Abstract
Intracellular mitogen-activated protein kinase (MAPK) signaling cascades likely play an important role in the pathogenesis of cardiac and vascular disease. A substantial amount of basic science research has defined many of the details of MAPK pathway organization and activation, but the role of individual signaling proteins in the pathogenesis of various cardiovascular diseases is still being elucidated. In this review, the role of the MAPKs extracellular signal-regulated kinase (ERK), C-jun N-terminal kinase (JNK) and p38 MAPK in cardiac hypertrophy, cardiac remodeling after myocardial infarction, atherosclerosis and vascular restenosis will be examined with attention paid to genetically-modified murine model systems and to the use of pharmacologic inhibitors of protein kinases. Despite the complexities of this field of research, attractive targets for pharmacological therapy are emerging.
Introduction: Intracellular Signal Transduction Pathways
The growth and survival of adult cardiomyocytes, smooth muscle cells and macrophages are regulated by extracellular ligands, growth factors, and cytokines that bind to cell surface receptors and activate intracellular signal transduction cascades. These signaling pathways control essential processes in all eukaryotic cells, including gene transcription, protein translation, cytoskeletal remodeling, endocytosis, cell metabolism, cell proliferation and survival. The analysis of the function of specific signaling proteins in cardiovascular pathophysiology is a major goal for biomedical researchers.
When extracellular growth factors, ligands or cytokines bind to cell surface receptors, conformational changes occur in these receptors altering the intracellular signaling potential of these proteins. In one of the best-understood examples, the extracellular dimeric platelet-derived growth factor ligand binds simultaneously to two single-pass PDGF receptor tyrosine kinases and this facilitates the clustering of the two receptors so that they are able to phosphorylate one another on tyrosine residues on their intracellular portions [1]. These phosphorylated intracellular tyrosine residues, in turn, are docking sites for proteins that are able to bind to phosphorylated motifs, such as the src-homology 2 (SH2)-domain containing protein Grb2 [2]. Grb2 is constitutively bound to the guanine nucleotide exchange factor SOS [3,4]. When Grb2 binds to phosphotyrosine motifs on the intracellular portion of receptor tyrosine kinases, it brings SOS in close proximity to the plasma membrane-tethered small GTPase ras and facilitates ras activation [5, 6, 7]. Ras is a master regulator of intracellular signaling cascades and it promotes the activation of mitogen-activated protein kinase cascades (MAPKs) as well as other signaling pathways.
In addition to the direct activation of receptor tyrosine kinases by growth factor-mediated receptor clustering, there are several alternative mechanisms for transmembrane receptor activation that result in ras activation. For example, direct activation of GPCRs by extracellular ligands can result in the transactivation of RTKs, via the metalloproteinase-mediated extracellular release of tethered growth factors, such as heparin binding epidermal growth factor, or by the action of intracellular signaling proteins, such as src family tyrosine kinases, on the intracellular portions of RTKs [8]. Furthermore, receptor tyrosine kinase (RTK) activation by growth factor binding can result in the transactivation of G-protein coupled receptors (GPCRs) that promote the activation of Gα and Gβγ subunits. Transactivation of GPCRs can either occur as a result of GPCR ligand production downstream of RTK activation, or because of complex formation between RTKs and GPCRs [9].
MAPK cascades are triple kinase pathways that include a MAPK kinase kinase (MKKK), a MAPK kinase (MKK) and a terminal MAPK. MAPK cascades may be organized in this fashion in order to promote signal amplification and fidelity [10]. Furthermore, scaffolding proteins help to cluster particular components of MAPK cascades in specific subcellular localizations [11]. The first MKKK to be well characterized was Raf-1, a proto-oncogene that is a serine/threonine protein kinase [12]. Raf-1, and its highly related family members A-Raf and B-Raf, binds directly to activated GTP-bound ras. Raf-1 binding to ras results in several complicated post-translational modifications of Raf-1, ultimately leading to full kinase activation [13]. Once fully activated, Raf-1 phosphorylates and activates MKK1 or MKK2 (also called MEK1/2). MKK1/2, in turn, phosphorylates ERK1 or ERK2 (ERK1/2) on a threonine and a tyrosine residue in its activation loop, leading to kinase activation. Fully activated ERK1/2 has a variety of substrates at the plasma membrane, in the cytosol and in the nucleus that regulates important aspects of cell physiology.
In addition to the Raf-MKK-ERK cascade, at least three other major MAPK cascades, the c-jun N-terminal kinase (JNK) cascade, the p38 MAPK cascade, and the big MAPK (ERK5) cascade play an important role in the regulation of cell physiology [10]. The ERK5 cascade will not be discussed further in this review because it has been characterized in fewer models of cardiovascular disease than the other three MAPK cascades. Activated GTP-bound ras indirectly promotes the activation of the JNK and p38 MAPK cascades, perhaps via the ability of ras to directly bind to and activate phosphatidylinositol-3’ kinase α (PI3Kα) that facilitates the activation of the rac GTPase in some cell types [14,15]. Activated rac, in turn, is able to bind to MEKK family members that are MKKKs for the JNK and p38 MAPK cascades [16]. While the Grb2-SOS-ras-PI3Kα-rac pathway plays an important role in the activation of the JNK and p38 MAPK cascades in some situations, there are other important stimuli that activate these cascades independent of ras, such as cell “stress” due to the accumulation of reactive oxygen species (ROS), hyperosmolarity, genotoxicity or dysfunction of the endoplasmic reticulum [17–20]. In the case of the JNK and p38 cascades, there are several MKKKs, such as MEKK1–4, ASK1, TAK1 and MLK3 [21–27]. The specific MKKK that regulates activation of the JNK or the p38 MAPK cascade is not known in many physiological contexts. There are two major MKKs in the JNK cascade, named MKK4 and MKK7. There are three genes that encode the JNK family members JNK1, JNK2 and JNK3. There are four splice variants of JNK1, four splice variants of JNK2, and two splice variants of JNK3 that lead to the production of 10 isoforms at either 46 or 54 kDa [28]. For the p38 MAPK cascade, there are several MKKKs, such as ASK1 and TAK1, two major MKKs, named MKK3 and MKK6. There are four genes encoding members off the p38 MAPK family, called p38α, p38β, p38γ and p38δ [29]. It is apparent that upstream activators of the JNK and p38 MAPK pathway are overlapping, especially at the level of MKKKs, and this has led to confusion about the independent regulation of these pathways. A leading model is that scaffolding proteins establish specificity by binding to unique combinations of MAPK pathway kinases and substrates in particular subcellular localizations [11,30,31].
The tremendous progress made in the identification and characterization of the components of the MAPK cascades has led to an explosion in translational research attempting to link these signaling pathways to cardiovascular disease. In this review, the role of the ERK MAPK, JNK and p38 MAPK cascades in the pathophysiology of cardiac hypertrophy, pathological remodeling after myocardial infarction, development of atherosclerosis, and vascular neointima formation will be discussed.
Signaling Mechanisms in Cardiac Hypertrophy: Background and MAPK Overexpression Studies
Most adult cardiomyocytes are unable to proliferate, and they respond to stress by enlarging due to increased protein synthesis or reduced protein degradation. If cardiomyocytes are stressed because of pressure overload, then the growth of individual cardiomyocytes is typically associated with abnormal gene expression and the increased deposition of extracellular matrix materials [32]. Extracellular matrix is primarily deposited in the heart by cardiac fibroblasts. When many cardiomyocytes get larger and extracellular matrix is deposited, the entire heart enlarges, resulting in the development of cardiac hypertrophy. Pressure overload-induced cardiac hypertrophy, also called ‘pathological’ cardiac hypertrophy, is associated with a very poor prognosis in humans and often contributes to the development of cardiac arrhythmias, diastolic dysfunction and congestive heart failure [33]. The growth of individual cardiomyocytes in response to pressure overload is characterized by the increased width of cells. Interestingly, in response to exercise or volume overload, cardiomyocytes enlarge but do not exhibit altered gene expression and this cardiomyocyte growth is not associated with extracellular matrix deposition [32]. The growth of individual cardiomyocytes in response to exercise is characterized by the increased length of cells. The enlargement of the heart that occurs in response to exercise or volume overload is called ‘physiological’ cardiac hypertrophy and is not associated with a poor prognosis in humans.
In most cases, pathologic cardiac hypertrophy develops as a consequence of pressure overload due to hypertension or valvular heart disease. To model cardiac hypertrophy in animals, surgical procedures were developed that mimic coarctation of the aorta that result in pressure overload [34, 35]. The development of these animal models of pressure overload, combined with the ability to genetically manipulate mice, has resulted in a series of important basic science discoveries that have implications towards the clinical management of patients.
When transverse aortic constriction (TAC) surgery is performed on mice, all three major MAPK pathways are activated in cardiac tissue [36]. In one study, JNK activation appeared to occur earliest and was fully activated within three hours of surgery, while ERK and p38 MAPK activation occurred within 7 days of surgery [36]. In another study, ERK1/2 and p38 MAPK activation occurred within 10 minutes of TAC [37]. Activation of ERK, JNK and p38 MAPK has been demonstrated in other animal model systems and also in humans with heart failure [38].
An important study in 1995 showed that cardiac-specific overexpression of an activated form of Harvey-ras (H-ras) in transgenic mice leads to cardiac hypertrophy and diastolic dysfunction [39]. In this work, a myosin light chain 2v promoter fragment was linked to a cDNA encoding the oncogenic Val12 mutant form of H-ras to create a construct that was used to generate several lines of transgenic mice. Mice that were homozygous for the MLC-ras transgene exhibited a marked 57.5% increase in left ventricular mass and in the left ventricular weight-to-body weight ratio when compared to nontransgenic control mice. Increased LV weight was associated with an increased average area of cardiomyocytes. No increase in cardiac fibrosis was observed in transgenic mice, however, myofibrillar disarray was observed on histologic analysis and atrial natriuretic factor mRNA levels were markedly increased. In addition, cardiac catheterization showed that diastolic function was depressed in MLC-ras transgenic mice [39].
In related work on the role of the ras-Raf-MKK-ERK signaling cascade, overexpression of an activated form of MKK1 (MEK1) was shown to lead to profound cardiac hypertrophy without fibrosis [40]. In this study, the cardiac-specific α-myosin heavy chain promoter was linked to a cDNA encoding an activated form of MKK1 (Ser217/221 to Glu) to create a construct that was used to generate several lines of transgenic mice. All lines of mice developed concentric left ventricular hypertrophy with increased cardiomyocyte cell size but without interstitial fibrosis. Indeed, cardiac hypertrophy was observed beginning at 3 weeks of age. ERK1/2 activation was increased in cardiac tissue obtained from MHC-MKK1 transgenic mice. Atrial natriuretic factor, brain natriuretic peptide, α-skeletal actin, and β-myosin heavy chain gene expression were all elevated in 8-week-old transgenic MHC-MKK1 mice. Isolated working heart preparations were used to evaluate cardiac function and this showed that MHC-MKK1 transgenic mice had increased systolic function, determined by dP/dtmax, and reduced diastolic function, determined by dP/dtmin [40].
