Abstract
Macrophages are strategically located throughout the body tissues, where they ingest and process foreign materials, dead cells and debris and recruit additional macrophages in response to inflammatory signals. They are highly heterogeneous cells that can rapidly change their function in response to local microenvironmental signals. In this Review, we discuss the four stages of orderly inflammation mediated by macrophages: recruitment to tissues; differentiation and activation in situ; conversion to suppressive cells; and restoration of tissue homeostasis. We also discuss the protective and pathogenic functions of the various macrophage subsets in antimicrobial defence, antitumour immune responses, metabolism and obesity, allergy and asthma, tumorigenesis, autoimmunity, atherosclerosis, fibrosis and wound healing. Finally, we briefly discuss the characterization of macrophage heterogeneity in humans.
The mononuclear phagocytic system is generated from committed haematopoietic stem cells located in the bone marrow. Macrophage precursors are released into the circulation as monocytes, and within a few days they seed tissues throughout the body, including the spleen, which serves as a storage reservoir for immature monocytes1. When monocytes migrate from the circulation and extravasate through the endothelium, they differentiate into macrophages or dendritic cells (DCs). Thus, the primary role of monocytes is to replenish the pool of tissue-resident macrophages and DCs in steady state and in response to inflammation. Monocytes, DCs and macrophages, along with neutrophils and mast cells, are ‘professional’ phagocytic cells. Professional phagocytes are distinguished from ‘non-professional’ phagocytes according to how effective they are at phagocytosis2. A major factor that differentiates professional and non-professional phagocytes is that professional phagocytes express a multitude of receptors on their surfaces that detect signals that are not normally found in healthy tissues. For example, scavenger receptors are responsible for binding apoptotic and necrotic cells, opsonized pathogens and cell debris. Moreover, professional phagocytes express Toll-like receptors (TLRs), but the interplay between phagocytic receptors (which initiate and assist in the mechanics of phagocytosis) and pattern recognition receptors (PRRs, such as TLRs, which detect ‘non-self ’ or ‘damage’) is complex. The interplay between these receptors is likely to involve synergistic and antagonistic interactions, including downstream signalling mechanisms within the phagocytic cell that remain largely unknown3,4.
Within the mononuclear phagocyte pool, macrophages are often distinguished from DCs by differential expression of surface makers such as F4/80 (which is encoded by EGF-like module containing, mucin-like, hormone receptor-like sequence 1 (Emr1) and is a useful marker of some but not all macrophages in the mouse), CD11b and CD18 (also known as MAC1), CD68 and Fc receptors (TABLE 1). However, few, if any, known marker combinations can definitively segregate macrophages from myeloid DCs at present because these populations exist on a continuum of development from common myeloid progenitors (BOX 1; TABLE 1).
Table 1.
Cell surface markers commonly used in macrophage research*
| Common name | Gene | Comments |
|---|---|---|
| CD11b | Itgam | Expressed on all myeloid lineage, including neutrophils |
| F4/80 | Emr1 | Expressed on most tissue macrophages in the mouse. Useful for IHC. Expression of Emr1 is regulated by numerous factors, including downregulation by interferon-γ101. Limited usefulness in humans as F4/80 is predominantly expressed on eosinophils170 |
| CD68 | Cd68 | Expressed on all macrophages. Useful for IHC, including human paraffin-embedded tissues |
| CSF1R | Csf1r | Expressed on all monocytic cells, including macrophages and osteoclasts |
| MAC2 (also known as galectin 3) | Lgals3 | Useful for IHC |
| CD11c | Itgax | Expressed on many monocytic-derived cells, including macrophages. Enriched in certain populations of dendritic cells |
| LY6G | Ly6g | Enriched on granulocytes. A useful marker system when used together with LY6C to determine relative amounts of granulocytes and monocytes or macrophages |
| LY6C | Ly6c1 | Enriched on monocytic myeloid lineages. A useful marker system when used together with LY6G to determine relative amounts of granulocytes and monocytes or macrophages. |
| IL-4Rα | Il4rα | Expressed on most macrophages, but also on lymphocytes and other cell types that are responsive to IL-4 and IL-13 |
| CD163 | Cd163 | Expressed on most tissue macrophages. Useful for IHC, including human paraffin-embedded tissues |
Csf1r, colony stimulating factor 1 receptor; Emr1, EGF-like module containing, mucin-like, hormone receptor-like sequence 1; IHC, immunohistochemistry; Il4rα, interleukin-4 receptor, alpha; Itgam, integrin alpha-M; Itgax, integrin alpha-X; Lgals3, lectin, galactose binding, soluble 3; Ly6, lymphocyte antigen 6.
Listed is a subset of markers for the mononuclear phagocyte system that is in widespread use.
Box 1 | Macrophages and dendritic cells.
A major problem in defining macrophages and myeloid dendritic cells (DCs) lies in the fact that they express common cell surface markers, as they both arise from common myeloid precursors. A widespread experimental method to separate DCs from macrophages is based on CD11c expression. However, most, if not all, macrophages express low (or intermediate) amounts of CD11c, and this complicates the interpretation of experiments with CD11c-based cell enrichment or depletion165,166. Moreover, although F4/80 is commonly used as a macrophage marker in the mouse, there are probably some cells classified as DCs that also express F4/80, as well as some macrophages that lack F4/80 expression. Thus, the cell surface marker-based separation strategies are only an enrichment for mononuclear phagocytes that have functional properties relative to DCs or macrophages (for example, antigen presentation capacity is relative to DCs rather than macrophages). Even then, ‘DCs’ and ‘macrophages’ isolated from the same organ can have identical stimulatory effects on naive T cells167, and these issues have been discussed at length168.
Another problem in characterizing myeloid cell populations stems from the existing nomenclature for DC and macrophage subsets. For example, TIP-DCs (tumour-necrosis factor/inducible nitric oxide synthase-producing DCs), which are an inflammatory population of newly recruited myeloid cells, can be identified by a surface marker combination of CD11c+CD11b+MHC class-IIhi. However, rather than ‘DCs’, these cells might be considered to be inflammatory macrophages that have been exposed to Toll-like receptor ligands and cytokines in situ, as macrophages express CD11c, and expression of MHC class-II is likely to be induced by local interferon-γ. Moreover, CD169+ subcapsular lymph node phagocytes are essential for tumour-derived antigen presentation in draining lymph nodes169, and their function (that is, good antigen presentation) is most closely associated with conventional DCs; however, they are called macrophages.
Despite these issues, macrophage and DC subset definition can be substantially refined. Lineages can best be defined by lineage-specific genes, as identified by conditional genetic deletion approaches. For example, ablation of basic leucine zipper transcriptional factor ATF-like 3 (BATF3) causes a complete deficiency in CD103+ DCs in the gut, whereas CD8+ DCs are ablated in the absence of interferon regulatory factor 8 (IRF8), nuclear factor interleukin-3-regulated protein (NFIL3) and at least six other transcription factors, while other mononuclear phagocytes remain intact1,53. Observations about the specificity of gene expression of transcription factors and cell surface proteins can be used as a platform for lineage tracing experiments: the success of CX3C-chemokine receptor 1–green fluorescent protein (CX3CR1–GFP) mice for detection of the circulating monocytes is an example of successful lineage tracing in myeloid cells, whereas notable advances have been made in dissecting the fine details of distinct origins and functional properties of the gut mononuclear phagocytes53,54,134.
In this Review, we provide an overview of the homeostatic, protective and pathogenic functions of the various macrophage subsets in health and disease, and discuss the current obstacles to the complete characterization of macrophage heterogeneity and effector function.
Tissue distribution of macrophages
Macrophages are divided into subpopulations based on their anatomical location and functional phenotype5 (FIG. 1). Specialized tissue-resident macrophages include osteoclasts (bone), alveolar macrophages (lung), histiocytes (interstitial connective tissue) and Kupffer cells (liver). The gut is populated with multiple types of macrophages and DCs, which have distinct phenotypes and functions, but work together to maintain tolerance to the gut flora and food (BOX 1). Secondary lymphoid organs also have distinct populations of macrophages that perform unique functions, including marginal zone macrophages in the spleen, which suppress innate and adaptive immunity to apoptotic cells6, and subcapsular sinus macrophages of lymph nodes (LNs), which clear viruses from the lymph and initiate antiviral humoral immune responses7,8. Distinct macrophage subpopulations also patrol so-called immune-privileged sites — such as the brain (microglia), eye and testes — where they are assumed to have central functions in tissue remodelling and homeostasis. These tissue-specific macrophage subpopulations ingest foreign materials and recruit additional macrophages from circulation during an infection or following injury.
Figure 1. Tissue macrophages perform important homeostatic functions.
Mononuclear phagocytes are generated from committed haematopoietic stem cells located in the bone marrow. Macrophage precursors are released into the circulation as monocytes and quickly migrate into nearly all tissues of the body, where they differentiate into mature macrophages. Various populations of mature tissue macrophages are strategically located throughout the body and perform important immune surveillance activities, including phagocytosis, antigen presentation and immune suppression.
