Abstract
Background. Middle East respiratory syndrome coronavirus (MERS-CoV) emerged in 2012, causing severe acute respiratory disease and pneumonia, with 44% mortality among 136 cases to date. Design of vaccines to limit the virus spread or diagnostic tests to track newly emerging strains requires knowledge of antigenic and serologic relationships between MERS-CoV and other CoVs.
Methods. Using synthetic genomics and Venezuelan equine encephalitis virus replicons (VRPs) expressing spike and nucleocapsid proteins from MERS-CoV and other human and bat CoVs, we characterize the antigenic responses (using Western blot and enzyme-linked immunosorbent assay) and serologic responses (using neutralization assays) against 2 MERS-CoV isolates in comparison with those of other human and bat CoVs.
Results. Serologic and neutralization responses against the spike glycoprotein were primarily strain specific, with a very low level of cross-reactivity within or across subgroups. CoV N proteins within but not across subgroups share cross-reactive epitopes with MERS-CoV isolates. Our findings were validated using a convalescent-phase serum specimen from a patient infected with MERS-CoV (NA 01) and human antiserum against SARS-CoV, human CoV NL63, and human CoV OC43.
Conclusions. Vaccine design for emerging CoVs should involve chimeric spike protein containing neutralizing epitopes from multiple virus strains across subgroups to reduce immune pathology, and a diagnostic platform should include a panel of nucleocapsid and spike proteins from phylogenetically distinct CoVs.
Keywords: MERS-CoV Vaccine Design, Diagnostics, Serology, Synthetic Genomics
Novel approaches are needed to respond rapidly to new emerging diseases, especially early in the epidemic, when prompt public health interventions can limit mortality and epidemic spread. Coronaviruses (CoVs) constitute a group of phylogenetically diverse enveloped viruses that have the largest plus-strand RNA genomes and replicate efficiently in most mammals [1, 2]. Human CoV (HCoV-229E, -OC43, -NL63, and -HKU 1) infections typically result in mild-to-severe upper and lower respiratory tract disease [3, 4]. SARS-CoV emerged in 2002–2003, causing acute respiratory distress syndrome with 10% mortality overall and up to 50% mortality among aged individuals [5]. Most recently, Middle East respiratory syndrome CoV (MERS-CoV) emerged in the Middle East in April 2012, manifesting as severe pneumonia, acute respiratory distress syndrome, and acute renal failure. The virus is still circulating and has caused 136 human infections with 58 deaths (mortality rate, approximately 44%) [6, 7].
Phylogenetic analysis groups CoVs into 4 genera—Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus—and for many mammalian CoVs, bats are considered reservoirs [6, 8, 9]. SARS-CoV is closely related to bat CoV (BtCoV) HKU 3 [1, 10–12], whereas MERS-CoV is closely related to Pipistrellus BtCoV HKU 5 and Tylonycteris BtCoV HKU 4 [9]. However, the serologic and antigenic relationship between strains is unclear. Given the vast number of genetically distinct CoVs, well-defined serologic and virologic reagents are needed to rapidly track MERS-CoV and other CoV infections in natural populations and to optimize vaccine and therapeutic designs early in an outbreak setting, especially within and between phylogenetic subgroups.
The spike (S) and nucleocapsid (N) proteins are major immunogenic components of CoVs and are produced in abundant quantities during infection. The S protein is the principle determinant of protective immunity and cross-species transmission in CoV [11]. Antibodies against S protein protect from homologous and heterologous SARS-CoV challenge in vivo [13], whereas N protein–specific immune responses may offer limited protection especially against low-dose challenge [13]. Therefore, antibodies against S and N protein have diagnostic and therapeutic potential [14, 15].
In this article, we use alphavirus replicon vaccine vectors to express a panel of recombinant S and N proteins from distantly related alphacoronaviruses and betacoronaviruses, including MERS-CoV and other subgroup 2c CoVs. Using mouse polyclonal antisera and recombinant proteins, we compare the cross-reactivity and neutralization titers of these antisera between distantly related human and bat CoVs. Our results indicate that the S glycoprotein but not the N protein is the major determinant of the neutralizing antibody response to MERS-CoV; that the N proteins of CoVs only cross-react within but not between subgroups; that little if any cross-neutralization or cross-reactivity exists between the S proteins of CoVs within subgroup 2c or any other subgroup; and that cross-neutralization and cross-reactive patterns were validated with the convalescent-phase serum sample from a patient infected with MERS-CoV Hu/England-N1/2012 and a donor panel of human antisera against 3 different HCoVs. Our approach provides critical reagents, antisera, and recombinant virus vaccines that allow for rapid diagnosis of and intervention against MERS-CoV and other zoonotic CoVs that emerge in the future.
MATERIALS AND METHODS
Viruses, Cells, and Plaque Assays
MERS-CoV Hu/England-N1/2012 and MERS-CoV Hu/SA-N1/2012 were cultured on Vero 81 cells and grown in Opti-Mem (Gibco, Carlsbad, CA) with 5% fetal clone serum (Hyclone, South Logan, UT) and gentamicin/kanamycin (Gibco). Viral growth assays in Vero and Calu-3 cells were performed as previously described [16].
Generation of Polyclonal Mouse Antisera, Neutralization Assays, and Western and Northern Blot Analysis
Genes encoding the indicated S and N proteins were synthesized from Bio Basic (Ontario, Canada) and packaged into Venezuelan equine encephalitis virus replicon particles (VRPs). Following vaccination, mouse polyclonal sera were generated from BALB/c mice, and neutralization assays involving MERS-CoV strains and SARS-CoV were as described previously [17]. For Western blots, VRP or virus-infected cell lysates and controls were prepared as described before in detail [8], and these blots were probed using the indicated mouse polyclonal sera. Vero cells inoculated with MERS-CoV isolates were harvested 12 hours after infection by means of Trizol reagent (Invitrogen) and were used to perform Northern blots [18].
