Abstract
We examined the association of social activity with cognitive decline in 1138 persons without dementia at baseline with a mean age of 79.6 (SD = 7.5) who were followed for up to 12 years (mean = 5.2; SD = 2.8). Using mixed models adjusted for age, sex, education, race, social network size, depression, chronic conditions, disability, neuroticism, extraversion, cognitive activity, and physical activity, more social activity was associated with less cognitive decline during average follow-up of 5.2 years (SD = 2.7). A one point increase in social activity score (range = 1–4.2; mean = 2.6; SD = 0.6) was associated with a 47% decrease in the rate of decline in global cognitive function (p < .001). The rate of global cognitive decline was reduced by an average of 70% in persons who were frequently socially active (score = 3.33, 90th percentile) compared to persons who were infrequently socially active (score = 1.83, 10th percentile). This association was similar across five domains of cognitive function. Sensitivity analyses revealed that individuals with the lowest levels of cognition or with mild cognitive impairment at baseline did not drive this relationship. These results confirm that more socially active older adults experience less cognitive decline in old age.
Keywords: Cognition, Cognitive reserve, Aging, Social behavior, Life Style, Risk reduction behavior, Longitudinal studies, Epidemiology
INTRODUCTION
Declines in memory and other cognitive abilities are a common feature of aging and associated with lowered quality of life, lack of functional independence (Gaugler, Duval, Anderson, & Kane, 2007; Tabbarah, Crimmins, & Seeman, 2002) and mortality (Schupf et al., 2005). Cognitive decline is the defining feature of Alzheimer’s disease and other dementias and often signals their onset (Backman, Jones, Berger, Laukka, & Small, 2005). Finding an effective population-based strategy to prevent or delay cognitive decline is an increasingly salient public health priority for our aging society. Evidence from longitudinal studies suggests that one potential modifiable risk factor for cognitive decline may be participation in social activity. Older adults who are more socially active experience less declines in cognitive abilities (Barnes, Mendes de Leon, Wilson, Bienias, & Evans, 2004; Bassuk, Glass, & Berkman, 1999; Ertel, Glymour, & Berkman, 2008; Fratiglioni, Paillard-Borg, & Winblad, 2004; Lovden, Ghisletta, & Lindenberger, 2005; Zunzunegui, Alvarado, Del Ser, & Otero, 2003) and have a reduced risk for dementia (Fabrigoule et al., 1995; Karp et al., 2005; Saczynski et al., 2006). However, despite these relatively consistent findings, many experts conclude there is still “inconclusive evidence” that social activity is a true risk factor for cognitive decline (Daviglus et al., 2010). Methodological issues such as a lack of robust cognitive assessment or short follow-up have been cited, and two alternative explanations for the findings have not been fully accounted for: reverse causation (social activity may be limited for some persons as a result of prior cognitive decline before observation) or unmeasured confounding. Therefore, further research that addresses these methodological limitations is still necessary.
To test the hypothesis that a higher level of social activity in later life was associated with reduced rate of cognitive decline, we used data from more than 1,100 older participants without dementia at baseline in the Rush Memory and Aging Project followed for an average of 5.2 (and up to 12) years. This analysis expands upon our previous cross-sectional finding of a relationship between social activity and cognitive function in this dataset (Krueger et al., 2009). Linear mixed effects models were used to investigate the association of social activity at baseline with initial level and annual rate of change in global cognitive function. We tested whether this association was affected by a large number of potentially confounding factors and whether the association was independent of the influence of other types of activities that have been linked to cognition in late life, physical and cognitive activity. We also examined whether the association of social activity and cognitive decline varied across five specific cognitive domains. Finally, we tested whether persons who were cognitively impaired at baseline were driving a relationship between social activity and cognitive decline (i.e., reverse causation) by repeating analyses after removing persons with the lowest levels of cognitive function and with diagnosed mild cognitive impairment (MCI) at baseline. As an additional test of reverse causation, we also tested whether initial cognitive function was related to change in social activity.