Phenotype and function of macrophage subsets
Because there is great overlap in surface marker expression between the different macrophage subsets9, a useful characterization approach has been to quantify specific gene expression profiles after cytokine or microbial stimulation10 (TABLE 2). Several macrophage subsets with distinct functions have been described. Classically activated macrophages (M1 macrophages) mediate defence of the host from a variety of bacteria, protozoa and viruses, and have roles in antitumour immunity. Alternatively activated macrophages (M2 macrophages) have anti-inflammatory function and regulate wound healing. ‘Regulatory’ macrophages can secrete large amounts of interleukin-10 (IL-10) in response to Fc receptor-γ ligation11,12. Tumour-associated macrophages (TAMs) suppress antitumour immunity, and myeloid-derived suppressor cells (MDSCs) are linked to TAMs and may be their precursors13. Although there are obvious differences among the M2 macrophage, regulatory macrophage, TAM and MDSC subsets, they all exhibit immune suppressive activity14. Consequently, when stimulated, macrophages suppressive activity14. Consequently, when stimulated, macrophages adopt context-dependent phenotypes that either promote or inhibit host antimicrobial defence, antitumour immunity and inflammatory responses. It is generally believed that macrophages represent a spectrum of activated phenotypes rather than discrete stable subpopulations13. Indeed, numerous studies have documented flexibility in their programming, with macrophages switching from one functional phenotype to another in response to the variable microenvironmental signals of the local milieu15–20.
Table 2.
Combinatorial marker systems for phenotyping activated macrophages*
| Marker type | Associated signalling molecules |
Gene (alternative names) | Comments |
|---|---|---|---|
| M2 markers | STAT6 phosphorylation in vivo and ex vivo without further perturbation | Relma (Fizz1, Retnla) | Highly induced by IL-4 and IL-13. Not expressed in humans |
| Socs2 | Highly induced by IL-4 and IL-13. Not macrophage-specific | ||
| Irf4 | Highly induced by IL-4 and IL-13. Not macrophage-specific | ||
| Chia (Amcase) | Highly induced by IL-4 and IL-13. Not macrophage-specific | ||
| Chi3l1 (Gp39, Ykl40) | Highly induced by IL-4 and IL-13. Not macrophage-specific | ||
| Chi3l2 (Ykl39) | Not expressed in mice | ||
| Chi3l3(Ym1) | Not expressed in humans. Can be highly induced by IL-4 and IL-13 in some situations | ||
| Cxcl13 | Chemokine linked to TH2 cell responses | ||
| Ccl12 | Chemokine linked to TH2 cell responses | ||
| Ccl24 | Chemokine linked to TH2 cell responses | ||
| Klf4 | Transcription factor induced by IL-4 in both mouse and human macrophages171 | ||
| M1 markers |
|
Marco | Calmodulin-associated. Also found in other activation scenarios |
| Socs3 | Induced by IL-10, IL-6 and many other factors | ||
| Nos2 | Not readily expressed in human macrophages | ||
| Il12b | Highly induced in M1 activation | ||
| Ptgs2 (Cox2) | Highly induced in M1 activation | ||
| Il23α (Il23p19) | Highly induced in M1 activation | ||
| Ido1 | Useful marker of human and mouse exposure to type 1 and 2 interferons | ||
| Context-dependent markers | Arg1 | Can be induced by the STAT6 or STAT3 pathways172,173 | |
| Il10 | Differentially produced by most, if not all macrophages174 | ||
| Mrc1 | Linked with M2 macrophages but widely expressed on many macrophage subsets |
Arg1, arginase 1; Ccl, CC-chemokine ligand; Chi3l, chitinase 3-like; Chia, chitinase, acidic; Cxcl13, CXC-chemokine ligand 13; Ido1, indoleamine 2,3-dioxygenase 1; Il, interleukin; Irf4, interferon regulatory factor 4; Klf4, Krüppel-like factor 4; Marco, macrophage receptor with collagenous structure; Mrc1, mannose receptor, C type 1; Nos2, nitric oxide synthase 2, inducible; Ptgs2, prostaglandin-endoperoxide synthase 2; Relma, resistin-like molecule alpha; Socs, suppressor of cytokine signalling; STAT, signal transducer and activator of transcription; TH2, T helper 2.
Shown are marker combinations that can be used to assign phenotypic characteristics to a mouse macrophage population. The use of multiple markers, especially when combined with assays for phosphorylated STATs, avoids the problems associated with markers, such as ARG1, that are widely expressed in either M1 or M2 polarized environments.
A notable exception is the infection of macrophages by Fransicella spp. — in this case, autocrine or paracrine IL-4 and IL-13 production is enforced by a myeloid differentiation primary response protein 88 (MYD88)-dependent pathway175, and the subsequent activation of STAT6 favours bacterial survival.
Macrophage activation states
A conventional approach for studying macrophage activation in vitro is the stimulation of cells (plated on plastic) with microbial agonists or cytokines and the measurement of effector cytokine production and changes in gene expression. However, macrophage responsiveness in vivo is different. Should the vast numbers of macrophages that inhabit the colon, liver and lungs respond so readily to external stimulation, then systemic cytokine production would be continuous. Therefore, tissue macrophages, as well as newly recruited monocytes, are subject to a hierarchy of activation states that ensure baseline tissue homeostasis is the ‘default’ and prevent constant inflammation, which is the underlying cause of numerous chronic diseases.
At steady state, tissue macrophages have intrinsic anti-inflammatory functions. For example, colonic macrophages spend their existence bathed in IL-10 and mute any inflammatory response to the gut flora and their products21,22. Disruption of the normal sources or quantities of IL-10 or IL-10 signalling in immune cells leads to massive inflammation in the gut23. Another specialized macrophage type that suppresses immune responses is the marginal zone macrophages of the spleen, which are required to reduce self-reactivity to apoptotic cells6. Depletion of marginal zone macrophages leads to the formation of DNA-specific antibodies and a systemic lupus erythematosus-like autoimmune syndrome.
An initial level of macrophage activation occurs when early warning signals trigger monocyte recruitment and in situ activation or when IL-4 induces in situ macrophage proliferation24. Tissue damage sensing is probably crucial at the second level of macrophage response, regardless of whether the damage is of a microbial nature. The mechanisms of tissue damage sensing have been discussed in recent reviews25,26. Beyond the initial activation and stimulation of macrophages, cooperative actions of multiple sensors, feedforward cytokine networks and inter-organ communication increase the output of monocytes and neutrophils driving inflammatory responses. Macrophage effectors work together in cell-intrinsic and cell-extrinsic networks27. For example, the production of interferon-γ (IFNγ) by T helper 1 (TH1) cells requires IL-12 production from activated mononuclear phagocytes; IFNγ then stimulates macrophages to activate the antimicrobial arsenal28.
A key component of the next layer of the macrophage response is the production of anti-inflammatory feedback mechanisms that encompass cell-intrinsic signalling feedback loops and cell-extrinsic mechanisms, such as the production of IL-10, which is an essential and non-redundant anti-inflammatory cytokine.
The final layer of macrophage response is the least clear and involves the final decision between chronic inflammation and re-establishment of homeostasis. The understanding of the underlying mechanisms that restore homeostasis after an inflammatory reaction underpins all research efforts related to chronic inflammatory diseases.
Macrophages and tissue homeostasis
Tissue surveillance and immunosuppression
Mature macrophages are strategically located throughout the body and perform an important immune surveillance function. They constantly survey their immediate surroundings for signs of tissue damage or invading organisms and are poised to stimulate lymphocytes and other immune cells to respond when danger signals are phagocytosed and/or detected by cell surface receptors. For example, when a macrophage ingests a pathogen, the pathogen becomes trapped in a phagosome, which then fuses with a lysosome unless prevented from doing so by pathogen-specific mechanisms. Within the fused phagolysosome, enzymes and toxic free radicals digest and destroy the pathogen. In addition to fighting infections, resident tissue macrophages are involved in maintaining healthy tissues by removing dead and dying cells and toxic materials. For example, alveolar macrophages facilitate the removal of allergens from the lung, whereas Kupffer cells in liver participate in the clearance of pathogens and toxins from the circulation. Tissue macrophages also suppress inflammation mediated by inflammatory monocytes, thereby ensuring that tissue homeostasis is restored following infection or injury. Indeed, important homeostatic functions have been assigned to the mononuclear phagocytes in almost every tissue of the body (FIG. 1).
Macrophages function as sentinel cells in the tissues
Because normal cells of the body must not be mistakenly removed or compromised, macrophages are selective of the material that they phagocytose. During and following phagocytosis, PRRs (including TLRs, C-type lectin receptors (CLRs), scavenger receptors, retinoic acid-inducible gene 1 (RIG1)-like helicase receptors (RLRs) and NOD-like receptors (NLRs)) recognize signals associated with invading pathogens, foreign substances (for example, silica or asbestos) and dead or dying cells1,5. Some PRRs (such as the mannose receptor, DC-specific ICAM3-grabbing non-integrin (DC-SIGN) and macrophage receptor with collagenous structure (MARCO)) function in pathogen binding and phagocytosis, whereas signalling PRRs (which include the TLRs, NLRs and RLRs) sense microbial products and aberrant self on the cell surface or in the cytoplasm of cells and activate transcriptional mechanisms that lead to phagocytosis, cellular activation and the release of cytokines, chemokines and growth factors29–31. Macrophages also express numerous secreted molecules, including complement and Fc receptors that bind opsonin molecules, C3b and antibodies, which activate the complement cascade and enhance the process of phagocytosis by tagging the pathogen surface. Thus, macrophages use various surface receptors and secreted molecules to monitor and respond to changes in their environment.
