Abstract
Macrophages display remarkable plasticity and can change their physiology in response to environmental cues. These changes can give rise to different populations of cells with distinct functions. In this Review we suggest a new grouping of macrophage populations based on three different homeostatic activities—host defence, wound healing and immune regulation. We propose that similarly to primary colours, these three basic macrophage populations can blend into various other ‘shades’ of activation. We characterize each population and provide examples of macrophages from specific disease states that have the characteristics of one or more of these populations.
Macrophages have long been considered to be important immune effector cells. Élie Metchnikoff, who won the Nobel Prize 100 years ago for his description of phagocytosis, proposed that the key to immunity was to “stimulate the phagocytes”(REF. 1). Since this discovery, immunologists have been occupied with the concept of macrophages as immune effector cells and with understanding how these cells participate in host defence. However, by focusing on the immune functions of macrophages, immunologists have ignored their vital homeostatic roles, which are independent of their involvement in immune responses.
Macrophages are prodigious phagocytic cells that clear approximately 2 × 1011 erythrocytes each day; this equates to almost 3 kg of iron and haemoglobin per year that is ‘recycled’ for the host to reuse. This clearance process is a vital metabolic contribution without which the host would not survive. Macrophages are also involved in the removal of cellular debris that is generated during tissue remodelling, and rapidly and efficiently clear cells that have undergone apoptosis. These processes occur independently of immune-cell signalling, and the removal of ‘effete’ or apoptotic cells seems to result in little or no production of immune mediators by unstimulated macrophages2. The receptors that mediate these homeostatic clearance processes include scavenger receptors, phosphatidyl serine receptors, the thrombospondin receptor, integrins and complement receptors3. In general, these receptors that mediate phagocytosis either fail to transduce signals that induce cytokine-gene transcription or actively produce inhibitory signals and/or cytokines, and most of the phagocytosis that occurs on a daily basis by macrophages is independent of other immune cells. Therefore, the primary role of macrophages is not to function an elite immune effector cell, but instead as a common ‘janitorial’ cell, the main function of which is to clear the interstitial environment of extraneous cellular material.
Necrosis that results from trauma or stress also generates cellular debris that must be cleared by macrophages. In contrast to the examples cited above, the clearance of this debris markedly alters the physiology of macrophages. In many cases the debris from necrosis is loaded with endogenous danger signals, such as heat-shock proteins, nuclear proteins (including HMGB1; high-mobility group box 1 protein), histones, DNA and other nucleotides, and components of the extracellular matrix that are cleaved by cellular proteases4. Phagocytosis of these components by macrophages leads to dramatic changes in their physiology, including alterations in the expression of surface proteins and the production of cytokines and pro-inflammatory mediators. The alterations in macrophage surface-protein expression in response to these stimuli could potentially be used to identify biochemical markers that are unique to these altered cells.
Box 1 | T-helper-17-cell responses and macrophages.
The cytokine environment that is generated by T helper 1 (TH1) or TH2 cells can have distinct effects on the physiology of macrophages. However, the contribution of TH17-cell-associated cytokines to macrophage biology is unclear. Similarly to interferon-γ (IFNγ) and interleukin-4 (IL-4), IL-17 is produced by cells of both the innate and adaptive immune response. In addition to TH17 cells, IL-17 can be rapidly produced by both γδ T cells and natural killer T (NKT) cells101. In mice, TH17 cells develop in the presence of transforming growth factor-β (TGFβ) and IL-6 (REFS 25,26,102), whereas in humans it is thought that IL-1β and IL-6 are necessary103. In both humans and mice, the cytokine IL-23 seems to be pivotal for the expansion of these cells24. As all of these cytokines are produced by macrophages, it is clear that these immune cells can influence TH17-cell development (FIG. 4). however, in the gut lamina propria macrophages can inhibit IL-17 production and instead give rise to regulatory T cells104.
The effect of IL-17 on macrophage physiology remains somewhat conjectural. There is some evidence that IL-17 can directly affect macrophage physiology. An early report105 suggested that IL-17 induced the production of pro-inflammatory cytokines by macrophages, but these studies have yet to be confirmed by careful quantitative analysis. It was recently shown that TH17 cells could drive osteoclastogenesis from monocyte precursor cells and confer bone-resorption properties to these cells106. Therefore, the interplay between TH17 cells and macrophages can be complex, and the cytokines that are produced by macrophages (or dendritic cells) can either induce or inhibit the differentiation of TH17 cells. In turn, the cytokines that are produced by TH17 cells can influence osteoclast physiology. however, there is little evidence suggesting that IL-17 directly alters the physiology of most tissue macrophages, especially when compared with the evidence showing that TH17 cells influence the migration and function of polymorphonuclear leukocytes.
Macrophages detect the endogenous danger signals that are present in the debris of necrotic cells through Toll-like receptors (TLRs)2,5,6, intracellular pattern-recognition receptors and the interleukin-1 receptor (IL-1R), most of which signal through the adaptor molecule myeloid differentiation primary-response gene 88 (MyD88)5. This function makes macrophages one of the primary sensors of danger in the host. Importantly, the stimulation of macrophages by cellular debris can occur in experimental animals that are devoid of lymphocytes, which confirms that these processes do not depend on adaptive immune responses2.
The response of macrophages to endogenous danger signals is only one example of the many different stimuli that trigger macrophage activation in tissues. Macrophages have remarkable plasticity that allows them to efficiently respond to environmental signals and change their phenotype, and their physiology can be markedly altered by both innate and adaptive immune responses. Indeed, since the work of Mackaness in the 1970s7 we have learned that changes in the physiology of macrophages in response to some environmental signals can provide them with enhanced antimicrobial activity. However, environmental signals do not always induce changes that increase macrophage immune function. In fact, both innate and adaptive immune responses can give rise to macrophages that are more susceptible to pathogenic infections and less equipped to produce cytokines that enhance the immune response.
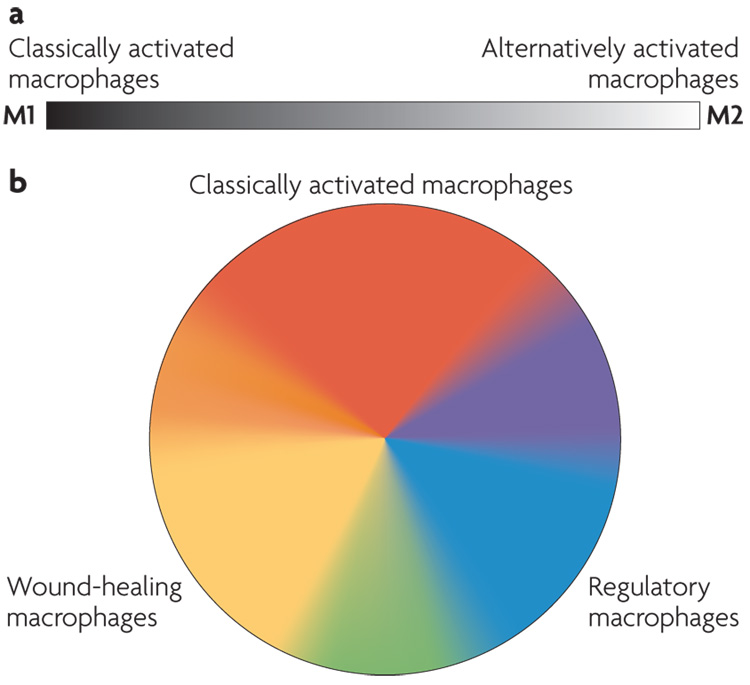
In an effort to emulate the T-cell literature, macrophages have been classified along what could be viewed as a linear scale, on which M1 macrophages represent one extreme and M2 macrophages represent the other (FIG. 1). In this classification, the M1 designation was reserved for classically activated macrophages and the M2 designation for alternatively activated macrophages8. However, the M2 designation has rapidly expanded to include essentially all other types of macrophage9. This classification persists despite a growing body of evidence indicating that the M2 designation encompasses cells with dramatic differences in their biochemistry and physiology10. We suggest that a more informative foundation for macrophage classification should be based on the fundamental macrophage functions that are involved in maintaining homeostasis. We propose three such functions: host defence, wound healing and immune regulation. classifying macrophages according to these functions provides three basic macrophage populations, analogous to the three primary colours in a colour wheel (FIG. 1). This classification also helps to illustrate how macrophages can evolve to exhibit characteristics that are shared by more than one macrophage population, analogous to secondary colours in a colour wheel. Furthermore, it brings the classically activated (or host defence) macrophages closer to the other two cell types, allowing for the development of macrophages that share characteristics of two populations. In fact, we think that there may be many different shades of activation that have yet to be identified, resulting in a ‘spectrum’ of macrophage populations based on their function.