Enzyme-Linked Immunosorbent Assay (ELISA) and Blocking Assay
An ELISA using indicated virus-infected cell lysates or antigens expressed from VRPs was performed as described previously [19], and the reactivity of mouse or human serum was determined using a chemiluminescent substrate. Blocking ELISA was performed by sequentially reacting plate-bound MERS-CoV lysate antigen with convalescent-phase serum obtained from a patient with MERS, followed by mouse polyclonal serum raised against VRP-packaged MERS-CoV N or S proteins. Blocking was expressed as the percentage reduction in the reactivity of the mouse serum alone.
RESULTS
Molecular Characterization of MERS-CoV Hu/England-N1/2012
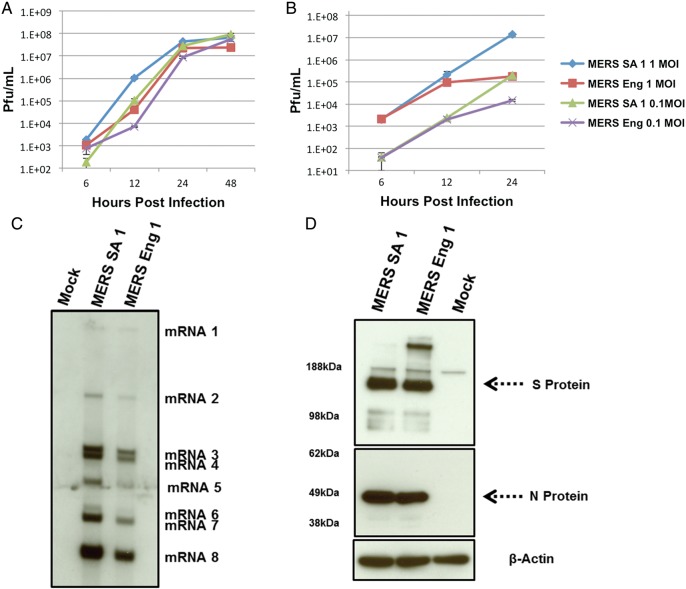
MERS-CoV Hu/England-N1/2012 (MERS Eng 1) was isolated from a 49-year-old patient with severe respiratory illness and was transferred to London for treatment [7]. Twenty-nine mutations in MERS Eng 1 at the amino acid level were identified and compared to the published sequence of MERS-CoV Hu/SA-N1/2012 (MERS SA 1; GenBank JX869059.2; Supplementary Figure S2). To identify whether these mutations altered virus growth, we analyzed the replication kinetics of the 2 isolates in Vero cells and a continuous epithelial cell line, Calu-3 (Figure 1A and 1B). Although the replication kinetics were slightly different between 2 isolates in Vero cells, peak viral titers were equivalent. In contrast, virus growth was markedly distinct in human Calu-3 cells and could have represented differences in strain-specific in vitro adaptation phenotypes or resulted from functional differences in the sensitivity to innate immune responses [20].
Figure 1.
Growth of Middle East respiratory syndrome coronavirus (MERS-CoV) Hu isolates at indicated multiplicity of infection (MOI) in Vero cells (A) and Calu-3 cells (B). Infected cultures were sampled in triplicates at times indicated, and viral titers (shown as plaque-forming units [PFU]/mL) were determined by plaque assay on Vero cells. Error bars indicate standard error of the mean. C, Northern blot analysis of RNA harvested 12 hours after infection from Vero cells infected with MERS-CoV Hu isolates at a MOI of 5. D, Western blots of lysates harvested 12 hours after infection from Calu-3 2B4 cells infected with MERS-CoV Hu isolates at a MOI of 5 that were probed with antisera to spike (S) and nucleocapsid (N) proteins. β-actin indicates loading control.
CoV replication and transcription involves production of a nested set of subgenomic messenger RNAs (mRNAs) [2], and previous reports predicted 10 open reading frames in MERS SA 1 and MERS Eng 1 [9]. Northern blot analysis identified 8 subgenomic mRNAs after infection in both viruses (Figure 1C). The observed nested set of subgenomic mRNA expression is consistent with observations for other CoVs [2, 9]. The open reading frames encoded by each mRNA in these isolates are detailed in Supplementary Table 1.
Serologic Relationships Among MERS-CoV Strains
VRPs function as efficient expression and vaccine platforms for a variety of antigens [13, 21]. We generated VRPs expressing MERS SA 1 S and N proteins and then immunized mice. N protein–specific antiserum recognized a discrete 50-kDa band at the predicted molecular weight in lysates from Vero cells (Supplementary Figure 1A) and Calu-3 cells (Figure 1D) infected with VRP-N or with the 2 different MERS-CoV isolates. For the most part, similar expression patterns were evident between VRPs and viruses; however, the N protein of MERS Eng 1 had a slightly lower molecular weight, which was consistent with amino acid deletions at positions S391 and I392 (Supplementary Figure 2).
Mouse anti-S serum identified an approximately 180-kDa S protein in VRP-S– or MERS-CoV–infected Vero cells (Supplementary Figure 1A). The observed molecular weights were consistent with the sizes of the S proteins of other CoVs [2, 22]. We noted similar results in Calu-3 cells (Figure 1D), and interestingly, an increased amount of a higher-molecular-weight form of S protein (glycosylated dimer) was noted in MERS Eng 1 in both cell lines. Antiserum against MERS SA 1 S and N proteins also recognized MERS Eng 1 in ELISAs (data not shown), and N and S proteins recognized the MERS-CoV Jordan isolate (Supplementary Figure 1B).