METHODS
Participants
Participants were enrolled in the Rush Memory and Aging Project, an ongoing longitudinal cohort study of common chronic conditions of aging (Bennett et al., 2005). Participants are older persons (age 65 or older) recruited from approximately 40 retirement and subsidized housing facilities in the Chicago metropolitan area. All participants signed an informed consent agreeing to annual clinical evaluation and organ donation at the time of death. The study was approved by the Institutional Review Board of Rush University Medical Center. The clinical evaluation has been described in detail previously (Bennett et al., 2005). The structured baseline evaluation included medical history, neurological and neuropsychological examinations. Annual follow-up evaluations were identical to the baseline evaluation in essential details, with examiners blinded to previous data. At each evaluation, diagnosis of dementia or Alzheimer’s disease was performed by an experienced clinician after review of all available data from the clinical evaluation.
Persons with dementia at baseline or who did not have valid data from at least one follow-up visit were excluded from analysis. At the time of these analyses, 1406 participants had completed a baseline evaluation. Of these, 111 persons met the criteria for dementia or did not have a valid dementia assessment and another 157 died before or had not yet reached their first follow-up. The remaining 1138 participants completed between 2 and 13 annual evaluations (mean = 6.2; SD = 2.7) evaluations. The mean age was 79.6 years (SD = 7.5), mean education was 14.5 years (SD = 3.2), and the mean score on the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) was 27.9 (SD = 2.1) at baseline; 74.3% were women and 87.3% were White, non-Hispanic.
Assessment of Late Life Social Activity
Frequency of social activity was assessed using a previously established scale (Bennett et al., 2005; Buchman et al., 2009) that asks how often during the past year participants engaged in six common types of activities that involve social interaction (1) go to restaurants, sporting events or teletract [off-track betting], or play bingo; (2) go on day trips or overnight trips; (3) do unpaid community or volunteer work; (4) visit relatives’ or friends’ houses; (5) participate in groups, such as senior center, Knights of Columbus, Rosary Society, or something similar; and (6) attend church or religious services. Participants rated how often they participated in each activity based on a five-point scale: (1) once a year or less; (2) several times a year; (3) several times a month; (4) several times a week; and (5) every day or almost every day. The items were summed and divided by the total number of items to obtain a composite measure of social activity with higher scores indicating more activity.
Assessment of Cognitive Function
Cognitive function was assessed annually using a battery of 21 tests, as previously described (Bennett et al., 2005). Scores on 19 tests were used to create summary indices of global cognitive function and 5 specific cognitive domains: episodic memory (7 tests: immediate and delayed recall of story A from Logical Memory, immediate and delayed recall of the East Boston Story, Word List Memory, Word List Recall, and Word List Recognition), semantic memory (3 tests: a 15-item version of the Boston Naming Test, Verbal Fluency, and a 15-item reading test), working memory (3 tests: Digit Span Forward, Digit Span Backward, and Digit Ordering), perceptual speed (4 tests: Symbol Digit Modalities Test, Number Comparison, and 2 indices from a modified version of the Stroop Neuropsychological Screening Test), and visuospatial ability (2 tests: a 15-item version of Judgment of Line Orientation and a 16-item version of Standard Progressive Matrices).
To obtain the summary indices, raw scores on each of the individual tests were converted to z scores using the baseline mean (SD) of the entire cohort. The Z scores of all 19 tests were averaged to compute the global cognitive function score. Summary scores for the five cognitive domains were derived by converting raw scores on each of the individual tests to Z scores using the mean (SD) of the entire cohort and then averaging the Z scores from tests in a specific domain. Psychometric information on these summary scores, including factor analytic support for the five domains, have been previously reported (Wilson et al., 2005).
Clinical diagnoses of dementia and MCI were performed using a three stage process including computer scoring of cognitive tests, clinical judgment by an experienced neuropsychologist, and diagnostic classification by an experienced clinician, as previously described (Bennett et al., 2005). Diagnosis of dementia and probable AD followed the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (McKhann et al., 1984). Diagnosis of MCI was rendered for individuals found to have cognitive impairment by the neuropsychologist but who did not meet the accepted criteria for dementia by the clinician (Bennett et al., 2005). Persons who did not meet criteria for MCI or dementia were classified as having no cognitive impairment.
