Abstract
Ocular inflammatory disorders disproportionately affect women, and the majority of affected women are of childbearing age. The role of sex or reproductive hormones has been proposed in many other inflammatory or autoimmune disorders, and findings from non-ocular autoimmune diseases suggest a complex interaction between sex hormones, genetic factors and the immune system. However, despite the age and sex bias, factors that influence this disparity are complicated and unclear. This review aims to evaluate the gender disparities in prevalence, incidence and severity of the most common infectious and non-infectious ocular inflammatory disorders.
Keywords: Disparity, gender, ocular inflammation, sex, uveitis
Introduction
Epidemiologic studies have confirmed clinical impressions that the prevalence of uveitis is, in general, higher in women than in men.1 The largest population-based study on uveitis also indicated that this difference increases with increasing age.1 Many ocular inflammatory diseases, whether limited to the eye (e.g. birdshot chorioretinitis) or associated with a systemic autoimmune disorder [e.g. systemic lupus erythematosus (SLE)], seem to affect females disproportionately more often. Recent findings indicate an interaction between sex hormones, the immune system, and genetic and epigenetic modifications in the pathogenesis and severity of these autoimmune diseases.2–5 Sex hormones are well known to influence immune function. Estrogen and androgen receptors are found on lymphocytes and synovial macrophages and can stimulate a Th2 immune response while attenuating Th1, B-cell and Th17 responses.3–5 Progesterone also suppresses Th1 and Th17 but induces regulatory T-cells.3–5 This may explain the observed quiescence of many autoimmune diseases during mid and late pregnancy.6,7 Prolactin, on the other hand, appears to promote pro-inflammatory behavior by inducing CD4+T-cells, decreasing apoptosis of B-cells and increasing immunoglobulin production.3,4 Additionally, the presence of two X chromosomes may play a role in the increased risk of developing various autoimmune disorders. The X chromosome encodes several genes which play a role in innate and adaptive immune responses in SLE and other autoimmune diseases. Failure of or skewed X inactivation during thymic development can lead to overexpression of risk genes, resulting in excessive X-coded proteins.2 Another possible explanation for sex disparity in the prevalence and incidence of autoimmune diseases is epigenetic modification. The direct implications of each of these factors have yet to be determined. This review will focus on ocular autoimmune diseases and sex disparities.
Non-Infectous Uveitides
Collagen Vascular Diseases and Rheumatoid Arthritis
Connective tissue disorders are, in general, more prevalent in women. SLE is a systemic inflammatory disorder of unknown etiology that affects the skin, kidneys, joints, lungs, CNS and the eye. Women, especially in the second and third decade of life, are affected more frequently than men. The most common ocular involvement is keratoconjunctivitis sicca (KCS); however, retinal vasculitis, choroidal vasculitis and scleritis can also occur.8,9 In a large cohort from Nepal, ocular involvement was present in almost half of the patients (47.3%), and the overwhelming majority (94.5%) of patients with ocular complications of SLE were females, with a female-to-male ratio of 17:1. The average age for females and males was similar (26.72 ± 10.29 years and 24.40 ± 5.40 years, respectively). The most common ocular manifestation was KCS (39.5%) followed by lupus retinopathy (21%).10 The typically cited female-to-male ratio of 9:1 in SLE is true for childbearing age. Prior to puberty, the ratio is much less impressive, which suggests the possible role of female reproductive or sex hormones.11 Women may also have different environmental exposures that may be culturally or occupationally determined. Gender-specific exposures such as hair-dye and cosmetics have been suggested, but no link has been shown to date.2 In a large prospective study of female nurses in USA [Nurses' Health Study (NHS)], early age at menarche or menopause, oral contraceptive use and post-menopausal hormone therapy were found to be associated with a higher risk of developing SLE, although the increased risk was modest.12 Breastfeeding was not associated with any significant risk.12 Other studies also suggest that exogenous estrogen may indeed be associated with a higher risk of developing SLE in a dose-dependent manner and may also be associated with increased disease severity.13,14 However, attempts to modify disease course with the use of androgen therapy has had little to no impact in multiple clinical trials.15,16 Genetic and epigenetic factors have also been proposed as an explanation for the higher incidence and prevalence of SLE in women. Active SLE patients have been shown to have demethylation and increased expression of CD40 ligand.17 Whether men have a more severe disease course is controversial, as several studies indicated contradictory results.2 The effect of pregnancy is also unclear, with studies indicating discordant results.18,19
Granulomatosis with polyangiitis (GPA), formerly known as Wegener's Granulomatosis, is a rare vasculitis of unknown etiology, with a mean age of onset at ∼50.20 It frequently affects upper and lower respiratory tracts, kidneys and the eye. Ophthalmic involvement occurs in 50% of cases and can lead to significant morbidity, with 8% of patients suffering vision loss. The most common manifestation is orbital disease, followed by episcleral and scleral inflammation. Uveitis is usually the least common ocular manifestation, occurring in <3% of all ophthalmic GPA patients.21 There appears to be no sex predilection in GPA or its ocular involvement; however, the disease severity and mortality may be higher among women.20
Sex disparity in rheumatoid arthritis (RA) is smaller than SLE, with a female-to-male ratio of 2–3:1.2 It has a peak incidence at age 45–55 years, which coincides with menopause, suggesting a relation with estrogen loss.2 A large prospective study of female nurses found no association between RA risk and menarche, parity or exogenous estrogen.22 However, other studies suggested a protective role of pregnancy or parity. Interestingly, after age 45 years the incidence among men approaches that of women, which suggests that decreased levels of androgen may also play a role. Androgen levels were also found to be inversely related to severity of the disease, which can explain the milder disease course in men with RA.24 Several post-menopausal hormone therapy trials found no significant effect on disease activity in women, while androgen-therapy showed modest effect as adjuvant treatment in men.24,25 Uveitis associated with RA is very rare, and data on sex and ocular complications of rheumatoid arthritis are lacking.
Juvenile Idiopathic Arthritis
Juvenile idiopathic arthritis (JIA) is the most common systemic disease associated with childhood uveitis. JIA diagnosis requires arthritis in at least one joint that persists for a minimum of 6 weeks in a child younger than 16 years of age. It has seven subtypes based on International League of Associations for Rheumatology (ILAR) classification: systemic JIA, oligoarticular JIA, rheumatoid factor (RF) negative polyarticular JIA, RF positive polyarticular JIA, psoriatic arthritis, enthesitis-related arthritis and other arthritis.26 Factors relevant to JIA-associated uveitis such as age at onset, ANA status or human leukocyte antigen (HLA)-B27 are not part of this classification system. The oligoarticular subtype constitutes approximately half of all JIA cases.
Juvenile idiopathic arthritis-associated uveitis (JIAU) is typically a non-granulomatous bilateral anterior uveitis. Oligoarticular subtype in ANA+ girls with younger age of onset have the highest risk of developing uveitis.27 Sex appears to play a significant role in the incidence and the severity of JIA. Overall, there are more girls with JIA than boys, with a ratio of 3:1–2:1, but the sex risk varies with JIA subtype. For example, female-to-male ratio in oligoarticular JIA is 2:1–3:1, in polyarticular JIA it is 3:1-4:1, and both sexes are almost equally affected in systemic JIA with a ratio of 1:1.27 World-wide cumulative incidence of JIA-associated uveitis among patients with JIA was 8.3 in 100 persons in one large meta-analysis.28 There is a significant geographic variability in the incidence and prevalence of JIA-associated uveitis, with the highest prevalence and incidence seen in Scandinavia, Germany, and USA and the lowest in East Asia and South Asia.28 A Canadian study which evaluated the incidence of JIA among different ethnicities indicated that, while ethnicity may be an important factor for incident JIA, with Northern European ethnicity conferring a higher risk, it was not a significant factor for incident JIA-associated uveitis. For the development of JIA-associated uveitis, age at onset and ANA were more important factors.29
It is not surprising that the cumulative incidence of JIAU is higher among girls, given that girls are more frequently affected in the most common subtype of JIA, oligoarticular JIA, which is also the subtype most commonly associated with uveitis. However, sex does not appear to be an independent risk factor once JIA subtype and age of onset are taken into account.28,29 This is supported by a nationwide registry study in Germany where sex was not found as an independent risk factor.30 Nevertheless, in children younger than 2 years of age, girls still appear to be at more risk for developing JIAU than boys.31 Whether sex plays a role in severity of JIAU has been the subject of debate. Male sex as a risk factor for poor visual outcome, as proposed by some studies, is controversial. While some studies supported sex as a risk factor for prognosis,32 others failed to show any association between sex and disease severity or outcomes.31
Sarcoidosis
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology characterized histopathologically by the presence of non-caseating granulomas. It manifests most commonly as bilateral hilar lymph-adenopathy but can also cause skin, joint or ocular inflammation. Prevalence is estimated at 10–20 per 100,000 33 in USA, and it affects both sexes, although with a slight female predominance. There are geographical and racial variations in prevalence. Familial clustering has been reported, suggesting a genetic basis for the disease. The majority of cases present between the age of 10–40 years; however, another peak incidence at age 50–60 years has also been noted, particularly in women.34 The disease is most common in Scandinavians and in African–Americans in USA.33 Lifetime incidence in USA is higher in women (1.3%) than in men (1.0%); incidence is also higher in African Americans (2.4%) compared to Caucasians (0.85%).33 African–Americans also appear to have a more severe and disseminated disease than Caucasians. Erythema nodosum, seen in 34% of cases, is more frequent in women and Caucasians.34 In a large population based registry study in Denmark, Jorgensen et al. found that a number of autoimmune diseases, including sarcoidosis, were associated with parity. Parous women were more likely to have Hashimoto's thyroiditis, Graves' disease, erythema nodosum, psoriasis and sarcoidosis. This was particularly notable in the first year after childbirth.35
Ocular involvement occurs in 20–60% of patients with systemic sarcoidosis, accounts for ∼10% of all uveitis cases, and can be the presenting symptom in ∼30% of patients with sarcoidosis.36 Uveitis and conjunctival nodules are the most common form of ocular involvement. Posterior uveitis occurs in ∼20%; however, posterior uveitis is the most common anatomic location in whites, especially in elderly female patients (Figure 1).36,37 Ocular sarcoidosis can have significant visual consequences, with ∼10% of patients experiencing blindness in at least one eye.36
Figure 1.

