Abstract
Background
Prenatal repair of myelomeningocele, the most common form of spina bifida, may result in better neurologic function than repair deferred until after delivery. We compared outcomes of in utero repair with standard postnatal repair.
Methods
We randomly assigned eligible women to undergo either prenatal surgery before 26 weeks of gestation or standard postnatal repair. One primary outcome was a composite of fetal or neonatal death or the need for placement of a cerebrospinal fluid shunt by the age of 12 months. Another primary outcome at 30 months was a composite of mental development and motor function.
Results
The trial was stopped for efficacy of prenatal surgery after the recruitment of 183 of a planned 200 patients. This report is based on results in 158 patients whose children were evaluated at 12 months. The first primary outcome occurred in 68% of the infants in the prenatal-surgery group and in 98% of those in the postnatal-surgery group (relative risk, 0.70; 97.7% confidence interval [CI], 0.58 to 0.84; P<0.001). Actual rates of shunt placement were 40% in the prenatal-surgery group and 82% in the postnatal-surgery group (relative risk, 0.48; 97.7% CI, 0.36 to 0.64; P<0.001). Prenatal surgery also resulted in improvement in the composite score for mental development and motor function at 30 months (P = 0.007) and in improvement in several secondary outcomes, including hindbrain herniation by 12 months and ambulation by 30 months. However, prenatal surgery was associated with an increased risk of preterm delivery and uterine dehiscence at delivery.
Conclusions
Prenatal surgery for myelomeningocele reduced the need for shunting and improved motor outcomes at 30 months but was associated with maternal and fetal risks. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT00060606.)
Spina bifida is the most common of congenital anomalies of the central nervous system that are compatible with life. The most frequent form is myelomeningocele, characterized by the extrusion of the spinal cord into a sac filled with cerebrospinal fluid, resulting in lifelong disability. Despite folic acid fortification, the incidence of myelomeningocele has stabilized at 3.4 per 10,000 live births in the United States.1 Liveborn infants with myelomeningocelehave a death rate of approximately 10%.2-4 Long-term survivors have major disabilities, including paralysis and bowel and bladder dysfunction. Damage to the spinal cord and peripheral nerves usually is evident at birth and is irreversible despite early postnatal surgical repair. The severity of the neurologic disability in the lower limbs is correlated with the level of the injury to the spinal cord.5
Nearly all infants who are born with myelomeningocele have the Arnold–Chiari II malformation, which includes a constellation of anomalies that include hindbrain herniation (downward displacement of the medulla, fourth ventricle, and cerebellum into the spinal canal), brain-stem abnormalities, low-lying venous sinuses, and a small posterior fossa. This malformation is also associated with hydrocephalus and developmental brain abnormalities. Hydrocephalus is treated by diverting cerebrospinal fluid to the peritoneal cavity by the surgical placement of a shunt, which then requires lifelong monitoring. Surgical revisions are common to address shunt failure or infection.6 The Arnold–Chiari II malformation has well-documented effects on motor, cranial-nerve, and cognitive functions, which are compounded by the adverse effects of hydrocephalus and shunting.5
Sequential ultrasonographic images that are obtained from fetuses with myelomeningocele suggest that insults to both the central and peripheral nervous systems may be progressive. Movement in the lower limbs may be lost, and hindbrain herniation and hydrocephalus may worsen during fetal gestation.7,8 In studies in animals, prenatal coverage of a spina bifida–like lesion preserved neurologic function and improved hindbrain herniation.9-13 These data suggest a “two-hit” hypothesis in which the final neurologic deficit results from a combination of failure of neural-tube formation and spinal cord injury resulting from prolonged exposure of neural elements to the intrauterine environment.
Human prenatal myelomeningocele repair by hysterotomy was first performed in 1997, and by 2003, more than 200 fetuses had undergone the procedure. Early data suggested a dramatic improvement in hindbrain herniation in comparison with historic controls but also showed an increased maternal risk, including preterm labor and uterine dehiscence, and a substantially increased risk of fetal or neonatal death and preterm birth.14,15 We performed the Management of Myelomeningocele Study (MOMS) to compare the safety and efficacy of prenatal repair of myelomeningocele with that of standard postnatal repair.
Methods
Recruitment and Study Population
We conducted the trial at three maternal–fetal surgery centers — the Children's Hospital of Philadelphia, Vanderbilt University, and the University of California, San Francisco — together with an independent data-coordinating center at George Washington University and with the Eunice Kennedy Shriver National Institute of Child Health and Human Development. All other fetalintervention centers in the United States agreed not to perform prenatal surgery for myelomeningocele while the trial was ongoing. The trial was approved by the institutional review board at each center. The study protocol, including the statistical analysis plan and full inclusion and exclusion criteria, is available with the full text of this article at NEJM.org.
Inclusion criteria were a singleton pregnancy, myelomeningocele with the upper boundary located between T1 and S1, evidence of hindbrain herniation, a gestational age of 19.0 to 25.9 weeks at randomization, a normal karyotype, U.S. residency, and maternal age of at least 18 years. Major exclusion criteria were a fetal anomaly unrelated to myelomeningocele, severe kyphosis, risk of preterm birth (including short cervix and previous preterm birth), placental abruption, a body-mass index (the weight in kilograms divided by the square of the height in meters) of 35 or more, and contraindication to surgery, including previous hysterotomy in the active uterine segment.