Assessment of Other Covariates
All covariates included in this analysis came from the baseline assessment. We quantified social network size with standard questions about the number of children, family, and friends seen at least once a month, as previously described (Bennett, Schneider, Tang, Arnold, & Wilson, 2006).
Depressive symptomatology was assessed with a 10-item version of the Center for Epidemiologic Studies Depression Scale (Kohout, Berkman, Evans, & Cornoni-Huntley, 1993) as previously described (Bennett et al., 2005). Persons were asked if they had experienced each of 10 symptoms during the past week and the score was the total number of items. The total number of seven self-reported chronic medical conditions (diabetes mellitus, hypertension, heart disease, cancer, thyroid disease, head injury, and stroke) was used as a measure of chronic illness, as described elsewhere (Wilson et al., 2002). Disability was assessed with the Katz scale, which asks about ability to independently perform six daily living activities: walking, bathing, dressing, eating, getting from bed to chair, and toileting (Katz & Akpom, 1976). The score was the number of activities that the person was unable to perform without help.
The personality traits of neuroticism, indicative of distress proneness, and extraversion, indicative of sociability, were measured using subscales from the NEO Five-Factor Inventory (Costa & McCrae, 1992). Participants rated agreement with each neuroticism or extraversion item (6 items each) and total scores were computed (range: 0 to 48) with higher scores indicating a higher level of each trait as previously described (Wilson et al., 2006).
Participants rated their current frequency of participation in nine cognitively stimulating activities (e.g., reading a book, visiting a library) on a five-point scale, with 5 indicating participation in the activity every day or approximately every day, and 1 indicating participation once a year or less (Wilson et al., 2005). We excluded two items that had social components (going to museums and concerts) and the remaining item scores were averaged to yield a summary measure of late life cognitive activity. Frequency of physical activity was assessed with questions adapted (McPhillips, Pellettera, Barrett-Connor, Wingard, & Criqui, 1989) from the 1985 Health Interview Survey (U.S. Public Health Service, 1985). Persons were asked if they had participated in each of five activities (e.g., walking for exercise, calisthenics) during the past 2 weeks, and if so, the number of times and mean time per occasion. Minutes in each activity were summed and divided by 120 to yield a summary measure of hours per week of physical activity, as described elsewhere (Wilson et al., 2002).
Income was measured using the “show-card” method. Participants were asked to select 1 of 10 levels of total family income listed on a card (Cornoni-Huntley, Brock, Ostfeld, Taylor, & Wallace, 1986). Consistent with other studies of older adults, 150 (13%) of the cohort included in this analysis did not provide income data.
Statistical Analysis
We first examined the relationship between social activity and covariates using both parametric and non-parametric tests of correlation and analysis of variance for categorical variables. We used linear mixed effects models to examine the relationship of change in cognitive function with social activity. Models were fit separately for global cognition and each of the five cognitive domains. Models were fit with a random effects term for intercept and follow-up time as well as fixed effects terms for time, age, sex, race, education, and interactions of time with these demographic variables. Terms for social engagement at baseline and the interaction of time and social engagement were added to test the association between social engagement and baseline level of cognition and rate of change. To examine the influence of covariates, terms were added for social networks, depressive symptoms, chronic conditions, disability, neuroticism, and extraversion. To examine if the influence of social activity on cognitive decline was independent of other activities, terms were added for cognitive activity and physical activity. Because of missing income data for some participants, a model adjusting for all previously mentioned variables plus income was fit separately.
