Abstract
The innate and adaptive immune responses have evolved distinct strategies for controlling different viral pathogens. Encephalomyocarditis virus (EMCV) is a picornavirus that can cause paralysis, diabetes, and myocarditis within days of infection. The optimal innate immune response against EMCV in vivo requires CD1d. Interaction of antigen-presenting cell CD1d with distinct natural killer T-cell (“NKT”) populations can induce rapid gamma interferon (IFN-γ) production and NK-cell activation. The T-cell response of CD1d-deficient mice (lacking all NKT cells) against acute EMCV infection was further studied in vitro and in vivo. EMCV persisted at higher levels in CD1d-knockout (KO) splenocyte cultures infected in vitro. Furthermore, optimal resistance to repeat cycles of EMCV infection in vitro was also shown to depend on CD1d. However, this was not reflected in the relative levels of NK-cell activation but rather by the responses of both CD4+ and CD8+ T-cell populations. Repeated EMCV infection in vitro induced less IFN-γ and alpha interferon (IFN-α) from CD1d-deficient splenocytes than with the wild type. Furthermore, the level of EMCV replication in wild-type splenocytes was markedly and specifically increased by addition of blocking anti-CD1d antibody. Depletion experiments demonstrated that dendritic cells contributed less than the combination of NK and NKT cells to anti-EMCV responses and that none of these cell types was the main source of IFN-α. Finally, EMCV infection in vivo produced higher levels of viremia in CD1d-KO mice than in wild-type animals, coupled with significantly less lymphocyte activation and IFN-α production. These results point to the existence of a previously unrecognized mechanism of rapid CD1d-dependent stimulation of the antiviral adaptive cellular immune response.
Critical elements of successful immune responses to acute challenge are activation of the innate immune system and subsequent adaptive immune responses, the latter mediated by antigen-specific T and B cells. Mammals have populations of T cells that specifically recognize endogenous as well as exogenous glyco- and phospholipids presented by nonpolymorphic major histocompatibility complex (MHC) class I-like CD1 molecules. CD1d is constitutively expressed on antigen-presenting cells (APC) and inducible on other cell types, whereas CD1a to -c are induced during APC differentiation (7, 8, 11, 14, 15, 24, 29, 47). Many CD1d-restricted T cells express NK-cell markers, such as CD161 (“NKT cells”), and a major subset uses an invariant T-cell-receptor (TCR) α chain (Vα14Jα18; formerly Vα14Jα281) and limited TCRβ chain repertoire (“iNKT”). CD1d-restricted T-cell subsets have been implicated as both positive and negative regulators of antipathogen immune responses (4, 15, 58) based on their unique ability to rapidly produce high levels of Th1- and Th2-type cytokines, including gamma interferon (IFN-γ) and interleukin 4 (IL-4), respectively, in response to CD1d (8, 34, 54).
The classic invariant murine CD1d-dependent T cells can be CD4+ or double negative (8, 12). Other CD1d-restricted T cells possess diverse TCR (4, 5, 15, 16, 20, 25, 26, 64). Essentially similar populations of CD1d-restricted T cells have been identified in humans (22, 23, 25, 27, 58) which are functionally and phenotypically homologous to the extent that iNKT from each species can recognize CD1d from the other and specifically respond to the same exogenous glycolipid antigen α-galactosylceramide (α-GalCer) (13). Therefore, murine models are widely used to define the role of CD1d-restricted T cells in various diseases, including in viral infections. It is thought that CD1d-restricted T cells play a role in linking the innate and adaptive arms of the immune system, and it has become clear that their capacity to participate in early immune responses confers the potential to mediate activities important in control of infectious agents. CD1d-restricted T cells can respond very rapidly to such infectious stimuli, are able to activate a variety of innate and adaptive immune effectors, and are now known to enhance resistance to certain groups of viruses, bacteria, and parasites (10, 11, 12, 15, 29, 34, 50, 55, 58, 59).
