Summary
Multiple Sclerosis (MS) is a chronic autoimmune inflammatory demyelinating disorder of the central nervous system (CNS), which causes severe disability and requires extensive medical attention and treatment. While the infiltration of pathogenic immune cells into the CNS leads to the formation of inflammatory lesions in its initial relapsing-remitting stage, late stages of MS are characterized by progressive neuronal loss and demyelination even without continued interaction with the peripheral immune compartment. Several genetic and environmental factors modulate and influence these processes on multiple levels. Genetic variants confer a predisposition for the development of MS, but are not accessible to therapeutic intervention as of today. However, migration studies suggest that environmental factors influence disease development, activity and progression. This article reviews mechanisms of disease pathogenesis in MS and their modulation by environmental factors such as geographical localization, the gut microbiome and the diet.
Introduction
Multiple Sclerosis (MS) is a chronic autoimmune disorder of the central nervous system (CNS) and among the top causes of disability in young adults in the United States of America and Europe1,2. In its initial phase, autoreactive immune cells enter the CNS and cause inflammatory lesions that lead to a broad variety of neurologic symptoms. These periodic relapses define the initial stage of MS (relapsing remitting MS, RRMS) and are followed by a progressive stage in 50% of the affected individuals, in which chronic disability progresses with or without superposition of relapses due to continued demyelination and neurodegeneration (secondary progressive MS, SPMS). In 15%, MS takes a progressive course from its beginning (primary progressive MS, PPMS)3,4.
In recent years, genetic factors have been identified and found to correlate with the presence or absence of disease as shown in twin and sibling studies: the concordance rate is about 25% in monozygotic as opposed to 5% in dizygotic twins, and having a sibling with MS increases the risk of disease about 20 to 40 fold5–7. Today, over 100 genetic risk loci have been identified and include HLA alleles (HLA-DRB1*1501, DR3, DR4), cytokines, chemokines and their receptors (e. g. IL12A, IL2RA, IL7RA, CCR5, CXCR5), transcription factors (e. g. SOCS1, STAT3, IRF8, EOMES), adhesion molecules (e. g. VCAM1), as well as micro-RNA genes (e. g. MIR1204, MIR1205, MIR1208, MIR3686), clearly underlining both the importance of the immune system and the multi-factorial nature of MS pathogenesis8,9. Interestingly, genetic variants associated with Vitamin D metabolism (CYP27B1) have been shown to increase the risk of MS as well10. This observation as well as the considerably weak genetic association of most of the genes discovered (3–6 fold increase of the relative risk of MS for HLADRB1*1501 and 0.8–1.2 fold for the other loci described7,11,12) provides evidence for the relevance of environmental factors.
Mechanisms of disease pathogenesis
From a pathophysiological point of view, the different stages and subsets of MS can be potentially influenced by several environmental stimuli. In the initial relapsing-remitting stage of MS (RRMS), a breakdown of the blood brain barrier (BBB) is linked to the invasion of the CNS by pathogenic adaptive immune cells, most importantly T and B cells 13. Thus, factors that influence immune cell priming in the periphery as well as macromolecules that require an open BBB to reach the CNS can influence these initial stages of MS. Examples of these factors discussed in this review are the influence of sunlight on melatonin levels and T-cell priming, direct interactions between the gut flora and T-cell priming in the gut associated lymphatic tissue, or cigarette smoke.
On the other hand, the secondary progressive stage that follows RRMS (SPMS) as well as PPMS are characterized by CNS-intrinsic processes behind an (almost) intact BBB and are driven by CNS-resident cells such as astrocytes, microglia, oligodendrocytes in addition to pro-inflammatory monocytes recruited from the periphery. The mechanisms involved range from the production of neurotoxic mediators such as TNF-α, the inhibition of reparative processes due to increased oxidative stress, mitochondrial dysfunction, toxic products from lipid metabolism such as sphingolipids up to hitherto unknown mechanisms13,14. Thus, factors provided to the environment have to be able to cross the BBB in order to influence the CNS-restricted processes that contribute to the pathogenesis of progressive MS. Little is known about the nature and mechanisms by which these factors affect local CNS-inflammation; however, these factors are starting to be identified including metabolic products generated by the gut microbiome, e.g., toll like receptor ligands15,16, or other immunomodulatory metabolites such as Indoxyl-3-sulfate17. These and other environmental factors can influence MS progression by acting on several targets as discussed below.