Figure 1. Colour wheel of macrophage activation.
a | A monochromatic depiction of the previous nomenclature showing the linear scale of the two macrophage designations, M1 and M2. b | The three populations of macrophages that are discussed in this article are arranged according to the three primary colours, with red designating classically activated macrophages, yellow designating wound-healing macrophages and blue designating regulatory macrophages. Secondary colours, such as green, may represent tumour-associated macrophages, which have many characteristics of regulatory macrophages but also share some characteristics of wound-healing macrophages. In obese individuals, wound-healing macrophages may transit towards a classically activated-macrophage phenotype.
In this Review we describe some of the physiological alterations that occur in macrophages in response to environmental cues, and the mechanisms by which these changes can be exploited by pathogens or pathological processes to the detriment of the host. In addition, we discuss how the characterization and manipulation of specific macrophage populations can be used for therapeutic purposes.
The origin and maintenance of macrophages
Macrophages are present in virtually all tissues. They differentiate from circulating peripheral-blood mononuclear cells (PBMcs), which migrate into tissue in the steady state or in response to inflammation11. These PBMcs develop from a common myeloid progenitor cell in the bone marrow that is the precursor of many different cell types, including neutrophils, eosinophils, basophils, macro phages, dendritic cells (Dcs) and mast cells. During monocyte development, myeloid progenitor cells (termed granulocyte/macrophage colony-forming units) sequentially give rise to monoblasts, pro-monocytes and finally monocytes, which are released from the bone marrow into the bloodstream11 (FIG. 2). Monocytes migrate from the blood into tissue to replenish long-lived tissue-specific macrophages of the bone (osteoclasts), alveoli, central nervous system (microglial cells), connective tissue (histiocytes), gastrointestinal tract, liver (Kupffer cells), spleen and peritoneum11.
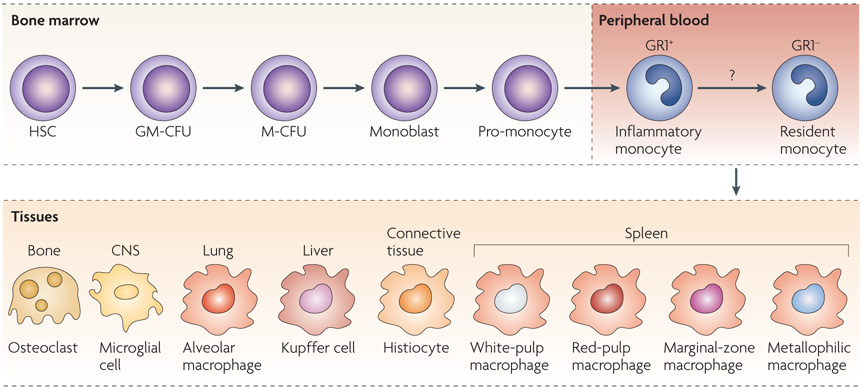
Figure 2. Monocyte heterogeneity.
Monocytes originate in the bone marrow from a common haematopoietic stem cell (HSC). They undergo differentiation steps during which they commit to the myeloid and then to a monocyte lineage. In response to macrophage colony-stimulating factor, they divide and differentiate into monoblasts and then pro-monocytes before becoming monocytes, which exit the bone marrow and enter the bloodstream. In mice, there is evidence of two distinct monocyte populations in the blood that have different phenotypes and biochemical signatures. GR1+CX3CR1low (CX3C-chemokine receptor 1) monocytes rapidly exit the blood, and for this reason they are referred to as ‘inflammatory’ monocytes. GR1− monocytes have been termed ‘resident’ monocytes to differentiate them from the other population. It remains unknown whether inflammatory monocytes mature into resident monocytes in the blood or whether these two cells represent distinct monocyte populations. Human monocytes can also be divided into two populations, but the designations inflammatory and resident do not apply to these populations. Monocytes migrate to different tissues, where they replenish tissue-specific macrophages. CNS, central nervous system; GM-CFU, granulocyte/macrophage colony-forming unit; M-CFU, macrophage colony-forming unit.
In the blood, monocytes are not a homogeneous population of cells, and there is substantial debate about whether specific monocyte populations give rise to specific tissue macrophages12. Although monocyte heterogeneity is not fully understood, one theory suggests that monocytes continue to develop and mature in the blood and they can be recruited to the tissue at various points during this maturation continuum13. The point at which they leave the blood may in fact define their function. In mice, two populations of monocytes from either end of this maturation continuum have been identified and termed as ‘inflammatory’ and ‘resident’ monocytes, based primarily on the amount of time they spend in the blood before migrating into tissues14. These two mouse monocyte populations can be differentiated based on the expression of cell-surface markers. Inflammatory monocytes in mice are defined as CCR2+ (CC-chemokine receptor 2), CX3CR1low (CX3C-chemokine receptor 1) and GR1+ (also known as Ly6), whereas resident monocytes are defined as CCR2−CX3CR1hiGR1− (REF. 14). The extrapolation of data from mice to humans becomes confusing because human monocytes, which can also be separated into two general categories based on their expression of cell-surface markers, seem to have distinct physiology from that of mouse monocytes15. In humans, most monocytes are CD14hiCD16− and are referred to as ‘classical’ monocytes16, or are CD14+CD16+ and are referred to as ‘non-classical’ monocytes16. Approximately 90% of human monocytes express the classical markers, whereas in mice the two populations of monocytes are approximately equally represented in the blood16.
In mice, the first monocytes that enter the bloodstream from the bone marrow are thought to have an inflammatory phenotype16. These cells then rapidly exit the bloodstream and develop into tissue macrophages or DCs17. One theory holds that monocytes that are not initially recruited to the tissue can further mature in the blood and eventually become part of a blood-resident population of monocytes that is thought to contribute to the integrity of the endothelial-cell lining of blood vessels and the maintenance of tissue-resident macrophage populations in the steady state18. Furthermore, these cells become the main source of inflammatory macrophages in tissues during inflammation and trauma, in which the migration of monocytes from the bloodstream is dramatically enhanced.
Although it is well-accepted that monocytes can replenish tissue-macrophage numbers, especially during inflammation, there are cases in which local proliferation of tissue-resident colony-forming cells can directly give rise to populations of mature macrophages in the tissue. The best example of this local proliferation might be the microglial cells of the central nervous system19. Several unresolved questions regarding monocyte heterogeneity remain, such as whether discrete monocyte populations give rise to specific macrophage populations in the tissue and whether alterations in monocyte markers can be exploited to diagnose diseases or to analyse disease resolution following treatment.
Macrophage populations
Macrophages can respond to endogenous stimuli that are rapidly generated following injury or infection. These early stimuli are typically produced by innate immune cells and can exert a marked, though usually transient, effect on the physiology of macrophages. Macrophages can also respond to signals that are produced by antigen-specific immune cells. These signals are more focused and prolonged than innate immune stimuli and generally give rise to longer-term alterations in macrophages20. To complicate matters, macrophages themselves can produce several factors that influence their own physiology. In the next section, we describe three populations of activated macrophage, each of which has a distinct physiology (FIG. 3). We propose that these populations can be generated in response to either innate or adaptive immune signals. In many ways, the response of macrophages to stress, tissue damage or other homeostatic processes can predict how these cells will respond during an adaptive immune response.
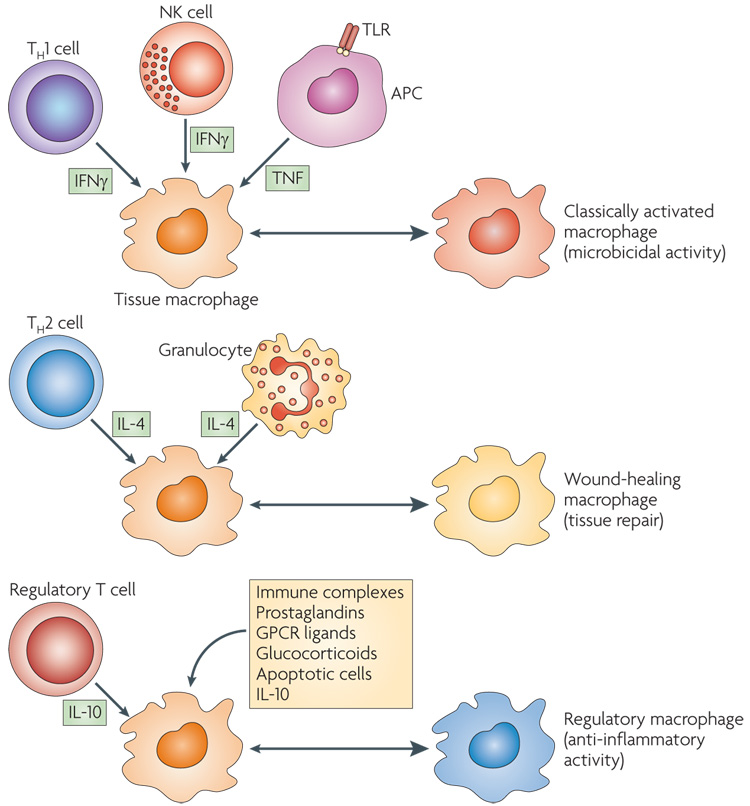
Figure 3. Cytokines produced by immune cells can give rise to macrophages with distinct physiologies.
