Summary
Therapeutic mouthwash (MW) is an adjunctive tool along with a regular oral hygiene routine of daily tooth brushing and daily flossing. Previous systematic reviews have demonstrated that it is effective against dental biofilm and gingival inflammation, for prevention of dental caries, and for managing one’s bad breath condition according to the active ingredients. MWs prevent the microorganisms from bacterial adhesion that corresponds to the initial step in biofilm formation.
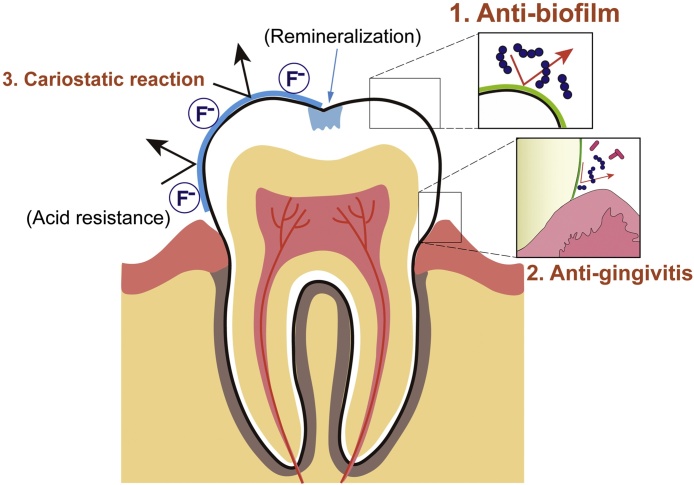
This review summarized the current state of evidence such as anti-biofilm, anti-gingivitis and cariostatic properties of MWs by evaluating systematic reviews from the past six years. The anti-biofilm property has been proven to be effective, with strong evidence of three main clinical efficacies.
The most commonly studied active agent was chlorhexidine gluconate (CHX), followed by essential oil (EO) and cetylpyridinium chloride. All the systematic reviews are in complete agreement that CHX and EO provide statistically significant improvements in terms of plaque and gingival indices. These effects have held up over the years as the number of studies has increased. While the use of fluoride MW is proven to be effective in improving the oral health of both children and adults, the quality of evidence is still regarded as low.
Keywords: Dental biofilm, Mouthwash, Chemical control, Gingivitis, Dental caries
1. Introduction
Dental biofilm is the primary etiology for dental caries, gingivitis, and periodontitis [1], [2], [3], [4], [5]. Unless biofilms are appropriately controlled, they accelerate their physiological heterogeneity and a series of complex interactions and cause tooth mineralization and systemic inflammation [6], [7], [8]. It is generally accepted that mechanical approaches such as tooth brushing and flossing are fundamental for the control of dental biofilms, because they can be applied without surgical intervention [8]. However, even in well-trained patients, adequate cleaning of hard-to-reach areas and of the gingival margin is difficult. Brushing and interdental cleaning is especially difficult for elderly patients with physical or mental limitations, malposed or isolated teeth, bridge-work, or orthodontic appliances [9]. In addition, even though flossing and interdental brushing have been considered to be important to oral health, systematic reviews and meta-analyses suggest that flossing in addition to tooth brushing may be associated with a small reduction in dental biofilm [10], [11], [12].
To supplement the effects of mechanical approaches, various antimicrobial agents are now incorporated into oral care products such as toothpastes and mouthwashes (MWs) [13], [14], [15]. For many years, MW has been the most frequently tested vehicle for antimicrobial compounds.
Variations of MWs are generally divided into cosmetic or therapeutic products. Cosmetic MW masks bad breath temporarily by a flavor composition that gives a fresh, invigorating feeling. Therapeutic MW, by contrast, has active ingredients intended to help mechanical control and maintain oral health. The efficacies of different formulations such as delmopinol (Del) [16], hexetidine [17], povidone-iodide [18], chlorhexidine gluconate (CHX) [19], [20], [21], essential oil (EO) [19], [20], [21], [22], cetylpyridinium chloride (CPC) [23], and hydrogen peroxide [24], have been reported in several systematic reviews. These reviews have shown that the MWs effected improvement on clinical parameters, including the plaque index and gingival inflammation, without causing any microbial resistance and alterations to microbial flora [25].
Anti-biofilm strategies have currently been developed against each stage of biofilm formation, because it becomes increasingly different to target biofilms as their formation progresses (Table 1) [26], [27]. Therapeutic MW acts as an anti-plaque and an anti-gingivitis agent, preventing the initial step of biofilm formation, that is, bacterial adhesion of microorganisms. Fluoride MW acts as a cariostatic agent, causing remineralization and increasing the acid resistance of dental hard tissue [28].
Table 1.
