Abstract
Alveolar capillary dysplasia with misalignment of the pulmonary veins (ACD/MPV) is a rare, fatal developmental lung disorder of neonates and infants. This review aims to address recent findings in the etiology and genetics of ACD/MPV and to raise awareness of this poorly known disease, which may also present as milder, unclassified forms. Successively discussed are what is known about the epidemiology, pathogenesis, pathophysiology, diagnostic indicators and approaches, genetic testing, treatment, and cases of delayed onset. The review concludes with suggestions for future directions to answer the many unknowns about this disorder.
Keywords: interstitial lung disease, persistent pulmonary hypertension of the newborn, alveolar air–blood barrier, FOXF1 transcription factor gene, comparative microarray analysis
The interstitial lung diseases of childhood, characterized by defective gas exchange, restrictive lung physiology, and diffuse involvement on lung imaging, include a heterogeneous group of conditions involving disordered development of the alveoli and distal airspaces (1, 2). Alveolar capillary dysplasia with misalignment of the pulmonary veins (ACD/MPV), the most common of these disorders, produces respiratory failure early in life and carries a mortality rate that approaches 100% (3). ACD/MPV is distinguished by immature lobular development and reduced capillary density, both of which indicate premature growth arrest. Distinct histological features allow for identification by a pathologist with a high index of suspicion and experience in diagnosing rare pediatric lung disorders.
Until recently, the primary clinical focus has been on early recognition and accurate diagnosis of ACD/MPV to avoid invasive and futile interventions for patients and their families. However, advances in our understanding of the genetics, pathology, and clinical features of the disease have begun to shed light on both the disease burden (revealing a potentially higher prevalence than originally thought) and underlying physiologic mechanisms. Furthermore, reports of infants presenting with fulminant symptoms of ACD/MPV well beyond the neonatal period, even as late as 7 months of age, have begun to emerge, challenging the established phenotype and offering the possibility that long-term survivors with milder forms of the disease may exist (4). DNA sequencing and comparative genomic hybridization have led to the identification of FOXF1 as one of the genes responsible for ACD/MPV and allowed for limited noninvasive diagnostic testing in some infants (5, 6).
As is true for most rare diseases, progress in understanding ACD/MPV depends on the identification of new cases and increasing awareness among health-care providers. Insights into the physiologic mechanisms underlying the development of ACD/MPV also may illuminate aspects of normal lung development that thus far have remained elusive. This review is presented with these considerations in mind.
Historical Perspective
MacMahon made the first mention of “congenital alveolar dysplasia of the lungs” in 1947, when he described a condition “involving both lungs uniformly or only a portion of a single lobe” associated with abnormalities in other organs (7).
The seminal case reported by Janney and colleagues in 1981 (8) described a term neonate who developed severe respiratory distress 12 hours after birth and a clinical course consistent with persistent fetal circulation. Despite the best treatment available at the time, the infant developed severe bradycardia and hypotension and died at 40 hours of life. Post mortem examination of the lungs showed “failure of formation and ingrowth of alveolar capillaries”, abnormal air–blood barriers, and anomalous veins in the bronchovascular bundles. Janney and her team designated this entity of persistent fetal circulation with abnormalities of pulmonary vascular development “congenital alveolar capillary dysplasia.”
Since these early descriptions, more than 100 cases of ACD/MPV have been reported worldwide. Most of them have been sporadic, de novo events, but approximately 10% involved siblings, suggesting a heritable form of the disease (9–11). For several reasons, these case reports almost certainly underestimate the true prevalence of ACD/MPV. First, the definitive diagnosis of ACD/MPV currently depends on histological examination of lung tissue on autopsy or ante mortem lung biopsy, and neither of these diagnostic modalities is universally pursued in the setting of a critically ill or dying newborn (12). Second, many infants born with ACD/MPV have associated malformations in other organ systems that may allow the lung pathology to go undetected. Third, the diagnosis may be missed by pathologists unfamiliar with the disorder (13–16). Last, evidence is mounting to suggest that a less severe phenotype compatible with prolonged survival might exist, although definitive diagnostic criteria for this group are yet to be established (17–19).
Epidemiology
The incidence or prevalence of ACD/MPV is not yet known, but there are clearly more cases than those that have been reported formally in the literature. Furthermore, it seems likely that some cases originally classified as idiopathic persistent pulmonary hypertension of the newborn (PPHN) may actually have been ACD/MPV (14, 20). A slight male predominance (60%) has appeared in reported cases (21, 22). No geographic pattern is apparent; cases have been distributed worldwide.
More than 90% of affected infants are born at term (17, 21), and in greater than 60% of cases, onset of cyanosis and respiratory failure occurs within 48 hours of birth (9, 23). In a recent review of 21 cases of ACD/MPV from England, Melly and colleagues (24) proposed that the severity of disruption in capillary density and lack of contact with the alveolar epithelium may be two major factors that determine clinical course and prognosis, and they ascribed the potential for survival beyond the neonatal period in a minority of patients to higher capillary density and apposition. The prevalence of this milder, potentially survivable phenotype is not known.
The number of infants with ACD/MPV treated with extracorporeal life support is unknown, but several case series cite infants with unexplained PPHN who fail to improve with full cardiopulmonary support (15, 25). Twenty-two percent of neonates referred for PPHN do not survive, according to 2010 Extracorporeal Life Support Organization registry data (26). Given the decrease in extracorporeal membrane oxygenation (ECMO) referrals for PPHN (27, 28) and the generally favorable outcomes, this group of nonsurvivors may include infants with ACD/MPV.
