ABSTRACT
We discovered a novel Betacoronavirus lineage A coronavirus, China Rattus coronavirus (ChRCoV) HKU24, from Norway rats in China. ChRCoV HKU24 occupied a deep branch at the root of members of Betacoronavirus 1, being distinct from murine coronavirus and human coronavirus HKU1. Its unique putative cleavage sites between nonstructural proteins 1 and 2 and in the spike (S) protein and low sequence identities to other lineage A betacoronaviruses (βCoVs) in conserved replicase domains support ChRCoV HKU24 as a separate species. ChRCoV HKU24 possessed genome features that resemble those of both Betacoronavirus 1 and murine coronavirus, being closer to Betacoronavirus 1 in most predicted proteins but closer to murine coronavirus by G+C content, the presence of a single nonstructural protein (NS4), and an absent transcription regulatory sequence for the envelope (E) protein. Its N-terminal domain (NTD) demonstrated higher sequence identity to the bovine coronavirus (BCoV) NTD than to the mouse hepatitis virus (MHV) NTD, with 3 of 4 critical sugar-binding residues in BCoV and 2 of 14 contact residues at the MHV NTD/murine CEACAM1a interface being conserved. Molecular clock analysis dated the time of the most recent common ancestor of ChRCoV HKU24, Betacoronavirus 1, and rabbit coronavirus HKU14 to about the year 1400. Cross-reactivities between other lineage A and B βCoVs and ChRCoV HKU24 nucleocapsid but not spike polypeptide were demonstrated. Using the spike polypeptide-based Western blot assay, we showed that only Norway rats and two oriental house rats from Guangzhou, China, were infected by ChRCoV HKU24. Other rats, including Norway rats from Hong Kong, possessed antibodies only against N protein and not against the spike polypeptide, suggesting infection by βCoVs different from ChRCoV HKU24. ChRCoV HKU24 may represent the murine origin of Betacoronavirus 1, and rodents are likely an important reservoir for ancestors of lineage A βCoVs.
IMPORTANCE While bats and birds are hosts for ancestors of most coronaviruses (CoVs), lineage A βCoVs have never been found in these animals and the origin of Betacoronavirus lineage A remains obscure. We discovered a novel lineage A βCoV, China Rattus coronavirus HKU24 (ChRCoV HKU24), from Norway rats in China with a high seroprevalence. The unique genome features and phylogenetic analysis supported the suggestion that ChRCoV HKU24 represents a novel CoV species, occupying a deep branch at the root of members of Betacoronavirus 1 and being distinct from murine coronavirus. Nevertheless, ChRCoV HKU24 possessed genome characteristics that resemble those of both Betacoronavirus 1 and murine coronavirus. Our data suggest that ChRCoV HKU24 represents the murine origin of Betacoronavirus 1, with interspecies transmission from rodents to other mammals having occurred centuries ago, before the emergence of human coronavirus (HCoV) OC43 in the late 1800s. Rodents are likely an important reservoir for ancestors of lineage A βCoVs.
INTRODUCTION
Coronaviruses (CoVs) infect a wide variety of animals, including humans, causing respiratory, enteric, hepatic, and neurological diseases of various severities. On the basis of genotypic and serological characterization, CoVs were traditionally classified into three distinct groups (1, 2). Recently, the Coronavirus Study Group of the International Committee on Taxonomy of Viruses (ICTV) has revised the nomenclature and taxonomy to reclassify the three CoV groups into three genera, Alphacoronavirus, Betacoronavirus, and Gammacoronavirus (3). Novel CoVs, which represent a novel genus, Deltacoronavirus, have also been identified (4–6). As a result of the ability to use a variety of host receptors and evolve rapidly through mutation and recombination, CoVs are able to adapt to new hosts and ecological niches, causing a wide spectrum of diseases (2, 7–12).
The severe acute respiratory syndrome (SARS) epidemic and identification of SARS-CoV-like viruses from palm civets and horseshoe bats in China have boosted interest in the discovery of novel CoVs in both humans and animals (13–20). It is now known that CoVs from all four genera can be found in mammals. Historically, alphacoronaviruses (αCoVs) and betacoronaviruses (βCoVs) have been found in mammals, while gammacoronaviruses (γCoVs) have been found in birds. However, recent findings also suggested the presence of γCoVs in mammals (5, 21, 22). Although deltacoronaviruses (δCoVs) are also mainly found in birds, potential mammalian δCoVs have been reported (4, 23). In particular, a δCoV closely related to sparrow CoV HKU17, porcine CoV HKU15, has been identified in pigs, which suggested bird-to-mammal transmission (4). On the basis of current findings, a model for CoV evolution was proposed, where bat CoVs are likely the gene source of Alphacoronavirus and Betacoronavirus and avian CoVs are the gene source of Gammacoronavirus and Deltacoronavirus (4). However, one notable exception to this model is Betacoronavirus lineage A.
The genus Betacoronavirus consists of four lineages, A to D. While human coronavirus (HCoV) OC43 and HCoV HKU1 belong to Betacoronavirus lineage A (20, 24–27), SARS-CoV belongs to Betacoronavirus lineage B and the recently emerged Middle East respiratory syndrome coronavirus (MERS-CoV) belongs to Betacoronavirus lineage C. No human CoV has yet been identified from Betacoronavirus lineage D. On the other hand, besides Alphacoronavirus, diverse bat CoVs have been found in Betacoronavirus lineage B (e.g., SARS-related Rhinolophus bat CoVs), lineage C (e.g., Tylonycteris bat CoV HKU4 and Pipistrellus bat CoV HKU5), and lineage D (e.g., Rousettus bat CoV HKU9) (8, 14, 15, 28–37), supporting the suggestion that bat CoVs are likely the ancestral origin of other mammalian CoVs in these lineages. However, no bat CoVs belonging to Betacoronavirus lineage A have yet been identified, despite the numerous surveillance studies on bat CoVs conducted in various countries over the years (38). Therefore, the ancestral origin of the mammalian lineage A βCoVs, such as HCoV OC43 and HCoV HKU1, remains obscure.
While HCoV OC43 is likely to have originated from zoonotic transmission, sharing a common ancestor with bovine coronavirus (BCoV) that dates back to 1890 (27, 30, 39), closely related CoVs belonging to the same species, Betacoronavirus 1, have also been found in various mammals, including pigs, horses, dogs, waterbucks, sable antelope, deer, giraffes, alpaca, and dromedary camels, suggesting a common ancestor in mammals with subsequent frequent interspecies transmission (40–47). Although no zoonotic origin of HCoV HKU1 has been identified, the virus is most closely related to mouse hepatitis virus (MHV) and rat coronavirus (RCoV), which together are now classified as murine coronavirus (3, 20, 42). We therefore hypothesize that rodent CoVs are the ancestral origin of Betacoronavirus lineage A. In this study, we tested samples from various rodent species in Hong Kong and southern China for the presence of lineage A βCoVs. A novel CoV, China Rattus coronavirus (ChRCoV) HKU24, was discovered from Norway rats in Guangzhou, China. Complete genome analysis showed that ChRCoV HKU24 represents a novel species within Betacoronavirus lineage A but possesses features that resemble those of both Betacoronavirus 1 and murine coronavirus. A high seroprevalence was also demonstrated among Norway rats from Guangzhou using Western blot analysis against ChRCoV HKU24 recombinant N protein and spike polypeptide. The present results suggest that ChRCoV HKU24 likely represents the murine origin of Betacoronavirus 1 and provides insights into the ancestor of Betacoronavirus lineage A.
MATERIALS AND METHODS
Sample collection.
All rodent samples were collected from January 2010 to August 2012 using procedures described previously (5, 14). Samples from southern China were collected from animal markets or restaurants. Samples from Hong Kong were collected from wild and street rodents by the Agriculture, Fisheries and Conservation Department and the Food and Environmental Hygiene Department of the Hong Kong Special Administrative Region (HKSAR), respectively. Alimentary tract samples were placed in viral transport medium containing Earle's balanced salt solution (Invitrogen, NY, USA), 20% glucose, 4.4% NaHCO3, 5% bovine albumin, 50,000 μg/ml vancomycin, 50,000 μg/ml amikacin, and 10,000 units/ml nystatin, before transportation to the laboratory for RNA extraction. The study was approved by the Committee on the Use of Live Animals for Teaching and Research, The University of Hong Kong, and the Institutional Review Board, The University of Hong Kong/Hospital Authority.
RNA extraction.
Viral RNA was extracted from the samples using a QIAamp viral RNA minikit (Qiagen, Hilden, Germany). The RNA was eluted in 60 μl of buffer AVE and was used as the template for reverse transcription-PCR (RT-PCR).
RT-PCR of the RdRp gene of CoVs using conserved primers and DNA sequencing.
Initial CoV screening was performed by amplifying a 440-bp fragment of the RNA-dependent RNA polymerase (RdRp) gene of CoVs using conserved primers (5′-GGTTGGGACTATCCTAAGTGTGA-3′ and 5′-CCATCATCAGATAGAATCATCATA-3′) designed by the use of multiple-sequence alignments of the nucleotide sequences of available RdRp genes of known CoVs (14, 20). Reverse transcription was performed using a SuperScript III kit (Invitrogen, San Diego, CA, USA). The PCR mixture (25 μl) contained cDNA, PCR buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl, 2 mM MgCl2, 0.01% gelatin), 200 μM each deoxynucleoside triphosphate, and 1.0 U Taq polymerase (Applied Biosystems, Foster City, CA, USA). The mixtures were amplified by 60 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min and a final extension at 72°C for 10 min in an automated thermal cycler (Applied Biosystems, Foster City, CA, USA). Standard precautions were taken to avoid PCR contamination, and no false-positive result was observed for the negative controls.
PCR products were gel purified using a QIAquick gel extraction kit (Qiagen, Hilden, Germany). Both strands of the PCR products were sequenced twice with an ABI Prism 3700 DNA analyzer (Applied Biosystems, Foster City, CA, USA), using the two PCR primers. The sequences of the PCR products were compared with known sequences of the RdRp genes of CoVs in the GenBank database.
Viral culture.
The three rodent samples positive for ChRCoV HKU24 by RT-PCR were subject to virus isolation in Huh-7.5 (human hepatoma), Vero E6 (African green monkey kidney), HRT-18G (human rectum epithelial), BSC-1 (African green monkey renal epithelial), RK13 (rabbit kidney), MDBK (bovine kidney), NIH 3T3 (mouse embryonic fibroblast), J774 (mouse macrophage), BHK-21 (baby hamster kidney), RK3E (rat kidney), RMC (rat kidney mesangial), RAW 264.7 (mouse macrophage), and primary SD rat lung cells as described previously (48, 49).
Real-time RT-PCR quantitation.
Real-time RT-PCR was performed on rodent samples positive for ChRCoV HKU24 by RT-PCR using previously described procedures (14). Reverse transcription was performed using the SuperScript III kit with random primers (Invitrogen, San Diego, CA, USA). cDNA was amplified in a LightCycler instrument with a FastStart DNA Master SYBR green I mix reagent kit (Roche Diagnostics GmbH, Mannheim, Germany) using specific primers (5′-ACAGGTTCTCCCTTTATAGATGAT-3′ and 5′-TCTCCTGTATAGTAGCAGAAGCAT-3′) targeting the RdRp gene of ChRCoV HKU24 using procedures described previously (14, 50). For quantitation, a reference standard was prepared using the pCRII-TOPO vector (Invitrogen, San Diego, CA, USA) containing the target sequence. Tenfold dilutions equivalent to 3.77 to 3.77 × 109 copies per reaction were prepared to generate concomitant calibration curves. At the end of the assay, PCR products (a 133-bp fragment of RdRp) were subjected to melting curve analysis (65 to 95°C, 0.1°C/s) to confirm the specificity of the assay. The detection limit of this assay was 3.77 copies per reaction.
Complete genome sequencing.
Three complete genomes of ChRCoV HKU24 were amplified and sequenced using the RNA extracted from the original alimentary tract samples as the templates. The RNA was converted to cDNA by a combined random priming and oligo(dT) priming strategy. The cDNA was amplified by degenerate primers designed by multiple-sequence alignments of the genomes of other CoVs for which complete genomes are available, using strategies described in our previous publications (14, 20, 35, 49) and the CoVDB CoV database (51) for sequence retrieval. Additional primers were designed from the results of the first and subsequent rounds of sequencing. These primer sequences are available on request. The 5′ ends of the viral genomes were confirmed by rapid amplification of cDNA ends (RACE) using a 5′/3′ RACE kit (Roche Diagnostics GmbH, Mannheim, Germany). Sequences were assembled and manually edited to produce the final sequences of the viral genomes.