Macrophages and tissue injury
An unanswered question in macrophage biology is whether resident mononuclear phagocyte populations of a given organ sufficiently respond to tissue stress and infection, or whether there is always a requirement for recruitment of new inflammatory cells. In many infections and tissue stress situations, the resident macrophage populations of organs such as the liver, lungs and gut are insufficient to mediate microbial control and subsequent tissue repair. Instead, monocytes enter the damaged organs and differentiate into a spectrum of mononuclear phagocytes. These newly recruited cells are pro-inflammatory, and therefore damaged tissues exist on an inflammatory tightrope where excessive production of inflammatory mediators must be balanced with the need to protect tissue integrity: this process can be considered as ‘orderly’ inflammation32. It is only recently that molecular links between bone marrow mobilization of effector monocytes and specific inflammatory reactions have been elucidated. Therefore, in this section we focus on a series of specific inflammatory responses that we consider to be informative of the general principles of orderly inflammation.
Monocyte recruitment and subsequent macrophage fate in tissues
‘Emergency myelopoiesis’ is the process of generating large pools of monocytes and neutrophils from cells in the bone marrow beyond the normal requirements of a healthy person. Tissue stress, including acute and chronic infection, as well as sterile inflammation, drives the production of monocytes and neutrophils in a process that is dependent on cytokines such as granulocyte colony stimulating factor (G-CSF) and chemokines including CC-chemokine ligand 2 (CCL2) and CCL5 (REF. 33) (FIG. 2). The increased production of monocytes and neutrophils is found in many different types of stress and can therefore be considered a common, conserved pathway. Moreover, the production of circulating MDSCs increases in cancer, but also in Crohn’s disease34, autoimmune disease35, transplantation tolerance36 and smouldering sepsis induced by caecal ligation and puncture (CLP)37. CLP-mediated induction of MDSCs is dependent on myeloid differentiation primary response protein 88 (MYD88)37 and therefore we might expect that the TLR and IL-1 receptor (IL-1R) common pathway, via MYD88, induces haematopoietic cytokines, such as G-CSF and granulocyte/macrophage colony stimulating factor (GM-CSF), that act on bone marrow precursors to increase the output of neutrophils and monocytes38,39. The MDSC pool that exits the bone marrow comprises mature and immature mixtures of monocytic and granulocytic cells, suggesting that either the capacity of the bone marrow to mature the cells is compromised or the bone marrow receives signals to expel the haematopoietic cells at an increased rate. This pathway is an example of long-range communication between the damaged site and the bone marrow to generate increased numbers of tissue macrophages.
Figure 2. Inter-organ communication is required for macrophage recruitment.
During infection and tissue stress, monocyte recruitment has a key role in providing the damaged tissues with adequate numbers of macrophages. The figure depicts an exemplar of the monocyte-to-macrophage recruitment and deposition process. Here, Leishmania major parasites that have infiltrated the skin after a sandfly bite elicit a weak local macrophage response that is insufficient to generate a protective response. The body compensates by depositing platelets on the parasite surface that release platelet-derived growth factor (PDGF). The local PDGF then increases the levels of CC-chemokine ligand 2 (CCL2), possibly by the stimulation of fibroblasts and other PDGF-responsive interstitial cells. CCL2 is a key monocyte attractant that causes monocyte efflux from the bone marrow and presumably the splenic monocyte reservoir. Extravasation of the monocytes is followed by differentiation into macrophages that phagocytose the parasites and present their antigens to T cells. Interferon-γ (IFNγ) production from T cells drives an M1 response that contains parasite growth. TH1, T helper 1.
A widely accepted view is that monocytes adopt two distinct fates after bone marrow exit1. One type of monocyte — which is defined by high expression of CX3C-chemokine receptor 1 (CX3CR1) and low expression of the myeloid marker lymphocyte antigen 6C (LY6C) (TABLE 1) — has a ‘patrolling’ function in and around the vascular endothelium1. Importantly, patrolling monocytes lack the expression of the chemokine receptor CC-chemokine receptor 2 (CCR2) and cannot respond to CCL2. A recent study has shown that the transcription factor NUR77 (encoded by nuclear receptor subfamily 4, group A, member 1 (Nr4a1)) is required for the development of patrolling monocytes40. By contrast, the LY6Chi monocyte pool is linked to inflammation, expresses CCR2 and can be rapidly mobilized1. The spleen harbours large numbers of LY6Chi monocytes in the subcapsular red pulp that rapidly emigrate to inflammatory sites41.
Multiple types of acute infections cause monocyte mobilization, including infection with influenza, Listeria monocytogenes, Toxoplasma gondii and fungi42–45. Recent results have revealed a surprising complexity to chemokine-induced monocyte recruitment. For example, in acute Citrobacter rodentium infection in the gut (a mouse model of severe Escherichia coli infection), the NLR protein nucleotide-binding oligomerization domain protein 2 (NOD2) in non-haematopoietic cells of the gut lamina propria is responsible for CCL2 production and the subsequent recruitment of large numbers of monocytes that flood the colon and become inflammatory macrophages46. This process is essential for bacterial clearance and for the restoration of tissue homeostasis because NOD2-deficient mice cannot clear the bacteria efficiently and thereby have increased bacterial loads and tissue damage.
CCL2 also drives monocyte recruitment in other settings. For example, when the protozoan parasite Leishmania major infects macrophages, it does not induce a strong inflammatory response and few, if any, chemokines and cytokines are made47. Nevertheless, L. major induces a strong inflammatory response at the infection site; it was shown that complement deposition on parasites induces platelets to accumulate at the infection site and release platelet-derived growth factor (PDGF), which stimulates local CCL2 production and thus creates a chemokine gradient to induce monocyte recruitment48 (FIG. 2). Moreover, monocyte recruitment can be initiated by low circulating amounts of TLR agonists that induce CCL2 production in bone marrow mesenchymal cells and drive inflammatory monocytes into circulation49. This mechanism presumably bypasses the splenic reservoir and is thus an example of the diverse mechanisms the body uses to produce sufficient monocytes and get them into circulation, and ultimately into tissues where they terminally differentiate into macrophages.
Differentiation of the recruited monocytes in situ
The fate of the recruited monocytes and their subsequent differentiation into macrophages is a key issue because inflammatory monocytes have the potential to cause tissue damage or even promote metastasis50. Monocytes quickly differentiate into macrophages and DCs at the site, and it remains unclear how their inflammatory activity is constrained, although IL-10 is likely to have an irreplaceable effect in suppressing activated macrophages at the damage site51.
We cannot assume that the circulating LY6Chi monocyte population is uniform. It is possible that the LY6Chi monocyte population consists of both inflammatory and regulatory populations that counter-balance each other, or the LY6Chi monocytes might convert into regulatory macrophages upon exposure to the non-inflammatory tissue mononuclear phagocytes. In this regard, it was recently shown that pre-emptive CSF1 treatment reduced graft-versus-host disease by expanding suppressive or regulatory macrophages52.
The gut has been fertile ground for research into the fate of recruited monocytes. Several groups have established that a population of gut macrophages is exclusively derived from the circulating monocyte pool, whereas another gut mononuclear phagocyte population, which is characterized by the expression of CD103, is a distinct population of resident gut DCs that have their own functional specializations in terms of promoting immune responses53,54.
During C. rodentium and T. gondii infection, the recruited monocyte population is essential to resolve acute inflammation, but must rapidly convert to an anti-inflammatory phenotype following interaction with the gut-resident macrophages in order to restrain excessive responses to the gut flora. Moreover, in the brain, it was recently shown that recruited pro-inflammatory immature LY6Chi myeloid cells convert in situ to regulatory populations that suppress T cell response55. Be it the gut or any other organ system, it remains unclear if and how monocytes differentiate at the damage site and how the overall number of mononuclear cells in an organ are controlled after homeostasis is re-established. It seems likely the underlying plasticity in myeloid lineages and conversion between pro- and anti-inflammatory activities will be a paradigm uncovered in numerous pathological scenarios.
In situ proliferation of macrophages
The textbook picture of macrophage differentiation from recruited monocytes was recently challenged by a study that demonstrated that tissue macrophages undergo massive proliferation in TH2-mediated inflammation24. In this scenario, IL-4 produced by TH2 cells is sufficient to cause local macrophage proliferation during helminth infections, resulting in increased numbers of M2 effector macrophages, which expel worms (FIG. 3). Furthermore, recruited M1 macrophages were induced to proliferate as long as sufficient IL-4 was present24. The signalling mechanism regulated by IL-4 to push macrophages into the cell cycle remains unclear, but may be related to the expression of macrophage-activating factor (MAF; also known as c-MAF) and MAFB transcription factors that suppress macrophage proliferation56.
Figure 3. In situ macrophage proliferation.
A recent discovery has shown that, contrary to previous thinking, macrophages can enter the cell cycle and proliferate locally. Thus far, in situ proliferation has been shown to be specific for T helper 2 (TH2)-type responses to worms. In the example shown, a nematode is recognized through unknown mechanisms that may involve basophils, nuocytes and other sentinel lymphocytes and granulocytes. a | Local secretion of interleukin-4 (IL-4) initiates macrophage proliferation in situ, followed by amplification of the IL-4 response, which is mediated by antigen-specific TH2 cells. b | The increase in macrophage numbers has been proposed to play an important part in both killing the worms and driving a resolving phase of the infection. The underlying mechanism of IL-4-induced proliferation may involve multiple signals from the IL-4 receptor (IL-4R), including activation of signal transducer and activator of transcription 6 (STAT6). Individually or collectively, these signals may repress macrophage-activating factor (MAF) and MAFB, causing entry into the cell cycle. c | IL-4 can also cause M1-polarized macrophages to enter the cell cycle. In this case, an M1-polarized macrophage receives dual polarizing signals that drive gene expression characteristic of both M1 and M2 macrophages. ARG1, arginase 1; MAPK, mitogen-activated protein kinase; NO, nitric oxide; PI3K, phosphoinositide 3-kinase; RELMα, resistin-like molecule-α; TNF, tumour necrosis factor.