The molecular mechanisms linking activated MKK1 to the development of cardiac hypertrophy in intact animals are not firmly established, however, there are several potential mechanisms that have been demonstrated in cultured cells. Activated MKK1 may promote cardiac hypertrophy by the phosphorylation and activation of the transcription factors Elk-1 and GATA-4 by ERK [41, 42]. Furthermore, activated MKK1 may promote hypertrophy via the activation of the cellular translational machinery. ERK can directly phosphorylate the TSC2 gene product tuberin on serine-664, and this leads to the activation of mammalian target of rapamycin (mTOR), a master regulator of protein synthesis [43]. Furthermore, additional ERK substrates, including 90 kDa ribosomal protein S6 kinase (p90RSK) and the MAPK-interacting kinases (Mnk1/2), are known to promote increased translation [44]. Activated p90RSK can also phosphorylate and inactivate tuberin, leading to mTOR activation [45]. Mnk1 can phosphorylate and activate the eukaryotic initiation factor 4E (eIF4E) [46]. Therefore, activated MKK1 may promote cardiac hypertrophy via ERK-mediated effects on cardiomyocyte transcription and translation.
While activation of the ras-Raf-MKK-ERK cascade in heart promotes cardiac hypertrophy, activation of the JNK cascade does not. Transgenic mice were generated with cardiac-specific overexpression of an activated form of MKK7 (Ser-281 and Thr-275 to Asp, called MKK7D) [47]. Transgenic MKK7D mice exhibited increased activation of both JNK1 and JNK2 in cardiac tissue, without activation of ERK1/2 or p38α. Transgenic MKK7D mice died at around 7 weeks of age with signs of congestive heart failure including edematous lungs and ascites. Although there was biatrial enlargement in transgenic MKK7D mice, there was no left ventricular hypertrophy and cardiomyocyte cell size was not increased. However, ANF and α-skeletal actin mRNA levels were increased in MKK7D cardiac tissue. Diastolic left ventricular filling was impaired in MKK7D transgenic mice. Interestingly, MKK7D mice also exhibited reduced connexin 43 protein levels and gap junction formation between cardiomyocytes in ventricular sections [47]. In related work, transgenic mice with cardiac-specific overexpression of an MKK7-JNK1 fusion protein were generated and were found to have normal ventricular weight at baseline, but were resistant to cardiac hypertrophy induced by overexpression of calcineurin A [48]. The ability of JNK1 activation to antagonize calcineurin A-induced cardiac hypertrophy may be explained by the ability of JNK1 to phosphorylate members of the nuclear factor of activated T cell transcription factor family (NFATs), there by preventing their nuclear translocation. Indeed, overexpression of JNK1 and MKK7 in cultured cardiomyocytes blocked calcineurin A-induced nuclear translocation of an NFATc3-green fluorescent protein fusion protein [48].
Activation of the p38 cascade in cardiac tissue does not promote cardiac hypertrophy as demonstrated by transgenic mice with cardiac-specific overexpression of activated mutant forms of MKK3 (MKK3bE) and MKK6 (MKK6bE) [49]. Both MKK3bE and MKK6bE transgenic mice exhibited increased p38 kinase activity in cardiac tissue. Both types of transgenic mice died between 5 and 7 weeks of age with signs of congestive heart failure. Neither MKK3bE or MKK6bE transgenic mice developed left ventricular hypertrophy or cardiomyocyte enlargement, however, they both developed biatrial enlargement. Prominent interstitial cardiac fibrosis was observed in both types of transgenic mice, and ANF, β-myosin heavy chain, and α-skeletal actin mRNA levels were increased. LV systolic function was depressed in MKK3bE mice with reduced LV wall thickness, but systolic function was not reduced in MKK6bE mice. Both types of transgenic mice exhibited increased diastolic chamber stiffness [49].
Taken together, overexpression studies lead to the model that the simultaneous activation of ERK, JNK and p38 MAPK in heart after pressure overload contribute to the development of pathological cardiac hypertrophy. In this model, ERK activation promotes the growth of cardiomyocytes, JNK activation leads to reduced gap junction formation, p38 MAPK activation promotes cardiac fibrosis, and activation of all three pathways promotes reduced diastolic compliance. Pressure overload also leads to the activation of the calcineurin A-NFAT pathway in heart that clearly plays a major role in promoting the growth of cardiomyocytes [32].
Signaling Mechanisms in Cardiac Hypertrophy: MAPK Loss-of-Function Studies
Overexpression studies in mice suggest that ras-mediated ERK activation plays an important role in the growth of individual cardiomyocytes, whereas p38 and JNK activation does not promote cardiac hypertrophy but instead promotes cardiac dysfunction. Loss-of-function studies in vivo correlate, in part, with overexpression studies.
Several studies investigated the role of the Raf-MKK-ERK cascade in the development of cardiomyocyte hypertrophy by use of pharmacologic inhibitors. In one study, rat neonatal cardiomyocytes were treated with endothelin-1 or phenylephrine in the presence of the selective and highly potent MKK1/2 inhibitor U0126, or the selective Raf family member inhibitor SB-386023 [50]. Ligand-induced protein synthesis, cardiomyocyte growth, sarcomeric organization, and expression of β-myosin heavy chain were all potently inhibited by U0126 or SB386023. In another study, phenylephrine- and endothelin 1-induced protein synthesis, measure by both radioactive methionine and radioactive leucine incorporation, was dramatically inhibitors by treatment of cardiomyocytes with the MKK1/2 inhibitors U0126 or PD184352 [51]. In an in vivo study, cardiac hypertrophy was induced in rats after administration of the NO synthase inhibitor L-NAME that resulted in the development of systemic hypertension [52]. L-NAME induced cardiac hypertrophy was blocked in animals that were treated with the MKK1/2 inhibitor PD98059. Therefore, experiments with pharmacologic inhibitors support a role of the Raf-MKK-ERK pathway in the development of cardiac hypertrophy.
Activation of MAPK cascades is often dependent on the action of the Grb2/SOS complex. To determine the role of Grb2 in the development of pathological cardiac hypertrophy, experiments were performed with Grb2 haplo-insufficient mice [53]. Grb2−/− mice do not survive early embryonic development, but Grb2+/− mice appear normal at birth and have normal cardiac structure and function at baseline. Grb2 haplo-insufficient mice were resistant to pressure-overload induced cardiac hypertrophy induced by transverse aortic constriction (TAC). Furthermore, Grb2+/− mice developed less interstitial cardiac fibrosis and exhibited reduced levels of ANF and β-myosin heavy chain mRNA in response to TAC when compared to wild type mice. In response to TAC, cardiac JNK and p38 MAPK activation was reduced in Grb2+/− mice, but ERK1/2 activation was normal. It is important to note that Grb2 may affect the activation of several non-MAPK signaling pathways, including the PI3Kα-Akt1 pathway and the PLCγ-Ca++ pathway, through complex interactions with other scaffolding proteins such as GAB1 [53].
To specifically investigate the role of Raf-1 in the development of cardiac hypertrophy, transgenic mice were generated with cardiac-specific overexpression of a dominant negative form of Raf-1 (DN-Raf) [54]. Cardiomyocytes express Raf-1, B-Raf and A-Raf and dominant negative Raf-1 likely inhibits all three family members [55]. Transgenic DN-Raf mice were resistant to TAC-induced cardiac hypertrophy and cardiomyocyte enlargement [54]. Furthermore, ERK1/2 activation was reduced in DN-Raf cardiac tissue 1 week after TAC, but p38 and JNK activation was unaffected. Pressure overload-induced ANF and β-myosin heavy chain gene induction was diminished in DN-Raf cardiac tissue. Interestingly, DN-Raf mice exhibited significantly increased mortality after TAC that was associated with increased cardiomyocyte apoptosis when compared to wild type mice [54].
To specifically analyze the role of Raf-1 in cardiac hypertrophy, mice with cardiac-specific targeted gene disruption of Raf-1 (Raf CKO) were produced [56]. Although Raf CKO mice exhibited a normal life expectancy, they developed abnormal cardiac function by 10 weeks of age with markedly reduced LV systolic function, LV dilatation, and reduced LV posterior wall thickness. Furthermore, Raf CKO mice exhibited increased cardiomyocyte apoptosis at 3 to 5 weeks of age. Cardiac signal transduction was abnormal at baseline in Raf CKO mice with increased ASK1, JNK and p38 MAPK activities. However, endothelin-1-induced MKK and ERK1/2 activation was not affected in Raf CKO cardiac tissue. The cardiac phenotype of Raf CKO mice was ameliorated by breeding with ASK1 knockout mice [56]. Taken together, these results suggest that Raf-1 inhibits ASK1 in an MKK/ERK-independent manner, and also suggest that B-Raf and A-Raf function in parallel with Raf-1 to promote ERK1/2 activation in heart.
To specifically examine the role of ERK1/2 in the development of cardiac hypertrophy, the cardiac phenotypes of ERK1−/− and ERK2+/− mice were analyzed [37]. ERK2 haplo-insufficient mice were examined because ERK2 comprises about 70% of total ERK protein in heart. ERK2−/− mice are nonviable and ERK1−/− ERK2+/− mice exhibit increased embryonic lethality. Both ERK1−/− and ERK2+/− mice exhibited a normal cardiac hypertrophic response to pressure overload by TAC. In addition, a transgenic mouse model was generated with cardiac-specific overexpression of dual-specificity phosphatase 6 (DUSP6) that is a relatively specific phosphatase for ERK1/2. DUSP6 transgenic mice exhibited reduced ERK1/2 activation in response to TAC, but developed a normal degree of left ventricular hypertrophy. However, DUSP6 transgenic mice exhibited more interstitial fibrosis and increased cardiomyocyte apoptosis after TAC when compared to nontransgenic mice [37]. While these findings suggest that ERK1/2 is not required for the growth of cardiomyocytes, cardiac-specific targeted disruption of ERK2 may provide more definitive information about this question.
