessed… this may cause participants and investigators to start guessing that they were in the placebo group. Relapse rate significantly higher in placebo group versus continuation group, and relapse rate high in placebo group; therefore, likely that blinding would be broken and influence outcome reporting. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | See 'Blinding of participants and personnel (performance bias).' |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "During this second phase of the maintenance study, 12 patients (eight from the treatment arm and four from the placebo group) dropped out of the study due to adverse events (one patient), low compliance (two patients) or refusal to undergo final endoscopy after 12 months (nine patients)" (results, p. 1403). Comment: predefined endpoints/outcomes were not outlined in study's methods section. It is unclear if results published represent primary or secondary outcomes. The short‐term phase had 22 participant dropouts (out of 164), the maintenance phase had 15 participant dropouts (out of 133) and once participants were randomized, the treatment arm had 4 dropouts and control arm had 8 dropouts. The reasons for dropout were not defined. The proportion of missing participants compared with the observed event rate for individual symptoms in the continuous PPI was very high (e.g. 8/49 dropouts and only 4/49 reporting heart burn at end of trial) (p. 1403). It is also important to note that a sample size and power calculation were not reported. Therefore, it is unclear how significant these dropout rates were. |
| Selective reporting (reporting bias) | Unclear risk |
Quote: "Results were evaluated using both 'per protocol' and 'intention‐to‐treat' analyses; the 95% confidence intervals (95% CI) were also calculated. The intention‐to‐treat population was defined as all patients initially enrolled who had taken at least one dose of study medication" (statistics, p. 1401). Comment:
|
| Other bias | Unclear risk | Unsure of source of funding (Pharmacia, Milano, Italy). |
Van der Velden 2010.
| Methods | Prospective, multicenter, double‐blind, randomized trial | |
| Participants | n = 203 Mean age 57 years 35% with esophagitis A* 19% esophagitis 7% with hiatus hernia 9% with GERD, reflux, or pyrosis |
|
| Interventions |
Intervention: placebo daily + on‐demand pantoprazole 20 mg orally daily as needed x 13 weeks Control: continuous pantoprazole 20 mg orally daily + placebo daily as needed x 13 weeks |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Outcomes used for review
|
|
| Notes | *Los Angeles (LA) Classification system of esophagitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomization was performed in sets of 6 (daily pantoprazole to daily placebo: 2 to 4) using a computer‐generated list, according to which an external party generated sealed sequentially numbered identical containers" (randomization, blinding, and sample size on p. 44). Comment: good technique that reduced bias. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Sets were sent to the general practitioners, where the nurse allocated the next available number of the set on entry into the trial" (randomization, blinding, and sample size on p. 44). Comment: good technique that reduced bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "The study was performed double‐blind; after completion of the study, unblinded data were analyzed" (randomization, blinding, and sample size on p. 44). Comment: outcomes were subjective and participant specific (e.g. symptoms, quality of life scores, etc.). Therefore, blinding of participants was crucial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "The study was performed double‐blind; after completion of the study, unblinded data were analyzed" (randomization, blinding, and sample size on p. 44). "...At week 5, participants were phoned to enquire about their well‐being. As patients, on their own initiative, reported the randomization arm they believed they were participating in, this was registered without discussion" (follow‐up, p. 45). Comment: the outcome of use of rescue medication could be influenced by unblinded assessment. As figure 3 showed, participants could either use < 2 pills/week, 2 to < 6 pills/week, or 6 or 7 pills/week. Participants were not randomized to these groups. It appeared that groups were created after trial was complete. It was unclear if these groups were predefined (no mention in methods). There was also no explanation/justification as to how the authors decided to make these groups. For satisfaction (i.e. inadequate relief dropouts) outcome: unblinded investigators could not influence bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: For symptom control and rescue medication: Figure 2. "Trial design. Patients leaving the study are shown in grey boxes together with reasons for discontinuation. IR = inadequate relief; AE = adverse event; OR = own request; O = other." For use of rescue medication outcome: Figure 3. The number of participants per dosage group were shown. Furthermore, the percentages of participants with a correct idea about the randomization arm they participated in were indicated (% correct), together with the numbers of participants who expressed this idea. IR = inadequate relief; AR = all other reasons. "The sample size calculation was based on the percentage of patients taking 6 or more tablets on‐demand per week. With assumptions of 25% in the daily pantoprazole and 60% in the daily placebo arm, at least 40 patients per arm were needed (α: 0.05, b: 0.1)…With the randomization ratio originally set at 1:2 and an expected drop‐out of 20% during run‐in, the number of patients needed in the study was at least 282" (randomization, blinding, and sample size calculation, p. 44). "In the daily pantoprazole arm, 52 patients completed the study (84%), 5 patients (8%) discontinued for inadequate relief and 5 discontinued for other reasons (fig. 2). In the daily placebo arm, 94 patients (67%) successfully completed the study, 34 patients (24%) discontinued for inadequate relief and 13 discontinued for other reasons (9%)" (results, p. 46). Comment: For symptom control and rescue medication: this outcome is well documented and reasons for dropout were clear. For author‐defined satisfaction outcome (% correct guessing of allocation): outcome not predefined in methods. Also did not report % correct guesses for those who dropped out (e.g. those who had inadequate relief). In addition, the authors of this study stated that 40 participants were required in each arm (p. 44), though a power was not stated. The ODT arm experienced a high dropout rate (33%) which is greater than anticipated (20%). |
| Selective reporting (reporting bias) | High risk |
Quote: "We did an intention to treat (ITT) analysis among patients adequately treated with daily pantoprazole in the run‐in, representing stable long‐term‐treated patients. Results of patients leaving the study after randomization for other reasons than inadequate relief were used in the analyses according to data at their final visit" (data analysis, p. 45). Comment: comparing methods to results, the outcomes that were to be reported were. However, it was unclear for some outcomes that ITT was used. For example, adequate relief (figure 3) did not include all participants (it excluded those with inadequate relief), therefore it appeared that more participants had relief than not. Participants were also analyzed to number of rescue pills used weekly (e.g. < 2 pills, 2 to < 6 pills, and 6 or 7 pills); however, participants were not randomized to these groups. These groups were created at study completion. Quote: "Primary outcome was the volume of rescue medication per week. Three dosage groups were created: 0 to 2 tablets/week [(al‐ most) quitting treatment], 2 to < 6/week (substantial reduction), 6‐7/week (ongoing daily treatment)" (outcome measure, p. 45). Comment: the authors also did not report the exact volume of medications used use weekly. For example, for those in the category of 2 to < 6 pills per week, it was unclear exactly how many participants used 2, 3, 4, or 5 pills weekly. Participants who use 2 pills weekly versus 5 pills weekly were not equivalent clinically. Quote: "These 141 patients entered the study using 6.3 DDD [defined daily dose] per week, whereas during the study, symptom control was achieved at a mean of 5.38 tablets of pantoprazole (20 mg) per week (table 2), which equals 2.7 DDD. This amounts to a reduction of 57%. As 20 mg of pantoprazole is defined as 0.5 DDD" (pharmacological dependency, p. 47 and Table 2). Comment: selective reporting was apparent in the quote above as defined daily dose was only reported for the placebo group (n = 141) and not the CT arm. Quote: "Secondary outcomes were final adjusted dosage, reported symptom control and generic quality of life (in randomization arms, as well as in dosage groups)" (p. 45). Comment: symptom control and quality of life not assessed separately. Interpretation of results was subjective as symptom control was inferred from the scales used (Quality of Life in Reflux and Dyspepsia and 36‐item Short Form). Note: the authors did not predefine how they would assess participant satisfaction. The % correct guess of which treatment the participant received was a marker for satisfaction according to the authors. However, % correct guess was not reported for dropouts. |
