Abstract
Introduction
Membranoproliferative glomerulonephritis type II or dense deposit disease (MPGN II/DDD) causes chronic renal dysfunction that progresses to end stage renal disease in about half of patients within 10 years of diagnosis. Deficiency of and mutations in the complement factor H (CFH) gene are associated with the development of MPGN II/DDD, suggesting that dysregulation of the alternative pathway of the complement cascade is important in disease pathophysiology.
Subjects
Patients with MPGN II/DDD were studied to determine whether specific allele variants of CFH and CFHR5 segregate preferentially with the MPGN II/DDD disease phenotype. The control group was compromised of 131 people in whom age related macular degeneration had been excluded.
Results
Allele frequencies of four single nucleotide polymorphisms in CFH and three in CFHR5 were significantly different between MPGN II/DDD patients and controls.
Conclusion
We have identified specific allele variants of CFH and CFHR5 associated with the MPGN II/DDD disease phenotype. While our data can be interpreted to further implicate complement in the pathogenesis of MPGN II/DDD, these associations could also be unrelated to disease pathophysiology. Functional studies are required to resolve this question.
Keywords: membranoproliferative glomerulonephritis type II, dense deposit disease, complement factor H, complement factor H‐related 5, end‐stage renal failure
The membranoproliferative glomerulonephritides are diseases of diverse and often obscure aetiology that account for 4% and 7% of primary renal causes of nephrotic syndrome in children and adults, respectively.1 Based on renal immunopathology and ultrastructural studies, three subtypes are recognised. Membranoproliferative glomerulonephritis (MPGN) types I and III are variants of immune complex mediated disease; MPGN II, in contrast, has no known association with immune complexes.2
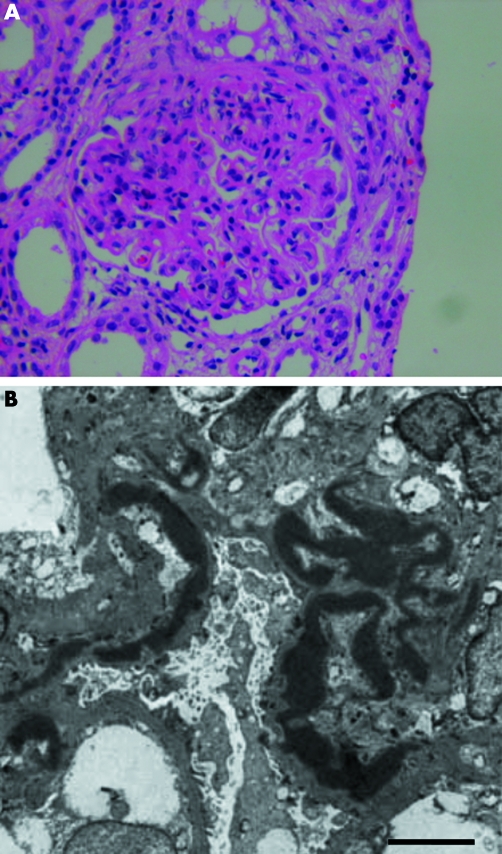
MPGN II accounts for <20% of cases of MPGN in children and only a fractional percentage of cases in adults.1,3,4 Both sexes are affected equally, with the diagnosis usually made in children between the ages of 5 and 15 years who present with non‐specific findings such as haematuria, proteinuria, acute nephritic syndrome, or nephrotic syndrome.2 More than 80% of patients with MPGN II are also positive for serum C3 nephritic factor (C3NeF), an autoantibody directed against C3bBb, the convertase of the alternative pathway of the complement cascade.5 C3NeF is found in up to half of people with MPGN types I and III and also in healthy individuals, making electron microscopic demonstration of dense deposits in the glomerular basement membrane (GBM) necessary for a definitive diagnosis of MPGN II.2 This morphological hallmark is so characteristic of MPGN II that the disease is more accurately referred to as dense deposit disease (DDD) (fig 1).
Figure 1 Light micrograph showing marked glomerular hypercellularity with dense intramembranous deposits that cause capillary wall thickening in a person with MPGN II/DDD. The deposits can form a segmental, discontinuous, or diffuse pattern in the lamina densa of the glomerular basement membrane (GBM). By light microscopy, they are eosinophilic and refractile, stain brightly with periodic acid‐Schiff and are highly osmophilic, which explains their electron dense appearance (A). Even by electron microscopy, the deposits lack substructure and appear as very dark homogeneous smudges (B). The exact composition of dense deposits remains unknown (bar = 5 um).
Spontaneous remissions of MPGN II/DDD are uncommon.3,4,6,7 The more common outcome is chronic deterioration of renal function leading to end stage renal disease (ESRD) in about half of patients within 10 years of diagnosis.7,8,9,10 In some patients, rapid fluctuations in proteinuria occur with episodes of acute renal deterioration in the absence of obvious triggering events; in other patients, the disease remains stable for years despite persistent proteinuria.
