Abstract
The primitive carotid-vertebrobasilar anastomoses are primitive embryonic cerebral vessels that temporarily provide arterial supply from the internal carotid artery to the longitudinal neural artery, the future vertebrobasilar artery in the hindbrain. Four types known are the trigeminal, otic, hypoglossal, and proatlantal intersegmental arteries. The arteries are accompanied by their corresponding nerves and resemble an intersegmental pattern. These vessels exist in the very early period of cerebral arterial development and rapidly involute within a week. Occasionally, persistence of the carotid to vertebrobasilar anastomosis is discovered in the adult period, and is considered as the vestige of the corresponding primitive embryonic vessel. The embryonic development and the segmental property of the primitive carotid-vertebrobasilar anastomoses are discussed. This is followed by a brief description of the persisting anastomoses in adults.
Keywords: carotid-vertebrobasilar anastomoses, trigeminal artery, otic artery, hypoglossal artery, proatlantal intersegmental artery
Introduction
In the early embryonic period, four transient vessels originating from the primitive internal carotid artery (ICA) and dorsal aorta known as the primitive carotid-vertebrobasilar anastomoses supply the future vertebrobasilar artery. These anastomoses are the trigeminal, otic, hypoglossal, and proatlantal intersegmental arteries. At this stage, the future vertebrobasilar artery is a paired vascular plexus in the ventral wall of the hindbrain, known as the longitudinal neural arteries. The anastomoses rapidly regress within a week with the development of the vertebrobasilar system. Occasionally, they are incidentally discovered in the adult, and are considered remnants of the primitive embryonic circulation. In this review, the embryology with emphasis on the segmental property of the primitive carotid-vertebrobasilar anastomoses is discussed. This is followed by a brief review of the persistent form of these vessels found in adults.
The Primitive Carotid-vertebrobasilar Anastomoses
Detailed morphological description of the primitive carotid-vertebrobasilar anastomosis in human embryo was first described by Padget in her landmark study published in 1948.1) Preceding her report, a few reports had suggested the existence of the segmental vessels in the embryonic hindbrain area.2–4) However, Padget was the first to define these anastomoses in detail. Her study has never been surpassed in quality since then, and virtually every discussion concerning the morphological development of the carotid-vertebrobasilar anastomoses stems from her study. Therefore, in this review article, the embryonic development of the carotid-vertebrobasilar anastomoses is based on the study by Padget. However, one must be aware of the fact that the morphological description of the development of the cerebral arteries in the human embryo is based on a single study with a relatively limited number of specimens.
Embryonic Development
At the earliest stage, when the embryo is in 3 mm length (Carnegie Stage 11), two branches sprout from the first aortic arch; the primitive internal carotid and the primitive trigeminal arteries. This is the earliest appearance of the trigeminal artery and it communicates with the primordial hindbrain channel (a vascular plexus on the surface of the hindbrain temporarily functioning as a vein of the head, later developing into the bilateral longitudinal neural arteries). At the same period, the primordial hindbrain channel is also supplied by two or three slender, irregularly placed vessels in the otic region.
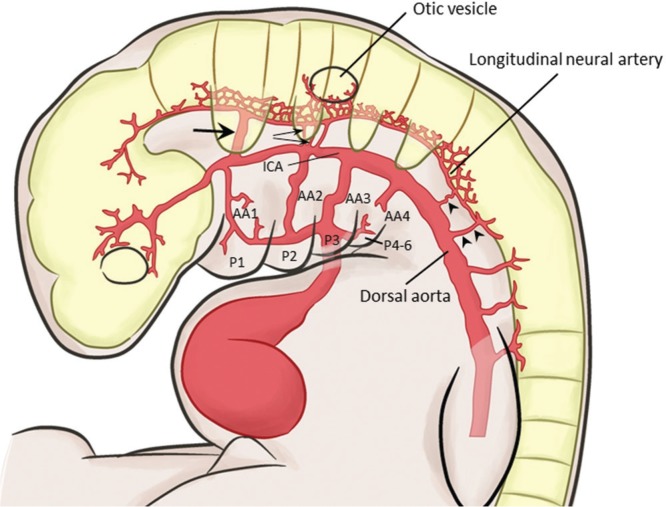
In an embryo of 4 mm (Carnegie Stage 12), the primitive trigeminal artery now arises from the primitive ICA instead from the aortic arch, and terminates in a fragment of the longitudinal neural artery (Fig. 1). Two or three primitive otic arteries are observed opposite the second aortic arch between the paired longitudinal neural and primitive internal carotid arteries. The otic arteries are seen only in the 3 to 4 mm stages during the development of the second aortic arch and are never observed in later stages. As soon as the second aortic arch regresses, the primitive hypoglossal and primitive proatlantal intersegmental (C1 segmental) arteries with their corresponding nerves supply the caudal end of the longitudinal neural arteries.
Fig. 1.
Vessels in an embryo of 4 mm. The primitive trigeminal artery (arrow) is arising from the primitive internal carotid artery (ICA). A few primitive otic arteries arise opposite to the second aortic arch (small arrows). The caudal end of the hindbrain is supplied by the primitive hypoglossal (arrowhead) and proatlantal intersegmental (double arrowheads) arteries. The primitive carotid-vertebrobasilar anastomoses communicate the primitive ICA and dorsal aorta with the longitudinal neural artery, and accompany their corresponding nerves. AA: aortic arch; P: pharyngeal arch.
In a slightly advanced embryo of 4 mm (Carnegie Stage 13), the primitive ICA bifurcates into two branches of equal caliber: the continuation of the ICA and the trigeminal artery. Although the distal ICA has formed its primary cranial and caudal portion (future posterior communicating artery), no definite connection between the ICA and the neural artery has developed at this stage. Therefore, the longitudinal neural arteries are primarily supplied by the trigeminal artery. At the caudal side, the neural artery is chiefly supplied by the proatlantal intersegmental artery.
In a 5–6 mm embryo, the caudal division of the ICA further develops and forms an anastomosis with the cranial end of the longitudinal neural artery to become the definitive posterior communicating artery. The posterior communicating artery then replaces the trigeminal artery as the source of blood supply to the cranial longitudinal neural artery. The caudal end of the longitudinal neural artery is still chiefly supplied by the proatlantal intersegmental artery.