Classically activated macrophages arise in response to interferon-γ (IFNγ), which can be produced during an adaptive immune response by T helper 1 (TH1) cells or CD8+ T cells (not shown) or during an innate immune response by natural killer (NK) cells, and tumour-necrosis factor (TNF), which is produced by antigen-presenting cells (APCs). Wound-healing (alternatively activated) macrophages arise in response to interleukin-4 (IL-4), which can be produced during an adaptive immune response by TH2 cells or during an innate immune response by granulocytes. Regulatory macrophages are generated in response to various stimuli, including immune complexes, prostaglandins, G-protein coupled receptor (GPCR) ligands, glucocorticoids, apoptotic cells or IL-10. Each of these three populations has a distinct physiology. Classically activated macrophages have microbicidal activity, whereas regulatory macrophages produce high levels of IL-10 to suppress immune responses. Wound-healing macrophages are similar to the previously described alternatively activated macrophages and have a role in tissue repair. TLR, Toll-like receptor.
Classically activated macrophages
The term classically activated has been used to designate the effector macrophages that are produced during cell-mediated immune responses. In the original characterization of activated macrophages, the combination of two signals, interferon-γ (IFNγ) and tumour-necrosis factor (TNF), resulted in a macrophage population that had enhanced microbicidal or tumoricidal capacity and secreted high levels of pro-inflammatory cytokines and mediators7,21. IFNγ can be produced by innate or adaptive immune cells; natural killer (NK) cells are an important innate early source of this cytokine. NK cells respond to stress and infections by producing IFNγ, which can prime macrophages to secrete pro-inflammatory cytokines, produce increased amounts of superoxide anions and oxygen and nitrogen radicals to increase their killing ability22. Therefore, innate immune mediators allow macrophages to provide better resistance against infection. The production of IFNγ by NK cells is generally transient and therefore cannot sustain a population of activated macrophages. consequently, an adaptive immune response is usually necessary to maintain classically activated macrophages and confer stable host defence against many intracellular microorganisms. This is typically provided by the sustained production of IFNγ by T helper 1 (TH1) cells. These T cells are antigen specific, but the microbicidal macrophages that they induce can kill indiscriminately.
Although there have been several excellent early reviews regarding classically activated macrophages and the immune responses that give rise to this cell type1,7,11,20,21, a few points about these cells deserve particular mention. First, classical activation was originally reported to require both IFNγ and TNF. Typically, a TLR ligand acting in a MyD88-dependent manner will induce the transcription of TNF, which can then cooperate with IFNγ in an autocrine manner to activate this macrophage population (FIG. 3). In addition to MyD88, some TLR ligands can also activate TIR-domain-containing adaptor protein inducing IFNβ (TRIF)-dependent pathways, which signal through IFN-regulatory factor 3 (IRF3) and result in IFNβ production23. This endogenously produced IFNβ can replace the IFNγ that is produced by NK cells and T cells and activate classically activated macrophages. Therefore, the original two-signal requirement for the activation of this macrophage population can be overcome by certain TLR agonists that induce both TNF and IFNβ.
Second, the pro-inflammatory cytokines that are produced by classically activated macrophages are an important component of host defence, but they can cause extensive damage to the host. For example, IL-1, IL-6 and IL-23 are produced by classically activated macrophages and have been associated with the development and expansion of TH17 cells24–26 (FIG. 4). These cells produce IL-17, a cytokine that is associated with high levels of polymorphonuclear leukocyte (PMN) recruitment to tissues, which can contribute to inflammatory autoimmune pathologies (reviewed in REF. 27) (BOX 1). conversely, the clearance of apoptotic PMNs by macrophages during inflammation can lead to an inhibition of inflammation, owing in part to the production of transforming growth factor-β (TGFβ)28.
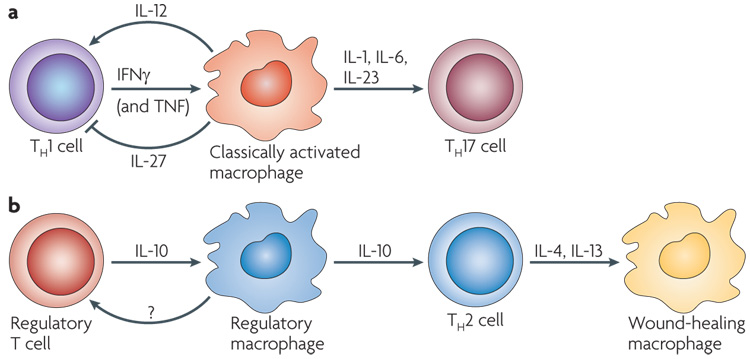
Figure 4. Interactions between macrophage and T cells.
a | Interferon-γ (IFNγ) produced by T helper 1 (TH1) cells or CD8+ T cells, along with tumour-necrosis factor (TNF) from antigen-presenting cells, can give rise to classically activated macrophages, which secrete interleukin-1 (IL-1), IL-6 and IL-23. These cytokines can give rise to TH17 cells, which can contribute to autoimmune responses. Classically activated macrophages also produce IL-12 to promote the differentiation of TH1 cells, but they can also produce IL-27, which inhibits various immune responses and negatively regulates TH1 and TH2 cells119. b | IL-10 produced by regulatory T cells can give rise to a population of regulatory macrophages, which act as antigen-presenting cells, produce IL-10 and can induce the expansion of TH2 cells. There is controversy about whether some regulatory macrophages can also promote the development of regulatory T cells. IL-4 and/or IL-13 produced by TH2 cells can promote the development of wound-healing macrophages, but these macrophages are poor antigen-presenting cells and may even inhibit T-cell proliferation.
The role of classically activated macrophages in host defence to intracellular pathogens has been well documented1,7,11,22. Indeed, mice lacking IFNγ expression are more susceptible to various bacterial, protozoal or viral infections, as are humans with genetic mutations in these signalling pathways29. However, there are some specific examples of the killing of intracellular microorganisms by classically activated macrophages that reveal the complex interplay between host and pathogen. Leishmania spp. are intracellular parasites that replicate primarily in tissue-resident macrophages. The stimulation of these cells with IFNγ and TNF before infection yields a population of macrophages that efficiently kills the parasite. However, stimulation of macrophages with IFNγ alone results in less efficient clearance of the parasite because Leishmania spp. are eukaryotic organisms that do not express TLR ligands and therefore do not trigger detectable TNF production. Stimulation of macrophages with exogenous TNF or with a TLR ligand, such as lipopolysaccharide (LPS), results in complete clearance of the parasite, thereby confirming the importance of TLR activation or TNF production in the development of classically activated macrophages. In simplified molecular terms, the panoply of genes that is triggered during the activation of classically activated macrophages is induced by a combination of transcription factors. These include signal transducer and activator of transcription (STAT) molecules, which are activated following IFNγ receptor ligation, and nuclear factor-κB (NFκB) and mitogen-activated protein kinases (MAPKs), which are activated in response to TLR or TNF receptor ligation21.
The second, unexpected role of classically activated macrophages in host defence against Leishmania spp. was observed when IFNγ was added to cultures of resting macrophages following infection by Leishmania donovani. It was shown that intracellular killing was decreased because the organism prevented efficient macrophage activation by interfering with IFNγ signalling30. Other intracellular pathogens can also disrupt or redirect IFNγ signalling in macrophages. Mycobacterium tuberculosis, for example, expresses a 19 kDa lipoprotein that inhibits the synthesis of several IFNγ-responsive proteins that are involved in antigen presentation31.
In summary, classically activated macrophages are products of a cell-mediated immune response. They can also be transiently generated in response to innate stimuli following stress or viral infections. Some pathogens have developed the ability to interfere with IFNγ signalling and prevent efficient macrophage activation. These classically activated macrophages are vital components of host defence, but their activation must be tightly controlled because the cytokines and mediators that they produce can lead to host-tissue damage. Indeed, classic ally activated macrophages are key mediators of the immunopathology that occurs during several autoimmune diseases, including rheumatoid arthritis32 and inflammatory bowel disease4.
Wound-healing macrophages
Similarly to classically activated macrophages, wound-healing macrophages can develop in response to innate or adaptive signals. One of the first innate signals to be released during tissue injury is thought to be IL-4 (REF. 33). Basophils and mast cells are important early sources of innate IL-4 production, although other granulocytes might also contribute34 (FIG. 3). In addition to injury, these cells can also produce IL-4 in response to chitin, a structural biopolymer that is found in some fungi and parasites35. This early IL-4 production rapidly converts resident macrophages into a population of cells that are programmed to promote wound healing; IL-4 stimulates arginase activity in macrophages, allowing them to convert arginine to ornithine, a precursor of polyamines and collagen, thereby contributing to the production of the extracellular matrix36.