Study Procedures
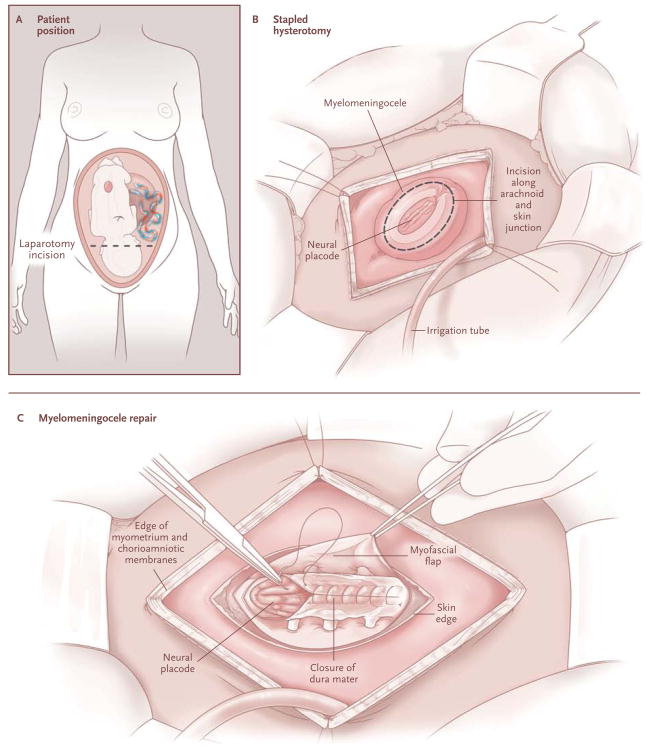
Women who were interested in the trial contacted the coordinating center, and if eligible, they were referred to one of the three clinical centers for evaluation and randomization after they provided written informed consent. Randomization to undergo either prenatal or postnatal surgery in a 1:1 ratio was accomplished on a secure Web site maintained by the coordinating center, with randomization sequences stratified according to center with the use of the simple urn method.16 For patients who were assigned to the prenatalsurgery group, myelomeningocele repair by study-approved surgeons was performed with the use of standardized operative techniques and perioperative management (Fig. 1; details are provided in the Supplementary Appendix, available at NEJM.org). Women in the prenatal-surgery group stayed nearby with a support person until cesarean delivery at 37 weeks of gestation (if still undelivered), whereas women in the postnatal-surgery group went home and returned to the center at 37 weeks for cesarean delivery and postnatal repair by the same surgical team.
Figure 1.
Prenatal Repair of Myelomeningocele.
All children were evaluated at 12 and 30 months of age on the basis of physical and neurologic examinations and developmental testing. The 12-month examination included radiography of the spine to determine the anatomical level of the lesion and magnetic resonance imaging of the head and spine. Trained independent pediatricians and psychologists who were unaware of study-group assignments and who reported directly to the coordinating center conducted the testing. Patients who were unable or unwilling to return to the center received a home visit by the follow-up team.
Primary Outcomes
Two primary outcomes were prespecified. The first outcome, at 12 months, was a composite of fetal or neonatal death or the need for a cerebrospinal fluid shunt (either placement of the shunt or meeting objective criteria for its placement) (for details, see the Supplementary Appendix). Because this trial was unmasked and criteria for shunt placement vary widely, an independent committee of neurosurgeons, who were unaware of study-group assignments, reviewed the clinical and radiologic data for each child to determine whether criteria for shunt placement were met.
The second primary outcome, at 30 months, was a composite score of the Mental Development Index of the Bayley Scales of Infant Development II and the child's motor function, with adjustment for lesion level. The anatomical level of the lesion was determined by an independent group of radiologists on the basis of the 12-month radiograph. An independent pediatrician determined the functional level of the lesion by assessing motorsensory and distal somatosensory function, a determination that was confirmed by videotape review by an independent expert. The difference between the functional level and anatomical level in vertebral segments was calculated. The Bayley scores were ranked across all infants, with fetal, neonatal, or infant deaths being assigned the lowest rank. The same procedure was separately conducted for the calculated difference between functional and anatomical level. The composite score for each infant consisted of the sum of the two ranks.17
Secondary Outcomes
Maternal, fetal, and neonatal secondary outcomes included surgical and pregnancy complications and neonatal morbidity and mortality. Infant secondary outcomes were radiographic appearance of components of the Chiari II malformation, as evaluated by independent radiologists; the time to the first shunt placement (or meeting the criteria for such placement); locomotion; the Psychomotor Development Index of the Bayley Scales; scores on the Peabody Developmental Motor Scales; the degree of functional impairment on the basis of physical examination; and the degree of disability, as measured by the WeeFIM (Functional Independence Measure for Children) instrument.