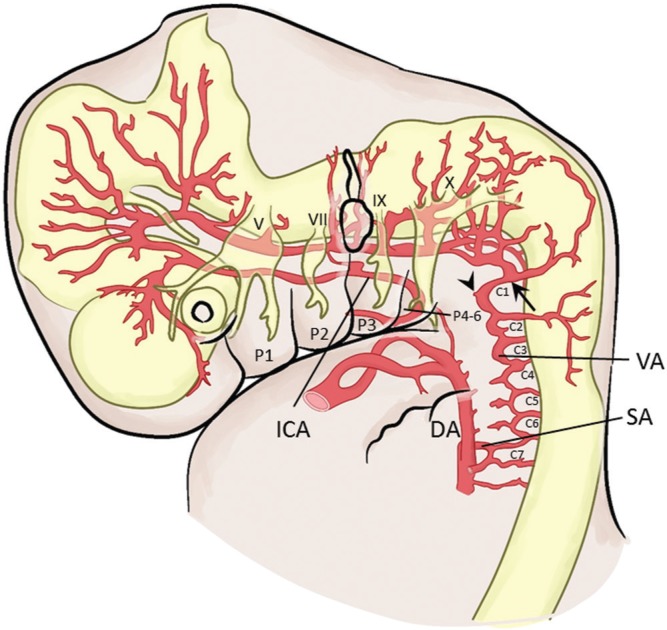
Development of the longitudinal neural artery into the basilar and vertebral arteries is almost complete in a 7–12 mm embryo. Fusion of first to sixth cervical segmental arteries and subsequent obliteration of the aortic ends results in the formation of the cervical vertebral artery. The cervical vertebral artery is then supplied by the seventh segmental artery, which is the future subclavian artery (Fig. 2). This new dorsal aorta to neural artery anastomosis (formation of the vertebral artery) replaces the role of the hypoglossal and proatlantal intersegmental arteries. Although the hypoglossal artery regresses, the distal portion of the proatlantal intersegmental artery remains as the transverse suboccipital part of the vertebral artery (Fig. 2).5)
Fig. 2.
Vessels in an embryo of 7–12 mm. Fusion of the first to sixth cervical segmental arteries (C1–C7) results in the formation of the vertebral artery. Note that the distal portion of the proatlantal intersegmental artery (PIA, C1) remains as the horizontal portion of the vertebral artery (arrow). Persistence of the proximal portion of the PIA results in the persistent PIA seen in adults (arrowhead). The muscular components of each pharyngeal arch are associated with its own cranial nerve. DA: dorsal aorta; ICA: internal carotid artery; P: pharyngeal arch; SA: subclavian artery; VA: vertebral artery.
In summary, in the early embryonic period, the blood to the hindbrain is supplied by four carotid-vertebrobasilar anastomoses: the trigeminal, otic, hypoglossal, and proatlantal intersegmental arteries. The major supply to the cranial and caudal hindbrain is from the trigeminal and proatlantal intersegmental artery, respectively. As the posterior communicating and vertebrobasilar arteries develop, blood supply to the hindbrain is replaced by these arteries and the primitive carotid-vertebrobasilar anastomoses rapidly regress in about a week. The first to regress is the otic artery, followed by the hypoglossal artery. The trigeminal and proatlantal intersegmental arteries persist to a slightly later period.
The Segmental Property of the Primitive Carotid-vertebrobasilar Anastomoses
The term “segmental” has become widely recognized after publication by Evans.3) The term was used for the regular dorsal branches of the aorta, particularly those which are connected by a secondary longitudinal anastomosis to form the vertebral artery (C1 to C6 cervical segmental arteries).5) The segmental artery is actually an intersegmental artery in that the vessel passes between the early embryonic somites. Another feature of the segmental artery is that the vessel is accompanied with the corresponding nerve.
Since the carotid-vertebrobasilar anastomoses originate from the dorsal aorta and ICA, and the ICA being formed from the third aortic arch and the distal dorsal aorta, these anastomoses may be broadly interpreted as dorsal aorta-longitudinal neural artery anastomoses (Fig. 1). As can be easily understood by studying Fig. 1, the spinal segmental arteries are dorsal aorta-neural artery anastomoses. The resemblance of the carotid-vertebrobasilar anastomoses to spinal segmental arteries has stimulated the interest of many investigators. For instance, Evans has made reference to the carotid-vertebrobasilar anastomoses as “presegmental branches of the dorsal aorta”, and De Vriese mentioned the possibility of the “cephalic segmental arteries” related to the fifth, eighth, and twelfth nerves.1,3) More recently, in their textbook, Lasjaunias et al. assumed a segmental property of the carotid-vertebrobasilar anastomoses related to the pharyngeal arch.6) However, segmentation of the occipitocervical region is not as straightforward as in the cervical spine, and simple extension of the segmental artery concept to this region is difficult. The difference between the occipitocervical region and spine is that multiple segmentation factors are related in the development of the occipital region, whereas that in the spine is simple. These factors include the occipital somites, pharyngeal and aortic arches, and the rhombomeres (segmentation of the hindbrain).7) The segmentation factors in the occipitocervical region will be discussed in association with the development of cranial nerves and arteries, as well as the homology of the occipitocervical and spinal structures.
Segmentation of the body starts in the cephalic region by the beginning of the third week. In that period, the mesodermal layer on each side of the neural tube begins to be organized into segments known as the occipital somites and proceeds cephalocaudally to develop the spinal somites. In the head region, occipital somites form in association with segmentation of the neural plate into neuromeres (rhombomeres in the hindbrain) and contribute to mesenchyme in the head.8) In the head region, the number of somite is generally agreed as being four.5,7,9,10) Some researchers advocate the existence of another segmental structure in human embryo rostral to the occipital somites called the somitomeres. However, substantial controversy remains over their existence, and will not be discussed in this review.
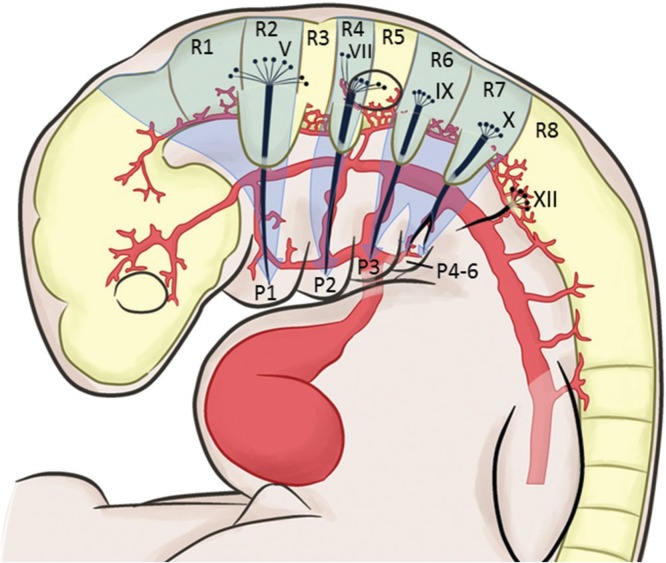
The pharyngeal arches appear in the fourth and fifth weeks of development and contribute to the formation of the face and neck. Each pharyngeal arch consists of an ectodermal surface, mesenchymal core, and an inner epithelium of endodermal origin. A distinct feature of the pharyngeal arch is that the core of each arch receives substantial numbers of neural crest cells of the hindbrain, which migrate into the arches to contribute to skeletal components of the face. The original mesoderm of the pharyngeal arches develops into the musculature of the face and neck. Thus, each pharyngeal arch is characterized by its own muscular components. The muscular components of each arch are associated with their own cranial nerve (Fig. 2). The muscle component of the first pharyngeal arch is innervated by the mandibular branch of the trigeminal artery; the second pharyngeal arch by the facial nerve; the third pharyngeal arch by the glossopharyngeal nerve; and the fourth and sixth pharyngeal arch by the superior and the recurrent branch of the vagal nerve (Fig. 3). In addition, each arch is associated with its own arterial component, the aortic arch (Fig. 1).8)
Fig. 3.