Self-renewal of tissue macrophages is an appealing concept because it would bypass the requirement for bone marrow-generated monocytes and thus allow local sites to develop an anti-inflammatory milieu that allows for wound repair. Presumably, the expanded population of M2 macrophages would also restrain excessive T cell responses by l-arginine depletion57. However, many questions about tissue macrophage self-renewal remain unanswered. It is unclear whether tissue macrophage self-renewal occurs generally in TH2-dominated inflammation. For example, do alveolar macrophages proliferate in asthma and allergic lung inflammation? Similarly, do tissue-resident macrophages proliferate at the sites of deep tissue TH2 responses, such as at sites of schistosome egg deposition in the liver and Trichinella spiralis worm invasion in muscle? Finally, as the gut harbours the largest population of macrophages in the body, do these cells self-renew to perpetuate the necessary numbers of anti-inflammatory macrophages, or do most originate from bone marrow-derived monocytes?
Macrophage activity in response to tissue injury or infection
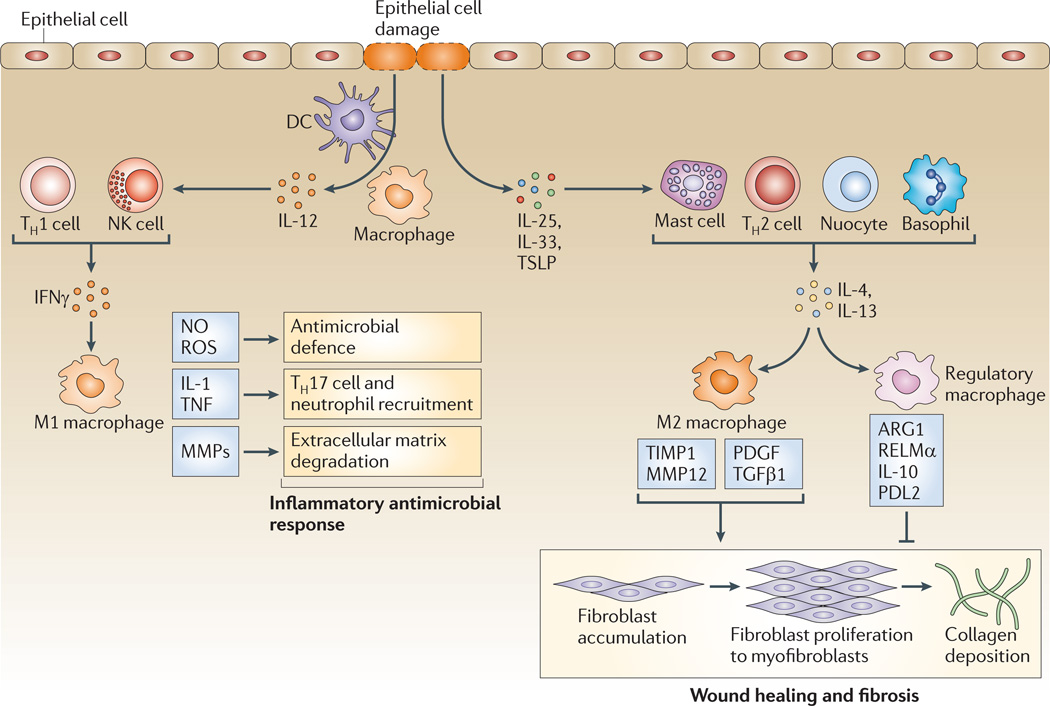
Following tissue injury or infection, the first-responder macrophages usually exhibit an inflammatory phenotype and secrete pro-inflammatory mediators such as tumour necrosis factor (TNF), nitric oxide (NO) and IL-1, which participate in the activation of various antimicrobial mechanisms, including oxidative processes that contribute to the killing of invading organisms51,58. Other mediators produced by activated macrophages include IL-12 and IL-23, which are decisive in influencing the polarization of TH1 and TH17 cells, which further drive inflammatory responses forward. Activated macrophages produce reactive oxygen and nitrogen intermediates, including NO and super-oxide, that are highly toxic for microorganisms but can also be highly damaging to neighbouring tissues and lead to aberrant inflammation32. Indeed, M1 macrophages are believed to participate in various chronic inflammatory and autoimmune diseases59 (FIG. 4). Therefore, pro-inflammatory and antimicrobial M1 macrophage responses must be controlled to prevent extensive collateral tissue damage to the host.
Figure 4. Distinct macrophage subsets regulate inflammation and wound healing.
When tissues are damaged, inflammatory mediators are released, triggering an antifibrinolytic-coagulation cascade that activates clotting and the development of a provisional extracellular matrix (ECM). Platelet activation and degranulation also promotes blood vessel dilation and increased permeability, allowing efficient recruitment of inflammatory monocytes to the site of tissue injury, where they differentiate into macrophages and become activated by various cytokines, such as interferon-γ (IFNγ), that are released from neighbouring inflammatory cells, including neutrophils, natural killer (NK) cells, resident tissue macrophages and T cells. Pattern recognition receptor engagement can also contribute to the activation of resident dendritic cells (DCs) and recruited monocytes. During this initial leukocyte migration phase, inflammatory macrophages often display an M1-like phenotype, producing nitric oxide (NO), reactive oxygen species (ROS), interleukin-1 (IL-1) and tumour necrosis factor (TNF), which are important components of the antimicrobial arsenal. Secretion of matrix metalloproteinases (MMPs) such as MMP2 and MMP9 by inflammatory M1 macrophages also helps to degrade the ECM, facilitating the recruitment of inflammatory cells to the site of tissue injury. If the tissue-damaging irritant persists, activated M1 cells can further exacerbate the inflammatory response by recruiting large numbers of T helper 17 (TH17) cells and neutrophils, leading to substantial tissue damage. The damaged epithelial cells also release alarmins, including IL-25, IL-33 and thymic stromal lymphopoietin (TSLP), which induce IL-4 and IL-13 secretion by a variety of innate and adaptive immune cells, including nuocytes, mast cells, basophils and TH2 cells. When the inflammatory stimulus or pathogen is eliminated, M1 cell activation diminishes, and the alarmins and TH2-type cytokines drive the conversion of the immune response into a wound healing response, which is characterized by the accumulation of M2 macrophages that promote wound healing and fibrosis through the production of MMPs (including MMP12, tissue inhibitor of metalloproteinases 1 (TIMP1), growth factors (including platelet-derived growth factor (PDGF)) and cytokines (such as transforming growth factor-β1 (TGFβ1)). In the final stages of a wounding response, macrophages take on a regulatory/suppressive phenotype, which is characterized by the expression of arginase 1 (ARG1), resistin-like molecule-α (RELMα), programmed death ligand 2 (PDL2) and IL-10, which have all been shown to facilitate the resolution of wound healing and restore homeostasis while limiting the development of fibrosis, in part by suppressing T cell proliferation and collagen synthesis by activated myofibroblasts. M2 macrophages also promote the resolution of wound healing by antagonizing inflammatory M1 responses.
Regulators of tissue repair
In addition to their innate phagocytic activity and role in antimicrobial immunity, macrophages are intimately involved in wound repair60,61 (FIG. 4). In contrast to pro-inflammatory and antimicrobial M1 macrophage responses, M2 macrophages exhibit potent anti-inflammatory activity and have important roles in wound healing and fibrosis62,63. They also antagonize M1 macrophage responses, which may be crucial for the activation of the wound healing response and for tissue homeostasis to be restored59. Recent studies have also shown that M1 macrophages can themselves ‘convert’ into anti-inflammatory macrophages with an M2 wound-healing phenotype64,65.
M2 macrophages produce growth factors that stimulate epithelial cells and fibroblasts, including transforming growth factor-β1 (TGFβ1) and PDGF66. Macrophage-derived TGFβ1 contributes to tissue regeneration and wound repair by promoting fibroblast differentiation into myofibroblasts, by enhancing expression of tissue inhibitors of metalloproteinases (TIMPs) that block the degradation of extracellular matrix (ECM) and by directly stimulating the synthesis of interstitial fibrillar collagens in myofibroblasts67,68. Macrophage-derived PDGF also stimulates the proliferation of activated ECM-producing myofibroblasts69.
M2 macrophages can also regulate wound healing independently of their interactions with myofibroblasts. Indeed, they produce matrix metalloproteinases (MMPs) and TIMPs that control ECM turnover70, they engulf and digest dead cells, debris and various ECM components that would promote tissue-damaging M1 macrophage responses66,71, and they secrete specific chemokines that recruit fibroblasts, TH2 cells and regulatory T (TReg) cells72,73. Moreover, M2 macrophages produce factors that induce myofibroblast apoptosis74, serve as antigen-presenting cells (APCs) that propagate antigen-specific TH2 and TReg cell responses (which promote wound healing while limiting the development of fibrosis75,76) and express immunoregulatory proteins (such as IL-10, resistin-like molecule-α (RELMα; also known as RETNLα or FIZZ1), chitinase-like proteins and arginase 1 (ARG1)) that have been shown to decrease the magnitude and duration of inflammatory responses and promote wound healing57,77–81 (FIG. 4).