Genome analysis.
The nucleotide sequences of the genomes and the deduced amino acid sequences of the open reading frames (ORFs) were compared to those of other CoVs for which complete genomes are available using the sequences in CoVDB (51). Phylogenetic tree construction was performed using the maximum likelihood method and PhyML software, with bootstrap values being calculated from 100 trees. Protein family analysis was performed using the PFAM and InterProScan tools (52, 53). Prediction of transmembrane domains was performed using the TMHMM server (54). The structure of the ChRCoV HKU24 N-terminal domain (NTD) was predicted using a web-based homology-modeling server, SWISS-MODEL. A BLASTp search against the sequences in the Protein Data Bank (PDB) was performed with the default parameters to find suitable templates for homology modeling. On the basis of the higher sequence identity, the QMEAN Z-score, coverage, and lower E value, the crystal structure of the BCoV NTD (PDB accession number 4H14) was selected as the template. The predicted structure was visualized using the Jmol viewer.
Estimation of divergence dates.
The divergence time was calculated on the basis of complete RdRp and HE gene sequence data using a Bayesian Markov chain Monte Carlo (MCMC) approach implemented in the BEAST (version 1.8.0) package, as described previously (49, 55, 56). One parametric model (Constant Size) and one nonparametric model (Bayesian Skyline) tree priors were used for inference. Analyses were performed under the SRD06 model and by the use of both a strict and a relaxed molecular clock. The MCMC run was 2 × 108 steps long with sampling every 1,000 steps. Convergence was assessed on the basis of the effective sampling size after a 10% burn-in using Tracer (version 1.5) software (55). The mean time of the most recent common ancestor (tMRCA) and the highest posterior density regions at 95% (HPDs) were calculated, and the best-fitting models were selected by use of a Bayes factor and marginal likelihoods implemented in Tracer (56). Bayesian skyline under a relaxed-clock model with an uncorrelated exponential distribution was adopted for making inferences, as Bayes factor analysis for the RdRp and hemagglutinin-esterase (HE) genes indicated that this model fitted the data better than the other models tested. The tree was summarized in a target tree by the Tree Annotator program included in the BEAST package by choosing the tree with the maximum sum of posterior probabilities (maximum clade credibility) after a 10% burn-in.
Cloning and purification of His6-tagged recombinant ChRCoV HKU24 nucleocapsid protein and spike polypeptide.
To produce fusion plasmids for protein purification, primers 5′-CTAGCTAGCATGTCTCATACGCCA-3′ and 5′-CTAGCTAGCTTATATTTCTGAGCTTCCC-3′ and primers 5′-CTAGCTAGCCAACCAATAGCAGATGTGTA-3′ and 5′-CTAGCTAGCTTATCTCTTGGCTCGCCATGT-3′ were used to amplify the nucleocapsid gene and a partial S1 fragment encoding amino acid residues 317 to 763 of the spike protein of ChRCoV HKU24, respectively, as described previously (31, 49, 57, 58). The sequences, coding for a total of 443 amino acid (aa) and 447 aa residues, respectively, were amplified and cloned into the NheI site of expression vector pET-28b(+) (Merck, KGaA, Darmstadt, Germany) in frame and downstream of the series of six histidine residues. The His6-tagged recombinant nucleocapsid protein and the spike polypeptide were expressed and purified using Ni-nitrilotriacetic acid affinity chromatography (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
Western blot analysis.
To detect the presence of antibodies against the ChRCoV HKU24 N protein and spike polypeptide in rodent sera and to test for possible cross antigenicity between ChRCoV HKU24 and other βCoVs, 600 ng of purified His6-tagged recombinant N protein or spike polypeptide of ChRCoV HKU24 was loaded into the well of a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel and subsequently electroblotted onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The blot was cut into strips, and the strips were incubated separately with 1:2,000, 1:4,000, or 1:8,000 dilutions of sera collected from rodents for which serum samples were available, human sera from two patients with HCoV OC43 infection, sera from two rabbits with rabbit coronavirus (RbCoV) HKU14 infection, and sera from two patients with SARS-CoV infection. The antigen-antibody interaction was detected with 1:4,000 horseradish peroxidase-conjugated anti-rat IgG, anti-human IgG, or anti-rabbit IgG (Zymed) and an ECL fluorescence system (GE Healthcare Life Sciences, Little Chalfont, United Kingdom) as described previously (14, 58).
Nucleotide sequence accession numbers.
The nucleotide sequences of the three genomes of ChRCoV HKU24 have been lodged within the GenBank sequence database under accession no. KM349742 to KM349744.
RESULTS
Identification of a novel CoV from Norway rats in China.
Of 91 alimentary tract samples from rodents in China, RT-PCR for a 440-bp fragment in the RdRp gene of CoVs was positive for a potentially novel CoV in 3 samples from Norway rats (Rattus norvegicus) from a restaurant in Guangzhou (Table 1). None of the 573 alimentary tract samples from rodents in Hong Kong, including those from Norway rats, was positive for CoVs. Sequencing results suggested that the potentially novel virus was most closely related to MHV with ≤85% nucleotide sequence identities and members of the species Betacoronavirus 1, including HCoV OC43, BCoV, equine coronavirus (ECoV) and porcine hemagglutinating encephalomyelitis virus, with ≤84% nucleotide sequence identities. Quantitative RT-PCR showed that the viral load in the positive samples ranged from 1.2 × 103 to 1.3 × 106 copies/g. Attempts to stably passage ChRCoV HKU24 in cell cultures were unsuccessful, with no cytopathic effect or viral replication being detected.
TABLE 1.
Detection of ChRCoV HKU24 in rodents by RT-PCR and serological studies by Western blotting
| Scientific name | Common name | No. of rodents tested | No. of rodents positive for the following/total no. of rodents tested (%): |
||
|---|---|---|---|---|---|
| ChRCoV HKU24 in alimentary tract samples by RT-PCR | ChRCoV HKU24 antibody by N-protein Western blot analysis | ChRCoV HKU24 antibody by S1 fragment Western blot analysis | |||
| Crocidura attenuata | Asian gray shrew | 5 | 0/5 (0) | NAc | NA |
| Niviventer fulvescens | Chestnut white-bellied rat | 97 | 0/97 (0) | NA | NA |
| Rattus andamanensis | Indochinese forest rat | 170 | 0/170 (0) | NA | NA |
| Rattus norvegicusa | Norway rat | 82 | 3/82 (3.6) | 60/74 (81.1) | 21/60 (35) |
| Rattus norvegicusb | Norway rat | 308 | 0/277 (0) | 15/31 (48.4) | 0/15 (0) |
| Rattus rattus | Black rat | 54 | 0/24 (0) | 4/30 (0.13) | 0/4 (0) |
| Rattus tanezumi | Oriental house rat | 9 | 0/9 (0) | 7/9 (77.8) | 2/7 (2.9) |
Norway rats from Guangzhou.
Norway rats from Hong Kong.
NA, not available.
Genome organization and coding potential of ChRCoV HKU24.
Complete genome sequence data for the three strains of ChRCoV HKU24 were obtained by assembly of the sequences of the RT-PCR products from the RNA directly extracted from the corresponding individual specimens. The three genomes shared >99% nucleotide sequence similarity. Their genome size was 31,234 bases, with the G+C content (40%) being closer to that of murine coronavirus than to that of Betacoronavirus 1 (Table 2). The genome organization was similar to that of other lineage A βCoVs and had the characteristic gene order 5′-replicase ORF1ab, hemagglutinin-esterase (HE), spike (S), envelope (E), membrane (M), nucleocapsid (N)-3′ (Table 2 and Fig. 1). Moreover, additional ORFs coding for nonstructural (NS) proteins NS2a, NS4, NS5, and N2 were found. A putative transcription regulatory sequence (TRS) motif, 5′-CUAAAC-3′, similar to that of αCoVs and the motif 5′-UCUAAAC-3′ in other lineage A βCoVs, was identified at the 3′ end of the leader sequence and preceded each ORF except the ORFs for the NS4, E, and N2 genes (Table 3) (26, 49, 59–61). However, there were base mismatches for HE and NS5, with alternative TRS motifs, 5′-CUGAAC-3′ and 5′-GUAAAC-3′, respectively, being detected.
TABLE 2.
Comparison of genomic features of ChRCoV HKU24 and other CoVs for which complete genome sequences are available and amino acid identities between the predicted proteins of ChRCoV HKU24 and the corresponding proteins of other CoVs
| Coronavirusa | Genomic features |
Pairwise amino acid sequence identity with the ChRCoV HKU24-R05005I sequenceb (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size (no. of bases) | G+C content | 3CLpro | RdRp | Hel | HE | S | E | M | N | |
| Alphacoronavirus | ||||||||||
| TGEV | 28,586 | 0.38 | 45.5 | 58.3 | 59.1 | 26.1 | 22.4 | 36.4 | 27.1 | |
| MCoV | 28,894 | 0.38 | 46.5 | 59.3 | 57.2 | 25.8 | 24.7 | 32.0 | 27.6 | |
| CCoV | 29,363 | 0.38 | 44.6 | 58.3 | 58.7 | 26.3 | 23.5 | 36.4 | 27.6 | |
| FIPV | 29,355 | 0.38 | 45.2 | 58.4 | 58.7 | 25.6 | 22.4 | 33.8 | 26.1 | |
| PRCV | 27,550 | 0.37 | 45.5 | 58.3 | 58.9 | 26.7 | 23.5 | 35.7 | 27.8 | |
| HCoV 229E | 27,317 | 0.38 | 44.4 | 56.3 | 57.7 | 26.9 | 26.5 | 32.5 | 26.9 | |