Statistical Analysis
We determined that the enrollment of 100 patients per study group would provide a power of more than 90% (with a two-sided type I error of 5%) to detect a 28% reduction in the first primary outcome, assuming an 80% shunt rate in the postnatal-surgery group among children who survived to 12 months of age,18 a 5% rate of death before shunt placement, and a 5% loss to follow-up. The reduction was calculated on the assumption of a 5% death rate and a 30% reduction in shunt placement (or meeting shunt criteria) among survivors in the prenatal-surgery group. This sample size was also sufficient to detect a difference of 0.5 SD of the composite score, with a power of more than 90%. Analyses were performed according to the intention-to-treat principle. We compared continuous variables using the Wilcoxon test and categorical variables using the chi-square test, Fisher's exact test, or the Cochran–Armitage test for trend. We analyzed the time to shunt placement (or meeting shunt criteria) using Kaplan–Meier survival curves and log-rank tests. An independent data and safety monitoring committee monitored the trial. A group sequential method was used to characterize the rate at which the type I error was spent; the chosen spending function was the Lan–DeMets characterization of the O'Brien–Fleming boundary.19
Four interim analyses were performed for each primary end point; at the fourth analysis, the critical values for the stopping boundary corresponded to a P value of 0.0233 for the 12-month outcome and 0.0123 for the 30-month outcome. For the first primary end point, we report the 97.7% confidence interval for the relative risk. For all secondary outcomes, a nominal P value of less than 0.05 was considered to indicate statistical significance, without adjustment for multiple comparisons. Relative risks and 95% confidence intervals are reported for dichotomous variables.
Results
Patients
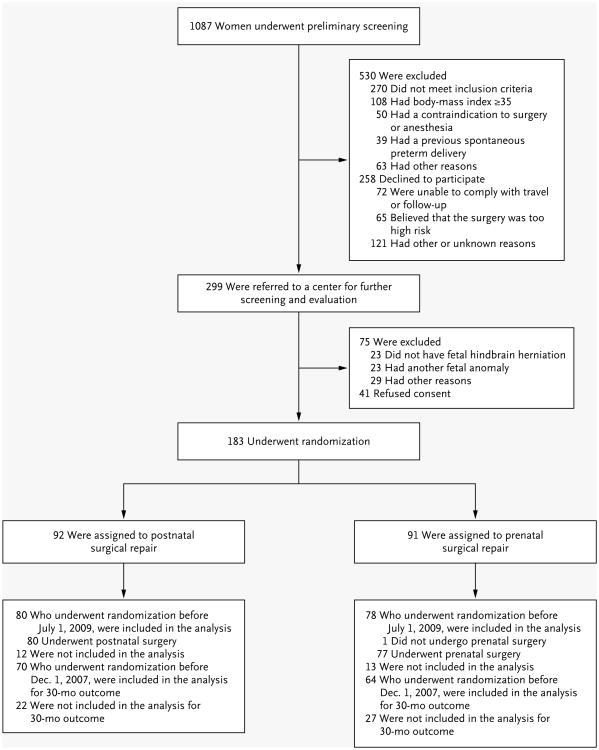
From February 2003 through December 2010, a total of 183 eligible women underwent randomization (Fig. 2). In the prenatal-surgery group, one woman chose postnatal surgery after randomization, and two women returned home for delivery. In the postnatal-surgery group, seven women chose not to return to the clinical center for delivery, and four were unable to return because of preterm labor or other complications.
Figure 2.
Enrollment and Outcomes.
The data and safety monitoring committee met on December 7, 2010, and recommended termination of the trial on the basis of efficacy of prenatal surgery. Of the 183 women who underwent randomization, this report is based on the findings in 158 women who underwent randomization before July 1, 2009 (i.e., the outcome cohort reviewed by the committee). For outcomes up to 30 months, the report is based on the findings in 134 women who underwent randomization before December 1, 2007.
Baseline characteristics were similar between groups, except that there were fewer female fetuses and the lesion level was more severe in the prenatal-surgery group (P = 0.02 for both comparisons) (Table 1).
Table 1.
Baseline Characteristics of the Study Population.*
| Characteristic | Prenatal Surgery (N = 78) | Postnatal Surgery (N = 80) |
|---|---|---|
| Fetal sex female — no. (%) | 35 (45) | 51 (64) |
| Gestational age at randomization — wk | 23.6±1.4 | 23.9±1.3 |
| Maternal age at screening — yr | 29.3±5.3 | 28.8±4.9 |
| Race or ethnic group — no. (%)† | ||
| White | 73 (94) | 74 (92) |
| Black | 1 (1) | 1 (1) |
| Hispanic | 2 (3) | 4 (5) |
| Other | 2 (3) | 1 (1) |
| Married or living with partner — no. (%) | 73 (94) | 74 (92) |
| Years of schooling — no. | 14.8±1.7 | 15.0±1.6 |
| Body-mass index at trial entry‡ | 26.2±3.7 | 25.9±3.9 |
| Current smoker — no. (%) | 6 (8) | 4 (5) |
| Either parent with familial history of neural-tube defect — no. (%) | 8 (10) | 14 (18) |
| Nullipara — no. (%) | 33 (42) | 36 (45) |
| Previous uterine surgery — no. (%) | 11 (14) | 8 (10) |
| Cervical length — mm | 38.9±7.3 | 39.7±5.7 |
| Anterior placenta — no. (%) | 36 (46) | 32 (40) |
| Lesion level on ultrasonography — no. (%) | ||
| Thoracic | 4 (5) | 3 (4) |
| L1–L2 | 21 (27) | 10 (12) |
| L3–L4 | 30 (38) | 45 (56) |
| L5–S1 | 23 (29) | 22 (28) |
| Lesion level L3 or lower on ultrasonography — no. (%) | 53 (68) | 67 (84) |
| Club foot on ultrasonography — no. (%) | 20 (26) | 15 (19) |
Plus–minus values are means ±SD. The only between-group comparisons that were significant were the female sex of the fetus and a lesion level of L3 or lower on ultrasonography (P = 0.02 for both comparisons). Percentages may not total 100 because of rounding.
Race or ethnic group was self-reported.
The body-mass index is weight in kilograms divided by the square of height in meters.
