Abstract
Introduction
Obesity continues to disproportionately affect medically vulnerable populations. Digital health interventions may be effective for delivering obesity treatment in low-resource primary care settings.
Methods
Track is a 12-month randomized controlled trial of a digital health weight loss intervention in a community health center system. Participants are 351 obese men and women aged 21 to 65 years with an obesity-related comorbidity. Track participants are randomized to usual primary care or to a 12-month intervention consisting of algorithm-generated tailored behavior change goals, self-monitoring via mobile technologies, daily self-weighing using a network-connected scale, skills training materials, 18 counseling phone calls with a Track coach, and primary care provider counseling. Participants are followed over 12 months, with study visits at baseline, 6, and 12 months. Anthropometric data, blood pressure, fasting lipids, glucose and HbA1C and self-administered surveys are collected. Follow-up data will be collected from the medical record at 24 months.
Results
Participants are 68% female and on average 50.7 years old with a mean BMI of 35.9 kg/m2. Participants are mainly black (54%) or white (33%); 12.5% are Hispanic. Participants are mostly employed and low-income. Over 20% of the sample has hypertension, diabetes and hyperlipidemia. Almost 27% of participants currently smoke and almost 20% score above the clinical threshold for depression.
Conclusions
Track utilizes an innovative, digital health approach to reduce obesity and chronic disease risk among medically vulnerable adults in the primary care setting. Baseline characteristics reflect a socioeconomically disadvantaged, high-risk patient population in need of evidence-based obesity treatment.
Keywords: Obesity, weight loss, mobile health, minority health, rural health, primary care, digital health
INTRODUCTION
Obesity continues to exact a considerable toll among medically vulnerable populations. Socioeconomic factors strongly pattern exposure to obesogenic environments, the adoption of obesogenic risk behaviors,1 and the limited availability of weight management resources.2,3 Racial/ethnic minority populations are overrepresented among the socioeconomically disadvantaged and these groups disproportionately bear the nation's obesity burden.4 Obesity increases risk of cardiovascular diseases, type 2 diabetes, some cancers, and several other chronic conditions.5-7 Racial/ethnic minority populations exhibit greater rates of adulthood weight gain8,9 and extreme obesity,1 both of which increase obesity-associated chronic disease risk7,10,11 and subsequent premature mortality.12,13
Extant clinical trial evidence shows that even modest weight losses (3-5%) reduce blood pressure, blood glucose, HbA1C, triglycerides, and LDL cholesterol,14-17 and prevent both diabetes and hypertension in predisposed individuals.18-20 Despite a greater need in medically vulnerable populations, obesity is often recalcitrant to treatment.21,22 Medically vulnerable populations are underrepresented in weight loss trials23,24 and most studies – even landmark trials – find smaller weight loss outcomes for socioeconomically disadvantaged and racial/ethnic minority participants.23 For example, blacks in the Diabetes Prevention Program (DPP) were less likely than whites to meet the trial's weight loss goals.25 Larger weight losses were observed for racial/ethnic minority participants in the Weight Loss Maintenance trial than in the DPP, but weight losses were smaller for racial/ethnic minority participants than for white participants.26
Moreover, the challenges of impacting obesity have limited the translation of efficacious behavioral treatments for obesity in the primary care setting. Nationally, only 20% of obese patients receive primary care provider (PCP) counseling for weight management.3 Racial/ethnic minorities are less likely than whites to be counseled.27-29 This is unfortunate because PCPs can be helpful agents of behavior change,15 particularly when PCP counseling is delivered with other behavioral weight management strategies, including social support and behavioral skills training.30,31
As such, there is a need for obesity treatment strategies integrated into primary care and aimed at those with highest risk – low-income, racial/ethnic minority adults with obesity and related comorbidities. We designed Track, a digital health approach to obesity treatment among this patient population. Designed to be integrated at low cost and with minimal additional effort by primary care clinics, our findings might inform obesity counseling reimbursement policies and clinical guidelines in primary care settings with high-risk patient populations.
METHODS/DESIGN
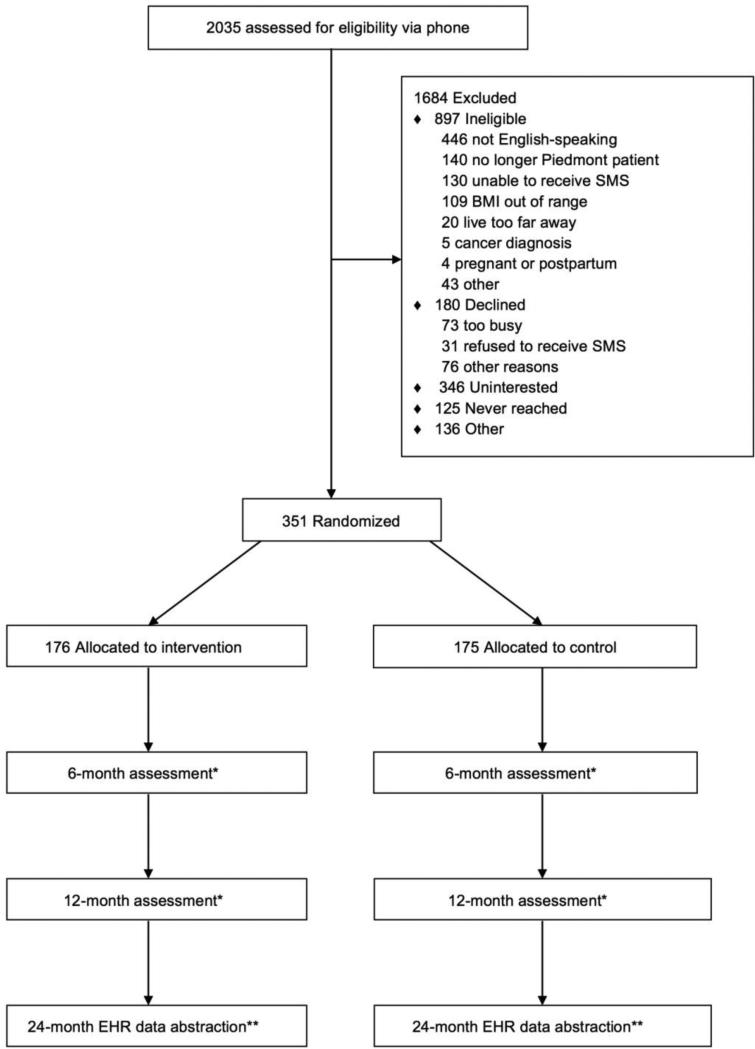
Track is a randomized controlled trial of a 12-month weight loss intervention for obese (BMI: 30.0-44.9 kg/m2) community health center patients with a diagnosis of hypertension, diabetes and/or hyperlipidemia. The primary outcome is weight change over 12 months; secondary outcomes include achievement of ≥ 3% weight loss over 12 months (based on the new obesity guidelines suggesting that a 3% weight loss is clinically meaningful14), changes in diet and physical activity and cardiometabolic risk factors, such as waist circumference, blood pressure, fasting lipids, glucose and HbA1C. We will also examine weight and blood pressure change at 24 months post randomization (Figure 1). All study procedures were approved by the Duke University Institutional Review Board (protocol #B0033) and the Piedmont Health Board of Advisors.