Cross-neutralization Patterns Across Strains
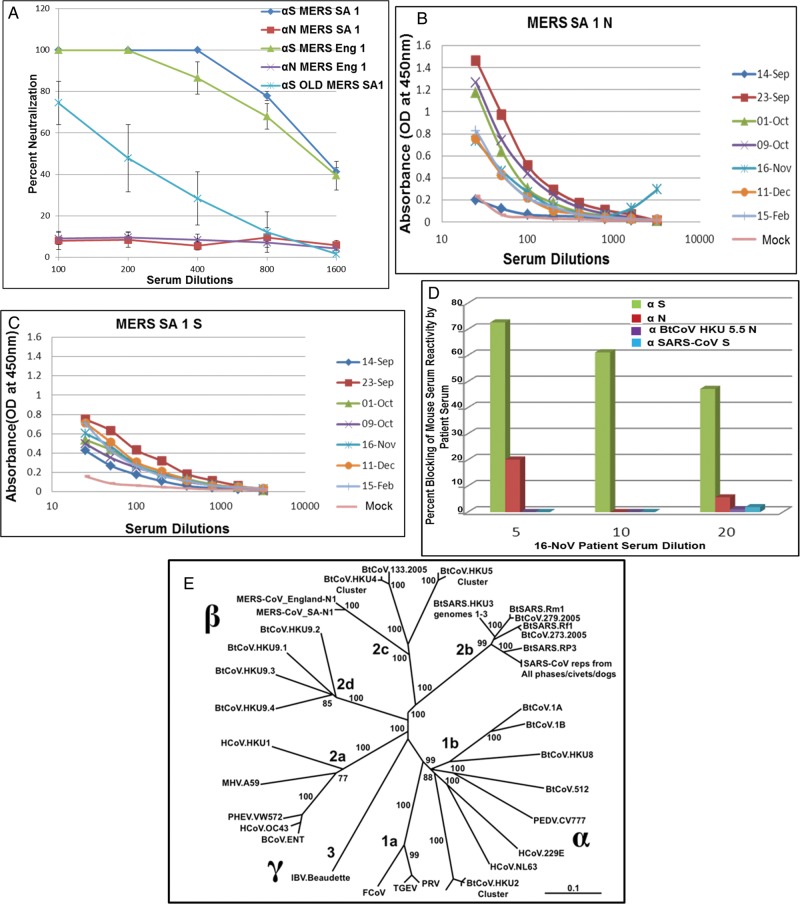
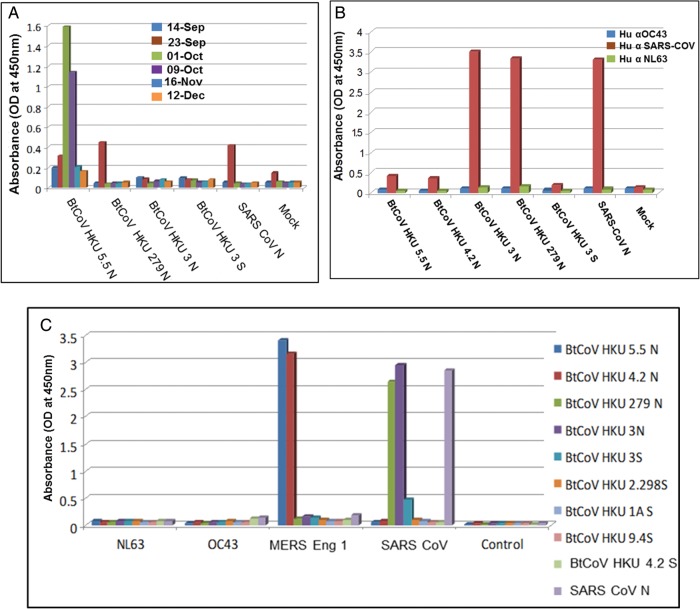
Plaque reduction neutralization tests (PRNT50) indicated complete neutralization of both MERS-CoV isolates (PRNT50 titer, approximately 1:1400 for each; Figure 2A) and the MERS-CoV Jordan isolate (Supplementary Figure1C) by VRP-S antiserum, whereas no neutralization was observed with N antiserum. Similar findings have been reported with SARS-CoV, as well as with other known human and animal CoVs [13, 23]. Interestingly, serum from aged mice vaccinated with VRP S showed a 6-fold reduction in PRNT50 titers (approximately 1:200), indicating that immunosenescence attenuates vaccine responses to MERS-CoV antigens, as was noted with SARS-CoV vaccines [13, 17]. Using serum from NA 01 patient, ELISA demonstrated high reactivity of the patient's serum to N and S antigens of MERS SA 1 expressed from VRPs (Figure 2B and 2C). Titers of antibody against N protein in patient serum peaked 3–5 weeks after onset of illness (which occurred on 3 September 2012) and waned thereafter, but the antibodies were still detected up to 5 months after illness onset. Titers of antibody against S protein were consistent from 3 weeks to 5 weeks after illness onset, after which they remained detectable. Most importantly, patient serum collected on 16 November 2012 (which contained high titers against S protein) outcompeted the binding of mouse S antiserum to intact virus in a blocking assay (Figure 2D). These data suggest that different/overlapping epitopes are recognized by human and mouse antisera following virus or VRP-S infection.
Figure 2.
A, Serum from each of 4 young and aged mice immunized with Venezuelan equine encephalitis virus replicons (VRPs) expressing spike (S) or nucleocapsid (N) proteins were tested in a plaque reduction neutralization test to neutralize Middle East respiratory syndrome coronavirus (MERS-CoV) Hu isolates. Error bars indicate standard error of the mean. B and C, NA01 patient sera collected at indicated dates after hospitalization were analyzed in an enzyme-linked immunosorbent assay, using cell lysates expressing S and N antigens from VRPs. D, Indicated dilutions of NA01 patient sera collected on November 16, 2012 were screened with 1:800 dilutions of mouse antisera to S, N, bat CoV (BtCoV) HKU 5.5 N, or SARS-CoV S in an in vitro competition assay for binding to MERS-CoV or SARS-CoV. E, The full-length genome sequences of 51 CoVs were downloaded from GenBank or PATRIC, aligned with ClustalX, and phylogenetically compared by maximum likelihood estimation, using 100 bootstraps. The tree shows that CoVs are divided into 3 distinct phylogenetic groups, defined as α, β, and γ. This taxonomic nomenclature replaced the former group 1, 2, and 3 designation, respectively. Classical subgroup clusters are marked as 2a–2d for the β-CoVs and as 1a and 1b for the α-CoVs. The tree was generated using maximum likelihood estimation with the PhyML package. The scale bar represents nucleotide substitutions. Only nodes with bootstrap support of >70% are labeled. Accession numbers and definitions of various CoV strains will be provided upon request.