Macrophages in disease
Adipose tissue macrophages in metabolic disorders
M2 macrophages have been found to regulate important metabolic functions82. These macrophages are induced by peroxisome proliferator activated receptor-γ (PPARγ) signalling and maintain adipocyte function, insulin sensitivity and glucose tolerance, which can prevent the development of diet-induced obesity and type 2 diabetes83,84. A recent paper suggested that IL-4-producing eosinophils are required to maintain M2 macrophages in healthy non-obese mice176. These studies suggest that as obesity progresses, adipose tissue-associated macrophages switch from an M2-like phenotype to a classically activated M1-like phenotype with potent pro-inflammatory activity82, with the NLRP3 inflammasome serving as the molecular switch by sensing obesity-associated danger signals85 (see REF. 86 for a review).
The role of M2 macrophages in allergy and asthma
M2 macrophages were originally described as suppressive cells because they inhibit the production of a wide variety of pro-inflammatory mediators87,88. However, the definition and function of M2 macrophages has been expanded, particularly in regards to their role in regulating TH2-type inflammatory responses, as in addition to downregulating pro-inflammatory responses, M2 macrophages are involved in the development of TH2-dependent immunity to some extracellular parasites and fungi89,90.
Numerous studies have identified roles for M2 macrophages in allergic responses driven by IL-4 and IL-13 (REF. 91). However, their function in allergy and asthma remains controversial, with some studies suggesting that M2 macrophages promote allergic inflammation and others indicating a suppressive role for these cells. A recent study suggested that M2 macrophages are required for the development of airway disease following infection with Sendai virus, which is a mouse parainfluenza virus92. The authors found that M2 macrophages secrete IL-13 and that their depletion significantly attenuated TH2-driven inflammation in the lung. M2 macrophages induced during rhinovirus infection have also been shown to exacerbate eosinophilic airway inflammation by producing the chemokine CCL11 (also known as eotaxin 1), which recruits eosinophils93. The epithelial-derived cytokine IL-33 has also been hypothesized to function as a major driver of eosinophilic airway inflammation because it promotes the differentiation of airway macrophages towards an M2 phenotype94,95.
Nevertheless, other studies have questioned the importance of macrophages in the development of allergic airway disease and instead support a role for another type of mononuclear phagocyte, CD11c+ DCs, in the development of eosinophilic inflammation and TH2-associated cytokine production in the lung96. Additional reports have also identified a suppressive role for M2 macrophages in allergy and asthma. Indeed, by facilitating the uptake and removal of fungal conidia, M2 macrophages have been shown to inhibit asthma symptoms associated with chronic fungal infections90. In contrast with M2 macrophages in mice infected with Sendai virus, M2 macrophages producing IL-13 mediated the resolution of respiratory syncitial virus-induced lung injury by reducing inflammation and epithelial damage97. Chitinase proteins expressed by M2 macrophages have also been proposed to suppress allergic inflammation by degrading or sequestering chitin, a potent and highly abundant allergen in the airway80. RELMα, which is expressed by M2 macrophages, eosinophils and epithelial cells, inhibits TH2-driven inflammation in the lung79,98. However, the specific contribution of M2 macrophages and the proteins they express to airway inflammation remains unclear, as the expression of many of these proteins is not exclusive to TH2 cytokine-stimulated macrophages. These studies emphasize the need to elucidate the functions of molecules expressed specifically by M2 macrophages (TABLE 2).
The role of macrophages in tumorigenesis
Distinct macrophage subsets have been linked with either protective or pathogenic roles in cancer99. A protective role in tumorigenesis has been described for M1 macrophages, which activate tumour-killing mechanisms and antagonize the suppressive activities of TAMs, MDSCs, M2 macrophages, regulatory macrophages and immature myeloid cells (which have all been shown to suppress adaptive tumour-specific immune responses and promote tumour growth, invasion, metastasis, stroma remodelling and angiogenesis100–105). M1 macrophages also amplify TH1 responses, providing a positive feedback loop in the antitumour response64.
By contrast, TAMs isolated from solid and metastatic tumours have a suppressive M2-like phenotype. Furthermore, accumulating evidence from many tumour models suggests that macrophages contribute to tumour progression, with increasing numbers of TAMs, MDSCs and immature monocytes correlating with poor outcomes106–108. These observations are also consistent with the tumour-promoting activities of IL-4 and IL-13, which also promote M2 macrophage differentiation109– 112. A novel population of forkhead box P3 (FOXP3)-expressing macrophages was also shown to display immunosuppressive properties and promote tumour growth113.
Importantly, IFNγ was recently shown to reverse the immunosuppressive and pro-tumoural properties of TAMs. So, IFNγ could potentially be administered locally to combat the generation and maintenance of immunosuppressive TAMs and thus boost protective M1 macrophage and T cell responses within the tumour microenvironment114. Moreover, blocking nuclear factor-κB (NF-κB) signalling can switch TAMs to an M1-like phenotype that is cytotoxic against tumour cells115. Natural killer T cells can also kill TAMs directly, providing an additional approach for targeting TAMs and promoting tumour-specific immunity116.
A major problem in the analysis of TAMs concerns how the cells are phenotyped and thus categorized. Diverse phenotypes have been attributed to TAMs, and this stems partly from differences in tumour types, donors and isolation techniques. Therefore, TAM phenotyping should rely on defining gene and protein expression profiles in vivo and ex vivo and on comparison of these profiles with the gene expression profiles of conventional macrophage subsets. Moreover, TAMs should be expected to exhibit the same plasticity as other macrophages following cytokine stimulation ex vivo. Undoubtedly, comprehensive profiling of TAMs from both mouse cancer models and human samples will be a key part of understanding the tumorigenesis process, as cancer researchers have increasingly recognized ‘inflammation’ as being inseparable from cancer itself117.
Contrasting roles for macrophage subsets in autoimmunity
M1-like macrophage-derived TNF, IL-18, IL-12 and IL-23 have been identified as important mediators in several chronic inflammatory and autoimmune diseases, including Crohn’s disease, rheumatoid arthritis, multiple sclerosis and autoimmune hepatitis118–120. For example, during experimental colitis, a subset of CX3CR1int LY6Chi GR1+ (glutathione reductase 1+) macrophages expressing TLR2, CCR2 and TNF was shown to promote inflammation in the colon121. Similarly, in patients with Crohn’s disease, researchers identified a population of CD14+ macrophages that are distinct from the normal intestinal macrophage pool and produce large amounts of pro-inflammatory cytokines, including IL-23 and TNF122,123. Because IL-23 and TNF mediate pathology in Crohn’s disease, these inflammatory macrophages have been hypothesized to contribute to pathogenesis of the disease. Nevertheless, other studies have shown that impaired pro-inflammatory cytokine production by macrophages can also contribute to Crohn’s disease by diminishing the capacity of macrophages to clear potentially pathogenic commensal bacteria from the lining of the bowel119.
Resident tissue macrophages also maintain homeostasis in the intestine by clearing apoptotic cells and debris, by promoting epithelial repair and by producing IL-10, which has been shown to maintain expression of FOXP3 in colonic TReg cells124,125. In a pathology as complex as Crohn’s disease, it is important to bear in mind that the principles of macrophage heterogeneity and plasticity also apply, and thus multiple macrophage populations are likely to have flexible pro- and anti-inflammatory (or homeostatic) effects in the gut and are subject to both temporal and anatomical effects.
Contrasting roles for different macrophage subsets have also been described in the pathogenesis of rheumatoid arthritis. For example, TNF produced by M1-like macrophages was shown to trigger cytokine production by synovial cells, leading to the development of chronic polyarthritis120. By contrast, macrophages producing reactive oxygen species were found to protect mice from arthritis by inhibiting T cell activation126.
Macrophages have also been identified as key regulators in demyelinating diseases of the central nervous system (CNS). Indeed, infiltrating M1-like macrophages are thought to contribute to axonal loss in multiple sclerosis and in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis127. Macrophages recruited to the CNS prime T cells to execute a TH1 effector programme in EAE128, whereas recruited myeloid cells producing IL-23 stimulate the production of GM-CSF by helper T cells, which regulates disease development and severity129,130. These observations suggest that macrophages could be targeted to prevent or reduce axonal loss in multiple sclerosis131. However, macrophages also have protective roles in multiple sclerosis by promoting T cell apoptosis and by expressing anti-inflammatory cytokines such as TGFβ1 and IL-10, which contribute to the termination of inflammation132. Moreover, a subset of macrophages expressing the inhibitory receptor CD200 (also known as OX2) has also been shown to prevent the onset of EAE in mice133. Finally, a population of monocyte-derived macrophages was shown to inhibit inflammation in a model of spinal cord injury, providing further evidence for a protective role for macrophages in the CNS134. Thus, macrophages have both protective and pathogenic roles in a wide variety of autoimmune and inflammatory diseases.