Pathogenesis
The pathogenesis of both ACD/MPV and its associated pulmonary hypertension remain to be fully explained. Cullinane and colleagues (13) were the first to suggest that the primary pulmonary vascular anomaly is a failure of fetal lung vascularization in response to an unknown teratogen. With the number of pulmonary capillaries drastically reduced, blood entering an individual lung unit can only drain via anomalous (“misaligned”) veins. The obstruction that is created leads to the occlusive changes observed in the pulmonary arterial circulation. Sirkin and colleagues (29) shared the view that an early antenatal insult leads to a deficient pulmonary capillary bed and a decreased bronchial generation count, and suggested a genetic defect could explain both the extrapulmonary findings and ACD/MPV occurrence in families. Others have proposed that the muscular thickening in the small pulmonary arteries may be the primary lesion, resulting from pulmonary vasoconstriction, with consequent failure of normal alveolar capillary development (30). However, this latter theory does not adequately explain the vascular changes observed in utero, because gas exchange does not involve the alveolar capillary interface before birth. Further identification of the genetic mechanisms involved in the pathogenesis of ACD/MPV will likely clarify the underlying defect in pulmonary vascular development that results in irreversible hypoxemia and pulmonary hypertension and explain the characteristic patterns of functional response to therapy.
Pathophysiology and Diagnosis
Physical and Laboratory Findings
Key diagnostic criteria are summarized in Tables 1 and 2. Infants with ACD/MPV usually present with minimal or no parenchymal lung disease. Profound hypoxemia, with partial pressures of oxygen in arterial blood less than 30 mm Hg, and severe metabolic acidosis consistent with pulmonary hypertensive crisis and right ventricular failure are nearly always present. However, to date, no routine laboratory tests can distinguish ACD/MPV from other causes of neonatal pulmonary hypertension. Blood gases may support the diagnosis, but threshold values that are pathognomonic for ACD/MPV have not been identified. ACD/MPV should always be considered in infants with idiopathic PPHN whose symptoms recur after successful weaning from ECMO (31, 32).
TABLE 1.
KEYS TO DIAGNOSIS
| Perinatal | Prenatal risk factors: none identified |
| Gestation: full term (> 90%) | |
| Apgar scores: not predictive (often 8 or 9 at 1 and 5 min) | |
| Symptom onset: 24–48 h after birth (> 60%) or weeks to months (delayed presentation) | |
| Presentation (variable) | Classic neonatal presentation |
| PPHN: tachypnea, cyanosis, respiratory distress | |
| Acute hypoxemic respiratory failure | |
| Idiopathic pulmonary hypertension | |
| Delayed presentation | |
| Asymptomatic or mild tachypnea at birth; ± transient oxygen requirement; may be evaluated for sepsis | |
| Course | Early (at symptom onset) |
| Transient response to pulmonary vasodilators, mechanical ventilation, extracorporeal life support | |
| Later (hours to weeks) | |
| Unremitting pulmonary hypertension | |
| Second course of ECMO not curative | |
| Uniformly fatal without lung transplantation | |
| Associated anomalies | Important diagnostic clue when present with unexplained PPHN |
| Most commonly: gastrointestinal, genitourinary, cardiac systems (detailed in Table 2) | |
| Imaging (nondiagnostic) | Chest radiograph: normal, hazy, or diffuse ground-glass pattern; pneumothoraces may be present early or develop late |
| Echocardiogram: pulmonary hypertension (right ventricular hypertrophy, bowing of IVS into LV, tricuspid regurgitation) | |
| PFO or PDA | |
| Cardiac catheterization: often no structural heart lesion | |
| Normal, delayed, or absent capillary blush phase | |
| Laboratory tests | Not pathognomonic |
| Pathology (gold standard) | Characteristic histology (detailed in Table 3) |
| Genetics | Approximately 10% of cases are familial |
| Microdeletions, point mutations in or upstream to the FOXF1 gene on chromosome 16q24.1 (∼ 40%) |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; IVS = intraventricular septum; LV = left ventricle; PDA = patent ductus arteriosus; PFO = patent foramen ovale; PPHN = persistent pulmonary hypertension of the newborn.
TABLE 2.
EXTRAPULMONARY ANOMALIES ASSOCIATED WITH ALVEOLAR CAPILLARY DYSPLASIA WITH MISALIGNMENT OF THE PULMONARY VEINS
| System | Examples (References) |
| Gastrointestinal | Intestinal malrotation (10, 14–16, 30, 34, 46, 63) |
| Esophageal atresia (14) | |
| Tracheoesophageal fistula (14, 15) | |
| Anal atresia (14, 51) | |
| Absent gallbladder (10, 14, 29, 34) | |
| Postpyloric ecstasy of the small intestine (34, 64) | |
| Omphalocele (23) | |
| Duodenal stenosis (14, 21, 34, 63) | |
| Meckel diverticulum (34, 59) | |
| Arteriovenous malformation of the liver (cavernous hemangioma) (10, 20) | |
| Volvulus of the small bowel (34, 63) | |
| Total absence or decreased number of ganglionic cells of the colon (10, 14, 15, 39, 63) | |
| Redundant colon (14, 15, 34, 63) | |
| Imperforate anus (10, 14, 20, 34) | |
| Annular pancreas (14, 30, 34) | |
| Asplenia (14, 22, 34, 43) | |
| Urogenital | Bilateral ureteropelvic junction obstruction with hydronephrosis (9, 10, 34) |
| Bilateral hydronephrosis, posterior urethral valves (10, 15, 34, 63) | |
| Bladder hypertrophy (10) | |
| Hydroureter (10) | |
| Stenosis of the distal ureters with hydronephrosis (34, 37, 65) | |
| Bicornuate uterus (8, 34, 63, 66) | |
| Cryptorchidism (9, 15, 34) | |
| Cardiovascular | Hypoplastic left heart syndrome, left outflow tract stenosis (10, 34, 40, 44) |
| Bicuspid aortic valve (21, 40) | |
| Patent ductus arteriosus (43, 44) | |
| Atrial septal defect (10, 43, 44) | |
| Atrioventricular septal defect, quadricuspid pulmonary valve (40) | |
| Pulmonary valvular and subvalvular stenosis (43) | |
| Malformed mitral and tricuspid valves (10, 59) | |
| Retroesophageal subclavian artery (34, 63) | |
| Continuation of the left superior vena cava with the coronary sinus (18, 34) | |
| Absent right umbilical artery (34) | |
| Cor triatriatum, nonobstructive (10, 34, 43, 46) |
Extrapulmonary findings are present in 50 to 80% of cases (10, 14, 21, 25, 30, 33, 34). Therefore, an infant with unexplained respiratory failure and structural abnormalities of the genitourinary, gastrointestinal, or cardiovascular systems should be evaluated for ACD/MPV (10, 33). Additionally, disruption of right–left asymmetry of the intrathoracic and/or intraabdominal organs has been reported in more than 25% of cases (10). ACD/MPV has occurred in infants with Down syndrome; the existence of one does not preclude the other (35).
