Abstract
Patients with systemic lupus erythematosus (SLE) have an increased expression of type I interferon (IFN) regulated genes because of a continuous production of IFN-α. The cellular and molecular background to this IFN-α production has started to be elucidated during the last years, as well as the consequences for the innate and adaptive immune systems. Plasmacytoid dendritic cells (pDC) activated by immune complexes containing nucleic acids secrete type I IFN in SLE. Type I IFN causes differentiation of monocytes to myeloid-derived dendritic cell (mDC) and activation of auto-reactive T and B cells. A new therapeutic option in patients with SLE is, therefore, inhibition of IFN-α, and recent data from a phase I clinical trial suggests that administration of neutralizing monoclonal antibodies against anti-IFN-α can ameliorate disease activity.
Introduction
The innate immune system acts as the first line of defence against invading micro-organisms, but it also has important functions in the regulation of the adaptive immune response. A key role of the innate immune system in the etiopathogenesis of systemic lupus erythematosus (SLE) has been emphasised during the last few years, because of the observation that a majority of patients with SLE display an ongoing production of type I interferons (IFNs) with an increased expression of type I IFN–regulated genes (an IFN signature).
Type I IFNs are normally produced by plasmacytoid dendritic cells (pDC) in response to viral infections, but in SLE, these cells are also induced to synthesize IFN via Toll-like receptor (TLR) ligation by endogenous derived nucleic acids. Type I IFN contributes to loss of tolerance and activation of autoreactive T and B cells with production of autoantibodies. In this review, we will give a brief overview of the role of the type I IFN system and the dendritic cells (DC) in the etiopathogenesis of SLE. In addition, we will discuss recent data indicating that inhibition of type I IFN may have beneficial effects in SLE.
The type I interferon system
The type I IFN system comprises the molecular and cellular players involved in type I IFN production and their downstream effects. The type I IFNs consist of a large number of proteins, which are encoded by a family of 17 genes; 13 genes for the different IFN-α subtypes and single genes for IFN-β, IFN-ω, IFN-κ and IFN-ε.1 Viral DNA or RNA are the typical activators of type I IFN production, and secreted IFNs act on the type I IFN receptor (IFNAR) on target cells and induce production of proteins that inhibit viral replication. Five of the ten human TLRs, namely TLR3, 4, 7, 8 and 9, mediate type I IFN gene transcription, and these receptors are expressed either on the cell surface (TLR4) or in the endosome (TLR3, 7, 8, 9).2 TLR3 is activated by double-stranded RNA (dsRNA), TLR7 and TLR8 by single-stranded RNA (ssRNA) and TLR9 by unmethylated CpG-rich DNA. In addition, there are nucleic acid sensors in the cytosol that can mediate IFN production. These include the DNA-binding protein DNA-dependent activator of IFN-regulatory factors (DAI)3 and the two RNA helicases RIG-I and Mda5.2 Activation of the TLRs or the cytosolic nucleic acid sensors lead to phosphorylation of several transcription factors, among which IFN regulatory factor (IRF) 3, IRF5 and IRF7 are most important.
Many different cell types produce type I IFN in small quantities in response to certain RNA viruses. On the contrary, the pDC, also termed the natural interferon–producing cell (NIPC), produces very large amounts of IFN-α in response to many different micro-organisms.4 Upon activation, one single pDC can synthesize up to 109 IFN-α molecules in 12 h, which is partly because of the expression of TLR7 and TLR9, as well as IRF3, IRF5 and IRF7.5 These cells represent less than 1% of the peripheral blood mononuclear cells (PBMC) but are efficiently recruited to sites of inflammation, where they perform their many different functions.