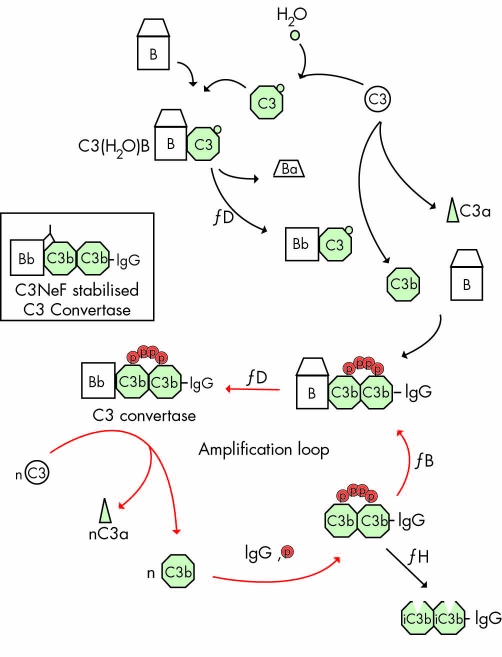
C3NeF persists throughout the disease course in more than 50% of patients with MPGN II/DDD.5 Its presence is typically associated with evidence of complement activation, such as a reduction in CH50, decrease in C3, increase in C3dg/C3d, and persistently high levels of activation of the alternative pathway of the complement cascade. C3, the most abundant complement protein in serum (∼1.2 mg/ml), normally undergoes low levels of continuous autoactivation by hydrolysis of its thioester in a process known as "tick over". C3 hydrolysis induces a large conformational protein change, making C3(H20) similar to C3b, a cleavage product of C3. C3(H20) associates with factor B to form C3(H20)Bb, which cleaves C3 to C3b in an amplification loop that consumes C3 and produces C3bBb2 (fig 2).
Figure 2 The alternative pathway of the complement cascade is systematically activated at a high level in patients with MPGN II/DDD. Normally, continuous low level activation of C3 occurs by spontaneous hydrolysis in a process known as tick over. C3 hydrolysis is associated with a large conformational protein change shown at the top of the diagram. The conformational change makes C3(H20) similar to C3b, a C3 cleavage product. The initial convertase, C3(H2O)Bb, activates C3 to form C3b. Although C3b has a fleeting half life, if it binds to IgG, cells, or basement membranes, it is protected from immediate inactivation. (C3b)2‐IgG complexes form in the fluid phase and bind properdin (P), which facilitates factor B binding and the generation of C3bBb, the convertase of the alternative pathway, shown here as a Bb(C3b)2‐IgG‐properdin complex. The amplification loop is depicted by the red arrows. C3NeF prolongs the half life of C3 convertase (inset). One mechanism to degrade C3 convertase is through its interaction with complement factor H, shown at the bottom right. Deficiency of and mutations in complement factor H are associated with MPGN II/DDD.
In MPGN II/DDD, C3NeF binds to C3bBb (or to the assembled convertase) to prolong the half life of this enzyme, resulting in persistent C3 consumption that overwhelms the normal regulatory mechanisms to control levels of C3bBb and complement activation.2 Normal control involves at least seven proteins, four of which are present in serum (complement factor H (CFH), complement factor H‐like protein 1 (CFHL1), complement factor I (CFI) and C4 binding protein (C4BP)) and three of which are cell membrane associated (membrane co‐factor protein (MCP, CD46), decay accelerating factor (DAF, CD55) and complement receptor 1 (CR1, CD35)).2,11,12
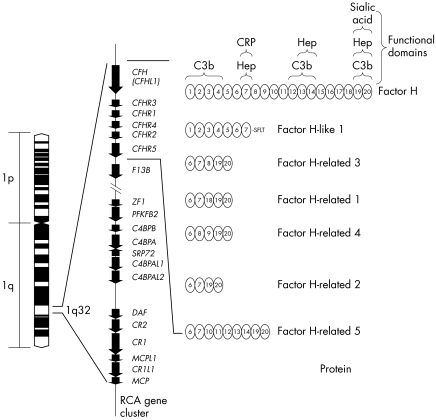
Of particular relevance to MPGN II/DDD is CFH, one of seven proteins in the CFH family. In pigs and mice, its deficiency is associated with the development of renal disease that is similar at the light and electron microscopic level to MPGN II/DDD, and in humans its deficiency as well as mutations in the CFH gene have been reported in patients with MPGN II/DDD11,13,14,15,16 (fig 3).
Figure 3 The regulators of complement activation is a gene cluster on chromosome 1. It includes genes that encode the seven proteins in the complement factor H family. Structurally, these proteins are similar, each being built on a motif of distinct functional domains called short consensus repeats (SCRs). CFH has 20 SCRs. The interacting partners with some of these SCRs has been determined and is shown on the top right (CRP, C reactive protein; Hep, heparin). Complement factor H‐like 1 (CFHL1) is a splice isoform of CFH, while complement factor H‐related proteins 1–5 (CFHR1–5) are each encoded by a unique gene (CFHR1–5). The SCRs of CFHR1–5 are similar to some of the SCRs in CFH, as denoted by the numbers in the ovals. For example, CFHR5 has nine SCRs, with the first two being similar to SCRs 6 and 7 of CFH and therefore having CRP and heparin binding properties. SCRs5–7 of CFHR5 have the numbers 12–14 within the corresponding ovals because these SCRs are similar to SCRs 12–14 of CFH and have C3b and heparin binding properties.
The other six members of the CFH family include CFHL1, which is a splice isoform of CFH, and five CFH related proteins encoded by distinct genes (CFHR1–5). There is little known about the latter five proteins, although they do show varying degrees of structural similarity to CFH.2 Most interesting in this group with respect to MPGN II/DDD is CFHR5, because it shows the highest similarity to CFH and has been demonstrated in renal biopsies of patients with other types of glomerulonephritis.2,17 In vitro studies have also shown that CFHR5 is present on surfaces exposed to complement attack, suggesting a possible role in the complement cascade.17
A possible relationship between CHF/CFHR5 and MPGN II/DDD is further strengthened by the observation that patients with MPGN II/DDD develop an ocular phenotype called drusen. Drusen result from the deposition of abnormal extracellular deposits in the retina within the ocular Bruch's membrane beneath the retinal pigment epithelium. The drusen of MPGN II/DDD are clinically and compositionally indistinguishable from drusen that form in age related macular degeneration (AMD),18,19,20 which is the most common form of visual impairment in the elderly.21,22 The single feature that distinguishes these two types of drusen is age of onset; drusen in MPGN II/DDD develop early, often in the second decade of life, while drusen in AMD are found in the elderly.