| Other bias | High risk |
Quote: "This study was funded by Nycomed BV, The Netherlands. Nycomed BV was not involved in patient selection, data collection, analysis or interpretation, or writing or submission of the manuscript. There are no further conflicts of interest" (p. 51). Comment: Interpretation of results was not intuitive. Symptom control and quality of life mean scores reported (but not standard deviation). Participants who were asymptomatic after the run‐in phase were randomized. Those who were not were excluded. Thus, PPI nonresponders were excluded from randomization. |
CT: continuous therapy; GERD: gastroesophageal reflux disease; GI: gastrointestinal; ITT: intention to treat; MDSL: mean daily symptom load; n: number of participants; NERD: nonerosive gastroesophageal reflux disease; ODT: on‐demand therapy; PP: per protocol; PPI: proton pump inhibitor.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Annibale 1998 | Did not exclude chronic NSAID use. |
| Archambault 1988 | No control arm (i.e. continuous/no change in PPI). |
| Bardhan 1990 | No control arm (i.e. continuous/no change in PPI). |
| Bardhan 1995 | No control arm (i.e. continuous/no change in PPI). |
| Bate 1989 | No control arm (i.e. continuous/no change in PPI). |
| Bate 1990 | No control arm (i.e. continuous/no change in PPI). |
| Bate 1995 | No control arm (i.e. continuous/no change in PPI). |
| Bate 1997 | No control arm (i.e. continuous/no change in PPI). |
| Bautista 2006 | Not an RCT. |
| Bayerdörffer 1993 | No control arm (i.e. continuous/no change in PPI). |
| Bigard 2005 | No control arm (i.e. continuous/no change in PPI). Both arms were deprescribing. |
| Björnsson 2006 | No control arm (i.e. continuous/no change in PPI). |
| Bytzer 2004 | No control arm (i.e. continuous/no change in PPI). Both arms were deprescribing. |
| Carlsson 1998 | No control arm (i.e. continuous/no change in PPI). |
| Chen 1993 | No control arm (i.e. continuous/no change in PPI). |
| Dabholkar 2011 | No control arm (i.e. continuous/no change in PPI). |
| Deboever 2003 | No control arm (i.e. continuous/no change in PPI). |
| Dent 1989 | No control arm (i.e. continuous/no change in PPI). |
| Dent 1994 | Did not exclude chronic NSAID use. |
| DeVault 2006 | No control arm (i.e. continuous/no change in PPI). |
| Earnest 1998 | No control arm (i.e. continuous/no change in PPI). |
| Eggleston 2009 | No control arm (i.e. continuous/no change in PPI). |
| Escourrou 1999 | No control arm (i.e. continuous/no change in PPI). |
| Farley 2000 | No control arm (i.e. continuous/no change in PPI). |
| Fass 2011 | No control arm (i.e. continuous/no change in PPI). |
| Fass 2012 | No control arm (i.e. continuous/no change in PPI). |
| Fennerty 2009 | No control arm (i.e. continuous/no change in PPI). |
| Goh 1995 | No control arm (i.e. continuous/no change in PPI). |
| Goh 2007 | No control arm (i.e. continuous/no change in PPI). |
| Gough 1996 | Did not exclude chronic NSAID use. |
| Hatlebakk 1997a | Did not exclude chronic NSAID use. |
| Hatlebakk 1997b | Did not exclude chronic NSAID use. |
| Havelund 1988 | No control arm (i.e. continuous/no change in PPI). |
| Hoshino 1995 | No control arm (i.e. continuous/no change in PPI). |
| Howden 2001 | No control arm (i.e. continuous/no change in PPI). |
| Howden 2009 | No control arm (i.e. continuous/no change in PPI). |
| Hsu 2015 | No control arm (i.e. continuous/no change in PPI). |
| Inadomi 2001 | Not an RCT. |
| Inadomi 2003 | No control arm (i.e. continuous/no change in PPI). |
| James 1994 | No control arm (i.e. continuous/no change in PPI). |
| Johnson 2001 | No control arm (i.e. continuous/no change in PPI). |
| Johnson 2010 | No control arm (i.e. continuous/no change in PPI). |
| Jones 1997 | No control arm (i.e. continuous/no change in PPI). |
| Juul‐Hansen 2008 | No control arm (i.e. continuous/no change in PPI). |
| Kaplan‐Machlis 2000 | No control arm (i.e. continuous/no change in PPI). |
| Kaspari 2005 | No control arm (i.e. continuous/no change in PPI). |
| Klinkenberg‐Knol 1987 | No control arm (i.e. continuous/no change in PPI). |
| Kovacs 2010 | No control arm (i.e. continuous/no change in PPI). |
| Labenz 2005 | No control arm (i.e. continuous/no change in PPI). |
| Lauristen 2003 | No control arm (i.e. continuous/no change in PPI). |
| Lind 1999 | No control arm (i.e. continuous/no change in PPI). |
| Lundell 1991 | No control arm (i.e. continuous/no change in PPI). |
| Metz 2003 | No control arm (i.e. continuous/no change in PPI). |
| Nagahara 2014 | No control arm (i.e. continuous/no change in PPI). |
| Niklasson 2010 | Not PPI indication. |
| Norman Hansen 2005 | No control arm (i.e. continuous/no change in PPI). |
| Peura 2009 | No control arm (i.e. continuous/no change in PPI). |
| Peura 2016 | No control arm (i.e. continuous/no change in PPI). |
| Ponce 2004 | Not an RCT. |
| Reimer 2010 | No control arm (i.e. continuous/no change in PPI). |
| Richter 2000 | No control arm (i.e. continuous/no change in PPI). |
| Richter 2004 | No control arm (i.e. continuous/no change in PPI). |
| Robinson 1995 | No control arm (i.e. continuous/no change in PPI). |
| Sandmark 1988 | No control arm (i.e. continuous/no change in PPI). |
| Scholten 2005 | No control arm (i.e. continuous/no change in PPI). |
| Sjöstedt 2005 | No control arm (i.e. continuous/no change in PPI). Both arms were deprescribing. |
| Sontag 1997 | No control arm (i.e. continuous/no change in PPI). |
| Sun 2015 | No control arm (i.e. continuous/no change in PPI). |
| Talley 2001 | No control arm (i.e. continuous/no change in PPI). |
| Talley 2002a | No control arm (i.e. continuous/no change in PPI). |
| Talley 2002b | No control arm (i.e. continuous/no change in PPI). |
| Tan 2011 | No control arm (i.e. continuous/no change in PPI). |
| Tsai 2004 | No control arm (i.e. continuous/no change in PPI). |
| Vakil 2001 | No control arm (i.e. continuous/no change in PPI). |
| Valenzuela 1991 | No control arm (i.e. continuous/no change in PPI). |
| Van Rensburg 1996 | No control arm (i.e. continuous/no change in PPI). |
| Van Rensburg 2008 | No control arm (i.e. continuous/no change in PPI). |
| Van Zyl 2000 | No control arm (i.e. continuous/no change in PPI). |
| Vantrappen 1988 | No control arm (i.e. continuous/no change in PPI). |
| Venables 1997 | No control arm (i.e. continuous/no change in PPI). |
| Vigneri 1995 | No control arm (i.e. continuous/no change in PPI). |
| Wulff 1989 | No control arm (i.e. continuous/no change in PPI). |
| Zwisler 2015 | No control arm (i.e. continuous/no change in PPI). |
NSAID: nonsteroidal anti‐inflammatory drug; PPI: proton pump inhibitor; RCT: randomized controlled trials.
Differences between protocol and review
The terminology of the primary outcome of "Inadequate symptom relief" was changed to "Lack of symptom control" to better reflect the subgroups of "treatment failure" and "inadequate symptom relief."
Several sensitivity analyses were outlined in the protocol; however, due to paucity of trials included within this review, these analyses were not completed.