Figure 1.
CONSORT diagram for Track Study
Setting
Track is conducted in four federally-qualified community health centers (CHCs) of Piedmont Health Services, Inc. (Piedmont). Piedmont is a private, non-profit community health system that operates health centers with Level 3 Patient-Centered Medical Home designation in a seven-county service area in central North Carolina. The four CHCs participating in Track are located in Carrboro, Burlington, and Prospect Hill, North Carolina. Patients are predominately racial/ethnic minority (70%), impoverished (96% with income <200% of the federal poverty level), and most are either uninsured, underinsured, or hold public insurance (45% uninsured, 32% Medicaid/S-CHIP, 6% Medicare). Registered dietitians are based at each health center. A meaningful use-compliant GE Centricity (CPS 12) electronic health record (EHR) is available at all Piedmont Health centers.
Participants
Participants include 351 men and women, aged 21 to 65 years, with a BMI of 30.0-44.9 kg/m2 and a weight ≤ 330 pounds (the weight limit for the digital scales used in the intervention) and a current diagnosis of hypertension, type 2 diabetes, and/or hyperlipidemia. Additional inclusion criteria are: at least two visits to a participating Piedmont center in the prior 12 months, North Carolina residency, and the ability to read and write in English. Participants must also have a mobile phone and be willing and able to send/receive 3-9 text messages per week. Exclusion criteria include: current pregnancy, being ≤ 12 months postpartum, cohabitation with another study participant, current employment by Piedmont, current participation in another obesity treatment study or a study involving physical activity, high blood pressure, diabetes, or high cholesterol, or plans to move outside of the study region within the next two years. Participants must not have had a cardiovascular event in the prior 6 months or a diagnosis of coronary obstructive pulmonary disease, congestive heart failure or tachycardia. Patients with history of a condition (e.g., cancer, schizophrenia, end stage renal disease) or medication (e.g., lithium, steroids, anti-psychotics) that would affect body weight, for which weight loss is contraindicated, or that might impact treatment are not eligible. Patients who have profound cognitive, developmental or psychiatric disorders or who have been hospitalized in a psychiatric facility in the prior 12 months are not eligible to participate.
Participant screening and recruitment
Recruitment of participants occurred between June 2013 and September 2014. Piedmont Health staff used electronic health record (EHR) data to generate lists of potentially eligible patients. Study staff then abstracted patients’ heights and weights from electronic health records to assess BMI eligibility and reviewed other information from the medical record to confirm eligibility. In order to best reflect the Piedmont patient population, we aimed to recruit a sample that was 25% male and 10% Hispanic.
Potentially eligible participants were sent invitation letters (signed by the health center medical director and the study principal investigator) and study brochures via postal mail. Patients could opt out of the study by calling the toll-free number provided in the recruitment letter. After one week, study staff called potentially eligible patients to invite participation, perform an initial eligibility assessment, and schedule a screening evaluation visit. Patients completed the informed consent process at the screening visit and eligible participants were then scheduled for a baseline study visit.
Randomization
Randomization occurred at the baseline visit, using a computer-based algorithm. The randomization algorithm allocated participants equally (1:1) across treatment arms, after accounting for CHC, gender and ethnicity (Hispanic vs. non-Hispanic) in order to ensure the equal representation of these characteristics across arms. The intervention design precluded blinding either patients or study coaches to treatment assignment.
Sample size
We hypothesize that there will be no change in the usual care group and a 2.6 kg reduction in weight in the intervention group, and that there will be an auto-correlation between baseline and follow up weight values of 0.55. Based on these values, using a 2-tailed test of differences at the alpha<0.05 level, we would have a power of 80% to detect a difference of 2.36 kg in weight with 140 complete cases per group. Based on our previous studies,32,33 we expect that 80% of all patients invited to participate in the study will complete the full protocol. Thus, we inflated the study sample to accommodate 20% attrition and aimed to enroll approximately 350 patients. All sample size calculations were conducted in PASS Version 11.
Treatment arms
Usual care
Usual care participants receive the current standard of care offered by their primary care providers. In order to optimize best practice, our team provides in-service trainings on obesity treatment at Piedmont provider meetings at least annually. These include trainings on counseling for weight loss, evidence for obesity treatment among medically vulnerable populations and the use of motivational interviewing during counseling. We provide self-help materials (NHLBI “Aim for a Healthy Weight”) to participants in the usual care arm at baseline and provide them with a collated list of community resources for healthful eating, physical activity, and weight management at 6-months post baseline. Control participants also receive quarterly newsletters that include seasonal-related health tips, and financial and safety information.
Weight loss intervention
Theoretical framework
Social Cognitive Theory (SCT)34,35 informed the intervention's design. From SCT, self-efficacy was selected as the primary psychosocial mediator that all aspects of the intervention were designed to target. There is strong and consistent evidence that self-efficacy is positively associated with weight loss intentions, initiation, and maintenance.36-38 Social Cognitive Theory also indicates that self-regulation can be facilitated through a number of processes that were built into the intervention, including self-monitoring,39-41 goal setting,38,42 and social support.43
Intervention design
The Track intervention contains five components (Table 1): 1) tailored behavioral goals (e.g., walk 10,000 steps/day, no sugary drinks, no fast food); 2) self-monitoring of these goals via interactive voice response (IVR) phone calls and SMS text messages; 3) daily self-weighing via a cellular-connected scale; 4) skills training materials in print and video; 5) 18 weight loss counseling coaching calls with a Piedmont registered dietitian; and 6) brief PCP-delivered weight loss counseling at medical visits.
Table 1.