Four recent studies have implicated specific allele variants of CFH with AMD, suggesting that subtle differences in CFH mediated regulation of the alternative complement pathway may play a role in a substantial proportion of AMD cases.23,24,25,26 One of these studies also showed that MPGN II/DDD and AMD patients segregate several of the same CFH risk alleles.23 In this study, we sought to refine the association of allele variations of CFH and CFHR5 with MPGN II/DDD.
Materials and methods
Patients and controls
Patients with biopsy proven MPGN II/DDD were ascertained in nephrology divisions and enrolled in this study under institutional review board approved guidelines. The control group was formed from ethnically but not age matched unrelated people in whom AMD had been excluded by ophthalmological examination.
Mutation screening and analysis
Coding and adjacent intronic regions of CFH and CFHR5 were PCR amplified for 35 cycles of denaturing at 94°C for 30 seconds, annealing at 61°C for 30 seconds, and extension at 70°C for 30 seconds. Product generation was verified by agarose gel electrophoresis, and amplicons were then bidirectionally sequenced in patients with MPGN II/DDD. All novel and reported SNPs identified through data mining (Ensembl database, dbSNP, Applied Biosystems) were typed in the control population by denaturing high performance liquid chromatography (DHPLC) (table 1). In brief, DHPLC analysis of each amplicon was performed at three different temperatures. Amplicons were analysed using Wavemaker software to estimate optimal temperature, run time, and acetonitrile gradient. Predicted temperatures were bracketed by +/−2°C to optimise sensitivity and maximise the likelihood that novel mutations would be detected.27
Table 1 Primers used to amplify CFH and CFHR5 coding sequences .
| Exon | Forward | Reverse |
|---|---|---|
| CFH | ||
| 1 | TGGGAGTGCAGTGAGAATTG | GCTAATGATGCTTTTCACAGGA |
| 2 | CCTGTGACTGTCTAGGCATTTT | TATGCCTGAATTATATCACTATTGCC |
| 3 | GCTTTGCTATGTTTAATTTTCCTT | AACTATGATGGAAATAATTAAATCTGG |
| 4 | TGCATATGCTGTTCATTTTC | GTCTTACATTAAAATATCTTAAAGTCTC |
| 5 | TTTCCTCCAATCTTATCCTGAG | CGTTCATTCTAAGGAATATCAGCA |
| 6 | CCTGATGGAAACAACATTTCTG | AACAGGGCCAGAAAAGTTCA |
| 7 | TGTTCATTTTAATGCCATTTTG | AGTTTTCGAAGTTGCCGAAA |
| 8 | CCTAGAAACCCTAATGGAATGTG | TGTTCAAGCAAAGTGACCAAA |
| 9 | TGAGCAAATTTATGTTTCTCATTT | ATGTCACCTTGTTTTACCAATGG |
| 10 | TGAATGCTTATGGTTATCCAGGT | AAAACCTGCAGGAACAAAGC |
| 11 | TCTTAGAATGGGAAATACTCAGATTG | TGGTTTTTCAGAAATTCATTTTCA |
| 12 | ATGTAAAATTAACTTTGGCAATGA | TTGCTGAAATAAGAATTAGAACTTTG |
| 13 | TGAATAAAAGAAGAAAATCTTTCCA | ATCTAAAACACATACATCATGTTTTCA |
| 14 | AAAACACATACATCATGTTTTCACAA | GATATGCCTCAACATTTCCAGTC |
| 15 | GTTGGTTTGATTCCTATCATTTG | TTGGAAAAGTAATAGGTATGTGTGTC |
| 16 | CTATGAGAATACAAGCCAAAAGTTC | TCTCTTGTGCTTCGTGTAAACAA |
| 17 | AACCCTTTGATTTTCATTCTTCA | TCAAAGTGAGGGGAATAATTGA |
| 18 | AATTTATGAGTTAGTGAAACCTGAAT | TCTTCATTCAAAGTGTAAGTGGTACC |
| 19 | ACAAAATGGCTAATATATTTTCTCAAG | TAATGTGTGGGCCCAGCC |
| 20 | CAAAATGAACACTAGGTGGAACC | ATTTTGGGGGAGTATAGCAGG |
| 21 | CTGTGTTTGCGTTTGCCTTA | TTCACGTGGCTGGAAAAATC |
| 22 | TTGAAAACCTGAAAGTCTATGAAGA | TCAATCATAAAGTGCACACCTTT |
| CFHR5 | ||
| 1 | CAGTCCCATTTCTGATTGTTCCA | GCTGAGGATAATTTGAAGGGG |
| 2 | GTGATTCATCGATGTAGCTCTTT | AATGACCAGAGGAGCCTGGAA |
| 3 | TGATGTCAGTTTTCAAAGTTTTCC | ACCACTCTCTCAGTTTTGCTAATTAT |
| 4 | CACATTAAATTTGTTTCTGCAATGA | AGAAGTGATGAAACAAGAATTTGA |
| 5 | CCATTTAAGCATTATTTATGGTTTC | AAACAGGACAGTTACTATTACTTTGCA |
| 6 | AAATATTTTCAGAGTAAGCACTCATTT | TTTATCATTTTGATTGGGATTGT |
| 7 | TGCAGATATTTTATTGACATAATTGTT | GTTGATCTTGTTGCTTCTTTACAAGA |
| 8 | CCATTTTCCTGAAACACTACCC | TCTGTTGCACTGTACCCCAA |
| 9 | AATTATTTGAATTTCCAGACACCTT | TTTTGGACTAATTTCATAGAATAACCC |
| 10 | CTTAAATGCAATTTCACTATTCTATGA | TAGCCATTATGTAGCC |
Haplotype analysis
Construction of block structures with distribution of haplotypes was completed using Haploview, a publicly available software program developed at the Whitehead Institute (http://www.broad.mit.edu/mpg/haploview/).28 Two datasets, one consisting of each control's sex and genotype, and the other describing marker information including SNP identification and chromosomal location, were assimilated in Excel files, which were loaded into the Haploview program. The output consisted of linkage disequilibrium (LD) plots and the corresponding population frequencies with crossover percentages.