The name originally given to macrophages that are generated in the presence of IL-4 was alternatively activated macrophages because they upregulated their expression of mannose receptor37. In many ways, however, this name may have led to some confusion in the field. First, the name implies that this is the only other (alternative) way that macrophages can be activated, and this is certainly not the case. Second, the term ‘activation’ is normally associated with host defence, and it now seems that rather than being important immune effector cells, they are actually more susceptible to some infections36,38.
Adaptive immune responses can also lead to the production of IL-4, and it is thought that this is the primary pathway for the development and maintenance of woundhealing macrophages. TH2-type immune responses are primarily induced in response to disturbances at mucosal surfaces35, and they are particularly important in the lung and intestines. These responses can also occur in non-mucosal tissues, especially in response to helminth infections39. The signature cytokines of a TH2-type immune response are IL-4 and IL-13. Macrophages treated in vitro with IL-4 and/or IL-13 fail to present antigen to T cells, produce minimal amounts of pro-inflammatory cytokines and are less efficient than classically activated macrophages at producing toxic oxygen and nitrogen radicals, and at killing intra cellular pathogens10. However, these cells secrete components of the extracellular matrix and therefore their primary function seems to be related to wound healing. These macrophages can also exert indirect regulatory effects on the immune response because the polyamines they produce can influence the production of cytokines and suppress the clonal expansion of neighbouring lymphocytes40.
The role of these macrophages in host defence and adaptive immunity remains somewhat enigmatic. Although there is clear evidence that alternatively activated macrophages can contribute to the clearance of helminths and nematodes41,42, evidence in support of direct microbicidal effects mediated by these cells and their secreted products is lacking. Several studies have shown that alternatively activated macrophages produce large amounts of chitinase and chitinase-like molecules, including YM1 (also known as CHI3L3) and YM2 (REF. 43), acidic mammalian chitinase (AMCase) and stabilin-interacting chitinase-like protein44,45 (TABLE 1). The initial suggestion was that these macrophage-derived chitinases might have a direct role in degrading the chitin-containing surfaces of some parasites, insects and fungi. However, YM1 and other molecules in this family were shown to lack chitin-degrading activity, which questioned this hypothesis46,47. Furthermore, the observation that these chitinase-like molecules have carbohydrate and matrix-binding activity is consistent with a role of these molecules in matrix reorganization and wound healing46,47. Therefore, focusing on a role for these cells in host defence may actually detract from their other important roles in tissue repair and homeostasis.
Table 1.
Characteristics and potential biomarkers for three macrophage populations
| Marker | Function | Expression | Ref. |
|---|---|---|---|
| Classically activated macrophages | |||
| IL-12 | Induces TH1-cell development | Induced by IFNγ | 107 |
| iNOS | Produces NO and citrulline from arginine to kill microorganisms | Depends on IFNγ | 108 |
| CCL15 | Attracts monocytes, lymphocytes and eosinophils | Upregulated by IFNγ | 109 |
| CCL20 | Chemoattractant for DC and T cells | Upregulated by IFNγ | 109 |
| CXCL9 | Involved in T-cell trafficking | Induced by IFNγ | 9 |
| CXCL10 | Attracts NK and T cells; signals through CXCR3 | Induced by IFNγ | 9 |
| CXCL11 | Attracts NK and T cells; signals through CXCR3 | Induced by IFNγ | 9 |
| Wound-healing macrophages | |||
| CCL18 | Attracts lymphocytes, immature DCs and monocytes | Induced by IL-4 | 110 |
| YM1 | Chitinase-like protein that can bind to extracellular matrix | Strongly induced by IL-4 | 111 |
| RELMα | Can promote deposition of extracellular matrix | Strongly induced by IL-4 | 111 |
| CCL17 | Attracts T cells and macrophages | Induced by IL-4 and suppressed by IFNγ | 112 |
| IL-27Rα | Inhibits pro-inflammatory cytokine production | Upregulated by IL-4 | 113 |
| IGF1 | Stimulates fibroblast proliferation and survival | Induced by IL-4 | 114 |
| CCL22 | Attracts TH2 cells and other CCR4-expressing cells | Induced by IL-4 | 115 |
| DCIR | C-type lectin containing an ITIM motif | Induced by IL-4 | 109 |
| Stabilin 1 | Endocytic receptor that may be involved in lysosomal sorting | Induced by IL-4 | 116 |
| Factor XIII-A | Can bind to extracellular matrix proteins and contribute to wound healing | Induced by IL-4 and suppressed by IFNγ | 117 |
| Regulatory macrophages | |||
| IL-10 | Potent anti-inflammatory cytokine | Induced by TLRs in combination with other stimuli | 61 |
| SPHK1 | Catalyses the conversion of sphingosine to sphingosine-1 phosphate | Induced by TLRs and immune complexes | 10 |
| LIGHT | Provides co-stimulatory signals for T cells through HVEM | Induced by TLRs and immune complexes | 10 |
| CCL1 | Attracts eosinophils and TH2 cells; binds CCR8 | Induced by TLRs in combination with several other stimuli | 118 |
CCL, CC-chemokine ligand; CCR8, CC-chemokine receptor 8; CXCL, CXC-chemokine ligand; CXCR, CXC-chemokine receptor; DC, dendritic cell; DCIR, DC immunoreceptor; HVEM, herpesvirus entry mediator; IFNγ, interferon-γ; IGF1, insulin-like growth factor 1; IL, interleukin; IL-27Rα, IL-27 receptor α-chain; iNOS, inducible nitric-oxide synthase; ITIM, immunoreceptor tyrosine-based inhibiting motif; NK, natural killer; NO, nitric oxide; RELMα, resistin-like molecule-α; SPHK1, sphingosine kinase 1; TH, T helper; TLR, Toll-like receptor.
Wound-healing macrophages can also be detrimental to the host when their matrix-enhancing activity is dysregulated, similarly to the dysregulated activity of classically activated macrophages in autoimmunity. The tissue fibrosis that occurs during chronic schistosomiasis has been attributed to the uncontrolled activation of wound-healing macrophages48. Macrophages that lacked expression of IL-4 receptor (IL-4R) failed to induce this pathology, and treatment with antibodies that are specific for IL-4 caused a reduction in fibrosis and a decrease in the accumulation of wound-healing macrophages48. This macrophage population has also been identified in the lungs of mice with experimental asthma49 and may contribute to airway remodelling in this disease.
Accumulating evidence indicates that IL-4- or IL-13-treated macrophages are more susceptible to some intracellular infections50–54. However, one caveat of these studies is that IFNγ and IL-4 cross-regulate each other, and the conditions under which IL-4 is generated are usually the conditions under which the production of IFNγ (which has an important role in pathogen clearance) is low. Therefore, it is difficult to determine whether IL-4 is directly or indirectly responsible for these observations. It is probable that the combination of high IL-4 and low IFNγ production can contribute to the intracellular growth of certain pathogens in macrophages by promoting the development of wound-healing rather than classically activated macrophages.
A striking example of the intracellular growth of a pathogen under TH2-polarized conditions is seen during infection with the facultative intracellular fungus Cryptococcus neoformans52. This organism grows at a higher rate in transgenic mice that overexpress IL-13 compared with wild-type mice, whereas IL-13-deficient mice are more resistant to infection. The overexpression of IL-13 in this study was associated with a population of macrophages that express YM1 and CD206 (REF. 52), two markers that had previously been associated with alternatively activated (or wound-healing) macrophages37,43 (TABLE 1). Furthermore, IL-4-induced polyamine biosynthesis can contribute to the intracellular growth of the parasite Leishmania major in macrophages51. Another example comes from recent studies showing that treatment of macrophages with IL-4 resulted in increased susceptibility to infection with M. tuberculosis because autophagy-mediated killing was inhibited50. In addition, the bacterium Francisella tularensis has recently been shown to grow in a macrophage population that expresses YM1 (REF. 53). Finally, classically activated macrophages in mice that are resistant to infection with Yersinia enterocolitica contribute to the clearance of the bacterium, whereas in susceptible mice, macrophages with a wound-healing phenotype allow for its persistence54.
Regulatory macrophages
Similarly to the two populations of macrophages described above, regulatory macrophages can arise following innate or adaptive immune responses. Although stress responses are not typically considered part of innate immunity, the hypothalamic–pituitary–adrenal (HPA) axis can exert marked effects on macrophages. Glucocorticoids are released by adrenal cells in response to stress and can inhibit macrophage-mediated host defence and inflammatory functions by inhibiting the transcription of pro-inflammatory cytokine genes and decreasing mRNA stability55, giving rise to a population of regulatory macrophages. Indeed, experimental hypophysectomy or adrenalectomy, which removes the source of glucocorticoids, can amplify and exacerbate mild pro-inflammatory signals. However, not all macrophage functions are adversely affected by glucocorticoids56; phagocytosis of apoptotic cells seems to remain largely intact if not increased in the presence of glucocorticoids57. The production of the regulatory cytokine TGFβ by macrophages following the phagocytosis of apoptotic cells in the presence of pro-inflammatory stimuli can also contribute to the immunoregulatory function of these macrophages28. Therefore, glucocorticoids can exert direct and indirect inhibitory affects on immune responses. Glucocorticoid-treated antigen-presenting cells either fail to present antigen to T cells, preferentially bias T-cell responses towards a TH2-cell phenotype28 or induce the development of regulatory T cells that can inhibit immune responses58.