To address the role of the JNK pathway in cardiac hypertrophy, mice with altered expression and activity of JNK proteins were investigated [48]. JNK1−/−, JNK2−/−, JNK1+/− JNK2−/−, and transgenic mice with cardiac-specific overexpression of dominant negative forms of JNK1 and JNK2 (MHC-DN-JNK1/2) were subjected to pressure overload by TAC. While JNK1−/− and JNK2−/− mice developed cardiac hypertrophy to a similar extent as wild type mice, JNK1+/− JNK2−/− mice and MHC-DN-JNK1/2 transgenic mice developed an exaggerated form of cardiac hypertrophy in response to TAC. Furthermore, 7 month-old JNK1+/− JNK2−/− mice and MHC-DN-JNK1/2 transgenic mice developed spontaneous cardiac hypertrophy with cardiomyocyte enlargement in the absence of pressure overload [48]. MHC-DN-JNK1/2 also exhibited increased cardiac NFAT activity measured by use of an NFAT binding element-luciferase reporter construct. This study suggests that JNK1/2 inhibits cardiac hypertrophy through phosphorylation and inactivation of NFAT transcription factors. Another group more recently examined the response of JNK1−/−, JNK2−/− and JNK3−/− mice to pressure overload by TAC [57]. While all three knockout mice developed cardiac hypertrophy to an extent indistinguishable from wild type mice, JNK1−/− mice exhibited an abnormal response to TAC manifested by reduced LV systolic function for several weeks after the surgery that eventually returned to normal. These findings suggest that JNK1 plays a protective role to maintain LV systolic function in the acute phase after pressure overload.
To address the role of p38 MAPK in cardiac hypertrophy, transgenic mice with cardiac-specific overexpression of a dominant negative forms of p38α or p38β were examined [53, 58]. Both DN-p38α and DN-p38β transgenic mice developed cardiac hypertrophy to a similar extent as nontransgenic mice, although there was a trend towards greater hypertrophy in DN-p38β mice. Furthermore, both DN-p38α and DN-p38β transgenic mice exhibited reduced interstitial fibrosis after TAC when compared to nontransgenic mice [53]. In another study, transgenic mice were examined cardiac-specific overexpression of dominant negative p38α, dominant negative MKK3, or dominant negative MKK6 [59]. In this study, DN-p38α, DN-MKK3 and DN-MKK6 transgenic mice all developed enhanced cardiac hypertrophy in response to pressure overload induced by TAC with increased cardiomyocyte size. Furthermore, all three types of transgenic mice developed spontaneous cardiac hypertrophy in the absence of pressure overload. Finally, cardiac NFAT activity was increased in DN-p38α cardiac tissue as measured by an NFAT-dependent luciferase reporter transgenic mouse [59]. This study suggested that p38α, similar to JNK1/2, inhibits cardiac hypertrophy through inactivation of NFAT transcription factors.
Mice with cardiac-specific disruption of the p38α gene had normal cardiac structure and function in the absence of experimental manipulation [60]. When subjected to pressure overload by TAC, p38α CKO mice developed cardiac hypertrophy to a similar but slightly greater extent than control p38αloxp/loxp α-MHC-Cre(−) mice. In addition, p38α CKO mice developed markedly increased cardiomyocyte apoptosis and fibrosis after TAC. One way to resolve the apparent inconsistencies between the various p38α loss-of-function studies is that there may be a biphasic dose-response curve to p38α activation in cardiomyocytes. In this model, low-level and/or transient p38α activation has an important anti-apoptotic function in cardiomyocytes, but high-level and/or sustained p38α activation promotes cell death. Presumably all cardiac p38α activity would be eliminated in the p38α CKO mouse, but only the high-level and/or sustained activity would be eliminated in the various dominant negative transgenic models. An alternative theory is that p38α has an anti-apoptotic function that is not dependent on kinase activity, similar to the ability of Raf-1 to bind to and inhibit ASK1.
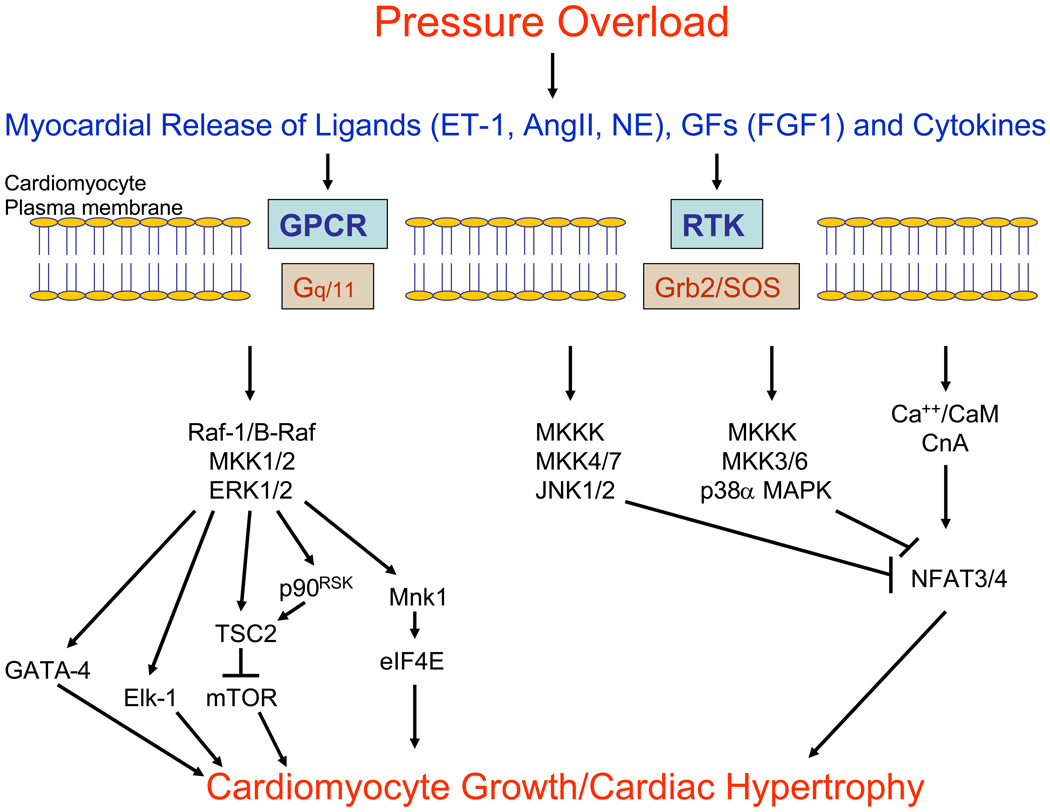
One current model of the role of intracellular signaling pathways in the development of pathological cardiac hypertrophy is that the Raf -MKK1/2-ERK1/2 cascade likely contributes to the growth of cardiomyocytes, but the calcineurin-NFAT pathway is dominant in this process (Figure 1) [32, 61]. Furthermore, the MKKK-MKK3/6-p38α MAPK and the MKKK-MKK4/7-JNK1/2 pathways both inhibit the growth of cardiomyocytes, and p38α/β promotes cardiomyocyte dysfunction. This model implies that inhibition of p38 MAPK in vivo may lead to increased cardiac hypertrophy with increased cardiac function and therefore may be useful for patients with dilated cardiomyopathy who have depressed cardiac contractility and ventricular wall thinning. On the other hand, inhibition of ERK1/2 in vivo may lead to regression of cardiac hypertrophy and may promote cardiomyocyte apoptosis and therefore would be useful in patients with hypertrophic cardiomyopathy with preserved systolic function.
Figure 1.
Model of the role of MAPK cascades in cardiomyocyte hypertrophy. In response to pressure overload, there is an intramyocardial release of ligands such as endothelin-1 (ET-1), angiotensin II (AngII) and norepinephrine (NE), growth factors such as fibroblast growth factor 1 (FGF1), and cytokines. These extracellular factors bind to and activate transmembrane G protein-coupled receptors (GPCR), receptor tyrosine kinases (RTK) and cytokine receptors (not depicted). Activated transmembrane receptors, in turn, directly activate intracellular signaling proteins, including G proteins (Gq/11) and the Grb2/SoS complex that promote activation of MAPK cascades and the Ca++/Calmodulin (CaM)-Calcineurin A (CnA)-NFAT3/4 cascade. Activation of these signaling cascades modulates the growth of cardiomyocytes in the manner depicted. Specifically, ERK MAPK phosphorylates a variety of targets that may contribute to cardiomyocyte growth, including the transcription factors Elk-1 and GATA4, and several proteins that regulate the translational machinery, including tuberin (TSC2 gene product), Mnk1 and p90RSK. On the other hand, JNK and p38 MAPK phosphorylate NFAT family members, resulting in inhibition of the calcineurin-NFAT hypertrophic pathway.
Role of MAPKs in Cardiac Remodeling After Myocardial Infarction
Myocardial infarction usually occurs when there is acute thrombosis in a coronary artery at the site of an atherosclerotic lesion, resulting in termination of blood flow to myocardium supplied by the affected artery. In the days and weeks after an acute myocardial infarction, pathological cardiac remodeling can occur that is characterized by cardiomyocyte death in the infarct border zone with resultant infarct extension, fibrosis at the site of infarct and in the unaffected myocardium, dilatation of the left ventricle, and hypertrophy of the unaffected myocardium [62]. Cardiac remodeling is thought to occur as a result of inflammatory mechanisms that are triggered by the necrotic myocardium, generation of reactive oxygen species by necrotic myocardium, and also because of increased wall stress in the infarct border zone. Although remodeling has acute adaptive features, such as the deposition of extracellular matrix in the infarct zone to prevent cardiac rupture, it eventually promotes functional decompensation and the development of heart failure.
The role of MAPKs in pathological cardiac remodeling has been investigated in several studies. In particular, p38 MAPK has been widely studied in this context. In the minutes after experimental myocardial infarction, ERK1/2, JNK1/2 and p38α MAPK are all activated in both the ischemic myocardium and unaffected portions of the left ventricle of mice and rats [63, 64]. Although the activation of various MAPKs in the unaffected myocardium is variable in the days following myocardial infarction, it appears that p38α MAPK is most consistently activated for several weeks after the initial insult [65–67]. In one study, transgenic mice with cardiac-specific expression of a dominant negative form of p38α MAPK were evaluated for their ability to remodel after experimental myocardial infarction [63]. DN-p38α mice and nontransgenic controls were subjected to left coronary artery ligation and 2-dimenstional transthoracic echocardiography performed two hours after surgery revealed nearly identical initial infarct sizes. Cardiac remodeling was evaluated 7 days after MI and DN-p38α exhibited a reduced area of scarring at the site of infarction with markedly reduced cardiomyocyte apoptosis in the infarct border zone. Furthermore, the left ventricular end-systolic volume was significantly reduced in DN-p38α mice when compared to nontransgenic mice 7 days after MI. One potential molecular mechanism for the reduction in pathological cardiac remodeling is that cardiac tissue from DN-p38α transgenic mice obtained 2 hours after LAD ligation exhibited reduced deamidation of the pivotal anti-apoptotic protein Bcl-XL [63]. Deamidation of Bcl-xL inhibits its anti-apoptotic function and may result in its degradation. In a related study, overexpression of DN-p38α in transgenic mouse heart tissue resulted in increased expression of Bcl-2 at baseline, which was increased after ischemia/reperfusion injury [68]. Taken together, these results suggest that activation of p38α MAPK promotes pathological remodeling by reducing the activity or expression of the anti-apoptotic family members Bcl-XL and Bcl-2.