| HCoV NL63 | 27,553 | 0.34 | 42.8 | 56.5 | 57.6 | 25.8 | 31.0 | 32.8 | 25.4 | |
| PEDV | 28,033 | 0.42 | 42.4 | 59.1 | 58.7 | 25.6 | 30.1 | 38.5 | 21.6 | |
| Rh-BatCoV HKU2 | 27,165 | 0.39 | 43.6 | 57.6 | 55.8 | 24.6 | 30.6 | 35.9 | 26.4 | |
| Mi-BatCoV 1A | 28,326 | 0.38 | 42.4 | 58.0 | 58.4 | 25.1 | 31.3 | 32.9 | 26.9 | |
| Mi-BatCoV HKU8 | 28,773 | 0.42 | 43.1 | 58.8 | 56.1 | 25.5 | 29.3 | 35.1 | 26.5 | |
| Sc-BatCoV 512 | 28,203 | 0.40 | 41.1 | 58.3 | 58.1 | 25.2 | 26.8 | 38.0 | 24.9 | |
| Ro-BatCoV HKU10 | 28,494 | 0.39 | 43.1 | 56.9 | 57.1 | 26.6 | 34.5 | 36.2 | 26.4 | |
| Hi-BatCoV HKU10 | 28,492 | 0.38 | 43.1 | 56.7 | 57.0 | 25.8 | 34.5 | 35.7 | 25.6 | |
| Betacoronavirus lineage A | ||||||||||
| Betacoronavirus 1 | ||||||||||
| HCoV OC43 | 30,738 | 0.37 | 85.8 | 91.8 | 93.5 | 70.1 | 67.1 | 78.6 | 88.7 | 74.0 |
| BCoV | 31,028 | 0.37 | 86.8 | 92.6 | 93.7 | 69.6 | 68.0 | 78.6 | 89.2 | 74.9 |
| PHEV | 30,480 | 0.37 | 86.5 | 92.0 | 93.7 | 68.9 | 67.0 | 77.4 | 89.2 | 74.0 |
| ECoV | 30,992 | 0.37 | 86.8 | 92.6 | 94.7 | 66.2 | 69.5 | 76.2 | 85.7 | 73.1 |
| SACoV | 30,995 | 0.37 | 86.8 | 92.6 | 93.7 | 69.6 | 68.2 | 80.5 | 89.6 | 74.9 |
| CRCoV | 31,028 | 0.37 | 86.5 | 92.3 | 93.5 | 69.9 | 67.6 | 77.4 | 90.0 | 74.7 |
| GiCoV | 30,979 | 0.37 | 86.8 | 92.6 | 93.7 | 69.6 | 68.4 | 78.6 | 89.6 | 74.9 |
| DcCoV UAE-HKU23 | 31,036 | 0.37 | 86.8 | 92.6 | 93.4 | 69.6 | 68.1 | 77.4 | 90.5 | 74.4 |
| Murine coronavirus | ||||||||||
| MHV | 31,357 | 0.42 | 82.8 | 90.3 | 90.5 | 39.9 | 63.8 | 63.9 | 82.7 | 67.9 |
| RCoV | 31,250 | 0.41 | 82.5 | 90.3 | 90.5 | 59.3 | 63.3 | 62.5 | 80.5 | 67.5 |
| HCoV HKU1 | 29,926 | 0.32 | 82.2 | 88.1 | 88.9 | 50.1 | 60.4 | 53.0 | 78.4 | 62.8 |
| RbCoV HKU14 | 31,084 | 0.38 | 86.8 | 92.5 | 94.7 | 69.9 | 67.9 | 74.2 | 91.3 | 73.9 |
| ChRCoV HKU24-R05009I | 31,234 | 0.40 | 100 | 100 | 99.8 | 99.8 | 100 | 100 | 100 | 100 |
| ChRCoV HKU24-R05010I | 31,324 | 0.40 | 100 | 100 | 100 | 99.8 | 100 | 100 | 100 | 100 |
| Betacoronavirus lineage B | ||||||||||
| SARS-CoV | 29,751 | 0.41 | 49.0 | 66.8 | 68.6 | 29.9 | 26.5 | 37.7 | 34.3 | |
| SARSr-Rh-BatCoV HKU3 | 29,728 | 0.41 | 48.4 | 66.7 | 68.8 | 29.5 | 26.5 | 38.1 | 34.1 | |
| Betacoronavirus lineage C | ||||||||||
| Ty-BatCoV HKU4 | 30,286 | 0.38 | 52.3 | 68.6 | 68.6 | 33.0 | 25.6 | 42.4 | 36.7 | |
| Pi-BatCoV HKU5 | 30,488 | 0.43 | 52.0 | 68.6 | 67.1 | 31.4 | 25.6 | 42.9 | 35.9 | |
| MERS-CoV | 30,107 | 0.41 | 53.3 | 68.7 | 67.1 | 31.9 | 29.3 | 43.3 | 37.7 | |
| Betacoronavirus lineage D | ||||||||||
| Ro-BatCoV HKU9 | 29,114 | 0.41 | 46.9 | 67.1 | 68.4 | 28.6 | 25.6 | 42.4 | 33.3 | |
| Gammacoronavirus | ||||||||||
| IBV | 27,608 | 0.38 | 43.9 | 62.0 | 59.8 | 27.2 | 21.6 | 31.5 | 27.6 | |
| BWCoV SW1 | 31,686 | 0.39 | 44.3 | 60.2 | 57.7 | 25.4 | 24.7 | 26.7 | 29.2 | |
| BdCoV HKU22 | 31,759 | 0.39 | 44.3 | 60.6 | 57.9 | 25.2 | 23.1 | 25.1 | 29.2 | |
| Deltacoroanvirus | ||||||||||
| BuCoV HKU11 | 26,476 | 0.39 | 37.5 | 51.1 | 48.9 | 26.3 | 25.6 | 28.9 | 24.5 | |
| ThCoV HKU12 | 26,396 | 0.38 | 38.0 | 51.8 | 48.4 | 26.2 | 23.6 | 30.6 | 22.1 | |
| MunCoV HKU13 | 26,552 | 0.43 | 38.5 | 53.1 | 50.3 | 26.0 | 21.3 | 28.8 | 21.7 | |
| PorCoV HKU15 | 25,421 | 0.43 | 40.4 | 52.2 | 49.0 | 25.6 | 25.3 | 26.9 | 24.2 | |
| WECoV HKU16 | 26,027 | 0.40 | 39.1 | 51.9 | 49.3 | 25.6 | 23.3 | 28.2 | 22.2 | |
| SpCoV HKU17 | 26,067 | 0.45 | 40.8 | 52.0 | 49.0 | 25.5 | 21.6 | 27.3 | 25.7 | |
| MRCoV HKU18 | 26,674 | 0.47 | 38.8 | 51.9 | 49.3 | 26.1 | 22.5 | 28.9 | 23.7 | |
| NHCoV HKU19 | 26,064 | 0.38 | 35.2 | 53.7 | 48.0 | 24.2 | 23.9 | 30.8 | 23.1 | |
| WiCoV HKU20 | 26,211 | 0.39 | 36.9 | 51.6 | 48.8 | 26.8 | 28.6 | 27.8 | 23.2 | |
| CMCoV HKU21 | 26,216 | 0.35 | 37.6 | 51.6 | 50.2 | 25.1 | 24.7 | 26.1 | 22.2 | |
Two of the strains of ChRCoV HKU24 characterized in this study are in bold. TGEV, porcine transmissible gastroenteritis virus; MCoV, mink coronavirus; CCoV, canine coronavirus; FIPV, feline infectious peritonitis virus; PRCV, porcine respiratory coronavirus; HCoV 229E, human coronavirus 229E; HCoV NL63, human coronavirus NL63; PEDV, porcine epidemic diarrhea virus; Rh-BatCoV HKU2, Rhinolophus bat coronavirus HKU2; Mi-BatCoV 1A, Miniopterus bat coronavirus 1A; Mi-BatCoV HKU8, Miniopterus bat coronavirus HKU8; Sc-BatCoV 512, Scotophilus bat coronavirus 512; Ro-BatCoV HKU10, Rousettus bat coronavirus HKU10; Hi-BatCoV HKU10, Hipposideros bat coronavirus HKU10; HCoV OC43, human coronavirus OC43; BCoV, bovine coronavirus; PHEV, porcine hemagglutinating encephalomyelitis virus; ECoV, equine coronavirus; SACoV, sable antelope CoV; CRCoV, canine respiratory coronavirus; GiCoV, giraffe coronavirus; DcCoV UAE-HKU23, dromedary camel coronavirus UAE-HKU23; MHV, murine hepatitis virus; RCoV, rat coronavirus; HCoV HKU1, human coronavirus HKU1; SARS-CoV, SARS coronavirus; SARSr-Rh-BatCoV HKU3; SARS-related Rhinolophus bat coronavirus HKU3; Ty-BatCoV HKU4, Tylonycteris bat coronavirus HKU4; Pi-BatCoV HKU5, Pipistrellus bat coronavirus HKU5; MERS-CoV, Middle East respiratory syndrome coronavirus; Ro-BatCoV HKU9, Rousettus bat coronavirus HKU9; IBV, infectious bronchitis virus; BWCoV SW1, beluga whale coronavirus SW1; BdCoV HKU22, bottlenose dolphin coronavirus HKU22; BuCoV HKU11, bulbul coronavirus HKU11; ThCoV HKU12, thrush coronavirus HKU12; MunCoV HKU13, Munia coronavirus HKU13; PorCoV HKU15, porcine coronavirus HKU15; WECoV HKU16, white-eye coronavirus HKU16; SpCoV HKU17, sparrow coronavirus HKU17; MRCoV HKU18, magpie robin coronavirus HKU18; NHCoV HKU19, night heron coronavirus HKU19; WiCoV HKU20, wigeon coronavirus HKU20; CMCoV HKU21, common moorhen coronavirus HKU21.
3CLpro, chymotrypsin-like protease; RdRp, RNA-dependent RNA polymerase; Hel, helicase; HE, hemagglutinin-esterase; S, spike; E, envelope; M, membrane; N, nucleocapsid.
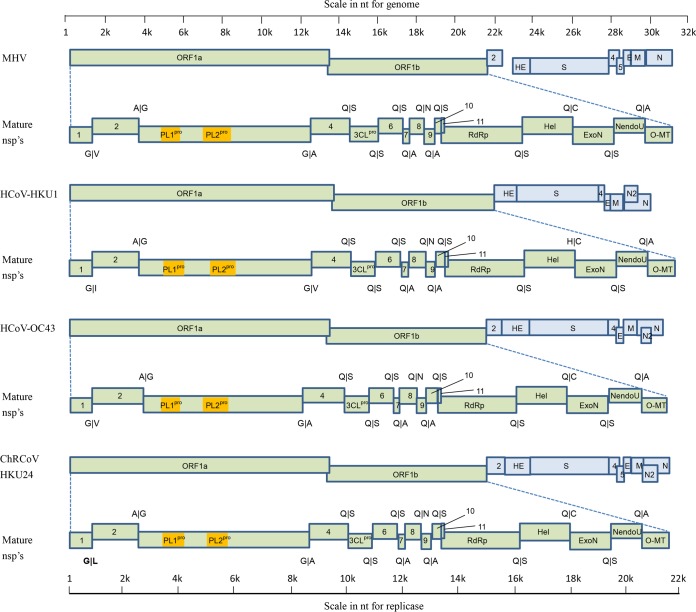
FIG 1.
Comparison of genome organizations of ChRCoV HKU24, MHV, HCoV OC43, and HCoV HKU1. Papain-like proteases 1 and 2 (PL1pro and PL2pro, respectively) are represented by orange boxes. The residues at the cleavage site are indicated above or below the boundary of each nonstructural protein. The unique cleavage site in ChRCoV HKU24 is in bold.
TABLE 3.
Coding potential and predicted domains in different proteins of ChRCoV HKU24
| ORF | Nucleotide positions (start-end) | No. of nucleotides | No. of amino acids | Frame(s) | Putative function or domaina | Amino acid positions | Putative TRS |
|
|---|---|---|---|---|---|---|---|---|
| Nucleotide position in genome | TRS sequence (distance [no. of bases] to AUG)b | |||||||
| 1ab | 213–21637 | 21,425 | 7141 | +3, +2 | 63 | CUAAAC(144)AUG | ||
| nsp1 | 213–950 | 738 | 246 | +3 | Unknown | 1–246 | ||
| nsp2 | 951–2714 | 1,764 | 588 | +3 | Unknown | 247–834 | ||
| nsp3 | 2715–8603 | 5,889 | 1,963 | +3 | Acidic domain, hydrophobic domain, ADRP, putative PLpro domain PL1pro, PL2pro | 835–2797 | ||
| nsp4 | 8604–10091 | 1,488 | 496 | +3 | Hydrophobic domain | 2798–3293 | ||
| nsp5 | 10092–11000 | 909 | 303 | +3 | 3CLpro | 3294–3596 | ||
| nsp6 | 11001–11861 | 861 | 287 | +3 | Hydrophobic domain | 3597–3883 | ||
| nsp7 | 11862–12128 | 267 | 89 | +3 | Unknown | 3884–3972 | ||
| nsp8 | 12129–12719 | 591 | 197 | +3 | Unknown | 3973–4169 | ||
| nsp9 | 12720–13049 | 330 | 110 | +3 | Unknown | 4170–4279 | ||
| nsp10 | 13050–13460 | 411 | 137 | +3 | Unknown | 4280–4416 | ||
| nsp11 | 13461–13505 | 45 | 14 | +3 | Unknown (short peptide at the end of ORF1a) | 4417–4430 | ||
| nsp12 | 13461–16243 | 2,783 | 928 | +2 | RdRp | 4417–5344 | ||
| nsp13 | 16244–18042 | 1,797 | 599 | +2 | Hel | 5345–5943 | ||
| nsp14 | 18041–19603 | 1,563 | 521 | +2 | ExoN, N7-MTase | 5944–6464 | ||
| nsp15 | 19604–20728 | 1,125 | 375 | +2 | NendoU | 6465–6839 | ||
| nsp16 | 20729–21637 | 909 | 302 | +2 | O-MT | 6840–7141 | ||
| NS2a | 21639–22469 | 831 | 276 | +3 | 21629 | CUAAAC(4)AUG | ||
| HE | 22484–23761 | 1,278 | 425 | +2 | Hemagglutinin domain | 129–266 | 22466 | CUGAAC(12)AUG |
| Cleavage site | Between 1 and 18 | |||||||
| Active site for neuraminate O-acetylesterase activity, FGDS | 38–41 | |||||||
| S | 23777–27853 | 4,077 | 1,358 | +2 | Type I membrane glycoprotein | 23771 | CUAAACAUG | |
| N-terminal domain | 16–299 | |||||||
| Cleavage site | Between 763 and 764 | |||||||
| Two heptad repeats | 1045–1079 (HR1), 1253–1285 (HR2) | |||||||
| Transmembrane domain | 1303–1325 | |||||||
| Cytoplasmic tail rich in cysteine residues | ||||||||
| NS4 | 27946–28356 | 411 | 136 | +1 | Transmembrane domain | 7–29 | ||
| NS5 | 28338–28652 | 315 | 104 | +3 | 28286 | GUAAAC(46)AUG | ||
| E | 28645–28893 | 249 | 82 | +1 | Two transmembrane domains | 13–37 and 38–82 | ||
| M | 28908–29603 | 696 | 231 | +3 | Three transmembrane domains | 26–45, 50–72, and 79–101 | 28899 | CUAAAC(3)AUG |
| N2 | 29596–30288 | 693 | 230 | +1 | ||||
| N | 29613–30944 | 1,332 | 443 | +3 | 29600 | CUAAAC(7)AUG | ||
ADRP, ADP-ribose 1″-phosphatase; PL1Pro and PL2Pro, papain-like protease 1 and papain-like protease 2, respectively; 3CLpro, 3C-like protease; RdRp, RNA-dependent RNA polymerase; Hel, helicase; ExoN, 3′-to-5′ exonuclease; N7-MTase, (guanine-N7)-methyltransferase; NendoU, nidoviral uridylate-specific endoribonuclease; O-MT, 2′-O-ribose methyltransferase.