Biopsy proven sarcoidosis-associated panuveitis diagnosed at age 52 years in an African-American female. Fundus photograph of the right eye shows retinal vasculitis, retinal vascular sheathing, old segmental exudates inferiorly and punched-out chorioretinal scars. Her vision (20/25 OU) is well preserved due to aggressive systemic immunomodulatory therapy.
The incidence of sarcoidosis is not strongly associated with sex, although poor visual prognosis has been found to be associated with female sex, as have advanced age, black race, systemic disease and posterior segment involvement.36 The large US multicenter study A Case Control Etiologic Study of Sarcoidosis (ACCESS) indicated some sexual disparities in sarcoidosis. Women were more likely to be affected by sarcoidosis and to have ocular and neurologic involvement, whereas men had a higher risk of hypercalcemia. Another study from Sweden also highlighted a slight female preponderance.34 Among ocular sarcoidosis patients, female predominance appears to be higher with a female-to-male ratio of 3:1; however, there are no sex differences in age at presentation.37,39
HLA-B27-Associated Uveitis
Human leukocyte antigen (HLA)-B27-associated anterior uveitis, the most common etiology of acute anterior uveitis, demonstrates clear sex preponderance; males are 2.5 times more likely to be affected than females.40 Although HLA-B27 is present in 5–7% of the general Caucasian population, ∼50% of acute anterior uveitis patients are HLA-B27 positive. The chance of an individual that carries HLA-B27 developing a spondyloarthropathy or eye disease is 1 in 4.40 HLA-B27-associated anterior uveitis is far less common in non-white populations. HLA-B27-associated diseases are characterized by enthesitis and negative rheumatoid factor (RF) and include ankylosing spondylitis (AS), reactive arthritis syndrome (formerly known as Reiter's syndrome), inflammatory bowel disease (ulcerative colitis and Crohn's disease) and psoriatic arthritis.40
Ankylosing spondylitis, the most common of the seronegative spondyloarthropathies, is characterized by back pain and progressive stiffness of the spine. The prevalence of AS varies among different ethnic groups (7.5% among non-Hispanic Whites versus 1.1% among African Americans). Even though early literature suggested overwhelming male predominance, today the male-to-female ratio is reported to be ∼2:1–3:1. It is believed that there is no significant difference in clinical or radiological manifestations of the disease between men and women; however, women may be more likely to have involvement of the cervical spine and peripheral joints.41 Uveitis, the most common extra-articular involvement, occurs in 25–40% of AS patients and is typically a non-granulomatous alternating anterior uveitis (one eye at a time). Conversely, AS can be responsible for ∼40% of all HLA-B27-associated anterior uveitis. There is no correlation between the joint disease activity and the ocular disease.42
In a large cohort of anterior uveitis, HLA-B27-associated anterior uveitis was more likely than HLA-B27-negative anterior uveitis to be associated with male sex and frequent recurrent episodes.43 In a Chinese cohort, in addition to male preponderance, a younger age of onset was observed among male patients with HLA-B27-associated uveitis compared to females. Interestingly, male sex was also associated with increased risk of vision loss in a recent cohort in USA,45 whereas another study from the Netherlands that focused on differences in outcomes based on gender indicated no gender differences in vision loss among HLA-B27-associated anterior uveitis patients.46 Both studies, as many others, confirmed a male preponderance.45,46
A recent study indicated that relative female sparing among HLA-B27 patients may be attributed to estrogens, which induce the constitutive synthesis of nitric oxide, the reduction in the expression of adhesion molecules (E-selectin) and the modulation of genes that promote proinflammatory cytokines, such as IL-1, IL-6 and TNF-α.47
Reactive arthritis, formerly known as Reiter's syndrome, is a mono- or oligoarticular arthritis that occurs days to weeks following an infection with organisms such as Chlamydia, Shigella, Yersinia, Salmonella, Campylobacter, Escherichia coli or Clostridium difficile.48 The classic triad consists of arthritis, urethritis and conjunctivitis. Ocular involvement occurs in ∼20% of patients. Conjunctivitis is seen more frequently than uveitis. Reactive arthritis predominantly affects men, and the disease may be less severe in affected women.49
Inflammatory bowel disease (IBD) and psoriatic arthritis (PsA) are rarer causes of uveitis. PsA affects women and men equally.50 IBD affects women slightly more, with a female-to-male ratio of 1.59 for Crohn's disease (CD) and 1.18 for ulcerative colitis (UC). This sex association suggests that hormonal factors may play a role in disease expression.51,52 Acute anterior uveitis is the most common form of ocular involvement for both UC (12.0%) and CD (22.4%). IBD patients who have uveitis and sacroiliitis tend to be HLA-B27 positive. PsA is also associated with anterior uveitis, although these patients tend to be older than average HLA-B27 positive uveitis patients. Twenty percent of both IBD and PsA patients have sacroiliitis.53 Similar to sarcoidosis, eryhthema nodosum in IBD patients is also associated with female sex as well as eye and joint involvement.54
Behçet Disease
Behçet disease (BD) is a rare immune-mediated small-vessel systemic vasculitis that manifests with oral and genital ulcerations, skin lesions and uveitis. Vascular, central nervous system and gastrointestinal involvement can also occur. The disease is more prevalent in certain geographic regions and among particular ethnic groups. The disease onset is characteristically in the third decade.55 BD is rare in USA but is more common in the Middle East, Mediterranean countries and Asia, particularly in regions along the “Silk Road”.56 The HLA-B51 allele is the most strongly associated genetic risk factor for BD. While genetic factors seem to play a role in the development of BD, there is a general consensus that unidentified environmental stimuli are probably necessary and that the disease is possibly triggered by an aberrant response to exogenous stimuli including both bacterial and viral infections.57
According to the International Study Group for BD guidelines, for a patient to be diagnosed with BD, the patient must have recurrent oral ulcers at least three times within a 12-month period along with two of the following four findings: recurrent genital ulcers, eye inflammation (anterior and/or posterior uveitis, cells in the vitreous and retinal vasculitis), skin lesions (including erythema nodosum, pseudofolliculitis and papulopustular lesions), and a positive pathergy test. The pathergy test has a specificity of 95–100%, but the results are often negative in American and European patients.58
Gender predilection in BD has been long debated. It has been proposed by several studies that the disease affects males more frequently and, possibly, more severely. Globally, males are affected more frequently than females though male-to-female ratios range from 0.36:1 to 4.9:1. According to the international collaborative study reports, the male-to-female ratio is 2.15:1.59–61 Whether the age of onset or severity of disease is different in men is also controversial. In an epidemiologic study from Iran, mean age of onset for males (31.2 years) did not differ significantly from that of females (33.1 years), and no significant sex predominance was found in prevalence or severity.62 Similarly, recent large population based studies from China and USA also dispute male predominance in Behçet's disease.63 A US population-based study showed a higher incidence and prevalence among women.61,64 Another study from the American Midwest showed a slight male preponderance among their American BD patients and a more prominent male predominance among those originally from the “Silk Road” region, although the difference was not significant.65
Oral ulcers, genital ulcers and skin lesions are usually more frequent and more severe among females, while males more commonly develop ocular lesions, vascular involvement and neurologic disease.63,66 It is possible that females with genital ulcers are less likely to seek medical care due to cultural implications, which may contribute to reported lower prevalence of BD among females in some countries. Erythema nodosum-like lesions are also more frequently observed in females.66 On the other hand, the disease may be significantly more severe, even fatal, in males. Numerous studies propose that the mortality rate in BD is considerably higher among young males in their 20s–40s.67
Ocular involvement occurs in 50–80% of BD patients. Male patients are more likely to develop ocular disease at a younger age and present with more severe disease. According to some studies, male sex is associated with more frequent posterior segment involvement and poorer visual outcomes (Figure 2).67–69 In an epidemiologic study, 56% of patients with BD developed ocular involvement at a mean age of 30 years, and ocular involvement was the first manifestation of BD in 8.6% of patients.70 Childhood Behçet's uveitis is also more common in males.71 Female sex has been associated with milder and non-recurrent forms of ocular involvement.72 Although there is no clear consensus on whether there is gender predilection in BD, there is overall agreement that isolated anterior uveitis, which has more favorable prognosis, is more commonly seen in women,73 and panuveitis, and hence poorer visual prognosis, is more frequent in men.71
Figure 2.
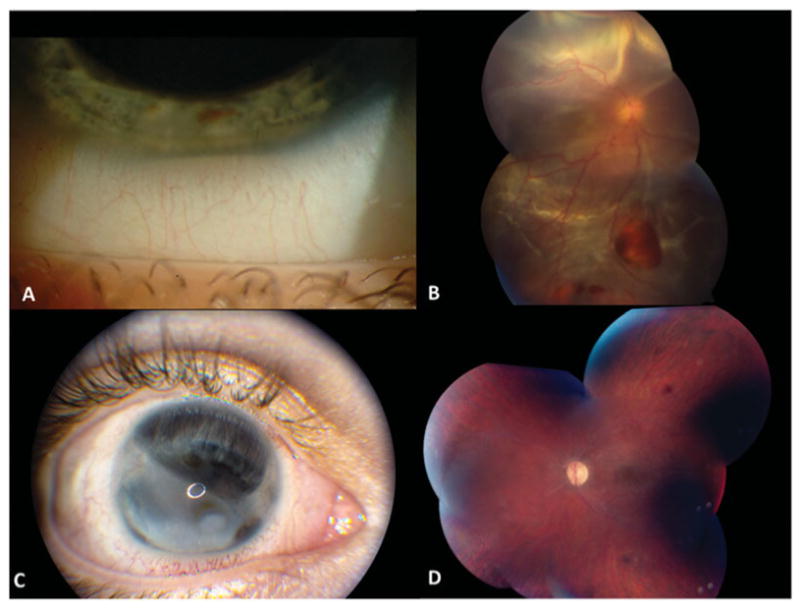
Italian male with Behcet's Disease (BD) and panuveitis diagnosed at age 15 years. The right eye which showed hypopyon uveitis (A) early in the disease course developed chronic retinal detachment (B) and eventually resulted in blindness with anterior and posterior segment complications (C) despite multiple attempts to treat medically and surgically. His left eye remains quiescent on long-term immunomodulatory therapy with good visual acuity (20/32) despite diffuse attenuation of retinal vasculature and thinning/atrophy of the retina (D).