Besides their antiviral properties, type I IFNs have profound immunomodulatory effects in the adaptive immune system. Thus, type I IFNs cause DC maturation and activation, with increased expression of major histocompatibility complex (MHC) class I and II molecules; chemokines and chemokine receptors; co-stimulatory molecules such as CD80, CD86; the B-lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL).6 This promotes development of helper T cells along the Th1 pathway, but cytotoxic T cells are in addition stimulated by type I IFNs because of an increase in DC cross-presentation and inhibition of T-cell apoptosis. Type I IFNs promote B-cell activation, differentiation, antibody production and Ig isotype class switching. Furthermore, type I IFN stimulates the production of several cytokines by natural killer (NK) cells and monocytes/macrophages/DCs, such as IFN-γ, IL-6, IL-10 and IL-15.6 Interestingly, type I IFNs also enhance the effects of IFN-γ and IL-6 and shift the effects of IL-10 from an anti-inflammatory to a more pro-inflammatory profile. Consequently, type I IFNs have many different effects on the immune system that could promote and sustain autoreactive immune responses.
The innate immune system and dendritic cells
The innate immune system, borne by cells such as granulocytes, NK cells, macrophages and proteins such as complement and cytokines, induces a variety of prompt reactions to infectious agents and other challenges. Dendritic cells (DCs) sit at the inter phase between innate and adaptive immune responses. They are a heterogeneous family of cells of haematopoietic origin specialized in capturing and processing antigens with the ultimate goal of presenting their peptides to lymphocytes and induce specific adaptive responses. DCs are found in all tissues including blood and lymphoid organs.7 In peripheral tissues, DCs are found in an immature stage and are highly efficient at capturing antigens. In response to activation signals, they mature into antigen-presenting cells. In addition to presenting peptides to T cells in the context of MHC Class I and Class II molecules, DCs present glycolipids and glycopeptides to T cells and NKT cells, as well as polypeptides to B cells. DCs can secrete a diversified panel of chemokines that attract different cell types at different times of the immune response. They also express a unique set of co-stimulatory molecules that permit the activation of naive T cells and thus allow the launching of primary immune responses.
Through the cytokines they secrete (i.e., IL-12, IL-23 or IL-10) and the surface molecules they express (i.e., OX40-L or ICOS-l), DCs can polarize naive T cells into Th1, Th2, Treg, Th17, etc.8 There are two main pathways of DC ontogeny from haematopoietic progenitor cells (HPCs). One pathway generates myeloid-derived DCs (mDCs), whereas another generates pDCs. Myeloid DCs are found in three compartments: 1) peripheral tissues, 2) secondary lymphoid organs and 3) blood. In the skin, two distinct types of mDCs are found in two distinct layers: Langerhans cells in the epidermis and interstitial DCs in the dermis. Plasmacytoid DCs circulate in the blood and migrate to secondary lymphoid organs by crossing high-endothelial venules. Blood myeloid DCs express TLR1, 2, 3, 5, 6, 8 and 10 but not TLR7 and 9.9
DCs can be activated by many agents, including microbes, dying cells, cells of the innate immune system and cells of the adaptive immune system. Pathogen-associated molecular patterns from microbes10 signal DCs through a variety of pattern-recognition receptors including TLRs;11 cell surface C-type lectins receptors and intracytoplasmic NOD-like receptors.12 Lysates of dying cells also induce the maturation of DCs,13 and some components of dying cells enhance antigen presentation by DCs leading to T-cell immunity.13,14 These endogenous activating molecules are collectively called damage-associated molecular pattern molecules.15 They include heat shock proteins, high-mobility group box 1 protein (HMGB1), β-defensin and uric acid. In the steady state, immature DCs also present self-antigens to lymphocytes in the absence of co-stimulation, thus, leading to peripheral tolerance.16
Cytokines secreted during specific immune responses against invading pathogens might interfere with DCs homeostasis and induce responses that can be responsible for tissue pathology. Indeed, alterations of DCs homeostasis have been directly implicated in various human diseases, including cancer, allergy, infections and autoimmune diseases such as type I diabetes, multiple sclerosis and SLE.8,17
The type I IFN system in SLE
Patients with SLE have increased serum levels of IFN-α, which correlate to both disease activity and severity.18 In addition, several clinical manifestations, such as skin rash, fever and leucopenia, as well as several markers of immune activation correlate to serum IFN-α levels.