Intervention Design
| Component | Mode of delivery | Frequency of engagement (over 12-month period) |
|---|---|---|
| Self-monitoring of obesity behavior change goals | IVR calls and text messages | Weekly |
| Behavior change goal feedback | IVR calls and text messages | Weekly |
| Self-weighing | Cellular network-connected scales | Daily |
| Self-weighing feedback | Text messages with weight loss progress | Weekly |
| Tailored skills training | Printed goal information Videos | As desired, but we suggest review every 8 weeks as goals change |
| Interpersonal counseling | Coaching calls | 18/year (weekly for calls 1-4, biweekly for calls 5-10, and monthly for calls 11-18) |
| PCP-delivered weight counseling | Weight counseling using updates integrated into EHR | Variable; at each PHS medical visit |
Behavior Change Goals
The intervention utilizes the interactive obesity treatment approach (iOTA), which aims to create an energy deficit for weight loss through the modification of routine obesogenic lifestyle behaviors.32,33,44-47 The iOTA goal library contains two dozen obesogenic behavior change goals (e.g., no fast food, no sugary drinks, eat at least 5 fruits and vegetables a day) that were selected based on their: 1) empirical support; 2) population relevance; 3) ease of self-monitoring; and 4) concreteness. At the baseline study visit, each intervention participant completes a short self-administered survey to assess level of engagement in various dietary, physical activity, and other weight control behaviors. A computer algorithm then uses this information to create a personalized ranking of all the goals in the library based on each participant's need to change each behavior, readiness and self-efficacy to change each behavior, and the potential caloric deficit promoted by the specific behavior change. The algorithm rank orders the goals and participants are asked to self-monitor their adherence to the top 3 goals for the first 8 weeks of the study. Then, starting at week 9, participants self-monitor the next 3 goals on their list in order to maintain motivation and facilitate goal mastery. Goals change every 8 weeks throughout the 12-month intervention period. At the 6-month study visit, participants complete the obesogenic behavior survey again to update their goal list.
All participants also receive a universal 4th goal that rotates at 8-week intervals. In the first interval, we assign a “no red zone foods” goal. To determine the “red zone foods,” we ask participants to select the foods they consume regularly (at least 3 days per week) from a list of commonly eaten, high-calorie foods and beverages (e.g., sodas, sweet tea, desserts, potato chips, pizza, hamburgers). This goal encourages participants to reduce the highest-calorie foods in their diet and maximize the caloric deficit. We provide all intervention participants with a list of “green zone” foods that they can substitute for their red zone foods. The other universal goals are: “practice portion control” and “walk 7-10,000 steps per day.” We provide all intervention participants with pedometers (Yamax SW-650/651 Digi-Walker) and a worksheet that includes both their current weight and their goal weight after 12 months. Their goal weight is 7% less than their current weight.
Self-monitoring
Regular self-monitoring is a robust predictor of weight loss,39,41,48 although adherence wanes over time.49 To enhance engagement potential,50 Track intervention participants self-monitor their behavior change progress weekly via interactive voice response (IVR) or SMS text messaging throughout the intervention period. The Track IVR system calls intervention participants weekly, requests self-monitoring data (by keypad) on participants’ 4 goals (e.g., How many days did you drink number sugary drinks last week?), then immediately provides automated tailored feedback (e.g., “You are doing better than last week. This week, try drinking flavored seltzer water instead of soda to save calories.”). Feedback messages describe trends in progress, reinforce successes, offer motivational strategies, and provide short skills training tips. We have hundreds of hours of audio content (recorded by professional actors) that the automated system pieces together seamlessly during the call.51 This means that participants hear a human voice (not a digitized voice) that invites self-monitoring and immediately delivers tailored feedback. The weekly IVR calls are 2-3 minutes in duration. Participants who do not respond to IVR attempts are sent a SMS message and are prompted to communicate their weekly tracking data via SMS. Participants who provide self-monitoring data via SMS also receive tailored feedback and a brief skills training message (Figure 2). We have a robust retry protocol that attempts to reach participants if the first IVR call or SMS text goes unanswered.
Figure 2.
Example of Track text message
Regular self-monitoring of body weight is supported by emerging evidence.40,52 As in our previous studies,53 we provide intervention participants with scales from BodyTrace, (BodyTrace, New York, NY) which transmit weight data directly to our systems through cellular networks; they do not need a computer, smartphone, or Internet connection. We ask participants to weigh themselves daily and we provide materials and weekly feedback sent via SMS to help participants interpret their daily weight fluctuations and to maintain progress towards their weight loss goal of 7% of initial body weight. Weights received from these scales are available for the coaches to review and to use in providing feedback to participants.
Skills training materials
After randomization and goal assignment at the baseline visit, intervention participants watch a 10-minute video that introduces the Track intervention components. Participants receive a binder and a DVD with additional videos that include descriptive and skills training materials specific to each Track goal. We give participants DVD players if they do not already own one. As participants’ goals change every 8 weeks, they can refer to these materials for continual skills training and behavior change tips.
Telephone counseling calls
Track coaches include 3 Piedmont registered dietitians (RDs) and 2 psychology graduate students. Intervention participants are assigned one coach at baseline and stay with that coach for the duration of the study. Coaches deliver a total of 18 counseling calls over the 12-month intervention period: weekly for calls 1-4, every two weeks for calls 5-10, and monthly for calls 11-18. Each counseling call lasts 20-30 minutes and is designed to enhance/sustain participant motivation, deliver in-depth behavioral skills training (e.g., a lesson on how to read a food label), and provide social support. The coaching calls favor a directive approach, but coaches are trained in the principles of motivational interviewing (MI)54 to counsel participants through any behaviors that they are ambivalent about changing.54 On each call, coaches: (1) review self-monitoring data (behavioral goals and daily weights) and reinforce its importance; (2) discuss barrier reduction strategies; (3) deliver skills training content and; (4) discuss community resources. In later sessions, coaches and patients collaboratively develop weight maintenance plans.
Coaches use a web-based application that presents data on each participant, allows for note taking, and provides access to the self-monitoring data for behavioral goals and a graph of daily weights over time. The system can record coaching and IVR calls and automatically stores process data (e.g., date/time, call disposition, duration). Track coaches participated in a 2-day training session at study start-up and receive biannual refresher trainings. They are trained to detect clinical information (emergent diagnoses, acute symptoms) that requires referral to the provider. Coach supervisors review coaching calls for adherence to protocol and deliver weekly coach supervision.