Finally, we conducted a series of sensitivity analyses to test whether reverse causality could influence our results. First, we removed persons who were most likely to have experienced cognitive decline due to preclinical dementia in the time before baseline. We repeated the fully adjusted model for global cognition after sequentially removing persons at the lowest levels of baseline cognitive function in 5 percentile increments up to the lowest 30%. We also repeated the fully adjusted model after excluding persons who were diagnosed with MCI at baseline. Because cognitive ability could still affect social activities within the “normal” range of cognitive performance, we also conducted an analysis of change in social activity as a function of cognitive function. We modeled change in social activity as a function of initial level of cognitive function using mixed effects models with terms for time, age, sex, education, race, and their interactions of all variables with time. To examine this association within the normal range of cognitive performance, observation time after a diagnosis of dementia over the course of follow-up was censored from analysis. Model validation was performed graphically and analytically and there was no evidence of nonlinearity or nonproportionality. Programming was done in SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Social activity scores ranged from 1 to 4.2 (mean = 2.6; SD = 0.6). More socially active persons had higher levels of global cognition at baseline (r = 0.30; p < .001) and were younger (r = −0.15; p < .001), more highly educated (r = 0.15; p < .001), less neurotic (r = −0.15; p < .001), more extraverted (r = 0.30; p < .001), and had fewer depressive symptoms (r = −0.10; p < .001), less disability (r = −0.17; p < .001), and larger social networks (r = 0.25; p < .001) than less socially active persons. Women were more socially active than men (mean = 2.60 vs. mean = 2.50, F = 6.7; p = .01). Chronic medical conditions and race (white vs. all others) were not significantly associated with social activity. Social activity was correlated with cognitive activity (r = 0.30; p < .001) and physical activity (r = 0.20; p < .001).
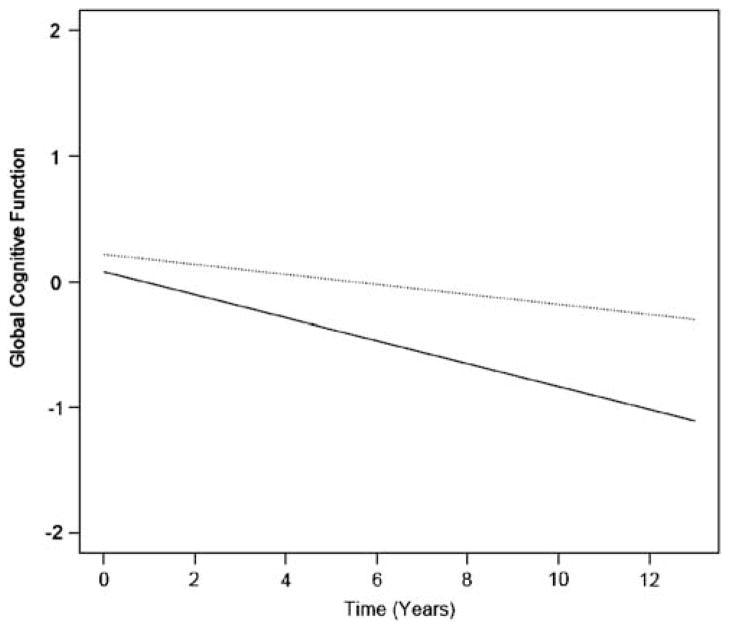
Participants were followed for an average of 5.2 years (SD = 2.8; range = 0.4–12.3). In an initial linear mixed effects model, we tested the relation of social activity to initial level of cognitive function and rate of change in cognitive function (Table 1, Model 1). In this model, there was an average decline of 0.074 unit per year on the global measure of cognition. Social activity was associated with higher baseline levels of global cognition and with a reduced rate of cognitive decline (as indicated by the statistically significant positive estimate for the social activity × time interaction term). In a fully adjusted model that included terms for covariates and other activity types, the association between social activity and initial level of cognitive function was attenuated almost by half, yet the association between social activity and rate of change in cognitive function was attenuated by less than 2% (Table 1; Model 2). In the fully adjusted model, on average, a one-point increase in social activity score was associated with a 0.034 unit reduction in rate of cognitive decline per year, or a 47% decrease in the annual rate of decline in global cognitive function. As shown in the figure, compared to a person who is infrequently socially active (score = 1.83; 10th percentile), rate of global cognitive decline was reduced by an average of 70% in a person who is frequently socially active (score = 3.33; 90th percentile). Separately, the fully adjusted model was repeated with a term for income in the subset with income data (n = 988) to more fully determine whether socioeconomic status accounted for the results. The association between social activity and change in cognition was essentially not affected by adjustment for income (estimate = 0.032; SE = 0.007; p < .001).