The process of biofilm development and anti-biofilm strategies.
| Stage of biofilm formation | Target | Strategy |
|---|---|---|
| 1. Bacterial adhesion | Adherence site | Coat tooth surfaces with molecules that block the attachment |
| 2. Coaggregation and matrix production | Surface receptor and metabolism of microorganisms | Coat surfaces with substances that interfere with matrix production |
| 3. Cell-to-cell signaling for multiplication | Quorum sensing | Deliver signal blockers to interrupt matrix formation |
| 4. Maturation | Microorganisms and extracellular matrix | Mechanical elimination and/or chemical controls |
The aim of this review is to present the current state of the evidence on the efficacies of MWs, focusing especially on reduction of dental biofilm and gingival inflammation. In this review, systematic reviews of the past six years are compared and summarized.
2. Study selection and data collection
The search for articles was conducted in MEDLINE, for publication dates from 2012 to December 20, 2017. To be included, a study had to be a systematic review of randomized clinical trials (RCTs) related to MWs. Search terms and results are summarized in Table 2. Abstracts and titles were then reviewed and categorized according to the issues brought up in this review.
Table 2.
A summary of search terms and results used in this study.
| Sequence no. | Terms and strategy (publication dates from 2012 to 2017) | Hits |
|---|---|---|
| #1 | Mouthwash and systematic review | 295 |
| #2 | Mouthwash and meta-analysis | 116 |
| #3 | Mouthrinse and systematic review | 32 |
| #4 | Mouthrinse and meta-analysis | 21 |
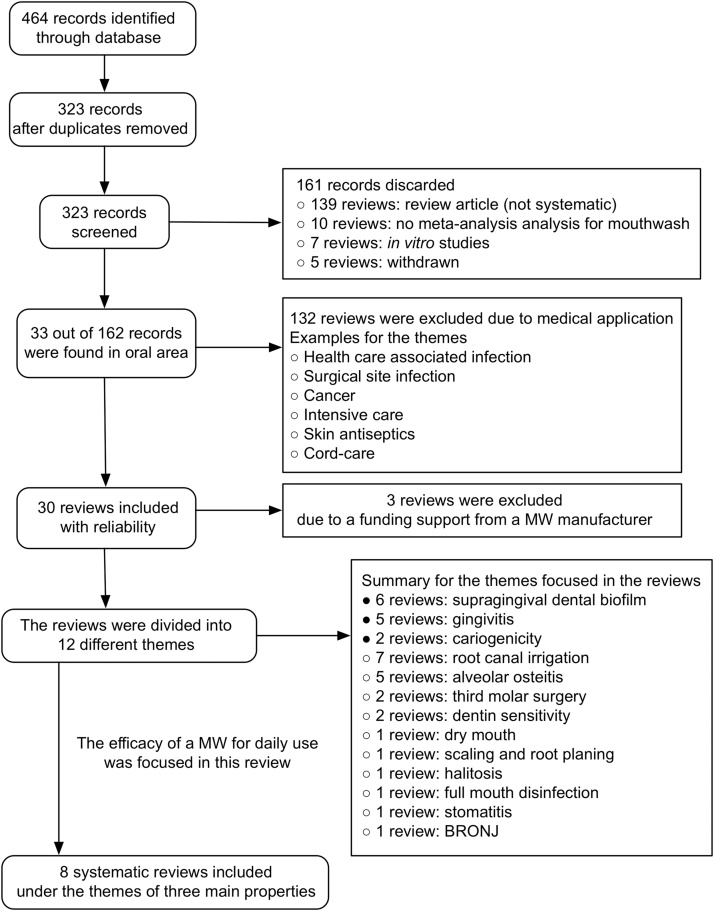
The following inclusion and exclusion criteria were used for data collection. A follow-up RCT of at least four weeks of daily use of MW was selected. If no such paper on a featured subject was found, an independent search by hand was conducted. An investigation funded by a MW manufacturer was not included. A single RCT or a meta-analysis using data from only one (the same) study group was also excluded. Flow diagram of the screening and selection process was summarized in Fig. 1.
Figure 1.
Flow diagram of the screening and selection process.
3. Current evidence of the effects of mouthwashes
3.1. Anti-dental biofilm properties
Six systematic reviews were found on this topic (Table 3) and the number of reviews was the highest of all those on clinical efficacies (Fig. 2). In the past, there were a lot of six-month RCTs on anti-dental biofilm and anti-gingivitis agents, because there was a general consensus, supported by established guidelines, that inhibitory and anti-dental biofilm activities of a given formulation had to be proven by RCTs conducted over the long term (more than six months) [36]. The evidence for a formulation’s safety without critical side-effects was also demanded.
Table 3.