Multiple Sclerosis
Multiple sclerosis (MS) is the most common inflammatory demyelinating disorder of the central nervous system and primarily affects young women of Northern European descent. Some studies suggested an association between latitude and MS prevalence74 while others revealed no such association.75
The disease is believed to develop from a complex interaction of genetic and environmental factors.76 MS is frequently diagnosed based on the presenting signs and symptoms, as well as MRI, evoked potentials and spinal fluid analyses. Well-defined risk factors for MS include Northern European descent, cigarette smoking, vitamin D insufficiency, Epstein–Barr Virus (EBV) infection,77,78 and having MHC class II HLA-DR alleles and interleukin (IL)-7 receptor gene polymorphisms.79,80
Parallel to many autoimmune disorders, the disease is more common in women of childbearing age. Globally, it is about twice as common in women than in men. Regionally, the median estimated female-to-male ratio ranges between 1.5 and 2.5, with the lowest incidence rates in the Western Pacific and Africa and the highest rate in Europe.81 MS commonly presents in younger adults in the second and third decades of life with peak age of onset occurring ∼30 years; however, the onset may rarely occur in childhood or after 50 years of age.82 Though there is no consensus, some reports suggest that MS onset in childhood is more common in females.82,83 In addition, MS presenting in adults over the age of 50 years more frequently affects males,82 suggesting a possible role of reproductive or sex hormones. Interestingly, a review of 28 epidemiologic studies found that female-to-male ratio of MS incidence increased from 1.4 to 2.3 between 1995 and 2000.84 A case–control study from Greece found a similar increase in the incidence of MS in females since 1980 that was concurrent with a population shift from rural to urban areas. Though the reason for increased incidence among women is not clear, urbanization was speculated as one of the reasons.85
Female preponderance in MS, similar to other autoimmune diseases, can be due to gonadal hormones, genetic differences or environmental exposures. The high prevalence of MS in females and the higher susceptibility during childbearing ages suggest a possible role of female hormones in the inflammatory and neurodegenerative process that characterizes MS. Earlier age at menarche has been associated with higher risk for developing MS, but parity has not been found to be associated in most studies. The effect of sex on clinical features of MS is not as clear as its effect on prevalence. Although female sex carries a higher risk of developing MS, it seems to provide a protective effect against developing progressive onset MS and cognitive decline.88,89 There have been studies indicating worse prognosis with progression to disability in men compared to women,88 although these findings have not been universal.89 In a study investigating risk factors for disability progression in a large MS population (2837 patients; 22,723 patient years), men progressed 38% more quickly than women, yet both required canes at similar ages.89
Pregnancy seems to offer reduced relapse rates and decreased neurological symptoms despite increased relapse rates in the postpartum period. Fertility treatments utilizing gonadotropin releasing hormone (GnRH) agonists may also increase the risk of relapse in MS patients, especially when it does not result in pregnancy90 Lastly, the presence of two X chromosomes was shown to increase susceptibility to MS in animal models independent of hormone levels.91
Multiple sclerosis-associated uveitis occurs in ∼1–10% of patients, precedes the diagnosis of MS in ∼25% of patients, and is typically a bilateral intermediate uveitis which can have granulomatous features in 56% of patients.92,93 Expectedly, the majority of MS-associated uveitis patients, ∼75%, are white females,93–95 but uveitis onset appears to occur at similar ages for both sexes. Women with intermediate uveitis of pars planitis type tend to be more likely than men to have MS, though this difference is not significant.95 There is also no correlation between the duration of uveitis and progression or severity of MS.94,96 It is important to note that anti-TNF therapy used for the treatment of uveitis can also be associated with development or exacerbation of MS,97 so MRI imaging should be obtained prior to starting anti-TNF therapy in patients with intermediate uveitis. In summary, gender predilection is quite clear in terms of incidence and prevalence of MS and MS-associated uveitis; however, it does not demonstrate as clear of an association in terms of disease course and prognosis.
Vogt–Koyanagi–Harada Syndrome
Vogt–Koyanagi–Harada syndrome (VKH) is a granulomatous inflammatory disorder characterized by bilateral, granulomatous panuveitis associated with dermatologic, neurologic and auditory involvement. VKH mainly affects pigmented individuals such as Asians, Hispanics, Native Americans and Middle Easterners.98
The pathogenic mechanism of the disease is believed to be T-lymphocyte-mediated attack on melanocytes in the skin, uvea, central nervous system and the inner ear.98 VKH syndrome has been associated with HLA genotypes; the strongest association has been reported for HLA-DRB1*04:05 haplotype.99 A sex stratification analysis among Mexican Mestizo healthy individuals and VKH patients showed that DRB1*04:05 and DRB1*04:04 were significantly increased in VKH patients and that these alleles were associated with female sex only.100 No significant sex difference in VKH prevalence was reported in Asian populations.101,102
VKH syndrome has four stages: prodromal, acute uveitic, convalescent and chronic recurrent. The prodromal stage is characterized by flulike symptoms, headache, nausea, meningismus, dysacusia and tinnitus. Most reports indicate that VKH syndrome tends to affect women more than men and may occur at all ages, including childhood.103
The acute uveitic stage is characterized by bilateral simultaneous or near simultaneous sudden onset decreased vision with bilateral granulomatous anterior uveitis. At this stage, variable degrees of vitritis, thickening of the posterior choroid with elevation of the peripapillary retinal choroidal layer, hyperemia and edema of the optic nerve, and multiple serous retinal detachments may be seen on ocular examination. Clinical manifestations during the acute phase of the disease are similar in all of the studied populations with some variations in ocular appearances during the convalescent stage. The convalescent stage is characterized by gradual depigmentation of the choroid, resulting in the classic orange-red discoloration, also known as “sunset glow” fundus appearance (Figure 3). Vitiligo, alopecia and poliosis are typically seen during this stage. The chronic recurrent stage is characterized by repeated flares of uveitis.
Figure 3.

A 40-year-old African-American female with Native American heritage with Vogt–Koyanagi–Harada Syndrome-associated panuveitis. Both eyes show retinal pigment epithelial changes and chorioretinal scars which resulted in “sunset glow fundus” appearance, particularly in the left eye.
There is no significant difference in gender distribution between different ethnic groups, although mean age at presentation seems to vary with Asians presenting at a significantly older age than all other groups. A poor visual outcome is associated with both a longer median duration of disease and greater number of recurrent episodes of inflammation. Younger patients were more likely to have better visual outcomes, but sex, race or ethnicity do not seem to have significant impact. Uveitis occurs more commonly in females, in general, though sex does not appear to have a significant impact on prognosis or visual outcomes.105 Higher VKH prevalence among females may be associated with genetic factors more than hormonal factors, although this has yet to be studied.
Sympathetic Ophthalmia
Sympathetic ophthalmia (SO) is a rare diffuse bilateral granulomatous uveitis following uveal trauma to one eye. It may develop from days to several years after a penetrating eye injury with ∼90% developing within 1 year following the injury. The eye sustaining the injury is the inciting eye and the fellow eye, developing inflammation days to years later, is called the sympathizing eye.106 SO has also been described following multiple ocular surgeries, most commonly vitreoretinal surgery.107 The etiology of SO is believed to be an autoimmune response against ocular antigens, specifically a delayed hypersensitivity to melanin-containing structures of the outer segments of the photoreceptor layer of the retina. There may be a genetic predisposition to developing SO following accidental or surgical ocular trauma. SO patients frequently express HLA-DR4 as well as HLA-DQw3 and HLA-DRw53.108
Sympathetic ophthalmia presents as a bilateral panuveitis with granulomatous features such as mutton-fat keratic precipitates, moderate to severe vitritis, retinal vasculitis, choroiditis, papillitis and circumpapillary choroidal lesions. White-yellowish choroidal lesions (clinical Dalen–Fuchs nodules) are common in the peripheral retina.109,110 In British and Irish populations, HLA-DRB1*04 and HLA-DQA1*03 haplotypes have been found to be associated with higher likelihood of developing SO and a more severe disease course.107
The likelihood of developing SO following surgery is similar between males and females, while trauma-induced SO is more common in males.111 There is no racial or age predisposition. There is male predominance and a biphasic age peak with the first peak in childhood and the second in the aging population. The former is likely secondary to accidental trauma and the latter due to ocular surgery.111–113 The reported male preponderance in SO prevalence is likely related to the risk for trauma itself. Interestingly, in a large cohort of SO patients, female sex was the only factor significantly associated with incident vision loss.114 There are no reports indicating that men are more likely than women to develop SO once trauma is encountered. In fact, recent reports indicate that both sexes are affected equally.113,115
White Dot Syndromes
The white dot syndromes are a heterogeneous group of rare idiopathic inflammatory disorders characterized by discrete, multiple, well-circumscribed, white spots at the level of the retina, outer retina, RPE and choroid. They include Acute Posterior Multifocal Placoid Pigment Epitheliopathy (APMPPE), Serpiginous Choroiditis, Multiple Evanescent White Dot Syndrome (MEWDS), Multifocal Choroiditis and Panuveitis (MCP), Punctate Inner Choroidopathy (PIC), Birdshot Retinochoroidopathy (BSRC), Acute Zonal Occult Outer Retinopathy (AZOOR) and rarer forms such as Acute Macular Neuroretinopathy, Unilateral Acute Idiopathic Maculopathy (UAIM) and Diffuse Subretinal Fibrosis (DSF).116 The etiology and pathogenesis of white dot syndromes remain unknown. In many cases, a prodromal viral syndrome can be recognized and some investigators have hypothesized an infectious trigger. A common feature of all of these syndromes is white-yellow chorioretinal spots or lesions, scotomas, photopsias and lack of systemic disease association. A female predilection has been shown in MEWDS, BSRC, MCP, PIC and AZOOR, whereas APMPPE is reported to be more common in males or to have equal sex distribution.116,117
APMPPE typically presents with bilateral acute painless and asymmetric loss of vision, scotomas and photopsias in otherwise healthy young adults. It typically affects individuals 20–40 years of age118 and typically develops after a viral illness. It is characterized by multiple large cream-colored placoid lesions at the level of RPE, scattered throughout the posterior pole; the fovea may or may not be involved.119 Lesions usually resolve within 2–6 weeks leaving behind pigmentary changes. Most cases are self-limiting, and permanent visual loss is rare.116 Serpiginous Choroiditis is a rare, bilateral, chronic, progressive, recurrent inflammatory disease of the RPE, choriocapillaris and choroid.120 Patients also show an increased frequency of HLA-B7.116 It is usually seen in Caucasians between 40 and 60 years of age with no sex predilection. Retinal examination shows gray-yellowish subretinal infiltrates ranging in size from one to several disc diameters and spreading centrifugally from the peripapillary region in a serpentine (“snake-like”) pattern.121 Active lesions reveal a leading edge which blocks early and stains late on fluorescein angiography and resolve with subsequent RPE and choriocapillaris atrophy.