Several studies employed pharmacologic inhibitors of p38 MAPK to evaluate the role of this protein kinase in cardiac remodeling after experimental myocardial infarction. In one study, rats were subjected to experimental myocardial infarction by ligation of the left anterior descending coronary artery [69]. On day 6 after surgery, mice were evaluated by echocardiography, and animals with anterior wall infarction affecting greater than 40% of the LV were randomized to receive RWJ-67657, a p38α and p38β inhibitor, or vehicle for three weeks. On day 27 after MI, animals were evaluated for the presence of cardiac remodeling. Echocardiography demonstrated that fractional shortening was significantly higher in animals that received RWJ when compared to animals treated with vehicle. Echocardiography also showed that infarct expansion was reduced in RWJ-treated animals. Cardiac catheterization showed that systolic function, measured by determining the dP/dtmax, and diastolic function, measured by determining the LVEDP, were both better preserved in animals that received RWJ after MI when compared to those that received vehicle [69]. Finally, cardiac fibrosis was reduced in the unaffected myocardium of animals treated with RWJ after MI. In another recent study with rats, animals were subjected to experimental MI, and then immediately begun on treatment with SB203580, a p38α and p38β inhibitor, or vehicle for 1 or 6 weeks [70]. At both 1 and 6 weeks after MI, treatment with SB203580 resulted in reduced myocardial fibrosis, reduced TNF-α levels and collagen I levels, and increased LV contractile function. In a mouse study, animals were subjected to experimental myocardial infarction and p38-inhibition therapy was begun two weeks later, after early cardiac remodeling was completed [71]. Mice were treated with SC-409, the angiotensin converting enzyme inhibitor (ACEI) enalapril, enalapril and SC-409 or vehicle for 12 weeks. At the end of the therapy period, mice treated with SC-409 exhibited an improved ejection fraction, increased cardiac output, decreased LV chamber dilatation, and reduced myocardial collagen deposition. Inhibition of p38 MAPK activity had a similar benefit as ACEI therapy in this mouse model system.
Analysis of mice haplo-insufficient for 14-3-3τ also demonstrated the role of p38α MAPK in post-infarction cardiac remodeling [72]. 14-3-3 proteins are intracellular dimeric phospho-serine binding proteins that inhibit activation of p38α MAPK and JNK1/2 by binding to ASK1 and other MKKKs, but they promote activation of the ERK cascade via complex interactions with Raf family members [73]. 14-3-3τ−/− mice did not survive embryonic development, but haplo-insufficient mice appeared normal at birth. Cardiac tissue from 14-3-3τ+/− mice exhibited increased basal activation of ASK1, p38α and JNK1/2, but reduced ERK1/2 activation [72]. Surgical ligation of the LAD was performed in 14-3-3τ−/− and wild type mice and both groups had similar initial infarct sizes by echocardiography 1 day after surgery. However, seven days after surgery, 14-3-3τ−/− mice had increased LV chamber dilatation, increased infarct size and increased cardiomyocyte apoptosis in the infarct border zone when compared to wild type mice. Furthermore, 14-3-3τ−/− mice exhibited significantly increased mortality in the days after MI, chiefly as a result of ventricular rupture at the site of myocardial infarction. Treatment of 14-3-3τ−/− mice with SB202190 improved the survival of these animals after MI [72].
The mechanisms by which p38α MAPK promotes pathological cardiac remodeling may include the induction of apoptosis, via modulation of Bcl-xL and Bcl-2 activity, and the production of inflammatory cytokines. Another possible explanation was recently elaborated in a study that showed that p38α can block cardiomyocyte mitosis [74]. In this study, microarray analysis of neonatal rat cardiomyocytes treated with SB203580 showed that p38 inhibition results in the upregulation of many cell cycle proteins, including cyclin A2, cyclin B, cdc2 and aurora B. Treatment of cultured adult rat cardiomyocytes with FGF1 and SB203580 induced mitosis. Analysis of mice with cardiac-specific disruption of p38α MAPK (MLC-2v-Cre p38α loxP/loxP) showed that these mice had a 92.3% increase in neonatal cardiomyocyte mitoses. In a second study, rats were subjected to experimental myocardial infarction, and animals were treated with SB203580 by intraperitoneal injection every 3 days for 1 month, a single injection of FGF1 plus self-assembling peptides into the infarct border zone immediately after coronary artery ligation, SB203580 plus FGF1, or vehicle alone [75]. Evidence of cardiomyocyte mitosis, measured by cyclin A and H3P staining, was increased within the infarct and infarct border zones of rats treated with SB203580 or SB203580/FGF1 for two weeks. Evidence of pathological cardiac remodeling was reduced in rats treated with SB203580 or SB203580/FGF1 two weeks after MI with treated animals exhibiting increased fractional shortening, reduced left ventricular scar volume, reduced ventricular muscle loss, and reduced thinning at the site of myocardial infarction [75]. When rats were analyzed three months after MI and two months after SB203580 injection were discontinued, the salutary effects of combined SB203580/FGF1 treatment on cardiac structure and function persisted, although the effects of SB203580 treatment alone were diminished, and this difference may be due to increased myocardial capillary density seen in rats treated with FGF1 [75].
An important MKKK in heart that can activate both the JNK1/2 and p38α MAPK pathways is ASK1. This stress-inducible MKKK is interesting because of its ability to be regulated by reactive oxygen species via thioredoxin [76]. Thioredoxin binds to and inhibits the activity of ASK1 in a reduction/oxygenation-sensitive matter. The concentration of reactive oxygen species is increased in unaffected myocardium following myocardial infarction in mice [77]. To evaluate the role of ASK1 in cardiac remodeling, one group subjected ASK1−/− and wild type mice to experimental myocardial infarction [78]. Although the initial infarct sizes were the same in both groups, ASK1−/− mice exhibited reduced cardiac remodeling, with reduced fibrosis in the border zone and remote myocardium, reduced diastolic LV dimension, improved fractional shortening, and reduced cardiomyocyte apoptosis in the border zone. Activation of JNK1/2 in the infarct border zone was reduced in ASK1−/− mice 2 and 7 days after MI, but p38α activation was not affected in knockout animals [78]. These results suggest that ASK1-JNK1/2 signaling promotes pathological cardiac remodeling after myocardial infarction.
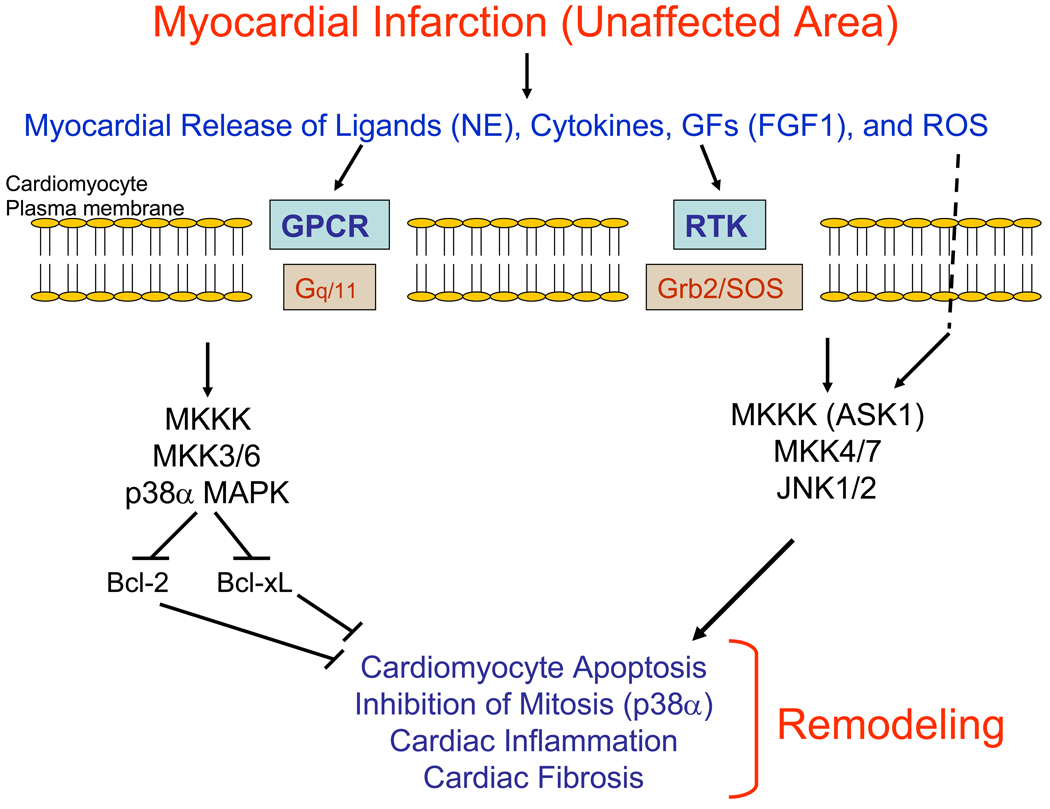
A current model of cardiac remodeling after MI is that the activation of the p38α MAPK and JNK1/2 cascades promotes fibrosis in the infarct area and unaffected myocardium, apoptosis in the infarct border zone with resultant infarct expansion, and left ventricular chamber dilatation (Figure 2). However, not all studies support this model of cardiac remodeling, and the role of the ERK1/2 cascade in this process is not well known [66, 79]. The use of pharmacologic agents to inhibit ASK1, p38α MAPK or JNK1/2 may reduce pathological cardiac remodeling after MI in humans.
Figure 2.
Model of the role of MAPK cascades in pathological cardiac remodeling after myocardial infarction. After myocardial infarction, there is a local release of ligands such as norepinephrine (NE), cytokines, growth factors such as FGF1, and reactive oxygen species (ROS) that leads to the activation transmembrane receptors and intracellular MAPK cascades. ROS may modify the activity of the MKKK ASK1 by blocking the ability of thioredoxin to inhibit ASK1. The activation of the MKKK-MKK3/6-p38α MAPK and the ASK1-MKK4/7-JNK1/2 cascades promotes pathological cardiac remodeling that includes cardiomyocyte apoptosis, inhibition of cardiomyocyte mitosis, inflammation and fibrosis. Specifically, p38α MAPK promotes cardiomyocyte mitosis by inhibiting the activity or expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL.
Role of MAPKs in Atherosclerotic Lesion Development
Atherosclerosis is a complex inflammatory condition characterized by the development of fatty deposits in the inner layers of arteries [80,81]. Atherosclerotic lesions contain many cell types, including smooth muscle cells, endothelial cells, and T lymphocytes, but always contain a large number of abnormal lipid-laden macrophages that are called foam cells. The development of atherosclerosis in humans is influenced by the presence of important risk factors, such as hypercholesterolemia, hypertension, diabetes mellitus, cigarette smoking and a family history of early atherosclerosis.