Statistical analysis
The χ2 test of independence was used to detect differences in SNP frequencies between patients with MPGNII/DDD and controls, and p⩽0.05 was considered significant. The LD plots for CFH and CFHR5 were created using the control population.
Results
Patients and controls
In total, 22 patients with biopsy proven MPGN II/DDD and 131 people without AMD participated in this study. Mean age of the control group was 78.4 years, reflecting our ascertainment criterion to exclude AMD.
CFH, CFHR5, and MPGN II/DDD
Allele frequencies of four of seven CFH SNPs genotyped in the MPGN II/DDD patient group and the control population showed a significant association with the MPGN II/DDD disease phenotype at p<0.05. These SNPs included exon 2 I62V, IVS 2−18insTT, exon 9 Y402H, and exon 10 A473A. Allele frequencies for exon 7 A307A, exon 13 Q672Q, and exon 18 D936E were not significantly different between groups (tables 2–4).
Table 2 CFH SNPs in patients segregating MPGN II/DDD.
| Ex2 | I62V | IVS2 | −18insTT | Ex7 | A307A | IVS7 | −53G→T | Ex9 | Y402H | Ex10 | A473A | Ex13 | Q672Q | IVS15 | −30C→A | Ex18 | D936E | IVS18 | −89T→C | Ex20 | N1050Y | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs800292 | rs1061147 | rs1061170 | rs2274700 | rs3753396 | rs1065489 | |||||||||||||||||
| MPGN2 | MPGN2 | MPGN2 | MPGN2 | MPGN2 | MPGN2 | MPGN2 | MPGN2 | MPGN2 | MPGN2 | MPGN2 | ||||||||||||
| GG | 20 | (T)9(T)9 | 20 | CC | 3 | GG | 8 | CC | 9 | GG | 18 | AA | 13 | CC | 8 | GG | 13 | TT | 19 | AA | 21 | |
| GA | 2 | (T)11(T)9 | 2 | CA | 10 | GT | 10 | CT | 10 | GA | 4 | AG | 9 | CA | 10 | GT | 9 | TC | 3 | AT | 1 | |
| AA | 0 | (T)11(T)11 | 0 | AA | 9 | TT | 4 | TT | 3 | AA | 0 | GG | 0 | AA | 4 | TT | 0 | CC | 0 | TT | 0 | |
| Sum | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | |||||||||||
| f1 | 0.95G | 0.95 (T)9 | 0.64A | 0.59G | 0.36Y | 0.90G | 0.80A | 0.57C | 0.80G | 0.93T | 0.98A | |||||||||||
| f2 | 0.05A | 0.05 (T)11 | 0.36C | 0.41T | 0.64H | 0.10A | 0.20G | 0.41A | 0.20T | 0.07C | 0.02T | |||||||||||
| At risk haplotype | G | 9 | A | G | C | G | A | C | G | T | A | |||||||||||
| MPGN2‐1 | G,G | 9,9 | A,C | G,T | C,T | G,G | G,A | C,A | G,T | T,T | A,A | |||||||||||
| MPGN2‐2 | G,G | 9,9 | C,C | T,T | T,T | G,G | G,A | C,A | G,T | T,T | A,A | |||||||||||
| MPGN2‐7 | G,G | 9,9 | A,C | G,T | C,T | G,G | G,A | C,A | G,T | T,T | A,A | |||||||||||
| MPGN2‐9 | G,G | 9,9 | A,C | G,T | C,T | G,G | G,A | C,A | G,T | T,T | A,A | |||||||||||
| MPGN2‐10 | G,G | 9,9 | A,C | G,T | C,T | G,G | A,A | C,A | G,T | T,T | A,A | |||||||||||
| MPGN2‐11 | G,G | 9,9 | A,C | G,T | C,T | G,A | A,A | C,A | G,G | T,C | A,T | |||||||||||
| MPGN2‐12 | G,G | 9,9 | A,A | T,T | C,C | G,G | A,A | C,C | G,G | T,T | A,A | |||||||||||
| MPGN2‐13 | G,G | 9,9 | A,C | G,T | C,T | G,G | G,A | C,A | G,T | T,T | A,A | |||||||||||
| MPGN2‐14 | G,G | 9,9 | A,C | G,T | C,T | G,G | G,A | C,A | G,T | T,T | A,A | |||||||||||
| MPGN2‐15 | G,G | 9,9 | A,A | G,G | C,C | G,G | A,A | C,C | G,G | T,T | A,A | |||||||||||
| MPGN2‐16 | G,G | 9,9 | C,C | T,T | T,T | G,A | G,A | A,A | G,T | T,C | A,A | |||||||||||
| MPGN2‐17 | G,G | 9,9 | A,A | G,G | C,C | G,G | A,A | C,C | G,G | T,T | A,A | |||||||||||
| MPGN2‐18 | G,G | 9,9 | A,C | G,T | C,T | G,G | G,A | C,A | G,G | T,T | A,A | |||||||||||
| MPGN2‐19 | G,G | 9,9 | A,A | G,G | C,C | G,G | A,A | C,C | G,G | T,T | A,A | |||||||||||
| MPGN2‐20 | G,G | 9,9 | A,A | G,G | C,C | G,G | A,A | C,C | G,G | T,T | A,A | |||||||||||
| MPGN2‐21 | G,A | 9,11 | C,C | T,T | T,T | G,A | G,A | A,A | G,T | T,T | A,A | |||||||||||
| MPGN2‐22 | G,G | 9,9 | A,A | G,G | C,C | G,G | A,A | C,C | G,G | T,T | A,A | |||||||||||
| MPGN2‐23 | G,G | 9,9 | A,A | G,G | C,C | G,G | A,A | C,C | G,G | T,T | A,A | |||||||||||