Taking these considerations into account, we will briefly summarize the current knowledge about the relevance of geographical factors and focus on more recent discoveries of specific risk factors such as the diet and commensal bacteria. All of these illuminate potential avenues for the development of novel therapeutic strategies for progressive stages of MS, for which there is currently no specific therapy, and potentially other neurologic disorders.
Geographic factors and sun exposure
One of the strongest pieces of evidence for the influence of environmental factors on the development of MS is the latitude gradient. First described in the 1960s18, it delineates the fact that the incidence of MS increases with the distance from the equator in both hemispheres. The magnitude of this gradient within the United States, Europe or Norway in terms of incidence rates has decreased in the last years19–21, which seems mostly due to an increase in disease incidence in females and in southern areas without change in northern areas22. Interestingly, in the southern hemisphere the latitude gradient is still strong23,24; the reason for this difference between hemispheres is unknown. However, even when correcting for population-specific differences in the prevalence of risk alleles for MS24, the effects of the environment are still detectable, with additional support provided by migration studies in which migrants from high to low risk areas reduce their MS incidence accordingly and in correlation to their age at moving (the younger at moving, the bigger the risk reduction)25. These observations emphasize the relevance of environmental factors in the pathogenesis of MS.
Obviously and by definition, the latitude gradient reflects differences in the exposure to sunlight of a given individual. Photoimmunologic effects have been described for several cell types (e.g. dendritic cells, B and T cells, monocytes) and inflammatory mediators (e.g. IL-10, IL-13, IL-33) relevant to MS pathogenesis, which are most likely acting in concert to suppress pro-inflammatory activity while promoting anti-inflammatory effects26. In addition, the UVB-dependent generation of bioactive Vitamin D and its relevance for MS have been studied over decades, since high serum levels of Vitamin D correlate with low incidence and disease activity, thus representing both a mechanistic explanation for the latitude gradient as well as a druggable target for MS27,28. However, even though recent clinical studies showed a decrease in inflammatory cytokine serum levels of Vitamin D treated patients29, an intervention study using Vitamin D2 failed to show effectiveness of the treatment, but, in contrast, demonstrated increased disease scores in the treatment group after the study period30. Thus, changes in Vitamin D levels might represent an epiphenomenon of sun exposure rather than an explanation for the latitude gradient or a druggable target for therapeutic intervention in MS.
In addition, seasonal differences in MS disease activity have been reported and are contradictory to a protective role for Vitamin D: other than predicted by the correlation between sun exposure and increased levels of protective Vitamin D31, which would suggest higher disease activity during low sun exposure seasons such as fall and winter, recent studies have demonstrated the opposite, namely an increase in disease activity during spring and summer32. A mechanistic explanation for this observation has been offered in a study correlating disease activity to melatonin levels. Melatonin, a biochemical product generated from the essential amino acid tryptophan, is synthesized in the pineal gland and secreted in response to darkness; while it is involved in the regulation of the circadian clock as well as immune responses, it follows a seasonal pattern with increased serum levels in fall and winter33. In MS, melatonin levels negatively correlate with disease activity, most likely via blockade of Th17 and induction of Tr1 differentiation via Nfil3 and Erk1/2, respectively34. Its therapeutic potential has been demonstrated in the animal model of MS (Experimental autoimmune encephalomyelitis, EAE)34–36 and in in vitro studies using peripheral blood mononuclear cells (PBMCs) from MS patients and healthy controls, in which melatonin improves impaired antioxidant defense through upregulation of sirtuin 1 and antioxidant enzymes37. Given its antioxidant properties, which are relevant to neurodegeneration and demyelination38, melatonin might be an interesting therapeutic target for the treatment of both active and progressive stages of MS and thus worthy of further experimental and clinical study.
The gut microbiome and dietary factors
Several additional environmental factors have been shown to influence MS pathogenesis. Most prominently, infection with Epstein-Barr virus (EBV) and the subsequent immune response to this pathogen as well as environmental toxins prevalent in cigarette smoke have been validated as independent environmental risk factors for MS. These have been recently discussed in detail elsewhere7,39,40. Thus, we will focus on an interesting and meaningful research area, which has found its way into MS research and promises to pave novel avenues for therapeutic intervention – namely the interaction between the gut microbiome, the diet and autoimmune inflammation in the CNS.