MEWDS is an uncommon inflammatory condition that typically affects otherwise healthy young myopic females between 20 and 50 years of age. Presenting symptoms include acute unilateral vision loss, photopsias, and central or paracentral scotomas. Approximately 30% of patients have a viral prodrome.122 Association with HLA-B51 has been reported.123 During the acute phase of the disease, multiple discrete small white dots are seen at the level of the RPE or deep retina in the perimacular area. The most characteristic feature is foveal granularity. Whether sex plays a role in prognosis is unclear; however, MEWDS is a self-limited disease with good visual recovery within 2–10 weeks.116
BSRC typically presents as a slow indolent disease characterized by small creamy lesions at the level of choroid scattered throughout the post-equatorial fundus, particularly in the nasal quadrant, with mild or no anterior uveitis and mild to moderate vitritis. Retinal vasculitis, CME and optic nerve inflammation can be seen at any phase of the disease course. BSRC has a high correlation with the HLA-A29 haplotype (93–96%), affects patients in their fourth to sixth decades, and is more common in Caucasians.116,124 An extensive review of the literature by Shah et al.124 indicated a slight female predominance (54.1%).
AZOOR was initially described in young myopic women between the ages of 20–50 years with apparently normal fundus findings, loss of outer retinal function, irreversible ERG abnormalities and permanent visual field loss.125 Subsequent papers also report an overrepresentation of women. Retina findings usually appear later in the disease and are characterized by attenuated retinal vessels and zonal loss of retinal pigment epithelium.116
MCP and PIC typically present as acute painless vision loss with associated photopsias, scotomas and visual field loss.126 They are more common in females, with PIC overwhelmingly more common in young myopic Caucasian females.127 Patients with MCP demonstrate multiple punched-out chorioretinal lesions, both in the posterior pole and the periphery, and vitritis, whereas PIC patients have minimal or no intraocular inflammation. Both diseases can be complicated by choroidal neovascular membranes which can cause severe vision loss (Figure 4).126,127
Figure 4.

Fundus photos of a 40-year-old myopic female with punctate inner choroidopathy (PIC) demonstrate a macular scar secondary to choroidal neovascularization (CNV) in the left eye and active macular lesions with late leakage on fluorescein angiogram in the right eye.
Tubulointerstitial Nephritis and Uveitis (TINU) Syndrome
Tubulointerstitial nephritis and uveitis (TINU) syndrome is a rare cause of uveitis described first by Dobrin et al. in 1975.128 It constitutes only 1–2% of all patients in specialized uveitis clinics, and there are just over 200 cases reported in the literature.129 TINU commonly presents with constitutional symptoms, proteinuria, hematuria and elevated serum creatinine. A renal biopsy is required for definitive diagnosis. Urine beta2-microglobulin and HLA-DRB1*0102 have been associated with TINU syndrome and can be helpful in diagnosis. There is no racial predilection. Prior to the 1990s, there was a reported significant female predominance, and only 18% of reported cases of TINU were males. Recently, more cases of TINU have been described in males, such that males comprise 34% of all TINU patients after the 1990s.130 The reasons for initial female predilection and recent increase in prevalence among males are not fully understood.
Infectious Uveitides
Sex differences could affect the frequency of infectious uveitides either because of cultural disparities in acquiring potential pathogens or because of sex-determined variations in biological response.131 In general, males in multiple species are felt to be more susceptible to pathogens than females.131 The most important differences in infectious uveitis would be predicted to occur in sexually transmitted diseases, because both behavior and physiology might influence risk of infection and uveitis.
Infectious Uveitis Caused by Sexually Transmitted Diseases
Syphilitic uveitis in HIV-infected patients is overwhelmingly reported in males: in pooled data from 101 patients, 97 were male.132 A small Chinese case series showed only a slight male predominance among 14 non-HIV patients with syphilitic uveitis;133 population data regarding sex prevalence was not given. In an international series and review of acute syphilitic posterior placoid uveitis, 8 of 37 HIV-negative patients were female (24.3%) again with unknown population frequencies.133,134 US population data in 2011 indicated 8.3 per 100,000 primary and secondary syphilis infections in men versus 1.0 per 100,000 in women (http://www.cdc.gov/std/stats11/tables.htm, accessed 1 Sept 2013), presumably based on exposure and social factors rather than biological susceptibility. Larger series of syphilitic uveitis in non-HIV patients with information regarding population frequencies of infection would be needed to determine the relative influences of immune status, behavior and sex in syphilitic uveitis.
Cytomegalovirus (CMV) infection is of particular interest, because it is both sexually transmitted135 and the cause of potentially blinding retinitis in AIDS. CMV can also be transmitted through household and day care contacts and breastfeeding, placing women at special risk. AIDS in USA predominantly affects men, but the frequency of AIDS-indicator infections in the early 1990s were related to the degree of immunosuppression and were equal in men and women;136 cytomegalovirus retinitis (CMVR) was not enumerated in this study. Declining proportions of men relative to women occurred in sequential multicenter studies of CMVR conducted from 1990 to 2000.137 Incident cases of CMVR in 2002 were statistically more common in women than in men in the large multicenter Longitudinal Study of the Ocular Complications of AIDS (LSOCA).137 This may have been a result of increasing numbers of women, minorities and heterosexuals with HIV infection. Being uninsured or untreated at study entry was also associated with more incident CMVR, suggesting economic disadvantage as an additional factor.137 Currently, sex equality in HIV testing and treatment is improving globally, which may alleviate health care disparities between men and women.138 In 2012, reanalysis of CMVR incidence among the 2002 study participants after long-term follow-up did not support a sex difference in incident CMVR.139 Although women in general are somewhat more likely to be infected with CMV than men (OR 1.17 [1.14–1.21]),140 HIV-positive women have been reported to have an increased risk (odds ratio 1.43) for cytomegalovirus disease only when HIV is non-sexually acquired, e.g. by injection drug use.141
Infectious Uveitis Caused by Non-Sexually Transmitted Diseases
In the same large HIV-infected LSOCA cohort, non-sexually transmitted ocular infections such as non-CMV herpes viruses (simplex, zoster), protozoa (toxoplasmosis and pneumocystis), Cryptococcus, and atypical mycobacterium had similar prevalence between men and women.142 The influence of biologic sex differences in the pathogenesis of ocular infections in immunocompromised individuals therefore appears to be small, although few cases of these infections were seen. For herpetic iridocyclitis in non-HIV-infected patients, there was a slight female predominance of 1.2:1 in a series of 111 Turkish patients with herpetic iridocyclitis.143 Herpes simplex 2 (HSV 2) infection is more common in women than in men in USA (http://www.cdc.gov/std/Herpes/STDFact-Herpes.htm, accessed 1 Sept 2013) and perhaps in Turkey, which may have influenced prevalence of the ocular infection, which was not typed as due to HSV 1 or 2. In herpes zoster ophthalmicus, no sex predilection was found in a Hawaiian cohort accrued from a large health plan.144 Fuchs uveitis, suspected of chronic viral infection in many cases, showed a slight male predominance in 166 Saudi patients and a slight female predominance in Chinese patients; whether this relates to population prevalence of the candidate viruses (rubella, cytomegalovirus, herpes) is unknown.145,146
Vertical Transmission of Infections Associated with Uveitis
Women also vertically transmit infections that may produce infectious uveitis in their offspring. Vertically transmissible infections with the potential for ocular involvement include syphilis, HSV 2, cytomegalovirus and toxoplasmosis. Syphilis surveillance and treatment of pregnant women is well-established in USA; there are currently ∼350 cases occurring annually or ∼8.5 per 100,000 live births (http://www.cdc.gov/std/stats11/tables/1.htm, accessed 1 Sept 2013). Maternal transmission of herpes simplex 2 is suspected of causing eventual necrotizing herpetic retinopathy in infants and children based on birth histories and serologic testing.147 Congenital CMV infection is more common than congenital HIV; seropositive women are more likely to be categorized as non-white and of a lower socioeconomic status.148 Virtually all of the 5–15% symptomatic infants born with congenital CMV infection will have ocular manifestations of chorioretinitis, optic atrophy or cortical visual defects.149 Both spontaneous healing and delayed reactivation of CMVR have been reported.150 Serologic rather than ocular screening is a more effective strategy for detection of congenital toxoplasmosis, because only 3.8–13.7% [95% C.I.] of mothers of children with congenital toxoplasmosis have chorioretinal lesions consistent with healed toxoplasmosis.151 Pre- and post-partum antibiotics in cases of first trimester maternal seroconversion can result in favorable visual outcomes.152 Post-natal treatment does not prevent the development of new fundus lesions in affected children as they age,153 but may reduce the incidence compared to untreated children.154
In summary, there are well-described sex differences in many of the uveitides; particularly those associated with systemic autoimmune diseases. In most instances, females are more commonly affected than males, although in some cases female sex portends a better prognosis. The causes of these differences are legion and, as of yet, poorly understood but include genetic, epigenetic, hormonal, immunological and environmental influences. However, lack of evaluation of outcomes with respect to gender in the literature poses a real problem in assessing the true impact of gender in each of these diseases. It is crucial to include gender as a risk factor in prospective and retrospective clinical research studies to enable better ascertainment of gender disparity in autoimmune diseases.