Contributions of authors
Conceiving the protocol: FJR, PM, CRF, KP, VW, BF.
Designing the protocol: FJR, TB, PM, CRF, KP, VW, BF.
Coordinating the protocol: FJR, TB, WT, LP, BF.
Designing search strategies: SG, Cochrane Upper GI and Pancreatic Diseases group.
Writing the protocol: FJR, TB.
Providing general advice on the protocol: WT, VW, CRF, PM, KP, BF.
Securing funding for the protocol: BF.
Data analysis: TB, FJR, WT, VW.
Writing the systematic review: TB, FJR, WT.
Sources of support
Internal sources
Bruyere Research Institute, Canada.
External sources
Ministry of Health and Long‐Term Care, Ontario, Health Services Research Fund, Canada.
Ontario Pharmacy Research Collaboration (OPEN), Canada.
Declarations of interest
TB: none known.
FJR: none known.
VW: none known.
CRF: none known.
PM: has received funding for lectures and a research chair has been funded, in part, by an unrestricted grant from AstraZeneca to McMaster University. PM is the joint coordinating editor of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group, however editorial decisions about this review were made by the other joint coordinating editor and independent peer reviewers.
KP: none known.
WT: none known.
BF: funding for preparation (including relevant systematic review) and evaluation of an evidence‐based PPI deprescribing guideline was provided to BF's research institute by the Government of Ontario, Canada. BF has received an honorarium to present a lecture overview of the guideline at the Ontario (Canada) Long‐Term Care Physicians' annual meeting.
All members and authors of this systematic review and the affiliated PPI‐deprescribing guideline team are required to complete a standardized declaration of interest form.
Joint first author
New
References
References to studies included in this review
Bayerdörffer 2016 {published data only}
- Bayerdörffer E, Bigard MA, Weiss W, Mearin F, Rodrigo L, Munoz JED, et al. Randomized, multicenter study: on‐demand versus continuous maintenance treatment with esomeprazole in patients with non‐erosive gastroesophageal reflux disease. BMC Gastroenterology 2016;16(48):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bour 2005 {published data only}
- Bour B, Staub J‐L, Chousterman M, Labayle F, Nalet B, Nouel O, et al. Long‐term treatment of gastro‐oesophageal reflux disease patients with frequent symptomatic relapses using rabeprazole: on‐demand treatment compared with continuous treatment. Alimentary Pharmacology & Therapeutics 2005;21(7):805‐12. [DOI] [PubMed] [Google Scholar]
Janssen 2005 {published data only}
- Janssen W, Meier E, Gatz G, Pfaffenberger B. Effects of pantoprazole 20 mg in mild gastroesophageal reflux disease: once‐daily treatment in the acute phase, and comparison of on‐demand versus continuous treatment in the long term. Current Therapeutic Research, Clinical and Experimental 2005;66(4):345‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2007 {published data only}
- Morgan DG, O'Mahony MFJ, O'Mahony WF, Roy J, Camacho F, Dinniwell J, et al. Maintenance treatment of gastroesophageal reflux disease: an evaluation of continuous and on‐demand therapy with rabeprazole 20 mg. Canadian Journal of Gastroenterology 2007;21(12):820‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilotto 2003 {published data only}
- Pilotto A, Leandro G, Franceschi M, Ageing and Acid‐Related Disease Study Group. Short‐ and long‐term therapy for reflux oesophagitis in the elderly: a multi‐centre, placebo‐controlled study with pantoprazole. Alimentary Pharmacology & Therapeutics 2003;17(11):1399‐406. [DOI] [PubMed] [Google Scholar]
Van der Velden 2010 {published data only}
- Velden AW, Wit NJ, Quartero AO, Grobbee DE, Numans ME. Pharmacological dependency in chronic treatment of gastroesophageal reflux disease: a randomized controlled clinical trial. Digestion 2010;81(1):43‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Annibale 1998 {published data only}
- Annibale B, Franceschi M, Fusillo M, Beni M, Cesana B, Delle Fave G. Omeprazole in patient with mild or moderate reflux esophagitis induces lower relapse rates than ranitidine during maintenance treatment. Hepato‐Gastroenterology 1998;45(21):742‐51. [PubMed] [Google Scholar]
Archambault 1988 {published data only}
- Archambault AP, Pare P, Bailey RJ, Navert H, Williams CN, Freeman HJ. Omeprazole (20 mg daily) versus cimetidine (1200 mg daily) in duodenal ulcer healing and pain relief. Gastroenterology 1988;94(5 Pt 1):1130‐4. [DOI] [PubMed] [Google Scholar]
Bardhan 1990 {published data only}
- Bardhan KD. Omeprazole or ranitidine for reflux oesophagitis: comment. National Medical Journal of India 1990;3(1):20‐1. [PubMed] [Google Scholar]
Bardhan 1995 {published data only}
- Bardhan KD, Hawkey CJ, Long RG, Morgan AG, Wormsley KG, Moules IK, et al. Lansoprazole versus ranitidine for the treatment of reflux oesophagitis. Alimentary Pharmacology & Therapeutics 1995;9(2):145‐51. [DOI] [PubMed] [Google Scholar]
Bate 1989 {published data only}
- Bate CM, Wilkinson SP, Bradby GV, Bateson MC, Hislop WS, Crowe JP, et al. Randomized, double blind comparison of omeprazole and cimetidine in the treatment of symptomatic gastric ulcer. Gut 1989;30(10):1323‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bate 1990 {published data only}
- Bate CM, Keeling PW, O'Morain C, Wilkinson SP, Foster DN, Mountford RA, et al. Comparison of omeprazole and cimetidine in reflux oesophagitis: symptomatic, endoscopic and histological evaluations. Gut 1990;31(9):968‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bate 1995 {published data only}
- Bate CM, Booth SN, Crowe JP, Mountford RA, Keeling PW, Hepworth‐Jones B, et al. Omeprazole 10 mg or 20 mg once daily in the prevention of recurrence of reflux oesophagitis. Gut 1995;36(4):492‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bate 1997 {published data only}
- Bate CM, Green JR, Axon AT, Murray FE, Tildesley G, Emmas CE, et al. Omeprazole is more effective than cimetidine for the relief of all grades of gastro‐oesophageal reflux disease‐associated heartburn, irrespective of the presence or absence of endoscopic oesophagitis. Alimentary Pharmacology & Therapeutics 1997;11(4):755‐63. [DOI] [PubMed] [Google Scholar]
Bautista 2006 {published data only}
- Bautista JM. The rising trend of on‐demand and intermittent proton pump inhibitor therapy in gastroesophageal reflux disease: what is the evidence?. Journal of Gastroenterology and Hepatology 2006;21(Suppl 5):S112‐4. [Google Scholar]
Bayerdörffer 1993 {published data only}
- Bayerdörffer E, Mannes GA, Sommer A, Höchter W, Weingart J, Hatz R, et al. Long‐term follow‐up after eradication of Helicobacter pylori with a combination of omeprazole and amoxycillin. Scandinavian Journal of Gastroenterology 1992;28(196):19‐25. [DOI] [PubMed] [Google Scholar]
Bigard 2005 {published data only}