While the importance of the gut microbiome, i.e., the “ecological community of commensal, symbiotic and pathogenic microorganisms”41,42, has been described some time ago for inflammatory bowel disease43, rheumathoid arthritis44 and type 1 diabetes45, only in 2011 was the essential role of the gut flora for the development of CNS inflammation discovered. Germ free mice were found to be resistant to spontaneous EAE, a striking notion, which was explained by a lack of local activation of T cells in the gut and subsequent deficient triggering of pathogenic antibody production by activated B cells46. Several follow-up studies have investigated the role of the gut microbiome using either germ free mice or mice treated orally with varying antibiotic cocktails47. While the first report from 2011 was verified and germ free mice showed either complete resistance or attenuation of EAE in several experimental paradigms46–48, using antibiotic cocktails to deplete gut microbiota has provided controversial results. Even though most of the antibiotic administration studies have shown an amelioration of EAE pointing to a disease promoting effect of gut bacteria49–51, experimental evidence suggests that certain bacterial strains have a beneficial effect on EAE development and protect from exacerbated disease, as shown in treatment experiments using the macrolide antibiotics clarithromycine and azithromycin or the cephalosporine cefuroxime52,53.
Underlining this notion, immunosuppressive mechanisms elicited by certain bacterial strains have recently been demonstrated: for example polysaccharide A from Bacteroides fragilis expands and increases the migratory capacity and suppressive activity of CD39-positive regulatory T cells through a Toll like receptor 2 (TLR2) dependent mechanism, suppressing the development of EAE as demonstrated in elegant studies by Kasper et al15,54–56. Interestingly, decreased serum levels of bacterial lipodipeptide, another TLR2 ligand, have been reported in MS patients as compared to healthy controls16. Along these lines, a recent study in pediatric MS showed perturbations in the gut microbiome of patients favoring increased glutathione metabolism and pro-inflammatory pathways associated with neurodegeneration57.
Of note, the interaction between the gut microbiome and the host seems to be based on reciprocity: T cell receptor transgenic mice lacking the TNF-α receptor 2 (TNFR2−/− 2D2) show changes in the gut microbiome and its interaction with pathogenic T cells, resulting in increased susceptibility to spontaneous CNS autoimmunity58. Taken together, both disease promoting and ameliorating mechanisms can be induced by the gut microbiota, which interact closely and mutually with the host immune system. The nature of those interactions seems to depend on the composition of the gut microbiome and the immunologic state of the host.
This concept also implies that highly selective experimental strategies will be needed to manipulate specific subsets or even single strains of bacteria as a therapeutic approach for MS. As an example of this experimental approach, the tryptophanase-positive bacterium Lactobacillus reuteri has been shown to metabolize dietary tryptophan to produce ligands for the aryl hydrocarbon receptor (AHR), which thereafter cause increased transcription of IL-22 to balance the local mucosal environment and protect from colonization with Candida albicans59. Thus, these and other immunoregulatory pathways may be targeted to mimic the beneficial effects of the commensal flora on CNS inflammation and neurodegeneration.
The interaction between the gut microbiota and the diet also impacts the development of autoimmunity. Several examples in support of this hypothesis have recently been reported: dietary intake and microbial production of long and middle chain fatty acids promote the differentiation of pathogenic T cells in the gut and their subsequent induction of inflammatory lesions in the brain. Conversely, short chain fatty acids lead to the expansion of protective regulatory T cell responses and ameliorate disease60. Moreover, germ free mice exhibit deficits in microglia maturation and function, which are due to the lack of short chain fatty acids (SCFA) generated from the diet via gut microbiota61.
In MS, several clinical trials are currently ongoing to test the effects of dietary intervention on the disease. So far, protective effects have been assigned to a Mediterranean diet, caloric restriction, fish oil, polyunsaturated fatty acids, reservatrol, vitamin D and biotin, among others62–66. However, most of these studies, even though promising, need further validation due to small sample size or lack of sufficient controls. High sodium chloride intake has also been demonstrated in several animal models to exacerbate disease severity by expanding pro-inflammatory T cells and macrophages as well as causing deficits in remyelination67–70. An observational study showed similar trends in human MS, suggesting high sodium intake worsens disease71. It will be exciting to verify these observations in multi-center prospective interventional clinical trials to define the role of dietary intervention in MS.