PCP-delivered weight counseling
Primary Care Providers (PCPs) in participating sites are asked to counsel Track intervention participants at all medical visits during the 12-month study period. All Piedmont PCPs received annual in-service trainings on weight loss counseling from study staff. To address the three major barriers to PCP weight counseling - insufficient training, provider confidence, and provider time - the Track intervention includes regular participant progress reports (called “Track Updates”) delivered to PCPs through the Piedmont EHR (Figure 3). The reports include participant status on his/her behavior change goals, weight change data (from BodyTrace scales) and feedback regarding the participant's adherence to self-monitoring. Providers are alerted to these updates through “pop-ups” that display upon opening an intervention participant's electronic medical record.
Figure 3.
Sample Track Update sent to PCPs via the EHR
These recommendations are structured to take no more than 2 minutes for delivery. Variation in the quality of PCP counseling is expected, but there is a minimum expectation that PCPs will reinforce the need for behavior change and endorse intervention participation at each visit. PCPs are asked to document episodes of study-related counseling in the Piedmont EHR. To determine frequency of PCP counseling, we will abstract counseling-relevant data from the visit notes at the conclusion of the intervention period.
We also provide PCPs with Track quarterly reports. These reports provide the PCPs with clinic-level data and feedback on their individual Track counseling rates. The study team reinforces PCP participation in Track by periodically presenting at medical staff meetings throughout the study period. We will determine PCP counseling through a variety of measures. We collect self-report survey data from all participants about their experiences with provider weight counseling over the past year. We also assess provider counseling during the 24-month EHR review after the intervention is complete. We will review visit notes from both intervention and control participants about weight counseling (both related to the Track study and general weight counseling) in order to determine provider adherence to patient counseling recommendations. We will also conduct key informant interviews at the end of the trial with providers and Piedmont Health leadership to further ascertain counseling adherence and to assess adoption potential.
Data collection
At the screening visit, which on average takes 1.5 hours to complete, research assistants orient participants to the study, gather informed consent, and collect anthropometric data to confirm BMI eligibility. As such, research assistants are not blinded to study allocation. Waist circumference, blood pressure, lipid panel, glucose and HbA1C measurements are also collected at this screening visit. Anthropometric and blood pressure data collection activities are conducted again at both the 6 and 12 month follow-up visits and finger prick blood samples for lipids, glucose and HbA1C measures are taken again at 12 months. Surveys are administered at the baseline visit and again at 6 and 12 months post-baseline. We will collect weight, blood pressure and lab data from the EHR at 24 months post-baseline. Participants were reimbursed $35 each at baseline, 6, and 12 months for their time.
Anthropometric data
After changing into hospital gowns, body height is measured to the nearest 0.1 cm using a calibrated wall-mounted stadiometer (Seca 222)55 and body weight is measured to the nearest 0.1 kg using a portable electronic scale (Seca Model 876).55 Waist circumference is measured to the nearest 0.1 cm using a vinyl, retractable tape measure (AccuFitness MyoTape) where circumference is measured horizontally from the highest point of the iliac crest at minimal respiration. Approximately 5% of the baseline waist circumference measurements were repeated by a second research assistant for quality assurance purposes, although the first measure is used in analysis.
Blood Pressure
The Omron HEM 907XL, a microprocessor controlled, noninvasive device that automatically measures systolic pressure, diastolic pressure, and pulse rate for adults, is used to measure blood pressure three times at 1-minute intervals after 1-2 minutes of quiet sitting. Participants are advised not to smoke or to consume any caffeine within 30 minutes prior to their study visits.
Cardiometabolic biomarkers
Participants are instructed to fast for at least eight hours prior to their screening and 12-month study visits. At the screening and at 12-month visits, we measure fasting glucose, lipid panels (Cholestech LDX; Cholestech Corporation, Hayward, CA, USA) and hemoglobin A1C (Siemens DCA Vantage Hemoglobin A1C Analyzer, Tarrytown, NY) using fingerstick blood specimens.
Survey data
Surveys are administered in English via computer using an online survey tool. Demographic variables collected at baseline include age, gender, race/ethnicity, nativity, marital status, parity, child height/weight, socioeconomic status, insurance status, occupational status, and educational attainment. We administered validated measures to assess a range of relevant constructs, described in Table 2.
Table 2.
Survey measures and descriptions administered in the Track Study
| Construct | Survey description |
|---|---|
| Health-related quality of life56 | The 5-item EuroQol (EQ-5D) instrument assesses mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The EuroQol visual analog scale (EQ-VAS) is similar to a health thermometer and is designed to measure self-rated health quality of life. |
| Physical activity57 | WHO's 18-item Global Physical Activity Questionnaire measures three domains in which physical activity is performed (occupational, transport-related, and leisure-time) assessing intensity, duration, and frequency. |
| Dietary intake58 | The 110-item Block Food Frequency Questionnaire is designed to estimate the usual and customary intake of a wide array of nutrients and food groups. |
| Self-reported medical conditions59 | The NHANES diabetes and hypertension tools measures self-reported experience with diabetes, and hypertension awareness and treatment, and control of high blood pressure and high cholesterol. |
| Tobacco use28 | A 3-item questionnaire from the National Health Interview Survey evaluates current smoking behaviors and previous quit attempts. |
| Sleep quality60,61 | The Pittsburgh Sleep Quality Index assesses sleep behaviors and disturbances in the previous month. |
| Depression63 | The 8-item Patient Health Questionnaire was designed to evaluate the presence of depressive symptoms with scores ranging from 0-24. Scores above 10 indicate moderate depression. |
| Negative live events62 | A 16-item questionnaire measures frequency of stressful life events. |
| Provider communication63-65 | This measures questions assess the nature of weight management counseling at previous doctor visit. |
| Medication adherence66 | A short, modified version of the Morisky Medication Adherence Scale measures medication adherence for diabetes or hypertension management. |
| Perceived risk modification67 | A three-item scale is used to analyze participant assessment of the risk-lowering effect of losing weight, improving diet and improving exercise on chronic disease. |
| Attitudes toward mental health treatment68 | Four items from the Collaborative Psychiatric Epidemiology Surveys (CPES) are used to determine willingness to engage in professional treatment for mental health or substance abuse issues. |
| Food security69 | A 6-item short form from the U.S. Household Food Security Survey determines household food security. |
| Perceived stress70 | The Jackson Heart Study measure is used to assess perceptions of stress experienced in the past 12 months. |
| Health literacy71 | The Newest Vital Sign health literacy tool is a 6-item questionnaire that measures participant health literacy |
| Self-weighing53 | Participants are asked how often they weighed themselves in the past month with seven response options ranging from never to more than once a day. |
| Technology use72 | As 15-item scale adapted from the Pew Research Center's Internet and American Life Project assessing the on use of and access to the internet and mobile technologies. |
| Importance of race/ethnicity to identity73 | The 4-item Importance to Identity subscale of the Collective Self Esteem-Race Specific scale is used to understand the importance of one's race or ethnicity to his/her sense of identity. |
| Identity-based health promotion74 | Survey items are adapted from the Oserman et al study used to assess identity-based motivation for a variety of health-related behaviors. |
| Medication use75 | The Morisky Medication-Taking Adherence Scale-MMAS is a 4-item generic, self-reported scale that measures medication-taking behaviors. |
Data analysis
The study is a patient-randomized two-arm parallel group, longitudinal trial. The primary analysis will be based on intention-to-treat principles. We will model observed data vs. time plots for all participants to discern general trends in study outcomes.