| MPGN2‐24 | G,G | 9,9 | A,A | G,G | C,C | G,G | A,A | C,C | G,G | T,T | A,A | |||||||||||
| MPGN2‐27 | G,A | 9,11 | A,C | G,T | C,T | G,A | A,A | C,A | G,G | T,T | A,A | |||||||||||
| MPGN2‐29 | G,G | 9,9 | A,A | G,G | C,C | G,G | A,A | A,A | G,G | T,T | A,A | |||||||||||
| MPGN2‐30 | G,G | 9,9 | A,C | G,T | C,T | G,G | A,A | A,A | G,G | T,C | A,A |
Allele frequencies (f1 and f2) and number of patients by genotype are shown.
Table 3 Comparison of CFH SNP frequencies in MPGN II/DDD patients versus controls (allele frequencies given as f1 and f2).
| SNP | MPGNII/DDD | Controls | p | ||
|---|---|---|---|---|---|
| f1 | f2 | f1 | f2 | ||
| Exon 2 I62V | 42 (G) | 2 (A) | 202 (G) | 60 (A) | 0.0051 |
| IVS2 ‐18insTT | 42 (short) | 2 (long) | 194 (short) | 68 (long) | 0.0018 |
| Exon 7 A307A | 16 (C) | 28 (A) | 88 (A) | 174 (C) | 0.72 |
| Exon 9 Y402H | 28 (H) | 16 (Y) | 88 (H) | 174 (Y) | 0.00014 |
| Exon 10 A473A | 40 (G) | 4 (A) | 74 (G) | 62 (A) | 0.000013 |
| Exon 13 Q672Q | 35 (A) | 9 (G) | 217 (A) | 41 (G) | 0.45 |
| Exon 18 D936E | 35 (D) | 9 (E) | 115 (D) | 19 (E) | 0.32 |
Table 4 Coding SNPs associated with MPGN II/DDD and the related short consensus repeat (SCR) of CFH.
| SNP | SCR | Function of SCR |
|---|---|---|
| Exon 2 I62V | 1 | Interaction with C3b |
| Exon 9 Y402H | 7 | Heparin binding |
| Interaction with CRP | ||
| Exon 10 A473A | 8 | Interaction with CRP |
CRP, C‐reactive protein.
Five CFHR5 SNPs were genotyped in the MPGN II/DDD patient group and control population, including one non‐synonymous SNP (exon 2 P46S), two promoter SNPs (−249T→C, −20T→C) and two intronic SNPs (IVS1+75T→A, IVS2+58C→T). Allele frequencies of three SNPs (exon 2P46S, −249T→C and −20T→C) were significantly different between groups at p<0.05 (tables 5 and 6).
Table 5 CFHR5 SNPs in patients segregating MPGN II/DDD.
| Promoter | −249T→C | Promoter | −20T→C | IVS1 | 75T→A | Ex 2 | P46S | IVS2 | 58C→T | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs9427661 | rs9427662 | rs3748557 | rs12097550 | rs12097550 | |||||||
| MPGN2 | MPGN2 | MPGN2 | MPGN2 | MPGN2 | |||||||
| TT | 21 | TT | 21 | TT | 16 | CC | 19 | CC | 16 | ||
| TC | 1 | TC | 1 | TA | 5 | CT | 3 | CT | 5 | ||
| CC | 0 | CC | 0 | AA | 1 | TT | 0 | TT | 1 | ||
| Sum | 22 | 22 | 22 | 19 | 22 | ||||||
| f1 | 0.98T | 0.98T | 0.84T | 0.93P | 0.84C | ||||||
| f2 | 0.02C | 0.02C | 0.16A | 0.07S | 0.16T | ||||||
| At risk haplotype | T | T | T | C | C | ||||||
| MPGN2‐02 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐03 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐07 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐09 | T,T | T,T | A,T | C,C | C,T | ||||||
| MPGN2‐10 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐11 | T,T | T,T | A,T | C,C | C,T | ||||||
| MPGN2‐12 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐13 | T,T | T,T | A,T | C,T | C,T | ||||||
| MPGN2‐14 | T,T | T,T | A,A | C,C | T,T | ||||||
| MPGN2‐15 | T,T | T,T | T,T | C,T | C,C | ||||||
| MPGN2‐16 | C,T | C,T | A,T | C,C | C,T | ||||||
| MPGN2‐17 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐18 | T,T | T,T | A,T | C,C | C,T | ||||||
| MPGN2‐19 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐20 | T,T | T,T | T,T | C,T | C,C | ||||||
| MPGN2‐21 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐22 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐23 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐24 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐27 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐29 | T,T | T,T | T,T | C,C | C,C | ||||||
| MPGN2‐30 | T,T | T,T | T,T | C,C | C,C |
Allele frequencies (f1 and f2) and number of patients by genotype are shown.