Table 1.
Gender distribution in common non-infectious ocular inflammatory disorders.
| Disease | Incidence and prevalence | Severity |
|---|---|---|
| Systemic lupus erythematosus | More common in women | May be more severe in men |
| Rheumatoid arthritis | More common in women | Comparable |
| Granulomatosis with polyangiitis | Equal | May be more severe in women |
| JIA-associated uveitis | More common in girls | Comparable, though there are studies that suggest more severe disease in males |
| Sarcoidosis and associated uveitis | Slight female preponderance | May be more severe in women (poor visual prognosis and posterior segment involvement more common in women) |
| HLA-B27-associated entities | ||
|
More common in men More common in men Comparable Slight male preponderance Slight female preponderance |
AS-women more likely to have cervical spine involvement AS associated uveitis-poor visual prognosis, frequent flare-ups associated with male gender ReA-milder disease in women EN in IBD associated with female gender |
| Tubulointerstitial nephritis and uveitis Multiple sclerosis Behçet disease |
More common in women More common in women Slight male preponderance (geographic variability) |
Comparable May have more disabling course in men More severe in men (poor visual prognosis and posterior segment involvement more common in men) |
| Vogt–Koyanagi–Harada syndrome | More common in women | Comparable |
| Sympathetic ophthalmia | More common in men | Females may be more likely to have vision loss |
| White dot syndromes | ||
| APMPPE | More in men or comparable | No clear data on severity and gender |
| Serpiginous choroiditis | Comparable | |
| MEWDS | More common in women | |
| MCP | ||
| PIC | ||
| BSRC | ||
| AZOOR | ||
JIA, juvenile idiopathic arthritis; AS, ankylosing spondylitis; EN, erythema nodosum; ReA, reactive arthritis; APMPPE, acute multifocal placoid pigment epitheliopathy; MEWDS, Multiple Evanescent White Dot Syndrome; MCP, multifocal choroiditis and panuveitis; PIC, punctate inner choroidopathy; BSRC, birdshot chorioretinopathy; AZOOR, acute zonal occult outer retinopathy
Acknowledgments
H.N.S., C.C.C., D.U. and A.F.'s work has been supported by the National Eye Institute Intramural Research Program. D.A.G. is supported by an unrestricted grant from Research to Prevent Blindness (NY).
Footnotes
This manuscript has not been published elsewhere and that it has not been submitted simultaneously for publication elsewhere.
Declaration of Interest: None of the authors have any commercial relationship with regards to the subject of this manuscript.
References
- 1.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Tedeschi SK, Bermas B, Costenbader KH. Sexual disparities in the incidence and course of SLE and RA. Clin Immunol. 2013;149:211–218. doi: 10.1016/j.clim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Wilder RL. Adrenal and gonadal steroid hormone deficiency in the pathogenesis of rheumatoid arthritis. J Rheumatol Suppl. 1996;44:10–12. [PubMed] [Google Scholar]
- 4.Cohen-Solal JF, Jeganathan V, Hill L, Kawabata D, Rodriguez-Pinto D, Grimaldi C, et al. Hormonal regulation of B-cell function and systemic lupus erythematosus. Lupus. 2008;17:528–532. doi: 10.1177/0961203308089402. [DOI] [PubMed] [Google Scholar]
- 5.Waite JC, Skokos D. Th17 response and inflammatory autoimmune diseases. Int J Inflamm. 2012;2012 doi: 10.1155/2012/819467. 819467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiam NP, Hall AJ, Stawell RJ, Busija L, Lim LL. The course of uveitis in pregnancy and postpartum. Br J Ophthalmol. 2013;97:1284–1288. doi: 10.1136/bjophthalmol-2013-303358. [DOI] [PubMed] [Google Scholar]
- 7.Kump LI, Cervantes-Castañeda RA, Androudi SN, Foster CS, Christen WG. Patterns of exacerbations of chronic non-infectious uveitis in pregnancy and puerperium. 7. Ocul Immunol Inflamm. 2006;14:99–104. doi: 10.1080/09273940500557027. [DOI] [PubMed] [Google Scholar]
- 8.Sivaraj RR, Durrani OM, Denniston AK, Murray PI, Gordon C. Ocular manifestations of systemic lupus erythematosus. Rheumatology (Oxford) 2007;46:1757–1762. doi: 10.1093/rheumatology/kem173. Review. [DOI] [PubMed] [Google Scholar]
- 9.Snyers B, Lambert M, Hardy JP. Retinal and choroidal vaso-occlusive disease in systemic lupus erythematosus associated with antiphospholipid antibodies. Retina. 1990;10:255–260. doi: 10.1097/00006982-199010000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Sitaula R, Shah DN, Singh D. The spectrum of ocular involvement in systemic lupus erythematosus in a tertiary eye care center in Nepal. Ocul Immunol Inflamm. 2011;19:422–425. doi: 10.3109/09273948.2011.610023. [DOI] [PubMed] [Google Scholar]
- 11.Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352–356. doi: 10.1097/00002281-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Costenbader KH, Feskanich D, Stampfer MJ, Karlson EW. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007;56:1251–1262. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- 13.Bernier MO, Mikaeloff Y, Hudson M, Suissa S. Combined oral contraceptive use and the risk of systemic lupus erythematosus. Arthritis Rheum. 2009;61:476–481. doi: 10.1002/art.24398. [DOI] [PubMed] [Google Scholar]
- 14.Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142(Pt 1):953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 15.Crosbie D, Black C, McIntyre L, Royle PL, Thomas S. Dehydroepiandrosterone for systemic lupus erythematosus. Cochrane Database Syst Rev. 2007:CD005114. doi: 10.1002/14651858.CD005114.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez-Nemegyei J, Cobarrubias-Cobos A, Escalante-Triay F, Sosa-Muñoz J, Miranda JM, Jara LJ. Bromocriptine in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled study. Lupus. 1998;7:414–419. doi: 10.1191/096120398678920334. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in Tcells from women with lupus. J Immunol. 2007;179:6352–6358. doi: 10.4049/jimmunol.179.9.6352. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Zhao Y, Song Y, Zhang W, Bian X, Yang J, et al. Pregnancy in women with systemic lupus erythematosus: a retrospective study of 111 pregnancies in Chinese women. J Matern Fetal Neonatal Med. 2012;25:261–266. doi: 10.3109/14767058.2011.572310. [DOI] [PubMed] [Google Scholar]
- 19.Ateka-Barrutia O, Khamashta MA. The challenge of pregnancy for patients with SLE. Lupus. 2013;22:1295–1308. doi: 10.1177/0961203313504637. [DOI] [PubMed] [Google Scholar]
- 20.Takala JH, Kautiainen H, Leirisalo-Repo M. Survival of patients with Wegener's granulomatosis diagnosed in Finland in 1981–2000. Scand J Rheumatol. 2010;39:71–76. doi: 10.3109/03009740903140701. [DOI] [PubMed] [Google Scholar]
- 21.Tarabishy AB, Schulte M, Papaliodis GN, Hoffman GS. Wegener's granulomatosis: clinical manifestations, differential diagnosis, and management of ocular and systemic disease. Surv Ophthalmol. 2010;55:429–444. doi: 10.1016/j.survophthal.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses' Health Study. Arthritis Rheum. 2004;50:3458–3467. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- 23.Nelson JL, Ostensen M. Pregnancy and rheumatoid arthritis. Rheum Dis Clin North Am. 1997;23:195–212. doi: 10.1016/s0889-857x(05)70323-9. [DOI] [PubMed] [Google Scholar]
- 24.Cutolo M. Androgens in rheumatoid arthritis: when are they effectors? Arthritis Res Ther. 2009;11:126. doi: 10.1186/ar2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall GM, Daniels M, Huskisson EC, Spector TD. A randomised controlled trial of the effect of hormone replacement therapy on disease activity in postmenopausal rheumatoid arthritis. Ann Rheum Dis. 1994;53:112–116. doi: 10.1136/ard.53.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petty RE, Southwood TR, Baum J, Bhettay E, Glass DN, Manners P, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991–1994. [PubMed] [Google Scholar]
- 27.Heiligenhaus A, Heinz C, Edelsten C, Kotaniemi K, Minden K. Review for disease of the year: epidemiology of juvenile idiopathic arthritis and its associated uveitis: the probable risk factors. Ocul Immunol Inflamm. 2013;21:180–191. doi: 10.3109/09273948.2013.791701. [DOI] [PubMed] [Google Scholar]
- 28.Carvounis PE, Herman DC, Cha S, Burke JP. Incidence and outcomes of uveitis in juvenile rheumatoid arthritis, a synthesis of the literature. Graefes Arch Clin Exp Ophthalmol. 2006;244:281–290. doi: 10.1007/s00417-005-0087-3. [DOI] [PubMed] [Google Scholar]
- 29.Saurenmann RK, Rose JB, Tyrrell P, Feldman BM, Laxer RM, Schneider R, et al. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: ethnicity as a risk factor. Arthritis Rheum. 2007;56:1974–1984. doi: 10.1002/art.22709. [DOI] [PubMed] [Google Scholar]