Imaging Studies
The chest radiograph may show diffuse haziness or subtle ground-glass opacities but is often interpreted as normal. Pneumothoraces have been reported frequently in patients with fulminant disease, but it is not entirely clear whether this feature is related to abnormalities in lung architecture or surfactant function, a consequence of aggressive ventilator treatment to reverse hypoxemia, or is some combination of the two. To date, there are no reports of computed tomography or magnetic resonance lung imaging in infants with documented ACD/MPV.
An echocardiogram is performed to exclude a cardiac cause for pulmonary hypertension. Although the images will typically demonstrate suprasystemic right ventricular pressures and right-to-left shunting through a patent ductus arteriosus or a patent foramen ovale, these findings are not specific for ACD/MPV.
The presence of delayed or absent capillary blush phase on pulmonary angiography has been reported in a small series of patients with ACD/MPV who underwent cardiac catheterization for evaluation of pulmonary hypertension (36). At present, this finding is only supportive and cannot be used as a substitute for histopathologic diagnosis.
Confirmatory Diagnosis
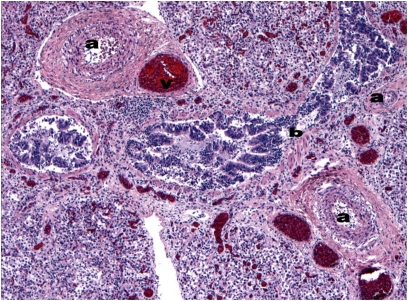
Currently, definitive diagnosis of ACD/MPV is based on histological examination of lung tissue by a pediatric pathologist with prior experience in diagnosing ACD/MPV. The characteristic histological features of ACD/MPV are listed in Table 3 and can be seen in Figure 1.
TABLE 3.
CONFIRMATORY DIAGNOSIS
| Histopathologic Features |
| Immature lobular development |
| Decreased number of pulmonary capillaries located away from the alveolar epithelium |
| Thickened alveolar septae |
| Medial hypertrophy of small pulmonary arteries and muscularization of distal arterioles |
| Malposition of pulmonary vein branches adjacent to pulmonary arteries (same adventitial sheath) |
| Lymphangiectasis (30%) |
Figure 1.
Hematoxylin and eosin staining of an autopsy specimen from an infant who presented at 5 weeks demonstrates the characteristic histologic features of ACD/MPV: small pulmonary arteries with medial hyperplasia (a), congested pulmonary veins malpositioned within the same adventitial sheath (v), and a dilated bronchiole (b). Additional findings, (i.e., neutrophilic infiltrate and hemorrhage) are consequences of the hypoxemic respiratory failure and aggressive ventilatory support. (Courtesy of the Weill Cornell Medical College Department of Pathology and Laboratory Medicine.)
Genetic Testing
In a recent published report (5), mutations or deletions in the FOXF1 transcription factor gene or deletions upstream to FOXF1 were identified in 40% of cases. Blood testing to screen for these defects is now available, but a negative result does not preclude the diagnosis, and histological examination of lung tissue remains the gold standard. Currently, the Department of Molecular and Human Genetics at Baylor College of Medicine in Houston is the only center known to be screening prenatally and postnatally for mutations and deletions. FOXF1 mutation analysis is a first step toward providing prenatal diagnosis for families at high risk.
Lung Biopsy
Thus far, approximately 90% of reported cases of ACD/MPV have been diagnosed on autopsy, and 10% of diagnoses have been made from lung tissue obtained during ante mortem lung biopsy. Although an infant may appear too unstable to tolerate an operative procedure, a positive biopsy provides important prognostic information and helps to dictate the care plan (37). Both open and thoracoscopic biopsy approaches have been used successfully (38).
The diagnostic yield from an operative lung biopsy is in large part dependent on surgical technique and proper handling of the tissue specimens. The Children's Interstitial Lung Disease (chILD) Pathology Cooperative Group, under the leadership of Dr. Claire Langston, developed a protocol for the handling of tissue obtained from operative lung biopsy (39). These comprehensive guidelines, which include recommendations for biopsy sites, sampling techniques, specimen size, tissue staining, and sample processing, are intended to standardize the approach to pathologic diagnosis to achieve optimal results.
It is likely that cases of ACD/MPV have been missed due to parents refusing autopsy or to reticence on the part of some health-care providers to advocate strongly for post mortem studies. As awareness of ACD/MPV among caretakers increases, it is anticipated that more infants will undergo ante mortem lung biopsy, yielding additional cases for study.
Differential Diagnosis
The following diagnoses should be excluded when evaluating an infant for ACD/MPV (22, 40–42):
Idiopathic PPHN, which is generally distinguished from ACD/MPV by its reversibility, most often resolving completely with pulmonary vasodilators, respiratory support, and/or ECMO;
Sepsis, pneumonia, and other infectious etiologies;
Primary respiratory disorders, including abnormalities of surfactant production (e.g., surfactant protein B deficiency, transporter abnormalities) or function (including hyaline membrane disease), pulmonary hypoplasia, congenital diaphragmatic hernia, and other rare diffuse interstitial lung disorders (e.g., acinar dysplasia, congenital alveolar dysplasia) (24);
Congenital cardiopulmonary diseases associated with severe cyanosis and/or pulmonary hypertension, most notably pulmonary venous stenosis and total anomalous pulmonary venous return. However, it should be remembered that cardiac lesions have been reported in up to 25% of children with ACD/MPV (10, 16, 34, 40, 43–45);
Neurologic disorders, including perinatal asphyxia and congenital neuromuscular disorders.
Genetics: Past and Future Approaches
The genetic etiology of ACD/MPV has remained elusive for decades (46). Pedigree analyses of ACD/MPV patients' families have suggested both autosomal dominant and recessive patterns of inheritance (10), thus implicating at least two causative genes.