Table 1.
Associations between social activity and initial level and annual rate of change in global cognition, from linear mixed effects models
| Model term | Model 1
|
Model 2
|
||
|---|---|---|---|---|
| Estimate (SE) | p Value | Estimate (SE) | p Value | |
| Time | −0.074 (0.004) | <.001 | −0.070 (0.004) | <.001 |
| Social activity | 0.156 (0.024) | <.001 | 0.085 (0.027) | .002 |
| Social activity × time | 0.035 (0.007) | <.001 | 0.034 (0.007) | <.001 |
Note. Model 1 adjusted for age, age × time, sex, sex × time, race, race × time, education, and education × time. Model 2 adjusted for same terms as model 1 plus: social networks, depression, chronic conditions, disability, neuroticism, extraversion, cognitive activity, and physical activity.
Fig. 1.
Estimated decline in global cognition for participants with high (dotted line: 90th percentile, score = 3.3) versus low (solid line: 10th percentile, score = 1.8) late life social activity. From linear mixed model adjusted for age, age × time, sex, sex × time, race, race × time, education, education × time, social networks, depression, chronic conditions, disability, neuroticism, extraversion, cognitive activity, and physical activity.
To examine whether social activity was related to specific cognitive abilities, we repeated the fully adjusted analysis with the five specific cognitive abilities. Social activity was not consistently associated with initial level of cognitive function across the cognitive abilities, but was consistently associated with reduced cognitive decline in all five cognitive abilities (Table 2).
Table 2.
Associations between social activity and initial level and annual rate of change in cognitive domains, from linear mixed effects models
| Model term | Episodic memory
|
Semantic memory
|
Working memory
|
Perceptual speed
|
Visuospatial ability
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | |
| Time | −0.055 (0.005) | <.001 | −0.060 (0.005) | <.001 | −0.068 (0.005) | <.001 | −0.105 (0.005) | <.001 | −0.044 (0.005) | <.001 |
| Social activity | 0.126 (0.037) | <.001 | 0.022 (0.032) | .27 | 0.049 (0.040) | .22 | 0.103 (0.039) | .009 | 0.059 (0.040) | .14 |
| Social activity × time | 0.028 (0.008) | <.001 | 0.031 (0.007) | <.001 | 0.031 (0.007) | <.001 | 0.036 (0.008) | <.001 | 0.024 (0.008) | .003 |
Note. All models adjusted for age, age × time, sex, sex × time, race, race × time, education, education × time, social networks, depression, chronic conditions, disability, neuroticism, extraversion, cognitive activity, and physical activity.
To examine whether reverse causation could account for these findings, we conducted a series of sensitivity analyses. First, we repeated the fully adjusted model for global cognition after removing persons who were the most severely cognitively impaired at baseline, and thus the most likely to have experienced previous cognitive decline and limited their social activities. We first sequentially removed 30% of persons with the lowest levels of global cognition at baseline in 5 percentile increments. The association between social activity and global cognitive decline remained robust. Thus, in a model where we removed the lowest 30th percentile of cognition at baseline that included only 748 participants, more frequent social activity was associated with reduced cognitive decline (estimate = 0.034; SE = 0.007; p < .001). We also repeated the fully adjusted model after removing 322 persons with MCI at baseline. In this model that included 766 participants with no cognitive impairment, the association was very similar to the estimate for the analysis including persons with MCI (estimate = 0.032; SE = 0.007; p < .001). Finally, we examined whether cognitive ability could still affect social activities within the normal range of cognitive performance using mixed effects models adjusted for age, sex, race, education, and their interactions with time, using all observation time before a diagnosis of dementia [average of 4.7 years, SD = 2.7, range = 0.4–12.1; 224 participants (19.7%) developed dementia over follow-up]. Global cognition at baseline was associated with less social activity at baseline (estimate = 0.254; SE = 0.037; p < .001), but it was not related to change in social activity (estimate = 0.004; SE = 0.009; p = .61).