- Bigard MA, Genestin E. Treatment of patients with heartburn without endoscopic evaluation: on‐demand treatment after effective continuous administration of lansoprazole 15 mg. Alimentary Pharmacology & Therapeutics 2005;22(7):635‐43. [DOI] [PubMed] [Google Scholar]
Björnsson 2006 {published data only}
- Björnsson E, Abrahamsson H, Simrén M, Mattsson N, Jensen C, Agerforz P, et al. Discontinuation of proton pump inhibitors in patients on long‐term therapy: a double‐blind, placebo‐controlled trial. Alimentary Pharmacology & Therapeutics 2006;24(6):945‐54. [DOI] [PubMed] [Google Scholar]
Bytzer 2004 {published data only}
- Bytzer P, Blum A, Herdt D, Dubois D. Six‐month trial of on‐demand rabeprazole 10 mg maintains symptom relief in patients with non‐erosive reflux disease. Alimentary Pharmacology & Therapeutics 2004;20(2):181‐8. [DOI] [PubMed] [Google Scholar]
Carlsson 1998 {published data only}
- Carlsson R, Dent J, Watts R, Riley S, Sheikh R, Hatlebakk J, et al. Gastro‐oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. European Journal of Gastroenterology & Hepatology 1998;10(2):119‐24. [PubMed] [Google Scholar]
Chen 1993 {published data only}
- Chen SP, Bei L, Wen SH. Study on omeprazole 20 mg twice weekly in prevention of duodenal ulcer relapse. Zhonghua Nei Ke za Zhi [Chinese Journal of Internal Medicine] 1993;32(8):538‐41. [PubMed] [Google Scholar]
Dabholkar 2011 {published data only}
- Dabholkar AH, Han C, Paris MM, Perez MC, Atkinson SN, Peura DA. The 12‐month safety profile of dexlansoprazole, a proton pump inhibitor with a dual delayed release formulation, in patients with gastro‐oesophageal reflux disease. Alimentary Pharmacology & Therapeutics 2011;33(3):366‐77. [DOI] [PubMed] [Google Scholar]
Deboever 2003 {published data only}
- Deboever G, Wagner C, Bourgeois N, Muls V, Belaiche J, Azerad M‐A, et al. Lansoprazole 15 mg OD is superior to ranitidine 300 mg OD in relieving heartburn and healing patients with (endoscopically confirmed) reflux oesophagitis grade I [Le lansoprazole 15 mg OD est superieur a la ranitidine 300 mg OD dans le soulagement du pyrosis et pour guerison des patient souffrant d'oesophagite de reflux de grade I (confirme par endoscopie)]. Acta Endoscopica 2003;33(4):573‐85. [Google Scholar]
Dent 1989 {published data only}
- Dent J, Hetzel DJ, Mackinnon MA, Reed WD, Narielvala FM. Evaluation of omeprazole in reflux oesophagitis. Scandinavian Journal of Gastroenterology 1989;24(166):76‐82. [DOI] [PubMed] [Google Scholar]
Dent 1994 {published data only}
- Dent J, Yeomans ND, Mackinnon M, Reed W, Narielvala FM, Hetzel DJ, et al. Omeprazole v ranitidine for prevention of relapse in reflux oesophagitis. A controlled double blind trial of their efficacy and safety. Gut 1994;35(5):590‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeVault 2006 {published data only}
- DeVault KR, Johanson JF, Johnson DA, Liu S, Sostek MB. Maintenance of healed erosive esophagitis: a randomized six‐month comparison of esomeprazole twenty milligrams with lansoprazole fifteen milligrams. Clinical Gastroenterology and Hepatology 2006;4(7):852‐9. [DOI] [PubMed] [Google Scholar]
Earnest 1998 {published data only}
- Earnest DL, Dorsch E, Jones J, Jennings DE, Greski‐Rose PA. A placebo‐controlled dose‐ranging study of lansoprazole in the management of reflux esophagitis. American Journal of Gastroenterology 1998;93(2):238‐43. [DOI] [PubMed] [Google Scholar]
Eggleston 2009 {published data only}
- Eggleston A, Katelaris PH, Nandurkar S, Thorpe P, Holtmann G. Clinical trial: the treatment of gastro‐oesophageal reflux disease in primary care ‐ prospective randomized comparison of rabeprazole 20 mg with esomeprazole 20 and 40 mg. Alimentary Pharmacology & Therapeutics 2009;29(9):967‐78. [DOI] [PubMed] [Google Scholar]
Escourrou 1999 {published data only}
- Escourrou J, Deprez P, Saggioro A, Geldof H, Fischer R, Maier C. Maintenance therapy with pantoprazole 20 mg prevents relapse of reflux oesophagitis. Alimentary Pharmacology & Therapeutics 1999;13(11):1481‐91. [DOI] [PubMed] [Google Scholar]
Farley 2000 {published data only}
- Farley A, Wruble LD, Humphries TJ. Rabeprazole versus ranitidine for the treatment of erosive gastroesophageal reflux disease: a double‐blind, randomized clinical trial. American Journal of Gastroenterology 2000;95(8):1894‐9. [DOI] [PubMed] [Google Scholar]
Fass 2011 {published data only}
- Fass R, Johnson DA, Orr WC, Han C, Mody R, Stern KN, et al. The effect of dexlansoprazole MR on nocturnal heartburn and GERD‐related sleep disturbances in patients with symptomatic GERD. American Journal of Gastroenterology 2011;106(3):421‐31. [DOI] [PubMed] [Google Scholar]
Fass 2012 {published data only}
- Fass R, Inadomi J, Han C, Mody R, O'Neil J, Perez MC. Maintenance of heartburn relief after step‐down from twice‐daily proton pump inhibitor to once‐daily dexlansoprazole modified release. Clinical Gastroenterology and Hepatology 2012;10(3):247‐53. [DOI] [PubMed] [Google Scholar]
Fennerty 2009 {published data only}
- Fennerty MB, Peura DA, Riff DS, Snoddy AM. Clinical trial: lansoprazole 15 or 30 mg once daily vs. placebo for treatment of frequent nighttime heartburn in self‐treating subjects. Alimentary Pharmacology & Therapeutics 2009;30(5):459‐68. [DOI] [PubMed] [Google Scholar]
Goh 1995 {published data only}
- Goh KL, Boonyapisit S, Lai KH, Chang R, Kang JY, Lam SK. Prevention of duodenal ulcer relapse with omeprazole 20 mg daily: a randomized double‐blind, placebo‐controlled study. Journal of Gastroenterology and Hepatology 1995;10(1):92‐7. [DOI] [PubMed] [Google Scholar]
Goh 2007 {published data only}
- Goh KL, Benamouzig R, Sander P, Schwan T. Efficacy of pantoprazole 20mg daily compared with esomeprazole 20mg daily in the maintenance of healed gastroesophageal reflux disease: a randomized, double‐blind comparative trial ‐ the EMANCIPATE study. European Journal of Gastroenterology & Hepatology 2007;19(3):205‐11. [DOI] [PubMed] [Google Scholar]
Gough 1996 {published data only}
- Gough AL, Long RG, Cooper BT, Fosters CS, Garrett AD, Langworthy CH. Lansoprazole versus ranitidine in the maintenance treatment reflux oesophagitis. Alimentary Pharmacology & Therapeutics 1996;10(4):529‐39. [DOI] [PubMed] [Google Scholar]
Hatlebakk 1997a {published data only}
- Hatlebakk JG, Berstad A. Lansoprazole 15 and 30 mg daily in maintaining healing and symptom relief in patients with reflux oesophagitis. Alimentary Pharmacology & Therapeutics 1997;11(2):365‐72. [DOI] [PubMed] [Google Scholar]
Hatlebakk 1997b {published data only}
- Hatlebakk JG, Berstad A. Prognostic factors for relapse of reflux oesophagitis and symptoms during 12 months of therapy with lansoprazole. Alimentary Pharmacology & Therapeutics 1997;11(6):1093‐9. [DOI] [PubMed] [Google Scholar]
Havelund 1988 {published data only}