However, not all patients with SLE have measurable serum levels of IFN-α, but most patients, especially early in the disease process, display an IFN signature in PBMC.19,20 The frequency of pDC in blood is reduced in patients with SLE, but activated such cells can be found in tissues where they produce IFN-α.21 The reason behind this activation of pDC is the propensity of immune complexes (IC) containing nucleic acid to trigger a type I IFN response in pDC. Such interferogenic ICs are internalised via the FcγRIIa expressed on pDC, reach the endosome and stimulate the relevant TLR with subsequent activation of transcription factors, which results in a massive IFN-α production.22 This mechanism for induction of type I IFN production has been demonstrated in vitro for both DNA- and RNA-containing IC,23 but RNA-containing ICs that trigger TLR7 seem to be especially potent as IFN-α inducers.24 Recent data suggests that HMGB1/RAGE interaction is necessary for the TLR9-induced IFN-α production by DNA-containing IC.25 The role of the TLR-independent pathways for type I IFN production in SLE is currently unclear.
Genetic studies have reported an association between SLE and genes involved in both the production and the effects of the type I IFNs. Thus, there is an association between polymorphisms in the interferon regulatory factor 5 (IRF5) and tyrosine kinase 2 (TYK2) genes and susceptibility to develop SLE.26 The transcription factor IRF5 is constitutively expressed in pDC and regulates type I IFN gene activation as outlined above, whereas the Janus kinase TYK2 binds to IFNAR and is required for signalling through this receptor.27 Recently, a haplotype in the third intron of STAT4 that encodes a transcription factor that transmits signals by type I IFN was also associated to susceptibility to SLE.28 Although these studies provide additional support for a fundamental role of the type I IFN system in SLE, it is important to note that the identified genes are involved in many different cellular functions that are critical in inflammation and immune regulation.27,29,30
DC alterations in SLE and effects on adaptive immune cells
SLE blood constitutes a DC-inducing environment because it promotes differentiation of healthy monocytes into mDCs. The DC-inducing property of SLE sera is mainly mediated through IFN-α.31 Indeed, blood SLE monocytes display DC like functions as they capture antigens and autoantigens and present them to CD4+ and CD8+ T cells. Thus, type I IFN–induced unabated DC activation could promote the expansion of autoreactive T cells. SLE DCs are characterized by their unique in-vitro ability to promote the differentiation of CD8+ T-lymphocyte in cytotoxic T lymphocytes (CTLs) able to generate nucleosomes and granzyme B-dependent autoantigens. Interestingly, terminally differentiated effector CD8+ T-lymphocytes (CCR7−, CD45RA+) are expanded in the blood of patients with SLE, and this expansion correlates with disease activity.32 These cells can induce direct tissue damage as they represent the main cell subset infiltrating the kidney in lupus nephritis, where they adopt a peri-glomerular localization. A direct correlation is found between the lupus nephritis activity score and the number of peri-glomerular infiltrating CD8+ T-lymphocytes.
Through their direct effect on B cells, type I IFNs enhance primary antibody responses to soluble proteins and induce the production of all subclasses of IgG in mice.33 IFN-α upregulates CD38, a germinal centre B-cell and plasma cell marker, on B lymphocytes and BAFF (B cell–activating factor) on monocytes and mDCs. BAFF in turn contributes to the survival of autoreactive B lymphocytes.34 In addition, IFN-α promotes the differentiation of activated B lymphocytes into plasmablasts. pDCs activated with viruses secrete IFN-α and IL-6, which permits plasmablasts to become antibody-secreting plasma cells.35 The same effect is observed when pDCs are activated with SLE ICs containing nucleic acids that bind TLRs.36,37 This could contribute to amplify the production of type I IFN and subsequently the differentiation of autoreactive plasma cells that would further secrete autoantibodies, thus, perpetuating this pathogenic loop.