Pregnancy and Neonatal Complications
There were no maternal deaths. Pregnancy complications were more common among women in the prenatal-surgery group (Table 2). Maternal morbidity and pregnancy complications that were related to prenatal surgery included oligohydramnios, chorioamniotic separation, placental abruption, and spontaneous membrane rupture. One third of women who underwent prenatal surgery had an area of dehiscence or a very thin prenatal uterine surgery scar at the time of delivery. Fetuses that were treated prenatally were born at an average gestational age of 34.1 weeks, and 13% were delivered before 30 weeks of gestation, whereas those in the postnatal-surgery group were born at an average of 37.3 weeks of gestation with none delivered before 30 weeks.
Table 2.
Maternal and Fetal or Neonatal Outcomes.*
| Outcome | Prenatal Surgery (N = 78) | Postnatal Surgery (N = 80) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| Maternal outcome | ||||
| Chorioamniotic membrane separation — no. (%) | 20 (26) | 0 | NA | <0.001 |
| Pulmonary edema — no. (%) | 5 (6) | 0 | NA | 0.03 |
| Modified biophysical profile <8 — no. (%)† | 13 (17) | 6 (8) | 2.22 (0.89–5.55) | 0.08 |
| Oligohydramnios — no. (%) | 16 (21) | 3 (4) | 5.47 (1.66–18.04) | 0.001 |
| Placental abruption — no. (%) | 5 (6) | 0 | NA | 0.03 |
| Gestational diabetes — no. (%) | 4 (5) | 5 (6) | 0.82 (0.23–2.94) | 1.00 |
| Chorioamnionitis — no. (%) | 2 (3) | 0 | NA | 0.24 |
| Preeclampsia or gestational hypertension — no. (%) | 3 (4) | 0 | NA | 0.12 |
| Spontaneous membrane rupture — no. (%) | 36 (46) | 6 (8) | 6.15 (2.75–13.78) | <0.001 |
| Spontaneous labor — no. (%) | 30 (38) | 11 (14) | 2.80 (1.51–5.18) | <0.001 |
| Blood transfusion at delivery — no. (%) | 7 (9) | 1 (1) | 7.18 (0.90–57.01) | 0.03 |
| Status of hysterotomy site at delivery — no./total no. (%) | ||||
| Intact, well-healed | 49/76 (64) | |||
| Very thin | 19/76 (25) | |||
| Area of dehiscence | 7/76 (9) | |||
| Complete dehiscence | 1/76 (1) | |||
| Fetal or neonatal outcome | ||||
| Bradycardia during fetal or neonatal repair — no. (%) | 8 (10) | 0 | NA | 0.003 |
| Perinatal death — no. (%) | 2 (3) | 2 (2) | 1.03 (0.14–7.10) | 1.00 |
| Gestational age at birth — wk | 34.1±3.1 | 37.3±1.1 | <0.001 | |
| Gestational age at birth — no. (%) | <0.001‡ | |||
| <30 wk | 10 (13) | 0 | ||
| 30–34 wk | 26 (33) | 4 (5) | ||
| 35–36 wk | 26 (33) | 8 (10) | ||
| ≥37 wk | 16 (21) | 68 (85) | ||
| Birth weight | ||||
| Mean — g | 2383±688 | 3039±469 | <0.001 | |
| Less than 3rd percentile — no. (%) | 0 | 2 (2) | NA | 0.50 |
| Less than 10th percentile — no. (%) | 3 (4) | 7 (9) | 0.45 (0.12–1.66) | 0.33 |
| Dehiscence at repair site — no./total no. (%) | 10/77 (13) | 5/80 (6) | 2.05 (0.73–5.73) | 0.16 |
| Apnea — no./total no. (%) | 28/77 (36) | 18/80 (22) | 1.62 (0.98–2.67) | 0.06 |
| Pneumothorax — no./total no. (%) | 1/77 (1) | 1/80 (1) | 1.05 (0.07–16.53) | 1.00 |
| Respiratory distress syndrome — no./total no. (%)§ | 16/77 (21) | 5/80 (6) | 3.32 (1.28–8.63) | 0.008 |
| Patent ductus arteriosus — no./total no.(%)¶ | 3/77 (4) | 0 | NA | 0.12 |
| Sepsis — no./total no. (%)║ | 4/77 (5) | 1/80 (1) | 4.16 (0.48–36.36) | 0.20 |
| Necrotizing enterocolitis — no./total no. (%)** | 1/77 (1) | 0 | NA | 0.49 |
| Periventricular leukomalacia — no./total no. (%) | 4/77 (5) | 2/80 (2) | 2.08 (0.39–11.02) | 0.44 |
| Foot deformity — no./total no. (%) | 39/78 (50) | 36/80 (45) | 1.11 (0.80–1.54) | 0.53 |
Plus–minus values are means ±SD. There were no instances of bronchopulmonary dysplasia, pulmonary interstitial emphysema, retinopathy of prematurity, pulmonary hypoplasia, grade 3 or 4 intraventricular hemorrhage, or confirmed seizures in either group. Data for neonatal outcomes are listed for 77 infants in the prenatal-surgery group, since 1 infant was stillborn. Additional rare adverse events are provided in the Supplementary Appendix, along with adverse events for 25 additional randomized patients and their offspring (median follow-up from randomization, 29.9 weeks) who underwent randomization on or after July 1, 2009. Percentages may not total 100 because of rounding. NA denotes not applicable.