- Havelund T, Laursen LS, Skoubo‐Kristensen E, Andersen BN, Pedersen SA, Jensen KB, et al. Omeprazole and ranitidine in treatment of reflux oesophagitis: double blind comparative trial. BMJ 1988;296(6515):89‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoshino 1995 {published data only}
- Hoshino E, Umeda N, Sano J, Miki K, Yahagi N, Oka M, et al. Lansoprazole for maintenance therapy of peptic ulcer disease: weekend full‐dose or everyday half‐dose administration?. Journal of Clinical Gastroenterology 1995;20(Suppl 2):S72‐4. [DOI] [PubMed] [Google Scholar]
Howden 2001 {published data only}
- Howden CW, Henning JM, Huang B, Lukasik N, Freston JW. Management of heartburn in a large, randomized, community‐based study: comparison of four therapeutic strategies. American Journal of Gastroenterology 2001;96(6):1704‐10. [DOI] [PubMed] [Google Scholar]
Howden 2009 {published data only}
- Howden CW, Larsen LM, Perez MC, Palmer R, Atkinson SN. Clinical trial: efficacy and safety of dexlansoprazole MR 60 and 90 mg in healed erosive oesophagitis ‐ maintenance of healing and symptom relief. Alimentary Pharmacology & Therapeutics 2009;30(9):895‐907. [DOI] [PubMed] [Google Scholar]
Hsu 2015 {published data only}
- Hsu PI, Lu CL, Wu DC, Kuo CH, Kao SS, Chang CC, et al. Eight weeks of esomeprazole therapy reduces symptom relapse, compared with 4 weeks, in patients with Los Angeles grade A or B erosive esophagitis. Clinical Gastroenterology and Hepatology 2015;13(5):859‐66. [DOI] [PubMed] [Google Scholar]
Inadomi 2001 {published data only}
- Inadomi JM, Jamal R, Murata GH, Hoffman RM, Lavezo LA, Vigil JM, et al. Step‐down management of gastroesophageal reflux disease. Gastroenterology 2001;121(5):1095‐100. [DOI] [PubMed] [Google Scholar]
Inadomi 2003 {published data only}
- Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step‐down from multiple‐ to single‐dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPI. American Journal of Gastroenterology 2003;98(9):1940‐4. [DOI] [PubMed] [Google Scholar]
James 1994 {published data only}
- James OF, Parry‐Billings KS. Comparison of omeprazole and histamine H2‐receptor antagonists in the treatment of elderly and young patients with reflux oesophagitis. Age and Ageing 1994;23(2):121‐6. [DOI] [PubMed] [Google Scholar]
Johnson 2001 {published data only}
- Johnson DA, Benjamin SB, Vakil NB, Goldstein JL, Lamet M, Whipple J, et al. Esomeprazole once daily for 6 months Is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double‐blind, placebo‐controlled study of efficacy and safety. American Journal of Gastroenterology 2001;96(1):27‐34. [DOI] [PubMed] [Google Scholar]
Johnson 2010 {published data only}
- Johnson D, Crawley JA, Hwang C, Krown B. Clinical trial: esomeprazole for moderate‐to‐severe nighttime heartburn and gastro‐oesophageal reflux disease‐related sleep disturbances. Alimentary Pharmacology & Therapeutics 2010;32(2):182‐90. [DOI] [PubMed] [Google Scholar]
Jones 1997 {published data only}
- Jones RH, Baxter G. Lansoprazole 30 mg daily versus ranitidine 150 mg b.d. in the treatment of acid‐related dyspepsia in general practice. Alimentary Pharmacology & Therapeutics 1997;11(3):541‐6. [DOI] [PubMed] [Google Scholar]
Juul‐Hansen 2008 {published data only}
- Juul‐Hansen P, Rydning A. On‐demand requirements of patients with endoscopy‐negative gastro‐oesophageal reflux disease: H2‐blocker vs. proton pump inhibitor. Alimentary Pharmacology & Therapeutics 2008;29(2):207‐12. [DOI] [PubMed] [Google Scholar]
Kaplan‐Machlis 2000 {published data only}
- Kaplan‐Machlis B, Spiegler GE, Zodet MW, Revicki DA. Effectiveness and costs of omeprazole vs ranitidine for treatment of symptomatic gastroesophageal reflux disease in primary care clinics in West Virginia. Archives of Family Medicine 2000;9(7):624‐30. [DOI] [PubMed] [Google Scholar]
Kaspari 2005 {published data only}
- Kaspari S, Kupcinskas L, Heinze H, Berghöfer P. Pantoprazole 20 mg on demand is effective in the long‐term management of patients with mild gastro‐oesophageal reflux disease. European Journal of Gastroenterology & Hepatology 2005;17(9):935‐41. [DOI] [PubMed] [Google Scholar]
Klinkenberg‐Knol 1987 {published data only}
- Klinkenberg‐Knol EC, Jansen JM, Festen HP, Meuwissen SG, Lamers CB. Double‐blind multicentre comparison of omeprazole and ranitidine in the treatment of reflux oesophagitis. Lancet 1987;1(8529):349‐51. [DOI] [PubMed] [Google Scholar]
Kovacs 2010 {published data only}
- Kovacs TO, Freston JW, Haber MM, Atkinson S, Hunt B, Peura DA. Long‐term quality of life improvement in subjects with healed erosive esophagitis: treatment with lansoprazole. Digestive Diseases and Sciences 2010;55(5):1325‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Labenz 2005 {published data only}
- Labenz J, Armstrong D, Lauritsen K, Katelaris P, Schmidt S, Schütze K, et al. Esomeprazole 20 mg vs. pantoprazole 20 mg for maintenance therapy of healed erosive oesophagitis: results from the EXPO study. Alimentary Pharmacology & Therapeutics 2005;22(9):803‐11. [DOI] [PubMed] [Google Scholar]
Lauristen 2003 {published data only}
- Lauritsen K, Devière J, Bigard MA, Bayerdörffer E, Mózsik G, Murray F, et al. Esomeprazole 20 mg and lansoprazole 15 mg in maintaining healed reflux oesophagitis: Metropole study results. Alimentary Pharmacology & Therapeutics 2003;17(3):331‐41. [DOI] [PubMed] [Google Scholar]
Lind 1999 {published data only}
- Lind T, Havelund T, Lundell L, Glise H, Lauritsen K, Pedersen SA, et al. On demand therapy with omeprazole for the long‐term management of patients with heartburn without oesophagitis ‐ a placebo‐controlled randomized trial. Alimentary Pharmacology & Therapeutics 1999;13(7):907‐14. [DOI] [PubMed] [Google Scholar]
Lundell 1991 {published data only}
- Lundell L, Backman L, Ekström P, Enander LK, Falkmer S, Fausa O, et al. Prevention of relapse of reflux esophagitis after endoscopic healing: the efficacy and safety of omeprazole compared with ranitidine. Scandinavian Journal of Gastroenterology 1991;26(3):248‐56. [DOI] [PubMed] [Google Scholar]
Metz 2003 {published data only}
- Metz DC, Bochenek WJ. Pantoprazole maintenance therapy prevents relapse of erosive oesophagitis. Alimentary Pharmacology & Therapeutics 2003;17(1):155‐64. [DOI] [PubMed] [Google Scholar]
Nagahara 2014 {published data only}
- Nagahara A, Hojo M, Asaoka D, Sasaki H, Watanabe S. A randomized prospective study comparing the efficacy of on‐demand therapy versus continuous therapy for 6 months for long‐term maintenance with omeprazole 20 mg in patients with gastroesophageal reflux disease in Japan. Scandinavian Journal of Gastroenterology 2014;49(4):409‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Niklasson 2010 {published data only}
- Niklasson A, Lindström L, Simrén M, Lindberg G, Björnsson E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: a double‐blind placebo‐controlled trial. American Journal of Gastroenterology 2010;105(7):1531‐7. [DOI] [PubMed] [Google Scholar]
Norman Hansen 2005 {published data only}