The etiopathogenesis of SLE
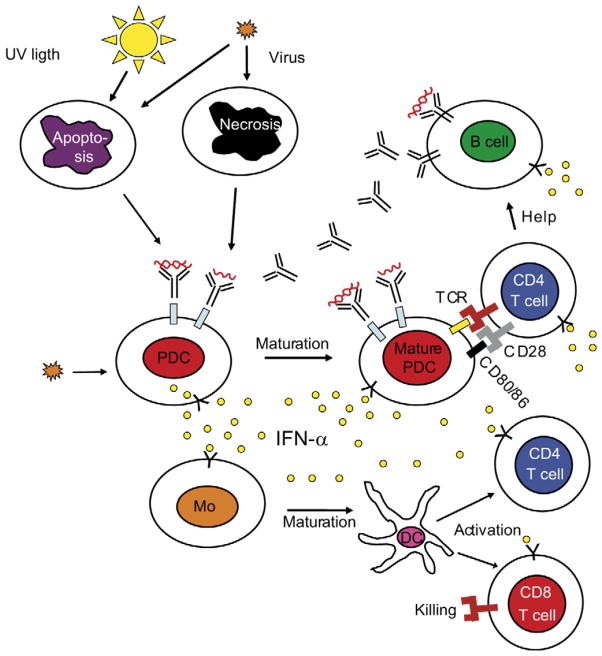
Alterations of the type I IFN system in SLE have been described more than 20 years ago. Furthermore, a plethora of literature describing the development of anti-nuclear antibodies, lupus symptoms and even full-blown SLE in humans treated with recombinant IFN-α is available. As reviewed above, type I IFNs induce and maintain the generation of mature DCs, which could tilt the fate of autoreactive T-lymphocytes that have escaped central tolerance from deletion to activation. Mature DCs activate cytotoxic CD8+ T cells that generate nucleosomes, which can be captured and presented by IFN-DCs.32 Together with IL-6, IFN promotes the differentiation of mature B cells into plasma cells. Thus, the effects of type I IFN on DCs, B and T cells could explain the break down of tolerance to nuclear antigens, autoantibody secretion and IC formation characteristic of SLE. A critical event in the etiopathogenesis is the formation of ICs containing DNA or RNA because such ICs can activate 1) B cells through the co-engagement of BCR and TLRs and 2) pDCs to secrete more IFN through the co-engagement of FcγR and TLRs. This results in an amplification of a pathogenic loop, where increased levels of autoantibodies are produced that generate more interferogenic ICs that sustain the type I IFN production (Figure 1).
Figure 1.
Schematic view of the etiopathogenesis of SLE. Viral infections induce type I interferon (IFN) production by plasmacytoid dendritic cells (pDC) and the release of autoantigens, which in susceptible individuals can break tolerance and cause production of autoantibodies against DNA and/or RNA containing autoantigens. These autoantigens together with autoantibodies will form immune complexes that can act as endogenous IFN inducers, which cause a continuous IFN-α production. Type I IFN will induce maturation of monocytes (Mo) into dendritic cells (DC), and activate B cells. IFN-induced mDCs activate both CD4 and CD8 T cells. All these events can explain the development of an autoimmune reaction as described in the text. Autoreactive B cells are also directly activated by immune complexes, which further increase the autoantibody production and sustain the autoimmune process. T-cell receptor (TCR).
In murine models of SLE, excessive IFN production only induces disease in certain genetic backgrounds, and epistatic interactions among several genes may be necessary for the disease to occur.38 This might apply to humans as well. The recently described polymorphisms in IFN-related genes (see above) may predispose to SLE by increasing the ability of pDC to release type I IFN and pro-inflammatory cytokines upon activation and by enhancing B-cell responses to these cytokines. Patients with SLE may display other genetic defects leading to alterations in autoreactive B-cell checkpoints that might be independent from IFN and DCs. These defects allow the survival of autoreactive clones into the peripheral compartment, as it has been described in children with SLE.39 Indeed, only a minor fraction of patients treated with IFN develop anti-nuclear/dsDNA antibodies, and even a smaller fraction develop SLE. Furthermore, healthy relatives of SLE patients often display antinuclear antibodies, but most of them do not develop SLE. IFN and/or IFN-DC might be necessary for the activation and differentiation of auto-reactive clones into autoantibody-secreting plasma cells, which will then further contribute to the production of IFN through IC formation and pDC activation.