Cross-reactive and Cross-neutralizing Antibody Responses Within and Across Alphacoronaviruses and Betacoronaviruses
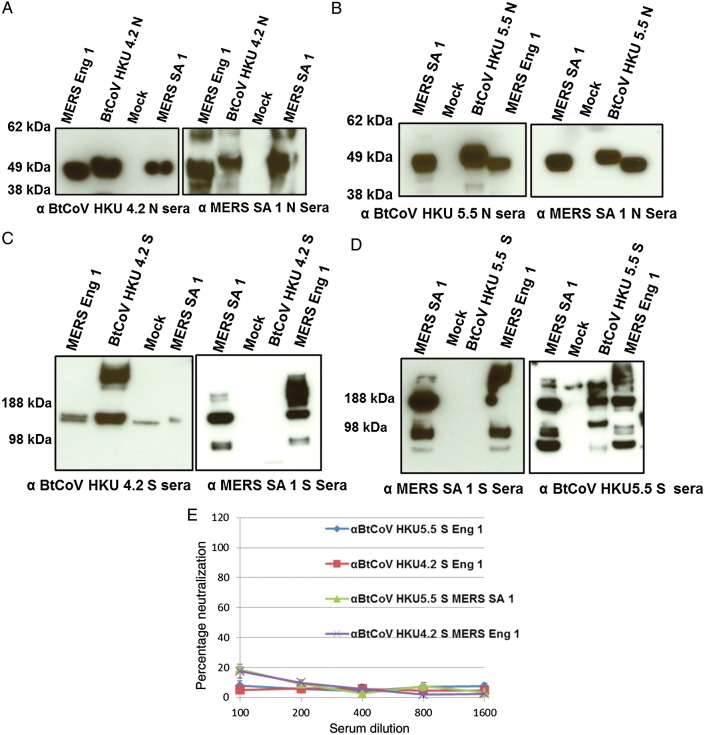
MERS SA 1 and MERS Eng 1 are closely related to BtCoV HKU 5 and BtCoV HKU 4 (Figure 2E) [6, 9, 24]. To evaluate antigenic relationships with the subgroup 2c betacoronaviruses, VRPs expressing S and N proteins of BtCoV HKU 4.2 and BtCoV HKU 5.5 were inoculated into mice. Antisera against both HKU 4.2 and 5.5 N proteins recognized the N proteins of both MERS-CoV isolates, whereas MERS SA 1 N antisera also detected the VRP-expressed HKU 4.2 and 5.5 N proteins, as revealed by Western blot (Figure 3A and 3B). We obtained similar results using ELISA and immunofluorescence assays (data not shown). In contrast, there was little if any observable cross-reactivity observed between MERS SA 1 S antisera with the VRP-expressed S proteins of HKU 4.2 and HKU 5.5, whereas antisera to HKU 5.5 S protein but not HKU 4.2 S protein recognized the S proteins of both MERS-CoV isolates (Figure 3C and 3D). We also measured serologic relationships using ELISA, which captures cross-reactivity to conformational epitopes, and confirmed these antigenic relationships (Figure 6C). Consistent with results of serologic tests, antisera against HKU 4.2 and HKU 5.5 S proteins did not cross-neutralize the MERS-CoV isolates. These data indicate that the N protein but not the S glycoprotein are antigenically conserved within the subgroup 2c betacoronaviruses evaluated in this panel.
Figure 3.
Western Blots showing cross-reactivity between nucleocapsid (N; A and B) and spike (S; C and D) proteins of Middle East respiratory syndrome coronavirus (MERS-CoV) Hu isolates and N and S proteins of bat CoV (BtCoV) HKU 4.2 and HKU 5.5 (E). Plaque reduction neutralization tests showing absence of cross-neutralization of MERS-CoV Hu isolates by antisera to BtCoV HKU 4.2 and 5.5 S proteins. Serum from groups of 4 mice immunized with Venezuelan equine encephalitis virus replicons was tested in this assay. Error bars indicate standard error of the mean. Note the cross-reactivity of antisera to BtCoV HKU 5.5 S protein to S proteins of MERS-CoV Hu isolates (D) but the absence of cross-neutralization.
Figure 6.
A, NA01 patient serum specimens collected at indicated dates were analyzed in an enzyme-linked immunosorbent assay (ELISA), using cell lysates expressing indicated antigens. B, Mouse antisera to the indicated antigens were screened in an ELISA. C, Human antisera to indicated CoVs were screened in an ELISA with cell lysates expressing indicated antigens.
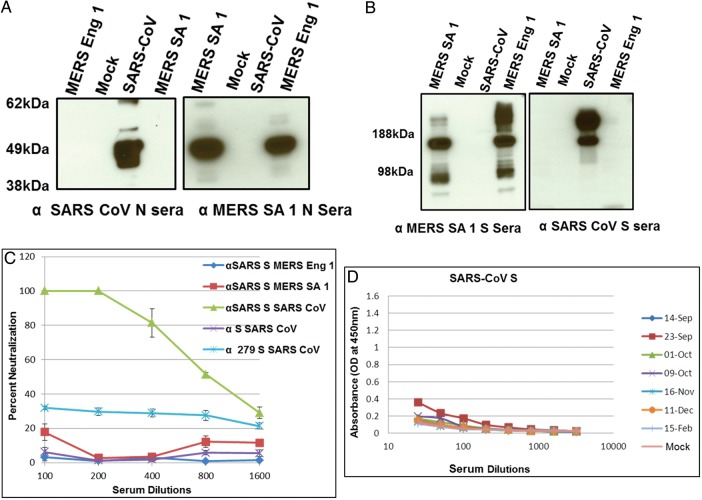
We then extended our analysis to the highly pathogenic SARS-CoV and related subgroup 2b betacoronaviruses. Polyclonal mouse sera to SARS-CoV or MERS SA 1 N or S proteins exhibited no cross-reactivity to the reciprocal strains (Figure 4A and 4B). We observed very low levels of cross-neutralization of MERS SA 1 by mouse antisera to SARS-CoV, using very high but not low concentrations of serum (Figure 4C), a finding that is consistent with a recent report [24]. Interestingly, ELISA results also showed very minimal cross-reactivity of the NA 01 patient sera obtained on 23 September 2012 to SARS-CoV S antigen (Figure 4D). Consistent with this observation, binding of mouse SARS-CoV S antiserum to SARS-CoV was not inhibited by NA01 patient sera in blocking assays (Figure 2D), indicating the absence of antibodies to SARS-CoV in the patient serum.