The modified biophysical profile is a test of fetal well-being that is calculated on the basis of results of ultrasonography evaluating the presence of fetal breathing, movement, and tone, along with the amniotic fluid index. The highest possible score is 8.
The between-group comparison was performed with the use of the Cochran–Armitage test for trend.
Respiratory distress syndrome was defined as a clinical diagnosis of the respiratory distress syndrome type I and the need for oxygen therapy (fraction of inspired oxygen, ≥0.40) at 24 hours of age or more.
Patent ductus arteriosus was reported if the infant was treated with medications or surgery.
Sepsis was defined as confirmation on blood culture, confirmed urinary tract infection, meningitis, or pneumonia.
Necrotizing enterocolitis was defined as a confirmed clinical diagnosis with any of the following findings observed on radiography, at the time of surgery, or at autopsy: unequivocal presence of intramural air, perforation, erythema and induration of the abdominal wall, intraabdominal abscess formation, or the formation of a stricture after an episode of suspected necrotizing enterocolitis.
The rates of adverse neonatal outcomes were generally similar between the two groups. However, one fifth of those in the prenatal-surgery group had evidence of the respiratory distress syndrome, which was probably caused by prematurity. There were no significant between-group differences in the rates of other complications of prematurity.
Two perinatal deaths occurred in each group. In the prenatal-surgery group, an intrauterine fetal death was diagnosed at 26 weeks (a stillbirth), and a neonatal death due to prematurity was diagnosed at 23 weeks; both deaths occurred on the fifth postoperative day. In the postnatal-surgery group, two neonates died, both with severe symptoms of the Chiari II malformation; both had received shunts.
First Primary Outcome
The first primary outcome, fetal or neonatal death or the need for a cerebrospinal fluid shunt by the age of 12 months, occurred in 68% of infants in the prenatal-surgery group and in 98% in the postnatal-surgery group (relative risk, 0.70; 97.7% confidence interval [CI], 0.58 to 0.84; P<0.001) (Table 3). Rates of actual shunt placement were 40% in the prenatal-surgery group and 82% in the postnatal-surgery group (P<0.001). (Additional details about the primary outcome are provided in the figure in the Supplementary Appendix.) At 12 months of age, the proportion of infants who had no evidence of hindbrain herniation was higher in the prenatal-surgery group (36%) than in the postnatal-surgery group (4%). Similarly, at 12 months, the prenatal-surgery group had a lower rate of moderate or severe hind-brain herniation (25%) than the postnatal-surgery group (67%), as well as lower rates of brain-stem kinking, abnormal fourth-ventricle location, and syringomyelia. There were no significant between group differences in the rates of identification of epidermoid cysts. Infants in the prenatal-surgery group underwent more procedures for delayed spinal cord tethering.
Table 3.
Infant Outcomes at 12 Months.*
| Outcome | Prenatal Surgery (N = 78) | Postnatal Surgery (N = 80) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| Primary outcome — no. (%) | 53 (68) | 78 (98) | 0.70 (0.58-0.84)† | <0.001 |
| Components of the primary outcome — no. (%) | <0.001 | |||
| Death before placement of shunt | 2 (3) | 0 | ||
| Shunt criteria met | 51 (65) | 74 (92) | ||
| Shunt placed without meeting criteria | 0 | 4 (5) | ||
| Placement of shunt — no. (%) | 31 (40) | 66 (82) | 0.48 (0.36–0.64) | <0.001 |
| Any hindbrain herniation — no./total no. (%) | 45/70 (64) | 66/69 (96) | 0.67 (0.56–0.81) | <0.001 |
| Degree of hindbrain herniation — no./total no. (%) | <0.001‡ | |||
| None | 25/70 (36) | 3/69 (4) | ||
| Mild | 28/70 (40) | 20/69 (29) | ||
| Moderate | 13/70 (19) | 31/69 (45) | ||
| Severe | 4/70 (6) | 15/69 (22) | ||
| Any brainstem kinking — no./total no. (%) | 14/70 (20) | 33/69 (48) | 0.42 (0.25–0.71) | <0.001 |
| Degree of brainstem kinking — no./total no. (%) | 0.001‡ | |||
| None | 56/70 (80) | 36/69 (52) | ||
| Mild | 4/70 (6) | 8/69 (12) | ||
| Moderate | 7/70 (10) | 17/69 (25) | ||
| Severe | 3/70 (4) | 8/69 (12) | ||
| Abnormal location of fourth ventricle — no./total no. (%) | 32/70 (46) | 49/68 (72) | 0.63 (0.47–0.85) | 0.002 |
| Location of fourth ventricle — no./total no. (%) | <0.001‡ | |||
| Normal | 38/70 (54) | 19/68 (28) | ||
| Low | 28/70 (40) | 29/68 (43) | ||
| At foramen magnum | 1/70 (1) | 8/68 (12) | ||
| Below foramen magnum | 3/70 (4) | 12/68 (18) | ||
| Syringomyelia — no./total no. (%) | 27/69 (39) | 39/67 (58) | 0.67 (0.47–0.96) | 0.03 |
| Epidermoid cyst — no./total no. (%) | 2/67 (3) | 1/66 (2) | 1.97 (0.18–21.20) | 1.00 |
| Surgery for tethered cord — no./total no. (%) | 6/77 (8) | 1/80 (1) | 6.15 (0.76–50.00) | 0.06 |
| Chiari decompression surgery — no./total no. (%) | 1/77 (1) | 4/80 (5) | 0.26 (0.03–2.24) | 0.37 |
| Shunt infection — no./total no. (%) | 5/77 (6) | 7/80 (9) | 0.73 (0.24–2.21) | 0.58 |
Percentages may not total 100 because of rounding.