Although research on atherosclerosis in the past focused on cholesterol and lipid metabolism, more recently scientists have concentrated on the cell biology of atherosclerosis including the molecular determinants of foam cell formation. In the response to injury hypothesis, damage to the arterial intima, perhaps because of hypertension, toxins in cigarette smoke, or the toxic effects of oxidized low density lipoprotein (oxLDL), leads to the recruitment, adherence and invasion of monocytes into the arterial intima [80, 81]. Once monocytes invade the arterial intima, they differentiate into macrophages and begin to take up lipids, especially modified forms of LDL to form foam cells. The molecular mechanisms that regulate foam cell formation are not yet well understood, although many important studies have investigated this issue. Mice are naturally resistant to the development of atherosclerosis despite high fat feeding, but the development of mice deficient for apolipoprotein E or the LDL receptor have provided important model systems for the in vivo analysis of atherosclerosis [82, 83]. A popular in vitro model system of foam cell formation is the treatment of peritoneal or bone marrow macrophages with copper (or myeloperoxidase)-modified oxLDL or acetylated-LDL [84].
The role of MAPK cascades in foam cell formation in vitro was recently investigated [85]. Mouse peritoneal macrophages were treated with oxLDL and ERK1/2, p38α MAPK and JNK1/2 were all activated within 15 minutes, with ERK1/2 activation occurring at the earliest time point. Peritoneal macrophages derived from CD36−/− mice were resistant to oxLDL-induced activation of JNK1/2, but still exhibited ERK1/2 and p38α MAPK activation [85]. CD36 is a transmembrane scavenger receptor, which with scavenger receptor A (SR-A) and other scavenger receptors, facilitates the uptake of oxLDL by macrophages [86]. CD36 binds to the src-family tyrosine kinase lyn and also to MEKK2 via its short carboxy-terminal cytoplasmic tail. Treatment of macrophages with the src inhibitor AG1879 or the JNK pathway inhibitor SP600125 blocked oxLDL-induced foam cell formation, but treatment of macrophages with the ERK pathway inhibitor U126 had no effect [85]. In related work, oxLDL-induced foam cell formation in the J774 macrophage cell line was found to be blocked by administration of the p38 MAPK inhibitor SB203580, but was not blocked by administration of the ERK pathway inhibitor PD98059 [87]. Furthermore, p38α MAPK and MKK6, but not ERK, were found to be required of oxLDL-stimulated CD36 expression in J774 cells.
To determine the specific role of JNK1 and JNK2 in the development of atherosclerosis, ApoE-null mice were bred with JNK1−/− or JNK2−/− mice and placed on a high fat diet [88]. JNK2−/− apoE−/− but not JNK1−/− apoE−/− mice were found to be resistant to the development of atherosclerosis after 14-weeks of high cholesterol feeding. Furthermore, apoE-null mice that were treated with the JNK1/2 pathway inhibitor SP600125 were found to develop significantly less atherosclerosis than apoE-null mice treated with vehicle. Lesions from animals with absent JNK2 did not have altered cellular composition, but were simply smaller in absolute size. Bone marrow transplantation from ApoE−/− donors into JNK2−/− apoE−/− recipients resulted in increased atherosclerotic lesion formation, while bone marrow transplantation from JNK2−/− apoE−/− donors into apoE−/− recipients resulted in reduced lesion formation, and both of these results suggested that the effect of JNK2 deficiency on lesion formation was due to the abnormal function of blood-borne cells, such as macrophages [88]. Indeed, JNK2−/− apoE−/− peritoneal macrophages were found to exhibit reduced oxLDL- and acLDL-induced foam cell formation in vitro when compared to apoE−/− macrophages. To determine the mechanism by which JNK2 promotes foam cell formation, investigators the status of scavenger receptor type A (SR-A) in JNK2−/− macrophages [88]. Serine phosphorylation of SR-A was markedly reduced in JNK2−/− macrophages, although total SR-A protein levels were increased. One model is that JNK2 phosphorylates SR-A in macrophages, and this promotes foam cell formation by facilitating the internalization of SR-A that is bound to modified LDL (Figure 3) [88].
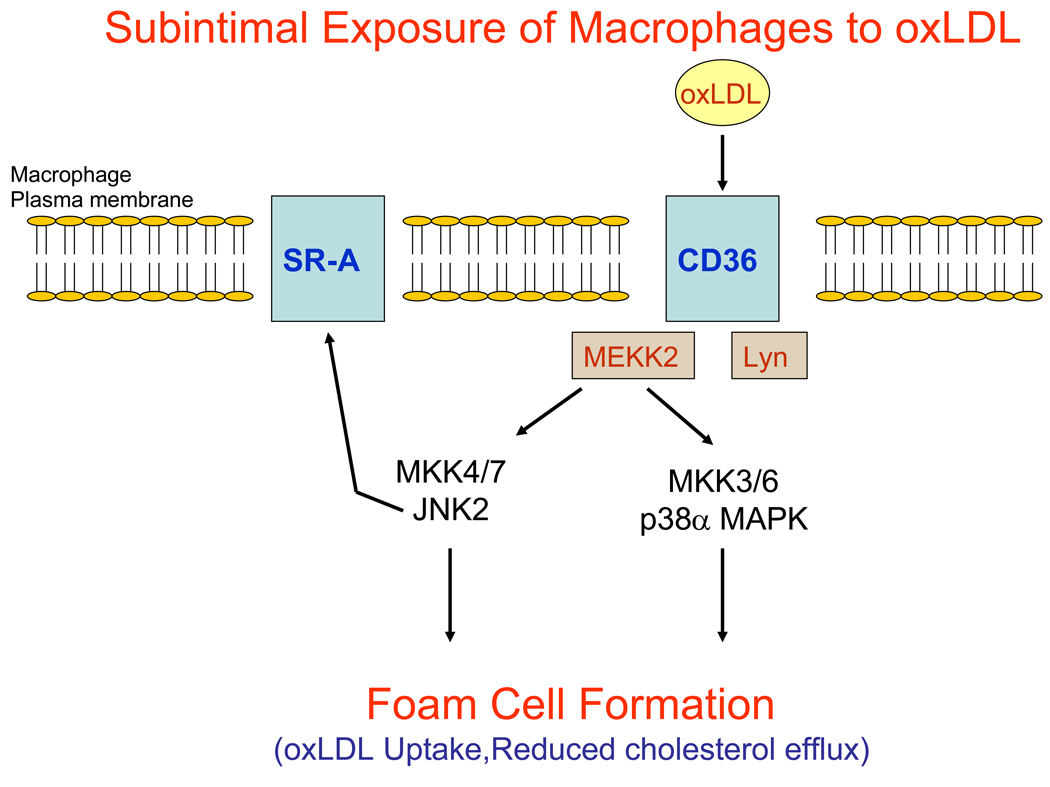
Figure 3.
Simplified model of macrophage foam cell formation in the arterial subintimal space. In response to vascular injury, monocytes are recruited to the intimal surface of arteries where they adhere and invade the vessel, differentiating into macrophages. In the subintimal space, macrophages are exposed to oxidized low density lipoprotein (oxLDL) that binds to the transmembrane protein CD36. Binding of oxLDL to CD36 triggers the activation of src-family kinases such as Lyn and MEKK2. Activation of Lyn and possibly MEKK2 leads to the activation of JNK2 and also p38α MAPK. Through largely undetermined mechanisms, activation of JNK2 and p38α MAPK promote the internalization of oxLDL either via CD36, scavenger receptor A (SR-A) or through other scavenger receptors. JNK2 promotes the phosphorylation of SR-A and this may lead to SR-A internalization while it is associated with modified LDL. Activation of MAPK cascades in macrophages may also modulate cholesterol efflux pathways.
Additional evidence about the role of MAPKs in atherosclerotic lesion development was provided by the analysis of Grb2+/− apoE−/− mice [89]. When these mice were placed on a high fat diet for 2 months, they were found to be highly resistant to atherosclerotic lesion formation when compared to apoE−/− mice. Furthermore, bone marrow transplantation from Grb2+/− apoE−/− donors into apoE−/− recipients resulted in reduced lesion formation when compared to apoE−/− mice transplanted with apoE−/− marrow. Activation of p38α MAPK and JNK in response to oxLDL treatment was reduced Grb2+/− apoE−/− cultured bone marrow macrophages when compared to apoE−/− macrophages [89]. The uptake of oxLDL was diminished in Grb2+/− apoE−/− bone marrow macrophages although the binding was unchanged. It is not apparent how Grb2-mediated signaling is affected by oxLDL binding to scavenger receptors and this is an area of active investigation.
A study evaluating mice deficient for ataxia telangectasia mutated (ATM) also showed that MAPKs regulate atherosclerotic lesion formation [90]. The finding that humans deficient for ATM have an increased risk for death due to ischemic heart disease led this group to investigate mice deficient for both ATM and apoE. ATM+/− apoE−/− mice developed metabolic syndrome on a high fat diet with hypertension, hyperglycemia, increased adiposity and increased aortic atherosclerosis when compared to apoE−/− mice on a similar diet. Furthermore, the aortae of ATM+/− apoE−/− mice exhibited increased JNK1/2 activation but reduced ERK1/2 and Akt1 activation. Bone marrow transplantation of ATM−/− apoE−/− marrow into apoE−/− recipients resulted in increased atherosclerotic lesion formation compared to animals transplanted with apoE−/− marrow, suggesting that blood-borne cells deficient for ATM conferred the atherosclerosis phenotype [90]. In addition, ATM−/− apoE−/− peritoneal macrophages exhibited increased JNK1/2 activation and increased lipoprotein lipase gene expression. These results suggest that ATM deficiency promotes JNK1/2 activation in macrophages leading to increased foam cell formation.
Taken together, foam cell formation appears to be dependent on the activation JNK2 and also perhaps p38α MAPK (Figure 3). Treatment of cultured macrophages with oxLDL results in the rapid activation of JNK, p38α MAPK and ERK, but treatment of macrophages with an ERK inhibitor does not block foam cell formation [85, 87]. Treatment of macrophages with src inhibitors, JNK inhibitors or p38 MAPK inhibitors does block oxLDL-induced foam cell formation [85, 87]. However, it is unclear whether oxLDL-mediated activation of JNK2 or p38α MAPK directly regulates the uptake of oxLDL by endocytosis of some other process. It is possible that JNK2 or p38α MAPK activity regulates the expression or activity of proteins required for internalization of oxLDL prior to oxLDL binding to the cell surface receptors. Nevertheless, JNK2 and p38α MAPK remain interesting targets for drug therapy to reduce atherosclerotic lesion formation.