Early (Abandoned) Candidate Genes: STRA6, BMP2, and AIMP1
Previously investigated candidate genes include the STRA6 (stimulated by retinoic acid 6) gene, which has been implicated in patients with ACD features and other malformations such as anophthalmia (47), the BMP2 (bone morphogenetic protein 2) gene, and the AIMP1 (previously known as EMAP II, endothelial monocyte-activating polypeptide II) gene. None of these genes was found to have been mutated in a group of patients with ACD/MPV; furthermore, the lung changes reported in those with STRA6 mutations did not include misalignment of pulmonary veins.
Genomic Deletions at 16q24.1
In 2009, Stankiewicz and colleagues (5) described the identification of different-sized overlapping submicroscopic genomic deletions in chromosomal region 16q24.1q24.2 in six unrelated patients with histopathologically verified sporadic ACD/MPV and other congenital malformations. These microdeletions were detected using array comparative genomic hybridization and ranged in size from approximately 100 kb to approximately 3.5 Mb. No deletions in this genomic region are found in different databases of genomic variants, indicating that these changes are pathogenic.
A Candidate Gene
The smallest region of overlap of the identified deletions at chromosomal region 16q24.1 contained the FOX transcription factor gene cluster, including FOXF1, FOXC2, and FOXL1. FOXF1 (Forkhead Box-F1, previously known as HFH-8 or FREAC-1) proved to be a very good candidate gene for ACD/MPV given its expression pattern (i.e., expressed in mesodermal endothelial and smooth muscle cells in the fetal and adult lung) and the abnormalities described in a previously reported murine model.
Animal Model of ACD
The murine model of FOXF1 deficiency was developed in Chicago in 2001 in the laboratory of Robert Costa, Ph.D. (48). Mouse embryos with homozygous (i.e., both copies) deficiency of Foxf1 all died by 8.5 days post coitum due to pulmonary vascular abnormalities. Interestingly, of the heterozygous mice (i.e., those with only one copy of the gene deficient), half lived without symptoms as normal mice, whereas the remainder had abnormal alveolar development and died with pulmonary hemorrhage in the setting of severe defects in alveolarization and vasculogenesis (49, 50). Their pulmonary histopathologic changes included some but not all of those seen in human infants with ACD/MPV, and because of this incomplete recapitulation, the connection to ACD/MPV was not made at the time.
To explain these unusual phenotypic findings, Kalinichenko and colleagues measured the levels of the Foxf1 protein in heterozygous Foxf1-deficient mice and observed that normal mice restored the decreased level of Foxf1 protein at the end of gestation, whereas affected mice did not. This was interpreted as “compensation” for the alveolarization defect (48).
FOXF1
To determine whether FOXF1 is pathogenic for ACD/MPV in humans, Stankiewicz and colleagues (5) sequenced FOXF1 in a cohort of 18 patients with ACD/MPV and other malformations collected at Baylor College of Medicine in Houston. This research team identified four different de novo heterozygous inactivating (frameshift, nonsense) point mutations in the coding sequence of FOXF1 in this group of unrelated patients with sporadic ACD/MPV, thus proving that FOXF1 is a pathogenic gene. In the remaining mutation-negative ACD/MPV cases, a 16q24 region-specific high-resolution oligonucleotide microarray analysis revealed three de novo deletions in 16q24.1 (5), further confirming that FOXF1 is a dosage-sensitive (haploinsufficient) gene, responsible for approximately 40% of ACD/MPV cases, representing a new genomic disorder. The presence of two upstream deletion copy number variants leaving FOXF1 intact, as well as the intriguing fact that all the analyzed deletions were of maternal inheritance, indicated that other molecular mechanisms, such as position effects and genomic imprinting, may operate at this FOX gene cluster locus.
In contrast to the association of point mutations in FOXF1 with bowel malrotation, microdeletions of FOXF1 were found in patients with ACD/MPV who also manifested hypoplastic left heart syndrome and gastrointestinal atresias, likely due to haploinsufficiency for the neighboring FOXC2 and FOXL1 genes. These differences reveal the phenotypic consequences of gene alterations in cis.
The additional anomalies, cardiac malformations, esophageal and anal atresia, renal and vertebral/axial malformations, which are variably present in up to 80% of patients with ACD/MPV with deficiency of FOXF1, are also common findings in patients with VACTERL (vertebral, anal, cardiac, tracheoesophageal, renal, and limb malformations) association. Thus, the identification of FOXF1 may contribute to unraveling the molecular bases of the VACTERL association.
Search for the Other ACD/MPV Gene(s)
Studies in humans and mice have revealed that the FOXF1 protein is involved in sonic hedgehog signaling, implying that other genes from this well-known and important pathway may be involved in the etiology of ACD/MPV.
Currently, the most promising approaches for novel gene identification are next-generation sequencing technologies that have been successfully applied for both whole-genome and whole-exome sequencing. These methodologies have already enabled identification of several new genes (51–53). Although still relatively expensive, these technologies will soon be used in sequencing some ACD/MPV samples from pedigrees that suggested both autosomal dominant and recessive inheritance. For families showing autosomal recessive pattern inheritance, the addition of single-nucleotide polymorphism microarrays may be of help in narrowing the critical region associated with ACD/MPV.
Identification of additional ACD/MPV genes would be broadly informative to the general biology and pathophysiology of human lung development. In particular, these data may contribute to a better understanding of the etiology of pulmonary hypertension, a characteristic feature of ACD/MPV and a common pulmonary disorder.
Treatment
The clinical approach to infants with ACD/MPV is no different than that for other neonates presenting with PPHN. However, the response to therapy is often minimal and/or not sustained, which may serve as an initial diagnostic clue.
Because the hypoxemia is so severe and unrelenting, many practitioners will attempt lung recruitment strategies, such as high-frequency ventilation or surfactant. However, infants with ACD/MPV usually present with minimal parenchymal lung disease. Lung recruitment strategies may induce respiratory alkalosis and a transient improvement in oxygenation, but may also contribute to rapid deterioration due to lung overdistension and associated compromise of right atrial filling. Recent case series have reported high rates of unilateral or bilateral pneumothorax, which could be another consequence of excessive airway pressures (20, 54).