Linear mixed modeling will be used to test the primary hypothesis.76 A linear mixed-effects modeling approach will be used to estimate changes in weight over time, adjusted for site, and test the primary hypothesis.76 The variables in the model will include a time main effect term, a treatment-by-time interaction term, and may include a fixed effect parameter to account for differences by site. Baseline weight will be retained as part of the response vector and the treatment groups will be constrained to a common intercept to reflect baseline equality of groups assumed by randomization. We will test for significant violation of this assumption before modeling. For the primary outcome, we will test the null hypothesis that the parameter on the interaction between treatment and 12-month change for the intervention is 0. Most additional outcomes are continuous and longitudinal and will be analyzed using similar models and assumptions, as described above. We plan to model binary, longitudinal outcomes using generalized estimating equation models. We will collect EHR data on weight and cardiometabolic risk marker data between 12-24 months post randomization. In order to optimize the quality of weight data collected in the health centers, we conducted a quality improvement initiative at Piedmont, conducting comprehensive training, and instituting a calibration protocol. Weights between 22-24 months will be used to examine long-term weight change outcomes. We will also model longitudinal data using all weight data available to examine weight change trajectories over time. Given that not all patients will have a visit exactly at 24 months, we will include weights collected between 22-24 months when analyzing weights one-year post intervention.
We used descriptive statistics examining both the frequency and average value for various measures to help characterize the sample (Table 3). For the cardiometabolic panel, measurements that were above or below the measuring range of the Cholestech LDX or the DCA Vantage Analyzer produced a range error upon reading. As such, we imputed either the highest or lowest possible readable value for those measurements of total cholesterol (n = 1), HDL cholesterol (n = 3), triglycerides (n = 4) and HbA1c (n = 2).
Table 3.
Baseline characteristics of the Track analytic sample (n=351)
| Variable | N (percent) |
|---|---|
| Gender | |
| Female | 239 (68.1) |
| Male | 112 (31.9) |
| Race | |
| Black or African American | 188 (53.6) |
| White | 115 (32.8) |
| American Indian or Native American | 9 (2.6) |
| Asian | 4 (1.1) |
| Unreported | 27 (7.7) |
| More than 1 race | 8 (2.3) |
| Ethnicity | |
| Hispanic or Latino | 44 (12.5) |
| Not Hispanic or Latino | 305 (86.9) |
| Unreported | 2 (0.6) |
| Education | |
| Less than high school graduate | 51 (14.5) |
| High school graduate or GED | 125 (35.6) |
| Some college or vocational/trade school | 97 (27.6) |
| Associate degree | 42 (12.0) |
| College graduate or post grad degree | 36 (10.3) |
| Annual household income | |
| 0 - $24,999 | 180 (51.3) |
| $25,000 - $34,999 | 56 (16.0) |
| $35,000 - $49,999 | 46 (13.1) |
| Over $50,000 | 26 (7.4) |
| Unknown or unreported | 43 (12.3) |
| People supported by this income: mean (SD) | 2.8 (1.5) |
| Living under 2014 U.S. Census poverty threshold | |
| Below | 104 (29.6) |
| Borderline | 56 (16.0) |
| Above | 144 (41.0) |
| Unknown | 47 (13.4) |
| Marital status | |
| Married or living with partner | 172 (49.0) |
| Not married or living with partner | 178 (50.7) |
| Unreported | 1 (0.3) |
| Current employment | |
| Yes, full- or part-time | 234 (66.7) |
| No | 110 (31.3) |
| Unreported | 7 (2.0) |
| Health insurance | |
| Yes | 176 (50.1) |
| No | 175 (49.9) |
| Current smoker | |
| Yes | 93 (26.5) |
| No | 257 (73.2) |
| Unreported | 1 (0.3) |
| Eligibility diagnosis | |
| Diabetes only | 12 (3.4) |
| Hypertension only | 103 (29.3) |
| Hyperlipidemia only | 32 (9.1) |
| Diabetes and hypertension | 42 (12.0) |
| Diabetes and hyperlipidemia | 20 (5.7) |
| Hypertension and hyperlipidemia | 69 (19.7) |
| Diabetes, hypertension and hyperlipidemia | 73 (20.8) |
| *Depression | |
| Yes | 67 (19.1) |
| No | 282 (80.3) |
| Unknown | 2 (0.6) |
| Mean (SD) | |
| Age (yrs) | 50.7 (8.9) |
| Weight (kg) | 99.3 (14.1) |
| BMI (kg/m2) | 35.9 (3.9) |
| Waist circumference (cm) | 114.7 (10.2) |
| Blood pressure: systolic (mmHg) | 130.0 (17.5) |
| Blood pressure: diastolic (mmHg) | 82.0 (11.7) |
| **Cardiometabolic markers | |
| Triglycerides (mg/dL), n=344 | 161.2 (104.6) |
| LDL (mg/dL), n=323 | 110.8 (32.9) |
| HDL (mg/dL), n=343 | 44.6 (14.9) |
| Total cholesterol (mg/dL), n=344 | 187.0 (38.3) |
| Fasting glucose (mg/dL), n=344 | 117.5 (49.1) |
| HbA1C (%, NGSP units), n=335 | 6.60 (1.7) |
Using the PHQ-8 scale with scores ranging from 0-24. Scores ≥ 10 are indicative of moderate depression
Using imputed values for measurements read outside the possible range.