- Norman Hansen A, Bergheim R, Fagertun H, Lund H, Moum B. A randomised prospective study comparing the effectiveness of esomeprazole treatment strategies in clinical practice for 6 months in the management of patients with symptoms of gastroesophageal reflux disease. International Journal of Clinical Practice 2005;59(6):665‐71. [DOI] [PubMed] [Google Scholar]
Peura 2009 {published data only}
- Peura DA, Riff DS, Snoddy AM, Fennerty MB. Clinical trial: lansoprazole 15 or 30 mg once daily vs. placebo for treatment of frequent nighttime heartburn in self‐treating subjects. Alimentary Pharmacology & Therapeutics 2009;30(5):459‐68. [DOI] [PubMed] [Google Scholar]
Peura 2016 {published data only}
- Peura D, Moigne A, Pollack C, Nagy P, Lind T. A 14‐day regimen of esomeprazole 20 mg/day for frequent heartburn: durability of effects, symptomatic rebound, and treatment satisfaction. Postgraduate Medical Journal 2016;128(6):577‐83. [DOI] [PubMed] [Google Scholar]
Ponce 2004 {published data only}
- Ponce J, Argüello L, Bastida G, Ponce M, Ortiz V, Garrigues V. On‐demand therapy with rabeprazole in nonerosive and erosive gastroesophageal reflux disease in clinical practice: effectiveness, health‐related quality of life, and patient satisfaction. Digestive Diseases and Sciences 2004;49(6):931‐6. [DOI] [PubMed] [Google Scholar]
Reimer 2010 {published data only}
- Reimer C, Bytzer P. Discontinuation of long‐term proton pump inhibitor therapy in primary care patients: a randomized placebo‐controlled trial in patients with symptom relapse. European Journal of Gastroenterology & Hepatology 2010;22(10):1182‐8. [DOI] [PubMed] [Google Scholar]
Richter 2000 {published data only}
- Richter JE, Bochenek W. Oral pantoprazole for erosive esophagitis: a placebo‐controlled, randomized clinical trial. American Journal of Gastroenterology 2000;95(11):3071‐80. [DOI] [PubMed] [Google Scholar]
Richter 2004 {published data only}
- Richter JE, Fraga P, Mack M, Sabesin SM, Bochenek W. Prevention of erosive oesophagitis relapse with pantoprazole. Alimentary Pharmacology & Therapeutics 2004;20(5):567‐75. [DOI] [PubMed] [Google Scholar]
Robinson 1995 {published data only}
- Robinson M, Sahba B, Avner D, Jhala N, Greski‐Rose PA, Jennings DE. A comparison of lansoprazole and ranitidine in the treatment of erosive oesophagitis. Alimentary Pharmacology & Therapeutics 1995;9(1):25‐30. [DOI] [PubMed] [Google Scholar]
Sandmark 1988 {published data only}
- Sandmark S, Carlsson R, Fausa O, Lundell L. Omeprazole or ranitidine in the treatment of reflux esophagitis. Results of a double‐blind, randomized, Scandinavian multicenter study. Scandinavian Journal of Gastroenterology 1988;23(5):625‐32. [DOI] [PubMed] [Google Scholar]
Scholten 2005 {published data only}
- Scholten T, Dekkers CP, Schütze K, Körner T, Bohuschke M, Gatz G. On‐demand therapy with pantoprazole 20 mg as effective long‐term management of reflux disease in patients with mild GERD: the ORION trial. Digestion 2005;72(2‐3):76‐85. [DOI] [PubMed] [Google Scholar]
Sjöstedt 2005 {published data only}
- Sjöstedt S, Befrits R, Sylvan A, Harthon C, Jörgensen L, Carling L, et al. Daily treatment with esomeprazole is superior to that taken on‐demand for maintenance of healed erosive oesophagitis. Alimentary Pharmacology & Therapeutics 2005;22(3):183‐91. [DOI] [PubMed] [Google Scholar]
Sontag 1997 {published data only}
- Sontag SJ, Robinson M, Roufail W, Hirschowitz BI, Sabesin SM, Wu WC, et al. Daily omeprazole surpasses intermittent dosing in preventing relapse of oesophagitis: a US multi‐centre double‐blind study. Alimentary Pharmacology & Therapeutics 1997;11(2):373‐80. [DOI] [PubMed] [Google Scholar]
Sun 2015 {published data only}
- Sun J, Yuan YZ, Hou XH, Zou DW, Lu B, Chen MH, et al. Esomeprazole regimens for reflux symptoms in Chinese patients with chronic gastritis. World Journal of Gastroenterology 2015;21(22):6965‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talley 2001 {published data only}
- Talley NJ, Lauritsen K, Tunturi‐Hihnala H, Lind T, Moum B, Bang C, et al. Esomeprazole 20 mg maintains symptom control in endoscopy‐negative gastro‐oesophageal reflux disease: a controlled trial of 'on‐demand' therapy for 6 months. Alimentary Pharmacology & Therapeutics 2001;15(3):347‐54. [DOI] [PubMed] [Google Scholar]
Talley 2002a {published data only}
- Talley N, Moore M, Sprogis A, Katelaris P. Randomised controlled trial of pantoprazole versus ranitidine for the treatment of uninvestigated heartburn in primary care. Medical Journal of Australia 2002;117:415‐9. [DOI] [PubMed] [Google Scholar]
Talley 2002b {published data only}
- Talley NJ, Venables TL, Green JR, Armstrong D, O'Kane KP, Giaffer M, et al. Esomeprazole 40 mg and 20 mg is efficacious in the long term management of patients with endoscopy‐negative gastro‐oesophageal reflux disease: a placebo‐controlled trial of on‐demand therapy for 6 months. European Journal of Gastroenterology & Hepatology 2002;14(8):857‐63. [DOI] [PubMed] [Google Scholar]
Tan 2011 {published data only}
- Tan VP, Wong WM, Cheung TK, Lai KC, Hung IF, Chan P, et al. Treatment of non‐erosive reflux disease with a proton pump inhibitor in Chinese patients: a randomized controlled trial. Journal of Gastroenterology 2011;46(7):906‐12. [DOI] [PubMed] [Google Scholar]
Tsai 2004 {published data only}
- Tsai HH, Chapman R, Shepherd A, McKeith D, Anderson M, Vearer D, et al. Esomeprazole 20 mg on‐demand is more acceptable to patients than continuous lansoprazole 15 mg in the long‐term maintenance of endoscopy‐negative gastro‐oesophageal reflux patients: the COMMAND Study. Alimentary Pharmacology & Therapeutics 2004;20(6):657‐65. [DOI] [PubMed] [Google Scholar]
Vakil 2001 {published data only}
- Vakil NB, Shaker R, Johnson DA, Kovacs T, Baerg RD, Hwang C, et al. The new proton pump inhibitor esomeprazole is effective as a maintenance therapy in GERD patients with healed erosive oesophagitis: a 6‐month, randomized, double‐blind, placebo‐controlled study of efficacy and safety. Alimentary Pharmacology & Therapeutics 2001;15:927‐35. [DOI] [PubMed] [Google Scholar]
Valenzuela 1991 {published data only}
- Valenzuela J, Berlin R, Snape W, Johnson T, Hirschowitz B, Colon‐Pagan J, et al. U.S. experience with omeprazole in duodenal ulcer‐multicenter double‐blind comparative study with ranitidine. Digestive Diseases and Sciences 1991;36(6):761‐8. [DOI] [PubMed] [Google Scholar]
Van Rensburg 1996 {published data only}
- Rensburg CJ, Honiball PJ, Grundling HD, Zyl JH, Spies SK, Eloff FP, et al. Efficacy and tolerability of pantoprazole 40 mg versus 80 mg in patients with reflux esophagitis. Alimentary Pharmacology & Therapeutics 1996;10(3):397‐401. [DOI] [PubMed] [Google Scholar]
Van Rensburg 2008 {published data only}
- Rensburg C, Berghöfer P, Enns R, Dattani ID, Maritz JF, Gonzalez Carro P, et al. Efficacy and safety of pantoprazole 20 mg once daily treatment in patients with ulcer‐like functional dyspepsia. Current Medical Research and Opinion 2008;24(7):2009‐18. [DOI] [PubMed] [Google Scholar]