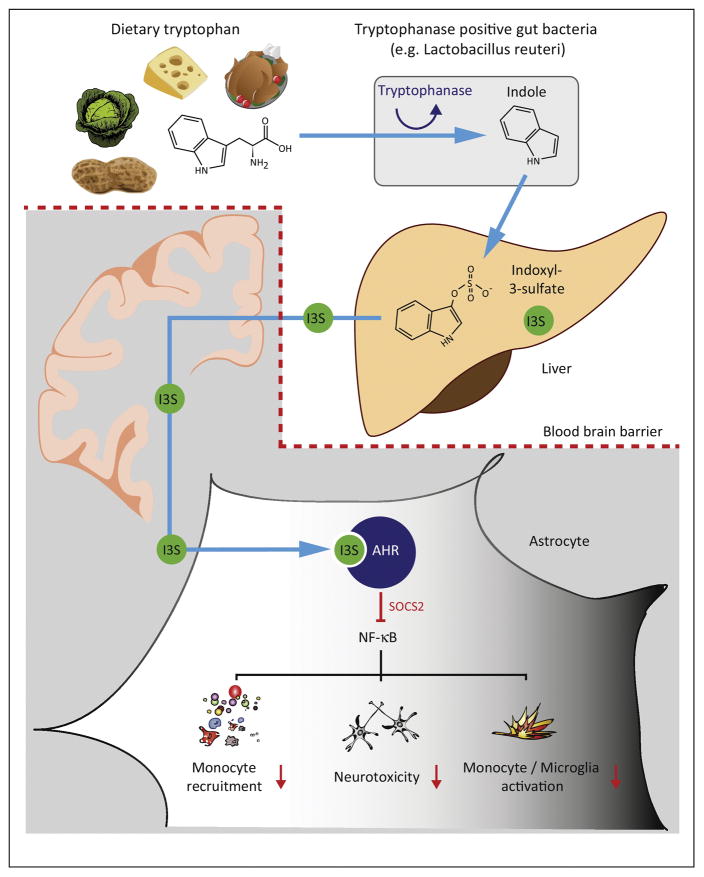
Most of the studies outlined above demonstrate alterations in the interaction of the gut microbiome, the diet and CNS immunopathology in a remote way, mostly by influencing immune cell priming or by dampening pro-inflammatory mediator production in the periphery, which lead to ameliorated disease as an indirect consequence. However, direct interaction between metabolites generated from the gut microbiota and the CNS exist as well. Our group has recently demonstrated a direct link between the generation of metabolites from dietary tryptophan and the subsequent amelioration of late stages of EAE. Mechanistically, tryptophanase-positive bacteria, such as Lactobacillus reuteri, generate indole from dietary tryptophan, which is metabolized by the host to indole-3-sulfate (I3S), indole-3-propionic acid (I3PA) and indole-3-aldehyde (I3A). Indole, I3S, I3PA, and I3A cross the blood brain barrier and suppress pro-inflammatory activities by activating AHR in astrocytes. Lack of dietary tryptophan or deficiency of AHR in astrocytes caused a failure to recover during chronic stages of EAE. Most importantly, we17 and others72,73 found that in MS and IBD patients AHR ligands in the peripheral blood were decreased and that similar mechanisms were relevant for inflammatory bowel disease74. These observations have several implications: first, dietary tryptophan and its metabolites influence local inflammation in the CNS. Second, the interaction of bacterial and host metabolism directly leads to the generation of an anti-inflammatory milieu in the CNS in a direct way, namely via metabolites generated cooperatively by gut microbes and the host. Third, MS patients seem to harbor deficits in the generation, uptake or stability of these anti-inflammatory metabolites, resulting in a decrease in their levels an in AHR-dependent immunoregulation. Taken together, these observations provide mechanistic insights relevant to MS progression, while they pave the way for the development of therapeutic interventions targeting the gut microbiome and metabolic pathways.
Conclusion
Apart from a multi-faceted genetic predisposition favoring the development of CNS autoimmunity, several environmental factors contribute to the development of disease as suggested both by the latitude gradient and migration studies. Among these, photoimmunologic effects lead to downmodulatory cell-based effects as well as the generation of Vitamin D and melatonin. While infections and environmental toxins, best documented for EBV and cigarette smoke, have been found to influence the disease course, we have only begun to appreciate the role of the gut microbiome and the diet in recent years. However, these factors do not only have an effect on early stages of the disease, but seem to influence chronic inflammation during late disease stages, when CNS intrinsic cells, such as astrocytes and microglia, are among the driving forces of axonal loss, demyelination and failure to recover. These novel observations provide starting points for further mechanistic studies dissecting the role of specific microbiota in the gut and dietary intervention. In the next decade, the examination of these factors offers hope for the development of innovative strategies for early and late stage MS and paves the avenue to increase our understanding of immunopathology in the interplay between the environment and CNS inflammation.