Track will also be evaluated using the RE-AIM planning and evaluation framework77 (Table 4). The RE-AIM framework addresses five issues related to both internal and external validity by comprehensively evaluating the success of interventions on issues key for translation from research to practice and dissemination: 1) Reach and representativeness of individuals who participate; 2) Effectiveness/Efficacy of the intervention on the primary outcomes at the individual level; 3) Adoption at the organizational/CHC level; 4) Implementation measured at the CHC provider/staff level; and 5) Maintenance at both the individual participant and provider level. Additionally, we collect intervention cost data for cost effectiveness analyses.
Table 4.
RE-AIM Measures and how they are applied in the Track Study.
| Domain | Description | Measure | Data Source(s) |
|---|---|---|---|
| Reach | Degree to which target population is reached by study activities | 1. % Eligible population contacted 2. % Who respond to contact 3. % Who participate/are excluded 4. Representativeness of study sample to target population |
1-4. Study database 1-4. PHS EHR |
| Efficacy | Improvement in study outcomes | 1. Change in weight and secondary outcomes measures | 1. In-person measurement |
| Adoption | Potential organizational uptake | 1. Patient intervention satisfaction 2. Intervention satisfaction among PHS PCPs, dietitians and administrators |
1. Survey 2. Qualitative key informant interviews among randomly selected patients (n=15), PCPs/dieticians (n=10), administrators (n=5) |
| Implementation | Degree to which intervention is implemented as intended | 1. Interventionist adherence to counseling protocol 2. PCP weight loss counseling 3. Secular trends in PCP counseling 4. Participant adherence to intervention |
1. Study database 2. PHS EHR 3. Patient survey (baseline, 6, 12mo) 4. Survey for all PCPs (12mo) |
| Maintenance | Can program outcomes be sustained over time? | 1. Weight change at 24 months | 1. PHS EHR (after QI initiative to improve clinic weight measurements) |
RESULTS
Baseline characteristics
We randomized 351 patients to either the intervention (n=176) or to usual care (n=175) treatment arms (Figure 1). At baseline, almost one-third (31.9%) of the sample is male (Table 4). Participants are an average of 50.7 (SD=8.9) years old with an average BMI of 35.9 (SD=3.9) kg/m2. Over half (53.6%) of participants self-identify as black and 12.5% as Hispanic. Participants are mostly employed either full- or part-time (68.0%) and are low-income – 66.3% have a total combined annual household income < $35,000; 29.6% live beneath the 2014 U.S. Census Bureau poverty threshold and 34.3% had received support from the Supplemental Nutrition Assistance Program (SNAP) (data not presented in table). Including themselves, participants report supporting an average of 2.8 (SD=1.5) persons with their household income. One-half (50.1%) of participants do not live with household partners. Similarly, one-half (50.1%) of the sample have a high school diploma, GED or less and only 10% have completed a 4-year college degree or higher. Half (49.9%) of study participants are uninsured.
One-fifth (20.8%) of the sample have all three diagnoses required for enrollment (hypertension, diabetes, and hyperlipidemia). Over one quarter (26.6%) of participants are current smokers. Almost one-fifth (19.4%) score above the PHQ clinical threshold for depression. Mean blood pressure measurements are in the prehypertensive range, while mean lipid levels are normal. Mean fasting blood glucose and hemoglobin A1c levels are elevated.
DISCUSSION
Few obesity treatment interventions have been successful in producing long-term, clinically meaningful outcomes among adults in primary care who are low-income, racially and ethnically diverse and who have obesity and related comorbidities. This is, at least in part, because the current “gold standard” for weight loss treatment consists of components (in-person coaching or group sessions, verbose skills training materials, copious diet logs) that are not readily testable in populations that face barriers to access and have low literacy and numeracy rates.
Track was designed to preserve the multi-component approach to obesity treatment in primary care, while overcoming many of the barriers to delivering the gold standard. For example, Track utilizes digital health technologies to facilitate self-monitoring, provide tailored feedback on progress, and integrate providers (both primary care providers and coaches), while increasing the scalability and, potentially, the cost effectiveness of treatment delivery.
We were successful in recruiting a sample that is composed of rural, middle-aged adults with obesity who are exceedingly socioeconomically disadvantaged. These patients are already exhibiting signs of significant cardiovascular disease risk; all were diagnosed with at least one obesity-related comorbidity and one-fifth of the sample already has hypertension, diabetes and hyperlipidemia. At baseline, many participants in our sample are current smokers (26.6%) and score above the clinical threshold for depression (19.4%). This group's exposure to adverse clinical, behavioral, and social determinants portends considerable risk for future cardiometabolic dysfunction. Notably, this is a group for which we have few evidence-based intervention approaches.
During the past decade, there has been increasing interest in determining how best to treat obesity in the primary care setting.78 These efforts have been promising, but their findings do not yet extend to medically vulnerable populations, who have the highest risk of obesity and related chronic disease. Progress in treating obesity in these groups has been slow, and the reliably smaller weight losses often observed in these populations suggest that more intensive approaches are necessary. However, new treatment approaches must also be cost efficient. Moreover, there is great opportunity for new treatments, given greater recognition of the need to treat obesity in primary care14 as well as the rapid expansion of federal, state, and private payer attention to obesity treatment. In Track, we have developed an innovative intervention approach specifically designed for medically vulnerable populations - one that incorporates several types of care providers in a manner in which they are most effective and links them with patients using a common digital health platform. Our model is designed to be scalable, sustainable, and cost effective, while improving obesity and related outcomes in a population that desperately needs effective treatment solutions.
ACKNOWLEDGEMENTS
We express deep gratitude to the administration and staff of Piedmont Health for their continued collaboration and participation in Track. In particular, we would like to thank Brian Toomey, MSW, Abigail DeVries, MD, Heather Miranda, RD, LDN, Marni Holder, RN, FNPBC, Ashley Brewer, RD, LDN, Kristen Norton, MA, RD, LDN, Diane Butler, RD, Jennifer Cunningham, and staff at the PHS health centers for their support. We are grateful to research assistants Jasmine Burroughs, Jacob Christy, Vanessa Da Costa, Jade Miller, and Vanessa Potter for their tireless efforts in data collection. We thank Hallie Davis-Penders for her assistance with manuscript preparation. Lastly, we would like to especially thank the women and men participating in Track.
FUNDING
This trial is funded by the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases, [R01DK078798]. The funder had no role in study design, data collection, data analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration
The trial is registered with clinicaltrials.gov NCT01827800.