Role of MAPKs in Restenosis After Percutaneous Arterial Intervention
Atherosclerotic vascular disease is often treated by percutaneous transluminal balloon angioplasty and/or by arterial stenting. Unfortunately, in many cases, neointima formation in the weeks and months after balloon angioplasty or stenting results in arterial restenosis with resultant morbidity and mortality. Neointima formation is primarily a disorder of smooth muscle cells with smooth muscle cell migration and proliferation and extracellular matrix deposition in the intima being important features of this disorder [91, 92]. Although the incidence of restenosis is markedly reduced when drug eluting stents are employed that release sirolimus or taxol, the occurrence of delayed in-stent thrombosis due to deficient endothelialization suggests that more complete information about the biology of neointima formation will be clinically useful.
Neointima formation may be triggered by the local release of growth factors, cytokines and ligands in response to balloon angioplasty or stent placement. The release of bioactive factors at the site of vascular intervention is thought to be a consequence of endothelial cell injury and denudation, stretch injury to cells throughout the vessel, and local deposition of fibrin and platelets [92]. Growth factors, cytokines and ligands that are released in response to vascular injury bind to receptors on the surface of smooth muscle cells promoting the activation of intracellular signaling cascades and leading to cell migration and proliferation. Balloon injury of the rat carotid artery results in the rapid activation of ERK1/2, p38α/β MAPK and JNK1/2 [93–95].
To address the role of MAPK cascades in neointima formation, mice that were haplo-insufficient for Grb2 were subjected to carotid injury by use of a beaded probe method [96]. Three weeks after injury, carotid arteries were examined for neointima formation and signaling pathway activation. Grb2+/− mice were resistant to carotid injury-induced neointima formation with dramatically reduced intimal smooth muscle cells. In addition, Grb2 haplo-insufficient mice exhibited reduced ERK, JNK and p38 MAPK activation in carotid artery extracts after probe injury [96].
To more specifically examine the role of ras activation in neointima formation, mice with smooth muscle cell-specific targeted disruption of the Nf1 gene that encodes neurofibromin, a GTPase that deactivates ras, were examined [97]. Mice with smooth muscle cell-specific Nf1 knockout (Nf1smKO) were subjected to carotid artery injury by external ligation. Four weeks after carotid ligation, arteries were examined and Nf1smKO mice were found to develop exaggerated neointima formation with a dramatically increased intima-to-media ratio. Cell proliferation and ERK1/2 activation in the neointima was significantly increased in Nf1smKO mice when compared to control animals [97]. The activation of p38 and JNK in Nf1smKO carotid arteries after injury was not examined in this study.
To specifically examine the role of p38α in the pathogenesis of neointima formation, compound transgenic mice with smooth muscle cell-specific inducible expression of dominant negative p38α were produced [98]. In these mice, tetracycline administration resulted in the expression of dominant negative p38α in the smooth muscle cells of the aorta and carotid arteries. Compound transgenic or control mice were treated with tetracycline for two weeks and then subjected to carotid injury by the beaded probe method. Mice expressing DN-p38α in SMCs were resistant to neointima formation after carotid injury when compared to control animals. To address the mechanism by which p38α promotes the proliferation of smooth muscle cells, PDGF stimulation was demonstrated to result in the p38α-dependent phosphorylation of the retinoblastoma protein (Rb), a master regulator of cell cycle progression. Furthermore, PDGF stimulation of cultured smooth muscle cells promoted the p38α-dependent expression of minichromosome maintenance protein 6 (MCM6), a protein required for DNA synthesis in the S phase of the cell cycle [98].
In one in vivo study that employed the HVJ liposome-mediated delivery of dominant negative forms of ERK1 and JNK1 to rat arteries, both reagents were found to be effective at inhibiting neointima formation after balloon injury of the common carotid artery [99]. Both dominant negative reagents were highly expressed in the carotid arteries, and the carotid intima-to-media ratio was decreased at both the 14 and 28 day time points after injury compared to control animals. Furthermore, liposome-mediated delivery of wild type forms of ERK1 and JNK1 increased neointima formation after balloon injury. [99].
Several animal studies of neointima formation employed pharmacologic inhibitors of MAPKs. In one early study, administration of PD98059, a MKK1 inhibitor, via the external application of a pluronic gel containing the compound, blocked medial smooth cell proliferation after balloon injury of the carotid artery, but did not block neointima formation [91]. However, ERK1/2 activation was not completely blocked in the carotid arteries by administration of the pluronic gel. In another study, administration of a novel oral MKK1 inhibitor PD0185625, to rats blocked carotid neointima formation at both 14 and 28 days after balloon injury. Administration of PD0185625 completely blocked ERK1/2 activation in balloon-injured carotid arteries and also inhibited BrDU incorporation in vascular smooth muscle cells [100]. Finally, administration of an oral p38α/β inhibitor FR167653 to rats significantly inhibited carotid neointima formation 14 days after balloon injury [101]. Furthermore, carotid artery production of the cytokine IL-1β was induced by balloon injury, consistent with p38α activation, but was blocked by FR167653 administration.
The results of animal studies investigating neointima formation after vascular injury suggest that ERK1/2, JNK1/2 and p38α all promote neointima formation and smooth muscle cell proliferation (Figure 4). The ability of p38α to promote VSMC proliferation dramatically contrasts with its ability to suppress mitosis in adult cardiomyocytes. This difference emphasizes the fact that MAPKs often have widely disparate biological roles in different cell types and in different physiological contexts.
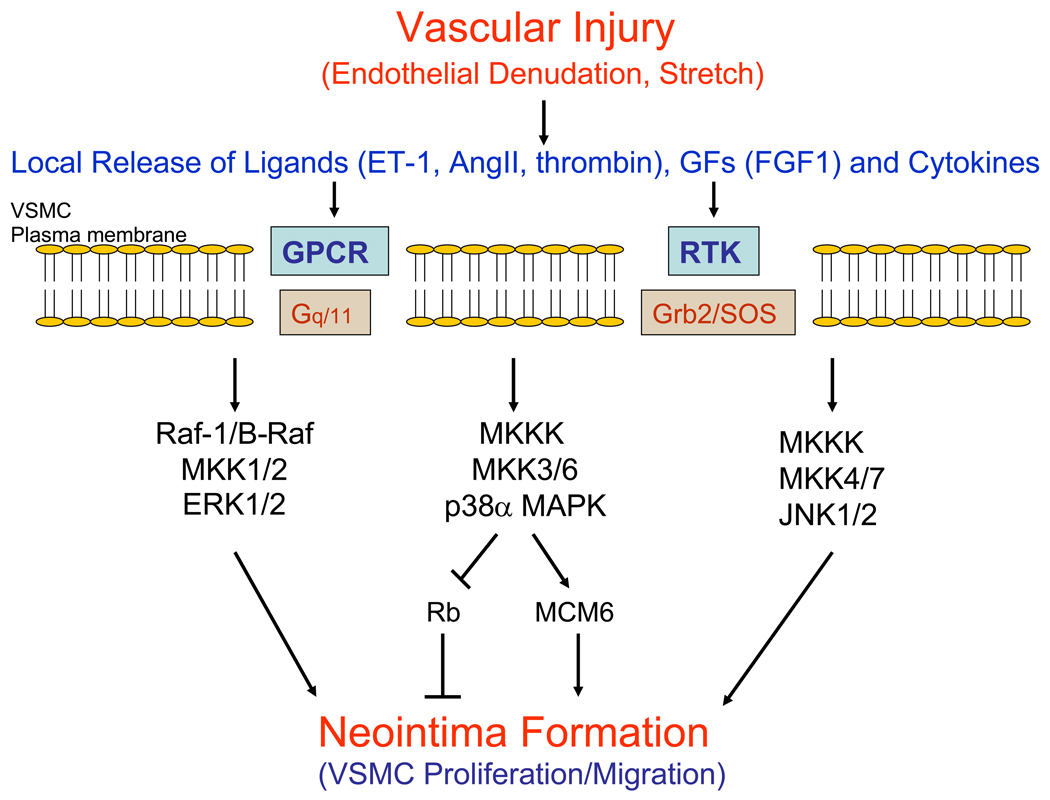
Figure 4.
Model of smooth muscle cell proliferation in neointima formation after vascular injury. In response to arterial injury that includes endothelial denudation and stretch, there is a local release of ligands such as endothelin-1 (ET-1), angiotensin II (AngII) and thrombin, growth factors (GFs), and cytokines that bind to transmembrane receptors. These receptors include G protein-coupled receptors (GPCRs), receptor tyrosine kinases (RTKs) and cytokine receptors (not depicted) that activate intracellular signaling proteins such as G proteins (Gq/11) and the Grb2/SOS complex. In turn, intracellular MAPK cascades are activated that ultimately leads to vascular smooth muscle cell migration (VSMC) into the intima and proliferation, resulting in neointima formation. The activation of p38α MAPK in vascular smooth muscle cells promotes the hyperphosphorylation of Rb and the expression of MCM6 that both contribute to cell proliferation.
Conclusion
Tremendous progress has been achieved in the past decade to characterize the role of MAPK cascades in various forms of cardiovascular disease. Although there are important inconsistencies and controversies within the field that should be resolved, four major themes emerge from this area of biomedical research. First, MAPK cascades modulate the hypertrophic response of the heart to pressure overload. Activation of the Raf-MKK-ERK1/2 cascade promotes the growth of individual cardiomyocytes, while activation of the MKKK-MKK4/7-JNK and MKKK-MKK3/6-p38 cascade antagonizes the growth of individual cardiomyocytes. Furthermore, activation of ERK1/2 promotes cardiomyocyte survival after pressure overload, while activation of p38α MAPK promotes cardiomyocyte contractile dysfunction. Second, MAPK cascades regulate cardiac remodeling after myocardial infarction. Activation of p38α MAPK and JNK1/2 promote pathological cardiac remodeling that includes cardiomyocyte apoptosis in the infarct border zone, infarct expansion, and fibrosis at the site of the infarction and in the unaffected myocardium. Third, MAPKs modulate atherosclerotic lesion formation via the regulation of macrophage foam cell formation. JNK2 and p38α activity are required for the uptake of oxLDL by macrophages in culture and JNK2 is required for atherosclerotic lesion development in vivo because of its ability to facilitate the transformation of macrophages into foam cells. Fourth, MAPK cascades play a pivotal role in the formation of neointima after vascular injury. Activation of ERK1/2, JNK1/2 and p38α MAPK in vascular smooth muscle cells is required for the formation of neointima after vascular injury.