Boldface indicates putative TRS sequences.
The coding potential and characteristics of putative nonstructural proteins (nsp's) of ORF1 of ChRCoV HKU24 are shown in Tables 3 and 4. The ORF1 polyprotein possessed 68.6 to 75.0% amino acid sequence identities to the amino acid sequences of the polyproteins of other lineage A βCoVs. It possessed a unique putative cleavage site, G/L, between nsp1 and nsp2, in contrast to the G/V found in the other lineage A βCoVs except HCoV HKU1, which possessed a G/I cleavage site (Table 4 and Fig. 1). Other predicted cleavage sites were mostly conserved between ChRCoV HKU24 and other lineage A βCoVs. However, the lengths of nsp1, nsp2, nsp3, nsp13, nsp15, and nsp16 in ChRCoV HKU24 differed from those of the corresponding nsp's in members of Betacoronavirus 1 and murine coronavirus, as a result of deletions or insertions.
TABLE 4.
Cleavage site used between nsp's in lineage A βCoVs
| nsp | Cleavage site |
|||||
|---|---|---|---|---|---|---|
| ChRCoV HKU24a | Betacoronavirus 1 | RbCoV HKU14 | MHV | RCoV | HCoV HKU1 | |
| nsp1/nsp2 | G/L | G/V | G/V | G/V | G/V | G/I |
| nsp2/nsp3 | A/G | A/G | A/G | A/G | A/G | A/G |
| nsp3/nsp4 | G/A | G/A | G/A | G/A | G/A | G/V |
| nsp4/nsp5 | Q/S | Q/S | Q/S | Q/S | Q/S | Q/S |
| nsp5/nsp6 | Q/S | Q/S | Q/S | Q/S | Q/S | Q/S |
| nsp6/nsp7 | Q/S | Q/S | Q/S | Q/S | Q/S | Q/S |
| nsp7/nsp8 | Q/A | Q/A | Q/A | Q/A | H/A | Q/A |
| nsp8/nsp9 | Q/N | Q/N | Q/N | Q/N | Q/N | Q/N |
| nsp9/nsp10 | Q/A | Q/A | Q/A | Q/A | Q/A | Q/A |
| nsp10/nsp12 | Q/S | Q/S | Q/S | Q/S | Q/S | Q/S |
| nsp12/nsp13 | Q/S | Q/S | Q/S | Q/S | Q/S | Q/S |
| nsp13/nsp14 | Q/C | Q/C | Q/C | Q/C | Q/C | H/C |
| nsp14/nsp15 | Q/S | Q/S | Q/S | Q/S | Q/S | Q/S |
| nsp15/nsp16 | Q/A | Q/A | Q/A | Q/A | Q/A | Q/A |
The unique cleavage site in ChRCoV HKU24 is in bold.
All lineage A βCoVs except HCoV HKU1 possess the NS2a gene between ORF1ab and the HE gene. Unlike RbCoV HKU14, in which the gene for NS2a is broken into several small ORFs (49), ChRCoV HKU24 is predicted to possess a single NS2a protein, as in other lineage A βCoVs. This NS2a protein displayed from 43.7 to 62.0% amino acid sequence identities to the NS2a amino acid sequences of members of Betacoronavirus 1 and 45.7 to 47.3% amino acid sequence identities to the NS2a amino acid sequences of members of murine coronavirus. Although the βCoV-specific NS2 protein has been shown to be nonessential for in vitro viral replication (62), cyclic phosphodiesterase domains have been predicted in the NS2 proteins of some CoVs and toroviruses, and a possible role in viral pathogenicity has been suggested in MHV (63, 64). In contrast to MHV and RCoV, such a domain was not found in ChRCoV HKU24.
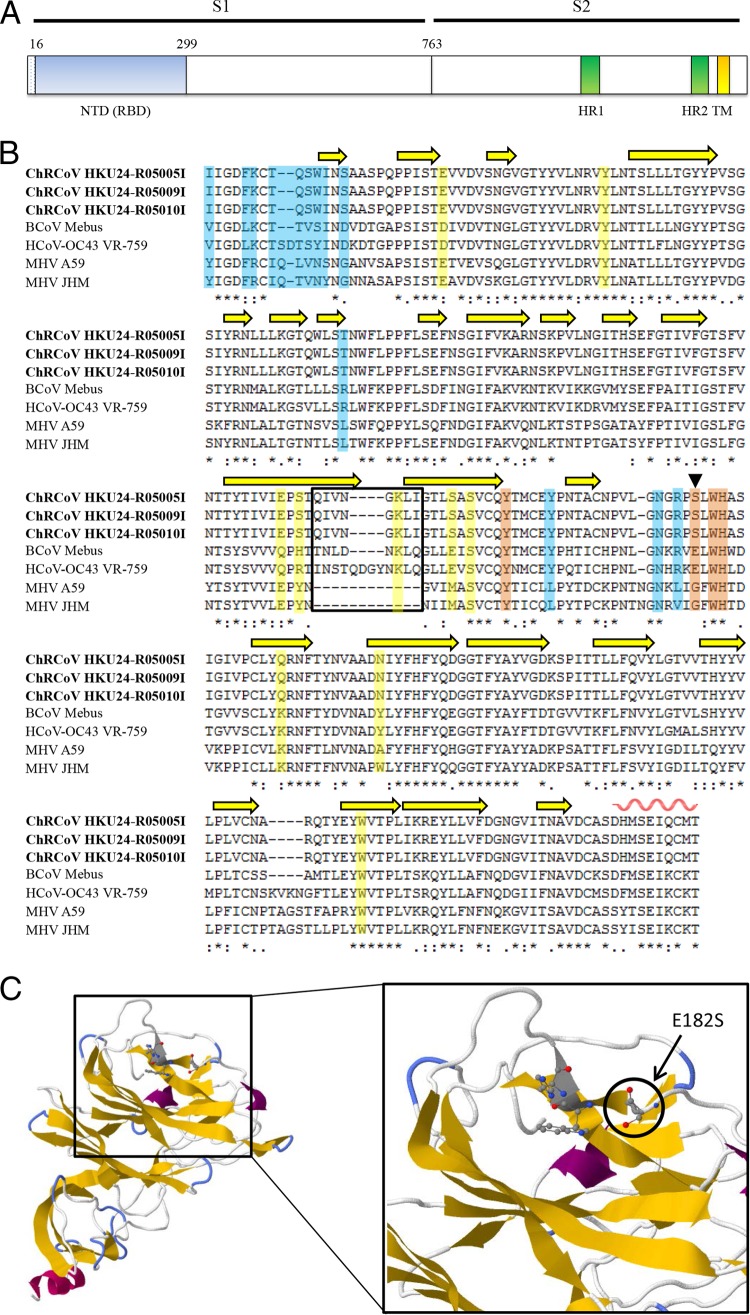
Similar to other CoV S proteins, the S protein of ChRCoV HKU24 is predicted to be a type I membrane glycoprotein, with most of the protein (residues 16 to 1302) being exposed on the outside of the virus and with a transmembrane domain (residues 1303 to 1325) occurring at the C terminus (Fig. 2). Two heptad repeats (HRs), important for membrane fusion and viral entry, were located at residues 1045 to 1079 (HR1) and 1253 to 1285 (HR2). The S protein of ChRCoV HKU24 possessed 66.7 to 69.6% amino acid sequence identities to the amino acid sequences of members of Betacoronavirus 1 and 62.4 to 64.3% amino acid sequence identities to the amino acid sequences of members of murine coronavirus. The amino acid sequence identities between the ChRCoV HKU24 NTD and the BCoV and MHV NTDs were 61 and 56%, respectively. BCoV and HCoV OC43 utilize N-acetyl-9-O-acetylneuramic acid as a receptor for the initiation of infection (65, 66). In contrast, MHV utilizes carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) as a receptor, and its receptor-binding domain does not bind sugars (10, 67, 68). Recent structural studies showed that, among the four critical sugar-binding residues in BoV, a Glu → Gly substitution which may explain the reduction in sugar-binding affinity was found in one residue in MHV. In ChRCoV HKU24, a Glu → Ser substitution is found at this position (Fig. 2). Comparison of the amino acid sequences between the S proteins of ChRCoV HKU24 and MHV showed that ChRCoV HKU24 possessed many amino acid substitutions in the region corresponding to the MHV NTD (Fig. 2). In particular, 12 of the 14 important contact residues at the MHV NTD/murine CEACAM1a (mCEACAM1a) interface were not conserved between ChRCoV HKU24 and MHV. Similar to the MHV and BCoV NTDs, the ChRCoV HKU24 NTD is also predicted, using homology modeling, to contain a core structure with a β-sandwich fold like that in human galectins (galactose-binding lectins) (10). Modeling showed that the β-sandwich core structure of ChRCoV HKU24 consists of one six-stranded β sheet and one seven-stranded β sheet that are stacked together through hydrophobic interactions (Fig. 2). In addition, the S protein of ChRCoV HKU24 possessed a unique predicted cleavage site, RAKR, among lineage A βCoVs.
FIG 2.
Predicted model of ChRCoV HKU24 spike protein and NTD using the SWISS-MODEL tool. (A) Predicted domain structure of ChRCoV HKU24 spike protein. NTD, N-terminal domain; RBD, receptor-binding domain; HR, heptad repeat; TM, transmembrane anchor. The signal peptide corresponds to residues 1 to 15 and is cleaved during molecular maturation. (B) Sequence alignment of the ChRCoV HKU24 NTD with the BCoV, HCoV OC43, and MHV NTDs, performed using the PROMALS3D program. The three strains of ChRCoV HKU24 characterized in this study are in bold. β strands are shown as yellow arrows, and the alpha helix is shown as a red wavy line. Loops 10 and 11 are boxed. The 14 contact residues at the MHV NTD/mCEACAM1a interface are highlighted in blue, the four BCoV critical sugar-binding residues are highlighted in brown, and the BCoV noncritical sugar-binding residues are highlighted in yellow. The location of the residue substitution that might decrease the sugar-binding affinity of BCoV NTD is marked by an inverted triangle. Asterisks, positions that have fully conserved residues; colons, positions that have strongly conserved residues; periods, positions that have weakly conserved residues. (C) Predicted structure of the ChRCoV HKU24 NTD constructed through homology modeling from BCoV NTD (4h14) and close-up view of the pocket above the β-sandwich core. The global model quality estimation score of 0.83 and QMEAN4 Z-score of −1.82 indicate reliable overall model quality.
Other predicted domains in the HE, S, NS4, NS5, E, M, and N proteins of ChRCoV HKU24 are summarized in Table 3 and Fig. 1. The NS4 of ChRCoV HKU24 shared 37 to 42% amino acid sequence identity to the NS4 proteins of members of murine coronavirus. In most members of Betacoronavirus 1, NS4 is split into smaller proteins. NS5 of ChRCoV HKU24 is homologous to NS5/NS5a of members of Betacoronavirus 1, having 47.7% to 51.4% amino acid sequence identities, but it has only 39.5% amino acid sequence identity to NS5 of members of MHV. Interestingly, NS5 is not found in the genome of RCoV. The absence of a preceding TRS upstream of the E of ChRCoV HKU24 suggests that the translation of this E protein may be cap independent via an internal ribosomal entry site (IRES), as demonstrated in MHV (69). Similarly, the E proteins of RCoV and HCoV HKU1 were also not preceded by a TRS. This is in contrast to the findings for members of Betacoronavirus 1, which possess a preceding TRS upstream of their E proteins (49, 61). Downstream of the N gene, the 3′ untranslated region contains a predicted bulged stem-loop structure of 69 nucleotides (nt; nucleotide positions 30944 to 31012) that is conserved in βCoVs (70). Overlapping with the bulged stem-loop structure by 5 nt, a conserved pseudoknot structure (nucleotide positions 31008 to 31059) that is important for CoV replication is found. Since nonstructural proteins in CoVs may possess unique functions for replication and virulence (71, 72), further studies are warranted to understand the potential function of the nsp's and NS proteins in ChRCoV HKU24.