Vantrappen 1988 {published data only}
- Vantrappen G, Rutgeerts L, Schurmans P, Coenegrachts JL. Omeprazole (40 mg) is superior to ranitidine in short‐term treatment of ulcerative reflux esophagitis. Digestive Diseases and Sciences 1988;33(5):523‐9. [DOI] [PubMed] [Google Scholar]
Van Zyl 2000 {published data only}
- Zyl JH, K Grundling H, Rensburg CJ, Retief FJ, O'Keefe SJ, Theron I, et al. Efficacy and tolerability of 20 mg pantoprazole versus 300 mg ranitidine in patients with mild reflux‐oesophagitis: a randomized, double‐blind, parallel, and multicentre study. European Journal of Gastroenterology & Hepatology 2000;12(2):197‐202. [DOI] [PubMed] [Google Scholar]
Venables 1997 {published data only}
- Venables T, Newland R, Patel A, Hole J, Wilcock C, Turbitt M. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro‐oesophageal reflux disease in general practice. Scandinavian Journal of Gastroenterology 1997;32:965‐73. [DOI] [PubMed] [Google Scholar]
Vigneri 1995 {published data only}
- Vigneri S, Termini R, Leandro G, Badalamenti S, Pantalena M, Savarino V, et al. A comparison of five maintenance therapies for reflux esophagitis. New England Journal of Medicine 1995;333(17):1106‐10. [DOI] [PubMed] [Google Scholar]
Wulff 1989 {published data only}
- Danish Omeprazole Study Group. Omeprazole and cimetidine in the treatment of ulcers of the body of the stomach: a double blind comparative trial. BMJ 1989;298(6674):645‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwisler 2015 {published data only}
- Zwisler JE, Jarbol DE, Lassen AT, Kragstrup J, Thorsgaard N, Schaffalitzky de Muckadell OB. Placebo‐controlled discontinuation of long‐term acid‐suppressant therapy: a randomised trial in general practice. International Journal of Family Medicine 2015; Vol. 2015:175436. [DOI: 10.1155/2015/175436] [DOI] [PMC free article] [PubMed]
Additional references
ACPG 2000
- Alberta Clinical Practice Guideline Working Group. Guideline for diagnosis and treatment of chronic undiagnosed dyspepsia in adults. The Alberta Clinical Guideline Program 2000; Vol. June:1‐10.
Ament 2012
- Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. American Family Physician 2012;86(1):66‐70. [PubMed] [Google Scholar]
Anthierens 2010
- Anthierens S, Tansens A, Petrovic M, Christiaens T. Qualitative insights into general practitioners views on polypharmacy. BMC Family Practice 2010;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armstrong 1999
- Armstrong TA, Coursin DB, Devlin J, Duke JS, Fish D, Gonzalez ER, et al. ASHP therapeutic guidelines on stress ulcer prophylaxis. American Journal of Health‐System Pharmacy. United States: American Society of Health‐Systems Pharmacy, 1999; Vol. 56, issue 4:347‐79.
Armstrong 2005
- Armstrong D, Marshall JK, Chiba N, Enns R, Fallone CA, Fass R, et al. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults ‐ update 2004. Canadian Journal of Gastroenterology 2005;19(1):15‐35. [DOI] [PubMed] [Google Scholar]
CADTH 2007
- Canadian Agency for Drugs and Technologies in Health. Economic models and conclusions for the treatment of dyspepsia; and gastroesophageal reflux disease‐related heartburn and the prevention of non‐steroidal anti‐inflammatory drug‐induced gastrointestinal complications, 2007. www.cadth.ca/sites/default/files/compus/pdf/compus_economic_report_april‐07_e.pdf (accessed 9 November 2015).
CDHF 2015
- Canadian Digestive Health Foundation. GERD, 2015. www.cdhf.ca/en/statistics (accessed 9 November 2015).
CHN 2013
- Canadian Healthcare Network. Top 100 Drugs. Pharmacy Practice 2013, issue March:1‐25.
Chubineh 2012
- Chubineh S, Birk J. Proton pump inhibitors: the good, the bad, and the unwanted. Southern Medical Journal 2012;105(11):613‐8. [DOI] [PubMed] [Google Scholar]
Claudene 2008
- Claudene GJ, Beatriz K, Joseph RS. Appropriate proton pump inhibitor use among older adults: a retrospective chart review. American Journal of Geriatric Pharmacotherapy 2008;6(5):249‐54. [DOI] [PubMed] [Google Scholar]
Dangler 2013
- Dangler M, Ochs L, White R. Assessing the appropriate use of proton pump inhibitors in a veteran outpatient population. Federal Practitioner 2013;30(5):21‐5. [Google Scholar]
Dent 2005
- Dent J, El‐Serag HB, Wallander MA, Johansson S. Epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut 2005;54(5):710‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dersimonian 1986
- Dersimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Donnellan 2004
Fohl 2011
- Fohl AL, Regal RE. Proton pump inhibitor‐associated pneumonia: not a breath of fresh air after all?. World Journal of Gastrointestinal Pharmacology and Therapeutics 2011;2(3):17‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forgacs 2008
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ (Clinical Research Ed.) 2008;336(7634):2‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadzhanova 2012
- Gadzhanova SV, Roughead EE, Mackson JM. Initiation and duration of proton pump inhibitors in the Australian veteran population. Internal Medicine Journal 2012;42(5):e68‐73. [DOI] [PubMed] [Google Scholar]
GESA 2011
- Gastroenterological Society of Australia. Gastro‐oesophageal Reflux Disease in Adults. 5th Edition. Mulgrave, Australia: Digestive Health Foundation, 2011:10. [Google Scholar]
Gisbert 2004
- Gisbert JP, Khorrami S, Carballo F, Calvet X, Gene E, Dominguez‐Munoz E. Meta‐analysis: Helicobacter pylori eradication therapy vs. antisecretory non‐eradication therapy for the prevention of recurrent bleeding from peptic ulcer. Alimentary Pharmacology & Therapeutics 2004;19(6):617‐29. [DOI] [PubMed] [Google Scholar]
Gnjidic 2012
- Gnjidic D, Couteur DG, Kouladjian L, Hilmer SN. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clinics in Geriatric Medicine 2012;28(2):237‐53. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- GRADE Working Group, McMaster University. GRADEpro. Version accessed 7 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Graves 1997
- Graves T, Hanlon JT, Schmader KE, Landsman PB, Samsa GP, Pieper CF, et al. Adverse events after discontinuing medications in elderly outpatients. Archives of Internal Medicine 1997;157(19):2205‐10. [PubMed] [Google Scholar]
Grime 2001
- Grime J, Pollock K, Blenkinsopp A. Proton pump inhibitors: perspectives of patients and their GPs. British Journal of General Practice 2001;51(470):703‐11. [PMC free article] [PubMed] [Google Scholar]
Haastrup 2014
- Haastrup P, Paulsen M, Begtrup L, Hansen J, Jarbol D. Strategies for discontinuation of proton pump inhibitors: a systematic review. Family Practice 2014;31(6):625‐30. [DOI] [PubMed] [Google Scholar]
Heidelbaugh 2010
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. American Journal of Managed Care 2010;16(9):e228‐34. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hilmer 2009