Rhombomeres and their relationship to the pharyngeal arches. The hindbrain has eight distinct segments called the rhombomeres (R1–R8). The rhombomeres give rise to motor nuclei to cranial nerves IV, V, VI, VII, IX, X, XI, and XII, as well as neural crest cells to the pharyngeal arches. Migration of the neural crest cells occur in three streams (arrows) to their designated pharyngeal arches. The neural crest cells provide guidance cues for the sensory cranial nerve fibers in the ganglia to establish connection with the corresponding rhombomeres. P: pharyngeal arch.
By the end of the fourth week of development, coinciding the pharyngeal and aortic arch formation, nuclei for all twelve cranial nerves has formed in the brain. At the same period, eight distinct segments called the rhombomeres (R1–R8) are established by neuroepithelial proliferation centers in the hindbrain (Fig. 3). The rhombomeres give rise to motor nuclei of cranial nerves IV, V, VI, VII, IX, X, XI, and XII. The rhombomere 2 give rise to the motor nucleus of the trigeminal nerve, rhombomere 4 to the facial nerve, rhombomere 6 to the glossopharyngeal nerve, rhombomere 7 to the vagal nerve, and rhombomere 8 to the hypoglossal nerve (Fig. 3). Innervation of the muscular structures by the cranial motor nerves is as discussed above (Fig. 2). The rhombomeres also give rise to neural crest cells to populate specific pharyngeal arches. These neural crest cells migrate in three streams: R1 and R2 as well as midbrain neural crest cells to pharyngeal arch 1; R4 to pharyngeal arch 2, R6 and R7 to pharyngeal arch 3–6 (Fig. 3 arrows). R3 and R5 do not participate in cell migration. The three streams provide neural axonal guidance cues for axons from sensory ganglia forming in the head and neck regions, including the trigeminal, geniculate, vestibuloacoustic, petrosal, and nodose ganglia. Axons from the trigeminal ganglion enter the hindbrain at R2; those from the geniculate and vestibuloacoustic at R4; and those from the petrosal and nodose at R6 and R7. Establishment of this segmental pattern appears to be directed by occipital somites beneath the overlying neuroepithelium. Motor neurons for cranial nuclei are within the brainstem, while sensory ganglia originate from ectodermal placodes and neural crest cells that are outside the brain. Thus, the organization of the cranial nerves is homologous to that of spinal nerves, although not all cranial nerves contain both motor and sensory fibers.8)
Now that the segmentation factors in the occipitocervical region at the time of the carotid-vertebrobasilar anastomoses formation have been individually discussed, attempts to relate these factors will be made. Muller and O’Rahilly have studied the relationships between the somitic, rhombomeric, and pharyngeal segmentation in 145 serially sectioned human embryos.7) In their study, they observed the following. (1) Occipital somites 1–4 are related to rhombomere 8 and are caudal to pharyngeal arch 4. (2) The trigeminal neural crest is related to rhombomere 2. (3) The otic vesicle is partly related to rhombomere 4 and the whole of rhombomere 5 and to pharyngeal arches 2 and 3. (4) The hypoglossal neural crest is related to rhombomere 8, pharyngeal arch 4, and present at the level of occipital somites 2–4 (Table 1). These observations indicate that rhombomere 8 is the rostral most neural structure that may retain the segmental property that resemble the spinal cord, because the occipital somites, units of vertebrate segmentation, are related only to rhombomere 8 and not to other cephalic segmental factors.
Table 1.
Association of trigeminal, otic, and hypoglossal neural crest to segmental structures in the early embryonic period
| Occipital somite | Pharyngeal arch | Rhombomere | |
|---|---|---|---|
| Trigeminal | NA | 1 | 2 |
| Otic | NA | 2, 3 | 4, 5 |
| Hypoglossal | 2-4 | 4 | 8 |
In addition, Muller and O’Rahilly make special reference to the hypoglossal nerve and artery.7) The hypoglossal nerve fibers are formed from the rhombomere 8 to innervate the occipital somites 2–4 (in Carnegie stages 12, 13), which show an obvious resemblance to a spinal nerve. At this stage, the somites 2–4 are supplied by intersegmental arteries, of which the names are unspecified.5,7) Later in stage 14, the multiple hypoglossal fibers form two stems before uniting as the hypoglossal nerves, so by that time resemblance to a spinal nerve is lost. The hypoglossal artery accompanies the hypoglossal nerve, together which comprise a significant landmark situated between occipital somites 3 and 4. Thus, the hypoglossal artery exists at the border of the occipitocervical junction (somites 3 and 4), and above the first cervical space (somites 4 and 5).
Taking all the discussions into account, the following may be deduced: (1) Rhombomere 8 may be the rostral most neural structure that retain the obvious segmental property; (2) The hypoglossal artery and nerve are segmental components associated with rhombomere 8 and occipital somites 2–4; (3) For the neural structure rostral to the occipital somites, existence of the segmental property is likely, on the basis of rhombomeric (segmental) neural crest cell migration, association of cranial nerves to specific rhombomere, and the resemblance of the cranial nerves to spinal nerves; (4) Primitive trigeminal and otic arteries may be segmental, provided that they are associated with rhombomere 2 and 4 structures, respectively (see Fig. 3).
The Persistent Primitive Carotid-vertebrobasilar Anastomoses
The four primitive carotid-vertebrobasilar anastomoses occasionally persist into the adult period and may be detected incidentally. Rarely, these anastomoses are associated with pathology. A brief review of each anastomosis is made in this section.
The persistent primitive trigeminal artery (Figs. 4 and 5)
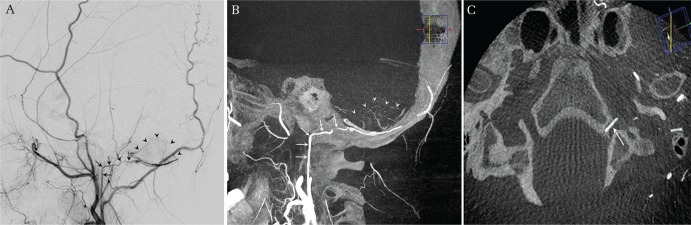
Fig. 4.
Persistent primitive trigeminal artery, lateral type. An incidentally discovered persistent primitive trigeminal artery (PTA) originating from the left internal carotid artery (ICA) in a patient who underwent clipping surgery for a ruptured anterior communicating artery aneurysm. Left ICA angiogram in AP (A) and lateral (B) views demonstrates a prominent PTA first coursing posterolaterally, then posteromedially to communicate with the upper basilar artery. CT-like axial image reconstruction of the three-dimensional rotational angiogram (3DRA) shows the PTA coursing near the apex of the petrous bone, reaffirming the lateral type PTA. Note that a small branch to the pons is originating from the PTA near the petrous apex in a magnified thin slice reconstructed axial 3DRA (arrow).