Table 6 Comparison of CFHR5 SNP frequencies in MPGN II/DDD patients versus controls (allele frequencies given as f1 and f2).
| SNP | MPGNII/DDD | Controls | p | ||
|---|---|---|---|---|---|
| f1 | f2 | f1 | f2 | ||
| Promoter −249T→C | 43 (T) | 1 (C) | 178 (G) | 28(A) | 0.033 |
| Promoter −20T→C | 43 (T) | 1 (C) | 178 (G) | 28(A) | 0.033 |
| IVS1 +75T→A | 37(T) | 7 (A) | 161 (A) | 41 (C) | 0.38 |
| Exon 2 P46S | 41 (P) | 3(S) | 205 (P) | 1 (S) | 0.00023 |
| IVS2 +58C→T | 37 (C) | 7 (T) | 158 (C) | 28 (T) | 0.28 |
Haploblocks
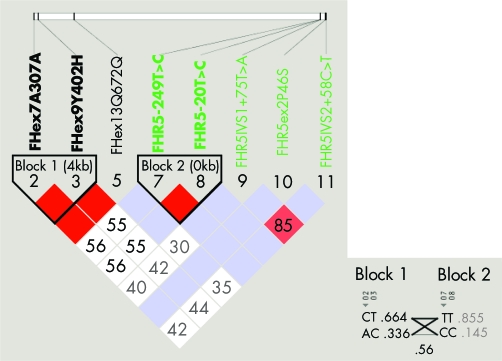
Haplotype blocks showed that A307A and Y402H are in linkage disequilibrium in CFH while −249T→C and −20T→C are in linkage disequilibrium in CFHR5 (fig 4).
Figure 4 Linkage disequilibrium plots show that A307A and Y402H are in linkage disequilibrium in CFH and that −249T→C and −20T→C are in linkage disequilibrium in CFHR5 (n = 103). Haplotype frequencies and crossover frequencies between blocks are shown to the right.
Discussion
The alternative pathway of complement represents an elegant system to protect humans from pathogens. Its central component, C3, circulates at a high concentration in plasma and is distributed throughout body fluids.29 Its activation creates a toxic local environment that damages foreign surfaces and results in the elimination of microbes. To prevent unrestricted complement activation, host cells and tissue surfaces downregulate the amplification loop using a combination of surface attached and membrane bound regulators of complement. Some host cells express a single membrane bound regulator of complement in high copy number, while other cells express several membrane bound regulators and also attach soluble fluid phase regulators. A few tissues lack membrane bound regulators and depend exclusively on the attachment of soluble regulators.2
In the kidney, endothelial and mesangial cells express two membrane bound regulators of complement, MCP and DAF.30,31 Podocytes express four: MCP, DAF, CR1, and CD59. Both mesangial cells and podocytes also secrete the soluble regulator CFH, which is upregulated in membranous nephropathy in response to complement activation and inflammation.32,33 CFH acts in an autocrine fashion by binding directly to the secreting mesangial cells and podocytes.
The GBM, in contrast, is unique. It lacks endogenous membrane bound regulators to protect it from complement mediated injury; however, its highly negatively charged surface binds and absorbs CFH.16 The dependency of the GBM on CFH for local complement control is consistent with the finding of pathological mutations in CFH in a few people with MPGN II/DDD.13,34
Our data identifying several allele variants of CFH and CFHR5 associated with MPGN II/DDD is consistent with the hypothesis that complement control plays a role in the pathogenesis of this disease. A comparison of our data with reported AMD data adds additional support, as the allele frequency for each of the identified at risk SNP variants we observed in CFH was higher in the MPGN II/DDD patient cohort than in the AMD patient cohort, and strong evidence implicates CFH in AMD.23,24,25,26 Although it is not known whether the amino acid changes in exons 2 and 9 of CFH impact function, these changes are found in domains that interact with C3b and heparin, and differences in C3b/C3d and heparin binding have been demonstrated with several amino acid changes in CFH that are associated with another renal disease, atypical haemolytic uraemic syndrome38 (tables 3 and 4).
With the exception of CFH, the function of other members of the factor H related family is largely unknown and their expression patterns have not been explored, however studies of CFHR5 have shown that it has properties similar to CFH, including heparin, CRP and C3b binding17 (fig 3). This similarity suggests that like CFH, CFHR5 could play a role in MPGN II/DDD. Consistent with this possibility is our finding of CFHR5 expression in renal biopsies from two patients with MPGN II/DDD (data not shown).
Our genotyping data show that some allele variants of CFHR5 preferentially associate with the MPGN II/DDD disease phenotype. Included are two SNPs in the promoter region of CFHR5, which could affect transcription, one by removing a binding site for C/EBPβ and the other by adding a GATA‐1 binding site. The other significant association changes a proline to serine in exon 2. As exons 1and 2 of CFHR5 encode a domain homologous to short consensus repeat 6 of CFH, which is integral to heparin and CRP binding, this change could affect complement activation and control.
Conclusion
Humans, pigs, and mice deficient in CFH develop MPGN II/DDD,11,13,14,15 implicating local dysfunction of the alternative pathway of the complement cascade in this disease. We have identified specific allele variants of CFH and CFHR5 associated with the MPGN II/DDD disease phenotype. While our data can be interpreted to further implicate complement in the pathogenesis of MPGN II/DDD, these associations could also be unrelated to disease pathophysiology. Functional studies are required to resolve this question.