DISCUSSION
In a large, community-based cohort of older persons who were free of dementia at the beginning of observation, more frequent social activity was associated with subsequent reduced rates of cognitive decline over an average of 5 years of follow-up. On average, the most socially active individuals (90th percentile) experienced only one quarter of the rate of cognitive decline experienced by the least socially active individuals (10th percentile). The association was found broadly across several cognitive abilities, including a measure of global cognition as well as five cognitive domains. The association was robust to adjustment for a wide array of potentially confounding variables including socioeconomic status, social network size, health, disability, affect, and personality, as well as other types of activity: physical and cognitive. Several sensitivity analyses did not support the alternate hypothesis of reverse causation, including removing the most cognitively impaired participants at baseline, as well as examining change in social activity as a function of initial cognition. These findings are consistent with the results of previous research finding that more socially active older adults experience less cognitive decline on average (Barnes et al., 2004; Bassuk et al., 1999; Ertel et al., 2008; Lovden et al., 2005; Zunzunegui et al., 2003). Some of these studies also tested for and found little evidence of reverse causation (Barnes et al., 2004; Ertel et al., 2008; Lovden et al., 2005). Thus, while not all studies are consistent with these inferences (Aartsen, Smits, van Tilburg, Knipscheer, & Deeg, 2002; Hultsch, Hertzog, Small, & Dixon, 1999), a substantial body of literature is developing that supports the notion that frequent social activity may help to prevent or delay cognitive decline in old age.
This study bolsters previous findings by addressing several important methodological challenges. First, this analysis had a relatively long follow-up, 5 years on average, and each participant was observed on an average of 6 evenly spaced one year intervals (including the baseline visit). This enhances the power to detect patterns of change in cognition. Second, rather than relying on a single instrument or one domain of cognition, this study had robust measures of cognitive function based on a standardized and well characterized battery of neuropsychological tests of separate cognitive abilities. Third, we were able to adjust for a wide variety of potential confounders in addition to the standard adjustments for demographics, education, health, and affect. This includes personality type, which could select for who is socially active in older age and has been shown to be related to cognitive impairment (Hultsch et al., 1999; Wilson et al., 2007). Furthermore, the observed associations were independent of related constructs such as the number of persons in one’s social network, as well as other types of activity, cognitive and physical, that have been shown to be related to reduced cognitive decline (Sofi et al., 2011; Wilson et al., 2003). Thus, while social activity is likely to include cognitive and physical components, this indicates that social activity is independently related to cognitive decline regardless of one’s level of cognitive or physical activity. Adjustment for all of these variables attenuated only the relationship between social activity and the initial level of cognitive function, but had almost no effect on the relationship between social activity and cognitive decline.
Finally, we tested for the influence of reverse causation and the results were strongly robust to this alternative scenario. The association withstood the removal of 30% of the cohort with the lowest levels of baseline cognition. This study was also uniquely able to report that the association was robust to the exclusion of persons clinically diagnosed with MCI at baseline. These results suggest that is very unlikely that persons who previously experienced cognitive decline (i.e., preclinical dementia) were driving the association between baseline social activity and subsequent change in cognition. Moreover, there was no evidence that cognitive ability was related to change in social activity within the normal (i.e., nondemented) range of cognitive function. Other strengths of this study include the use of a large, well characterized community-based cohort of older persons free of dementia at baseline and a high rate of follow-up participation (over 94%) and the use of an established measure of late life social activity that has been correlated with other health outcomes (Buchman et al., 2009; James, Boyle, Buchman, & Bennett, 2011).