Figure 4.
Western Blots showing no cross-reactivity between nucleocapsid (N; A) and spike (S; B) proteins of Middle East respiratory syndrome coronavirus (MERS-CoV) Hu isolates and SARS-CoV. C, Plaque reduction neutralization tests showing the absence of cross-neutralization of MERS-CoV Hu isolates by antisera to SARS-CoV S and of SARS-CoV by antisera to MERS-CoV/SA-1/2012 S protein and BtCoV 279 S protein. Note that antisera to SARS-CoV S neutralize SARS-CoV. Serum from groups of 4 mice immunized with Venezuelan equine encephalitis virus replicons was tested in this assay and error bars indicate standard error of the mean. D, Enzyme-linked immunosorbent assay results showing the absence of reactivity of NA01 patient sera to SARS-CoV S antigen.
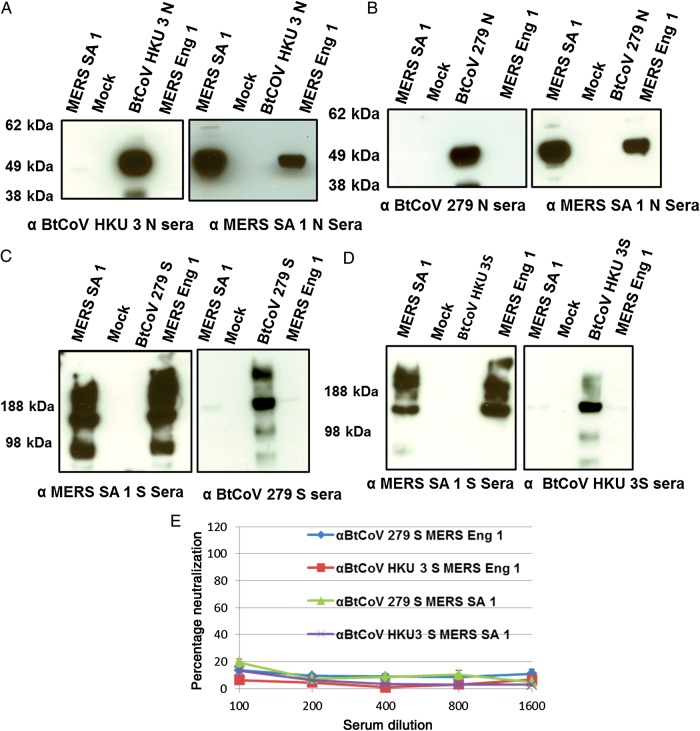
Consonant with these findings, no cross-reactivity was observed with antisera against the VRP-expressed N or S glycoproteins of BtCoV HKU 3 and 279 and the MERS-CoV isolates (Figure 5A–D). Furthermore, no cross-neutralization of MERS-CoV isolates by HKU 3S antiserum was observed, although this serum has previously been shown to neutralize a synthetically resurrected HKU 3 variant encoding the SARS S glycoprotein receptor binding domain [23]. Interestingly, we observed very low levels of cross-neutralization of SARS-CoV by BtCoV 279 S antiserum (Figure 4C).
Figure 5.
Western blots showing cross-reactivity between nucleocapsid (N; A and B) and spike (S; C and D) proteins of Middle East respiratory syndrome coronavirus (MERS-CoV) Hu isolates and N and S proteins of BtCoV 279 and HKU 3. E, Plaque reduction neutralization tests showing absence of cross-neutralization of MERS-CoV Hu isolates by antisera to BtCoV 279S and HKU 3 S proteins. Serum from groups of 4 mice immunized with Venezuelan equine encephalitis virus replicons was tested in this assay. Error bars indicate standard error of the mean.
To further elucidate the antigenic relationships between the S glycoproteins of alphacoronaviruses and the MERS-CoV isolates, we expressed and generated mouse antisera to BtCoV 1A and BtCoV HKU 2 (group 1b alphacoronaviruses), using the VRP platforms. Despite efficient recombinant S glycoprotein expression (Figure 7A and 7B), none of the recombinant S glycoproteins were recognized by MERS SA 1 S antisera. Antisera against BtCoV1A and HKU 2 S glycoproteins had little if any cross-reactivity with and did not neutralize MERS-CoV (Figure 7A–C).
Figure 7.
Western blots showing the cross-reactivity of spike (S) proteins of bat coronavirus (BtCoV) HKU 2.298 (A) and BtCoV 1A (B) with Middle East respiratory syndrome coronavirus (MERS-CoV) Hu isolates. C, Plaque reduction neutralization tests showing the absence of cross-neutralization of MERS-CoV Hu isolates by antisera. Serum from groups of 4 mice immunized with Venezuelan equine encephalitis virus replicons was tested in this assay. Error bars indicate standard error of the mean.
Antigenic Relationships Among the HCoVs
We next analyzed the antigenic relationships between VRP-derived mouse serum with the following representative HCoVs from each subgroup, using ELISA (Figure 6C): MERS Eng 1, from subgroup 2c; SARS-CoV, from subgroup 2b; HCoV-NL63, from subgroup 1b; and HCoV-OC43, from subgroup 2a. MERS Eng 1 was recognized by antisera targeting the N but not the S glycoprotein of viruses within the subgroup 2c betacoronaviruses. Likewise, SARS-CoV was only recognized by antisera to N but not S glycoproteins of viruses with the subgroup 2b betacoronaviruses. None of the antiserum screened reacted with HCoV-NL63 (subgroup 1b) or HCoV-OC43 (subgroup 2a). Although BtCoV HKU 2 is genetically close to HCoV-NL63, we did not observe any cross-reactivity within the S glycoprotein.