Most infants with ACD/MPV will develop progressive hypotension due to right ventricular failure and/or refractory hypoxemia. Because the hypoxemia and pulmonary hypertension cannot be effectively reversed, cardiotonic agents have only a minimal or transient effect in infants with ACD/MPV.
Although transient responses to pulmonary vasodilator therapy can be observed in infants with ACD/MPV, no infant has been reported to have a sustained response to any available pulmonary vasodilator. In a study of five infants with documented ACD/MPV, all responded to inhaled nitric oxide with improved oxygenation (>200 mm Hg) and reduced pulmonary artery pressures, but deterioration and death followed within hours (25). Other reports have demonstrated a similar pattern of response to inhaled or intravenous prostacyclin (55, 56), suggesting that the pattern of initial improvement followed by deterioration may be specific to the anomalous vasculature of ACD/MPV. To date, there are no reports of sildenafil use in infants with ACD/MPV.
ECMO is used at some point in the clinical course in most case series of ACD/MPV, and a report from the U.K. ECMO experience found that ACD/MPV was diagnosed in 14% of babies who died after ECMO support. This was almost certainly an underestimate, as ACD/MPV was documented in 33% of babies who had full autopsy examinations (12). At least one additional report showed that ACD/MPV was found in the majority of infants with idiopathic PPHN who did not respond to ECMO (six of seven infants) (20). The registry maintained by the Extracorporeal Life Support Organization indicates that more than 20% of infants with idiopathic PPHN do not respond to ECMO support, which should prompt investigation for ACD/MPV (20, 57).
Because none of the supportive therapies described above has changed the expected mortality due to ACD/MPV, lung transplantation is currently the only option that might prolong survival. Although successful lung transplantation in infants with ACD/MPV has not been reported, achieving early diagnosis by lung biopsy is an essential first step, followed by strategies that sufficiently prolong survival. A recent report demonstrated survival of an infant for more than 3 months after presentation in the neonatal period (58), indicating that lung transplantation may provide effective treatment for carefully selected affected infants.
Late Presenters and Long-Term Survivors
Reports of infants presenting with clinical features of ACD/MPV beyond the neonatal period have begun to emerge. Thus far, four reports of infants presenting with fulminant disease at 5 weeks, 7 weeks, 12 weeks, and 7 months have been published (4, 18, 19, 59). Although two of them presented with symptoms serious enough to warrant brief periods of respiratory observation in a neonatal intensive care unit before discharge (4, 59), the remaining two appeared asymptomatic and were discharged home as well babies (18, 19). With such limited information, it is difficult to estimate the number of patients with delayed presentation of ACD/MPV, but it is certainly greater than the four mentioned above. Until more specific diagnostic tools become available, similarities in lung pathology and clinical findings should increase diagnosis using the same approach to lung tissue sampling in late presenters as for those who present early.
The pathophysiology underlying the development of ACD/MPV has yet to be fully characterized, and mechanisms that might explain delayed presentation are even less clear. How infants with structural defects at the level of the air–blood barrier can remain virtually asymptomatic for weeks or months is one of the urgent questions now under investigation. The findings of Melly and colleagues (24) suggest that variations in disease severity and timing of presentation correspond to alterations in capillary density and contact with the alveolar epithelium. Better lobular development and patchy involvement have also been cited to explain the delay in onset of symptoms (9, 30, 60), but this remains controversial. In fact, thus far, the histological appearance of lung tissue taken from early and late presenters has been strikingly similar (4). As such, mechanistic explanations for delayed presentation of ACD/MPV will require the identification of triggers for the acute deterioration that signals the onset of fulminant disease. One of the most plausible explanations suggests that the onset of symptoms, whether early or delayed, results from vasoconstriction of a hypertrophied arterial bed (30), which then triggers a pulmonary hypertensive crisis. Interestingly, viral upper respiratory infections, known to trigger pulmonary hypertension in those at risk, were reported in two of the late presenters. Regardless, the remarkable overlap in clinical course and outcome of infants with advanced disease, whether the onset is early or delayed, requires further investigation.
There appears to be no survival advantage in presenting late. Once symptoms develop, the infant with delayed presentation of ACD/MPV experiences the same downward spiral that results in death.
A better understanding of the genetic and physiologic mechanisms that govern the ACD/MPV phenotype and timing of presentation may lead to expanding the current case definition to include long-term survivors. Of note, a recent case report in the British Journal of Radiology (61) described a 21-year-old woman with newly diagnosed pulmonary hypertension and “misplaced pulmonary arteries” located in the interlobular septae (in addition to ones that were located normally in the bronchovascular bundles). Although disputed, the authors claim that the histological findings are similar to those described in newborns with PPHN and may represent a forme fruste of the vascular anomaly seen in ACD/MPV. If replicated, their findings would lend support to the hypothesis that milder, survivable forms of the disorder exist.
Future Directions
Advances in ACD/MPV genetics research, combined with support from federal health-related research agencies, such as the Centers for Disease Control and Prevention and the National Institutes of Health, are providing an impetus for ongoing investigation into several aspects of this rare disease. Steadfast commitment by researchers and rare disease coalitions, academic research networks such as the Children's Interstitial Lung Disease Research Network (chILDRN), parent advocacy groups, and others, along with encouraging developments in the areas of genetic technology (i.e., the availability of oligonucleotide comparative genomic hybridization and exome sequencing) and electronic database development (with the impending launch of a state-of-the-art interactive international electronic database for rare lung diseases in children) should accelerate the rate at which further progress will be made.
With this foundation, future activities should place emphasis on the following goals:
To identify additional genes or genetic mechanisms operating at the FOXF1 locus involved in the pathogenesis of ACD/MPV, which may elucidate complex phenotype–genotype relationships;
To define regulatory mechanisms that determine phenotype, disease severity, timing of presentation, natural history, and outcomes;
To refine existing animal models of ACD/MPV (49, 62) for the purposes of developing prenatal and postnatal diagnostic tests and exploring new approaches to treatment;
To increase awareness and disseminate information about ACD/MPV across a broad spectrum of health-care providers;
Through the above activities, to identify a greater number of cases that can generate reliable and valid data for the purposes of developing accurate diagnostic criteria and preferred treatment practices.