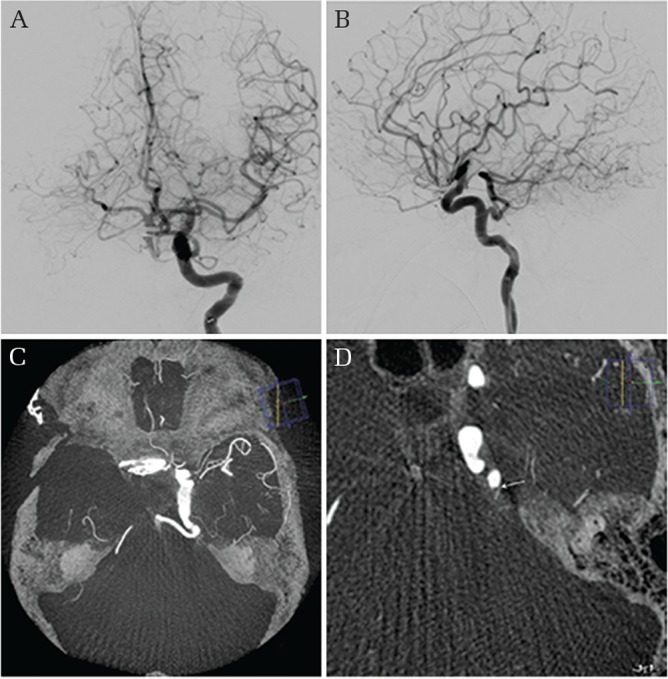
Fig. 5.
Persistent primitive trigeminal artery variant. Left internal carotid artery angiogram (A) and three-dimensional rotational angiogram (B) in lateral views show an incidental small PTA variant originating from the cavernous internal carotid artery supplying the left posterior inferior cerebellar artery territory.
The persistent primitive trigeminal artery (PTA) is the most common persistent primitive carotid-vertebrobasilar anastomoses with an incidence of approximately 0.5–0.7%.11,12)
Detailed anatomy of the PTA is based on sporadic cadaveric dissection case reports.13–21) The PTA commonly originates either from the posterolateral or posteromedial wall of the intracavenous ICA. Less commonly, the PTA may arise from the petrous ICA before entering the cavernous sinus. Intracavernously, the PTA takes either a lateral/petrosal or a medial/sphenoidal course, and categorized as lateral or medial type. The lateral type PTA arises from the posterolateral aspect of the C4 segment of the ICA, courses lateral to the abducens nerve, and pierces the dura just medial to the sensory root of the trigeminal nerve (Fig. 4).18) The medial type originates from the posteromedial aspect of the C4 ICA, courses medial to the abducens nerve, and pierces the dura of the dorsum sellae.18) After piercing the dura, the PTA joins the distal third of the basilar artery. The frequency of the lateral and medial types had been reported to be approximately equal for each type. However, two recent large studies have demonstrated 11 times higher incidence for the lateral type.11,12)
Occasionally, the PTA lacks communication with the basilar artery and directly supplies one of the cerebellar arteries (superior, anterior inferior, or posterior inferior cerebellar artery). This type of PTA is called the PTA variant (Fig. 5).
During its course, the PTA may give rise to branches to its related structures. Branches from a PTA in an adult anatomic specimen have been reported in only nine cases (Table 2). Interestingly, only the lateral type PTA was associated with the trigeminal nerve root and the neural structures. The medial type PTA was only associated with the branches of the menigohypophyseal trunk (MHT) and never with the branches to the neural structures. Additionally, Uchino et al. have demonstrated two lateral type PTAs, but no medial type PTA, supplying the superior cerebellar artery (neural structures) on magnetic resonance angiography (MRA).12) Suttner et al. hypothesized that the lateral and medial type PTA may be of different carotid-vertebrobasilar anastomosis origin, and that the branches of the MHT may be the remnant of the medial type PTA.19) Some authorities support this view as well, stating that the lateral artery of the clivus is the remnant of the trigeminal artery.22) Considering that the embryonic primitive trigeminal artery supplies the trigeminal ganglion and the hindbrain, the lateral type PTA, with its trigeminal and pontine branches, may represent the true remnant of the primitive trigeminal artery.19) At present, the definitive answer to what becomes of the primitive trigeminal artery is a subject of debate.
Table 2.
Branches of the persistent primitive trigeminal artery in relation to its course
| Series | Course | Branches |
|---|---|---|
| Yamada, et al.14) | Lateral | Trigeminal root, pontine perforating branches, AICA |
| Inoue, et al.15) | Medial | Inferior hypophyseal, tentorial, clival |
| Kida, et al.16) | Lateral | Trigeminal root, pontine perforating branches, AICA |
| Ohshiro et al.17) | Lateral | At the origin: MHT, AICS Intradural: trigeminal root, pontine perforating branches |
| Salas, et al.18) | Lateral | Pontine perforating branches |
| Suttner, et al.19) | Medial | Inferior hypophyseal, clival |
| Arakawa, et al.20) | Lateral | Trigeminal root, pontine perforating branches, AICA |
| Lateral | Trigeminal root, pontine perforating branches, AICA | |
| Tubbs, et al.21) | Lateral | At the origin: inferior hypophyseal |
AICA: anterior inferior cerebellar artery, AICS: artery of the inferior cavernous sinus, MHT: meningohypophyseal trunk.
The clinical relevance of the PTA or PTA variant to various vascular pathologies is largely considered not significant.23) Based on analysis with a large number of MRA, the association of PTA/PTA variant with intracranial aneurysm, arteriovenous malformation, Moyamoya disease, Sturge-Weber syndrome, and mental retardation was not identified.11,12) Rarely, PTA and its variant is associated with cranial nerve compression syndrome such as trigeminal neuralgia or oculomotor palsy.24–27) Of note is the association of PTA with a much higher frequency (12–16%) than expected in children with PHACE syndrome (acronym indicating malformative abnormalities occurring with infantile hemangioma in the face: P = posterior fossa; H = hemangioma; A = arterial; C = cardiac; E = eye. PHACES: S = sternal cleft). The association of PTA together with cardiovascular and structural brain anomalies is thought to be part of an embryonic error in vaculogenesis.23,28)
The Persistent Primitive Otic Artery
The persistent primitive otic artery (POA) is an extremely rare vessel that has been convincingly reported only eight times to date.29–34) In fact, the very existence of this vessel is in question. Unlike the trigeminal, hypoglossal, or proatlantal intersegmental arteries, which are strongly associated with the rhombencephalon and spinal cord, the primitive otic artery is associated with the otic placode.35) In addition, Lasjaunias and associates have denied its existence due to the fact that the eighth cranial nerve remains in the petrous bone, and that the phylogenic evidence showing dorsal aorta to longitudinal neural artery anastomosis at this level is lacking.6)
A rational diagnostic criteria set by Lie is as follows. (1) The POA should arise in the lateral portion of the petrous canal, close to the medial turn (Fig. 6, compare with Fig. 4). (2) The POA should run through the internal auditory meatus. (3) The POA should join the basilar artery at a caudal point.36) Many previous reports have misdiagnosed a low-lying PTA or a stapedial artery remnant37) as the POA. Using modern diagnostic modalities such as MRI/MRA and three-dimensional rotational angiogram, more accurate diagnosis is possible and the ongoing controversy over the POA may be clarified.