Regulatory macrophages can also arise during the later stages of adaptive immune responses, the primary role of which seems to be to dampen the immune response and limit inflammation59. There are many different ways to generate regulatory macrophages, but a single molecular mechanism that mediates this phenotypic switch has yet to be identified, although the MAPK extracellular-signal-regulated kinase (ERK) has emerged as a potential candidate60.
We first identified a population of regulatory macrophages following their in vitro stimulation with a TLR agonist in the presence of IgG immune complexes61. This combination of stimuli led to the development of a population of macrophages that produced high levels of the immunosuppressive cytokine IL-10. In addition to immune complexes, other factors can provide a signal for the differentiation of regulatory macrophages, including prostaglandins62, apoptotic cells3, IL-10 (REF. 9) and some ligands for G-protein-coupled receptors (GPcRs)63 (FIG. 3). Other inducers of regulatory macrophages may include adenosine63, dopamine, histamine, sphingosine 1-phosphate, melanocortin, vasoactive intestinal peptide, adiponectin and Siglec-9 (REFS 64–70). Some tumour-associated macrophages may share characteristics with this regulatory macrophage population71 (see later).
Although there can be subtle differences among the regulatory macrophage subpopulations that are generated by different stimuli, some characteristics are common to all. For example, one characteristic that seems to be shared by most of these regulatory cells is the need for two stimuli to induce their anti-inflammatory activity. The first signal (for example, immune complexes, prostaglandins, adenosine or apoptotic cells) generally has little or no stimulatory function on its own. However, when combined with a second stimulus, such as a TLR ligand, the two signals reprogramme macrophages to produce IL-10 (REF. 10), the production of which is the most important and reliable characteristic of regulatory macrophages. In addition to IL-10 production, these regulatory macrophages also downregulate IL-12 production61; therefore, the ratio of IL-10 to IL-12 could be used to define regulatory macrophages. Because IL-10 can inhibit the production and activity of various pro-inflammatory cytokines, these regulatory macrophages are potent inhibitors of inflammation, despite the fact that they retain the ability to produce many pro-inflammatory cytokines. This indicates that the presence of regulatory macrophages could negatively correlate with vaccine protection, which requires the induction of pro-inflammatory cytokines. Consequently, one potential therapeutic intervation could be to manipulate macro phage populations during vaccination, for example, to minimize the induction of regulatory macrophages.
Unlike wound-healing macrophages, these regulatory macrophages do not contribute to the production of the extracellular matrix, and many of these regulatory cells express high levels of co-stimulatory molecules (CD80 and CD86) and therefore can present antigens to T cells10. So, there are clear functional, as well as biochemical differences between regulatory and wound-healing macrophages (TABLE 1). Because there are subtle differences between the various subpopulations of regulatory macrophages, it will be important to develop reliable biomarkers for each subpopulation so that individual subpopulations could be targeted with cell- and disease-specific therapeutics.
Regulatory macrophages can also be exploited by parasitic, bacterial and viral pathogens. In many cases these pathogens mimic some of the stimuli mentioned above. For example, the amastigote stage of intracellular protozoan Leishmania spp. binds host IgG and engages the macrophage Fc receptor for IgG (FcγR) on entry into these cells. This engagement, and the activation of downstream signalling pathways, induces the development of regulatory macrophages that can be permissive to intracellular growth72. In another example, African trypanosomes change their main surface antigen to escape the humoral immune response, resulting in the generation of a robust antibody response and the formation of immune complexes. These immune complexes can bind to macrophage FcγR and induce regulatory macrophages, which might inhibit immune responses to subsequent clones of the parasites73. Furthermore, the oedema toxin from the bacterium Bacillus anthracis is a GPCR ligand, and its activation could result in the development of regulatory macrophages and enhance bacterial spread74. In addition, its lethal toxin can inhibit MAPK activation in macrophages, thereby interfering with the production of pro-inflammatory cytokines75. Infection with Coxiella burnetti results in host-cell apoptosis, and the uptake of apoptotic cells by macrophages renders these cells permissive to intracellular bacterial growth76. Finally, antibody-dependent enhancement (ADE), which is observed during infection with Dengue virus or Ross River virus (RRV), has recently been correlated with the emergence of a macrophage population that during RRV infection produces low levels of pro-inflammatory cytokines but increased levels of IL-10 (REF. 77). Therefore, there is a growing list of pathogens that either interfere with macrophage activation or specifically induce the development of regulatory macrophages. In either case, the result is the same: defective pathogen killing and enhanced survival and spread of these microorganisms.
In summary, both innate and adaptive signals can influence macrophage physiology, and these alterations allow macrophages to participate in homeostastic processes, such as tissue remodelling and wound healing, as well as in host defence. However, each of these alterations can have potentially dangerous consequences if not appropriately regulated. For example, classically activated macrophages can cause damage to host tissues, predispose surrounding tissue to neoplastic transformation and influence glucose metabolism by promoting insulin resistance (see later). Macrophages that are normally involved in wound healing can promote fibrosis, exacerbate allergic responses and be exploited by pathogens for intracellular survival. Regulatory macrophages can contribute to the progression of neoplasia (see later), and the high levels of IL-10 that these cells produce can predispose the host to infection.
Macrophage plasticity and disease
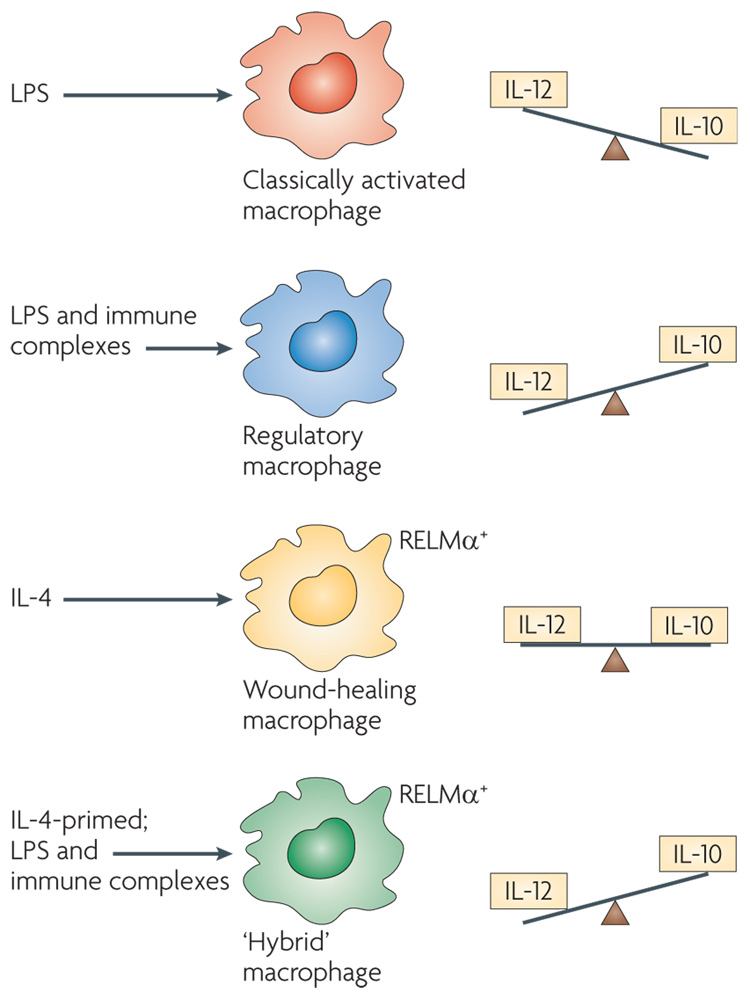
The plasticity of macrophages has resulted in a great deal of confusion regarding the identity of individual macrophage populations. Unlike T cells, which undergo extensive epigenetic modifications during differentiation (that is, chromatin is marked for the expression of specific genes), macrophages seem to retain their plasticity and respond to environmental signals78. Therefore, reliance on a single biochemical marker to identify a macrophage population can be problematic. For example, macrophages that have been treated with IL-4 can assume a wound-healing phenotype and express the antigen resistin-like molecule-α (ReLMα; also known as FIZZ1)43 (FIG. 5). The addition of immune complexes to cultures of these IL-4-primed cells converts them into a population of macrophages that produces high levels of IL-10 and low levels of IL-12 (REF. 10), a phenotype that is similar to regulatory macro phages. However, because ReLMα expression persists in these cells for some time, these macrophages could be mistaken for wound-healing cells if ReLMα were the only marker used to identify them. These ‘hybrid’ macrophages would exhibit characteristics of both regulatory and wound-healing macrophages.