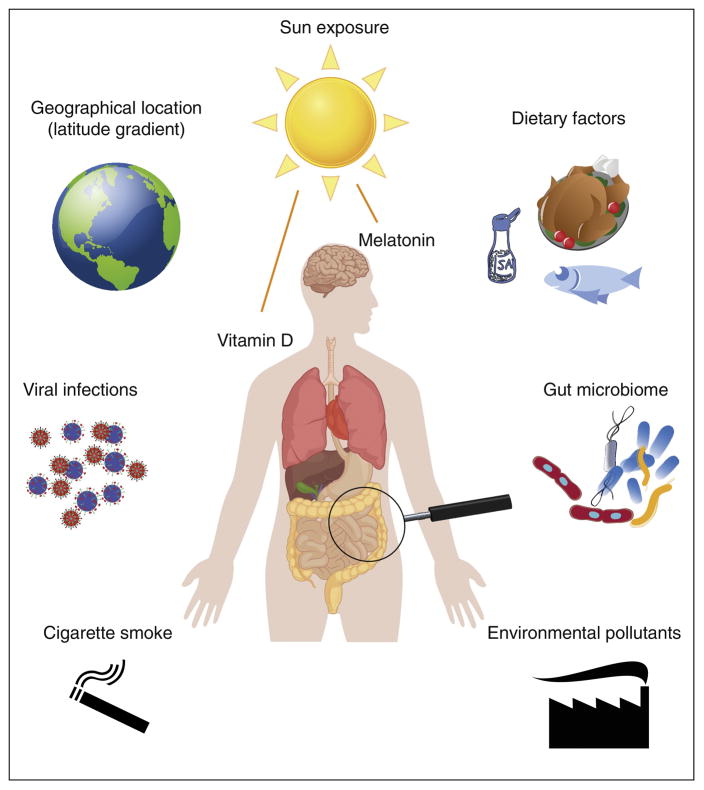
Figure 1. Enironmental factors relevant for Multiple Sclerosis.
Several environmental factors have been demonstrated to influence incidence and course of MS.
Figure 2. Metabolites of dietary tryptophan control autoimmune inflammation in the CNS.
The essential amino acid tryptophan is broken down to Indole by tryptophanase-positive, ampicillin-sensitive, vancomycin-resistant bacteria in the gut. One of the bacterial strains exhibiting these features is Lactobacillus reuteri. Thereafter, Indole is metabolized to Indoxyl-3-sulfate (I3S) and other metabolites in the liver and released into the circulation. These indole derivatives are capable of crossing even the intact blood brain barrier and enter into the CNS, where they bind and activate the cytosolic Aryl hydrocarbon receptor (AHR). AHR interacts with SOCS2 to inhibit NF-κB activation and nuclear translocation. Ultimately, NF-κB dependent pro-inflammatory pathways, including monocyte recruitment, neurotoxicity, and activation of monocytes and microglia are inhibitied. Together, this pathways confers down-modulatory actions on acute and chronic inflammation in the CNS and integrates dietary and microbial signals into CNS inflammation.
Highlights.
Environmental factors influence the development and course of Multiple Sclerosis.
Infections, pollutants and low sun exposure are independent risk factors for MS.
The interaction of the diet and gut microbiota shapes peripheral immune responses.
AHR agonists generated by bacterial and host metabolism reach the CNS.
These AHR agonists dampen autoimmune inflammation in the CNS via AHR in astrocytes.