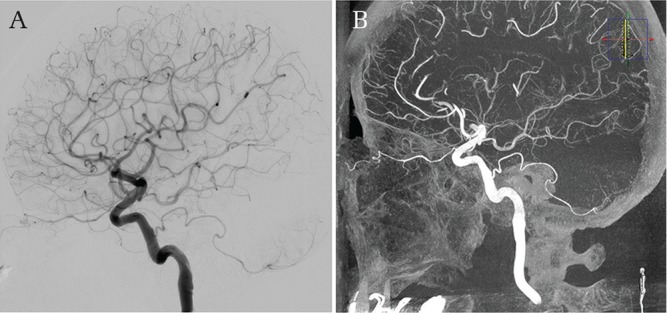
Fig. 6.
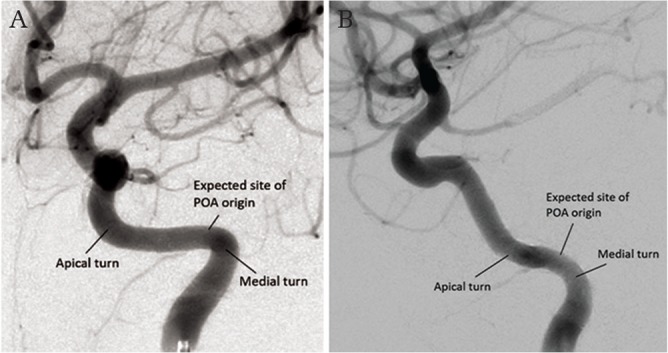
Origin of the persistent primitive otic artery. The persistent primitive otic artery (POA) should arise in the lateral portion of the petrous canal, close to the medial turn. The expected origin of the POA is indicated in an internal carotid artery angiogram in AP (A) and lateral (B) views. Compare with the origin of the persistent primitive trigeminal artery in Fig. 4.
The Persistent Primitive Hypoglossal Artery (Figs. 7 and 8)
Fig. 7.
Persistent primitive hypoglossal artery. An incidental persistent primitive hypoglossal artery (PHA) was discovered during an angiographic workup for a left cavernous internal carotid artery aneurysm. Right common carotid artery angiogram in AP (A) and lateral (B) views demonstrates a large PHA originating from the ICA at the C2 level (arrow). The ipsilateral vertebral artery was absent (not shown) and the contralateral was hypoplastic (small arrows). CT-like axial image reconstruction of the three-dimensional rotational angiogram shows the PHA passing through the right hypoglossal canal (C, arrow).
Fig. 8.
Persistent primitive hypoglossal artery variant. Right external carotid angiogram (A) and three-dimensional rotational angiogram (3DRA) (B) in lateral views show the hypoglossal branch of the ascending pharyngeal artery (arrows) partially supplying the right posterior inferior cerebellar artery territory (arrowheads). This vessel was considered a persistent primitive hypoglossal artery (PHA) variant. The patient underwent angiographic workup for a left cerebellar hemorrhage that was judged unrelated to the PHA variant. Note the PHA variant traversing the right hypoglossal canal in the axially reconstructed 3DRA image (C, arrow).
The persistent primitive hypoglossal artery (PHA) is the second most common persistent carotid-vertebrobasilar anastomosis with an incidence ranging from 0.027% to 0.29%.38,39) The PHA usually arises from the posterior side of the cervical ICA between the C1 and C3 levels and traverses the hypoglossal canal to form the vertebrobasilar artery. The diagnosis of PHA is made using the criteria revised by Brismar: (1) The PHA leaves the ICA as a large extracranial branch; (2) The PHA passes through hypoglossal canal; (3) The PHA gives rise to the trunk of the basilar artery.38) The most important factor of these is considered to be the passage of the PHA through the hypoglossal canal (Fig. 7).
In rare occasions, the PHA may originate from the external carotid artery (ECA) with nine such cases reported to date.40–46) Lasjaunias and colleagues presumed that the remnant of the primitive hypoglossal artery in adult was the ascending pharyngeal artery.6) Provided that their theory is correct, PHA of ECA origin is reasonable. An extremely rare PHA known as the PHA variant has been reported in a few occasions.47–50) In this variant, a vessel originating from the ICA or the ECA travels through the hypoglossal canal to directly supply the posterior inferior cerebellar artery region without an interposing vertebral artery (Fig. 8).
Historically, the contralateral vertebral artery is reported to be hypoplastic in one third of cases.51) In a recent large study published by Uchino et al. including over 2,000 patients and using CT angiography and MRA as the diagnostic modality, the same observation was affirmed.44)
In clinical practice, it is important to recognize that the PHA is the major supplier of the posterior circulation that may greatly influence the pathophysiology and treatment selection of the disease.52–55) In addition, as the ascending pharyngeal artery may represent the remnant of the PHA, one must be aware of the potential anastomosis between this artery and the vertebrobasilar system as well as the ICA when planning an embolization.
The Persistent Primitive Proatlantal Intersegmental Artery
The persistent primitive proatlantal intersegmental artery (PIA) originates from the common carotid artery bifurcation, external carotid artery, or internal carotid artery at the C2–4 cervical levels. The PIA then joins the vertebral artery at the suboccipital region and traverses the foramen magnum. Of these features, the only reliable identification criterion is the passage of the proatlantal artery through the foramen magnum.56) Two types are classified.57) The type 1 PIA arises from the posterior ICA and courses between the C1 arch and the occiput before entering the foramen magnum. The type 2 PIA originates from the proximal ECA and travel between the C1–2 interspace to join the vertebral artery. Thus, type 1 and 2 PIA may be regarded as persistence of the C1 and C2 segmental arteries, respectively.6)
Angiographically, the PIA is often confused with the PHA, but the differentiation can be made in the lateral view at the suboccipital region. In this region, the PIA follows an identical course to the normal vertebral artery. On the other hand, the PHA, entering the skull through the hypoglossal canal, lacks the dorsal sweep in the suboccipital region (Fig. 9).58)
Fig. 9.
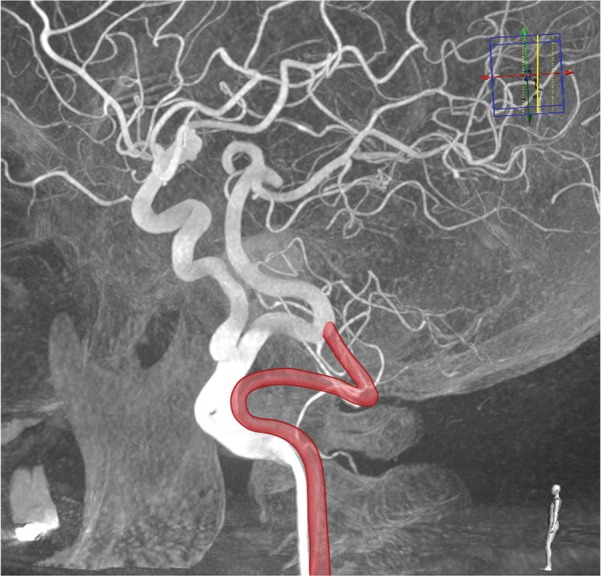
Course of the persistent primitive proatlantal intersegmental artery. Three-dimensional rotational angiogram of a right common carotid artery in lateral view in a patient with a persistent primitive hypoglossal artery (PHA). Added is a schematic drawing of the course of the persistent primitive proatlantal intersegmental artery (PIA) in the suboccipital region. Note the dorsal course of the PIA compared to the PHA.