Acknowledgements
We are indebted to the people with MPGNII/DDD whose participation has made this research possible. We are also grateful for the participation of people who served as control subjects for this study.
Abbreviations
AMD - age related macular degeneration
C3NeF - C3 nephritic factor
C4BP - C4 binding protein
CFH - complement factor H
CFHL1 - complement factor H‐like protein 1
CFI - complement factor I
CR1 - complement receptor 1
DAF - decay accelerating factor
DDD - dense deposit disease
DHPLC - denaturing high performance liquid chromatography
ESRD - end stage renal disease
GBM - glomerular basement membrane
LD - linkage disequilibrium
MCP - membrane co‐factor protein
MPGN I/II/III - membranoproliferative glomerulonephritis types I/II/III
SNP - single nucleotide polymorphism
Footnotes
Competing interests: there are no competing interests
References
- 1.Orth S R, Ritz E. The nephrotic syndrome. New Engl J Med 19983381202–1211. [DOI] [PubMed] [Google Scholar]
- 2.Appel G B, Cook H T, Hageman G, Jennette J C, Kashgarian M, Kirschfink M, Lambris J D, Lanning L, Lutz H U, Meri S, Rose N R, Salant D J, Sethi S, Smith R J H, Smoyer W, Tully H F, Tully S P, Walker P, Welsh M, Würzner R, Zipfel P F. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol 2005161392–1403. [DOI] [PubMed] [Google Scholar]
- 3.Habib R, Gubler M C, Loriat C, Maiz H B, Levy M. Dense deposit disease. A variant of membranoproliferative glomerulonephrtitis. Kidney Int 19757204–215. [DOI] [PubMed] [Google Scholar]
- 4.Habib R, Antignac C, Hinglais N, Gagnadoux M F, Broyer M. Glomerular lesions in the transplanted kidney in children. Am J Kidney Diseas 198710198–207. [DOI] [PubMed] [Google Scholar]
- 5.Schwertz R, Rother U, Anders D, Gretz N, Scharer K, Kirschfink M. Complement analysis in children with idiopathic membranoproliferative glomerulonephritis: a long‐term follow‐up. Pediatr Allergy Immunol 200112166–172. [DOI] [PubMed] [Google Scholar]
- 6.Cameron J S, Turner D R, Heaton J, Williams D G, Ogg C S, Chantler C, Haycock G B. Idiopathic mesangiocapillary glomerulonephritis. Comparison of types I and II in children and adults and long‐term prognosis. Am J Med 198374175–192. [DOI] [PubMed] [Google Scholar]
- 7.Barbiano di Belgiojoso G, Tarantino A, Colasanti G, Bazzi C, Guerra L, Durante A. The prognostic value of some clinical and histological parameters in membranoproliferative glomerulonephritis. Nephron 197719250–258. [DOI] [PubMed] [Google Scholar]
- 8.Droz D, Noel L H, Barbanel C, Grunfeld J P. Évolution a long terme des glomérulonéphrites membranoproliferative de l'adulte: remissionspontanée durable chez 13 malades avec étude de biopsies rénales itératives dans 5 cas. Neprhrologie 198236–11. [PubMed] [Google Scholar]
- 9.McEnery P T. Membranoproliferative glomerulonephritis: The Cincinnati experience cumulative renal survival from 1957 to 1989. J Pediatr 1990116S109–S114. [DOI] [PubMed] [Google Scholar]
- 10.Swainson C P, Robson J S, Thomson D, MacDonald M K. Mesangiocapillary glomerulonephritis: A long‐term study of 40 cases. J Pathol 1983141449–468. [DOI] [PubMed] [Google Scholar]
- 11.Meri S, Pangburn M K. Regulation of alternative pathway complement activation by glycosaminoglycans: specificity of the polyanion binding site on factor H. Biochem Biophys Res Commun 199419852–59. [DOI] [PubMed] [Google Scholar]
- 12.Pascual M, Steiger G, Sadallah S, Paccaud J P, Carpentier J L, James R, Schifferli J A. Identification of membrane‐bound CR1 (CD35) in human urine: evidence for its release by glomerular podocytes. J Exp Med 199479889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dragen‐Durey M A, Fremeaux‐Bacchi V, Loirat C, Blouin J, Niaudet P, Deschenes G, Coppo P, Herman Fridman W, Weiss L. Heterozygous and homozygous factor H deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol 200415787–795. [DOI] [PubMed] [Google Scholar]
- 14.Jansen J H, Hogasen K, Harboe M, Hovig T. In situ complement activation in porcine membranoproliferative glomerulonephritis type II. Kidney Int 199853331–349. [DOI] [PubMed] [Google Scholar]
- 15.Pickering M C, Cook H T, Warren J, Bygrave A E, Moss J, Walport M J, Botto M. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet 200231424–428. [DOI] [PubMed] [Google Scholar]
- 16.Zipfel P F, Smith R J H, Heinen S. The role of complement in membranoproliferative glomerulonephritis. In: Zipfel, ed: Complement and kidney disease. Germany: Birkhauser 2005199–221.