Figure 5. The plasticity of activated macrophages.
Classically activated macrophages produce high levels of interleukin-12 (IL-12) and modest levels of IL-10. By contrast, regulatory macrophages produce high levels of IL-10 and low levels of IL-12. Macrophages treated with IL-4 (that is, wound-healing macrophages) produce low levels of these cytokines, but express resistin-like molecule-α (RELMα) intracellularly, a marker that is not expressed by the other macrophage populations. Treatment of IL-4-primed macrophages with lipopolysaccharide (LPS) and immune complexes results in a hybrid phenotype in which the cells continue to express RELMα (similarly to wound-healing macrophages) but also produce high levels of IL-10 (similarly to regulatory macrophages).
Numerous studies have examined the stability and longevity of activated macrophages within the host. Several in vivo studies suggest that the phenotype of a macrophage population can change over time. It is not clear whether this phenotypic alteration is the result of de-differentiation of the original macrophages back to the resting state or of the migration of a new population of macrophages into the tissue site where they replace the original cells. Regardless of the mechanism, there are some cases in which a phenotypic switch in the macrophage population occurs over time and is associated with pathology. Two specific examples of this phenotypic switch are discussed in detail below.
Cancer: switching from classically activated to regulatory macrophages
The role of macrophages in cancer has been controversial and many aspects remain unresolved. early evidence showed that macrophage surveillance mechanisms are essential for preventing the growth of transformed or pre-transformed cells, and there is evidence showing that activated macrophages can kill transformed cells in vitro79. However, there is also evidence that macrophage depletion has little effect on the host’s susceptibility to cancer and in some cases may even be beneficial to the host (for reviews, see REFS 80,81). We propose that macrophages can have contrasting roles in cancer depending on their phenotype.
Classically activated macrophages have the potential to contribute to the earliest stages of neoplasia82, primarily because the free radicals that they produce can lead to the DNA damage; this causes mutations that can predispose host cells to transformation. An anecdotal example of this macrophage-mediated induction of tumorigenesis is the neoplasia that is associated with old tuberculosis scars in the lungs of previously infected patients. Classically activated macrophages accumulate in these scars, damage host tissue and induce cellular transformation. This scenario is in contrast to early in vitro studies, which convincingly showed that classically activated macrophages were cytotoxic to tumour cells but not to normal cells and therefore suggested that classically activated macrophages contributed to the early eradication of transformed cells83. Thus, although there is controversy about the roles of macrophages in the earliest stages of cancer, there seems to be general agreement that these cells resemble classically activated macrophages that have an inflammatory phenotype.
However, as tumours progress and grow, the tumour microenvironment markedly influences tumour-associated macrophages. These macrophages change their physiology and take on a phenotype that more closely resembles regulatory macrophages84. The tumour-derived agents that induce the development of these regulatory macrophages have not been identified, but candidates include prostaglandins, hypoxia, extracellular nucleotides, apoptotic cells, hyaluronan fragments and IgG85–88, which may work synergistically within the tumour microenvironment. Recent studies indicate that the induction of a suppressive macrophage population in tumours may occur through the MyD88-dependent activation of NFκB89. Irrespective of the stimulus, these tumour-associated macrophages produce high levels of IL-10, can inhibit immune responses to neo-antigens that are expressed by tumour cells and can de-activate neighbouring macrophages71. Recent studies also suggest that regulatory macrophages can contribute to angiogenesis and thereby promote tumour growth90.
A phenotypic characterization that encompasses all tumour-associated macrophages has yet to emerge, but it is clear that they exhibit several characteristics of regulatory macrophages, including the production of high levels of IL-10 but little or no IL-12 (REFS 71,90). However, they also seem to be defective in TNF production and they may suppress the activity of antigen-presenting cells. Therefore, although these cells share many characteristics of regulatory macrophages, they also have some of the characteristics of wound-healing macrophages. Thus, the original classically activated macrophages that might have participated in tumour formation can progressively differentiate to a regulatory phenotype and eventually become cells that share the characteristics of both regulatory and wound-healing macrophages (FIG. 1). The identification of biochemical markers on tumour-associated macrophages has the potential to identify only those suppressive macrophages that have been reprogrammed by the tumour. Therefore, these biochemical markers may lead to the development of experimental approaches that specifically target these cells for deletion and, consequently, restore immune responses in the tumour following therapy. Furthermore, this approach may be more effective than attempting to identify tumour-specific antigens that often vary throughout the course of disease and are inconsistently expressed across different groups of patients.
Obesity: switching from wound-healing to inflammatory macrophages
In healthy, non-obese humans, macrophages in the adipose tissue seem to function in an analogous way to wound-healing macrophages: they produce little to no pro-inflammatory cytokines but express arginase, which can inhibit nitric-oxide production and lead to polyamine production91. These cells also seem to support adipocyte function and to maintain sensitivity to insulin91. The nuclear receptor peroxisome-proliferator-activated receptor-γ (PPARγ) seems to be an important regulator of this macrophage phenotype92, and there have been several reports correlating the alternative activation state of macrophages with PPARγ activation93. Therefore, adipose-associated macrophages in non-obese humans exhibit an anti-inflammatory phenotype, which is analogous to the wound-healing macrophages described above. However, macrophages that are associated with adipose tissue in obese humans have a dramatically different physiology.
It is generally agreed that obesity is associated with chronic inflammation, and adipose-tissue-associated macrophages can act as sources of pro-inflammatory cytokines94. In obese individuals, macrophages accumulate in adipose tissue over time, and the cytokines that they produce can lead to insulin resistance and type 2 diabetes91,94. The inflammatory response that is mediated by macrophages during obesity might be analogous to the necrotic clearance mechanisms described earlier. In non-obese individuals, the low level of adipocyte turnover fails to induce the production of pro-inflammatory cytokines and, in fact, the clearance of apoptotic cells by adipose-tissue-associated macrophages may promote the maintenance of the anti-inflammatory state. By contrast, obesity has been associated with extensive adipocyte necrosis, which can result in the release of many activators of innate immune cells from necrotic cells95. In response to these stimuli, macrophages in adipose tissue secrete cytokines, especially TNF and IL-6, and chemokines, such as CC-chemokine ligand 2 (CCL2; previously known as MCP1). TNF and IL-6 can interfere with insulin signalling in adipocytes, leading to type 2 diabetes96. CCL2 can recruit additional macrophages to adipose tissue, thereby propagating chronic inflammation. Therefore, the population of adipose-tissue-associated macrophages progressively changes from a wound-healing-like phenotype to one that more closely resembles classically activated macrophages. The overexpression of procoagulant proteins by these inflammatory macrophages could also contribute to the atherogenic and cardiovascular risks that constitute part of obesity-associated metabolic syndrome96. Thus, the phenotypic switch from a quiescent population of macrophages in adipose tissue to an inflammatory population could be the cause of subsequent pathology, and the identification of macrophage populations within this tissue could also be used to predict the cardiovascular and metabolic complications that might develop.
It is tempting to speculate that a similar ‘switch’ in macrophage physiology may also occur during atherosclerosis. Atherosclerotic lesions are formed following the deposition of lipids to areas of intimal thickening in atherosclerosis-prone blood vessels97. Macrophages gradually accumulate in this area, which is enriched in oxidized low-density lipoprotein (LDL) and extracellularmatrix proteoglycans, and adhere to the areas of intimal thickening in a PPARγ-dependent manner98. As atherosclerotic lesions progress, macrophages assume an inflammatory phenotype and produce cytokines that are associated with classical activation99. The stimulus for this switch is not known, but potential candidates include high levels of inflammatory extracellular-matrix fragments, oxidized LDL and cellular debris4. The production of pro-inflammatory cytokines by plaque-resident macrophages is the reason why atherosclerosis is considered to be an inflammatory disease and it may explain the association between other inflammatory diseases, such as rheumatoid arthritis, and atherosclerosis100. It is therefore possible that both obesity and atherosclerosis are associated with macrophages that have undergone similar phenotypic switches, that is, from quiescent macrophages to inflammatory cells. Identification of the biochemical markers that are expressed by these macrophages could help to identify the later stages of disease progression.
Conclusion
In this Review we describe three populations of macrophages that have distinct physiologies and we anticipate an expansion of this classification. The plasticity of macrophages makes the task of assigning specific biochemical markers to each population difficult; analogous to assigning a colour to a chameleon. However, the potential for understanding macrophage heterogeneity is enormous because these cells can be biomarkers of diseases and have the potential to be used as surrogate markers of protection following drug treatment or even vaccination. Therefore, future studies to assign definitive biochemical markers to each of the different macrophage populations are needed so that individual populations of macrophages can be manipulated, selectively depleted or targeted by cell-specific therapeutics.
Acknowledgements
This work is supported in part by National Institutes of Health grants AI49388 and AI55576.