Macrophage subsets in atherosclerosis
It has been appreciated for quite some time that atherosclerosis is both a lipid disorder and inflammatory disease, with macrophages having a central role135. In atherosclerosis, it is thought that macrophages lodge in the intima and subintima of arteries, eventually leading to the formation of obstructive atherosclerotic plaques that are prone to rupture, leading to thrombosis, myocardial infarction or stroke. Studies have suggested that TH1 cells contribute to the development of atherosclerosis by producing IFNγ136, which stimulates the differentiation of highly activated macrophages, termed foam cells, that promote the formation of unstable lesions137. These pathogenic macrophages also express higher levels of scavenger receptors and CD36, which augments the uptake of modified forms of low-density lipoprotein138–140.
By contrast, TH2-associated cytokines, particularly IL-10, seem to have a protective role, as they block the formation of pathogenic M1-like macrophages in atherosclerotic plaques141. Although hypercholesterolaemia was initially hypothesized to be the primary stimulus for the recruitment of macrophages into the arterial wall, immunological and mechanical injuries, as well as bacterial and viral infections, are likely to contribute to the pathogenesis of atherosclerosis137. Toxic blood lipids, such as oxidized low-density lipoproteins (cholesterol) are removed by macrophages as part of their general homeostatic scavenging function139. Therefore, because macrophages facilitate the clearance of cholesterol, they could be viewed as having a protective role in atherosclerosis and lipid homeostasis.
However, hypercholesterolaemic mice that are deficient in macrophages were found to be highly resistant to developing atherosclerosis, suggesting that macrophages primarily have a pathogenic role in the disease142. Depletion of CD11b+ macrophages after plaque formation is, by contrast, less protective, suggesting that monocytes and macrophages are involved in the genesis but not maintenance of atherosclerosis143. Nevertheless, some reports have suggested that decreases in plaque size and regression of atherosclerosis correlates with macrophages emigrating from the plaque135,144. Thus, devising strategies that facilitate the depletion or inactivation of pathogenic M1-like macrophages from actively growing plaques could emerge as a useful therapy for atherosclerosis145,146.
Macrophage subsets in the pathogenesis of fibrosis
Studies have suggested that progressive fibrotic diseases, such as idiopathic pulmonary fibrosis (IPF), hepatic fibrosis and systemic sclerosis, are tightly regulated by macrophages61. ‘Pro-fibrotic’ macrophages produce various mediators, including TGFβ1, PDGF and insulin-like growth factor 1, that directly activate fibroblasts, and therefore these cells are intimately involved in wound healing (FIG. 4). These secreted proteins regulate the proliferation, survival and activation status of myofibroblasts, which control ECM deposition147–149. Pro-fibrotic macrophages also produce their own MMPs and TIMPs, which regulate inflammatory cell recruitment and ECM turnover70. In addition, they secrete various pro-fibrotic cytokines and chemokines, including IL-1β, which was identified as a potent pro-fibrotic mediator in the lung150,151. IL-1β stimulates TH17 cells to produce IL-17, which was identified as an important inducer of bleomycin-induced pulmonary fibrosis, a fibrotic disorder with characteristics that are similar to those of IPF152. Furthermore, macrophages function as APCs and promote TH2 responses153, which have been shown to induce and activate the pro-fibrotic cytokine TGFβ1 in macrophages through an IL-13- and MMP9-dependent mechanism62,154.
Nevertheless, although macrophages are clearly required for the initiation and maintenance of fibrosis, other studies have suggested that they are also involved in the suppression, resolution and reversal of fibrosis155. Indeed, macrophages phagocytose dead cells and cellular debris, which can help to reduce the danger signals that contribute to the production of pro-inflammatory and pro-fibrotic mediators. Moreover, they engulf and digest ECM components and stimulate the production of collagen-degrading MMPs in other inflammatory cells, including myofibroblasts and neutrophils70. The production of IL-10, RELMα and ARG1 by M2-like macrophages has been shown to suppress fibrosis57,79,156. Thus, with their potential to both induce and inhibit fibrosis, macrophages and the factors they express are integrated into all stages of the fibrotic process (FIG. 4). To better understand the pathogenesis of fibrosis, we therefore need to identify the specific macrophage subsets that promote, inhibit and reverse fibrosis and elucidate the contributions of the unique mediators that are expressed by each population.
Together, these examples illustrate how inflammatory and suppressive macrophages are crucially involved in the progression and resolution of disease. They also demonstrate the complex and often opposing roles of different macrophage subsets in health and disease. A more detailed understanding of the mechanisms that regulate the activation and deactivation of human macrophages is likely to lead to the development of more effective strategies for treating various important inflammatory diseases157.
Human macrophages
An important question in understanding the evolution of immune systems concerns the functions of macrophages after the advent of the lymphocyte-based non-self discrimination system. As we have stressed here, immunosuppression is a common trait of all tissue-resident macrophages, and so it seems plausible that control of T cell proliferation and interaction with TReg cells is a recently acquired function that is necessary for tissue homeostasis. All of these properties of macrophages can be readily dissected in mouse models, which leads us to consider the role of macrophages in humans. Here, differences to rodents are apparent in both the types of pathogens that infect humans and the effector molecules that are deployed by macrophages to control infections. Homotropic pathogens, including Mycobacterium tuberculosis, Mycobacterium leprae, Shigella flexneri, Plasmodium falciparum and numerous viruses such as measles and dengue virus, are predominantly or only found in humans. The long lifespan of humans compared to rodents is likely to be a driver of types of immune responses that are needed to control pathogens; the time lag until sexual maturity means that humans need to survive for decades to ensure their children are self-sufficient. For example, prevention of collateral tissue damage and oncogenic somatic mutations may be a factor in human evolutionary fitness compared to shorter-lived animals that quickly produce the next generation. The extrapolation of rodent models in order to understand homotropic pathogens has, however, not kept pace with the need for relevant systems for dissecting human macrophage-based immunity14.
Although murine M1- and M2-polarized macrophage subsets are relatively easy to distinguish based on combinatorial gene expression profiles (TABLE 2), the identification of equivalent subsets in humans has been more challenging. The basic problem is that panels of markers for in vitro-generated human macrophage subsets do not exist or cannot be agreed upon (TABLE 2). One approach to solve this problem is to ablate transcription factors that establish bias in macrophage phenotypes. For example, interferon regulatory factor 5 (IRF5) seems to be crucial for human M1 macrophage gene expression158. Therefore systematic gene expression profiling in IRF5-deficient human macrophages (or in other macrophage populations in which polarization is genetically fixed or biased) stimulated with different cytokines and TLR agonists might reveal panels of genes that associate with polarized subsets.
Moreover, neither ARG1 nor inducible nitric oxide synthase (iNOS) is expressed by in vitro polarized human macrophages stimulated with IL-4 or IFNγ, respectively, in amounts comparable with those expressed by mouse macrophages. So, the discrepancies in arginine-metabolizing enzyme expression are at the centre of an intense debate on similarities between the human and mouse macrophage subsets and their expected functions14. In addition, other effector pathways have undergone major evolutionary changes compared to rodents. For example, the p47 immunity-related GTPase (IRG) family has 20 members in mice but only two in humans (IRGM and IRGC)159,160. It has been shown that IRGM is involved in the protective anti-mycobacterial autophagy response, and variants of IRGM are strongly associated with Crohn’s disease pathogenesis and anti-bacteria autophagy responses161. It is reasonable to postulate that the pool of effector molecules will be more diverse from species to species as pathogens seek to exploit new niches. This controversial area has been extensively discussed162–164, but remains an area ripe for new discoveries, as evolutionary comparisons can be made between model organism and human macrophages to uncover the underlying effector mechanisms of pathogen control and elimination.
Perspectives
Macrophage research undergoes periods of intense activity and continuously provides informative insights for immunologists. Although much current research has focused on the signalling pathways that regulate inflammatory mediator production and subset development, new issues have arisen that need to be resolved within the contexts of normal homeostasis and acute or chronic disease. We identify three areas of research as paramount for further work.
First, the regulation of macrophages in the tissues remains unclear. For instance, it is only in the past few months that M2 macrophage proliferation in situ has been discovered. We also do not understand how homeostasis is restored after infection, how the response to damaged tissues is resolved and what mechanisms are involved in the layered hierarchy of macrophage activation in situ. Indeed, the number and diversity of signals and the magnitude of the response required to switch macrophages into a pro-inflammatory state remains unclear. How is the fate of recruited monocytes regulated? And what happens to excess macrophages in the tissues following deposition of vast numbers of newly recruited monocytes?
The second area of research that requires development is the underlying mechanisms that regulate the plasticity and stability of macrophage populations. As we have described here, most investigators agree that macrophages are highly plastic, yet the assays used to assign phenotypes require further development and standardization. In our view, new work on the transcription factors and epigenetic changes responsible for macrophage plasticity combined with better marker systems will advance the field. This type of work will help to better define macrophage subsets at a molecular level and provide the foundation that is needed to generate new genetic tools, which will finally allow us to interrogate the function of macrophage subsets in vivo.
Finally, the third area concerns the relationship between human macrophages and their cognate animal-derived model systems. This is perhaps the area of work with the biggest potential, as the chasm between understanding mouse and human macrophages is wide.
Acknowledgements
Work in P.J.M.’s laboratory is supported by The Hartwell Foundation, US National Institutes of Health (NIH) CORE grant P30 CA21765 and the American Lebanese Syrian Associated Charities. T.A.W. is supported by the Intramural Program of the US National Institute of Allergy and Infectious Diseases, NIH.
Glossary
- Mononuclear phagocytic system
This system consists of bone-marrow-derived cells (monocytes, macrophages and dendritic cells) that have different morphologies and are mainly responsible for phagocytosis, cytokine secretion and antigen presentation.