Therapeutic consequences
The emerging data indicating a causal relationship between SLE and activated type I IFN system suggest that downregulation of this system could be a therapeutic approach. This assumption is supported by the observation that two effective therapeutic agents in SLE, chloroquine and glucocorticoids, inhibit IFN-α production by NIPC/pDC40 or downregulate the IFN signature.19 In addition, type I IFNAR-knock-out experimental murine lupus models have a markedly reduced SLE disease.41,42 The prime therapeutic target in SLE may be IFN-α and neutralizing monoclonal antibodies (Mab) against anti-IFN-α are currently being tested. Recently, results from a phase I clinical trial using a single injection of an anti-IFN-α Mab in patients with SLE were reported.43 There was a dose-dependent inhibition of type I IFN–inducible genes in both peripheral blood and skin biopsies, as well a reduction in clinical disease activity. No safety problems appeared during this short-term study. A more profound inhibition of the type I IFN effects can be expected if IFNAR is blocked because this approach will prevent signalling by all type I IFNs. The pDC could also be directly targeted using human Mabs directed against specific markers such as BDCA-2 or BDCA-4 because ligation of these molecules inhibits IFN-α production.44
Other possible therapeutic approaches include elimination of the DNA or RNA in endogenous IFN-α inducers. Several methods can be used to reduce the amount of endogenous IFN-α inducers. DNase treatment is one option, and this has been tried in SLE patients, with the rationale to eliminate pathogenic dsDNA/anti-dsDNA antibody ICs. However, no clear therapeutic effect was noted, but this could in theory be due to remaining RNA-containing ICs that is perhaps more important for the induction of IFN-α in SLE in vivo. The action of interferogenic IC on pDC can be prevented by blockade of FcγRIIa by specific antibodies37 or by inhibition of TLRs by oligodeoxyribonucleotide or oligoribonucleotide TLR antagonists.45 Signalling molecules downstream the TLRs, such as MyD88, IRAK-1, IRAK-4, IRF-5 and IRF-7 or molecules used by the IFNAR, for instance Tyk2 or STAT4, also represent potential therapeutic targets. As some of these molecules have diverse functions in the immune system, their inhibition could have beneficial effects besides those directly linked to type I IFN.
As adequate type I IFN production is critical in the response to certain pathogens, especially viruses, inhibition of this system could potentially increase the risk of developing severe infections.
How this would compare to the risk associated to currently used therapies, such as high dose steroids and/or cytotoxic drugs, needs further evaluation.
Acknowledgments
LR is supported by grants from the Alliance for Lupus Research, the Swedish Research Council for Medicine, the Swedish Rheumatism Association, the King Gustaf V 80-year Foundation and Ulla and Roland Gustafsson Foundation. VP is supported by Baylor Health Care System Foundation, the Alliance for Lupus Research, The National Institutes of Health (AR054083-01 CORT and AR055503-01 CORT) and the Mary Kirkland Foundation.