Glossary
- Apoptosis
A common form of cell death (also known as programmed cell death) that can be caused by many physiological and developmental stimuli. Apoptosis involves cell shrinkage, chromatin condensation in the periphery of the nucleus, cell-membrane blebbing and DNA fragmentation into multiples of ~ 180 base pairs. eventually, the cell breaks up into many membrane-bound apoptotic bodies, which are phagocytosed by neighbouring cells.
- Necrosis
A form of cell death that frequently results from toxic injury, hypoxia or stress. Necrosis involves the loss of cell integrity and the release of cell contents into the interstitium. This form of cell death usually occurs together with inflammation. Depending on the context, the self antigens that are released by necrotic cells could become immunogenic.
- Extracellular matrix
Secreted products of many cell types that form an organized scaffold for cell support.
- Classically activated macrophage
A macrophage that is activated through Toll-like receptors and interferon-γ. These cells exhibit enhanced killing of intracellular microorganisms, increased secretion of cytokines and mediators, and higher expression of co-stimulatory molecules.
- Alternatively activated macrophage
A macrophage that is activated by interleukin-4 (IL-4) or IL-13 and expresses arginase-1, mannose receptor (CD206) and IL-4 receptor-α. Pathogen-associated molecular patterns that are expressed by helminths may also drive the alternative activation of macrophages.
- Superoxide anions
Anions that are produced by NADPH oxidase in phagocytes and can dismutate to hydrogen peroxide, which can be converted into ferryl or hydroxyl radicals by the fenton reaction.
- Osteoclastogenesis
A process whereby haematopoietic stem cells differentiate into multinucleated osteoclasts with bone-resorbing activity.
- Chitinase
An enzyme that breaks down the glycosidic bonds in chitin.
- Autophagy
An evolutionarily conserved process during which acidic double-membrane vacuoles sequester intracellular contents (such as damaged organelles and macromolecules) and target them for degradation through fusion to secondary lysosomes.
- Hypothalamic–pituitary–adrenal axis
A major part of the neuroendocrine system, which controls reactions to stress and regulates digestion, energy use, sexuality and the immune system (generally through an immunosuppressive action).
- Glucocorticoids
A group of compounds that belongs to the corticosteroid family. These compounds can be naturally produced (hormones) or can be synthetic. They affect metabolism and have anti-inflammatory and immunosuppressive effects. Some synthetic glucocorticoids (for example, dexamethasone) are used as chemotherapeutic drugs.
- Immune complexes
Antigen–antibody complexes that bind to and crosslink Fcγ receptors.
- Prostaglandins
Lipid mediators that are derived from arachidonic acid through the cyclooxygenase pathway. Bergstrom, Samuelsson and Vane won the Nobel prize in Medicine in 1982 for showing that aspirin-like compounds could inhibit prostaglandin synthesis.
- G-protein-coupled receptor
A receptor that is composed of seven membrane-spanning helical segments, which are connected by extracellular and intracellular loops. These receptors associate with G proteins, which are a family of trimeric intracellular-signalling proteins that have specific β-and γ-chains, and one of several α-chains.
- Tumour-associated macrophage
A cell that differentiates from circulating blood monocytes that have infiltrated tumours. Tumour-associated macrophages constitute an important component of the tumour microenvironment and can have positive or negative effects on tumorigenesis (that is, tumour promotion or immunosurveillance, respectively).
- Antibody-dependent enhancement
A phenomenon that was originally described in Dengue virus infection in which the presence of antibody enhances viral infection and increases disease severity.
- Neoplasia
New growths or tumours, which can be either benign or malignant. Derived from the Greek for new formations.
- Angiogenesis
The development of new blood vessels from existing ones. Angiogenesis is frequently associated with tumour development and metastasis.
- Adipose tissue
Loose connective tissue consisting primarily of adipocytes, the major function of which is to store energy in the form of fat.
- Insulin resistance
Failure of cells to appropriately respond to insulin-mediated induction of glucose uptake, resulting in increased blood sugar levels and possibly type 2 diabetes.
- Metabolic syndrome
A combination of disorders, including hypertension, obesity, high blood glucose levels and dyslipidaemia, that can result in cardiovascular disease.
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene CCL2 | CCR2 | CX3CR1 | FIZZ1 | GR1 | IFNγ | IL-1 | IL-4 | IL-6 | IL-10 | IL-12 | IL-13 | IL-17 | IL-23 | MyD88 | TGFβ | TNF | YM1
FURTHER INFORMATION
David M. Mosser’s homepage: http://www.life.umd.edu/cbmg/faculty/mosser/mosserlab.htm
References
- 1.Nathan C. Metchnikoff’s legacy in 2008. Nature Immunol. 2008;9:695–698. doi: 10.1038/ni0708-695. [DOI] [PubMed] [Google Scholar]
- 2.Kono H, Rock KL. How dying cells alert the immune system to danger. Nature Rev. Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erwig LP, Henson PM. Immunological consequences of apoptotic cell phagocytosis. Am. J. Pathol. 2007;171:2–8. doi: 10.2353/ajpath.2007.070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J. Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CJ, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nature Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 6.Park JS, et al. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 7.Mackaness GB. Cellular immunity and the parasite. Adv. Exp. Med. Biol. 1977;93:65–73. doi: 10.1007/978-1-4615-8855-9_5. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S. Alternative activation of macrophages. Nature Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 9.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692.In this paper the authors proposed that the M1 and M2 designation for macrophages should be primarily based on the ratio of IL-12 to IL-10 production.
- 10.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249.This work shows that alternatively activated macrophages are biochemically and functionally distinct from regulatory macrophages.
- 11.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 12.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunderkotter C, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 14.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2.This study shows that distinct monocyte populations exist, and have different cell-surface markers and homing capacities.
- 15.Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- 16.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 17.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 18.Auffray C, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 19.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 20.Gordon S. The macrophage: past, present and future. Eur. J. Immunol. 2007;37:S9–S17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 21.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262.This work shows that mice lacking the TLR adaptor molecule TRIF are defective in TLR3- and TLR4-mediated IFNγ production.
- 24.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112.This work shows that the phagocytosis of apoptotic cells by macrophages is associated with the production of TGFβ, which inhibits the production of pro-inflammatory cytokines.
- 29.Filipe-Santos O, et al. Inborn errors of IL-12/23- and IFN-γ-mediated immunity: molecular, cellular, and clinical features. Semin. Immunol. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Nandan D, Reiner NE. Attenuation of γ interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania donovani: selective inhibition of signaling through Janus kinases and Stat1. Infect. Immun. 1995;63:4495–4500. doi: 10.1128/iai.63.11.4495-4500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pai RK, Convery M, Hamilton TA, Boom WH, Harding CV. Inhibition of IFN-γ-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 2003;171:175–184. doi: 10.4049/jimmunol.171.1.175. [DOI] [PubMed] [Google Scholar]
- 32.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr. Opin. Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 33.Loke P, et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J. Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926.This study shows that the production of IL-4 and IL-13 is a rapid innate immune response to tissue injury.
- 34.Brandt E, Woerly G, Younes AB, Loiseau S, Capron M. IL-4 production by human polymorphonuclear neutrophils. J. Leukoc. Biol. 2000;68:125–130. [PubMed] [Google Scholar]
- 35.Reese TA, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746.This work demonstrates that chitin induces the production of IL-4 by eosinophils and basophils.
- 36.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287.This is the initial observation that IL-4 upregulates the expression of macrophage mannose receptor, thereby giving rise to the term alternative activation.
- 38.Raes G, Beschin A, Ghassabeh GH, De BP. Alternatively activated macrophages in protozoan infections. Curr. Opin. Immunol. 2007;19:454–459. doi: 10.1016/j.coi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Wilson MS, et al. Immunopathology of schistosomiasis. Immunol. Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordeiro-da-Silva A, et al. Immunological alterations induced by polyamine derivatives on murine splenocytes and human mononuclear cells. Int. Immunopharmacol. 2004;4:547–556. doi: 10.1016/j.intimp.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Anthony RM, et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao A, et al. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 2008;135:217–225. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raes G, et al. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J. Leukoc. Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 44.Kzhyshkowska J, et al. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood. 2006;107:3221–3228. doi: 10.1182/blood-2005-07-2843. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Z, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 46.Bleau G, Massicotte F, Merlen Y, Boisvert C. Mammalian chitinase-like proteins. EXS. 1999;87:211–221. doi: 10.1007/978-3-0348-8757-1_15. [DOI] [PubMed] [Google Scholar]
- 47.Fusetti F, et al. Structure of human chitotriosidase. Implications for specific inhibitor design and function of mammalian chitinase-like lectins. J. Biol. Chem. 2002;277:25537–25544. doi: 10.1074/jbc.M201636200. [DOI] [PubMed] [Google Scholar]
- 48.Hesse M, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J. Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533.This study shows that arginase-1 production by alternatively activated macrophages depends on TH2-type cytokines and inversely correlates with nitric oxide production.