- Phagocytosis
A process that is used by cells to internalize large particles, such as debris, apoptotic cells and pathogens, into phagosomes.
- Osteoclasts
Multinucleated giant cells of the monocyte lineage that are responsible for bone resorption. Osteoclasts degrade bone matrix and solubilize calcium from bone. Defects in their differentiation and a decrease in their number lead to bone osteopetrosis. Conversely, an increase in their number or function induces bone osteoporosis, indicating that osteoclasts have a pivotal role in bone homeostasis.
- Alveolar macrophages
Resident macrophages of the lung that are exposed to alveolar lumen and phagocytose inhaled particles (such as dust or allergens) and microorganisms.
- Kupffer cells
Large, stellate- or pyramidal-shaped, specialized macrophages that line the sinusoidal vessels of the liver. They regulate local immune responses, and remove microbial particles, endotoxin and other noxious substances that penetrate the portal venous system.
- Microglia
Phagocytic cells of myeloid origin that are involved in the innate immune response in the central nervous system. Microglia are considered to be the brain-resident macrophages.
- M1 macrophages
A macrophage subset that is activated by Toll-like receptor ligands (such as lipopolysaccharide) andinterferon-γ. M1 macrophages express pro-inflammatory cytokines and inducible nitric-oxide synthase, among others.
- M2 macrophages
A macrophage subset that is stimulated by interleukin-4 (IL-4) or IL-13. M2 macrophages express arginase 1, the mannose receptor CD206 and the IL-4 receptor α-chain, among others.
- Tumour-associated macrophages (TAMS)
An important component of the tumour microenvironment. These cells differentiate from circulating blood monocytes that have infiltrated tumours. They can have positive or negative effects on tumorigenesis (that is, tumour promotion or immunosurveillance, respectively).
- Myeloid-derived suppressor cells
(MDSCs). A group of immature CD11b+GR1+ cells, which include precursors of macrophages, granulocytes, dendritic cells and myeloid cells. Through direct interactions and secreted components, they negatively regulate T cell function.
- Opsonin molecules
Proteins that bind to the surface of a particle and enhance its uptake by a phagocyte. Opsonins include IgG and complement activation fragments (including C4b, C3b, iC3b, C3dg and C3d).
- Sterile inflammation
Inflammation that occurs in the absence of any microorganisms, as a result of tissue damage. In a similar way to microbe-induced inflammation, sterile inflammation is marked by the recruitment of neutrophils and macrophages and the production of pro-inflammatory cytokines and chemokines.
- Extracellular matrix (ECM)
Secreted products of many cell types that form an organized scaffold for cell support.
- Inflammasome
A molecular complex of several proteins that upon assembly cleaves pro-interleukin-1, thereby producing active interleukin-1
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Peter J. Murray’s homepage: http://www.stjude.org/murray.
Thomas A. Wynn’s homepage: http://www.niaid.nih.gov/LabsAndResources/labs/aboutlabs/lpd/immunopathogenesissection/pages/wynn.aspx
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Peter J. Murray, Email: peter.murray@stjude.org.
Thomas A. Wynn, Email: twynn@niaid.nih.gov.
References
- 1.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani B, Rabinovitch M, Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG) J. Exp. Med. 1972;135:780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15:243–250. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- 4.Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nature Rev. Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 6.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117:5403–5412. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 7.Iannacone M, et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079–1083. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junt T, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nature Rev. Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cros J, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutterwala FS, Noel GJ, Salgame P, Mosser DM. Reversal of proinflammatory responses by ligating the macrophage Fcγ receptor type I. J. Exp. Med. 1998;188:217–222. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 2011;89:557–563. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagemann T, et al. “Re-educating” tumor-associated macrophages by targeting NF-κB. J. Exp. Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. This study shows that activation of NF-κB by IL-1R and MYD88 signalling is required to maintain the immunosuppressive function of TAMs. In the absence of NF-κB signalling, TAMs adopt a ‘classically’ activated phenotype and kill tumour cells
- 16.Rutschman R, et al. Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J. Immunol. 2001;166:2173–2177. doi: 10.4049/jimmunol.166.4.2173. [DOI] [PubMed] [Google Scholar]
- 17.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 18.Mylonas KJ, Nair MG, Prieto-Lafuente L, Paape D, Allen JE. Alternatively activated macrophages elicited by helminth infection can be reprogrammed to enable microbial killing. J. Immunol. 2009;182:3084–3094. doi: 10.4049/jimmunol.0803463. [DOI] [PubMed] [Google Scholar]
- 19.Stout RD, et al. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 20.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 24. Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. A study convincingly showing that tissue macrophages undergo rapid in situ proliferation in response to IL-4
- 25.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature Rev. Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nature Rev. Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 27. Nish S, Medzhitov R. Host defense pathways: role of redundancy and compensation in infectious disease phenotypes. Immunity. 2011;34:629–636. doi: 10.1016/j.immuni.2011.05.009. An elegant exposition of the complexities associated with the understanding the host–pathogen interplay
- 28.Borden EC, et al. Interferons at age 50: past, current and future impact on biomedicine. Nature Rev. Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 31. Osorio F, Reis ESC. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity. 2011;34:651–664. doi: 10.1016/j.immuni.2011.05.001. A concise up-to-date summary of the role of C-type lectin receptors in pathogen-specific immune responses
- 32.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haile LA, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 35.Cripps JG, Gorham JD. MDSC in autoimmunity. Int. Immunopharmacol. 2011;11:789–793. doi: 10.1016/j.intimp.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia MR, et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J. Clin. Invest. 2010;120:2486–2496. doi: 10.1172/JCI41628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delano MJ, et al. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 39.Semerad CL, Poursine-Laurent J, Liu F, Link DC. A role for G-CSF receptor signaling in the regulation of hematopoietic cell function but not lineage commitment or differentiation. Immunity. 1999;11:153–161. doi: 10.1016/s1074-7613(00)80090-4. [DOI] [PubMed] [Google Scholar]
- 40. Hanna RN, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C− monocytes. Nature Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. An important study, which identified the orphan nuclear receptor NR4A1 as a master transcription factor that regulates the differentiation and survival of ‘patrolling’ LY6C− monocytes.
- 41. Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. This study used a series of fascinating and technically challenging experiments to define the spleen as a monocyte reservoir
- 42.Aldridge JR, Jr, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc. Natl Acad. Sci. USA. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunay IR, Sibley LD. Monocytes mediate mucosal immunity to Toxoplasma gondii. Curr. Opin. Immunol. 2010;22:461–466. doi: 10.1016/j.coi.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serbina NV, et al. Distinct responses of human monocyte subsets to Aspergillus fumigatus conidia. J. Immunol. 2009;183:2678–2687. doi: 10.4049/jimmunol.0803398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 46.Kim YG, et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang S, Kim CC, Batra S, McKerrow JH, Loke P. Delineation of diverse macrophage activation programs in response to intracellular parasites and cytokines. PLoS Negl. Trop. Dis. 2010;4:e648. doi: 10.1371/journal.pntd.0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goncalves R, Zhang X, Cohen H, Debrabant A, Mosser DM. Platelet activation attracts a subpopulation of effector monocytes to sites of Leishmania major infection. J. Exp. Med. 2011;208:1253–1265. doi: 10.1084/jem.20101751. A demonstration of a complex interaction between a parasite, platelets and local stimulation of CCL2 to draw monocytes to an infection site
- 49.Shi C, et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating Toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. A fascinating study that identifies the origin of metastasis-associated macrophages, which promote the extravasation, seeding and growth of tumour cells.