Although this analysis attempted to account for reverse causation and confounding, due to its observational study design, these two alternate scenarios cannot be ruled out completely. However, if social activity does have a causal role in delaying or preventing cognitive decline, the exact mechanisms are unknown. A commonly endorsed possibility is that social activity challenges older adults to participate in complex interpersonal exchanges, which could promote or maintain efficient neural networks in a case of “use it or lose it” (Hultsch et al., 1999). This could be achieved through the building of a cognitive reserve capacity that buffers the brain against the manifestation of cognitive impairment, even in the face of underlying neuropathology (Stern, 2002; Valenzuela & Sachdev, 2006). Social activity could also provide meaningful social roles and a sense of purpose in old age (Berkman, 2000), which could have direct neurohormonal influences on the brain including the reduction of the stress response (Fratiglioni et al., 2004). Finally, although we controlled for physical activity, social activity also requires a degree of physical activity above and beyond regular exercise and walking which could enhance cardiopulmonary fitness, leading to vascular changes in the brain and cerebral oxygenation that might protect against neuropathology (Fratiglioni et al., 2004).
A limitation of this study was the assessment of social activity through self-report, which may be subject to recall bias particularly for persons with cognitive difficulties. Another limitation that may limit the generalizability of these findings to the general older population is the use of a mostly white volunteer cohort who agreed to annual evaluations and post-mortem organ donation. Additionally, the population was on average close to 80 years of age at baseline, thus potentially representing a cohort of “healthy survivors” who may be more socially active than the general population of persons aged 65 and older. Finally, we did not have the capacity to examine social activity before old age, and thus we were not able to take into account the influence of timing and duration of social activity along the life course. Current social activity in old age may reflect life-long patterns of behavior, changes in lifestyle initiated in later life, or a combination of both. The data here do not allow for inferences regarding the time-dependency of this association, nor do they address whether adopting new social activities in old age is an effective strategy in maintaining cognitive health. The Experience Corp, an intervention trial to increase engagement in social and other activities in older adults, has shown promising results over a short (months to a few years) period (Carlson et al., 2008; Fried et al., 2004), yet the greatest effect on cognitive health is likely achieved through a lifetime of social activity. Despite a recent expert review stating that there is inconclusive evidence of risk factors for cognitive decline based mostly on observational data (Plassman, Williams, Burke, Holsinger, & Benjamin, 2010), well-designed observational studies are valuable in establishing the cumulative effects of lifetime of social activity on cognitive health. This study provides convincing evidence that a socially active lifestyle can help to prevent cognitive decline in old age.
Acknowledgments
We are indebted to the participants and the staff of the Rush Memory and Aging Project and the Rush Alzheimer’s Disease Center for this work, and to Sue Leurgans, PhD, for biostatistical consultation. This work was supported by the Illinois Department of Public Health, the National Institute on Aging grants R01AG17917, R01AG024871, R01AG22018, and the Robert C. Borwell Endowment Fund.
Footnotes
The authors had no conflicts of interest influencing this manuscript.
References
- Aartsen MJ, Smits CH, van Tilburg T, Knipscheer KC, Deeg DJ. Activity in older adults: Cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2002;57(2):153–162. doi: 10.1093/geronb/57.2.p153. [DOI] [PubMed] [Google Scholar]
- Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology. 2005;19(4):520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of Internal Medicine. 1999;131(3):165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurology. 2006;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- Berkman LF. Which influences cognitive function: Living alone or being alone? Lancet. 2000;355(9212):1291–1292. doi: 10.1016/S0140-6736(00)02107-3. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Wilson RS, Fleischman DA, Leurgans S, Bennett DA. Association between late-life social activity and motor decline in older adults. Archives of Internal Medicine. 2009;169(12):1139–1146. doi: 10.1001/archinternmed.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Saczynski JS, Rebok GW, Seeman T, Glass TA, McGill S, Fried LP. Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience Corps(R) Gerontologist. 2008;48(6):793–801. doi: 10.1093/geront/48.6.793. [DOI] [PubMed] [Google Scholar]