Serum from patients infected with SARS-CoV, HCoV-NL63, or HCoV-OC43 was screened against the N proteins from representative subgroup 2c and 2d betacoronaviruses. Consistent with our previous findings, human serum to SARS-CoV recognized BtCoV HKU 3N, BtCoV 279N, and SARS-CoV N (subgroup 2b) but did not recognize N proteins from other subgroups (Figure 6B). Similarly, there was no cross-reactivity of the human antisera from HCoV-NL63 (subgroup 1b) and HCoV-OC43 (subgroup 2a) infections with any of the viral antigens within the panel. Serum collected from the patient infected with MERS-CoV NA01 showed cross-reactive binding only to BtCoV HKU 5.5 N (subgroup 2c), and little if any cross-reactivity was noted outside the subgroup (Figure 6A), apart from very low, transient cross-detection of BtCoV 279 N and from cross-reactivity to SARS-CoV S and N recombinant proteins on a single day (23 September 2012).
DISCUSSION
Emerging respiratory CoVs offer a considerable threat to the health of global populations and the economy. Platforms for generating well-characterized molecular reagents and recombinant viruses are needed to detect and control the emergence of new strains, especially early in an outbreak, before the development of type-specific serologic reagents and therapeutics. Here, we characterized the genome organization, subgenomic mRNA expression, and protein expression patterns of 2 isolates of MERS-CoV. Using alphavirus replicon particles and synthetic gene design, we assembled a panel of recombinant proteins from and donor antisera against phylogenetically distant alphacoronaviruses and betacoronaviruses to evaluate the antigenic relationships between strains and to inform vaccine design. MERS-CoV is a highly pathogenic respiratory CoV of humans, causing acute respiratory distress syndrome, with mortality rates approaching 44%. CoV primer sets were not successful in diagnosing the etiology of the Jordan outbreak in April 2012, demonstrating a critical need for paneled reagent sets of recombinant proteins and sera that allow for serologic evaluations of cases, contact cases, and asymptomatic infections, using Western blot or ELISA-based techniques [25]. This article is also the first report that describes the serologic characterization of MERS-CoV and CoV reagents. An advantage of the VRP platform is that it can also function as a vaccine vector, affording the rapid production of candidate vaccines against newly emerged strains [23]. Using SARS-CoV and MERS-CoV as models, we clearly demonstrated that S protein–based recombinant vaccines elicit robust neutralization responses in young and aged rodent models. Because VRP-S vectors against SARS-CoV protected young and aged animals [17], we have developed a recombinant S vectored vaccine that could likely prove successful in preventing heterologous MERS-CoV infection in aged individuals, but this remains to be tested.
MERS Eng 1 replicated to lower titers than SA1 in Calu-3 cells. As the 2 viruses have different passage histories in vitro, tissue culture adaptive mutations may account for these differences, as reported with many SARS-CoV isolates [26]. Alternatively, 29 amino acid differences have been described, most of which reside in the replicase polyprotein (Supplementary Figure 2) and may affect replication efficiency. In addition, the S glycoprotein of MERS Eng 1 differs from that of MERS SA 1 by 2 amino acids, L506F and Q1020H (Supplementary Figure 2), which may account for the increased amount of the higher-molecular-weight form of S protein in MERS Eng 1 (Figure 1D and Supplementary Figure 1A). Recent studies indicate that TMPRSS2 likely plays important roles in viral entry by enhancing fusogenic potential through proteolytic processing of MERS-CoV S glycoprotein [22]. In addition, we identified a unique mutation, T1015N, in the MERS SA 1 isolate but not in the MERS Eng 1 isolate and showed that this mutation is responsible for increased in vitro fitness and for plaque morphology [20]. It is possible that the presence or absence of 1 or more of the S glycoprotein mutations in MERS Eng 1 may result in the slower growth phenotype in Calu3 cells.
Alphavirus VRPs have considerable potential as recombinant virus vaccine platforms in the absence of preexisting immunity [27–30]. We demonstrate efficient expression of several CoV S and N structural proteins both in vitro and in vivo, resulting in robust serologic responses in vaccinated mice. Antiserum to VRP-S glycoprotein but not to VRP-N protein neutralized both isolates of MERS-CoV. Furthermore, we and others have demonstrated that vaccine-induced immunopathology observed after challenge is minimized in VRP-S protein–based vaccines, partly because of the T-helper type 1–biased immune response and high neutralization titers elicited by VRP vectors [13, 31]. Importantly, vaccination of aged mice demonstrated that immunosenescence contributes to a reduction in the magnitude of the antibody response to MERS-CoV S glycoprotein, an important point to be considered in candidate vaccine designs. To date, wild-type and VRP 3526–coated VRP-S vaccines represent one of the few vaccine platforms that functioned well in aged animals, in addition to recombinant subunit–based vaccines and poxvirus-vectored vaccines [21, 32–34]. In SARS-CoV pathogenesis, increased age-related susceptibility is linked to increased prostaglandin D2 expression; it remains uncertain whether increased prostaglandin D2 levels have contributed to reduced vaccine performance, as well [35]. Because we have not observed MERS-CoV replication in immunocompetent and immunocompromised mice, these vectors must be tested directly in primates [36]. The safety of the VRP platform has been demonstrated in high-risk human populations and immunosenescent nonhuman primates [27, 28, 37, 38], and we believe that these vectors will be efficacious in healthcare workers and target populations infected with MERS-CoV.