- 30.Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K, German Uveitis in Childhood Study Group Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology (Oxford) 2007;46:1015–1019. doi: 10.1093/rheumatology/kem053. [DOI] [PubMed] [Google Scholar]
- 31.Saurenmann RK, Levin AV, Feldman BM, Laxer RM, Schneider R, Silverman ED. Risk factors for development of uveitis differ between girls and boys with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:1824–1828. doi: 10.1002/art.27416. [DOI] [PubMed] [Google Scholar]
- 32.Kalinina Ayuso V, Ten Cate HA, van der Does P, Rothova A, de Boer JH. Male gender as a risk factor for complications in uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2010;149:994–999. e5. doi: 10.1016/j.ajo.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Thomas KW, Hunninghake GW. Sarcoidosis. JAMA. 2003;289:3300–3303. doi: 10.1001/jama.289.24.3300. [DOI] [PubMed] [Google Scholar]
- 34.Hillerdal G, Nöu E, Osterman K, Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29–32. doi: 10.1164/arrd.1984.130.1.29. [DOI] [PubMed] [Google Scholar]
- 35.Jørgensen KT, Pedersen BV, Nielsen NM, Jacobsen S, Frisch M. Childbirths and risk of female predominant and other autoimmune diseases in a population-based Danish cohort. J Autoimmun. 2012;38:J81–J87. doi: 10.1016/j.jaut.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Umur KA, Tayfun B, Oguzhan O. Different ophthalmologic manifestations of sarcoidosis. Curr Opin Ophthalmol. 2012;23:477–484. doi: 10.1097/ICU.0b013e328358c7a6. [DOI] [PubMed] [Google Scholar]
- 37.Birnbaum AD, Oh FS, Chakrabarti A, Tessler HH, Goldstein DA. Clinical features and diagnostic evaluation of biopsy-proven ocular sarcoidosis. Arch Ophthalmol. 2011;129:409–413. doi: 10.1001/archophthalmol.2011.52. [DOI] [PubMed] [Google Scholar]
- 38.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Jr, Bresnitz EA, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(Pt 1):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 39.Lobo A, Barton K, Minassian D, du Bois RM, Lightman S. Visual loss in sarcoid-related uveitis. Clin Experiment Ophthalmol. 2003;31:310–316. doi: 10.1046/j.1442-9071.2003.00666.x. [DOI] [PubMed] [Google Scholar]
- 40.Wakefield D, Chang JH, Amjadi S, Maconochie Z, Abu El-Asrar A, McCluskey P. What is new HLA-B27 acute anterior uveitis? Ocul Immunol Inflamm. 2011;19:139–144. doi: 10.3109/09273948.2010.542269. [DOI] [PubMed] [Google Scholar]
- 41.Lee W, Reveille JD, Weisman MH. Women with ankylosing spondylitis: a review. Arthritis Rheum. 2008;59:449–454. doi: 10.1002/art.23321. [DOI] [PubMed] [Google Scholar]
- 42.Niederer R, Danesh-Meyer H. Uveitis screening: HLAB27 antigen and ankylosing spondylitis in a New Zealand population. N Z Med J. 2006;119:U1886. [PubMed] [Google Scholar]
- 43.Torres S, Borges S, Artiles A. HLA-B27 and clinical features of acute anterior uveitis in Cuba. Ocul Immunol Inflamm. 2013;21:119–123. doi: 10.3109/09273948.2012.748080. [DOI] [PubMed] [Google Scholar]
- 44.Zheng MQ, Wang YQ, Lu XY, Wang YL, Mao LP, Gu YF, et al. Clinical Analysis of 240 Patients with HLA-B27 Associated Acute Anterior Uveitis. Yan Ke Xue Bao. 2012;27:169–172. doi: 10.3969/j.issn.1000-4432.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Loh AR, Acharya NR. Incidence rates and risk factors for ocular complications and vision loss in HLA-B27-associated uveitis. Am J Ophthalmol. 2010;150:534–542. e2. doi: 10.1016/j.ajo.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braakenburg AM, de Valk HW, de Boer J, Rothova A. Human leukocyte antigen-B27-associated uveitis: long-term follow-up and gender differences. Am J Ophthalmol. 2008;145:472–479. doi: 10.1016/j.ajo.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Miayamoto N, Mandai M, Suzuma I, Suzuma K, Kobayashi K, Honda Y. Estrogen protects against cellular infi ltration by reducing the expressions of E-selectin and IL-6 in endotoxin-induced uveitis. J Immunol. 1999;163:374–379. [PubMed] [Google Scholar]
- 48.Braun J, Kingsley G, van der Heijde D, Sieper J. On the difficulties of establishing a consensus on the definition of and diagnostic investigations for reactive arthritis. Results and discussion of a questionnaire prepared for the 4th International Workshop on Reactive Arthritis, Berlin, Germany, July 3–6, 1999. J Rheumatol. 2000;27:2185–2192. [PubMed] [Google Scholar]
- 49.Sampaio-Barros PD, Bortoluzzo AB, Conde RA, Costallat LT, Samara AM, Bértolo MB. Undifferentiated spondyloarthritis: a longterm followup. J Rheumatol. 2010;37:1195–1199. doi: 10.3899/jrheum.090625. [DOI] [PubMed] [Google Scholar]
- 50.Brockbank J, Gladman D. Diagnosis and management of psoriatic arthritis. Drugs. 2002;62:2447–2457. doi: 10.2165/00003495-200262170-00004. [DOI] [PubMed] [Google Scholar]
- 51.Munkholm P, Langholz E, Nielsen OH, Kreiner S, Binder V. Incidence and prevalence of Crohn's disease in the county of Copenhagen, 1962–1987: a sixfold increase in incidence. Scand J Gastroenterol. 1992;27:609–614. doi: 10.3109/00365529209000127. [DOI] [PubMed] [Google Scholar]
- 52.Loftus EV, Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Ulcerative colitis in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gut. 2000;46:336–343. doi: 10.1136/gut.46.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basic and clinical science course. Intraocular Inflammation and Uveitis. Section 9 2013-2014. American Academy of Ophthalmology; pp. 120–123. [Google Scholar]
- 54.Farhi D, Cosnes J, Zizi N, Chosidow O, Seksik P, Beaugerie L, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore) 2008;87:281–293. doi: 10.1097/MD.0b013e318187cc9c. [DOI] [PubMed] [Google Scholar]
- 55.Gül A. Behçet's disease: an update on the pathogenesis. Clin Exp Rheumatol. 2001;19(Suppl 24):S6–S12. [PubMed] [Google Scholar]
- 56.Verity DH, Marr JE, Ohno S, Wallace GR, Stanford MR. Behçet's disease, the Silk Road and HLA-B51: historical and geographical perspectives. Tissue Antigens. 1999;54:213–220. doi: 10.1034/j.1399-0039.1999.540301.x. [DOI] [PubMed] [Google Scholar]
- 57.Gül A. Behçet's disease as an autoinflammatory disorder. Curr Drug Targets Inflamm Allergy. 2005;4:81–83. doi: 10.2174/1568010053622894. [DOI] [PubMed] [Google Scholar]
- 58.Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 59.Davatchi F, Shahram F, Chams-Davatchi C, Shams H, Nadji A, Akhlaghi M, et al. Behcet's disease: from East to West. Clin Rheumatol. 2010;29:823–833. doi: 10.1007/s10067-010-1430-6. [DOI] [PubMed] [Google Scholar]
- 60.Mahr A, Belarbi L, Wechsler B, Jeanneret D, Dhote R, Fain O, et al. Population-based prevalence study of Behçet's disease: differences by ethnic origin and low variation by age at immigration. Arthritis Rheum. 2008;58:3951–3959. doi: 10.1002/art.24149. [DOI] [PubMed] [Google Scholar]
- 61.Calamia KT, Wilson FC, Icen M, Crowson CS, Gabriel SE, Kremers HM. Epidemiology and clinical characteristics of Behçet's disease in the US: a population-based study. Arthritis Rheum. 2009;61:600–604. doi: 10.1002/art.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soheilian M, Heidari K, Yazdani S, Shahsavari M, Ahmadieh H, Dehghan M. Patterns of uveitis in a tertiary eye care center in Iran. Ocul Immunol Inflamm. 2004;12:297–310. doi: 10.1080/092739490500174. [DOI] [PubMed] [Google Scholar]
- 63.Wang LY, Zhao DB, Gu J, Dai SM. Clinical characteristics of Behçet's disease in China. Rheumatol Int. 2010;30:1191–1196. doi: 10.1007/s00296-009-1127-9. [DOI] [PubMed] [Google Scholar]
- 64.See LC, Kuo CF, Chou IJ, Chiou MJ, Yu KH. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: a Taiwan population-based study. Semin Arthritis Rheum. 2013;43:381–386. doi: 10.1016/j.semarthrit.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Saleh OA, Birnbaum AD, Tessler HH, Goldstein DA. Behçet uveitis in the American midwest. Ocul Immunol Inflamm. 2012;20:12–17. doi: 10.3109/09273948.2011.630550. [DOI] [PubMed] [Google Scholar]
- 66.Alpsoy E, Zouboulis CC, Ehrlich GE. Mucocutaneous lesions of Behcet's disease. Yonsei Med J. 2007;48:573–585. doi: 10.3349/ymj.2007.48.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seyahi E, Yazici H. Prognosis in Behcet's Syndrome. In: Yazici Y, Yazici H, editors. Behcet's syndrome. New York: Springer; 2010. pp. 285–295. [Google Scholar]
- 68.Cho YJ, Kim WK, Lee JH, Byeon SH, Koh HJ, Kwon OW, et al. Visual prognosis and risk factors for korean patients with behcet uveitis. Ophthalmologica. 2008;222:344–350. doi: 10.1159/000146080. [DOI] [PubMed] [Google Scholar]
- 69.Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V, et al. The long-term mortality and morbidity of Behçet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore) 2003;82:60–76. doi: 10.1097/00005792-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Krause L, Köhler AK, Altenburg A, Papoutsis N, Zouboulis CC, Pleyer U, et al. Ocular involvement in Adamantiades-Behçet's disease in Berlin, Germany. Graefes Arch Clin Exp Ophthalmol. 2009;247:661–666. doi: 10.1007/s00417-008-0983-4. [DOI] [PubMed] [Google Scholar]