Phylogenetic analyses.
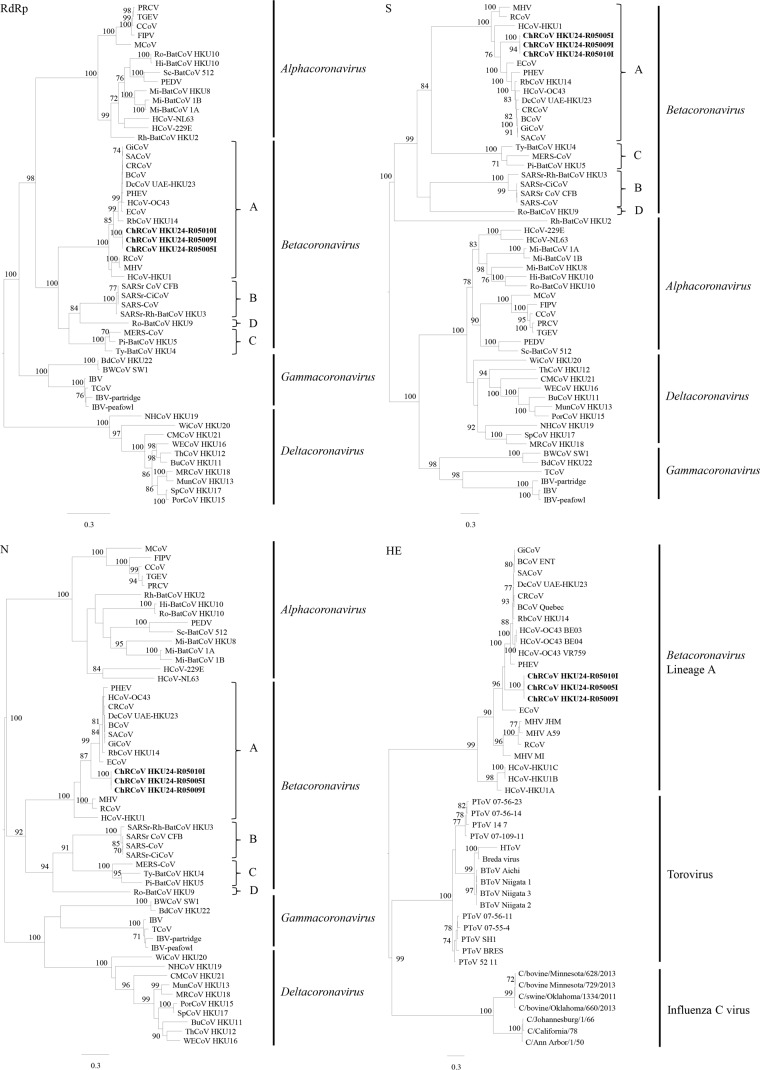
Phylogenetic trees constructed using the amino acid sequences of the RdRp, S, and N proteins of ChRCoV HKU24 and other CoVs are shown in Fig. 3, and the corresponding pairwise amino acid sequence identities are shown in Table 2. For all three genes, the three ChRCoV HKU24 strains formed a distinct cluster among lineage A βCoVs, occupying a deep branch at the root of members of the species Betacoronavirus 1 and being the most closely related to members of the species Betacoronavirus 1. Comparison of the amino acid sequences of the seven conserved replicase domains for CoV species demarcation (3), ADP-ribose 1″-phosphatase (ADRP), nsp5 (3C-like protease [3CLpro]), nsp12 (RdRp), nsp13 (Hel), nsp14 (exonuclease [ExoN]), nsp15 (nidoviral uridylate-specific endoribonuclease [NendoU]), and nsp16 (2′-O-ribose methyltransferase [O-MT]), showed that these domains of ChRCoV HKU24 possessed 69.5 to 81.7%, 82.2 to 86.8%, 88.1 to 92.6%, 88.9 to 94.8%, 80.2 to 88.7%, 70.1 to 79.5%, and 83.8 to 89.7% amino acid identities to those of other lineage A βCoVs, respectively (Table 5). Based on the present results, we propose a novel species, ChRCoV HKU24, to describe this virus under Betacoronavirus lineage A and distinguish it from RCoV.
FIG 3.
Phylogenetic analyses of the RdRp, S, N, and HE proteins of ChRCoV HKU24. The trees were constructed by the maximum likelihood method using the WAG+I+G substitution model and bootstrap values calculated from 100 trees. Bootstrap values below 70% are not shown. Nine hundred twenty-eight, 1,358, 443, and 425 aa positions in RdRp, S, N, and HE, respectively, were included in the analyses. The scale bar represents 0.3 substitution per site. The three strains of ChRCoV HKU24 characterized in this study are in bold. Definitions of the abbreviations are provided in footnote a of Table 2.
TABLE 5.
Pairwise comparisons of Coronaviridae-wide conserved domains in replicase polyprotein 1ab between ChRCoV HKU24 and other lineage A betacoronaviruses
| Replicase polyprotein domain | Pairwise amino acid sequence identity with the ChRCoV HKU24 sequence (%) |
|||
|---|---|---|---|---|
| Betacoronavirus 1 | RbCoV HKU14 | Murine coronavirus | HCoV HKU1 | |
| nsp3 (ADRP) | 74.8–81.7 | 74.8 | 69.5–70.2 | 71 |
| nsp5 (3CLpro) | 85.8–86.8 | 86.8 | 82.5–82.8 | 82.2 |
| nsp12 (RdRp) | 91.8–92.6 | 92.5 | 90.3 | 88.1 |
| nsp13 (Hel) | 93.4–94.8 | 94.7–94.8 | 90.5–90.7 | 88.9–89.1 |
| nsp14 (ExoN) | 86.4–88.7 | 88.7 | 83.9–84.1 | 80.2 |
| nsp15 (NendoU) | 77.6–79.2 | 79.5 | 72.0–73.6 | 70.1 |
| nsp16 (O-MT) | 88.7–89.7 | 89.1 | 83.8–85.1 | 84.1 |
HE proteins are glycoproteins that mediate reversible attachment to O-acetylated sialic acids by acting as both lectins and receptor-destroying enzymes which aid viral detachment from sugars on infected cells (68, 73). Related HEs have been found in influenza C viruses, toroviruses, and lineage A βCoVs, but not other CoVs. It has been suggested that HEs of lineage A βCoVs have arisen from an influenza C virus-like HE fusion protein, likely as a result of relatively recent lateral gene transfer events (73). Phylogenetic analysis of the HE proteins of lineage A βCoVs, toroviruses, and influenza C viruses showed that they fell into three separate clusters (Fig. 3). The HE of ChRCoV HKU24 also forms a deep branch at the root of all members of the species Betacoronavirus 1 except ECoV and is distinct from members of murine coronavirus. Previous studies have demonstrated the heterogeneity of gene expression of HE proteins among different MHV strains (74). Since the HE of ChRCoV HKU24 is not preceded by a perfectly matched TRS, further studies are required to determine if it is expressed and functional.
Estimation of divergence dates.
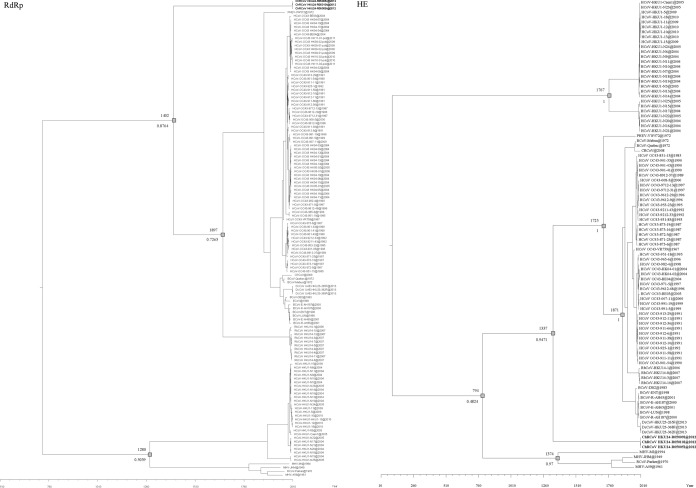
Using the uncorrelated relaxed-clock model on complete RdRp gene sequences, the date of tMRCA of ChRCoV HKU24, members of Betacoronavirus 1, and RbCoV HKU14 was estimated to be 1402 (HPDs, 918.05 to 1749.91) (Fig. 4). The date of divergence between HCoV OC43 and BCoV was estimated to be 1897 (HPDs, 1826.15 to 1950.05), consistent with results from previous molecular clock studies (27). Using the uncorrelated relaxed-clock model on complete HE gene sequences, the date of tMRCA of ChRCoV HKU24, members of Betacoronavirus 1, and RbCoV HKU14 was estimated to be 1337 (HPDs, 724.59 to 1776.78) (Fig. 4). The date of divergence between HCoV OC43 and BCoV was estimated to be 1871 (HPDs, 1764.55 to 1944.37). The estimated mean substitution rates of the RdRp and HE data sets were 1.877 × 10−4 and 4.016 × 10−4 substitution per site per year, respectively, which are comparable to previous estimates for other lineage A βCoVs (26, 27, 39).
FIG 4.
Estimation of tMRCA of the ChRCoV HKU24 strains, BCoV/HCoV OC43, and ChRCoV HKU24/members of Betacoronavirus 1/RbCoV HKU14 on the basis of the complete sequences of the RdRp and HE genes. The mean estimated dates (above the branch) and Bayesian posterior probabilities (below the branch) are labeled and are represented by gray squares. The taxa are labeled with their sampling dates.
Serological studies.
Western blot analysis using recombinant ChRCoV HKU24 N protein was performed using sera from 144 rodents for which serum samples were available, sera from two patients with HCoV OC43 infection, sera from two rabbits with RbCoV HKU14 infection, and sera from two patients with SARS-CoV infection. Among the sera tested from 74 Norway rats from Guangzhou for which serum samples were available, 60 (81.1%) were positive for antibody against recombinant ChRCoV HKU24 N protein with prominent immunoreactive bands of about 50 kDa (Table 1 and Fig. 5). These 60 positive samples included the 3 serum samples collected from the three Norway rats positive for ChRCoV HKU24 in their alimentary tract samples. In addition, 15 (48.4%) of 31 Norway rats from Hong Kong were also positive for antibody against recombinant ChRCoV HKU24 N protein, although the virus was not detected in alimentary tract samples from these rats. Moreover, 7 (77.8%) of 9 oriental house rats but only 4 (0.13%) of 30 black rats were positive for antibody against recombinant ChRCoV HKU24 N protein. Possible cross antigenicity between ChRCoV HKU24 and other βCoVs, including lineage A and B βCoVs, was found. Sera from two patients with HCoV OC43 infection, sera from two rabbits with RbCoV HKU14 infection, and sera from two patients with SARS-CoV infection were also positive for antibody against recombinant ChRCoV HKU24 N protein by Western blot assay (Fig. 5).
FIG 5.
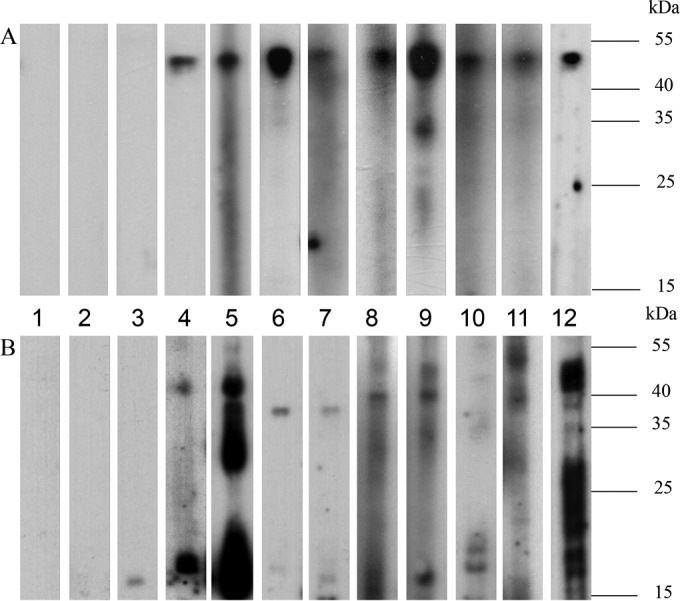
Western blot analysis for antibodies against purified His6-tagged recombinant ChRCoV HKU24 N protein (∼50-kDa) (A) and spike polypeptide (∼50-kDa) (B) in rodent serum samples and serum samples from other animals or humans infected by different βCoVs, including HCoV OC43 (Betacoronavirus lineage A), RbCoV HKU14 (Betacoronavirus lineage A), and SARS-CoV (Betacoronavirus lineage B). Lanes: 1, negative control; 2, oriental house rat serum sample negative for antibody against the ChRCoV HKU24 N protein and spike polypeptide; 3, Norway rat serum sample negative for antibody against the ChRCoV HKU24 N protein and spike polypeptide; 4, oriental house rat serum sample positive for antibody against the ChRCoV HKU24 N protein and spike polypeptide; 5, Norway rat serum sample positive for antibody against the ChRCoV HKU24 N protein and spike polypeptide; 6 and 7, serum samples from rabbits infected by RbCoV HKU14; 8 and 9, serum samples from patients with HCoV OC43 infection; 10 and 11, serum samples from patients with SARS-CoV infection; 12, positive control (anti-His antibody).