The relative risk for the composite primary outcome is reported with a 97.7% confidence interval.
The between-group comparison was performed with the use of the Cochran–Armitage test for trend.
Second Primary Outcome
The second primary outcome — a score derived from the Bayley Mental Development Index and the difference between the functional and the anatomical level of the lesion at 30 months — was significantly better in the prenatal-surgery group than in the postnatal-surgery group (P = 0.007). (Ranges of scores and implications of higher scores are provided in Table 4.) There were two deaths(one in each group) between 12 and 30 months of age; the death in the prenatal-surgery group was from coxsackie septicemia, and that in the postnatal-surgery group was from complications of chemotherapy for choroid plexus carcinoma.
Table 4.
Outcomes of Children at 30 Months.*
| Outcome | Prenatal Surgery (N = 64) | Postnatal Surgery (N = 70) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| Primary outcome score | 148.6±57.5 | 122.6±57.2 | 0.007 | |
| Primary outcome components | ||||
| Bayley Mental Development Index† | 89.7±14.0 | 87.3±18.4 | 0.53 | |
| Difference between motor function and anatomical levels‡ | 0.58±1.94 | —0.69±1.99 | 0.001 | |
| Bayley Mental Development Index — no./total no. (%)† | ||||
| ≥50 | 60/62 (97) | 59/67 (88) | 1.10 (1.00–1.21) | 0.10 |
| ≥85 | 46/62 (74) | 45/67 (67) | 1.10 (0.88–1.38) | 0.38 |
| Difference between motor function and anatomical levels — no./total no. (%)‡ | 0.002§ | |||
| ≥Two levels better | 20/62 (32) | 8/67 (12) | ||
| One level better | 7/62 (11) | 6/67 (9) | ||
| No difference | 14/62 (23) | 17/67 (25) | ||
| One level worse | 13/62 (21) | 17/67 (25) | ||
| ≥Two levels worse | 8/62 (13) | 19/67 (28) | ||
| Bayley Psychomotor Development Index† | ||||
| Mean | 64.0±17.4 | 58.3±14.8 | 0.03 | |
| ≥50 — no./total no. (%) | 29/62 (47) | 23/67 (34) | 1.36 (0.89–2.08) | 0.15 |
| ≥85 — no./total no. (%) | 10/62 (16) | 4/67 (6) | 2.70 (0.89–8.17) | 0.06 |
| Peabody Developmental Motor Scales¶ | ||||
| Stationary score | 7.4±1.1 | 7.0±1.2 | 0.04 | |
| Locomotion score | 3.0±1.8 | 2.1±1.5 | 0.002 | |
| Object manipulation score | 5.1±2.6 | 3.7±2.1 | <0.001 | |
| Walking independently on examination — no./total no. (%) | 26/62 (42) | 14/67 (21) | 2.01 (1.16–3.48) | 0.01 |
| Walking status — no./total no. (%) | 0.03 | |||
| None | 18/62 (29) | 29/67 (43) | ||
| Walking with orthotics or devices | 18/62 (29) | 24/67 (36) | ||
| Walking without orthotics | 26/62 (42) | 14/67 (21) | ||
| WeeFIM score║ | ||||
| Self-care | 20.5±4.2 | 19.0±4.2 | 0.02 | |
| Mobility | 19.9±6.4 | 16.5±5.9 | 0.003 | |
| Cognitive | 23.9±5.2 | 24.1±5.9 | 0.67 |
Plus–minus values are means ±SD. Listed are data for 134 of 136 patients who underwent randomization before December 1, 2007; data for 2 patients were not available. Before 30 months, there were 5 deaths (2 in the prenatal-surgery group and 3 in the postnatal-surgery group), so data for those infants are not included in any category except the primary-outcome score. Percentages may not total 100 because of rounding.
On the Bayley Scales of Infant Development II, the Mental Development Index and the Psychomotor Development Index are both scaled to have a population mean (±SD) of 100±15, with a minimum score of 50 and a maximum score 150. Higher scores indicate better performance.
For the difference between the motor-function level and the anatomical level, positive values indicate function that is better than expected on the basis of the anatomical level.
The between-group comparison was performed with the use of the Cochran–Armitage test for trend.
On the Peabody Developmental Motor Scales, the mean (±SD) score was 10±3, with a minimum score of 0 and a maximum score of 20. Higher scores indicate better performance.
On the WeeFIM evaluation, the score on the self-care measurement ranges from 8 to 56, and scores on the mobility and cognitive measurements range from 5 to 35, with higher scores indicating greater independence.
Secondary Outcomes
In post hoc analyses, infants in the prenatal-surgery group were more likely to have a level of function that was two or more levels better than expected according to the anatomical level (32% vs. 12%, P = 0.005) and less likely to have a level of function that was two or more levels worse than the expected level (13% vs. 28%, P = 0.03) than were infants in the postnatal-surgery group. Although the ability to walk is dependent on lesion level, children in the prenatal-surgery group were more likely than those in the postnatal-surgery group to be able to walk without orthotics or devices (42% vs. 21%, P = 0.01). On both the Bayley and Peabody motor scales, the prenatal-surgery group had better motor function than the postnatal-surgery group, even though those in the prenatal-surgery group had more severe anatomical levels of lesions. Parent-reported selfcare and mobility, as measured by the WeeFIM instrument, were significantly better in the prenatal-surgery group. There were no significant between-group differences in cognitive scores.