- 71.Tugal-Tutkun I, Urgancioglu M. Childhood-onset uveitis in Behçet disease:a descriptive study of 36 cases. Am J Ophthalmol. 2003;136:1114–1119. doi: 10.1016/s0002-9394(03)00791-8. [DOI] [PubMed] [Google Scholar]
- 72.Matsuo T, Itami M. Recurrent versus non-recurrent or no eye involvement in Behcet's disease. Ocul Immunol Inflamm. 2005;13:73–77. doi: 10.1080/09273940490518928. [DOI] [PubMed] [Google Scholar]
- 73.Wakefield D, Cunningham ET, Jr, Tugal-Tutkun I, Khairallah M, Ohno S, Zierhut M. Controversies in Behçet disease. Ocul Immunol Inflamm. 2012;20:6–11. doi: 10.3109/09273948.2011.649153. [DOI] [PubMed] [Google Scholar]
- 74.Simpson S, Jr, Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:1132–1141. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 75.Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 76.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 77.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 78.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 79.International Multiple Sclerosis Genetics Consortium. Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 80.Lundmark F, Duvefelt K, Iacobaeus E, Kockum I, Wallström E, Khademi M, et al. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat Genet. 2007;39:1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 81.Dua T, Rompani P. Atlas: multiple sclerosis resources in the world, 2008. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 82.Compston A, Confavreux C, Lassmann H, McDonald I, Miller D, Noseworthy J, et al. McAlpine's multiple sclerosis. 4th. Philadelphia: Churchill Livingstone/Elsevier; 2006. pp. 113–272. [Google Scholar]
- 83.Tintoré M, Arrambide G. Early onset multiple sclerosis: the role of gender. J Neurol Sci. 2009;286:31–34. doi: 10.1016/j.jns.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 84.Alonso A, Hernán MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology. 2008;71:129–135. doi: 10.1212/01.wnl.0000316802.35974.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kotzamani D, Panou T, Mastorodemos V, Tzagournissakis M, Nikolakaki H, Spanaki C, et al. Rising incidence of multiple sclerosis in females associated with urbanization. Neurology. 2012;78:1728–1735. doi: 10.1212/WNL.0b013e31825830a9. [DOI] [PubMed] [Google Scholar]
- 86.Simone IL, Carrara D, Tortorella C, Liguori M, Lepore V, Pellegrini F, et al. Course and prognosis in early-onset MS: comparison with adult-onset forms. Neurology. 2002;59:1922–1928. doi: 10.1212/01.wnl.0000036907.37650.8e. [DOI] [PubMed] [Google Scholar]
- 87.Lević ZM, Dujmović I, Pekmezović T, Jarebinski M, Marinković J, Stojsavljević N, et al. Prognostic factors for survival in multiple sclerosis. Mult Scler. 1999;5:171–178. doi: 10.1177/135245859900500306. [DOI] [PubMed] [Google Scholar]
- 88.Bergamaschi R. Prognostic factors in multiple sclerosis. Int Rev Neurobiol. 2007;79:423–447. doi: 10.1016/S0074-7742(07)79019-0. [DOI] [PubMed] [Google Scholar]
- 89.Tremlett H, Devonshire V. Is late-onset multiple sclerosis associated with a worse outcome? Neurology. 2006;67:954–959. doi: 10.1212/01.wnl.0000237475.01655.9d. [DOI] [PubMed] [Google Scholar]
- 90.D'hooghe MB, D'Hooghe T, De Keyser J. Female gender and reproductive factors affecting risk, relapses and progression in multiple sclerosis. Gynecol Obstet Invest. 2013;75:73–84. doi: 10.1159/000346319. [DOI] [PubMed] [Google Scholar]
- 91.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, et al. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zein G, Berta A, Foster CS. Multiple sclerosis-associated uveitis. Ocul Immunol Inflamm. 2004;12:137–142. doi: 10.1080/09273940490895344. [DOI] [PubMed] [Google Scholar]
- 93.Chen L, Gordon LK. Ocular manifestations of multiple sclerosis. Curr Opin Ophthalmol. 2005;16:315–320. doi: 10.1097/01.icu.0000179804.49842.e2. [DOI] [PubMed] [Google Scholar]
- 94.Le Scanff J, Sève P, Renoux C, Broussolle C, Confavreux C, Vukusic S. Uveitis associated with multiple sclerosis. Mult Scler. 2008;14:415–417. doi: 10.1177/1352458507083444. [DOI] [PubMed] [Google Scholar]
- 95.Raja SC, Jabs DA, Dunn JP, Fekrat S, Machan CH, Marsh MJ, et al. Pars planitis: clinical features and class II HLA associations. Ophthalmology. 1999;106:594–599. doi: 10.1016/S0161-6420(99)90122-7. [DOI] [PubMed] [Google Scholar]
- 96.Biousse V, Trichet C, Bloch-Michel E, Roullet E. Multiple sclerosis associated with uveitis in two large clinic-based series. Neurology. 1999;52:179–181. doi: 10.1212/wnl.52.1.179. [DOI] [PubMed] [Google Scholar]
- 97.Li SY, Birnbaum AD, Goldstein DA. Optic neuritis associated with adalimumab in the treatment of uveitis. Ocul Immunol Inflamm. 2010;18:475–481. doi: 10.3109/09273948.2010.495814. [DOI] [PubMed] [Google Scholar]
- 98.Bordaberry MF. Vogt-Koyanagi-Harada disease: diagnosis and treatments update. Curr Opin Ophthalmol. 2010;21:430–435. doi: 10.1097/ICU.0b013e32833eb78c. [DOI] [PubMed] [Google Scholar]
- 99.Yodmuang T, Rothova A, Kunavisarut P, Pathanapitoon K. Vogt-Koyanagi-Harada disease in Thailand. Ocul Immunol Inflamm. 2012;20:419–422. doi: 10.3109/09273948.2012.723780. [DOI] [PubMed] [Google Scholar]
- 100.Aláez C, Flores-A H, Concha del Río LE, Munguía A, Rodríguez A, García D, et al. Major histocompatibility complex and strong human leukocyte antigen-DRB1 and gender association with Vogt-Koyanagi-Harada syndrome in Mexican Mestizos. Hum Immunol. 2011;72:1198–1203. doi: 10.1016/j.humimm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Chee SP, Jap A, Bascal K. Spectrum of Vogt-Koyanagi-Harada disease in Singapore. Int Ophthalmol. 2007;27:137–142. doi: 10.1007/s10792-006-9009-6. [DOI] [PubMed] [Google Scholar]
- 102.Fang W, Yang P. Vogt-koyanagi-harada syndrome. Curr Eye Res. 2008;33:517–523. doi: 10.1080/02713680802233968. [DOI] [PubMed] [Google Scholar]
- 103.Damico FM, Kiss S, Young LH. Vogt-Koyanagi-Harada disease. Semin Ophthalmol. 2005;20:183–190. doi: 10.1080/08820530500232126. [DOI] [PubMed] [Google Scholar]
- 104.Concha del Río LE, Arellanes-García L. Vogt-Koyanagi-Harada disease in the developing world. Int Ophthalmol Clin. 2010;50:189–199. doi: 10.1097/IIO.0b013e3181d26a6f. [DOI] [PubMed] [Google Scholar]
- 105.Read RW, Rao NA, Cunningham ET. Vogt-Koyanagi-Harada disease. Curr Opin Ophthalmol. 2000;11:437–442. doi: 10.1097/00055735-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 106.Sen HN, Nussenblatt RB. Sympathetic ophthalmia: what have we learned? Am J Ophthalmol. 2009;148:632–633. doi: 10.1016/j.ajo.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kilmartin DJ, Wilson D, Liversidge J, Dick AD, Bruce J, Acheson RW, et al. Immunogenetics and clinical phenotype of sympathetic ophthalmia in British and Irish patients. Br J Ophthalmol. 2001;85:281–286. doi: 10.1136/bjo.85.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davis JL, Mittal KK, Freidlin V, Mellow SR, Optican DC, Palestine AG, et al. HLA associations and ancestry in Vogt-Koyanagi-Harada disease and sympathetic ophthalmia. Ophthalmology. 1990;97:1137–1142. doi: 10.1016/s0161-6420(90)32446-6. [DOI] [PubMed] [Google Scholar]
- 109.Marak GE., Jr Recent advances in sympathetic ophthalmia. Surv Ophthalmol. 1979;24:141–156. doi: 10.1016/0039-6257(79)90018-3. [DOI] [PubMed] [Google Scholar]
- 110.Damico FM, Kiss S, Young LH. Sympathetic ophthalmia. Semin Ophthalmol. 2005;20:191–197. doi: 10.1080/08820530500232100. [DOI] [PubMed] [Google Scholar]
- 111.Liddy L, Stuart J. Sympathetic ophthalmia in Canada. Can J Ophthalmol. 1972;7:157–159. [PubMed] [Google Scholar]
- 112.Albert DM, Diaz-Rohena R. A historical review of sympathetic ophthalmia and its epidemiology. Surv Ophthalmol. 1989;34:1–14. doi: 10.1016/0039-6257(89)90125-2. [DOI] [PubMed] [Google Scholar]
- 113.Chan CC, Roberge RG, Whitcup SM, Nussenblatt RB. 32 cases of sympathetic ophthalmia. A retrospective study at the National Eye Institute, Bethesda, Md., from 1982 to 1992. Arch Ophthalmol. 1995;113:597–600. doi: 10.1001/archopht.1995.01100050065032. [DOI] [PubMed] [Google Scholar]
- 114.Galor A, Davis JL, Flynn HW, Jr, Feuer WJ, Dubovy SR, Setlur V, et al. Sympathetic ophthalmia: incidence of ocular complications and vision loss in the sympathizing eye. Am J Ophthalmol. 2009;148:704–710. e2. doi: 10.1016/j.ajo.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 115.Castiblanco CP, Adelman RA. Sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 2009;247:289–302. doi: 10.1007/s00417-008-0939-8. [DOI] [PubMed] [Google Scholar]
- 116.Basic and Clinical Science Course. Intraocular Inflammation and Uveitis. Section 9 2013-2014. American Academy of Ophthalmology; pp. 147–172. [Google Scholar]