- Hilmer SN, Gnjidic D. The effects of polypharmacy in older adults. Clinical Pharmacology and Therapeutics 2009;85(1):86‐8. [DOI] [PubMed] [Google Scholar]
Iyer 2008
Jiang 2013
- Jiang Y‐X, Chen Y, Kong X, Tong Y‐L, Xu S‐C. Maintenance treatment of mild gastroesophageal reflux disease with proton pump inhibitors taken on‐demand: a meta‐analysis. Hepato‐gastroenterology 2013;60(125):1077‐82. [DOI] [PubMed] [Google Scholar]
Katz 2013
Laine 2012
- Laine L, Jensen DM. Management of patients with ulcer bleeding. American Journal of Gastroenterology 2012;107(3):345‐61. [DOI] [PubMed] [Google Scholar]
Lee 2004
- Lee TJ, Fennerty MB, Howden CW. Systematic review: is there excessive use of proton pump inhibitors in gastro‐oesophageal reflux disease?. Alimentary Pharmacology & Therapeutics 2004;20(11‐12):1241‐51. [DOI] [PubMed] [Google Scholar]
Leri 2013
- Leri F, Ayzenberg M, Voyce SJ, Klein A, Hartz L, Smego RA. Four‐year trends of inappropriate proton pump inhibitor use after hospital discharge. Southern Medical Association 2013;106(4):270‐3. [DOI] [PubMed] [Google Scholar]
Linsky 2013
- Linsky A, Simon SR. Reversing gears: discontinuing medication therapy to prevent adverse events. JAMA Internal Medicine 2013;173(7):524‐5. [DOI] [PubMed] [Google Scholar]
Lundell 1999
- Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45(2):172‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malfertheiner 2009
- Malfertheiner P, Chan Francis KL, McColl Kenneth EL. Peptic ulcer disease. Lancet 2009;374(9699):1449‐61. [DOI] [PubMed] [Google Scholar]
Molloy 2010
- Molloy D, Molloy A, O'Loughlin C, Falconer M, Hennessy M. Inappropriate use of proton pump inhibitors. Irish Journal of Medical Science 2010;179(1):73‐5. [DOI] [PubMed] [Google Scholar]
Murphy 2008
- Murphy CE, Stevens AM, Ferrentino N, Crookes BA, Hebert JC, Freiburg CB, et al. Frequency of inappropriate continuation of acid suppressive therapy after discharge in patients who began therapy in the surgical intensive care unit. Pharmacotherapy 2008;28(8):968‐76. [DOI] [PubMed] [Google Scholar]
Pace 2005
- Pace F, Negrini C, Wiklund I, Rossi C, Savarino V. Quality of life in acute and maintenance treatment of non‐erosive and mild erosive gastro‐oesophageal reflux disease. Alimentary Pharmacology & Therapeutics 2005;22:349‐56. [DOI] [PubMed] [Google Scholar]
Pace 2008
- Pace F, Bianchi Porro G. On‐demand PPI therapy in GERD. Current Treatment Options in Gastroenterology 2008;11:35‐42. [DOI] [PubMed] [Google Scholar]
Raghunath 2005
- Raghunath AS, Hungin APS, Cornford CS, Featherstone V. Use of proton pump inhibitors: an exploration of the attitudes, knowledge and perceptions of general practitioners. Digestion 2005;72(4):212‐8. [DOI] [PubMed] [Google Scholar]
Reeve 2012
- Reeve E, Wiese MD. Difficulties reducing inappropriate prescribing of proton pump inhibitors in the elderly. Drugs & Aging 2012;29(11):925‐8. [DOI] [PubMed] [Google Scholar]
Reeve 2013
- Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs & Aging 2013;30(10):793‐807. [DOI] [PubMed] [Google Scholar]
Reeve 2015
- Reeve E, Andrews JM, Wiese MD, Hendrix I, Roberts MS, Shakib S. Feasibility of a patient‐centered deprescribing process to reduce inappropriate use of proton pump inhibitors. Annals of Pharmacotherapy 2015;49(1):29‐38. [DOI] [PubMed] [Google Scholar]
Reimer 2009a
- Reimer C, Bytzer P. Clinical trial: long‐term use of proton pump inhibitors in primary care patients ‐ a cross sectional analysis of 901 patients. Alimentary Pharmacology & Therapeutics 2009;30(7):725‐32. [DOI] [PubMed] [Google Scholar]
Reimer 2009b
- Reimer C, Sondergaard B, Hilsted L, Bytzer P. Proton‐pump inhibitor therapy induces acid‐related symptoms in health volunteers after withdrawal of therapy. Gastroenterology 2009;137(1):80‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Scott 2013
- Scott IA, Gray LC, Martin JH, Pillans PI, Mitchell CA. Deciding when to stop: towards evidence‐based deprescribing of drugs in older populations. Evidence‐Based Medicine 2013;18(4):121‐4. [DOI] [PubMed] [Google Scholar]
Spirt 2006
- Spirt MJ, Stanley S. Update on stress ulcer prophylaxis in critically ill patients. Critical Care Nurse 2006;26(1):18‐29. [PubMed] [Google Scholar]
Talley 2005
- Talley NJ, Vakil N. Guidelines for the management of dyspepsia. American Journal of Gastroenterology 2005;100(10):2324‐37. [DOI] [PubMed] [Google Scholar]
Thompson 2013
- Thompson W, Farrell B. Deprescribing: what is it and what does the evidence tell us?. Canadian Journal of Hospital Pharmacy 2013;66(3):201‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tougas 1999
- Tougas G, Chen Y, Hwang P, Liu MM, Eggleston A. Prevalence and impact of upper gastrointestinal symptoms in the Canadian population: findings from the DIGEST study. American Journal of Gastroenterology 1999;94(10):2845‐54. [DOI] [PubMed] [Google Scholar]
Vakil 2006
- Vakil N, Zanten SV, Kahrilas P, Dent J, Jones R, Bianchi LK, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence‐based consensus. American Journal of Gastroenterology 2006;101(8):1900‐20. [DOI] [PubMed] [Google Scholar]
Van Herwaarden 2014
- Herwaarden N, Broeder AA, Jacobs W, Maas A, Bijlsma JWJ, Vollenhoven RF, et al. Down‐titration and discontinuation strategies of tumor necrosis factor–blocking agents for rheumatoid arthritis in patients with low disease activity. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD010455.pub2] [DOI] [PubMed] [Google Scholar]
Veldhuyzen van Zanten 2005
- Veldhuyzen van Zanten SJO, Bradette M, Chiba N, Armstrong D, Barkun A, Flook N, et al. Evidence‐based recommendations for short‐ and long‐term management of uninvestigated dyspepsia in primary care: an update of the Canadian Dyspepsia Working Group (CanDys) clinical management tool. Canadian Journal of Gastroenterology 2005;19(5):285‐303. [DOI] [PubMed] [Google Scholar]
Wahab 2012
- Wahab MSA, Nyfort‐Hansen K, Kowalski SR. Inappropriate prescribing in hospitalised Australian elderly as determined by the STOPP criteria. International Journal of Clinical Pharmacy 2012;34(6):855‐62. [DOI] [PubMed] [Google Scholar]
Wallace 2011
- Wallace JL, Sharkey KA. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. In: Brunton LL, Chabner BA, Knollman CB editor(s). Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th Edition. New York: McGraw‐Hill, 2011:1309‐22. [Google Scholar]
Zacny 2005
- Zacny J, Zamakhshary M, Sketris I, Veldhuyzen van Zanten S. Systematic review: the efficacy of intermittent and on‐demand therapy with histamine H2‐receptor antagonists or proton pump inhibitors for gastro‐oesophageal reflux disease patients. Alimentary Pharmacology & Therapeutics 2005;21:1299‐312. [DOI] [PubMed] [Google Scholar]
Zarowitz 2011
- Zarowitz B. The challenge of discontinuing proton pump inhibitors. Geriatric Nursing 2011;32(4):276‐8. [DOI] [PubMed] [Google Scholar]