The impact of CD1d-restricted T cells varies in different viral infections, where they contribute to resistance against certain but not all virus groups. For example, CD1d-restricted T cells are not essential for resistance to lymphocytic choriomeningitis virus (LCMV), murine cytomegalovirus, vaccinia virus, or severe acute respiratory syndrome-coronavirus and heterosubtypic immunity to influenza strains (6, 9, 28, 38, 59, 60). However, treatment with iNKT-cell activator, α-GalCer, protects mice from infection with the diabetogenic strain of the encephalomyocarditis virus (EMCV-D) (21), a picornavirus that can cause acute paralysis, diabetes, and myocarditis (2, 18, 19, 63). Significantly, BALB/c, mixed, and C57BL/6 CD1d-deficient mice are more susceptible to EMCV-D (21, 22). Resistance to EMCV-D infection depends on rapid induction of a CD1d-dependent innate immune response with IL-12 release, apparently through CD1d-restricted T-cell activation of APC, and consequent NK-cell activation (22). CD1d-restricted T cells appear to stimulate CD8 T-cell responses against respiratory syncytial virus, but interestingly, the reverse has been found in the case of LCMV (32, 33). Optimal resistance to herpes simplex viruses and a hepatitis B virus infection model also requires populations of CD1d-restricted T cells (1, 3, 30), and α-GalCer treatment was shown to be beneficial in murine cytomegalovirus and hepatitis B virus infection models (35, 60). iNKT cells are eliminated early in human immunodeficiency virus (HIV) and simian immunodeficiency virus infections and stay depressed after LCMV infection, although not in hepatitis C (37, 40, 41, 55-57). Finally, there is evidence for antiviral roles of human iNKT cells (36, 45, 46) and most recently for viral countermeasures involving CD1d down-regulation by the human viruses Kaposi’s sarcoma-associated herpesvirus and HIV (17, 31, 49). Mechanisms by which CD1d and/or CD1d-restricted T cells can confer antiviral responses have yet to be fully elucidated (9-12, 15, 25, 29, 50, 55, 58, 59).
Here we assessed the immunological response to EMCV in vitro in order to delineate the critical components underlying CD1d-mediated resistance to model acute viral infection. Under these conditions, NK cells were strongly stimulated in response to EMCV without any major difference between wild-type (WT) and CD1d-knockout (KO) cultures. However, there were clear differences in viral replication in vitro between wild-type and CD1d-KO mice, amplified when using experimental protocols that provided for multiple cycles of EMCV infection. Differences observed in the immune response to EMCV infection in vitro included higher levels of B-cell activation in CD1d-deficient cultures and lower levels of CD1d-KO T-cell activation. The latter effect, again, was strongest when these cultures were submitted to secondary viral infection. In agreement with these results, the difference between the levels of viral replication and IFN-γ and alpha interferon (IFN-α) production in wild-type and CD1d-KO splenocyte cultures was also most notable in such systems. EMCV replication in vitro was specifically increased in primary and secondary infection of wild-type splenocytes treated with anti-CD1d antibody to levels similar to those observed in CD1d-KO cultures. Notably, depletion of dendritic cells (DC) from the cultures had only a modest effect on viral replication, IFN levels, and lymphocyte activation, whereas depletion of NK as well as NKT cells resulted in a marked increase in viral titer and hyper T-cell activation.
The results of experimental EMCV infection in vivo largely corroborated the results of the in vitro studies. CD1d-KO animals supported viral replication to higher levels than wild-type mice, and their lymphocyte populations showed significantly lower levels of activation, which was also reflected in markedly lower levels of systemic IFN-α production.
Collectively, these observations point to the existence of a rapid CD1d-mediated mechanism of antiviral cellular immune response independent of immediate innate response by NK cells. This antiviral response, which may include CD1d-restricted-T-cell antigen recognition, was maximal with multiple cycles of viral replication in vitro and early during EMCV infection in vivo. The CD1d-dependent antiviral response may provide for amplified protection against infection of susceptible cells by virus which escapes NK-cell control of the initial round of infection, thereby linking innate and adaptive antiviral responses.
MATERIALS AND METHODS
Animals.
Mice deficient in both CD1d genes were prepared as described previously (22). CD1d-KO mice were further backcrossed for a total of 12 generations to the C57BL/6J background. Age-matched male (4- to 5-week-old) wild-type C57BL/6 (Jackson Labs. or Taconic) controls were used.
Cells and cell lines.
Splenocyte cultures were prepared from spleens that were minced, strained through a 70-μM filter, treated with red cell lysis buffer (Sigma), and seeded at 106 cells/ml in 24-well plates. Cultures were grown in complete RPMI, with 10% fetal calf serum and IL-2 (20 IU/ml; NBRMP, NCI) added at initiation. The mouse L929 cell line was obtained from ATCC and was grown in Dulbecco's modified Eagle medium (DMEM) with 10% fetal calf serum. Mixed L929/splenocyte cultures were incubated in a RPMI-DMEM mixture (1:1 ratio) during the first 24 h of passage and then transferred to complete RPMI.