Our results indicate the presence of strongly cross-reactive epitopes in the N protein within a particular subgroup but not between subgroups. Under identical conditions, little cross-reactivity or conservation of cross-neutralizing epitopes was observed between S proteins within and across subgroups. Similar studies showing strong conservation of cross-reactive epitopes between N proteins, but to a lesser extent between S proteins of the subgroup 2a CoVs, has been reported [39, 40]. Importantly, the pattern of serologic and antigenic relationship observed using the mouse antisera was recapitulated using the human antiserum to 4 different CoVs. Neutralization assays demonstrated little if any conservation of cross-neutralizing epitopes between S glycoproteins of CoVs within and across subgroups. In particular, the absence of cross-neutralization of MERS-CoV isolates by antiserum to HKU 4 or HKU 5 S glycoprotein and of SARS-CoV by antiserum to the HKU 3 or BtCoV 279 S glycoprotein suggests very limited conservation or, possibly, the deliberate masking of conserved cross-neutralizing sites within a subgroup. Although speculative, these cross-neutralization relationships suggest that at least 3 antigenically distinct CoVs could emerge from zoonotic viruses circulating within subgroup 1a/b, 2b, and 2c reservoirs and then simultaneously circulate in humans. These findings are evidence that vaccine design for any new emerging CoV should either focus on the development of chimeric S glycoproteins containing neutralizing epitopes from multiple strains within or across subgroups or on the development of new paradigms in structure-guided antigen design that improve the presentation of broadly neutralizing epitopes. Regions of S glycoprotein are interchangeable between CoVs within and across subgroups, rendering viable recombinant viruses [23]. Inclusion of N protein in such chimeric vaccines may broaden the protective response, although this remains to be tested using lethal challenge viruses. Such a vaccine might provide robust protection against several homologous and heterologous viruses within or across genoclusters.
After the SARS-CoV epidemic and in stark contrast to the situation with emerging influenza viruses such as influenza A(H7N9), the research and biomedical communities failed to develop broadly applicable biopreparedness platforms for rapid response against future emerging CoV threats. Because CoVs have demonstrated an accelerating pattern of zoonotic emergence since the 1980s [25, 41], our data indicate that an appropriate diagnostic platform should include a large panel of phylogenetically distinct CoV S and N structural proteins, which must be validated using larger panels of antisera against other HCoVs in the general population. While molecular-based platforms like polymerase chain reaction and deep sequencing offer clear advantages in early detection of active infections, public health response platforms would be strengthened by the availability of recombinant proteins and subgroup- and type-specific antisera that can track subclinical infections, determine the prevalence of infection in populations, and identify hospital-acquired infections. A recent report identified subclinical cases of MERS-CoV infection through reverse-transcription polymerase chain reaction, and the screen using the panel of recombinant proteins described here would have provided more-specific information about the presence of other CoVs in these cases [42]. The VRP platform we describe not only yields high-level expression of key recombinant proteins across the alphacoronaviruses and betacoronaviruses, it also provides the first candidate vaccine vectors with the potential to augment the T-helper type 1–based immune responses to MERS-CoV infection and to reduce associated immune pathology. The VRP 3526–associated approach is also applicable to improving the public health response to and the control of future outbreaks of other highly pathogenic emerging infectious diseases due to CoV in human populations.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the University of North Carolina–Chapel Hill genome analysis facility and Dr Mark Heise at Carolina Vaccine Institute, for providing sequencing services and sharing laboratory space for animal experiments, respectively; Dr Michael Cooper at the Armed Forces Health Surveillance Center, Dr Emad Mohareb at Navy Medical Research Unit 3, and Dr Kanta Subbarao at the National Institute of Allergy and Infectious Diseases, for providing us with MERS-CoV Hu/Jordan-N3/2012.
Financial support. This work was supported by the National Institutes of Allergy and Infectious Diseases, National Institute of Health (grants AI085524, AI057157, and U19 AI107810) and Public Health of England (formerly, Health Protection Agency, England).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Graham RL, Baric RS. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. J Virol. 2010;84:3134–46. doi: 10.1128/JVI.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyrc K, Sims AC, Dijkman R, et al. Culturing the unculturable: human coronavirus HKU1 infects, replicates, and produces progeny virions in human ciliated airway epithelial cell cultures. J Virol. 2010;84:11255–63. doi: 10.1128/JVI.00947-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–73. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–9. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 6.Lau SK, Li KS, Tsang AK, et al. Genetic characterization of Betacoronavirus lineage C viruses in bats revealed marked sequence divergence in the spike protein of Pipistrellus bat coronavirus HKU5 in Japanese pipistrelle: implications on the origin of the novel Middle East respiratory syndrome coronavirus. J Virol. 2013;87:8638–50. doi: 10.1128/JVI.01055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermingham A, Chand MA, Brown CS, et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:20290. [PubMed] [Google Scholar]
- 8.Huynh J, Li S, Yount B, et al. Evidence supporting a zoonotic origin of human coronavirus strain NL63. J Virol. 2012;86:12816–25. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Boheemen S, de Graaf M, Lauber C, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3:e00473-12. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller MA, Raj VS, Muth D, et al. Human coronavirus EMC does not require the SARS-coronavirus receptor and maintains broad replicative capability in mammalian cell lines. MBio. 2012;3:e00515-12. doi: 10.1128/mBio.00515-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolles M, Donaldson E, Baric R. SARS-CoV and emergent coronaviruses: viral determinants of interspecies transmission. Curr Opin Virol. 2011;1:624–34. doi: 10.1016/j.coviro.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlman S, Zhao J. Human coronavirus EMC is not the same as severe acute respiratory syndrome coronavirus. MBio. 2013;4:e00002-13. doi: 10.1128/mBio.00002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deming D, Sheahan T, Heise M, et al. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3:e525. doi: 10.1371/journal.pmed.