- Cornoni-Huntley JB, Brock DB, Ostfeld AM, Taylor JO, Wallace RB. NIH Publication No. 86–2443. Washington, DC: US Department of Health and Human Services; 1986. Established populations for epidemiologic studies of the elderly resource data book. [Google Scholar]
- Costa P, McCrae R. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Jr, Cox NJ, Trevisan M. National Institutes of Health State-of-the-Science Conference statement: Preventing alzheimer disease and cognitive decline. Annals of Internal Medicine. 2010;153(3):176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- Ertel KA, Glymour MM, Berkman LF. Effects of social integration on preserving memory function in a nationally representative US elderly population. American Journal of Public Health. 2008;98(7):1215–1220. doi: 10.2105/AJPH.2007.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrigoule C, Letenneur L, Dartigues JF, Zarrouk M, Commenges D, Barberger-Gateau P. Social and leisure activities and risk of dementia: A prospective longitudinal study. Journal of the American Geriatrics Society. 1995;43(5):485–490. doi: 10.1111/j.1532-5415.1995.tb06093.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Fried LP, Carlson MC, Freedman M, Frick KD, Glass TA, Hill J, Zeger S. A social model for health promotion for an aging population: Initial evidence on the Experience Corps model. Journal of Urban Health. 2004;81(1):64–78. doi: 10.1093/jurban/jth094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler JE, Duval S, Anderson KA, Kane RL. Predicting nursing home admission in the U.S: A meta-analysis. BMC Geriatrics. 2007;7:13. doi: 10.1186/1471-2318-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14(2):245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- James BD, Boyle PA, Buchman AS, Bennett DA. Relation of late-life social activity with incident disability among community-dwelling older adults. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2011;66:467–473. doi: 10.1093/gerona/glq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dementia and Geriatric Cognitive Disorders. 2005;21(2):65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- Katz S, Akpom CA. A measure of primary socio-biological functions. International Journal of Health Services. 1976;6(3):493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. Journal of Aging and Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Krueger KR, Wilson RS, Kamenetsky JM, Barnes LL, Bienias JL, Bennett DA. Social engagement and cognitive function in old age. Experimental Aging Research. 2009;35(1):45–60. doi: 10.1080/03610730802545028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovden M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psychology and Aging. 2005;20(3):423–434. doi: 10.1037/0882-7974.20.3.423. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. American Journal of Preventive Medicine. 1989;5(2):65–72. [PubMed] [Google Scholar]
- Plassman BL, Williams JW, Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine. 2010;153(3):182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Pfeifer LA, Masaki K, Korf ES, Laurin D, White L, Launer LJ. The effect of social engagement on incident dementia. American Journal of Epidemiology. 2006;163:433–440. doi: 10.1093/aje/kwj061. [DOI] [PubMed] [Google Scholar]
- Schupf N, Tang MX, Albert SM, Costa R, Andrews H, Lee JH, Mayeux R. Decline in cognitive and functional skills increases mortality risk in nondemented elderly. Neurology. 2005;65(8):1218–1226. doi: 10.1212/01.wnl.0000180970.07386.cb. [DOI] [PubMed] [Google Scholar]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, Macchi C. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. Journal of Internal Medicine. 2011;269:107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Tabbarah M, Crimmins EM, Seeman TE. The relationship between cognitive and physical performance: MacArthur Studies of Successful Aging. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2002;57(4):M228–M235. doi: 10.1093/gerona/57.4.m228. [DOI] [PubMed] [Google Scholar]
- US Public Health Service. 1985 Health Interview Survey. Hyattsville, MD: National Center for Health Statistics; 1985. [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: A non-parametric systematic review. Psychological Medicine. 2006;36(8):1065–1073. doi: 10.1017/S0033291706007744. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society. 2005;11(4):400–407. [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61(6):812–816. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Buchman AS, Arnold SE, Shah RC, Tang Y, Bennett DA. Harm avoidance and disability in old age. Experimental Aging Research. 2006;32(3):243–261. doi: 10.1080/03610730600699142. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Bennett DA. Loneliness and risk of Alzheimer disease. Archives of General Psychiatry. 2007;64(2):234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Journal of the American Medical Association. 2002;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Zunzunegui MV, Alvarado BE, Del Ser T, Otero A. Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2003;58(2):S93–S100. doi: 10.1093/geronb/58.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]