Acknowledgments
Research in the Quintana laboratory is supported by the National Institutes of Health, the National Multiple Sclerosis Society, the International Progressive MS Alliance and the American Cancer Society. V. R. received support from Mallinckrodt Pharmaceuticals and Deutsche Forschungsgemeinschaft (DFG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Dilokthornsakul P, et al. Multiple sclerosis prevalence in the United States commercially insured population. Neurology. 2016;86:1014–1021. doi: 10.1212/WNL.0000000000002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kingwell E, et al. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol. 2013;13:128. doi: 10.1186/1471-2377-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lublin FD, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89:225–240. doi: 10.1016/j.mayocp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Willer CJ, et al. Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci U S A. 2003;100:12877–12882. doi: 10.1073/pnas.1932604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebers GC, Sadovnick AD, Risch NJ. A genetic basis for familial aggregation in multiple sclerosis. Canadian Collaborative Study Group. Nature. 1995;377:150–151. doi: 10.1038/377150a0. [DOI] [PubMed] [Google Scholar]
- 7.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8:602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •8.Bashinskaya VV, Kulakova OG, Boyko AN, Favorov AV, Favorova OO. A review of genome-wide association studies for multiple sclerosis: classical and hypothesis-driven approaches. Hum Genet. 2015;134:1143–1162. doi: 10.1007/s00439-015-1601-2. This review gives an overview over of the approaches of genome-wide association studies (GWAS) and hypothesis-driven approaches for the detection of risk loci for MS. [DOI] [PubMed] [Google Scholar]
- 9.Welter D, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramagopalan SV, et al. Rare variants in the CYP27B1 gene are associated with multiple sclerosis. Ann Neurol. 2011;70:881–886. doi: 10.1002/ana.22678. [DOI] [PubMed] [Google Scholar]
- 11.Patsopoulos NA, et al. Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Ann Neurol. 2011;70:897–912. doi: 10.1002/ana.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadovnick AD. Genetic background of multiple sclerosis. Autoimmunity reviews. 2012;11:163–166. doi: 10.1016/j.autrev.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015;14:406–419. doi: 10.1016/S1474-4422(14)70305-9. [DOI] [PubMed] [Google Scholar]
- 14.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nature communications. 2014;5:4432. doi: 10.1038/ncomms5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrokhi V, et al. Bacterial lipodipeptide, Lipid 654, is a microbiome-associated biomarker for multiple sclerosis. Clin Transl Immunology. 2013;2:e8. doi: 10.1038/cti.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••17.Rothhammer V, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016 doi: 10.1038/nm.4106. This research article shows direct effects of tryptophan metabolites generated by commensal bacteria and host enzymes on the activation of anti-inflammatory pathways in astrocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acheson ED, Bachrach CA, Wright FM. Some comments on the relationship of the distribution of multiple sclerosis to latitude, solar radiation, and other variables. Acta Psychiatr Scand Suppl. 1960;35:132–147. doi: 10.1111/j.1600-0447.1960.tb08674.x. [DOI] [PubMed] [Google Scholar]
- 19.Wallin MT, Page WF, Kurtzke JF. Multiple sclerosis in US veterans of the Vietnam era and later military service: race, sex, and geography. Ann Neurol. 2004;55:65–71. doi: 10.1002/ana.10788. [DOI] [PubMed] [Google Scholar]
- 20.Alonso A, Hernan MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology. 2008;71:129–135. doi: 10.1212/01.wnl.0000316802.35974.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berg-Hansen P, Moen SM, Harbo HF, Celius EG. High prevalence and no latitude gradient of multiple sclerosis in Norway. Mult Scler. 2014;20:1780–1782. doi: 10.1177/1352458514525871. [DOI] [PubMed] [Google Scholar]
- 22.Koch-Henriksen N, Sorensen PS. Why does the north-south gradient of incidence of multiple sclerosis seem to have disappeared on the northern hemisphere? J Neurol Sci. 2011;311:58–63. doi: 10.1016/j.jns.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Taylor BV, et al. Latitudinal variation in incidence and type of first central nervous system demyelinating events. Mult Scler. 2010;16:398–405. doi: 10.1177/1352458509359724. [DOI] [PubMed] [Google Scholar]
- 24.Simpson S, Jr, Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:1132–1141. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 25.McLeod JG, Hammond SR, Kurtzke JF. Migration and multiple sclerosis in immigrants to Australia from United Kingdom and Ireland: a reassessment. I. Risk of MS by age at immigration. Journal of neurology. 2011;258:1140–1149. doi: 10.1007/s00415-010-5898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh-Wakefield F, Byrne SN. Photoimmunology and Multiple Sclerosis. Curr Top Behav Neurosci. 2015;26:117–141. doi: 10.1007/7854_2014_359. [DOI] [PubMed] [Google Scholar]