As discussed earlier, the primitive proatlantal intersegmetal artery, together with the primitive trigeminal artery, is the major feeder of the longitudinal neural artery. As such, it is surprising that we rarely come across the PIA. This may be attributable to the fact that the distal PIA becomes incorporated into the horizontal segment of the vertebral artery in normal subjects.5) Some authors assume that the PIA has close links with the horizontal portion of the occipital artery.6) They hypothesized that the occipital artery may be the remnants of the first two segmental arteries on the basis of the following reasons: (1) Identical course of the occipital artery and the first two segmental arteries; (2) Different varieties of origin of the occipital artery from the vertebral artery; (3) Constantly present C1 and C2 segmental arteries originating from the occipital artery (Fig. 10). However, it is noteworthy that the PIA actually is the proximal C1 segment of the C1 segmental artery extending from the dorsal aorta to the horizontal atlanto-occipital portion (Fig. 2 arrowhead).34)
Fig. 10.
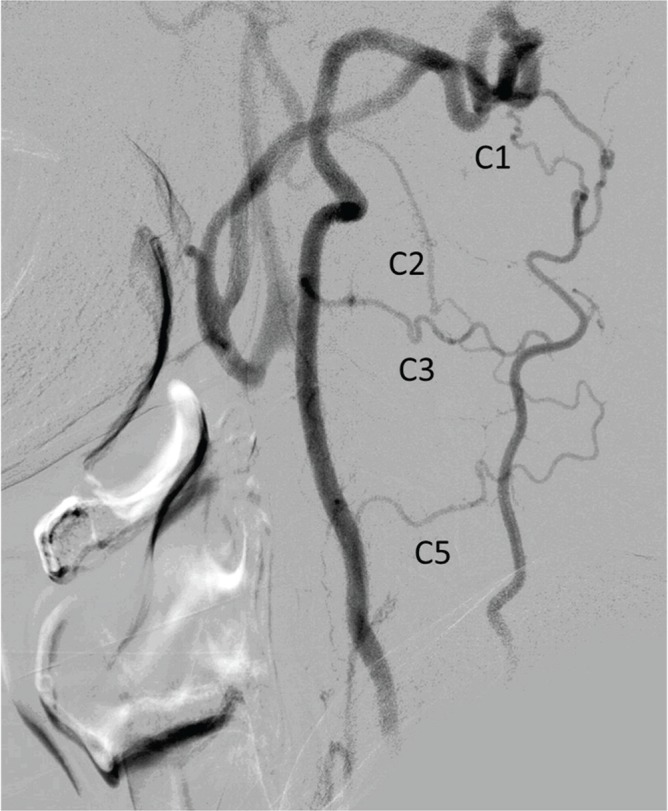
Cervical segmental arteries. Left subclavian artery angiogram in lateral view in a patient with severe left common carotid and left vertebral artery stenosis. The left occipital and vertebral arteries are reconstituted via the C1, C2, C3, and C5 segmental arteries. Anastomosis between the occipital and vertebral arteries is prominent at the C1 level, which may represent an embryonic link between the two vessels via the primitive proatlantal intersegmental artery. Note the C1 and C2 segmental artery reconstituting the occipital artery.
In clinical practice, anastomosis between the occipital artery and vertebral artery through the cervical segmental artery becomes most apparent in steno-occlusive disease of the carotid or vertebral arteries (Fig. 10). In normal subjects, the anastomoses may not be observed. However, anastomoses between the two arteries invariably exist, and caution should be given to the potential risk of thromboembolism when planning an embolization in this region.
Unclassified Persistent Primitive Carotid-vertebrobasilar Anastomoses
Recently, a few reports describing previously unknown carotid-vertebrobasilar anastomoses were published.59–61) Ranchod AI et al. and Ryu et al. reported a large carotid-vertebrobasilar anastomosis between the cervical ICA and the intracranial vertebral artery passing through the jugular foramen. The authors of both papers discuss the possible involvement of the jugular branch (one of the branch of the neuromeningeal trunk) of the ascending pharyngeal artery in the persistence of this vessel. This explanation seems feasible in light of the fact that the neuromeningeal trunk of the ascending pharyngeal artery is thought to be the remnant of the primitive hypoglossal artery by some researchers.6)
Kirkland et al. described a robust anomalous vessel originating from the ICA that traversed a foramen medial to the hypoglossal canal to become the sole contributor to the vertebral artery. An unknown variant of PHA or PIA was proposed for this artery and they named this vessel a ‘transclival artery’. However, the primitive carotid-vertebrobasilar anastomoses are named according to the nerve with which each vessel accompanies, and this nomenclature may not be appropriate. To the author’s best speculation, this vessel may be a PHA that traverses a separate foramen from the hypoglossal nerve, just as the accessory meningeal artery sometimes course through the foramen of Vesalius instead of the foramen ovale. Further study to clarify the associated nerve to this new vessel is warranted.
Conclusion
The embryology of the primitive carotid-vertebrobasilar anastomoses with special attention to the segmental property of these vessels was reviewed. This was followed by a brief review of the persistent form of the primitive carotid-vertebrobasilar anastomoses in adults.
Acknowledgment
I thank Hisako Namba for preparing all the illustrations in this review.
Footnotes
Conflicts of Interest Disclosure
The author has no personal financial or institutional interest in any of the drugs, materials, or devices described in this article. The author has not received any financial support in conjunction with the generation of the manuscript. No grant was received to generate the paper.
The author has registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.