Although these four themes suggest therapeutic targets for the development of pharmacologic agents to treat cardiovascular disease, there are many important limitations of the studies reviewed above that merit discussion. In some cases, these limitations explain the conflicting results obtained by different research groups. First, studies that examined the activation of MAPK cascades in cells were limited by the fact that they all examined the pan-cellular activation of signaling proteins. In other words, variations in the subcellular activation of various pathways, such as whether a MAPK was active in the nucleus versus the cytosol, were not examined in any of the studies discussed. This may be a significant limitation since activation of a pathway in one location often has a different biological effect than activation in another part of the cell [102]. Second, studies that examined the activation of MAPK cascades typically did not consider the integrated quantity of pathway activation. It is apparent that the length of time and quantity of activation of a particular MAPK is critical for determining the biological response [103]. Third, studies that employed overexpression of activated forms of a kinase are limited by the fact that the overexpressed kinase may be targeted to atypical subcellular locations, that the overexpressed kinase may phosphorylate non-ideal or nonspecific substrates, and that the kinase is continuously active and does not reflect the normal timing of pathway activation. Fourth, global gene knockout studies are limited by the lack of tissue specificity of the knockout and the possibility that an observed cardiovascular phenotype may be due to disruption of the gene in other tissues. Furthermore, when a gene is disrupted in a continuous fashion from the onset of embryonic development, compensatory pathways are often up-regulated that mitigate or alter the phenotype. Fifth, tissue specific knockout studies are limited by the toxicity of the Cre recombinase and the variability of gene disruption within cells in a specific tissue [104]. Sixth, studies that use pharmacologic agents are limited by the frequent off-target effects of these agents. Finally, loss-of-function studies with protein kinases that utilize pharmacologic agents answer different questions than loss-of-function studies that employ gene disruption technology or loss-function studies that involve the expression of a dominant negative mutant. Many protein kinases are multifunctional proteins that have non-kinase activities. For example, Raf-1 is both a protein kinase and an ASK1 inhibitor: if the kinase function of Raf-1 is inhibited with a small molecule inhibitor, the ability of Raf-1 to bind to and inhibit ASK1 remains [56]. In contrast, targeted disruption of the Raf-1 gene disrupts both functions of Raf-1. Overexpression of a dominant negative mutant inhibits the kinase activity of native Raf-1 but also likely potentiates its ability to inhibit ASK1.
Despite all of these caveats about the scientific investigation of MAPK pathway function in cardiovascular disease, promising targets have been identified. It will be exciting to translate some of these discoveries to the clinical realm in order to reduce the horrible burden of cardiovascular disease.
Acknowledgements
The author was supported by grants from the Burroughs Wellcome Fund, the Longer Life Foundation, and the National Institutes of Health (HL057278, HL061567, HL076770).
References
- 1.Ueno H, Colbert H, Escobedo JA, Williams LT. Inhibition of PDGF beta receptor signal transduction by coexpression of a truncated receptor. Science. 1991;252:844–848. doi: 10.1126/science.1851331. [DOI] [PubMed] [Google Scholar]
- 2.Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 3.Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- 4.Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci PG, et al. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- 5.Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;7:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 6.Chardin P, Camonis JH, Gale NW, van Aelst L, Schlessinger J, Wigler MH, Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993;260:1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- 7.Skolnik EY, Batzer A, Li N, Lee CH, Lowenstein E, Mohammadi M, Margolis B, Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993;260:1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- 8.Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat. Rev. Mol. Cell. Biol. 2003;4:651–657. doi: 10.1038/nrm1173. [DOI] [PubMed] [Google Scholar]
- 9.Delcourt N, Bockaert J, Marin P. GPCR-jacking: from a new route in RTK signaling to a new concept in GPCR activation. Trends Pharmacol. Sci. 2007;28:602–607. doi: 10.1016/j.tips.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 11.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 12.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell. Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 13.Muslin AJ. Role of raf proteins in cardiac hypertrophy and cardiomyocyte survival. Trends Cardiovasc. Med. 2005;15:225–229. doi: 10.1016/j.tcm.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 15.Tsakiridis T, Taha C, Grinstein S, Klip A. Insulin activates a p21-activated kinase in muscle cells via phosphatidylinositol 3-kinase. J. Biol. Chem. 1996;271:19664–19667. doi: 10.1074/jbc.271.33.19664. [DOI] [PubMed] [Google Scholar]
- 16.Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 17.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 18.Liu ZG, Baskaran R, Lea-Chou ET, Wood LD, Chen Y, Karin M, Wang JY. Three distinct signalling responses by murine fibroblasts to genotoxic stress. Nature. 1996;384:273–276. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Seimiya H, Naito M, Mashima T, Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K, Tsuruo T. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18:173–180. doi: 10.1038/sj.onc.1202276. [DOI] [PubMed] [Google Scholar]
- 20.Foltz IN, Gerl RE, Wieler JS, Luckach M, Salmon RA, Schrader JW. Human mitogen-activated protein kinase kinase 7 (MKK7) is a highly conserved c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activated by environmental stresses and physiological stimuli. J. Biol. Chem. 1998;273:9344–9351. doi: 10.1074/jbc.273.15.9344. [DOI] [PubMed] [Google Scholar]
- 21.Yan M, Dai T, Deak JC, Kyriakis JM, Zon LI, Woodgett JR, Templeton DJ. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 22.Wang XS, Diener K, Jannuzzi D, Trollinger D, Tan TH, Lichenstein H, Zukowski M, Yao Z. Molecular cloning and characterization of a novel protein kinase with a catalytic domain homologous to mitogen-activated protein kinase kinase kinase. J. Biol. Chem. 1996;271:31607–31611. doi: 10.1074/jbc.271.49.31607. [DOI] [PubMed] [Google Scholar]
- 23.Ellinger-Ziegelbauer H, Brown K, Kelly K, Siebenlist U. Direct activation of the stress-activated protein kinase (SAPK) and extracellular signal-regulated protein kinase (ERK) pathways by an inducible mitogen-activated protein Kinase/ERK kinase kinase 3 (MEKK) derivative. J. Biol. Chem. 1997;272:2668–2674. doi: 10.1074/jbc.272.5.2668. [DOI] [PubMed] [Google Scholar]
- 24.Gerwins P, Blank JL, Johnson GL. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J. Biol. Chem. 1997;272:8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- 25.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 27.Tibbles LA, Ing YL, Kiefer F, Chan J, Iscove N, Woodgett JR, Lassam NJ. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 1996;15:7026–7035. [PMC free article] [PubMed] [Google Scholar]
- 28.Davis RJ. Signal transduction by the c-Jun N-terminal kinase. Biochem. Soc. Symp. 1999;64:1–12. doi: 10.1515/9781400865048.1. [DOI] [PubMed] [Google Scholar]
- 29.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 30.Whitmarsh AJ. The JIP family of MAPK scaffold proteins. Biochem. Soc. Trans. 2006;34:828–832. doi: 10.1042/BST0340828. [DOI] [PubMed] [Google Scholar]
- 31.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 32.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell. Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 33.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlöf B LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 34.Rockman HA, Wachhorst SP, Mao L, Ross J., Jr ANG II receptor blockade prevents ventricular hypertrophy and ANF gene expression with pressure overload in mice. Am. J. Physiol. 1994;266:H2468–H2475. doi: 10.1152/ajpheart.1994.266.6.H2468. [DOI] [PubMed] [Google Scholar]
- 35.Dorn GW, 2nd, Robbins J, Ball N, Walsh RA. Myosin heavy chain regulation and myocyte contractile depression after LV hypertrophy in aortic-banded mice. Am. J. Physiol. 1994;267:H400–H405. doi: 10.1152/ajpheart.1994.267.1.H400. [DOI] [PubMed] [Google Scholar]
- 36.Esposito G, Prasad SV, Rapacciuolo A, Mao L, Koch WJ, Rockman HA. Cardiac overexpression of a G(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH(2)-terminal kinase activity in in vivo pressure overload. Circulation. 2001;103:1453–1458. doi: 10.1161/01.cir.103.10.1453. [DOI] [PubMed] [Google Scholar]
- 37.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc. Natl. Acad. Sci. U.S.A. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 39.Hunter JJ, Tanaka N, Rockman HA, Ross J, Jr, Chien KR. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J. Biol. Chem. 1995;270:23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 40.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babu GJ, Lalli MJ, Sussman MA, Sadoshima J, Periasamy M. Phosphorylation of elk-1 by MEK/ERK pathway is necessary for c-fos gene activation during cardiac myocyte hypertrophy. J. Mol. Cell. Cardiol. 2000;32:1447–1457. doi: 10.1006/jmcc.2000.1185. [DOI] [PubMed] [Google Scholar]
- 42.Purcell NH, Darwis D, Bueno OF, Muller JM, Shule R, Molkentin JD. Extracellular signal-regulated kinase 2 interacts with and is negatively regulated by the LIM-only protein FHL2 in cardiomyocytes. Mol. Cell. Biol. 2004;24:1081–1095. doi: 10.1128/MCB.24.3.1081-1095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma L, Chen Z, Erdjument-Bromage H, Temptst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Proud CG. Methods for studying signal-dependent regulation of translation factor activity. Meth. Enzymol. 2007;431:113–142. doi: 10.1016/S0076-6879(07)31007-0. [DOI] [PubMed] [Google Scholar]
- 45.Rolfe M, McLeod LE, Pratt PF, Proud CG. Activation of protein synthesis in cardiomyocytes by the hypertrophic agent phenylephrine requires the activation of ERK and involves phosphorylation of tuberous sclerosis complex 2 (TSC2) Biochem. J. 2005;388:973–984. doi: 10.1042/BJ20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahalingam M, Cooper JA. Phosphorylation of mammalian eIF4E by Mnk1 and Mnk2: tantalizing prospects for a role in translation. Prog. Mol. Subcell. Biol. 2001;27:131–142. [PubMed] [Google Scholar]
- 47.Petrich BG, Eloff BC, Lerner DL, Kovacs A, Saffitz JE, Rosenbaum DS, Wang Y. Targeted activation of c-Jun N-terminal kinase in vivo induces restrictive cardiomyopathy and conduction defects. J. Biol. Chem. 2004;279:15330–15338. doi: 10.1074/jbc.M314142200. [DOI] [PubMed] [Google Scholar]