- 17.Murphy B, Georgiou T, Machet D, Hill P, McRae J. Factor H‐related protein‐5: a novel component of human glomerular immune deposits. Am J Kid Dis 20023924–27. [DOI] [PubMed] [Google Scholar]
- 18.Mullins R F, Aptsiauri N, Hageman G S. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement. Eye 200115390–395. [DOI] [PubMed] [Google Scholar]
- 19.Hageman G S, Luthert P J, Victor Chong N H, Johnson L V, Anderson D H, Mullins R F. An integrated hypothesis that considers drusen as biomarkers of immune‐mediated processes at the RPE‐Bruch's membrane interface in aging and age‐related macular degeneration. Prog Retin Eye Res 200120705–732. [DOI] [PubMed] [Google Scholar]
- 20.Anderson D H, Mullins R F, Hageman G S, Johnson L V. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol 2002134411–431. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Peto T, Bird A, Vannewkirk M R. The epidemiology of age‐related macular degeneration. Am J Ophthalmol 2004137486–495. [DOI] [PubMed] [Google Scholar]
- 22.van Leeuwen R, Klaver C C, Vingerling J R, Hofman A, de Jong P T. Epidemiology of age‐related maculopathy: a review. Eur J Epidemiol 200318845–854. [DOI] [PubMed] [Google Scholar]
- 23.Hageman G S, Anderson D H, Johnson L V, Hancox L S, Taiber A J, Hardisty L I, Hageman J L, Stockman H A, Borchardt J D, Gehrs K M, Smith R J, Silvestri G, Russell S R, Klaver C C, Barbazetto I, Chang S, Yannuzzi L A, Barile G R, Merriam J C, Smith R T, Olsh A K, Bergeron J, Zernant J, Merriam J E, Gold B, Dean M. Allikmets R. Common haplotype in the complement regulatory gene, factor H (HF1/CFH), predisposes individuals to age‐related macular degeneration. Proc Nat Acad Sci 20051027227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards A O, Ritter III R, Abel K J, Manning A, Panhuysen C, Farrer L A. Complement factor H polymorphism and age‐related macular degeneration. Science 2005308421–424. [DOI] [PubMed] [Google Scholar]
- 25.Haines J L, Hauser M A, Schmidt S, Scott W K, Olson L M, Gallins P, Spencer K L, Kwan S Y, Noureddine M, Gilbert J R, Schnetz‐Boutaud N, Agarwal A, Postel E A, Pericak‐Vance M A. Complement factor H variant increases the risk of age‐related macular degeneration. Science 2005308419–421. [DOI] [PubMed] [Google Scholar]
- 26.Klein R J, Zeiss C, Chew E Y, Tsai J Y, Sackler R S, Haynes C, Henning A K, Sangiovanni J P, Mane S M, Mayne S T, Bracken M B, Ferris F L, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age‐related macular degeneration. Science 2005308385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasad S, Kölln K A, Cucci R A, Trembath R C, Van Camp G, Smith R J H. Pendred syndrome and DFNB4 — Mutation screening of SLC26A4 by denaturing high‐performance liquid chromatography and the identification of seven novel mutations. Am J Med Genet 2004124A1–9. [DOI] [PubMed] [Google Scholar]
- 28.Barrett J C, Fry B, Maller J, Daly M J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 200421263–265. [DOI] [PubMed] [Google Scholar]
- 29.Walport M J. Complement. First of two parts. N Engl J Med 20013441058–1066. [DOI] [PubMed] [Google Scholar]
- 30.van den Dobbelsteen M E, Verhasselt V, Kaashoek J G, Timmerman J J, Schroeijers W E, Verweij C L, van der Woude F J, van Es L A, Daha M R. Regulation of C3 and factor H synthesis of human glomerular mesangial cells by IL‐1 and interferon‐gamma. Clin Exp Immunol 199495173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timmerman J J, van der Woude F J, van Gijlswijk‐Janssen D J, Verweigj C L, van Es L A, Daha M R. Differential expression of complement components in human fetal and adult kidneys. Kidney Int 199649730–740. [DOI] [PubMed] [Google Scholar]
- 32.Angaku M. Complement regulatory proteins in glomerular diseases. Kidney Int 1998541419–1428. [DOI] [PubMed] [Google Scholar]
- 33.Bao L, Spiller O B, St John PL, Haas M, Hack BK, Ren G, Cunningham PN, Doshi M, Abrahamson DR, Morgan BP, Quigg RJ. Decay‐accelerating factor expression in the rat kidney is restricted to the apical surface of podocytes. Kidney Int 2002622010–2021. [DOI] [PubMed] [Google Scholar]
- 34.Ault B H, Schmidt B Z, Fowler N L, Kashtan C E, Ahmed A E, Vogt B A, Colten H R. Human factor H deficiency. Mutations in framework cysteine residues and block in H protein secretion and intracellular catabolism. J Biol Chem 199727225168–25175. [DOI] [PubMed] [Google Scholar]
- 35.Colville D, Guymer R, Sinclair R A, Savige J. Visual impairment caused by retinal abnormalities in mesangiocapillary (membranoproliferative) glomerulonephritis type II (“dense deposit disease”). Am J Kidney Dis 200342E2–E5. [DOI] [PubMed] [Google Scholar]
- 36.Duvall‐Young J, Short C D, Raines M F, Gokal R, Lawler W. Fundus changes in mesangiocapillary glomerulonephritis type II: clinical and fluorescein angiographic findings. Br J Ophthalmol 198973900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holz F G, Pauleikhoff D, Klein R, Bird A C. Pathogenesis of lesions in late age‐related macular disease. Am J Ophthalmol 2004137504–510. [DOI] [PubMed] [Google Scholar]
- 38.Manuelian T, Hellwage J, Meri S, Caprioli J, Noris M, Heinen S, Jozsi M, Neumann H P H, Remuzzi G, Zipfel P F. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest 20031111181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]