References
- 1).Padget DH: The development of the cranial arteries in the human embryo. Contrib Embryol 32: 205–261, 1948 [Google Scholar]
- 2).De Vriese B: Sur la signification morphologique des arteres cerebrales. Arch de Biol 21: 357–457, 1905 [Google Scholar]
- 3).Evans HM: The development of the vascular system. In Keibel F, Mall FP. (eds): Manual of Human Embryology. Philadelphia & London, J.B. Lippincott, 1910, pp. 570–709 [Google Scholar]
- 4).Tandler J: Zur entwicklungsgeschichte der kopfarterien bei den mammalia. Morphol Jahrb 30: 275–373, 1902 [Google Scholar]
- 5).Padget DH: Designation of the embryonic intersegmental arteries in reference to the vertebral artery and subclavian stem. Anat Rec 119: 349–356, 1954 [DOI] [PubMed] [Google Scholar]
- 6).Lasjaunias P, Berenstein A, Ter Brugge KG: The pharyngo-occipital system. In Lasjaunias P, Berenstein A, Ter Brugge KG. (eds): Surgical Neuroangiography, Volume 1, Clinical Vascular Anatomy and Variations, ed 2 Berlin, Springer-Verlag, 2001, pp. 165–224 [Google Scholar]
- 7).Müller F, O’Rahilly R: Segmentation in staged human embryos: the occipitocervical region revisited. J Anat 203: 297–315, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Sadler TW: Langman’s medical embryology, ed 11 Baltimore, Lippincott Williams & Wilkins, 2010 [Google Scholar]
- 9).Bardeen CR: Early development of the cervical vertebrae and the base of the occipital bone in man. Am J Anat 8: 181–186, 1908 [Google Scholar]
- 10).Sensenig EC: The development of the occipital and cervical segments and their associated structures in human embryos. Contrib Embryol 36: 141–151, 1957 [Google Scholar]
- 11).O’uchi E, O’uchi T: Persistent primitive trigeminal arteries (PTA) and its variant (PTAV): analysis of 103 cases detected in 16,415 cases of MRA over 3 years. Neuroradiology 52: 1111–1119, 2010 [DOI] [PubMed] [Google Scholar]
- 12).Uchino A, Saito N, Okada Y, Kozawa E, Mizukoshi W, Inoue K, Takahashi M: Persistent trigeminal artery and its variants on MR angiography. Surg Radiol Anat 34: 271–276, 2012 [DOI] [PubMed] [Google Scholar]
- 13).Quain R: Anatomy of the arteries of the human body and its applications to pathology and operative surgery. London, Taylor and Walton, 1844, p. 507 [Google Scholar]
- 14).Yamada M, Kodama K, Kawai K, Okamoto K: [The anterior inferior cerebellar artery as a branch of the internal carotid artery – its relation to the trigeminal artery]. Kaibogaku Zasshi 58: 112, 1983. (Japanese) [Google Scholar]
- 15).Inoue T, Rhoton AL, Theele D, Barry ME: Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery 26: 903–932, 1990 [DOI] [PubMed] [Google Scholar]
- 16).Kida MY, Ishida H, Dodo Y, Ikeda K, Kobayashi D, Nonaka M: A case of the persistent trigeminal artery. Sapporo Med J 59: 429–633, 1990. (Japanese) [Google Scholar]
- 17).Ohshiro S, Inoue T, Hamada Y, Matsuno H: Branches of the persistent primitive trigeminal artery—an autopsy case. Neurosurgery 32: 144–148, 1993 [DOI] [PubMed] [Google Scholar]
- 18).Salas E, Ziyal IM, Sekhar LN, Wright DC: Persistent trigeminal artery: an anatomic study. Neurosurgery 43: 557–561; discussion 561–562, 1998 [DOI] [PubMed] [Google Scholar]
- 19).Suttner N, Mura J, Tedeschi H, Ferreira MA, Wen HT, de Oliveira E, Rhoton AL, Jr: Persistent trigeminal artery: a unique anatomic specimen – analysis and therapeutic implications. Neurosurgery 47: 428–434, 2000 [DOI] [PubMed] [Google Scholar]
- 20).Arakawa T, Koizumi M, Terashima T, Honma S, Kawai K, Kodama K, Miki A: Two anatomical autopsy cases of direct communication between a persistent primitive trigeminal artery and an anterior inferior cerebellar artery. Ann Anat 189: 489–498, 2007 [DOI] [PubMed] [Google Scholar]
- 21).Tubbs RS, Shoja MM, Salter EG, Oakes WJ: Cadaveric findings of persistent fetal trigeminal arteries. Clin Anat 20: 367–370, 2007 [DOI] [PubMed] [Google Scholar]
- 22).Lasjaunias P, Berenstein A, Ter Brugge KG: The cavernous sinus region. In Lasjaunias P, Berenstein A, Ter Brugge KG. (eds): Surgical Neuroangiography, Volume 1, Clinical Vascular Anatomy and Variations, ed 2 Berlin, Springer-Verlag, 2001, pp. 387–425 [Google Scholar]
- 23).Meckel S, Spittau B, McAuliffe W: The persistent trigeminal artery: development, imaging anatomy, variants, and associated vascular pathologies. Neuroradiology 55: 5–16, 2013 [DOI] [PubMed] [Google Scholar]
- 24).Morita A, Fukushima T, Miyazaki S, Shimizu T, Atsuchi M: Tic douloureux caused by primitive trigeminal artery or its variant. J Neurosurg 70: 415–419, 1989 [DOI] [PubMed] [Google Scholar]
- 25).Tamura Y, Shimano H, Kuroiwa T, Miki Y: Trigeminal neuralgia associated with a primitive trigeminal artery variant: case report. Neurosurgery 52: 1217–1219; discussion 1219–1220, 2003 [PubMed] [Google Scholar]
- 26).Yamada Y, Kondo A, Tanabe H: Trigeminal neuralgia associated with an anomalous artery originating from the persistent primitive trigeminal artery. Neurol Med Chir (Tokyo) 46: 194–197, 2006 [DOI] [PubMed] [Google Scholar]
- 27).Bosco D, Consoli D, Lanza PL, Plastino M, Nicoletti F, Ceccotti C: Complete oculomotor palsy caused by persistent trigeminal artery. Neurol Sci 31: 657–659, 2010 [DOI] [PubMed] [Google Scholar]
- 28).Metry D, Heyer G, Hess C, Garzon M, Haggstrom A, Frommelt P, Adams D, Siegel D, Hall K, Powell J, Frieden I, Drolet B, PHACE Syndrome Research Conference : Consensus statement on diagnostic criteria for PHACE syndrome. Pediatrics 124: 1447–1456, 2009 [DOI] [PubMed] [Google Scholar]
- 29).Tomsick TA, Lukin RR, Chambers AA: Persistent trigeminal artery: unusual associated abnormalities. Neuroradiology 17: 253–257, 1979 [DOI] [PubMed] [Google Scholar]
- 30).Reynolds AF, Stovring J, Turner PT: Persistent otic artery. Surg Neurol 13: 115–117, 1980 [PubMed] [Google Scholar]
- 31).Luh GY, Dean BL, Tomsick TA, Wallace RC: The persistent fetal carotid-vertebrobasilar anastomoses. AJR Am J Roentgenol 172: 1427–1432, 1999 [DOI] [PubMed] [Google Scholar]
- 32).Patel AB, Gandhi CD, Bederson JB: Angiographic documentation of a persistent otic artery. ANJR Am J Neuroradiol 24: 124–126, 2003 [PMC free article] [PubMed] [Google Scholar]