It is through the accomplishment of the above goals that ACD/MPV may transform from a uniformly lethal disorder into one that is treatable and/or preventable.
Summary
ACD/MPV is a rare, lethal, neonatal developmental lung disorder that results in abnormal alveolar air–blood barriers for gas exchange, severe hypoxemia, and pulmonary hypertension. It may be distinguished from idiopathic PPHN by an incomplete response to interventions known to be effective in PPHN, the presence of associated anomalies of the gastrointestinal, genitourinary, and cardiovascular systems, and occasionally by onset beyond the early neonatal period. Histologic evaluation of lung tissue is the current gold standard for diagnosis and is most often performed at autopsy. Early ante mortem surgical lung biopsy can also provide the diagnosis, but the index of suspicion among health-care providers must be high. The FOXF1 gene has been identified as being responsible for ACD/MPV in 40% of cases examined, and additional candidate genes and potential defects are being investigated. We recommend development of an expanded registry to improve our understanding of the ACD/MPV phenotype, pathophysiology, and genetics.
Acknowledgments
The authors thank Dr. Claire Langston and Dr. Stefan Worgall for their help in preparing this manuscript.
Footnotes
Funding for this review was provided through a cooperative agreement between the U.S. Centers for Disease Control and Prevention and The 3 Angels Memorial Fund for ACD Research, grant #3 U50DD000478–01W1, National Institutes of Health grants HL101975–01 (P.S.), and HL 54705–14 and HL102235–01 (R.H.S.).
Originally Published in Press as DOI: 10.1164/rccm.201010-1697CI on March 11, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Fan LL, Deterding RR, Langston C. Pediatric interstitial lung disease revisited. Pediatr Pulmonol 2004;38:369–378 [DOI] [PubMed] [Google Scholar]
- 2.Hagood JSChildren's Interstitial Lung Disease (ChILD): eMedicine Pediatrics: General Medicine. 2009. [Accessed 2010 August 15]. Available from: http://emedicine.medscape.com/article/1003631-overview
- 3.Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, Brody AS, Nogee LM, Trapnell BC, Langston C, et al. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med 2007;176:1120–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed S, Ackerman V, Faught P, Langston C. Profound hypoxemia and pulmonary hypertension in a 7-month-old infant: late presentation of alveolar capillary dysplasia. Pediatr Crit Care Med 2008;9:e43–e46 [DOI] [PubMed] [Google Scholar]
- 5.Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, Ou Z, Wiszniewska J, Driscoll DJ, Maisenbacher MK, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet 2009;84:780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu S, Shao L, Kilbride H, Zwick D. Haploinsufficiency of FOXF1 and FOXC2 genes associated with lethal alveolar capillary dysplasia and congenital heart disease. Am J Med Genet 2010;152A:1257–1262 [DOI] [PubMed] [Google Scholar]
- 7.MacMahon HE. Congenital alveolar dysplasia of the lungs. Am J Pathol 1948;24:919–931 [PMC free article] [PubMed] [Google Scholar]
- 8.Janney CG, Askin FB, Kuhn C. Congenital alveolar capillary dysplasia–an unusual cause of respiratory distress in the newborn. Am J Clin Pathol 1981;76:722–727 [DOI] [PubMed] [Google Scholar]
- 9.Boggs S, Harris MC, Hoffman DJ, Goel R, McDonald-McGinn D, Langston C, Zackai E, Ruchelli E. Misalignment of pulmonary veins with alveolar capillary dysplasia: affected siblings and variable phenotypic expression. J Pediatr 1994;124:125–128 [DOI] [PubMed] [Google Scholar]
- 10.Sen P, Thakur N, Stockton DW, Langston C, Bejjani BA. Expanding the phenotype of alveolar capillary dysplasia (ACD). J Pediatr 2004;145:646–651 [DOI] [PubMed] [Google Scholar]
- 11.Licht C, Schickendantz S, Sreeram N, Arnold G, Rossi R, Vierzig A, Mennicken U, Roth B. Prolonged survival in alveolar capillary dysplasia syndrome. Eur J Pediatr 2004;163:181–182 [DOI] [PubMed] [Google Scholar]
- 12.Cassidy J, Smith J, Goldman A, Haynes S, Smith E, Wright C, Haworth S, Davis P, Firmin R, Kasem K, et al. The incidence and characteristics of neonatal irreversible lung dysplasia. J Pediatr 2002;141:426–428 [DOI] [PubMed] [Google Scholar]
- 13.Cullinane C, Cox PN, Silver MM. Persistent pulmonary hypertension of the newborn due to alveolar capillary dysplasia. Pediatr Pathol 1992;12:499–514 [DOI] [PubMed] [Google Scholar]
- 14.Antao B, Samuel M, Kiely E, Spitz L, Malone M. Congenital alveolar capillary dysplasia and associated gastrointestinal anomalies. Fetal Pediatr Pathol 2006;25:137–145 [DOI] [PubMed] [Google Scholar]
- 15.Chelliah BP, Brown D, Cohen M, Talleyrand AJ, Shen-Schwarz S. Alveolar capillary dysplasia–a cause of persistent pulmonary hypertension unresponsive to a second course of extracorporeal membrane oxygenation. Pediatrics 1995;96:1159–1161 [PubMed] [Google Scholar]
- 16.Rabah R, Poulik JM. Congenital alveolar capillary dysplasia with misalignment of pulmonary veins associated with hypoplastic left heart syndrome. Pediatr Dev Pathol 2001;4:167–174 [DOI] [PubMed] [Google Scholar]
- 17.Al-Hathlol K, Phillips S, Seshia MK, Casiro O, Alvaro RE, Rigatto H. Alveolar capillary dysplasia. Report of a case of prolonged life without extracorporeal membrane oxygenation (ECMO) and review of the literature. Early Hum Dev 2000;57:85–94 [DOI] [PubMed] [Google Scholar]