Discussion
As compared with postnatal surgery, prenatal surgery for myelomeningocele that was performed before 26 weeks of gestation decreased the risk of death or need for shunting by the age of 12 months and also improved scores on a composite measure of mental and motor function, with adjustment for lesion level, at 30 months of age. Prenatal surgery also improved several secondary outcomes, including the degree of hindbrain herniation associated with the Chiari II malformation, motor function (as measured by the difference between the neuromotor function level and anatomical lesion level), and the likelihood of being able to walk independently, as compared with postnatal surgery.
Despite having more severe lesions and a nearly 13% incidence of preterm delivery before 30 weeks, the prenatal-surgery group had significantly better outcomes than the postnatal-surgery group. The improvements were probably associated with the timing of the repair, which may have permitted more normal nervous-system development prenatally. Reductions in rates of shunt placement (or need for shunting) in the prenatal-surgery group were probably due to the reduction in rates of hindbrain herniation and improved flow of cerebrospinal fluid. In the case of infants with low lumbar and sacral lesions, in whom less impairment in lower-limb function may be predicted, the normalization of hindbrain position and the minimization of the need for postnatal placement of a cerebrospinal fluid shunt may be the primary indication for surgery.
Potential benefits of prenatal surgery must be balanced against the risks of prematurity and maternal morbidity. Prenatal surgery was associated with higher rates of preterm birth, intraoperative complications, and uterine-scar defects apparent at delivery, along with a higher rate of maternal transfusion at delivery. Chorioamniotic separation, which increases the risk of premature membrane rupture,20 was observed on ultrasonography in one fourth of women after prenatal surgery. Preterm labor leading to early delivery, placental abruption, and pulmonary edema associated with tocolytic therapy are wellknown complications of prenatal surgery. The assessment of the hysterotomy site at the time of delivery revealed thinning or an area of dehiscence in more than one third of the women. Since uterine dehiscence and rupture in a subsequent pregnancy are recognized risks of prenatal surgery,21 mothers who undergo prenatal surgery must understand that all subsequent pregnancies should be delivered by cesarean before the onset of labor.
Several aspects of the prenatal-surgery technique that was used in this trial warrant comment. All surgeons used a stapling device with absorbable staples for uterine entry. This approach minimizes blood loss and, in contrast with the use of metal staples, does not impair subsequent fertility.21-23 In all cases, a multidisciplinary team of experts followed a standard protocol to perform fetal surgery. The results of this trial should not be generalized to patients who undergo procedures at less experienced centers or who do not meet the eligibility criteria. For example, a body-mass index of 35 or more was an exclusion criterion for safety reasons, even though obesity is common among women carrying a fetus with myelomeningocele. Although the prenatal-surgery group had better outcomes than the postnatal-surgery group, not all infants benefited from the early intervention, and some had a poor neuromotor outcome. Finally, for the children in this study, continued follow-up is needed to assess whether the early benefits are durable and to evaluate the effect of prenatal intervention on bowel and bladder continence, sexual function, and mental capacity.
Previous cohort studies have suggested improved outcomes with prenatal surgery for myelomeningocele.24-28 However, since comparisons between infants who were treated in utero and historical controls are subject to substantial bias, results from a randomized trial were needed to confirm benefits and inform risks. In our study, prenatal surgery for myelomeningocele reduced the need for shunting and improved motor outcomes at 30 months, but the early intervention was associated with both maternal and fetal morbidity.
Supplementary Material
Acknowledgments
Supported by grants (U10 HD041666, U01HD041665, U10 HD041667, and U10 HD041669) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by grants (UL1-RR-024134, UL1-RR-024131, and UL1-RR-024975) from the Clinical and Translational Science Awards, National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the women and their children and families who participated in this study; the Society for Maternal–Fetal Medicine for its support throughout trial recruitment; and practitioners in the fetal-therapy community for their cooperation in withholding prenatal surgery during the study.