- 51.Bosschaerts T, et al. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-γ and MyD88 signaling. PLoS Pathog. 2010;6:e1001045. doi: 10.1371/journal.ppat.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashimoto D, et al. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J. Exp. Med. 2011;208:1069–1082. doi: 10.1084/jem.20101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bogunovic M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. These authors identify an IL-10-producing population of CD11b+F4/80+CD11c− macrophages that promote the differentiation of FOXP3+ regulatory T cells and suppress TH17 cell responses
- 55.Zhu B, et al. Plasticity of Ly-6Chi myeloid cells in T cell regulation. J. Immunol. 2011;187:2418–2432. doi: 10.4049/jimmunol.1100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aziz A, Soucie E, Sarrazin S, Sieweke MH. MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science. 2009;326:867–871. doi: 10.1126/science.1176056. This study showed that the transcription factors MAF and MAFB inhibit self-renewal of functionally differentiated monocytes and macrophages
- 57. Pesce JT, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. This study identifies ARG1 as the key mediator of the suppressive function of M2 macrophages
- 58.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 59.Sindrilaru A, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J. Clin. Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wynn TA. Fibrotic disease and the TH1/TH2 paradigm. Nature Rev. Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao W, Hong H, Kawakami Y, Lowell CA, Kawakami T. Regulation of myeloproliferation and M2 macrophage programming in mice by Lyn/Hck, SHIP, and Stat5. J. Clin. Invest. 2008;118:924–934. doi: 10.1172/JCI34013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 65.Arnold L, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barron L, Wynn TA. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G723–G728. doi: 10.1152/ajpgi.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts AB, et al. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl Acad. Sci. USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J. Leukoc. Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 69.Shimokado K, et al. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985;43:277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- 70.Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atabai K, et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J. Clin. Invest. 2009;119:3713–3722. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 73.Imai T, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int. Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 74.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 75.Fiorentino DF, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 76.Savage ND, et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFβ-1. J. Immunol. 2008;181:2220–2226. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 77.Herbert DR, et al. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J. Immunol. 2010;184:6438–6446. doi: 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sutherland TE, Maizels RM, Allen JE. Chitinases and chitinase-like proteins: potential therapeutic targets for the treatment of T-helper type 2 allergies. Clin. Exp. Allergy. 2009;39:943–955. doi: 10.1111/j.1365-2222.2009.03243.x. [DOI] [PubMed] [Google Scholar]
- 79.Pesce JT, et al. Retnla (Relmα/Fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reese TA, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.London A, et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J. Exp. Med. 2011;208:23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. This study, along with reference 84, shows that diet-induced obesity can lead to a shift in the activation state of adipose-associated macrophages from an M2 phenotype (induced by PPARγ) in lean animals to an M1 phenotype that contributes to insulin resistance
- 84.Odegaard JI, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chawla A, et al. Macrophage-mediated inflammation in metabolic disease. Nature Rev. Immunol. 2011 Oct 10; doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Waal Malefyt R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J. Immunol. 1993;151:6370–6381. [PubMed] [Google Scholar]
- 88.Gordon S. Alternative activation of macrophages. Nature Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 89.Anthony RM, et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhatia S, et al. Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype. PLoS ONE. 2011;6:e15943. doi: 10.1371/journal.pone.0015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prasse A, et al. IL-10-producing monocytes differentiate to alternatively activated macrophages and are increased in atopic patients. J. Allergy Clin. Immunol. 2007;119:464–471. doi: 10.1016/j.jaci.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 92.Kim EY, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nature Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nagarkar DR, et al. Rhinovirus infection of allergen-sensitized and -challenged mice induces eotaxin release from functionally polarized macrophages. J. Immunol. 2010;185:2525–2535. doi: 10.4049/jimmunol.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J. Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 95.Kurowska-Stolarska M, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 96.van Rijt LS, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shirey KA, et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4Rα-, TLR4-, and IFN-β-dependent. Mucosal Immunol. 2010;3:291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nair MG, et al. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Andreu P, et al. FcRγ activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 102.Nardin A, Abastado JP. Macrophages and cancer. Front. Biosci. 2008;13:3494–3505. doi: 10.2741/2944. [DOI] [PubMed] [Google Scholar]
- 103.Yang XD, et al. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nature Med. 2011;17:87–95. doi: 10.1038/nm.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sierra JR, et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J. Exp. Med. 2008;205:1673–1685. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kryczek I, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Imtiyaz HZ, et al. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steidl C, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N. Engl. J. Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahn GO, et al. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl Acad. Sci. USA. 2010;107:8363–8368. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.DeNardo DG, et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Terabe M, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R–STAT6 pathway. Nature Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 111.Gocheva V, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–11751. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 113. Zorro Manrique S, et al. Foxp3-positive macrophages display immunosuppressive properties and promote tumor growth. J. Exp. Med. 2011 Jun 13; doi: 10.1084/jem.20100730. This study identified FOXP3 expression in a subset of macrophages; this macrophage subset exhibits a distinct gene expression profile and may contribute to immunoregulation
- 114.Duluc D, et al. Interferon-γ reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int. J. Cancer. 2009;125:367–373. doi: 10.1002/ijc.24401. [DOI] [PubMed] [Google Scholar]
- 115. Fong CH, et al. An antiinflammatory role for IKKβ through the inhibition of"classical” macrophage activation. J. Exp. Med. 2008;205:1269–1276. doi: 10.1084/jem.20080124. This study identified a new role for inhibitor of NF-κB kinase-β (IKKβ) in the regulation of macrophage activation by showing that deletion of IKKβ in the myeloid lineage confers resistance to infection with group B Streptococcus through increased production of IL-12, iNOS and MHC class II by macrophages
- 116.Song L, et al. Vα24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J. Clin. Invest. 2009;119:1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 118.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith AM, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J. Exp. Med. 2009;206:1883–1897. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawane K, et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 121.Platt AM, Bain CC, Bordon Y, Sester DP, Mowat AM. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J. Immunol. 2010;184:6843–6854. doi: 10.4049/jimmunol.0903987. [DOI] [PubMed] [Google Scholar]
- 122.Kamada N, et al. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J. Immunol. 2009;183:1724–1731. doi: 10.4049/jimmunol.0804369. [DOI] [PubMed] [Google Scholar]
- 123.Kamada N, et al. Unique CD14+ intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J. Clin. Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith PD, et al. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4:31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gelderman KA, et al. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J. Clin. Invest. 2007;117:3020–3028. doi: 10.1172/JCI31935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hendriks JJ, Teunissen CE, de Vries HE, Dijkstra CD. Macrophages and neurodegeneration. Brain Res. Brain Res. Rev. 2005;48:185–195. doi: 10.1016/j.brainresrev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 128.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Codarri L, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nature Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 130.El-Behi M, et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nature Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α–PU.1 pathway. Nature Med. 2011;17:64–70. doi: 10.1038/nm.2266. This study identified miR-124 both as a key regulator of microglia quiescence in the central nervous system and as a previously unknown modulator of monocyte and macrophage activation
- 132.Kiefer R, Kieseier BC, Stoll G, Hartung HP. The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog. Neurobiol. 2001;64:109–127. doi: 10.1016/s0301-0082(00)00060-5. [DOI] [PubMed] [Google Scholar]
- 133.Hoek RM, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 134.Shechter R, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nature Rev. Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gupta S, et al. IFN-γ potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nature Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 138.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J. Clin. Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Acton S, et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 140.Suzuki H, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 141.Pinderski LJ, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ. Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 142.Smith JD, et al. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl Acad. Sci. USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stoneman V, et al. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ. Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 146.Erbay E, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nature Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nagaoka I, Trapnell BC, Crystal RG. Upregulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J. Clin. Invest. 1990;85:2023–2027. doi: 10.1172/JCI114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nagaoka I, Trapnell BC, Crystal RG. Regulation of insulin-like growth factor I gene expression in the human macrophage-like cell line U937. J. Clin. Invest. 1990;85:448–455. doi: 10.1172/JCI114458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor β1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc. Natl Acad. Sci. USA. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Invest. 2001;107:1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gasse P, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J. Clin. Invest. 2007;117:3786–3799. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilson MS, et al. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 154.Lee CG, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J. Exp. Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wilson MS, et al. IL-13Rα2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J. Clin. Invest. 2007;117:2941–2951. doi: 10.1172/JCI31546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aouadi M, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Krausgruber T, et al. IRF5 promotes inflammatory macrophage polarization and TH1–TH17 responses. Nature Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. These authors show that IRF5 expression in macrophages is reversibly induced by inflammatory stimuli and contributes to the plasticity of macrophage polarization, thus identifying IRF5 as a transcriptional repressor
- 159.Bekpen C, et al. Death and resurrection of the human IRGM gene. PLoS Genet. 2009;5:e1000403. doi: 10.1371/journal.pgen.1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bekpen C, Xavier RJ, Eichler EE. Human IRGM gene"to be or not to be”. Semin. Immunopathol. 2010;32:437–444. doi: 10.1007/s00281-010-0224-x. [DOI] [PubMed] [Google Scholar]
- 161.Deretic V. Autophagy in infection. Curr. Opin. Cell Biol. 2010;22:252–262. doi: 10.1016/j.ceb.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bogdan C. Species differences in macrophage NO production are important. Nature Immunol. 2002;3:102. doi: 10.1038/ni0202-102a. [DOI] [PubMed] [Google Scholar]
- 163.Fang FC, Nathan CF. Man is not a mouse: reply. J. Leukoc. Biol. 2007;81:580. doi: 10.1189/jlb.1206715. [DOI] [PubMed] [Google Scholar]
- 164.Nathan C. Role of iNOS in human host defense. Science. 2006;312:1874–1875. doi: 10.1126/science.312.5782.1874b. author reply 1874–1875. [DOI] [PubMed] [Google Scholar]
- 165.Manicassamy S, et al. Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Murphy KM. Comment on “;Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine”. Science. 2011;333:405. doi: 10.1126/science.1198277. author reply 405. [DOI] [PubMed] [Google Scholar]
- 167.Denning TL, et al. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory t cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J. Immunol. 2011;187:733–747. doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hume DA. Macrophages as APC and the dendritic cell myth. J. Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 169.Asano K, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34:85–95. doi: 10.1016/j.immuni.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 170.Hamann J, et al. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur. J. Immunol. 2007;37:2797–2802. doi: 10.1002/eji.200737553. [DOI] [PubMed] [Google Scholar]
- 171. Liao X, et al. Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. This paper identified Krüppel-like factor 4 (KLF4) as a crucial regulator of macrophage polarization. KLF4 cooperates with STAT6 to induce M2 and inhibit M1 macrophage activation via sequestration of co-activators required for NF-κB activation
- 172. El Kasmi KC, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nature Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. These authors identify a novel TLR-dependent pathway for the induction of ARG1 expression in mouse macrophages that leads to decreased NO production and increased susceptibility to intracellular infections
- 173.Qualls JE, et al. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci. Signal. 2010;3:ra62. doi: 10.1126/scisignal.2000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nature Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 175.Shirey KA, Cole LE, Keegan AD, Vogel SN. Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J. Immunol. 2008;181:4159–4167. doi: 10.4049/jimmunol.181.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]