EMCV virus preparation, virus titration, and infection in vitro and in vivo.
EMCV virus was grown and titrated on L929 cultures. Viral stocks were prepared on day 1 postinfection (later time points resulted in a lower titer), cleared of cell debris, filtered (0.45 μM), tested in plaque-forming assay, and stored at −80°C. For plaque-forming assay, 24- to 48-h-old L929 cultures were treated with various (usually 10−3 to 10−6) viral dilutions for 1 h in phosphate-buffered saline, covered by complete DMEM with 1% agarose, and upon solidification incubated at 37°C. Plaques were counted at 3 days by staining viable cells with 1% Neutral Red. The same assay was used to determine the viral titer in infected splenocyte culture supernatants. For the infection of fresh splenocytes in vitro, 1 × 106 cells per well in 24-well plates were infected with EMCV-D (multiplicity of infection [MOI] = 0.1 to 3 PFU per cell) as previously described (18, 19, 22).
For the in vivo experiments, test animals (4 to 6 weeks) were infected intraperitoneally with 500 PFU of diabetogenic EMCV strain (kindly provided by N. Bigley, Dayton, OH) as described previously (22) and observed for 2 to 7 days. Mice were bled for systemic cytokine and virus titer measurements as noted above, and their spleen cell populations were analyzed via fluorescence-activated cell sorting (FACS).
Both in vitro and in vivo experiments were repeated at least three times in every series, with the exception of those involving anti-mouse CD1d antibody, which were performed twice. The soluble CD1d antibody 1B1 and isotype control (BD-Biosciences, La Jolla, CA) were used at a concentration of 50 μg/ml. Representative data from individual replicate wells or animals or means (± standard deviation [SD]) for each experimental group are shown, and Student's t test was used as indicated.
Measurement of cytokine expression in vitro and in vivo.
IFN-α and IFN-γ levels in splenocyte cultures and sera of experimentally infected animals were determined by standard enzyme-linked immunosorbent assay using commercial kits (R & D, Minneapolis, MN, and PBL Biomedical Laboratories, Piscataway, NJ, respectively) according to manufacturers' recommendations. Culture medium samples or serum samples were taken at different time points and if not tested immediately were stored at −80°C. Limit of detection in splenocyte supernatants, which were assayed undiluted, was 1 pg/ml for IFN-γ and 5 pg/ml for IFN-α. Animal serum samples were assayed at several dilutions. Results were individual representative or means (± SD).
FACS analysis.
Splenocytes were stained with different combinations of fluorescein isothiocyanate-conjugated anti-mouse CD69, phycoerythrin (PE)-conjugated anti-mouse NK1.1, CD8, and CD19, and CyChrome (Cy7)-conjugated anti-mouse CD3 (all reagents from BD-Pharmingen, La Jolla, CA). Matched isotype antibodies from rat and hamster were used as controls. Cells were stained, washed, and resuspended in Hanks balanced salt solution-0.1% mouse serum buffer containing 0.1% sodium azide and analyzed by using an FC500 flow cytometer (Coulter). Gating was on viable leukocytes. Means (± SD) and/or individual representative results were shown or tabulated.
FACS sorting.
Splenocytes were stained, washed, resuspended in sterile medium containing 0.1% mouse serum, and sorted by using a MoFlo flow cytometer (Cytomation, Boulder, CO). Antimouse monoclonal antibodies (MAb) used were PE-conjugated anti-DC 33D1 (61) and CD11c or NK1.1 and Ly49b (“NK/NKT”) (BD-Pharmingen). Gating was done on viable leukocytes using PE-labeled isotype control.
RESULTS
Primary infection of splenocyte cultures with EMCV.