Summary for reduction of plaque indices.
| Solutions | N | Weighted mean difference (95% Cl) | Index | Compared control | Follow-up periods | Reference |
|---|---|---|---|---|---|---|
| CHX | 12 | −1.45 (−1.00 to −1.90) | QHI | Baseline | 4–6 weeks | [29] |
| 4 | −0.78 (−1.07 to −0.49) | TQHI | Placebo rinse | 6 months | [30] | |
| 3 | −0.640 (−0.756 to −0.524) | TQHI | Placebo rinse | 6 months | [31] | |
| 2 | −0.208 (−0.351 to −0.065) | PI | Placebo rinse | 6 months | [31] | |
| 17 | −0.362 (−0.571 to −0.153)a | QHI or TQHI | Baseline | ≥4 weeks | [32] | |
| 4 | −0.39 (−0.70 to −0.08) | PI | Baseline | ≥4 weeks | [33] | |
| 9 | −0.67 (−0.82 to −0.52) | QHI | Baseline | ≥4 weeks | [33] | |
| EO | 9 | −0.86 (−1.05 to −0.68) | TQHI | Placebo rinse | 6 months | [30] |
| 9 | −0.827 (−1.053 to −0.600) | TQHI | Placebo rinse | 6 months | [31] | |
| 16 | −0.265 (−0.405 to −0.124)a | QHI or TQHI | Baseline | ≥4 weeks | [32] | |
| 14 | −0.86 (−1.05 to −0.66) | QHI | Placebo rinse | 6 months | [34] | |
| CPC | 6 | −0.41 (−0.65 to −0.17) | TQHI | Placebo rinse | 6 months | [30] |
| 3 | −0.465 (−0.631 to −0.299) | TQHI | Placebo rinse | 6 months | [31] | |
| 8 | −0.112 (−0.273 to 0.029)a | TQHI | Baseline | ≥4 weeks | [32] | |
| Del | 2 | −0.24 (−0.67 to 0.19)* | TQHI | Placebo rinse | 6 months | [30] |
| 3 | −0.144 (−0.231 to −0.058) | TQHI | Placebo rinse | 6 months | [31] | |
| 4 | −0.173 (−0.853 to 0.507)a | TQHI | Baseline | 4 weeks | [32] | |
| AmF/SnF | 2 | −0.079 (−0.260 to 0.101)* | TQHI | Placebo rinse | 6 months | [31] |
| 2 | −0.195 (−0.335 to −0.054) | PI | Placebo rinse | 6 months | [31] |
N: number of studies, CHX: chlorhexidine gluconate mouthwash, EO: essential oils-containing mouthwash (listerine antiseptic), CPC: cetylpyridinium chloride mouthwash, Del: delmopinol mouthwash, AmF: amine fluoride mouthwash, SnF: stannous fluoride mouthwash, PI: plaque-index Silness & Löe, QHI: Quigley–Hein plaque index, TQHI: Turesky modification of the Quigley and Hein plaque index.
Expressed as the summary relative difference meaning a percentage change from the baseline.
No significant difference (p > 0.05).
Figure 2.
Schematic diagram showing clinical effects of MWs. Anti-biofilm property (1) has been proven to be effective with strong evidence, followed by anti-gingivitis (2) and cariostatic reaction (3).
The most commonly studied active agent was CHX, followed by EO and CPC. The mechanism of CHX’s action is thought to be due to membrane disruption [37]. All the reviews had similar inclusion criteria, such as RCTs of at least four weeks, (basically six months’) follow-up of daily use, and patients diagnosed with mild gingivitis. All the RCTs were performed by a randomized, controlled, observer-blind, parallel-group clinical trial and a toothbrush and toothpaste were provided to all subjects for the duration of the study. Therefore, the influence by the active ingredient contained in a dentifrice was controlled. All the authors concluded that there was strong evidence to support the efficacy of MWs containing CHX and EO as anti-dental biofilm and anti-gingivitis agents. The weighted mean differences (WMDs) calculated across different reviews varied due to differences in the plaque index used for the estimation and the follow-up period. When comparing MW containing EO to the placebo control in the test of reduction of dental biofilm, the WMDs at six months were almost the same (−0.83 to −0.86). These values were similar to those of a previous estimation (−0.83) [22], indicating that the effect of EO has been maintained over the years as the number of studies has increased. Although two of these systematic reviews were from the same study group [30], [31], the others were carried out by different authors. Boyle et al. [32] have also reported the percentage reduction of dental biofilm for EO at six months in a meta-analysis of 35 RCTs. The reduction (35%) was almost as much as that (32%) reported by Haas et al. [34]. Taken together, the finding that EO provides a significant improvement in the plaque index at six months is highly reliable.
Escribano et al. [30] and Haas et al. [34] also conducted mixed comparisons among products. No statistically significant difference was found when comparing CHX and EO (WMD = −0.09, p = 0.58) [30]. In contrast, a previous meta-analysis had shown that CHX had significantly better effects on dental biofilm control than did EO (WMD = 0.19; p = 0.0009) [21]. On the other hand, the relative differences at three months and six months reported by Boyle et al. [32] were 31.6% and 36% for CHX and 24% and 35% for EO, respectively. The reduction rate due to EO was lower than that due to CHX at three months, whereas that at six months was quite similar. There is no consensus on which active agent is superior for dental biofilm inhibition at this time.
Whereas MWs containing CPC also produced a significant reduction in dental biofilm accumulation, the reduction ratio was smaller than those due to CHX and EO (Table 3). Mixed comparisons between CHX and CPC, and between EO and CPC, yielded −0.37 and −0.46 as WMDs, respectively. A similar result was presented by Haps et al. [23]. The authors evaluated MWs with CPC as an active agent, and they came to the conclusion that CPC provided a small but significant benefit in the control of dental biofilm. CPC appeared to be less effective for dental biofilm reduction in comparison with CHX and EO. The effect of Del was small, and one of three reviews showed no significant difference compared with a placebo rinse (Table 3). The MW containing amine fluoride (AmF) and stannous fluoride (SnF) was less effective at preventing dental biofilm accumulation and one of two reviews showed no significant difference.