- 117.Abu-Yaghi NE, Hartono SP, Hodge DO, Pulido JS, Bakri SJ. White dot syndromes: a 20-year study of incidence, clinical features, and outcomes. Ocul Immunol Inflamm. 2011;19:426–430. doi: 10.3109/09273948.2011.624287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heath JD, Spalton DJ. Acute posterior multifocal placoid pigment epitheliopathy. Int Ophthalmol Clin. 1995;35:93–105. doi: 10.1097/00004397-199503520-00009. [DOI] [PubMed] [Google Scholar]
- 119.Gass JD. Acute posterior multifocal placoid pigment epitheliopathy. Arch Ophthalmol. 1968;80:177–185. doi: 10.1001/archopht.1968.00980050179005. [DOI] [PubMed] [Google Scholar]
- 120.Abrez H, Biswas J, Sudharshan S. Clinical profile, treatment, and visual outcome of serpiginous choroiditis. Ocul Immunol Inflamm. 2007;15:325–335. doi: 10.1080/09273940701375162. [DOI] [PubMed] [Google Scholar]
- 121.Jumper JM, McDonald R, Johnson RN, Ai E, Fu AD. Serpiginous choroiditis. In: Ryan SJ, editor. Retina. chapter 105. Vol. 2. St Louis, MO: Elsevier Mosby; 2006. pp. 1811–1820. [Google Scholar]
- 122.dell'Omo R, Pavesio CE. Multiple evanescent white dot syndrome (MEWDS) Int Ophthalmol Clin. 2012;52:221–228. doi: 10.1097/IIO.0b013e31826647ed. [DOI] [PubMed] [Google Scholar]
- 123.Borruat FX, Herbort CP, Spertini F, Desarnaulds AB. HLA typing in patients with multiple evanescent white dot syndrome (MEWDS) Ocul Immunol Inflamm. 1998;6:39–41. doi: 10.1076/ocii.6.1.39.8084. [DOI] [PubMed] [Google Scholar]
- 124.Shah KH, Levinson RD, Yu F, Goldhardt R, Gordon LK, Gonzales CR, et al. Birdshot chorioretinopathy. Surv Ophthalmol. 2005;50:519–541. doi: 10.1016/j.survophthal.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 125.Gass JD. Acute zonal occult outer retinopathy. Donders Lecture: The Netherlands Ophthalmological Society, Maastricht, Holland, June 19, 1992. J Clin Neuroophthalmol. 1993;13:79–97. [PubMed] [Google Scholar]
- 126.Folk JC, Walker JD. Multifocal choroiditis with panuveitis, diffuse subretinal fibrosis, and punctate inner choroidopathy. In: Ryan SJ, editor. Retina. 4th. USA: Elsevier Mosby; 2006. pp. 1771–1783. [Google Scholar]
- 127.Gerstenblith AT, Thorne JE, Sobrin L, Do DV, Shah SM, Foster CS, et al. Punctate inner choroidopathy: a survey analysis of 77 persons. Ophthalmology. 2007;114:1201–1204. doi: 10.1016/j.ophtha.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 128.Dobrin RS, Vernier RL, Fish AL. Acute eosinophilic interstitial nephritis and renal failure with bone marrow-lymph node granulomas and anterior uveitis. A new syndrome Am J Med. 1975;59:325–333. doi: 10.1016/0002-9343(75)90390-3. [DOI] [PubMed] [Google Scholar]
- 129.Mackensen F, Billing H. Tubulointerstitial nephritis and uveitis syndrome. Curr Opin Ophthalmol. 2009;20:525–531. doi: 10.1097/ICU.0b013e3283318f9a. [DOI] [PubMed] [Google Scholar]
- 130.Mandeville JT, Levinson RD, Holland GN. The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol. 2001;46:195–208. doi: 10.1016/s0039-6257(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 131.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 132.Tucker JD, Li JZ, Robbins GK, Davis BT, Lobo AM, Kunkel J, et al. Ocular syphilis among HIV-infected patients: a systematic analysis of the literature. Sex Transm Infect. 2011;87:4–8. doi: 10.1136/sti.2010.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang P, Zhang N, Li F, Chen Y, Kijlstra A. Ocular manifestations of syphilitic uveitis in Chinese patients. Retina. 2012;32:1906–1914. doi: 10.1097/IAE.0b013e3182509796. [DOI] [PubMed] [Google Scholar]
- 134.Eandi CM, Neri P, Adelman RA, Yannuzzi LA, Cunningham ET., Jr International Syphilis Study Group. Acute syphilitic posterior placoid chorioretinitis: report of a case series and comprehensive review of the literature. Retina. 2012;32:1915–1941. doi: 10.1097/IAE.0b013e31825f3851. [DOI] [PubMed] [Google Scholar]
- 135.Staras SA, Flanders WD, Dollard SC, Pass RF, McGowan JE, Jr, Cannon MJ. Influence of sexual activity on cytomegalovirus seroprevalence in the United States, 1988–1994. Sex Transm Dis. 2008;35:472–479. doi: 10.1097/OLQ.0b013e3181644b70. [DOI] [PubMed] [Google Scholar]
- 136.Farizo KM, Buehler JW, Chamberland ME, Whyte BM, Froelicher ES, Hopkins SG, et al. Spectrum of disease in persons with human immunodeficiency virus infection in the United States. JAMA. 1992;267:1798–1805. [PubMed] [Google Scholar]
- 137.Jabs DA, Van Natta ML, Kempen JH, Reed Pavan P, Lim JI, Murphy RL, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002;133:48–61. doi: 10.1016/s0002-9394(01)01322-8. [DOI] [PubMed] [Google Scholar]
- 138.Carael M, Marais H, Polsky J, Mendoza A. Is there a gender gap in the HIV response? Evaluating national HIV responses from the United Nations General Assembly Special Session on HIV/AIDS country reports. J Acquir Immune Defic Syndr. 2009;52(Suppl 2):S111–S118. doi: 10.1097/QAI.0b013e3181baeec2. [DOI] [PubMed] [Google Scholar]
- 139.Sugar EA, Jabs DA, Ahuja A, Thorne JE, Danis RP, Meinert CL, Studies of the Ocular Complications of AIDS Research Group Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2012;153:1016–1024. doi: 10.1016/j.ajo.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 141.Fleming PL, Ciesielski CA, Byers RH, Castro KG, Berkelman RL. Gender differences in reported AIDS-indicative diagnoses. J Infect Dis. 1993;168:61–67. doi: 10.1093/infdis/168.1.61. [DOI] [PubMed] [Google Scholar]
- 142.Gangaputra S, Drye L, Vaidya V, Thorne JE, Jabs DA, Lyon AT. Studies of the Ocular Complications of AIDS (SOCA) Research Group. Non-cytomegalovirus ocular opportunistic infections in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 2013;155:206–212. e5. doi: 10.1016/j.ajo.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tugal-Tutkun I, Otük-Yasar B, Altinkurt E. Clinical features and prognosis of herpetic anterior uveitis: a retrospective study of 111 cases. Int Ophthalmol. 2010;30:559–565. doi: 10.1007/s10792-010-9394-8. [DOI] [PubMed] [Google Scholar]
- 144.Borkar DS, Tham VM, Esterberg E, Ray KJ, Vinoya AC, Parker JV, et al. Incidence of herpes zoster ophthalmicus: results from the Pacific Ocular Inflammation Study. Ophthalmology. 2013;120:451–456. doi: 10.1016/j.ophtha.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Al-Mansour YS, Al-Rajhi AA, Al-Dhibi H, Abu El-Asrar AM. Clinical features and prognostic factors in Fuchs' uveitis. Int Ophthalmol. 2010;30:501–509. doi: 10.1007/s10792-010-9379-7. [DOI] [PubMed] [Google Scholar]
- 146.Yang P, Fang W, Jin H, Li B, Chen X, Kijlstra A. Clinical features of Chinese patients with Fuchs' syndrome. Ophthalmology. 2006;113:473–480. doi: 10.1016/j.ophtha.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 147.Silva RA, Berrocal AM, Moshfeghi DM, Blumenkranz MS, Sanislo S, Davis JL. Herpes simplex virus type 2 mediated acute retinal necrosis in a pediatric population: case series and review. Graefes Arch Clin Exp Ophthalmol. 2013;251:559–566. doi: 10.1007/s00417-012-2164-8. [DOI] [PubMed] [Google Scholar]
- 148.Cannon MJ. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol. 2009;46(Suppl 4):S6–S10. doi: 10.1016/j.jcv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 149.Ghekiere S, Allegaert K, Cossey V, Van Ranst M, Cassiman C, Casteels I. Ophthalmological findings in congenital cytomegalovirus infection: when to screen, when to treat? J Pediatr Ophthalmol Strabismus. 2012;49:274–282. doi: 10.3928/01913913-20120710-03. [DOI] [PubMed] [Google Scholar]
- 150.Coors LE, Spencer R. Delayed presentation of cytomegalovirus retinitis in an infant with severe congenital cytomegalovirus infection. Retina. 2010;30(4 Suppl):S59–S62. doi: 10.1097/IAE.0b013e3181c7018d. [DOI] [PubMed] [Google Scholar]
- 151.Noble AG, Latkany P, Kusmierczyk J, Mets M, Rabiah P, Boyer K, et al. Toxoplasmosis Study Group Chorioretinal lesions in mothers of children with congenital toxoplasmosis in the National Collaborative Chicago-based, Congenital Toxoplasmosis Study. Sci Med (Porto Alegre) 2010;20:20–26. [PMC free article] [PubMed] [Google Scholar]
- 152.Brézin AP, Thulliez P, Couvreur J, Nobré R, Mcleod R, Mets MB. Ophthalmic outcomes after prenatal and postnatal treatment of congenital toxoplasmosis. Am J Ophthalmol. 2003;135:779–784. doi: 10.1016/s0002-9394(02)01704-x. [DOI] [PubMed] [Google Scholar]
- 153.Garcia-Méric P, Franck J, Dumon H, Piarroux R. Management of congenital toxoplasmosis in France: current data. Presse Med. 2010;39:530–538. doi: 10.1016/j.lpm.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 154.Phan L, Kasza K, Jalbrzikowski J, Noble AG, Latkany P, Kuo A, et al. Toxoplasmosis Study Group Longitudinal study of new eye lesions in children with toxoplasmosis who were not treated during the first year of life. Am J Ophthalmol. 2008;146:375–384. doi: 10.1016/j.ajo.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