References
- 1.Hardy MP, Owczarek CM, Jermiin LS, Ejdeback M, Hertzog PJ. Characterization of the type I interferon locus and identification of novel genes. Genomics. 2004;84:331–345. doi: 10.1016/j.ygeno.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Akira SS, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Takaoka A, Wang Z, Choi MK, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 4.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald-Bocarsly P, Feng D. The role of type I interferon production by dendritic cells in host defense. Biochimie. 2007;89:843–855. doi: 10.1016/j.biochi.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janeway CA, Yagi J, Conrad PJ, et al. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R, Janeway CA. Decoding the patterns of self and non-self by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 12.Ueno H, Klechevsky E, Morita R, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 13.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 14.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 16.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Nat Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Bengtsson A, Sturfelt G, Truedsson L, et al. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not antiretroviral antibodies. Lupus. 2000;9:664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- 19.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blomberg S, Eloranta ML, Cederblad B, Nordlind K, Alm GV, Rönnblom L. Presence of cutaneous interferon-a producing cells in patients with systemic lupus erythematosus. Lupus. 2001;10:484–490. doi: 10.1191/096120301678416042. [DOI] [PubMed] [Google Scholar]
- 22.Rönnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54:408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 23.Lövgren T, Eloranta ML, Båve U, Alm GV, Rönnblom L. Induction of interferon-a production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 24.Lövgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Rönnblom L. Induction of interferon-a by immune complexes or liposomes containing systemic lupus erythematosus autoantigen-and Sjögren’s syndrome autoantigen-associated RNA. Arthritis Rheum. 2006;54:1917–1927. doi: 10.1002/art.21893. [DOI] [PubMed] [Google Scholar]
- 25.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 26.Sigurdsson S, Nordmark G, Göring HH, et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindler C, Levy DE, Decker T. JAK–STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 28.Remmers EF, Pleng RM, Lee AT, et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 2007;357:977–986. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan MH. STAT4: a critical regulator of inflammation in vivo. Immunol Res. 2005;31:231–242. doi: 10.1385/IR:31:3:231. [DOI] [PubMed] [Google Scholar]
- 30.Hu G, Mancl ME, Barnes BJ. Signaling through IFN regulatory factor-5 sensitizes p53-deficient tumors to DNA damage-induced apoptosis and cell death. Cancer Res. 2005;65:7403–7412. doi: 10.1158/0008-5472.CAN-05-0583. [DOI] [PubMed] [Google Scholar]
- 31.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 32.Blanco P, Pitard V, Viallard JF, Taupin JL, Pellegrin JL, Moreau JF. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:201–211. doi: 10.1002/art.20745. [DOI] [PubMed] [Google Scholar]
- 33.Le Bon A, Thompson C, Kamphuis E, et al. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176:2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 34.Litinskiy MB, Nardelli B, Hilbert DM, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 36.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. FcgRIIa is expressed on natural IFN-a-producing cells (plasmacytoid dendritic cells) and is required for the IFN-a production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 38.Lauwerys BR, Wakeland EK. Genetics of lupus nephritis. Lupus. 2005;14:2–12. doi: 10.1191/0961203305lu2052oa. [DOI] [PubMed] [Google Scholar]
- 39.Yurasov S, Wardemann H, Hammersen J, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lebon P. Inhibition of herpes simplex virus type 1-induced interferon synthesis by monoclonal antibodies against viral glycoprotein D and by lysosomotropic drugs. J Gen Virol. 1985;66:2781–2786. doi: 10.1099/0022-1317-66-12-2781. [DOI] [PubMed] [Google Scholar]
- 41.Santiago-Raber ML, Baccala R, Haraldsson KM, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braun D, Geraldes P, Demengeot J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 43.Wallac DJ, Petri M, Olsen N, et al. MEDI-545, an anti-interferon alpha monoclonal antibody, shows evidence of clinical activity in systemic lupus erythematosus. Arthritis Rheum. 2007;56:S526–S527. [Abstract 1325] [Google Scholar]
- 44.Blomberg S, Eloranta ML, Magnusson M, Alm GV, Rönnblom L. Expression of the markers BDCA-2 and -4 and production of interferon-a by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2524–2532. doi: 10.1002/art.11225. [DOI] [PubMed] [Google Scholar]
- 45.Lafyatis R, Marshak-Rothstein A. Toll-like receptors and innate immune responses in systemic lupus erythematosus. Arthritis Res Ther. 2007;9:222–228. doi: 10.1186/ar2321. [DOI] [PMC free article] [PubMed] [Google Scholar]