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockx B, Corti D, Donaldson E, et al. Structural basis for potent cross-neutralizing human monoclonal antibody protection against lethal human and zoonotic severe acute respiratory syndrome coronavirus challenge. J Virol. 2008;82:3220–35. doi: 10.1128/JVI.02377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan RW, Chan MC, Agnihothram S, et al. Tropism and innate immune responses of the novel human betacoronavirus lineage C virus in human ex vivo respiratory organ cultures. J Virol. 2013;87:6604–14. doi: 10.1128/JVI.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Josset L, Menachery VD, Gralinski LE, et al. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. MBio. 2013;4:e00165-13. doi: 10.1128/mBio.00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheahan T, Whitemore A, Long K, et al. Successful vaccination strategies that protect aged mice from lethal challenge from influenza virus and heterologous severe acute respiratory syndrome coronavirus. J Virol. 2011;85:217–30. doi: 10.1128/JVI.01805-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaldson EF, Sims AC, Graham RL, Denison MR, Baric RS. Murine hepatitis virus replicase protein nsp10 is a critical regulator of viral RNA synthesis. J Virol. 2007;81:6356–68. doi: 10.1128/JVI.02805-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Höschler K, Gopal R, Andrews N, et al. Cross-neutralisation of antibodies elicited by an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine in healthy adults against H5N1 clade 2 strains. Influenza Other Respi Viruses. 2007;1:199–206. doi: 10.1111/j.1750-2659.2007.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scobey T, Yount BL, Sims AC, et al. Reverse genetics with a full-length infectious cDNA of the Middle East respiratory syndrome coronavirus. Proc Natl Acad Sci U S A. 2013;110:16157–62. doi: 10.1073/pnas.1311542110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheahan T, Whitmore A, Long K, et al. Successful vaccination strategies that protect aged mice from lethal challenge from influenza virus and heterologous severe acute respiratory syndrome coronavirus. J Virol. 2011;85:217–30. doi: 10.1128/JVI.01805-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gierer S, Bertram S, Kaup F, et al. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J Virol. 2013;87:5502–11. doi: 10.1128/JVI.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker MM, Graham RL, Donaldson EF, et al. Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. Proc Natl Acad Sci U S A. 2008;105:19944–9. doi: 10.1073/pnas.0808116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan KH, Chan JF, Tse H, et al. Cross-reactive antibodies in convalescent SARS patients’ sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. J Infect. 2013;67:130–40. doi: 10.1016/j.jinf.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giménez LG, Rojas J, Rojas A, Mendoza J, Camacho AG. Development of an enzyme-linked immunosorbent assay-based test with a cocktail of nucleocapsid and spike proteins for detection of severe acute respiratory syndrome-associated coronavirus-specific antibody. Clin Vaccine Immunol. 2009;16:241–5. doi: 10.1128/CVI.00252-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vega VB, Ruan Y, Liu J, et al. Mutational dynamics of the SARS coronavirus in cell culture and human populations isolated in 2003. BMC Infect Dis. 2004;4:32. doi: 10.1186/1471-2334-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slovin SF, Kehoe M, Durso R, et al. A phase I dose escalation trial of vaccine replicon particles (VRP) expressing prostate-specific membrane antigen (PSMA) in subjects with prostate cancer. Vaccine. 2013;31:943–9. doi: 10.1016/j.vaccine.2012.11.096. [DOI] [PubMed] [Google Scholar]
- 28.Wecker M, Gilbert P, Russell N, et al. Phase I safety and immunogenicity evaluations of an alphavirus replicon HIV-1 subtype C gag vaccine in healthy HIV-1-uninfected adults. Clin Vaccine Immunol. 2012;19:1651–60. doi: 10.1128/CVI.00258-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fillis CA, Calisher CH. Neutralizing antibody responses of humans and mice to vaccination with Venezuelan encephalitis (TC-83) virus. J Clin Microbiol. 1979;10:544–9. doi: 10.1128/jcm.10.4.544-549.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesh RB, Gajdusek DC, Garruto RM, Cross JH, Rosen L. The distribution and prevalence of group A arbovirus neutralizing antibodies among human populations in Southeast Asia and the Pacific islands. Am J Trop Med Hyg. 1975;24:664–75. doi: 10.4269/ajtmh.1975.24.664. [DOI] [PubMed] [Google Scholar]
- 31.Tseng CT, Sbrana E, Iwata-Yoshikawa N, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7:e35421. doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolles M, Deming D, Long K, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85:12201–15. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Yehuda A, Ehleiter D, Hu AR, Weksler ME. Recombinant vaccinia virus expressing the PR/8 influenza hemagglutinin gene overcomes the impaired immune response and increased susceptibility of old mice to influenza infection. J Infect Dis. 1993;168:352–7. doi: 10.1093/infdis/168.2.352. [DOI] [PubMed] [Google Scholar]
- 34.Asanuma H, Hirokawa K, Uchiyama M, et al. Immune responses and protection in different strains of aged mice immunized intranasally with an adjuvant-combined influenza vaccine. Vaccine. 2001;19:3981–9. doi: 10.1016/s0264-410x(01)00129-3. [DOI] [PubMed] [Google Scholar]
- 35.Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–30. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munster VJ, de Wit E, Feldmann H. Pneumonia from human coronavirus in a macaque model. N Engl J Med. 2013;368:1560–2. doi: 10.1056/NEJMc1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fine DL, Roberts BA, Teehee ML, et al. Venezuelan equine encephalitis virus vaccine candidate (V3526) safety, immunogenicity and efficacy in horses. Vaccine. 2007;25:1868–76. doi: 10.1016/j.vaccine.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 38.Fine DL, Roberts BA, Terpening SJ, Mott J, Vasconcelos D, House RV. Neurovirulence evaluation of Venezuelan equine encephalitis (VEE) vaccine candidate V3526 in nonhuman primates. Vaccine. 2008;26:3497–506. doi: 10.1016/j.vaccine.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 39.Dea S, Verbeek AJ, Tijssen P. Antigenic and genomic relationships among turkey and bovine enteric coronaviruses. J Virol. 1990;64:3112–8. doi: 10.1128/jvi.64.6.3112-3118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogue BG, King B, Brian DA. Antigenic relationships among proteins of bovine coronavirus, human respiratory coronavirus OC43, and mouse hepatitis coronavirus A59. J Virol. 1984;51:384–8. doi: 10.1128/jvi.51.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao Z, Liu L, Du L, et al. Potent and persistent antibody responses against the receptor-binding domain of SARS-CoV spike protein in recovered patients. Virol J. 2010;7:299. doi: 10.1186/1743-422X-7-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Memish ZA, Zumla AI, Assiri A. Middle east respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369:884–6. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.