AUTHORS’ CONTRIBUTIONS
PF managed study design and execution and drafted the manuscript for publication. EL and DS coordinated intervention design and contributed to drafting of the manuscript. BB consulted on data safety and execution of the study. EP and SA participated in study design and conducted statistical analysis. LS, HB, HM, and AD participated in study conceptualization and design. GB conceived of the study, acquired study funding, led study design and supervised its coordination and drafted the manuscript for publication. All authors read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
DS and GGB have equity in Scale Down, LLC that produces mobile applications for weight loss. GGB serves on the scientific advisory board of Nutrisystem. The remaining authors declare that they have no conflicting interests.
REFERENCES
- 1.Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in adults: United States, 2005-2008. NCHS data brief. 2010;(50):1–8. [PubMed] [Google Scholar]
- 2.Sinclair J, Lawson B, Burge F. Which patients receive advice on diet and exercise? Do certain characteristics affect whether they receive such advice? Canadian family physician Medecin de famille canadien. 2008;54(3):404–412. [PMC free article] [PubMed] [Google Scholar]
- 3.Bleich SN, Pickett-Blakely O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient education and counseling. 2011;82(1):123–129. doi: 10.1016/j.pec.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS data brief. 2013;(131):1–8. [PubMed] [Google Scholar]
- 5.Kahng SK, Dunkle RE, Jackson JS. The relationship between the trajectory of body mass index and health trajectory among older adults - Multilevel modeling analyses. Res Aging. 2004;26(1):31–61. [Google Scholar]
- 6.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer WHO . IARC Handbooks of Cancer Prevention: Weight Control and Physical Activity. International Agency for Research on Cancer; Lyon, France: 2002. [Google Scholar]
- 8.Carpenter CL, Ross RK, Paganini-Hill A, Bernstein L. Lifetime exercise activity and breast cancer risk among post-menopausal women. British journal of cancer. 1999;80(11):1852–1858. doi: 10.1038/sj.bjc.6690610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. Jama. 2002;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 10.Hu FB, Stampfer MJ, Solomon C, et al. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;134(2):96–105. doi: 10.7326/0003-4819-134-2-200101160-00009. [DOI] [PubMed] [Google Scholar]
- 11.Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County Study. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27(4):514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 12.Visscher TL, Kromhout D, Seidell JC. Long-term and recent time trends in the prevalence of obesity among Dutch men and women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(9):1218–1224. doi: 10.1038/sj.ijo.0802016. [DOI] [PubMed] [Google Scholar]
- 13.Flegal KM, Williamson DF, Pamuk ER, Rosenberg HM. Estimating deaths attributable to obesity in the United States. American journal of public health. 2004;94(9):1486–1489. doi: 10.2105/ajph.94.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Khaodhiar L, Blackburn GL. International Textbook of Obesity. John Wiley & Sons, Ltd; Chichester, UK: 2001. [Google Scholar]
- 16.Lewis CE, Jacobs DR, Jr., McCreath H, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. American journal of epidemiology. 2000;151(12):1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 17.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 20.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134(1):1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 21.Wadden TA, Stunkard AJ. Handbook of Obesity Treatment. 1st Edition The Guilford Press; 2002. [Google Scholar]
- 22.Glenny AM, O'Meara S, Melville A, Sheldon TA, Wilson C. The treatment and prevention of obesity: a systematic review of the literature. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1997;21(9):715–737. doi: 10.1038/sj.ijo.0800495. [DOI] [PubMed] [Google Scholar]
- 23.Osei-Assibey G, Kyrou I, Adi Y, Kumar S, Matyka K. Dietary and lifestyle interventions for weight management in adults from minority ethnic/non-White groups: a systematic review. Obes Rev. 2010;11(11):769–776. doi: 10.1111/j.1467-789X.2009.00695.x. [DOI] [PubMed] [Google Scholar]
- 24.Yancey AK, Kumanyika SK, Ponce NA, et al. Population-based interventions engaging communities of color in healthy eating and active living: a review. Preventing chronic disease. 2004;1(1):A09. [PMC free article] [PubMed] [Google Scholar]
- 25.Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. Jama. 2008;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 27.Sonnenfeld N, Schappert SM, Lin SX. Racial and ethnic differences in delivery of tobacco-cessation services. American journal of preventive medicine. 2009;36(1):21–28. doi: 10.1016/j.amepre.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Cokkinides VE, Halpern MT, Barbeau EM, Ward E, Thun MJ. Racial and ethnic disparities in smoking-cessation interventions: analysis of the 2005 National Health Interview Survey. American journal of preventive medicine. 2008;34(5):404–412. doi: 10.1016/j.amepre.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Livaudais JC, Kaplan CP, Haas JS, Perez-Stable EJ, Stewart S, Jarlais GD. Lifestyle behavior counseling for women patients among a sample of California physicians. Journal of women's health (2002) 2005;14(6):485–495. doi: 10.1089/jwh.2005.14.485. [DOI] [PubMed] [Google Scholar]
- 30.Svetkey LP, Pollak KI, Yancy WS, Jr., et al. Hypertension improvement project: randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension. 2009;54(6):1226–1233. doi: 10.1161/HYPERTENSIONAHA.109.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosworth HB, Olsen MK, Neary A, et al. Take Control of Your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient education and counseling. 2008;70(3):338–347. doi: 10.1016/j.pec.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett GG, Foley P, Levine E, et al. Behavioral treatment for weight gain prevention among black women in primary care practice: a randomized clinical trial. JAMA internal medicine. 2013;173(19):1770–1777. doi: 10.1001/jamainternmed.2013.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett GG, Warner ET, Glasgow RE, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Archives of internal medicine. 2012;172(7):565–574. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bandura A. Self-efficacy : the exercise of control. W.H. Freeman; New York: 1997. [Google Scholar]
- 35.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 36.Linde JA, Rothman AJ, Baldwin AS, Jeffery RW. The impact of self-efficacy on behavior change and weight change among overweight participants in a weight loss trial. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2006;25(3):282–291. doi: 10.1037/0278-6133.25.3.282. [DOI] [PubMed] [Google Scholar]
- 37.Richman RM, Loughnan GT, Droulers AM, Steinbeck KS, Caterson ID. Self-efficacy in relation to eating behaviour among obese and non-obese women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(6):907–913. doi: 10.1038/sj.ijo.0801606. [DOI] [PubMed] [Google Scholar]