- 49.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor α1 and the type II IL-4 receptor in asthma pathogenesis. Proc. Natl Acad. Sci. USA. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris J, et al. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 51.Kropf P, et al. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005;19:1000–1002. doi: 10.1096/fj.04-3416fje. [DOI] [PubMed] [Google Scholar]
- 52.Muller U, et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 2007;179:5367–5377. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- 53.Shirey KA, Cole LE, Keegan AD, Vogel SN. Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J. Immunol. 2008;181:4159–4167. doi: 10.4049/jimmunol.181.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tumitan AR, Monnazzi LG, Ghiraldi FR, Cilli EM, hado de Medeiros BM. Pattern of macrophage activation in Yersinia-resistant and Yersinia-susceptible strains of mice. Microbiol. Immunol. 2007;51:1021–1028. doi: 10.1111/j.1348-0421.2007.tb03986.x. [DOI] [PubMed] [Google Scholar]
- 55.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nature Rev. Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann. N. Y. Acad. Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 1999;162:3639–3646. [PubMed] [Google Scholar]
- 58.Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann. NY Acad. Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- 59.Mosser DM. The many faces of macrophage activation. J. Leukoc. Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 60.Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcγR ligation leads to chromatin modifications at the IL-10 locus. J. Immunol. 2005;175:469–477. doi: 10.4049/jimmunol.175.1.469.This paper shows that activation of ERK is required for the hypersecretion of IL-10 by regulatory macrophages.
- 61.Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fcγ receptors. J. Immunol. 2001;166:6861–6868. doi: 10.4049/jimmunol.166.11.6861. [DOI] [PubMed] [Google Scholar]
- 62.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J. Exp. Med. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol. Ther. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasko G, Szabo C, Nemeth ZH, Deitch EA. Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a β-adrenoceptor-mediated mechanism. J. Neuroimmunol. 2002;122:34–39. doi: 10.1016/s0165-5728(01)00459-3. [DOI] [PubMed] [Google Scholar]
- 65.Sirois J, Menard G, Moses AS, Bissonnette EY. Importance of histamine in the cytokine network in the lung through H2 and H3 receptors: stimulation of IL-10 production. J. Immunol. 2000;164:2964–2970. doi: 10.4049/jimmunol.164.6.2964. [DOI] [PubMed] [Google Scholar]
- 66.Weigert A, et al. Tumor cell apoptosis polarizes macrophages role of sphingosine-1-phosphate. Mol. Biol. Cell. 2007;18:3810–3819. doi: 10.1091/mbc.E06-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lam CW, Perretti M, Getting SJ. Melanocortin receptor signaling in RAW264.7 macrophage cell line. Peptides. 2006;27:404–412. doi: 10.1016/j.peptides.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 68.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide enhance IL-10 production by murine macrophages: in vitro and in vivo studies. J. Immunol. 1999;162:1707–1716. [PubMed] [Google Scholar]
- 69.Huang H, Park PH, McMullen MR, Nagy LE. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J. Gastroenterol. Hepatol. 2008;1:S50–S53. doi: 10.1111/j.1440-1746.2007.05284.x. [DOI] [PubMed] [Google Scholar]
- 70.Ando M, Tu W, Nishijima K, Iijima S. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem. Biophys. Res. Commun. 2008;369:878–883. doi: 10.1016/j.bbrc.2008.02.111. [DOI] [PubMed] [Google Scholar]
- 71.Biswas SK, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428.In this study, tumour-associated macrophages are profiled by microarray analysis.
- 72.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 2005;201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baetselier PD, et al. Alternative versus classical macrophage activation during experimental African trypanosomosis. Int. J. Parasitol. 2001;31:575–587. doi: 10.1016/s0020-7519(01)00170-9. [DOI] [PubMed] [Google Scholar]
- 74.Kim C, et al. Antiinflammatory cAMP signaling and cell migration genes co-opted by the anthrax bacillus. Proc. Natl Acad. Sci. USA. 2008;105:6150–6155. doi: 10.1073/pnas.0800105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agrawal A, Pulendran B. Anthrax lethal toxin: a weapon of multisystem destruction. Cell Mol. Life Sci. 2004;61:2859–2865. doi: 10.1007/s00018-004-4251-4. [DOI] [PubMed] [Google Scholar]
- 76.Benoit M, Barbarat B, Bernard A, Olive D, Mege JL. Coxiella burnetii, the agent of Q fever, stimulates an atypical M2 activation program in human macrophages. Eur. J. Immunol. 2008;38:1065–1070. doi: 10.1002/eji.200738067. [DOI] [PubMed] [Google Scholar]
- 77.Mahalingam S, Lidbury BA. Suppression of lipopolysaccharide-induced antiviral transcription factor (STAT-1 and NF-κB) complexes by antibody-dependent enhancement of macrophage infection by Ross River virus. Proc. Natl Acad. Sci. USA. 2002;99:13819–13824. doi: 10.1073/pnas.202415999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stout RD, et al. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 79.Klimp AH, de Vries EG, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit. Rev. Oncol. Hematol. 2002;44:143–161. doi: 10.1016/s1040-8428(01)00203-7. [DOI] [PubMed] [Google Scholar]
- 80.Teng MW, Swann JB, Koebel CM, Schreiber RD, Smyth MJ. Immune-mediated dormancy: an equilibrium with cancer. J. Leukoc. Biol. 2008;84:988–993. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- 81.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912.This work correlates macrophage recruitment into tumours with an angiogenic switch and a poor prognosis.
- 82.Swann JB, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc. Natl Acad. Sci. USA. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romieu-Mourez R, et al. Distinct roles for IFN regulatory factor (IRF)-3 and IRF-7 in the activation of antitumor properties of human macrophages. Cancer Res. 2006;66:10576–10585. doi: 10.1158/0008-5472.CAN-06-1279. [DOI] [PubMed] [Google Scholar]
- 84.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J. Leukoc. Biol. 2008;84:623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu CH, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J. Biol. Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 86.Knowles HJ, Harris AL. Hypoxia and oxidative stress in breast cancer. Hypoxia and tumourigenesis. Breast Cancer Res. 2001;3:318–322. doi: 10.1186/bcr314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuang DM, et al. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110:587–595. doi: 10.1182/blood-2007-01-068031. [DOI] [PubMed] [Google Scholar]
- 88.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 89.Hagemann T, et al. “Re-educating” tumor-associated macrophages by targeting NF-κB. J. Exp. Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin EY, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 91.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 93.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr. Pharm. Des. 2008;14:1225–1230. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 94.Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol. Lett. 2007;112:61–67. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 95.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 96.Bastard JP, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 97.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu. Rev. Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 98.Fernandez AZ. Peroxisome proliferator-activated receptors in the modulation of the immune/inflammatory response in atherosclerosis. PPAR Res. 2008;2008:285842. doi: 10.1155/2008/285842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin-Fuentes P, et al. Individual variation of scavenger receptor expression in human macrophages with oxidized low-density lipoprotein is associated with a differential inflammatory response. J. Immunol. 2007;179:3242–3248. doi: 10.4049/jimmunol.179.5.3242. [DOI] [PubMed] [Google Scholar]
- 100.Haque S, Mirjafari H, Bruce IN. Atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Curr. Opin. Lipidol. 2008;19:338–343. doi: 10.1097/MOL.0b013e328304b65f. [DOI] [PubMed] [Google Scholar]
- 101.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. γδ T cells: an important source of IL-17. Curr. Opin. Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mangan PR, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 103.costa-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nature Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 104.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 105.Jovanovic DV, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J. Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 106.Sato K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 108.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 109.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 110.Kodelja V, et al. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 α with a Th2-associated expression pattern. J. Immunol. 1998;160:1411–1418. [PubMed] [Google Scholar]
- 111.Raes G, et al. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Dev. Immunol. 2002;9:151–159. doi: 10.1080/1044667031000137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wirnsberger G, Hebenstreit D, Posselt G, Horejs-Hoeck J, Duschl A. IL-4 induces expression of TARC/CCL17 via two STAT6 binding sites. Eur. J. Immunol. 2006;36:1882–1891. doi: 10.1002/eji.200635972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ruckerl D, Hessmann M, Yoshimoto T, Ehlers S, Holscher C. Alternatively activated macrophages express the IL-27 receptor α chain WSX-1. Immunobiology. 2006;211:427–436. doi: 10.1016/j.imbio.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 114.Wynes MW, Riches DW. Induction of macrophage insulin-like growth factor-I expression by the Th2 cytokines IL-4 and IL-13. J. Immunol. 2003;171:3550–3559. doi: 10.4049/jimmunol.171.7.3550. [DOI] [PubMed] [Google Scholar]
- 115.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 116.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 117.Torocsik D, Bardos H, Nagy L, Adany R. Identification of factor XIII-A as a marker of alternative macrophage activation. Cell. Mol. Life Sci. 2005;62:2132–2139. doi: 10.1007/s00018-005-5242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sironi M, et al. Differential regulation of chemokine production by Fcγ receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2) J. Leukoc. Biol. 2006;80:342–349. doi: 10.1189/jlb.1005586. [DOI] [PubMed] [Google Scholar]
- 119.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol. Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]