3.2. Anti-gingivitis property
Five systematic reviews on this issue were found. A summary of results for gingivitis indices is listed in Table 4. The authors investigated MWs containing CHX and EO and concluded that there was strong evidence to support the efficacy of these as anti-gingivitis agents. The WMDs varied under different conditions, such as gingival index, comparable control and duration. In addition, it was found that the anti-gingivitis effect of CHX was slightly different from its reported effect in previous systematic reviews. For example, the relative differences in CHX at three months and six months were 14.6% and 17.8%, respectively [32]. In contrast, the reduction in gingivitis due to CHX at six months evaluated by Gunsolley in 2006 was 28.7% [19]. The results for EO were similar to those found in previous systematic reviews. The WMDs in EO at six months ranged from −0.133 to −0.24 for GI and −0.52 to −0.537 for MGI. The WMDs estimated by Stoeken et al. [22] in 2007 were −0.14 for PI and −0.41 for MGI.
Table 4.
Summary for reduction of gingival indices.
| Solutions | N | Weighted mean difference (95% Cl) | Index | Compared control | Follow-up periods | Reference |
|---|---|---|---|---|---|---|
| CHX | 10 | −0.21 (−0.11 to −0.31) | GI | Baseline | 4–6 weeks | [29] |
| 4 | −0.185 (−0.285 to −0.086) | GI | Placebo rinse | 6 months | [31] | |
| 19 | −0.223 (−0.412 to −0.034)a | GI or MGI | Baseline | ≥4 weeks | [32] | |
| 9 | −0.32 (−0.42 to −0.23) | GI | Baseline | ≥4 weeks | [33] | |
| EO | 2 | −0.133 (−0.194 to −0.072) | GI | Placebo rinse | 6 months | [31] |
| 8 | −0.537 (−0.764 to −0.311) | MGI | Placebo rinse | 6 months | [31] | |
| 16 | −0.203 (−0.312 to −0.093)a | GI or MGI | Baseline | ≥4 weeks | [32] | |
| 11 | −0.52 (−0.67 to −0.37) | MGI | Placebo rinse | 6 months | [34] | |
| 3 | −0.24 (−0.46 to −0.01) | GI | Placebo rinse | 6 months | [34] | |
| CPC | 3 | −0.344 (−0.627 to −0.062) | GI | Placebo rinse | 6 months | [31] |
| 2 | −0.357 (−0.483 to −0.231) | MGI | Placebo rinse | 6 months | [31] | |
| 8 | −0.126 (−0.312 to 0.059)*, a | GI or MGI | Baseline | ≥4 weeks | [32] | |
| Del | 2 | −0.038 (−0.145 to −0.069)* | MGI | Placebo rinse | 6 months | [31] |
| 3 | −0.014 (−2.337 to 2.308)a, * | GI or MGI | Baseline | ≥4 weeks | [32] | |
| AmF/SnF | 2 | −0.248 (−0.427 to −0.069) | GI | Placebo rinse | 6 months | [31] |
GI: gingival index (Löe & Silness), MGI: modified gingival index.
Expressed as the summary relative difference meaning a percentage change from the baseline.
There was no significant difference (p > 0.05).
One of the reasons for the slight differences in anti-dental biofilm and anti-gingivitis effects of CHX, as presented in the systematic reviews, may be the concentration of CHX in the MW. Various types MWs are now available with concentrations of CHX of 0.05%, 0.06%, 0.1%, 0.12%, and 0.2% [33]. Almost of all the systematic reviews analyzed the RCTs using CHX at concentrations higher than 0.1% as the same group. There has been no systematic review comparing the efficacy at various concentrations in the past six years. Berchier et al. evaluated the effects on dental biofilm accumulation and periodontal parameters of 0.12% compared with 0.2% CHX MW [38]. The authors deduced a small but significant difference in favor of the 0.2% CHX concentration; nonetheless, the clinical relevance of this difference is probably negligible. However, the latest clinical study demonstrated that 0.2% CHX had a statistically significant greater effect in preventing dental biofilm than did the 0.12% and 0.06% solutions. Haydari et al. have compared the inhibiting effect on dental biofilm and gingivitis of commercial products containing 0.2%, 0.12%, and 0.06% CHX using RCT [39]. The commercial MW containing 0.2% CHX resulted in statistically significantly lower plaque scores than did the 0.12 and 0.06% MWs after 21 days’ use, whereas no statistically significant difference was found between the effects of the latter two concentrations. The consensus on the difference in clinical efficacy of CHX at various concentrations may be updated by future investigations.