- 18.Abdallah HI, Karmazin N, Marks LA. Late presentation of misalignment of lung vessels with alveolar capillary dysplasia. Crit Care Med 1993;21:628–630 [DOI] [PubMed] [Google Scholar]
- 19.Shankar V, Haque A, Johnson J, Pietsch J. Late presentation of alveolar capillary dysplasia in an infant. Pediatr Crit Care Med 2006;7:177–179 [DOI] [PubMed] [Google Scholar]
- 20.Tibballs J, Chow CW. Incidence of alveolar capillary dysplasia in severe idiopathic persistent pulmonary hypertension of the newborn. J Paediatr Child Health 2002;38:397–400 [DOI] [PubMed] [Google Scholar]
- 21.Alameh J, Bachiri A, Devisme L, Truffert P, Rakza T, Riou Y, Manouvrier S, Lequien P, Storme L. Alveolar capillary dysplasia: a cause of persistent pulmonary hypertension of the newborn. Eur J Pediatr 2002;161:262–266 [DOI] [PubMed] [Google Scholar]
- 22.Montgomery V, Buchino JJ. Clinical pathologic conference: a newborn infant with pulmonary hypertension. J Pediatr 1998;133:157–161 [DOI] [PubMed] [Google Scholar]
- 23.Gerrits LC, De Mol AC, Bulten J, Van der Staak FH, Van Heijst AFJ. Omphalocele and alveolar capillary dysplasia: a new association. Pediatr Crit Care Med 2010;11:e36–e37 [DOI] [PubMed] [Google Scholar]
- 24.Melly L, Sebire NJ, Malone M, Nicholson AG. Capillary apposition and density in the diagnosis of alveolar capillary dysplasia. Histopathology 2008;53:450–457 [DOI] [PubMed] [Google Scholar]
- 25.Steinhorn RH, Cox PN, Fineman JR, Finer NN, Rosenberg EM, Silver MM, Tyebkhan J, Zwass MS, Morin FC. Inhaled nitric oxide enhances oxygenation but not survival in infants with alveolar capillary dysplasia. J Pediatr 1997;130:417–422 [DOI] [PubMed] [Google Scholar]
- 26.Extracorporeal Life Support Organization ECLS Registry Report, International Summary. Ann Arbor, MI: Extracorporeal Life Support Organization; 2010. pp. 1–34 [Google Scholar]
- 27.Dalton H, Rycus P, Conrad S. Update on extracorporeal life support 2004. Semin Perinatol 2005;29:24–33 [DOI] [PubMed] [Google Scholar]
- 28.Roy B, Rycus P, Conrad S, Clark R. The changing demographics of neonatal extracorporeal membrane oxygenation patients reported to the Extracorporeal Life Support Organization (ELSO) Registry. Pediatrics 2000;106:1334–1338 [DOI] [PubMed] [Google Scholar]
- 29.Sirkin W, O'Hare BP, Cox PN, Perrin D, Cutz E, Silver MM. Alveolar capillary dysplasia: lung biopsy diagnosis, nitric oxide responsiveness, and bronchial generation count. Pediatr Pathol Lab Med 1997;17:125–132 [PubMed] [Google Scholar]
- 30.Cater G, Thibeault DW, Beatty EC, Kilbride HW, Huntrakoon M. Misalignment of lung vessels and alveolar capillary dysplasia: a cause of persistent pulmonary hypertension. J Pediatr 1989;114:293–300 [DOI] [PubMed] [Google Scholar]
- 31.Fisher JC, Stolar CJH, Cowles RA. Extracorporeal membrane oxygenation for cardiopulmonary failure in pediatric patients: is a second course justified?. J Surg Res 2008;148:100–108 [DOI] [PubMed] [Google Scholar]
- 32.Meehan JJ, Haney BM, Snyder CL, Sharp RJ, Acosta JM, Holcomb GW. Outcome after recannulation and a second course of extracorporeal membrane oxygenation. J Pediatr Surg 2002;37:845–850 [DOI] [PubMed] [Google Scholar]
- 33.Roeleveld PP, Martin J, Chow CW, Duke T. A neonate with coexisting congenital cystic adenomatoid malformation of the lung and alveolar capillary dysplasia: a case report with review of literature. Pediatr Crit Care Med 2008;9:e10–e13 [DOI] [PubMed] [Google Scholar]
- 34.Merchak A, Lueder G, White F, Cole F. Alveolar capillary dysplasia with misalignment of pulmonary veins and anterior segment dysgenesis of the eye: a report of a new association and review of the literature. J Perinatol 2001;21:327–330 [DOI] [PubMed] [Google Scholar]
- 35.Galambos C. Alveolar capillary dysplasia in a patient with Down's syndrome. Pediatr Dev Pathol 2006;9:254–255, author reply 256 [DOI] [PubMed] [Google Scholar]
- 36.Hintz SR, Vincent JA, Pitlick PT, Fineman JR, Steinhorn RH, Kim GE, Benitz WE. Alveolar capillary dysplasia: diagnostic potential for cardiac catheterization. J Perinatol 1999;19:441–446 [DOI] [PubMed] [Google Scholar]
- 37.Eulmesekian P, Cutz E, Parvez B, Bohn D, Adatia I. Alveolar capillary dysplasia: a six-year single center experience. J Perinat Med 2005;33:347–352 [DOI] [PubMed] [Google Scholar]
- 38.Singh SA, Ibrahim T, Clark DJ, Taylor RS, George DH. Persistent pulmonary hypertension of newborn due to congenital capillary alveolar dysplasia. Pediatr Pulmonol 2005;40:349–353 [DOI] [PubMed] [Google Scholar]
- 39.Langston C, Patterson K, Dishop MK, Askin F, Baker P, Chou P, Cool C, Coventry S, Cutz E, Davis M, et al. A protocol for the handling of tissue obtained by operative lung biopsy: recommendations of the chILD pathology co-operative group. Pediatr Dev Pathol 2006;9:173–180 [DOI] [PubMed] [Google Scholar]
- 40.Roth W, Bucsenez D, Bläker H, Berger I, Schnabel PA. Misalignment of pulmonary vessels with alveolar capillary dysplasia: association with atrioventricular septal defect and quadricuspid pulmonary valve. Virchows Arch 2006;448:375–378 [DOI] [PubMed] [Google Scholar]