Western blot analysis using recombinant ChRCoV HKU24 spike polypeptide was performed to verify the specificity of antibodies against ChRCoV HKU24 N protein using positive rodent sera and sera from two patients with HCoV OC43 infection, sera from two rabbits with RbCoV HKU14 infection, and sera from two patients with SARS-CoV infection. Among the sera from the 60 Norway rats positive for antibodies against the ChRCoV HKU24 N protein, 21 were positive for antibodies against the ChRCoV HKU24 spike polypeptide with prominent immunoreactive bands of about 50 kDa (Table 1 and Fig. 5). However, serum samples from the three Norway rats positive for ChRCoV HKU24 in their alimentary tract samples were negative for anti-ChRCoV HKU24 spike polypeptide antibody. Of the seven oriental house rats positive for antibodies against the ChRCoV HKU24 N protein, two were positive for antibodies against the ChRCoV HKU24 spike polypeptide. However, serum samples from the 4 black rats and 15 Norway rats from Hong Kong positive for antibodies against the ChRCoV HKU24 N protein were negative for antibodies against the ChRCoV HKU24 spike polypeptide. In contrast to N protein, no cross antigenicity between the ChRCoV HKU24 spike polypeptide and sera positive for antibodies against other βCoVs, including lineage A and B βCoVs, was detected. Sera from two patients with HCoV OC43 infection, sera from two rabbits with RbCoV HKU14 infection, and sera from two patients with SARS-CoV infection were all negative for antibody against recombinant ChRCoV HKU24 spike polypeptide by Western blot assay (Fig. 5).
DISCUSSION
We discovered a novel lineage A βCoV, ChRCoV HKU24, from Norway rats in southern China. Betacoronavirus lineage A comprises the traditional group 2 CoVs, including members of murine coronavirus and Betacoronavirus 1, HCoV HKU1, and RbCoV HKU14. ChRCoV HKU24 possessed <90% amino acid sequence identities to the amino acid sequences of all other lineage A βCoVs in five of the seven conserved replicase domains used for CoV species demarcation by ICTV (3), supporting the suggestion that ChRCoV HKU24 belongs to a separate species. The genome of ChRCoV HKU24 also possesses features distinct from those of other lineage A βCoVs, including a unique putative nsp1/nsp2 cleavage site and a unique putative cleavage site in S protein. Phylogenetically, its position at the root of Betacoronavirus 1, being distinct from the positions of murine coronavirus and HCoV HKU1, suggests that ChRCoV HKU24 may represent the murine ancestor for Betacoronavirus 1, after branching off from the common ancestor of murine coronavirus and HCoV HKU1. Interestingly, the genome of ChRCoV HKU24 possessed features that resemble those of the genomes of both Betacoronavirus 1 and murine coronavirus. It is more similar to Betacoronavirus 1 than murine coronavirus by the higher sequence identities in most predicted proteins, including NS2a, NS5, and S. On the other hand, it is more similar to murine coronavirus than to Betacoronavirus 1 in terms of its G+C content, the presence of a single NS4, and the absence of a TRS upstream of the E gene. Therefore, it is most likely that ChRCoV has evolved from the ancestor of murine coronavirus to infect other mammals, resulting in the generation of Betacoronavirus 1 with the acquisition of a TRS for the E gene. The tMRCAs of ChRCoV HKU24, members of Betacoronavirus 1, and RbCoV HKU14 were estimated to be 1402 (HPDs, 918.05 to 1749.91) and 1337 (HPDs, 724.59 to 1776.78) using complete RdRp and HE gene analysis, respectively, suggesting that interspecies transmission from rodents to other mammals occurred at least several centuries ago before the emergence of HCoV OC43 in humans in about the 1890s.
Western blot assays based on recombinant ChRCoV HKU24 N protein and spike polypeptide showed a high seroprevalence of ChRCoV HKU24 infection among Norway rats from Guangzhou. We evaluated the cross-reactivities of both N protein and spike polypeptide assays using sera from infections caused by other lineage A βCoVs, HCoV OC43 in humans and RbCoV HKU14 in rabbits, as well as SARS-CoV, a lineage B βCoV. Cross-reacting antibodies against N proteins were observed, a finding which is in line with previous findings on cross-reactivity between N proteins of different βCoVs (49, 57). In contrast, no cross-reactivities between spike polypeptides were detected, supporting the specificity of CoV spike polypeptide-based assays and their ability to rectify cross-reactivities (57, 58). Using the present assays, 60 of 74 Norway rats from Guangzhou were positive for antibodies against the ChRCoV HKU24 N protein, and among these rats, 21 were positive for antibodies against the ChRCoV HKU24 spike polypeptide, indicating that these 21 rats had previously been infected with ChRCoV HKU24. Interestingly, the three Norway rats positive for ChRCoV HKU24 in their alimentary tract samples were positive for antibodies against ChRCoV HKU24 N protein but negative for antibodies against ChRCoV HKU24 spike polypeptide. This is likely due to a delay in mounting neutralizing antibodies against spike protein during acute infection in these three rats, where antibodies against N protein may arise earlier as a result of the high abundance and antigenicity of CoV N proteins, or the response to the N protein may be a result of cross-reactions to N proteins from other βCoVs. The finding is also in keeping with previous findings on SARS-related Rhinolophus bat CoV, in which a negative correlation between the viral load and neutralizing antibody titer was observed (14). Besides Norway rats, antibodies against ChRCoV HKU24 N protein and spike polypeptide were also detected in two oriental house rats from Guangzhou, although antibodies against spike polypeptide were relatively weak. This suggests possible cross-species infection with ChRCoV HKU24 or cross-reactivity from a very close lineage A βCoV. Four black rats and 15 Norway rats in Hong Kong were also positive for antibodies against the ChRCoV HKU24 N protein but not the spike polypeptide. This suggests a possible past infection by another βCoV(s) and cross-reactivity between the N protein(s) of that βCoV(s) and the N protein of ChRCoV HKU24. More studies with diverse rodent species from China and other countries are required to determine the natural reservoir and host range of ChRCoV HKU24 and other murine lineage A βCoVs.
The present results extend our knowledge on the evolutionary origin of CoVs. While birds are important sources for γCoVs and δCoVs, bats host diverse αCoVs and βCoVs that may be the ancestral origins of various mammalian CoVs, including human CoVs. For human αCoVs, both HCoV NL63 and HCoV 229E likely originated from bat CoVs. HCoV NL63 has been shown to share a common ancestry with αCoVs from the North American tricolored bat, with the most recent common ancestor between these viruses occurring from approximately 563 to 822 years ago (75). Moreover, immortalized lung cell lines derived from this bat species allowed replication of HCoV NL63, supporting potential zoonotic-reverse zoonotic transmission cycles between bats and humans. HCoV 229E also shared a common ancestor with diverse αCoVs from leaf-nosed bats in Ghana, with the most recent common ancestor dating to 1686 to 1800 (76). However, no complete genomes are available for the putative bat ancestors of HCoV NL63 and HCoV 229E. For human βCoVs, SARS-CoV and MERS-CoV are also known to share common ancestors with bat CoVs. Soon after the SARS epidemic, horseshoe bats in China were found to be the reservoir for SARS-CoV-like viruses, which were postulated to have jumped from bats to civets and, later, humans (8, 14, 15). A recent study also reported the isolation of a SARS-like bat CoV in Vero E6 cells and the ability of this bat virus to use the angiotensin-converting enzyme 2 (ACE2) from humans, civets, and Chinese horseshoe bats for cell entry (77). MERS-CoV belongs to Betacoronavirus lineage C, which was known to consist of only two bat viruses, Tylonycteris bat CoV HKU4 and Pipistrellus bat CoV HKU5, before the MERS epidemic (35–37). This has led to the speculation that bats may be the zoonotic origin of MERS-CoV. However, recent evidence supports dromedary camels as the immediate source of human MERS-CoV (78–80). Nevertheless, a conspecific virus from a South African Neoromicia capensis bat has been found to share 85% nucleotide sequence identity to the nucleotide sequence of the MERS-CoV genome, suggesting the acquisition of MERS-CoV by camels from bats in sub-Saharan Africa, from where camels on the Arabian peninsula are imported (81). In contrast, there has been no evidence that bats are the origin of human lineage A βCoVs, such as HCoV OC43 and HCoV HKU1. HCoV OC43, being closely related to BCoV, is believed to have emerged relatively recently from bovine-to-human transmission in about 1890 (27, 30, 39). Both viruses belonged to the promiscuous CoV species Betacoronavirus 1, which consists of many closely related mammalian CoVs, implying a low threshold for cross-mammalian species transmission and a complex evolutionary history among these viruses (40–47, 49). However, the ancestral origin of members of Betacoronavirus 1 remains elusive. As for HCoV HKU1, no recent zoonotic ancestor has yet been identified, although the virus is most closely related to members of murine coronaviruses (20, 42). Although rodents constitute approximately 40% of all mammalian species, murine coronavirus has been the only CoV species known to exist in rodents. This is in contrast to the large diversity of CoVs found in bats, which make up another 20% of all species of mammals (6, 33, 36). The present results suggest that rodents may be an important reservoir for lineage A βCoVs and may harbor other ancestral viruses of Betacoronavirus 1 and HCoV HKU1 (Fig. 6). Nevertheless, many mysteries about the evolution of lineage A βCoVs remain unresolved, such as the origin of their HE proteins. For example, both toroviruses and influenza C viruses can be found in bovine and porcine samples. Further studies are required to determine if the HE proteins of potential rodent CoV ancestors of Betacoronavirus lineage A may have been acquired from cattle or pigs.
FIG 6.
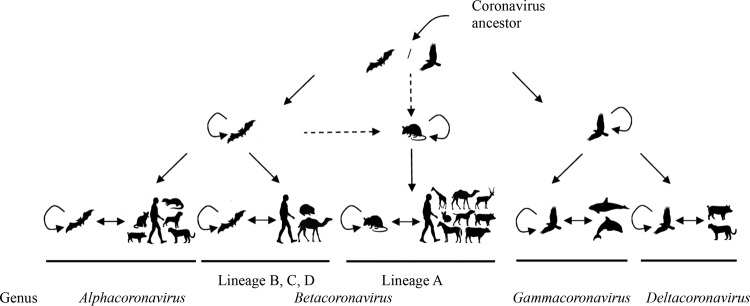
Evolution of CoVs from their ancestors in bat, bird, and rodent hosts to virus species that infect other animals. Dashed arrows, possible routes of transmission from bats or birds to rodents before establishment of Betacoronavirus lineage A.
The potential pathogenicity and tissue tropism of ChRCoV HKU24 remain to be determined. While CoVs are associated with a wide spectrum of diseases in animals, some CoVs, especially those from bats, were detected in apparently healthy individuals without obvious signs of disease (8, 14, 15, 31, 33). The detection of ChRCoV HKU24 in the alimentary tract samples of Norway rats suggested a possible enteric tropism. However, the three positive rats did not show obvious signs of disease. MHV, the prototype CoV most extensively studied before the SARS epidemic, can cause a variety of neurological, hepatic, gastrointestinal, and respiratory diseases in mice, depending on the strain tropism and route of inoculation. The virus, originally isolated from a mouse with spontaneous encephalomyelitis, causes disseminated encephalomyelitis with extensive destruction of myelin and focal necrosis of the liver in experimentally infected mice (82–84). Strain MHV A59 is primarily hepatotropic, while strain MHV JHM is neurotropic. Enterotropic strains can spread quickly as a result of the high level of excretion in feces and cause significant environmental contamination in animal houses. Respiratory tract-tropic or polytropic strains, although uncommon, are the strains that commonly contaminate cell lines. As for RCoV, it causes diseases primarily in the respiratory tract, with strain sialodacryoadenitis virus (SDAV) being more associated with upper respiratory tract, salivary and lacrimal gland, and eye infections and strain RCoV Parker causing pneumonia in experimentally infected rats (85, 86). Further investigations are required to study the tissue tropism and pathogenicity of ChRCoV HKU24 in Norway rats and other potential rodent reservoirs.