For MWs containing CPC at concentrations of more than 0.05%, significant benefits were observed for MGI (WMD = −0.357) and GI (WMD = −0.344) [31]. However, Boyle et al. [32] reported that CPC trials did not suggest a meaningful impact of this product (WMD = −0.126; 95% confidence interval (CI): −0.312 to 0.059). They also reported that relative reduction rates of CPC at three months and six months were 11.2% (95% Cl: −35.5 to +13.1) and 13.4% (95% Cl: −43.3 to +16.5), respectively. The use of EO over six months compared with CPC resulted in statistically lower levels of gingivitis, suggesting that EO was more efficacious than CPC [34].
There was no data on the comparison between CHX and EO for anti-gingivitis efficacy. Van Leeuwen et al. reported in a previous review that no significant difference was found between EO and CHX in the reduction of gingival inflammation [21].
Del mouthwashes showed no significant effect across two systematic reviews. Significant effects were found for rinses with AmF/SnF (Table 4).
Van Leeuwen et al. also reported a systematic review on the effects of a MW containing EO compared with its alcohol vehicle solution control (V-Sol) [35]. The results were that EO provided a significantly greater reduction than did V-Sol of dental biofilm and gingival inflammation. Furthermore, the V-Sol proved to be no different from a water-based control, meaning that the antiseptic effect of the hydro-alcohol solution seems negligible. However, this systematic review covered only one specific product of one manufacturer.
Escribano et al. conducted mixed comparisons among CHX, EO, and EO without alcohol [30]. No statistically significant differences were found when comparing CHX to EO without alcohol (WMD = −0.08; Cl: −0.6 to 0.44), and EO to EO without alcohol (WMD = 0.001; Cl: −0.46 to 0.46). However, this meta-analysis was conducted with the data set of only a single RCT using EO without alcohol.
Within the limitations of these analyses, further investigations will be needed for judging whether the alcohol vehicle enhances anti-biofilm properties or not.
3.3. Cariostatic property
MWs containing fluoride are recommended as part of a caries-preventive strategy for individuals at high risk of caries, such as patients undergoing orthodontic treatment or patients with hyposalivary function [40]. Fluoride MWs contain a variety of fluoride compounds, such as sodium fluoride, SnF, sodium monofluorophosphate, and AmF [41].
On this issue, two systematic reviews were found. A systematic review by Marinho et al. of 35 trials involving 15,305 children and adolescents concluded that supervised regular use of a fluoride MW by children and adolescents was associated with a large reduction in caries increment in permanent teeth [42]. Prevented fraction, which is the difference in mean caries increments between treatment and control groups expressed as a percentage of the main increment in the control group, was used as a primary outcome measure. There was a reported 27% decrease in the DMFS (95% Cl: 23–30%) and a 23% decrease in the DMFT (95% Cl: 18–29%) in permanent teeth using fluoride MW. None of the trials reported acute adverse symptoms during treatment. The authors excluded studies where the intervention consisted of use of any other caries-preventive agent or procedure such as other fluoride-based measures, chlorhexidine, sealants, oral hygiene interventions, xylitol chewing gums. In fact, these caries-preventive effects were at individual use of fluoride MW without a fluoridated dentifrice.
However, since almost all the people already use a fluoridated dentifrice daily, additional effect in the prevention of dental caries is still unclear. In a previous systematic review performed by Marinho et al., the size of the effect of a fluoride MW in combination with a fluoride toothpaste was compared to toothpaste used alone [43]. The DMFS prevented fraction pooled estimated from the random-effects meta-analysis of five trials (n = 2738) combined was only 0.07 (95% Cl: 0.0–0.13) with no significant difference (p = 0.06). Another systematic review conducted by Twetman et al. has also concluded that sodium fluoride MW may have an anti-caries effect in children with limited background of fluoride exposure, while its additional effect in children with daily use of fluoride toothpaste could be questioned [44].
Additionally, there was a systematic review to assess the ability to prevent root caries. Wierichs et al. evaluated results of clinical studies investigating chemical agents intended to reduce the initiation of root caries [45]. Changes were calculated for DMFRS/DFRS (decayed, missing, or filled root surfaces) and the root caries index. The results were that rinsing the mouth with a fluoride MW (225–900 ppm F−) significantly reduced DMFRS/DFRS (mean differences (MD) = −0.18; 95% CI: −0.35 to −0.01) compared with rinsing with a placebo. However, the inhibition effect was much lower than 38% silver diamine fluoride (SDF) (MD = −0.33; 95% CI: −0.39 to −0.28). The authors concluded that regular use of dentifrices containing 5000 ppm F− and SDF or CHX varnishes applied quarterly by professionals seemed to be efficacious in decreasing initiation and progression of root caries, respectively. In addition, as stated in the conclusion by the authors, the results should be interpreted with caution, due to the low number of clinical trials (four in total), the high risk of bias within studies, and the limiting grade of evidence.
While there are also some clinical trials for the effectiveness of fluoride MWs at preventing root caries, the quality of evidence is still to be regarded as low [45]. Although the use of fluoride MW is effective in improving the oral health of both children and adults, further studies are needed for its positive recommendation to all people.