- 41.Kunig AM, Parker TA, Nogee LM, Abman SH, Kinsella JP. ABCA3 deficiency presenting as persistent pulmonary hypertension of the newborn. J Pediatr 2007;151:322–324 [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Latif ME, Oei J, Ward M, Wills EJ, Tobias V, Lui K. Galvanised by a respiratory distress diagnosis. Arch Dis Child Educ Pract Ed 2008;93:112–119 [DOI] [PubMed] [Google Scholar]
- 43.Garola R, Thibeault D. Alveolar capillary dysplasia, with and without misalignment of pulmonary veins: an association of congenital anomalies. Am J Perinatol 1998;15:103–107 [DOI] [PubMed] [Google Scholar]
- 44.Lane J, Siwik E, Preminger T, Stork E, Spector M. Prospective diagnosis of alveolar capillary dysplasia in infants with congenital heart disease. Am J Cardiol 1999;84:618–620 [DOI] [PubMed] [Google Scholar]
- 45.Taborosi B, Tödt-Pingel I, Kayser G, Dittrich S. A rare case of aortic coarctation and ventricular septal defect combined with alveolar capillary dysplasia. Pediatr Cardiol 2007;28:319–323 [DOI] [PubMed] [Google Scholar]
- 46.Langston C. Misalignment of pulmonary veins and alveolar capillary dysplasia. Pediatr Pathol 1991;11:163–170 [DOI] [PubMed] [Google Scholar]
- 47.Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nürnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet 2007;80:550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalinichenko VV, Lim L, Shin B, Costa RH. Differential expression of forkhead box transcription factors following butylated hydroxytoluene lung injury. Am J Physiol Lung Cell Mol Physiol 2001;280:L695–L704 [DOI] [PubMed] [Google Scholar]
- 49.Kalinichenko VV, Lim L, Stolz DB, Shin B, Rausa FM, Clark J, Whitsett JA, Watkins SC, Costa RH. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev Biol 2001;235:489–506 [DOI] [PubMed] [Google Scholar]
- 50.Mahlapuu M, Enerbäck S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 2001;128:2397–2406 [DOI] [PubMed] [Google Scholar]
- 51.Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, Huff CD, Shannon PT, Jabs EW, Nickerson DA, et al. Exome sequencing identifies the cause of a Mendelian disorder. Nat Genet 2010;42:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lupski JR, Reid JG, Gonzaga-Jauregui C, Rio Deiros D, Chen DCY, Nazareth L, Bainbridge M, Dinh H, Jing C, Wheeler DA, et al. Whole-genome sequencing in a patient with Charcot-Marie-Tooth neuropathy. N Engl J Med 2010;362:1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoischen A, van Bon BWM, Gilissen C, Arts P, van Lier B, Steehouwer M, de Vries P, de Reuver R, Wieskamp N, Mortier G, et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet 2010;42:483–485 [DOI] [PubMed] [Google Scholar]
- 54.Michalsky MP, Arca MJ, Groenman F, Hammond S, Tibboel D, Caniano DA. Alveolar capillary dysplasia: a logical approach to a fatal disease. J Pediatr Surg 2005;40:1100–1105 [DOI] [PubMed] [Google Scholar]
- 55.Kelly LK, Porta NFM, Goodman DM, Carroll CL, Steinhorn RH. Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr 2002;141:830–832 [DOI] [PubMed] [Google Scholar]
- 56.Parker TA, Ivy DD, Kinsella JP, Torielli F, Ruyle SZ, Thilo EH, Abman SH. Combined therapy with inhaled nitric oxide and intravenous prostacyclin in an infant with alveolar-capillary dysplasia. Am J Respir Crit Care Med 1997;155:743–746 [DOI] [PubMed] [Google Scholar]
- 57.Fliman P, deRegnier R, Kinsella J, Reynolds M, Rankin L, Steinhorn RH. Neonatal extracorporeal life support: impact of new therapies on survival. J Pediatr 2006;148:595–599 [DOI] [PubMed] [Google Scholar]
- 58.Kinugasa H, Horigome H, Sugiura M, Saito T, Iijima T, Matsui A. Intravenous prostacyclin combined with inhaled nitric oxide therapy for an infant with alveolar capillary dysplasia. Pediatr Int 2002;44:525–527 [PubMed] [Google Scholar]
- 59.Shehata BM, Abramowsky CR. Alveolar capillary dysplasia in an infant with trisomy 21. Pediatr Dev Pathol 2005;8:696–700 [DOI] [PubMed] [Google Scholar]
- 60.Butler M, Ursell P, Wung J, Stolar C. Misalignment of the pulmonary veins with alveolar capillary dysplasia as a cause of persistent neonatal pulmonary hypertension [abstract]. In 8th Annual Children's National Medical Center ECMO Symposium; Breckenridge, CO; 1992:76 [Google Scholar]
- 61.Marshall GB, Silva CIS, English JC, Levy RD, Müller NL. Misplaced pulmonary arteries in an adult patient with pulmonary hypertension. Br J Radiol 2010;83:e5–e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han RNN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia?. Circ Res 2004;94:1115–1123 [DOI] [PubMed] [Google Scholar]
- 63.Vassal H, Malone M, Petros A, Winter R. Familial persistent pulmonary hypertension of the newborn resulting from misalignment of the pulmonary vessels (congenital alveolar capillary dysplasia). J Med Genet 1998;35:58–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haraida S, Lochbühler H, Heger A, Nerlich A, Diebold J, Wiest I, Müller-Höcker J, Löhrs U. Congenital alveolar capillary dysplasia: rare cause of persistent pulmonary hypertension. Pediatr Pathol Lab Med 1997;17:959–975 [PubMed] [Google Scholar]
- 65.Wagenvoort C. Misalignment of lung vessels: a syndrome causing persistent pulmonary hypertension. Hum Pathol 1986;17:727–730 [DOI] [PubMed] [Google Scholar]
- 66.Cluroe A. Test and teach. Number eighty eight: Part 2. Explanation and diagnosis: alveolar capillary dysplasia. Pathology 1998;30:205–206 [PubMed] [Google Scholar]