- 33).Zhang CW, Xie XD, Yang ZG, Wang CH, You C, Mao BY, He M, Sun H: Giant cavernous aneurysm associated with a persistent trigeminal artery and persistent otic artery. Korean J Radiol 10: 519–522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Raybaud C: Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg Clin N Am 21: 399–426, 2010 [DOI] [PubMed] [Google Scholar]
- 35).Bhattacharya JJ, Lamin S, Thammaroj J: Otic or mythic? ANJR Am J Neuroradiol 25: 160–162, 2004 [PMC free article] [PubMed] [Google Scholar]
- 36).Lie TA: Congenital Anomalies of the Carotid Arteries. Amsterdam, Excerpta Medica Foundation, 1968, pp. 70–75 [Google Scholar]
- 37).Osbourne A: Diagnostic Cerebral Angiography, ed 2 Philadelphia, Lippincott Williams & Wilkins, 1999 [Google Scholar]
- 38).Brismar J: Persistent hypoglossal artery, diagnostic criteria. Report of a case. Acta Radiol Diagn (Stockh) 17: 160–166, 1976 [DOI] [PubMed] [Google Scholar]
- 39).Uchino A, Saito N, Okada Y, Kozawa E, Nishi N, Mizukoshi W, Inoue K, Nakajima R, Takahashi M: Persistent hypoglossal artery and its variants diagnosed by CT and MR angiography. Neuroradiology 55: 17–23, 2013 [DOI] [PubMed] [Google Scholar]
- 40).Welten RJ, Eikelboom BC, Ackerstaff RG, Ludwig JW: A persistent hypoglossal artery arising from the external carotid artery. Eur J Vasc Surg 2: 269–272, 1988 [DOI] [PubMed] [Google Scholar]
- 41).Nakamura M, Kobayashi S, Yoshida T, Kamagata M, Sasaki T: Persistent external carotid-vertebrobasilar anastomosis via the hypoglossal canal. Neuroradiology 42: 821–823, 2000 [DOI] [PubMed] [Google Scholar]
- 42).Meguro T, Terada K, Hirotsune N, Nishino S, Asano T: Unusual variant of persistent primitive hypoglossal artery. Br J Radiol 80: e314–316, 2007 [DOI] [PubMed] [Google Scholar]
- 43).Lee EJ, Chang HW, Cho CH, Kim E, Lee SK, Kwon JH: Rare variant of persistent primitive hypoglossal artery in magnetic resonance angiography. Surg Radiol Anat 32: 801–804, 2010 [DOI] [PubMed] [Google Scholar]
- 44).Uchino A, Saito N: Persistent hypoglossal artery arising from the external carotid artery diagnosed by MR angiography. Surg Radiol Anat 33: 543–545, 2011 [DOI] [PubMed] [Google Scholar]
- 45).Nanto M, Takado M, Ohbuchi H, Mandai A, Osaka Y, Nakahara Y, Tenjin H: Rare variant of persistent primitive hypoglossal artery, arising from the external carotid artery. Neurol Med Chir (Tokyo) 52: 513–515, 2012 [DOI] [PubMed] [Google Scholar]
- 46).Sabouri S, Ebrahimzadeh SA, Rahimian N: Unusual variant of persistent primitive hypoglossal artery diagnosed by CT angiography: a case report and literature review. Clin Neuroradiol 24: 59–63, 2014 [DOI] [PubMed] [Google Scholar]
- 47).Murayama Y, Fujimoto N, Matsumoto K: Bilateral persistent primitive hypoglossal arteries associated with a large ruptured aneurysm on one side. Surg Neurol 24: 498–502, 1985 [DOI] [PubMed] [Google Scholar]
- 48).Andoh K, Tanohata K, Moriya N, Hagiwara H, Lee J, Sato M, Yoshida T, Nagashima T: The posterior inferior cerebellar artery arising from the extracranial segment of the internal carotid artery via the hypoglossal canal without an interposed segment of the basilar artery: a persistent primitive hypoglossal artery variant. Clin Imaging 25: 86–89, 2001 [DOI] [PubMed] [Google Scholar]
- 49).Lasjaunias P, Guibert-Tranier F, Braun JP: The pharyngo-cerebellar artery or ascending pharyngeal artery origin of the posterior inferior cerebellar artery. J Neuroradiol 8: 317–325, 1981 [PubMed] [Google Scholar]
- 50).Kim JT, Heo SH, Lee SH, Choi SM, Park MS, Kim BC, Yoon W, Kim MK, Cho KH: An uncommon anastomosis of the posterior inferior cerebellar artery and the external carotid artery with the patent vertebrobasilar system. Br J Radiol 82: e171–174, 2009 [DOI] [PubMed] [Google Scholar]
- 51).Resche F, Resche-Perrin I, Robert R, De Kersaint-Gilly A, Duveau D, Lajat Y: [The hypoglossal artery. A new case report - Review of the literature]. J Neuroradiol 7: 27–43, 1980 [PubMed] [Google Scholar]
- 52).Ouriel K, Green RM, DeWeese JA: Anomalous carotid-basilar anastomoses in cerebrovascular surgery. J Vasc Surg 7: 774–777, 1988 [DOI] [PubMed] [Google Scholar]
- 53).Pyun HW, Lee DH, Kwon SU, Lee JH, Choi CG, Kim SJ, Suh DC: Internal carotid artery stenosis with ipsilateral persistent hypoglossal artery presenting as a multiterritorial embolic infarction: a case report. Acta Radiol 48: 116–118, 2007 [DOI] [PubMed] [Google Scholar]
- 54).Kanazawa R, Ishihara S, Okawara M, Ishihara H, Kohyama S, Yamane F: A successful treatment with carotid arterial stenting for symptomatic internal carotid artery severe stenosis with ipsilateral persistent primitive hypoglossal artery: case report and review of the literature. Minim Invasive Neurosurg 51: 298–302, 2008 [DOI] [PubMed] [Google Scholar]
- 55).Elhammady MS, Başkaya MK, Sonmez OF, Morcos JJ: Persistent primitive hypoglossal artery with retrograde flow from the vertebrobasilar system: a case report. Neurosurg Rev 30: 345–349, 2007 [DOI] [PubMed] [Google Scholar]
- 56).Pasco A, Papon X, Bracard S, Tanguy JY, Ter Minassian A, Mercier P: Persistent carotid-vertebrobasilar anastomoses: how and why differentiating them? J Neuroradiol 31: 391–396, 2004 [DOI] [PubMed] [Google Scholar]
- 57).Lasjaunias P, Théron J, Moret J: The occipital artery. Anatomy--normal arteriographic aspects--embryological significance. Neuroradiology 15: 31–37, 1978 [DOI] [PubMed] [Google Scholar]
- 58).Hutchinson NA, Miller JD: Persistent proatlantal artery. J Neurol Neurosurg Psychiatr 33: 524–527, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Ranchod AI, Gora S, Swartz RN, Andronikou S, Mngomezulu V: A rare carotid-basilar anastomosis traversing the jugular foramen: origin and clinical implications. Interv Neuroradiol 17: 347–350, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Ryu B, Anami H, Ishikawa T, Inoue T, Sugiura M, Kawamata T: Extremely rare persistent primitive artery passing through the jugular foramen with symptomatic ipsilateral carotid artery stenosis. Acta Neurochir (Wien) 158: 1925–1929, 2016 [DOI] [PubMed] [Google Scholar]
- 61).Kirkland JD, Dahlin BC, O’Brien WT: The transclival artery: a variant persistent carotid-basilar arterial anastomosis not previously reported. J Neurointerv Surg 9: e11, 2017 [DOI] [PubMed] [Google Scholar]