- 27.Simon KC, Munger KL, Ascherio A. Vitamin D and multiple sclerosis: epidemiology, immunology, and genetics. Curr Opin Neurol. 2012;25:246–251. doi: 10.1097/WCO.0b013e3283533a7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munger KL, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 29.Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, Mansourian M. Short-term effect of high-dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. Neuroimmunomodulation. 2015;22:400–404. doi: 10.1159/000439278. [DOI] [PubMed] [Google Scholar]
- 30.Stein MS, et al. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology. 2011;77:1611–1618. doi: 10.1212/WNL.0b013e3182343274. [DOI] [PubMed] [Google Scholar]
- 31.Rosecrans R, Dohnal JC. Seasonal vitamin D changes and the impact on health risk assessment. Clin Biochem. 2014;47:670–672. doi: 10.1016/j.clinbiochem.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Spelman T, et al. Seasonal variation of relapse rate in multiple sclerosis is latitude dependent. Ann Neurol. 2014;76:880–890. doi: 10.1002/ana.24287. [DOI] [PubMed] [Google Scholar]
- 33.Brzezinski A. Melatonin in humans. The New England journal of medicine. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- ••34.Farez MF, et al. Melatonin Contributes to the Seasonality of Multiple Sclerosis Relapses. Cell. 2015;162:1338–1352. doi: 10.1016/j.cell.2015.08.025. This research article demonstrates that disease activity in MS negatively correlates with melatonin levels and thus provides an explanation for lower incidence of MS relapses during autumn and winter. Mechanistically, melatonin suppresses Th17 differentiation via Nfil3 while promoting Tr1 differentiation and IL-10 production via Erk1/2 and Ror-α. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen SJ, et al. Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2016;31:169–177. doi: 10.1016/j.intimp.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez-Sanchez N, et al. Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav Immun. 2015;50:101–114. doi: 10.1016/j.bbi.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Emamgholipour S, Hossein-Nezhad A, Sahraian MA, Askarisadr F, Ansari M. Evidence for possible role of melatonin in reducing oxidative stress in multiple sclerosis through its effect on SIRT1 and antioxidant enzymes. Life Sci. 2016;145:34–41. doi: 10.1016/j.lfs.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Kaur C, Ling EA. Antioxidants and neuroprotection in the adult and developing central nervous system. Curr Med Chem. 2008;15:3068–3080. doi: 10.2174/092986708786848640. [DOI] [PubMed] [Google Scholar]
- ••39.Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14:263–273. doi: 10.1016/S1474-4422(14)70267-4. This review gives an overview of the most relevant published work on environmental risk factors for MS based on their statistic validity. IgG seropositivity to Epstein-Barr virus nuclear antigen (EBNA), infectious mononucleosis and smoking were identified as most valid risk factors. [DOI] [PubMed] [Google Scholar]
- 40.van der Mei IA, Simpson S, Jr, Stankovich J, Taylor BV. Individual and joint action of environmental factors and risk of MS. Neurol Clin. 2011;29:233–255. doi: 10.1016/j.ncl.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Lederberg J, MA ‘Ome Sweet’ Omics - a genealogical treasury of word. Scientist. 2001;15:8. [Google Scholar]
- 42.Group NHW, et al. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaahtovuo J, Munukka E, Korkeamaki M, Luukkainen R, Toivanen P. Fecal microbiota in early rheumatoid arthritis. J Rheumatol. 2008;35:1500–1505. [PubMed] [Google Scholar]
- 45.Knip M, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. The New England journal of medicine. 2010;363:1900–1908. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berer K, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 47.Wekerle H, Berer K, Krishnamoorthy G. Remote control-triggering of brain autoimmune disease in the gut. Curr Opin Immunol. 2013;25:683–689. doi: 10.1016/j.coi.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popovic N, et al. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol. 2002;51:215–223. doi: 10.1002/ana.10092. [DOI] [PubMed] [Google Scholar]
- 50.Yokote H, et al. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. The American journal of pathology. 2008;173:1714–1723. doi: 10.2353/ajpath.2008.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochoa-Reparaz J, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 52.Wang D, Lu Z, Hu L, Zhang Y, Hu X. Macrolide antibiotics aggravate experimental autoimmune encephalomyelitis and inhibit inducible nitric oxide synthase. Immunol Invest. 2009;38:602–612. doi: 10.1080/08820130903062194. [DOI] [PubMed] [Google Scholar]