References
- 1.Boulet SL, Yang Q, Mai C, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol. 2008;82:527–32. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- 2.Shurtleff DB, Luthy DA, Nyberg DA, et al. Meningomyelocele: management in utero and post natum. Ciba Found Symp. 1994;181:270–86. doi: 10.1002/9780470514559.ch16. [DOI] [PubMed] [Google Scholar]
- 3.Hunt GM. The median survival time in open spina bifida. Dev Med Child Neurol. 1997;39:568. [PubMed] [Google Scholar]
- 4.Manning SM, Jennings R, Madsen JR. Pathophysiology, prevention and potential treatment of neural tube defects. Ment Retard Dev Disabil Res Rev. 2000;6:6–14. doi: 10.1002/(SICI)1098-2779(2000)6:1<6::AID-MRDD2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell LE, Adzick NS, Melchionne J, et al. Spina bifida. Lancet. 2004;364:1885–95. doi: 10.1016/S0140-6736(04)17445-X. [DOI] [PubMed] [Google Scholar]
- 6.Caldarelli M, Di Rocco C, La Marca F. Shunt complications in the first postoperative year in children with meningomyelocele. Childs Nerv Syst. 1996;12:748–54. doi: 10.1007/BF00261592. [DOI] [PubMed] [Google Scholar]
- 7.Korenromp MJ, Van Gool JD, Bruinese HW, Kriek R. Early fetal movements in myelomeningocele. Lancet. 1986;1:917–8. doi: 10.1016/s0140-6736(86)91022-6. [DOI] [PubMed] [Google Scholar]
- 8.Sival DA, Begeer JH, Staal-Schreinemachers AL, et al. Perinatal motor behaviour and neurological outcome in spina bifida aperta. Early Hum Dev. 1997;50:27–37. doi: 10.1016/s0378-3782(97)00090-x. [DOI] [PubMed] [Google Scholar]
- 9.Heffez DS, Aryanpur J, Rotellini NA, et al. Intrauterine repair of experimental surgically created dysraphism. Neurosurgery. 1993;32:1005–10. doi: 10.1227/00006123-199306000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Meuli M, Meuli-Simmen C, Hutchins GM, et al. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat Med. 1995;1:342–7. doi: 10.1038/nm0495-342. [DOI] [PubMed] [Google Scholar]
- 11.Meuli M, Meuli-Simmen C, Yingling CD, et al. Creation of myelomeningocele in utero: a model of functional damage from spinal cord exposure in fetal sheep. J Pediatr Surg. 1995;30:1028–33. doi: 10.1016/0022-3468(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 12.Paek BW, Farmer DL, Wilkinson CC, et al. Hindbrain herniation develops in surgically created myelomeningocele but is absent after repair in fetal lambs. Am J Obstet Gynecol. 2000;183:1119–23. doi: 10.1067/mob.2000.108867. [DOI] [PubMed] [Google Scholar]
- 13.Bouchard S, Davey MG, Rintoul NE, et al. Correction of hindbrain herniation and anatomy of the vermis following in utero repair of myelomeningocele in sheep. J Pediatr Surg. 2003;38:451–8. doi: 10.1053/jpsu.2003.50078. [DOI] [PubMed] [Google Scholar]
- 14.Tulipan N, Bruner JP, Hernanz-Schulman M, et al. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatr Neurosurg. 1999;31:183–8. doi: 10.1159/000028859. [DOI] [PubMed] [Google Scholar]
- 15.Sutton LN, Adzick NS, Bilaniuk LT, et al. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA. 1999;282:1826–31. doi: 10.1001/jama.282.19.1826. [DOI] [PubMed] [Google Scholar]
- 16.Wei LJ. An application of an urn model to the design of sequential controlled trials. J Am Stat Assoc. 1978;73:559–63. [Google Scholar]
- 17.O'Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–87. [PubMed] [Google Scholar]
- 18.Rintoul NE, Sutton LN, Hubbard AM, et al. A new look at myelomeningoceles: functional level, vertebral level, shunting, and the implications for fetal intervention. Pediatrics. 2002;109:409–13. doi: 10.1542/peds.109.3.409. [DOI] [PubMed] [Google Scholar]
- 19.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
- 20.Wilson RD, Johnson MP, Crombleholme TM, et al. Chorioamniotic membrane separation following open fetal surgery: pregnancy outcome. Fetal Diagn Ther. 2003;18:314–20. doi: 10.1159/000071972. [DOI] [PubMed] [Google Scholar]
- 21.Farrell JA, Albanese CT, Jennings RW, et al. Maternal fertility is not affected by fetal surgery. Fetal Diagn Ther. 1999;14:190–2. doi: 10.1159/000020918. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RD, Lemerand K, Johnson MP, et al. Reproductive outcomes in subsequent pregnancies after a pregnancy complicated by open maternal-fetal surgery (1996-2007) Am J Obstet Gynecol. 2010;203:209.e1–209.e6. doi: 10.1016/j.ajog.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Adzick NS, Harrison MR, Glick PL, et al. Fetal surgery in the primate. III. Maternal outcome after fetal surgery. J Pediatr Surg. 1986;21:477–80. doi: 10.1016/s0022-3468(86)80215-9. [DOI] [PubMed] [Google Scholar]
- 24.Johnson MP, Sutton LN, Rintoul N, et al. Fetal myelomeningocele repair: short-term clinical outcomes. Am J Obstet Gynecol. 2003;189:482–7. doi: 10.1067/s0002-9378(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 25.Johnson MP, Gerdes M, Rintoul N, et al. Maternal-fetal surgery for myelomeningocel: neurodevelopmental outcomes at 2 years of age. Am J Obstet Gynecol. 2006;194:1145–52. doi: 10.1016/j.ajog.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 26.Danzer E, Finkel R, Gerdes M, et al. The relationship of seizure activity and chronic epilepsy in early infancy and short-term neurodevelopmental outcome following fetal myelomeningocele closure. Neuropediatrics. 2010;41:140–3. doi: 10.1055/s-0030-1263164. [DOI] [PubMed] [Google Scholar]
- 27.Danzer E, Gerdes M, Bebbington MW, et al. Lower extremity neuromotor function and short-term ambulatory potential following in utero myelomeningocele surgery. Fetal Diagn Ther. 2009;25:47–53. doi: 10.1159/000197359. [DOI] [PubMed] [Google Scholar]
- 28.Danzer E, Finkel RS, Rintoul NE, et al. Reversal of hindbrain herniation after maternal-fetal surgery for myelomeningocele subsequently reduces the incidence and severity of brainstem dysfunction and cranial nerve compression. Neuropediatrics. 2008;39:359–62. doi: 10.1055/s-0029-1202835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.