3.4. Other points
One factor to be considered in recommending the use of MW is side-effects. Although CHX and EO are proven to be more effective in MW for reduction of dental biofilm and gingival inflammation than mechanical dental biofilm control either alone, considerable side-effects have been reported. James et al. concluded that rinsing with MW containing CHX for four weeks or longer caused extrinsic tooth staining [29]. In addition, other adverse effects, such as calculus build-up, transient taste disturbance and effects on the oral mucosa, were reported in the included studies. Strydonck et al. reported that staining was the most common observation in 19 out of 30 articles [33]. Also, increased calculus formation (six articles) and change of taste sensation (seven articles) were frequently observed. Other less frequent complaints were a burning sensation, hypersensitivity, mucosal lesions, and anaesthetized sensation. For these reasons, MW containing CHX is indicated in particular clinical situations for short periods of time. A MW with a low concentration (0.05% or 0.06%) of CHX is currently marketed for long term daily use. However, there is still lack of evidence on the effects on anti-dental biofilm and anti-gingivitis and any adverse effects associated with the use of CHX.
Although the side-effects of MW containing EO were not stated in the systematic reviews selected in this article, previous investigations have reported them [47], [48], [49]. The most common side-effects of alcohol-containing EO MW were complaints about its poor taste and oral irritation [46], [47]. High-risk populations such as children, alcohol addicts, patients with genetic deficiencies in ethanol metabolism or with oral cancer, and smokers should use alcohol-free MWs for the maintenance of oral health [49].
These side-effects must be taken into consideration when recommending effective antimicrobial MWs for patient use.
4. Conclusions
In conclusion, anti-biofilm properties have been proven to be effective, with strong evidence of three main clinical efficacies. MWs containing CHX and EO provide significant reductions in dental biofilm and gingivitis scores. These effects have been maintained over the years as the number of studies has increased. While the use of fluoride MW has been proven to be effective in improving the oral health of both children and adults, the quality of evidence should still to be regarded as low.
Although MW provides some clinical benefits, mechanical elimination is still essential and is the basic approach for the control of dental biofilms. Chemical control is an alternative or adjunctive method when elimination using dental instruments proves difficult. The aim of the chemical approach is to prevent biofilm accumulation rather than eradication, while still preserving the benefits of the normal resident oral microflora.
Conflict of interest
No potential conflicts of interest are disclosed.
Acknowledgement
This work was supported, in part, by Grant-in-Aid for Scientific Research (grant nos. 15H05021, 16K15785, 16K20451, 26305034 and 26462876) from the Japan Society for the Promotion of Science.
References
- 1.Pitts N.B., Zero D.T., Marsh P.D., Ekstrand K., Weintraub J.A., Eamos-Gomez F. Dental caries. Nat Rev Dis Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi N., Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 3.Kuboniwa M., Lamont R.J. Subgingical biofiflm formation. Periodontology 2000. 2010;52:38–52. doi: 10.1111/j.1600-0757.2009.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noiri Y., Li L., Ebisu S. The localization of periodontal-disease-associated bacteria in human periodontal pockets. J Dent Res. 2001;80:1930–1934. doi: 10.1177/00220345010800101301. [DOI] [PubMed] [Google Scholar]
- 5.Page R.C., Offenbacher S., Schroeder H.E., Seymour G.J., Kornman K.S. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontology 2000. 1997;14:216–248. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 6.Marsh P.D. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204–211. doi: 10.1159/000077756. [DOI] [PubMed] [Google Scholar]; Marsh P.D., Moter A., Devine D.A. Dental plaque biofilms. Periodontology 2000. 2011;55:16–35. doi: 10.1111/j.1600-0757.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- 7.Hasan A., Palmer R.M. A clinical guide to periodontology: pathology of periodontal disease. Br Dent J. 2014;216:457–461. doi: 10.1038/sj.bdj.2014.299. [DOI] [PubMed] [Google Scholar]
- 8.Socransky S.S., Haffajee A.D. Dental biofilms: difficult therapeutic targets. Periodontology 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 9.Santos A. Evidence-based control of plaque and gingivitis. J Clin Periodontol. 2003;30(Suppl. 5):13–16. doi: 10.1034/j.1600-051x.30.s5.5.x. [DOI] [PubMed] [Google Scholar]