Elucidating the receptor of ChRCoV HKU24 will be important to understand the mechanism of host adaptation and interspecies transmission from rodents to other mammals. The higher sequence identity to Betacoronavirus 1 than to murine coronavirus of the S protein and NTD of ChRCoV HKU24 is in line with the findings for other regions of the genome. Homology modeling showed that the conformation of the sugar-binding loop in the BCoV NTD is conserved in the ChRCoV HKU24 NTD. Moreover, 3 of the 4 critical sugar-binding residues in BCoV but only 2 of the 14 contact residues at the MHV NTD/mCEACAM1a interface are conserved in ChRCoV HKU24. While it remains to be ascertained if ChRCoV HKU24 may utilize sugar or CEACAM1 as a receptor, its predicted NTD appears to resemble that of BCoV more than that of MHV. On the basis of the presence of a β-sandwich fold in the NTDs of MHV and BCoV, it has been proposed that CoV NTDs may have originated from a host galectin with sugar-binding functions but evolved new structural features in MHV for binding to CEACMA1 (10, 87). If rodents are indeed the host origin for Betacoronavirus lineage A, including Betacoronavirus 1, it would be interesting to study the sugar-binding activity of NTDs of different rodent βCoVs to understand their evolutionary history. Although some lineage A βCoVs, such as Betacoronavirus 1 and MHV, can replicate in cell lines such as BSC-1 and HRT-18, attempts to isolate ChRCoV HKU24 from the three positive samples were unsuccessful. Future studies to isolate the virus from more rodent samples will allow characterization of its receptor usage and pathogenicity.
ACKNOWLEDGMENTS
We thank the following for facilitation of and assistance with sample collection: Wing-Man Ko, Secretary for the Food and Health Bureau; Vivian Lau, Kwok-Hau Sin, and M. C. Yuen of FEHD; Alan C. K. Wong, Siu-Fai Leung, Thomas Hon-Chung Sit, Howard Kai-Hay Wong, Chung-Tong Shek, and Joseph W. K. So of AFCD. We are grateful for the generous support of Carol Yu, Richard Yu, Hui Hoy, and Hui Ming in the genomic sequencing platform.
The views expressed in this paper are those of the authors only and do not represent the opinion of FEHD, AFCD, or the government of the HKSAR.
This work is partly supported by a Research Grant Council grant, University Grant Council; the Committee for Research and Conference Grants, the Strategic Research Theme Fund, and the University Development Fund, The University of Hong Kong; the Health and Medical Research Fund of the Food and Health Bureau of HKSAR; and the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease for the HKSAR Department of Health.
REFERENCES
- 1.Brian DA, Baric RS. 2005. Coronavirus genome structure and replication. Curr Top Microbiol Immunol 287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai MM, Cavanagh D. 1997. The molecular biology of coronaviruses. Adv Virus Res 48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Groot RJ, Baker SC, Baric R, Enjuanes L, Gorbalenya A, Holmes KV, Perlman S, Poon L, Rottier PJ, Talbot PJ, Woo PC, Ziebuhr J. 2011. Coronaviridae, p 806–828 InKing AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Academic Press, London, United Kingdom. [Google Scholar]
- 4.Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC, Wang M, Zheng BJ, Chan KH, Yuen KY. 2012. Discovery of seven novel mammalian and avian coronaviruses in Deltacoronavirus supports bat coronaviruses as the gene source of Alphacoronavirus and Betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and Deltacoronavirus. J Virol 86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo PC, Lau SK, Lam CS, Lai KK, Huang Y, Lee P, Luk GS, Dyrting KC, Chan KH, Yuen KY. 2009. Comparative analysis of complete genome sequences of three avian coronaviruses reveals a novel group 3c coronavirus. J Virol 83:908–917. doi: 10.1128/JVI.01977-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo PC, Lau SK, Huang Y, Yuen KY. 2009. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med (Maywood) 234:1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- 7.Herrewegh AA, Smeenk I, Horzinek MC, Rottier PJ, de Groot RJ. 1998. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J Virol 72:4508–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau SK, Li KS, Huang Y, Shek CT, Tse H, Wang M, Choi GK, Xu H, Lam CS, Guo R, Chan KH, Zheng BJ, Woo PC, Yuen KY. 2010. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol 84:2808–2819. doi: 10.1128/JVI.02219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo PC, Lau SK, Yip CC, Huang Y, Tsoi HW, Chan KH, Yuen KY. 2006. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. J Virol 80:7136–7145. doi: 10.1128/JVI.00509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng G, Sun D, Rajashankar KR, Qian Z, Holmes KV, Li F. 2011. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc Natl Acad Sci U S A 108:10696–10701. doi: 10.1073/pnas.1104306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Du L, Liu C, Wang L, Ma C, Tang J, Baric RS, Jiang S, Li F. 2014. Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc Natl Acad Sci U S A 111:12516–21521. doi: 10.1073/pnas.1405889111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh SH, Wang HY, Tsai CY, Kao CL, Yang JY, Liu HW, Su IJ, Tsai SF, Chen DS, Chen PJ, National Taiwan University SARS Research Team . 2004. Characterization of severe acute respiratory syndrome coronavirus genomes in Taiwan: molecular epidemiology and genome evolution. Proc Natl Acad Sci U S A 101:2542–2547. doi: 10.1073/pnas.0307904100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, Butt KM, Wong KL, Chan KW, Lim W, Shortridge KF, Yuen KY, Peiris JS, Poon LL. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 14.Lau SK, Woo PC, Li KS, Huang Y, Tsoi HW, Wong BH, Wong SS, Leung SY, Chan KH, Yuen KY. 2005. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A 102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, Zhang J, McEachern J, Field H, Daszak P, Eaton BT, Zhang S, Wang LF. 2005. Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 16.Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Penaranda S, Bankamp B, Maher K, Chen MH, Tong S, Tamin A, Lowe L, Frace M, DeRisi JL, Chen Q, Wang D, Erdman DD, Peret TC, Burns C, Ksiazek TG, Rollin PE, Sanchez A, Liffick S, Holloway B, Limor J, McCaustland K, Olsen-Rasmussen M, Fouchier R, Gunther S, Osterhaus AD, Drosten C, Pallansch MA, Anderson LJ, Bellini WJ. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 18.Fouchier RA, Hartwig NG, Bestebroer TM, Niemeyer B, de Jong JC, Simon JH, Osterhaus AD. 2004. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci U S A 101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, Wertheim-van Dillen PM, Kaandorp J, Spaargaren J, Berkhout B. 2004. Identification of a new human coronavirus. Nat Med 10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, Wong BH, Poon RW, Cai JJ, Luk WK, Poon LL, Wong SS, Guan Y, Peiris JS, Yuen KY. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonassen CM, Kofstad T, Larsen IL, Lovland A, Handeland K, Follestad A, Lillehaug A. 2005. Molecular identification and characterization of novel coronaviruses infecting graylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anas platyrhynchos). J Gen Virol 86:1597–1607. doi: 10.1099/vir.0.80927-0. [DOI] [PubMed] [Google Scholar]
- 22.Mihindukulasuriya KA, Wu G, St Leger J, Nordhausen RW, Wang D. 2008. Identification of a novel coronavirus from a beluga whale by using a panviral microarray. J Virol 82:5084–5088. doi: 10.1128/JVI.02722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong BQ, Liu W, Fan XH, Vijaykrishna D, Tang XC, Gao F, Li LF, Li GJ, Zhang JX, Yang LQ, Poon LL, Zhang SY, Peiris JS, Smith GJ, Chen H, Guan Y. 2007. Detection of a novel and highly divergent coronavirus from Asian leopard cats and Chinese ferret badgers in southern China. J Virol 81:6920–6926. doi: 10.1128/JVI.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau SK, Woo PC, Yip CC, Tse H, Tsoi HW, Cheng VC, Lee P, Tang BS, Cheung CH, Lee RA, So LY, Lau YL, Chan KH, Yuen KY. 2006. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol 44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo PC, Lau SK, Tsoi HW, Huang Y, Poon RW, Chu CM, Lee RA, Luk WK, Wong GK, Wong BH, Cheng VC, Tang BS, Wu AK, Yung RW, Chen H, Guan Y, Chan KH, Yuen KY. 2005. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis 192:1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau SK, Lee P, Tsang AKL, Yip CCY, Tse H, Lee RA, So LY, Lau YL, Chan KH, Woo PCY, Yuen KY. 2011. Molecular epidemiology of human coronavirus OC43 reveals evolution of different genotypes over time and recent emergence of a novel genotype due to natural recombination. J Virol 85:11325–11337. doi: 10.1128/JVI.05512-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vijgen L, Keyaerts E, Moës E, Thoelen I, Wollants E, Lemey P, Vandamme AM, Van Ranst M. 2005. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J Virol 79:1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominguez SR, O'Shea TJ, Oko LM, Holmes KV. 2007. Detection of group 1 coronaviruses in bats in North America. Emerg Infect Dis 13:1295–1300. doi: 10.3201/eid1309.070491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gloza-Rausch F, Ipsen A, Seebens A, Göttsche M, Panning M, Felix Drexler J, Petersen N, Annan A, Grywna K, Müller M, Pfefferle S, Drosten C. 2008. Detection and prevalence patterns of group I coronaviruses in bats, northern Germany. Emerg Infect Dis 14:626–631. doi: 10.3201/eid1404.071439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau SK, Woo PC, Li KS, Huang Y, Wang M, Lam CS, Xu H, Guo R, Chan KH, Zheng BJ, Yuen KY. 2007. Complete genome sequence of bat coronavirus HKU2 from Chinese horseshoe bats revealed a much smaller spike gene with a different evolutionary lineage from the rest of the genome. Virology 367:428–439. doi: 10.1016/j.virol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau SK, Poon RWS, Wong BHL, Wang M, Huang Y, Xu H, Guo R, Li KSM, Gao K, Chan KH, Zheng BJ, Woo PCY, Yuen KY. 2010. Coexistence of different genotypes in the same bat and serological characterization of Rousettus bat coronavirus HKU9 belonging to a novel Betacoronavirus subgroup. J Virol 84:11385–11394. doi: 10.1128/JVI.01121-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Poon LL, Chu DK, Chan KH, Wong OK, Ellis TM, Leung YH, Lau SK, Woo PC, Suen KY, Yuen KY, Guan Y, Peiris JS. 2005. Identification of a novel coronavirus in bats. J Virol 79:2001–2009. doi: 10.1128/JVI.79.4.2001-2009.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang XC, Zhang JX, Zhang SY, Wang P, Fan XH, Li LF, Li G, Dong BQ, Liu W, Cheung CL, Xu KM, Song WJ, Vijaykrishna D, Poon LL, Peiris JS, Smith GJ, Chen H, Guan Y. 2006. Prevalence and genetic diversity of coronaviruses in bats from China. J Virol 80:7481–7490. doi: 10.1128/JVI.00697-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong S, Conrardy C, Ruone S, Kuzmin IV, Guo X, Tao Y, Niezgoda M, Haynes L, Agwanda B, Breiman RF, Anderson LJ, Rupprecht CE. 2009. Detection of novel SARS-like and other coronaviruses in bats from Kenya. Emerg Infect Dis 15:482–485. doi: 10.3201/eid1503.081013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo PC, Wang M, Lau SK, Xu H, Poon RW, Guo R, Wong BH, Gao K, Tsoi HW, Huang Y, Li KS, Lam CS, Chan KH, Zheng BJ, Yuen KY. 2007. Comparative analysis of twelve genomes of three novel group 2c and group 2d coronaviruses reveals unique group and subgroup features. J Virol 81:1574–1585. doi: 10.1128/JVI.02182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo PC, Lau SK, Li KS, Poon RW, Wong BH, Tsoi HW, Yip BC, Huang Y, Chan KH, Yuen KY. 2006. Molecular diversity of coronaviruses in bats. Virology 351:180–187. doi: 10.1016/j.virol.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau SK, Li KS, Tsang AK, Lam CS, Ahmed S, Chen H, Chan KH, Woo PC, Yuen KY. 2013. Genetic characterization of Betacoronavirus lineage C viruses in bats reveals marked sequence divergence in the spike protein of Pipistrellus bat coronavirus HKU5 in Japanese pipistrelle: implications for the origin of the novel Middle East respiratory syndrome coronavirus. J Virol 87:8638–8650. doi: 10.1128/JVI.01055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drexler JF, Corman VM, Drosten C. 2014. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res 101:45–56. doi: 10.1016/j.antiviral.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vijgen L, Keyaerts E, Lemey P, Maes P, Van Reeth K, Nauwynck H, Pensaert M, Van Ranst M. 2006. Evolutionary history of the closely related group 2 coronaviruses: porcine hemagglutinating encephalomyelitis virus, bovine coronavirus, and human coronavirus OC43. J Virol 80:7270–7274. doi: 10.1128/JVI.02675-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alekseev KP, Vlasova AN, Jung K, Hasoksuz M, Zhang X, Halpin R, Wang S, Ghedin E, Spiro D, Saif LJ. 2008. Bovine-like coronaviruses isolated from four species of captive wild ruminants are homologous to bovine coronaviruses, based on complete genomic sequences. J Virol 82:12422–12431. doi: 10.1128/JVI.01586-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasoksuz M, Alekseev K, Vlasova A, Zhang X, Spiro D, Halpin R, Wang S, Ghedin E, Saif LJ. 2007. Biologic, antigenic, and full-length genomic characterization of a bovine-like coronavirus isolated from a giraffe. J Virol 81:4981–4990. doi: 10.1128/JVI.02361-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo PC, Lau SK, Wernery U, Wong EY, Tsang AK, Johnson B, Yip CC, Lau CC, Sivakumar S, Cai JP, Fan RY, Chan KH, Mareena R, Yuen KY. 2014. Novel betacoronavirus in dromedaries of the Middle East, 2013. Emerg Infect Dis 20:560–572. doi: 10.3201/eid2004.131769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guy JS, Breslin JJ, Breuhaus B, Vivrette S, Smith LG. 2000. Characterization of a coronavirus isolated from a diarrheic foal. J Clin Microbiol 38:4523–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erles K, Toomey C, Brooks HW, Brownlie J. 2003. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsunemitsu H, el-Kanawati ZR, Smith DR, Reed HH, Saif LJ. 1995. Isolation of coronaviruses antigenically indistinguishable from bovine coronavirus from wild ruminants with diarrhea. J Clin Microbiol 33:3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mengeling WL, Boothe AD, Ritchie AE. 1972. Characteristics of a coronavirus (strain 67N) of pigs. Am J Vet Res 33:297–308. [PubMed] [Google Scholar]
- 47.Jin L, Cebra CK, Baker RJ, Mattson DE, Cohen SA, Alvarado DE, Rohrmann GF. 2007. Analysis of the genome sequence of an alpaca coronavirus. Virology 365:198–203. doi: 10.1016/j.virol.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li IW, Chan KH, To KW, Wong SS, Ho PL, Lau SK, Woo PC, Tsoi HW, Chan JF, Cheng VC, Zheng BJ, Chen H, Yuen KY. 2009. Differential susceptibility of different cell lines to swine-origin influenza A H1N1, seasonal human influenza A H1N1, and avian influenza A H5N1 viruses. J Clin Virol 46:325–330. doi: 10.1016/j.jcv.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Lau SK, Woo PC, Yip CC, Fan RY, Huang Y, Wang M, Guo R, Lam CS, Tsang AK, Lai KK, Chan KH, Che XY, Zheng BJ, Yuen KY. 2012. Isolation and characterization of a novel Betacoronavirus subgroup A coronavirus, rabbit coronavirus HKU14, from domestic rabbits. J Virol 86:5481–5496. doi: 10.1128/JVI.06927-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau SK, Chan KH, Yip CCY, Ng TK, Tsang OTY, Woo PCY, Yuen KY. 2009. Confirmation of the first Hong Kong case of human infection by novel swine-origin influenza A (H1N1) virus diagnosed using ultrarapid, real-time reverse transcriptase PCR. J Clin Microbiol 47:2344–2346. doi: 10.1128/JCM.00924-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang Y, Lau SK, Woo PC, Yuen KY. 2008. CoVDB: a comprehensive database for comparative analysis of coronavirus genes and genomes. Nucleic Acids Res 36:D504–D511. doi: 10.1093/nar/gkm754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apweiler R, Attwood TK, Bairoch A, Bateman A, Birney E, Biswas M, Bucher P, Cerutti L, Corpet F, Croning MD, Durbin R, Falquet L, Fleischmann W, Gouzy J, Hermjakob H, Hulo N, Jonassen I, Kahn D, Kanapin A, Karavidopoulou Y, Lopez R, Marx B, Mulder NJ, Oinn TM, Pagni M, Servant F, Sigrist CJ, Zdobnov EM. 2001. The InterPro database, an integrated documentation resource for protein families, domains and functional sites. Nucleic Acids Res 29:37–40. doi: 10.1093/nar/29.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer EL. 2002. The Pfam protein families database. Nucleic Acids Res 30:276–280. doi: 10.1093/nar/30.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonnhammer EL, von Heijne G, Krogh A. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol 6:175–182. [PubMed] [Google Scholar]
- 55.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suchard MA, Weiss RE, Sinsheimer JS. 2001. Bayesian selection of continuous-time Markov chain evolutionary models. Mol Biol Evol 18:1001–1013. doi: 10.1093/oxfordjournals.molbev.a003872. [DOI] [PubMed] [Google Scholar]
- 57.Woo PC, Lau SK, Wong BHL, Chan KH, Hui WT, Kwan GSW, Peiris JSM, Couch RB, Yuen KY. 2004. False positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid enzyme-linked immunosorbent assay due to HCoV-OC43 and HCoV-229E rectified by Western blotting with recombinant SARS-CoV spike polyprotein. J Clin Microbiol 42:5885–5888. doi: 10.1128/JCM.42.12.5885-5888.2004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Woo PC, Lau SK, Tsoi HW, Chan KH, Wong BH, Che XY, Tam VK, Tam SC, Cheng VC, Hung IF, Wong SS, Zheng BJ, Guan Y, Yuen KY. 2004. Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia. Lancet 363:841–845. doi: 10.1016/S0140-6736(04)15729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeong YS, Repass JF, Kim YN, Hwang SM, Makino S. 1996. Coronavirus transcription mediated by sequences flanking the transcription consensus sequence. Virology 217:311–322. doi: 10.1006/viro.1996.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu HY, Ozdarendeli A, Brian DA. 2006. Bovine coronavirus 5′-proximal genomic acceptor hotspot for discontinuous transcription is 65 nucleotides wide. J Virol 80:2183–2193. doi: 10.1128/JVI.80.5.2183-2193.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Guy JS, Snijder EJ, Denniston DA, Timoney PJ, Balasuriya UB. 2007. Genomic characterization of equine coronavirus. Virology 369:92–104. doi: 10.1016/j.virol.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwarz B, Routledge E, Siddell SG. 1990. Murine coronavirus nonstructural protein NS2 is not essential for virus replication in transformed cells. J Virol 64:4784–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Haan CA, Masters PS, Shen X, Weiss S, Rottier PJ. 2002. The group-specific murine coronavirus genes are not essential, but their deletion, by reverse genetics, is attenuating in the natural host. Virology 296:177–189. doi: 10.1006/viro.2002.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snijder EJ, Bredenbeek PJ, Dobbe JC, Thiel V, Ziebuhr J, Poon LL, Guan Y, Rozanov M, Spaan WJ, Gorbalenya AE. 2003. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol 331:991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schultze B, Herrler G. 1992. Bovine coronavirus uses N-acetyl-9-O-acetylneuraminic acid as a receptor determinant to initiate the infection of cultured cells. J Gen Virol 73:901–906. doi: 10.1099/0022-1317-73-4-901. [DOI] [PubMed] [Google Scholar]
- 66.Vlasak R, Luytjes W, Spaan W, Palese P. 1988. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc Natl Acad Sci U S A 85:4526–4529. doi: 10.1073/pnas.85.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams RK, Jiang GS, Holmes KV. 1991. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A 88:5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Langereis MA, van Vliet AL, Boot W, de Groot RJ. 2010. Attachment of mouse hepatitis virus to O-acetylated sialic acid is mediated by hemagglutinin-esterase and not by the spike protein. J Virol 84:8970–8974. doi: 10.1128/JVI.00566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thiel V, Siddell SG. 1994. Internal ribosome entry in the coding region of murine hepatitis virus mRNA 5. J Gen Virol 75:3041–3046. doi: 10.1099/0022-1317-75-11-3041. [DOI] [PubMed] [Google Scholar]
- 70.Goebel SJ, Hsue B, Dombrowski TF, Masters PS. 2004. Characterization of the RNA components of a putative molecular switch in the 3′ untranslated region of the murine coronavirus genome. J Virol 78:669–682. doi: 10.1128/JVI.78.2.669-682.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Netland J, Ferraro D, Pewe L, Olivares H, Gallagher T, Perlman S. 2007. Enhancement of murine coronavirus replication by severe acute respiratory syndrome coronavirus protein 6 requires the N-terminal hydrophobic region but not C-terminal sorting motifs. J Virol 81:11520–11525. doi: 10.1128/JVI.01308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brockway SM, Lu XT, Peters TR, Dermody TS, Denison MR. 2004. Intracellular localization and protein interactions of the gene 1 protein p28 during mouse hepatitis virus replication. J Virol 78:11551–11562. doi: 10.1128/JVI.78.21.11551-11562.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeng Q, Langereis MA, van Vliet AL, Huizinga EG, de Groot RJ. 2008. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc Natl Acad Sci U S A 105:9065–9069. doi: 10.1073/pnas.0800502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yokomori K, Banner LR, Lai MM. 1991. Heterogeneity of gene expression of the hemagglutinin-esterase (HE) protein of murine coronaviruses. Virology 183:647–657. doi: 10.1016/0042-6822(91)90994-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huynh J, Li S, Yount B, Smith A, Sturges L, Olsen JC, Nagel J, Johnson JB, Agnihothram S, Gates JE, Frieman MB, Baric RS, Donaldson EF. 2012. Evidence supporting a zoonotic origin of human coronavirus strain NL63. J Virol 86:12816–12825. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pfefferle S, Oppong S, Drexler JF, Gloza-Rausch F, Ipsen A, Seebens A, Müller MA, Annan A, Vallo P, Adu-Sarkodie Y, Kruppa TF, Drosten C. 2009. Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerg Infect Dis 15:1377–1384. doi: 10.3201/eid1509.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL. 2013. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, Godeke GJ, Jonges M, Farag E, Diab A, Ghobashy H, Alhajri F, Al-Thani M, Al-Marri SA, Al Romaihi HE, Al Khal A, Bermingham A, Osterhaus AD, AlHajri MM, Koopmans MP. 2014. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis 14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reusken CB, Haagmans BL, Müller MA, Gutierrez C, Godeke GJ, Meyer B, Muth D, Raj VS, Smits-De Vries L, Corman VM, Drexler JF, Smits SL, El Tahir YE, De Sousa R, van Beek J, Nowotny N, van Maanen K, Hidalgo-Hermoso E, Bosch BJ, Rottier P, Osterhaus A, Gortázar-Schmidt C, Drosten C, Koopmans MP. 2013. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis 13:859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, Madani TA. 2014. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 81.Corman VM, Ithete NL, Richards LR, Schoeman MC, Preiser W, Drosten C, Drexler JF. 2014. Rooting the phylogenetic tree of Middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J Virol 88:11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bailey OT, Pappenheimer AM, Cheerer FS, Daniels JB. 1949. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. II. Pathology. J Exp Med 90:195–212. doi: 10.1084/jem.90.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheever FS, Daniels JB, Pappenheimer AM, Bailey OT. 1949. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. J Exp Med 90:181–194. doi: 10.1084/jem.90.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun N, Perlman S. 1995. Spread of a neurotropic coronavirus to spinal cord white matter via neurons and astrocytes. J Virol 69:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miura TA, Wang J. 2007. Rat coronaviruses infect rat alveolar type I epithelial cells and induce expression of CXC chemokines. Virology 369:288–298. doi: 10.1016/j.virol.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhatt PN, Percy DH, Jonas AM. 1972. Characterization of the virus of sialodacryoadenitis of rats: a member of the coronavirus group. J Infect Dis 126:123–130. doi: 10.1093/infdis/126.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peng G, Xu L, Lin YL, Chen L, Pasquarella JR, Holmes KV, Li F. 2012. Crystal structure of bovine coronavirus spike protein lectin domain. J Biol Chem 287:41931–41938. doi: 10.1074/jbc.M112.418210. [DOI] [PMC free article] [PubMed] [Google Scholar]