- 53.Mor F, Cohen IR. Beta-lactam antibiotics modulate T-cell functions and gene expression via covalent binding to cellular albumin. Proc Natl Acad Sci U S A. 2013;110:2981–2986. doi: 10.1073/pnas.1215722110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Telesford KM, et al. A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39(+)Foxp3(+) T cells and Treg function. Gut Microbes. 2015;6:234–242. doi: 10.1080/19490976.2015.1056973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, et al. A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes. 2014;5:552–561. doi: 10.4161/gmic.29797. [DOI] [PubMed] [Google Scholar]
- 56.Ochoa-Reparaz J, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- •57.Tremlett H, et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol. 2016 doi: 10.1111/ene.13026. This is an exciting pilot study in pediatric MS demonstrating alterations in the gut microbiome of MS patients as compared to healthy controls. In MS patients, the altered microbiome is enriched in pathways relevant for neurodegeneration and affected by immunomodulatory treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller PG, Bonn MB, Franklin CL, Ericsson AC, McKarns SC. TNFR2 Deficiency Acts in Concert with Gut Microbiota To Precipitate Spontaneous Sex-Biased Central Nervous System Demyelinating Autoimmune Disease. J Immunol. 2015;195:4668–4684. doi: 10.4049/jimmunol.1501664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zelante T, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- •60.Haghikia A, et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. This research article shows the differential influence of dietary long and short fatty acids on Th1, Th17, and Treg differentiation locally in the gut and their subsequent relevance for disease induction in EAE. [DOI] [PubMed] [Google Scholar]
- •61.Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. Host microbiota influence microglia homeostasis and response to immunologic challenge via a peripheral mechanism involving short-chain fatty acids, underlining the “remote control” of the diet and the microbiome over CNS resident cell types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sedaghat F, Jessri M, Behrooz M, Mirghotbi M, Rashidkhani B. Mediterranean diet adherence and risk of multiple sclerosis: a case-control study. Asia Pac J Clin Nutr. 2016;25:377–384. doi: 10.6133/apjcn.2016.25.2.12. [DOI] [PubMed] [Google Scholar]
- 63.Riccio P, et al. Anti-inflammatory nutritional intervention in patients with relapsing-remitting and primary-progressive multiple sclerosis: A pilot study. Exp Biol Med (Maywood) 2016;241:620–635. doi: 10.1177/1535370215618462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riccio P, Rossano R. Nutrition facts in multiple sclerosis. ASN Neuro. 2015;7 doi: 10.1177/1759091414568185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sedel F, et al. High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord. 2015;4:159–169. doi: 10.1016/j.msard.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Hoare S, et al. Higher intake of omega-3 polyunsaturated fatty acids is associated with a decreased risk of a first clinical diagnosis of central nervous system demyelination: Results from the Ausimmune Study. Mult Scler. 2016;22:884–892. doi: 10.1177/1352458515604380. [DOI] [PubMed] [Google Scholar]
- 67.Jorg S, et al. High salt drives Th17 responses in experimental autoimmune encephalomyelitis without impacting myeloid dendritic cells. Exp Neurol. 2016;279:212–222. doi: 10.1016/j.expneurol.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Hucke S, et al. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J Autoimmun. 2016;67:90–101. doi: 10.1016/j.jaut.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Kleinewietfeld M, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu C, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •71.Farez MF, Fiol MP, Gaitan MI, Quintana FJ, Correale J. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:26–31. doi: 10.1136/jnnp-2014-307928. In this observational study, high sodium intake is associated with an increase in disease activity as measured by clinical and radiological criteria, underlining experimental results in EAE. [DOI] [PubMed] [Google Scholar]
- 72.Amirkhani A, et al. Interferon-beta affects the tryptophan metabolism in multiple sclerosis patients. Eur J Neurol. 2005;12:625–631. doi: 10.1111/j.1468-1331.2005.01041.x. [DOI] [PubMed] [Google Scholar]
- 73.Monteleone I, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248. 248, e231. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- ••74.Lamas B, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605. doi: 10.1038/nm.4102. This research article shows the mutual interaction between the diet, the host and the gut microbiota and its impact on inflammatory bowel disease. Together with ref. 17, it describes a novel immunomodulatory mechanism involving ligands to AHR generated from dietary tryptophan by microbiota and host metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]