- 10.Berchier C.E., Slot D.E., Haps S., Van der Weijden G.A. The efficacy of dental floss in addition to a toothbrush on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg. 2008;6:265–279. doi: 10.1111/j.1601-5037.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- 11.Sambunjak D., Nickerson J.W., Poklepovic T., Johnson T.M., Imai P., Tugwell P. Flossing for the management of periodontal diseases and dental caries in adults. Cochrane Database Syst Rev. 2011;12 doi: 10.1002/14651858.CD008829.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Sälzer S., Slot D.E., Van der Weijden F.A., Dörfer C.E. Efficacy of inter-dental mechanical plaque control in managing gingivitis—a meta-review. J Clin Periodontol. 2015;42(Suppl. 16):S92–105. doi: 10.1111/jcpe.12363. [DOI] [PubMed] [Google Scholar]
- 13.Brading M.G., Marsh P.D. The oral environment: the challenge for antimicrobials in oral care products. Int Dent J. 2003;53(6 Suppl. 1):353–362. doi: 10.1111/j.1875-595x.2003.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 14.Marsh P.D. Controlling the oral biofilm with antimicrobials. J Dent. 2010;38(Suppl. 1):S11–S15. doi: 10.1016/S0300-5712(10)70005-1. [DOI] [PubMed] [Google Scholar]
- 15.Marsh P.D. Contemporary perspective on plaque control. Br Dent J. 2012;212:601–606. doi: 10.1038/sj.bdj.2012.524. [DOI] [PubMed] [Google Scholar]
- 16.Addy M., Moran J., Newcombe R.G. Meta-analysis of studies of 0.2% delmopinol mouth rinse as an adjunct to gingival health and plaque control measures. J Clin Periodontol. 2007;34:58–65. doi: 10.1111/j.1600-051X.2006.01013.x. [DOI] [PubMed] [Google Scholar]
- 17.Afennich F., Slot D.E., Hossainian N., Van der Weijden G.A. The effect of hexetidine mouthwash on the prevention of plaque and gingival inflammation: a systematic review. Int J Dent Hyg. 2011;9:182–190. doi: 10.1111/j.1601-5037.2010.00478.x. [DOI] [PubMed] [Google Scholar]
- 18.Sahrmann P., Puhan M.A., Attin T., Schmidlin P.R. Systematic review on the effect of rinsing with povidone–iodine during nonsurgical periodontal therapy. J Periodontal Res. 2010;45:153–164. doi: 10.1111/j.1600-0765.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- 19.Gunsolley J.C. A meta-analysis of six-month studies of antiplaque and antignigivitis agents. J Am Dent Assoc. 2006;137:1649–1657. doi: 10.14219/jada.archive.2006.0110. [DOI] [PubMed] [Google Scholar]
- 20.Gunsolley J.C. Clinical efficacy of antimicrobial mouthrinses. J Dent. 2010;38(Suppl. 1):S6–10. doi: 10.1016/S0300-5712(10)70004-X. [DOI] [PubMed] [Google Scholar]
- 21.Van Leeuwen M.P., Slot D.E., Van der Weijden G.A. Essential oils compared to chlorhexidine with respect to plaque and parameters of gingival inflammation: a systematic review. J Periodontal. 2011;82:174–194. doi: 10.1902/jop.2010.100266. [DOI] [PubMed] [Google Scholar]
- 22.Stoeken J.E., Paraskevas S., van der Weijden G.A. The long-term effect of a mouthrinse containing essential oils on dental plaque and gingivitis: a systematic review. J Periodontol. 2007;78:1218–1228. doi: 10.1902/jop.2007.060269. [DOI] [PubMed] [Google Scholar]
- 23.Haps S., Slot D.E., Berchier C.E., Van der Weijden G.A. The effect of cetylpyridinium chloride-containing mouth rinses as adjuncts to toothbrushing on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg. 2008;6:290–303. doi: 10.1111/j.1601-5037.2008.00344.x. [DOI] [PubMed] [Google Scholar]
- 24.Hossainian N., Slot D.E., Afennich F., Van der Weijden G.A. The effects of hydrogen peroxide mouthwashes on the prevention of plaque and gingival inflammation: a systematic review. Int J Dent Hyg. 2011;9:171–181. doi: 10.1111/j.1601-5037.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 25.Sreenivasan P., Gaffar A. Antiplaque biocides and bacterial resistance: a review. J Clin Periodontol. 2002;29:965–974. doi: 10.1034/j.1600-051x.2002.291101.x. [DOI] [PubMed] [Google Scholar]
- 26.Takenaka S., Ohshima H., Ohsumi T., Okiji T. Current and future strategies for the control of mature oral biofilms- Shift from a bacteria-targeting to a matrix-targeting approach. J Oral Biosci. 2012;54:173–179. [Google Scholar]
- 27.Costerton J.W., Stewart P.S. Battling biofilms. Sci Am. 2001;285:74–81. doi: 10.1038/scientificamerican0701-74. [DOI] [PubMed] [Google Scholar]
- 28.Rošin-Grget K., Peroš K., Sutej I., Bašić K. The cariostatic mechanisms of fluoride. Acta Med Acad. 2013;42:179–188. doi: 10.5644/ama2006-124.85. [DOI] [PubMed] [Google Scholar]
- 29.James P., Worthington H.V., Parnell C., Harding M., Lamont T., Cheung A. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev. 2017;3 doi: 10.1002/14651858.CD008676.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escribano M., Figuero E., Martín C., Tobías A., Serrano J., Roldán S. Efficacy of adjunctive anti-plaque chemical agents: a systematic review and network meta-analyses of the Turesky modification of the Quigley and Hein plaque index. J Clin Periodontol. 2016;43:1059–1073. doi: 10.1111/jcpe.12616. [DOI] [PubMed] [Google Scholar]