- 38.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6(1):67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 39.Levitsky DA, Garay J, Nausbaum M, Neighbors L, Dellavalle DM. Monitoring weight daily blocks the freshman weight gain: a model for combating the epidemic of obesity. International journal of obesity (2005) 2006;30(6):1003–1010. doi: 10.1038/sj.ijo.0803221. [DOI] [PubMed] [Google Scholar]
- 40.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. The American journal of clinical nutrition. 1997;66(2):239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 41.Boutelle KN, Kirschenbaum DS, Baker RC, Mitchell ME. How can obese weight controllers minimize weight gain during the high risk holiday season? By self-monitoring very consistently. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 1999;18(4):364–368. doi: 10.1037//0278-6133.18.4.364. [DOI] [PubMed] [Google Scholar]
- 42.Strecher V. Internet methods for delivering behavioral and health-related interventions (eHealth). Annual review of clinical psychology. 2007;3:53–76. doi: 10.1146/annurev.clinpsy.3.022806.091428. [DOI] [PubMed] [Google Scholar]
- 43.Wing RR, Jeffery RW. Benefits of recruiting participants with friends and increasing social support for weight loss and maintenance. J Consult Clin Psychol. 1999;67(1):132–138. doi: 10.1037//0022-006x.67.1.132. [DOI] [PubMed] [Google Scholar]
- 44.Bennett GG, Herring SJ, Puleo E, Stein EK, Emmons KM, Gillman MW. Web-based weight loss in primary care: a randomized controlled trial. Obesity (Silver Spring, Md.) 2010;18(2):308–313. doi: 10.1038/oby.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greaney ML, Quintiliani LM, Warner ET, et al. Weight management among patients at community health centers: The Be Fit Be Well Study. Obesity Management. 2009;5:222–228. [Google Scholar]
- 46.Foley P, Levine E, Askew S, et al. Weight gain prevention among black women in the rural community health center setting: the Shape Program. BMC public health. 2012;12:305. doi: 10.1186/1471-2458-12-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinberg DM, Levine EL, Askew S, Foley P, Bennett GG. Daily text messaging for weight control among racial and ethnic minority women: randomized controlled pilot study. Journal of medical Internet research. 2013;15(11):e244. doi: 10.2196/jmir.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardeman W, Griffin S, Johnston M, Kinmonth AL, Wareham NJ. Interventions to prevent weight gain: a systematic review of psychological models and behaviour change methods. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24(2):131–143. doi: 10.1038/sj.ijo.0801100. [DOI] [PubMed] [Google Scholar]
- 49.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett GG, Glasgow RE. The delivery of public health interventions via the Internet: actualizing their potential. Annual review of public health. 2009;30:273–292. doi: 10.1146/annurev.publhealth.031308.100235. [DOI] [PubMed] [Google Scholar]
- 51.Steinberg DM, Levine EL, Lane I, et al. Adherence to self-monitoring via interactive voice response technology in an eHealth intervention targeting weight gain prevention among Black women: randomized controlled trial. Journal of medical Internet research. 2014;16(4):e114. doi: 10.2196/jmir.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 53.Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. The efficacy of a daily self-weighing weight loss intervention using smart scales and e-mail. Obesity (Silver Spring, Md.) 2013;21(9):1789–1797. doi: 10.1002/oby.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller WRRS . Motivational Interviewing: Preparing People to Change Addictive Behavior. Guilford Press; New York, NY: 1991. [Google Scholar]
- 55.CDC CFDCAP, NCHS NCFHS National Health and Nutrition Examination Protocol. 2007:1–102. [Google Scholar]
- 56.Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. Journal of public health (Oxford, England) 2005;27(2):156–164. doi: 10.1093/pubmed/fdi025. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization. WHO STEPS surveillance manual: the WHO STEPwise approach to chronic disease risk factor surveillance. Geneva: World Health Organization. 2005 [Google Scholar]
- 58.Block Dietary Data Systems. www.nutritionquest.com.
- 59.CDC 2011-2012 National Health and Nutrition Examination Survey: Items taken from the Blood Pressure-BPQ. www.cdc.gov/nchs/data/nhanes/nhanes_11_12/bpq.pdf.
- 60.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 61.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of affective disorders. 2009;114(1-3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 62.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 63.Lin CT, Albertson GA, Schilling LM, et al. Is patients' perception of time spent with the physician a determinant of ambulatory patient satisfaction? Archives of internal medicine. 2001;161(11):1437–1442. doi: 10.1001/archinte.161.11.1437. [DOI] [PubMed] [Google Scholar]
- 64.Stewart AL, Napoles-Springer A, Perez-Stable EJ. Interpersonal processes of care in diverse populations. The Milbank quarterly. 1999;77(3):305–339, 274. doi: 10.1111/1468-0009.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. J Pers Soc Psychol. 1996;70(1):115–126. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- 66.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. Journal of clinical hypertension (Greenwich, Conn.) 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Stotland S, Zuroff DC. A new measure of weight locus of control: the Dieting Beliefs Scale. Journal of personality assessment. 1990;54(1-2):191–203. doi: 10.1080/00223891.1990.9673986. [DOI] [PubMed] [Google Scholar]
- 68.Alegria M, Jackson JS, Kessler RC, Takeuchi D. Collaborative Psychiatric Epidemiology Surveys (CPES), 2001-2003 [United States] 2007.
- 69.Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the Household Food Security Scale. American journal of public health. 1999;89(8):1231–1234. doi: 10.2105/ajph.89.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Payne TJ, Wyatt SB, Mosley TH, et al. Sociocultural methods in the Jackson Heart Study: conceptual and descriptive overview. Ethn Dis. 2005;15(suppl 6):S6–38. [PubMed] [Google Scholar]
- 71.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. The Annals of Family Medicine. 2005;3(6):514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.The Pew Research Center's Internet and American Life Project: Items taken from the Spring Change Assessment Pew Internet and American Life Project [Google Scholar]
- 73.Luhtanen R, Crocker J. A collective self-esteem scale: Self-evaluation of one's social identity. Pers Soc Psychol B. 1992;18(3):302–318. [Google Scholar]
- 74.Oyserman D, Fryberg SA, Yoder N. Identity-based motivation and health. J Pers Soc Psychol. 2007;93(6):1011–1027. doi: 10.1037/0022-3514.93.6.1011. [DOI] [PubMed] [Google Scholar]
- 75.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 76.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Vol. 998. John Wiley & Sons; 2004. [Google Scholar]
- 77.Glasgow RE, Klesges LM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Education Research. 2006;21(5):688–694. doi: 10.1093/her/cyl081. [DOI] [PubMed] [Google Scholar]
- 78.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. Journal of general internal medicine. 2009;24(9):1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]