- 48.Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 2003;22:5079–5089. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao RP, Kass DA, Wang Y. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yue TL, Gu JL, Wang C, Reith AD, Lee JC, Mirabile RC, Kreutz R, Wang Y, Maleeff B, Parsons AA, Ohlstein EH. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J. Biol. Chem. 2000;275:37895–37901. doi: 10.1074/jbc.M007037200. [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Proud CG. Ras/Erk signaling is essential for activation of protein synthesis by Gq protein-coupled receptor agonists in adult cardiomyocytes. Circ. Res. 2002;91:821–829. doi: 10.1161/01.res.0000041029.97988.e9. [DOI] [PubMed] [Google Scholar]
- 52.Sanada S, Node K, Minamino T, Takashima S, Ogai A, Asanuma H, Ogita H, Liao Y, Asakura M, Kim J, Hori M, Kitakaze M. Long-acting Ca2+ blockers prevent myocardial remodeling induced by chronic NO inhibition in rats. Hypertension. 2003;41:963–967. doi: 10.1161/01.HYP.0000062881.36813.7A. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, Wang Y, Muslin AJ. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J. Clin. Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 55.Hindley A, Kolch W. xtracellular signal regulated kinase (ERK)/mitogen activated protein kinase (MAPK)-independent functions of Raf kinases. J. Cell. Sci. 2002;115:1575–1581. doi: 10.1242/jcs.115.8.1575. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi O, Watanabe T, Nishida K, Kashiwase K, Higuchi Y, Takeda T, Hikoso S, Hirotani S, Asahi M, Taniike M, Nakai A, Tsujimoto I, Matsumura Y, Miyazaki J, Chien KR, Matsuzawa A, Sadamitsu C, Ichijo H, Baccarini M, Hori M, Otsu K. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J. Clin. Invest. 2004;114:937–943. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tachibana H, Perrino C, Takaoka H, Davis RJ, Naga Prasad SV, Rockman HA. JNK1 is required to preserve cardiac function in the early response to pressure overload. Biochem. Biophys. Res. Commun. 2006;343:1060–1066. doi: 10.1016/j.bbrc.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Huang S, Sah VP, Ross J, Jr., Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 59.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, Uno Y, Kashiwase K, Taniike M, Nakai A, Matsumura Y, Miyazaki J, Sudo T, Hongo K, Kusakari Y, Kurihara S, Chien KR, Takeda J, Hori M, Otsu K. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol. Cell. Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007;116:1413–1423. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 63.Ren J, Zhang S, Kovacs A, Wang Y, Muslin AJ. Role of p38alpha MAPK in cardiac apoptosis and remodeling after myocardial infarction. J Mol Cell Cardiol. 2005;38:617–623. doi: 10.1016/j.yjmcc.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida K, Yoshiyama M, Omura T, Nakamura Y, Kim S, Takeuchi K, Iwao H, Yoshikawa J. Activation of mitogen-activated protein kinases in the non-ischemic myocardium of an acute myocardial infarction in rats. Jpn. Circ. J. 2001;65:808–814. doi: 10.1253/jcj.65.808. [DOI] [PubMed] [Google Scholar]
- 65.Tenhunen O, Soini Y, Ilves M, Rysä J, Tuukkanen J, Serpi R, Pennanen H, Ruskoaho H, Leskinen H. p38 Kinase rescues failing myocardium after myocardial infarction: evidence for angiogenic and anti-apoptotic mechanisms. FASEB J. 2006;20:1907–1909. doi: 10.1096/fj.05-5618fje. [DOI] [PubMed] [Google Scholar]
- 66.Matsumoto-Ida M, Takimoto Y, Aoyama T, Akao M, Takeda T, Kita T. Activation of TGF-beta1-TAK1-p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H709–H715. doi: 10.1152/ajpheart.00186.2005. [DOI] [PubMed] [Google Scholar]
- 67.Qin F, Liang MC, Liang CS. Progressive left ventricular remodeling, myocyte apoptosis, and protein signaling cascades after myocardial infarction in rabbits. Biochim. Biophys. Acta. 2005;1740:499–513. doi: 10.1016/j.bbadis.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 68.Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, Molkentin JD. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J. Biol. Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- 69.See F, Thomas W, Way K, Tzanidis A, Kompa A, Lewis D, Itescu S, Krum H. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J. Am. Coll. Cardiol. 2004;44:1679–1689. doi: 10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 70.Yin H, Zhang J, Lin H, Wang R, Qiao Y, Wang B, Liu F. p38 Mitogen-Activated Protein Kinase Inhibition Decreases TNFalpha Secretion and Protects Against Left Ventricular Remodeling in Rats with Myocardial Ischemia. Inflammation. 2007 Oct 18; doi: 10.1007/s10753-007-9050-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 71.Liu YH, Wang D, Rhaleb NE, Yang XP, Xu J, Sankey SS, Rudolph AE, Carretero OA. Inhibition of p38 mitogen-activated protein kinase protects the heart against cardiac remodeling in mice with heart failure resulting from myocardial infarction. J. Card. Fail. 2005;11:74–81. doi: 10.1016/j.cardfail.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Lau JM, Jin X, Ren J, Avery J, DeBosch BJ, Treskov I, Lupu TS, Kovacs A, Weinheimer C, Muslin AJ. The 14-3-3tau phosphoserine-binding protein is required for cardiomyocyte survival. Mol. Cell. Biol. 2007;27:1455–1466. doi: 10.1128/MCB.01369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xing H, Zhang S, Weinheimer C, Kovacs A, Muslin AJ. 14-3-3 proteins block apoptosis and differentially regulate MAPK cascades. EMBO J. 2000;19:349–358. doi: 10.1093/emboj/19.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–25606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ. Res. 2000;87:392–398. doi: 10.1161/01.res.87.5.392. [DOI] [PubMed] [Google Scholar]
- 78.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci U S A. 2003;100:15883–15888. doi: 10.1073/pnas.2136717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frantz S, Behr T, Hu K, Fraccarollo D, Strotmann J, Goldberg E, Ertl G, Angermann CE, Bauersachs J. Role of p38 mitogen-activated protein kinase in cardiac remodelling. Br. J. Pharmacol. 2007;150:130–135. doi: 10.1038/sj.bjp.0706963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glass CK, Witztum JL. Atherosclerosis. the road ahead Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 81.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 82.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 84.Tiwari RL, Singh V, Barthwal MK. Macrophages: An elusive yet emerging therapeutic target of atherosclerosis. Med. Res. Rev. 2007 Nov 14; doi: 10.1002/med.20118. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 85.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silverstein RL, Febbraio M. CD36 and atherosclerosis. Curr. Opin. Lipidol. 2000;11:483–491. doi: 10.1097/00041433-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 87.Zhao M, Liu Y, Wang X, New L, Han J, Brunk UT. Activation of the p38 MAP kinase pathway is required for foam cell formation from macrophages exposed to oxidized LDL. APMIS. 2002;110:458–468. doi: 10.1034/j.1600-0463.2002.100604.x. [DOI] [PubMed] [Google Scholar]
- 88.Ricci R, Sumara G, Sumara I, Rozenberg I, Kurrer M, Akhmedov A, Hersberger M, Eriksson U, Eberli FR, Becher B, Borén J, Chen M, Cybulsky MI, Moore KJ, Freeman MW, Wagner EF, Matter CM, Lüscher TF. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 89.Proctor BM, Ren J, Chen Z, Schneider JG, Coleman T, Lupu TS, Semenkovich CF, Muslin AJ. Grb2 is required for atherosclerotic lesion formation. Arterioscler. Thromb. Vasc. Biol. 2007;27:1361–1367. doi: 10.1161/ATVBAHA.106.134007. [DOI] [PubMed] [Google Scholar]
- 90.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, Semenkovich CF. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4:377–389. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Koyama H, Olson NE, Dastvan FF, Reidy MA. Cell replication in the arterial wall: activation of signaling pathway following in vivo injury. Circ. Res. 1998;82:713–721. doi: 10.1161/01.res.82.6.713. [DOI] [PubMed] [Google Scholar]
- 92.Yu PJ, Ferrari G, Pirelli L, Gulkarov I, Galloway AC, Mignatti P, Pintucci G. Vascular injury and modulation of MAPKs: a targeted approach to therapy of restenosis. Cell Signal. 2007;19:1359–1371. doi: 10.1016/j.cellsig.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 93.Tanaka K, Oda N, Iwasaka C, Abe M, Sato Y. Induction of Ets-1 in endothelial cells during reendothelialization after denuding injury. J. Cell Physiol. 1998;176:235–244. doi: 10.1002/(SICI)1097-4652(199808)176:2<235::AID-JCP2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 94.Lai K, Wang H, Lee WS, Jain MK, Lee ME, Haber E. Mitogen-activated protein kinase phosphatase-1 in rat arterial smooth muscle cell proliferation. J. Clin. Invest. 1996;98:1560–1567. doi: 10.1172/JCI118949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu Y, Cheng L, Hochleitner BW, Xu Q. Activation of mitogen-activated protein kinases (ERK/JNK) and AP-1 transcription factor in rat carotid arteries after balloon injury. Arterioscler. Thromb. Vasc. Biol. 1997;17:2808–2816. doi: 10.1161/01.atv.17.11.2808. [DOI] [PubMed] [Google Scholar]
- 96.Zhang S, Ren J, Khan MF, Cheng AM, Abendschein D, Muslin AJ. Grb2 is required for the development of neointima in response to vascular injury. Arterioscler Thromb Vasc Biol. 2003;23:1788–1793. doi: 10.1161/01.ATV.0000085015.49110.85. [DOI] [PubMed] [Google Scholar]
- 97.Xu J, Ismat FA, Wang T, Yang J, Epstein JA. NF1 regulates a Ras-dependent vascular smooth muscle proliferative injury response. Circulation. 2007;116:2148–2156. doi: 10.1161/CIRCULATIONAHA.107.707752. [DOI] [PubMed] [Google Scholar]
- 98.Muslin AJ. unpublished observations [Google Scholar]
- 99.Izumi Y, Kim S, Namba M, Yasumoto H, Miyazaki H, Hoshiga M, Kaneda Y, Morishita R, Zhan Y, Iwao H. Gene transfer of dominant-negative mutants of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase prevents neointimal formation in balloon-injured rat artery. Circ. Res. 2001;88:1120–1126. doi: 10.1161/hh1101.091267. [DOI] [PubMed] [Google Scholar]
- 100.Gennaro G, Ménard C, Michaud SE, Deblois D, Rivard A. Inhibition of vascular smooth muscle cell proliferation and neointimal formation in injured arteries by a novel, oral mitogen-activated protein kinase/extracellular signal-regulated kinase inhibitor. Circulation. 2004;110:3367–3371. doi: 10.1161/01.CIR.0000147773.86866.CD. [DOI] [PubMed] [Google Scholar]
- 101.Ohashi N, Matsumori A, Furukawa Y, Ono K, Okada M, Iwasaki A, Miyamoto T, Nakano A, Sasayama S. Role of p38 mitogen-activated protein kinase in neointimal hyperplasia after vascular injury. Arterioscler. Thromb. Vasc. Biol. 2000;20:2521–2526. doi: 10.1161/01.atv.20.12.2521. [DOI] [PubMed] [Google Scholar]
- 102.Tsujita Y, Muraski J, Shiraishi I, Kato T, Kajstura J, Anversa P, Sussman MA. Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11946–11951. doi: 10.1073/pnas.0510138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chalmers CJ, Gilley R, March HN, Balmanno K, Cook SJ. The duration of ERK1/2 activity determines the activation of c-Fos and Fra-1 and the composition and quantitative transcriptional output of AP-1. Cell Signal. 2007;17:695–704. doi: 10.1016/j.cellsig.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, Pu WT, Izumo S, Jay PY. Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J. Card. Fail. 2006;12:392–398. doi: 10.1016/j.cardfail.2006.03.002. [DOI] [PubMed] [Google Scholar]