- 31.Serrano J., Escribano M., Roldán S., Martín C., Herrera D. Efficacy of adjunctive anti-plaque chemical agents in managing gingivitis: a systematic review and meta-analysis. J Clin Periodontol. 2015;(Suppl. 16):S106–S138. doi: 10.1111/jcpe.12331. [DOI] [PubMed] [Google Scholar]
- 32.Boyle P., Koechlin A., Autier P. Mouthwash use and the prevention of plaque, gingivitis and caries. Oral Dis. 2014;(Suppl. 1):1–68. doi: 10.1111/odi.12187. [DOI] [PubMed] [Google Scholar]
- 33.Van Strydonck D.A., Slot D.E., Van der Velden U., Van der Weijden F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol. 2012;39:1042–1055. doi: 10.1111/j.1600-051X.2012.01883.x. [DOI] [PubMed] [Google Scholar]
- 34.Haas A.N., Wagner T.P., Muniz F.W., Fiorini T., Cavagni J., Celeste R.K. Essential oils-containing mouthwashes for gingivitis and plaque: meta-analyses and meta-regression. J Dent. 2016;55:7–15. doi: 10.1016/j.jdent.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Van Leeuwen M.P., Slot D.E., Van der Weijden G.A. The effect of an essential-oils mouthrinse as compared to a solution on plaque and gingival inflammation: a systematic review and meta-analysis. Int J Dent Hyg. 2014;12:160–167. doi: 10.1111/idh.12069. [DOI] [PubMed] [Google Scholar]
- 36.Council on dental therapeutics: guidelines for acceptance of chemotherapeutic products for the control of supragingival dental plaque and gingivitis. J Am Dent Assoc. 1986;112:529–532. doi: 10.1016/s0002-8177(86)24021-0. [DOI] [PubMed] [Google Scholar]
- 37.Jones C.G. Chlorhexidine: is it still the gold standard? Periodontology 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 38.Berchier C.E., Slot D.E., Van der Weijden G.A. The efficacy of 0.12% chlorhexidine mouthrinse compared with 0.2% on plaque accumulation and periodontal parameters: a systematic review. J Clin Periodontol. 2010;37:829–839. doi: 10.1111/j.1600-051X.2010.01575.x. [DOI] [PubMed] [Google Scholar]
- 39.Haydari M., Bardakci A.G., Koldsland O.C., Aass A.M., Sandvik L., Preus H.R. Comparing the effect of 0.06%-, 0.12% and 0.2% chlorhexidine on plaque, bleeding and side effects in an experimental gingivitis model: a parallel group, double masked randomized clinical trial. BMC Oral Health. 2017;17:118. doi: 10.1186/s12903-017-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Mullane D.M., Baez R.J., Jones S., Lennon M.A., Petersen P.E., Rugg-Gunn A.J. Fluoride and oral health community. Dent Health. 2016;33:69–99. [PubMed] [Google Scholar]
- 41.Pitts N., Duckworth R.M., Marsh P., Mutti B., Parnell C., Zero D. Post-brushing rinsing for the control of dental caries: exploration of the available evidence to establish what advice we should give our patients. Br Dent J. 2012;212:315–320. doi: 10.1038/sj.bdj.2012.260. [DOI] [PubMed] [Google Scholar]
- 42.Marinho V.C., Chong L.Y., Worthington H.V., Walsh T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2016;7 doi: 10.1002/14651858.CD002284.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marinho V.C., Higgins J.P., Sheiham A., Logan S. Combinations of topical fluoride (toothpastes, mouthrinses, gels, varnishes) versus single topical fluoride for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2004;(1) doi: 10.1002/14651858.CD002781.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Twetman S., Petersson L., Axelsson S., Dahlgren H., Holm A.K., Källestål C. Caries-preventive effect of sodium fluoride mouthrinses: a systemayic review of controlled clinical trials. Acta Odontol Scand. 2004;62:223–230. doi: 10.1080/00016350410001658. [DOI] [PubMed] [Google Scholar]
- 45.Wierichs R.J., Meyer-Lueckel H. Systematic review on noninvasive treatment of root caries lesions. J Dent Res. 2015;94:261–271. doi: 10.1177/0022034514557330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Twetman S., Keller M.K. Fluoride rinses, gels and foams: an update of controlled clinical trials. Caries Res. 2016;50(Suppl. 1):38–44. doi: 10.1159/000439180. [DOI] [PubMed] [Google Scholar]
- 47.FDI Commission: mouthrinses and dental caries. Int Dent J. 2002;52:337–345. [PubMed] [Google Scholar]
- 48.Vlachojannis C., Winsauer H., Chrubasik S. Effectiveness and safety of a mouthwash containing essential oil ingredients. Phytother Res. 2013;27:685–691. doi: 10.1002/ptr.4762. [DOI] [PubMed] [Google Scholar]
- 49.Overholser C.D., Jr. Longitudinal clinical studies with antimicrobial mouthrinses. J Clin Periodontol. 1988;15:517–519. doi: 10.1111/j.1600-051x.1988.tb01024.x. [DOI] [PubMed] [Google Scholar]