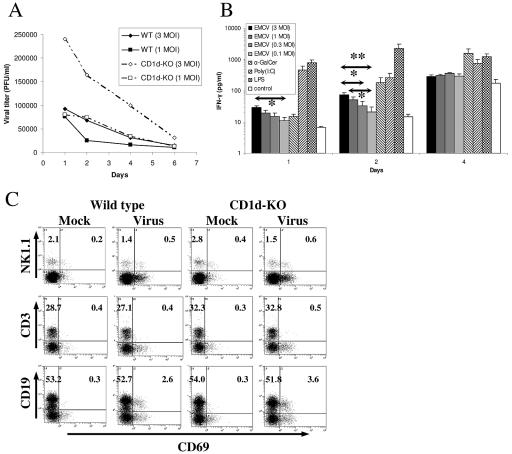
Previous results have shown that optimal resistance to EMCV infection (21, 22), as well as that by certain other pathogens, depends upon CD1d-restricted T cells. Therefore, we further investigated mechanisms of resistance to EMCV infection in vitro. Primary infection of mouse splenocytes with EMCV resulted in a rapid decrease of viral titer in infected cultures (Fig. 1A), as previously reported (19, 42). This was inversely correlated with increased levels of IFN-γ production, which in turn was viral dose dependent (Fig. 1B). Control pharmacological and mitogenic stimuli induced more-potent IFN-γ release. Both lipopolysaccharide (LPS) and poly(I:C) induced robust IFN-γ levels on days 1 and 2 from WT (Fig. 1B) and CD1-KO (not shown) splenocytes. iNKT-cell-specific synthetic lipid antigen α-GalCer stimulated delayed IFN-γ specifically only in WT cultures, which reached very high levels comparable to those of LPS and poly(I:C) by day 4 (Fig. 1B).
FIG. 1.
Primary EMCV infection of wild-type and CD1d-KO mouse splenocytes. (A) EMCV replication in vitro. Freshly isolated splenocytes from wild-type and CD1d-KO C57BL/6 mice were infected by EMCV using a MOI infectious units/splenocyte) of 1.0 or 3.0. Virus titers were determined as described in Materials and Methods. Data shown are from cultures of individual representative animals. (B) IFN-γ production in vitro. Splenocytes from wild-type C57BL/6 mice were infected with different EMCV doses or stimulated with α-galactosylceramide (100 ng/ml), poly(I:C), or LPS (both 5 μg/ml). Mean IFN-γ production was determined by enzyme-linked immunosorbent assay. *, P < 0.05; **, P < 0.01. (C) Activation of NK, T, and B cells after EMCV infection as measured by CD69 expression. Sample FACS profiles from individual representative animals are shown. FACS was performed on the day after infection by EMCV using a MOI of 1.0. Absolute percentages of inactive and activated (CD69+) NK1.1+, CD3+, and CD19+ cells within the viable leukocyte gate are shown. Quadrant gates were based on isotype staining.
Noticeably, a higher level of virus titer was maintained through several days in splenocytes from CD1d-KO mice at both MOI shown (Fig. 1A). This difference, however, was not reflected in the levels of NK cells, B cells, or T cells expressing activation marker CD69 after infection (Fig. 1C; Table 1), nor in IFN-γ release, which did not differ between WT (Fig. 1A) and CD1d-KO mice (not shown). In all cases, robust day-1 NK-cell (NK1.1+ CD3−) and B-cell (CD19+) activation in response to EMCV infection was consistently observed, coupled with very modest but reproducible T-cell (CD3+) activation (Fig. 1C; Table 1). Both T-cell and B-cell activation declined with time, starting with day 2, while NK-cell response consistently dropped on day 2 but then rose somewhat by day 3 (Table 1). The late NK-cell activation response at least partly reflected CD69 expression by NK cells in mock-infected cultures, which increased progressively, although staying below levels in virus-infected cultures. Elevated levels of NK-cell CD69 expression in virus-infected and control cultures (Table 1) also reflected generally increasing IFN-γ levels (Fig. 1B).
TABLE 1.
Cellular dynamics in uninfected and EMCV-infected splenocyte cultures from wild-type and CD1d-KO micea
| Day postinfection | Mouse | % Activated NK cells
|
% Activated T cells
|
% Activated B cells
|
|||
|---|---|---|---|---|---|---|---|
| Uninfected | Infected | Uninfected | Infected | Uninfected | Infected | ||
| 0 (2 h) | WT-1 | 2.6 | 4.7 | 1.4 | 1.8 | 2.6 | 4.0 |
| WT-2 | 2.4 | 4.3 | 1.5 | 1.3 | 2.1 | 3.6 | |
| CD1d-KO-1 | 5.3 | 6.4 | 1.5 | 1.2 | 2.2 | 2.9 | |
| 1 | WT-1 | 13.8 | 25.7 | 1.5 | 1.9 | 3.1 | 7.3 |
| WT-2 | 8.6 | 25.2 | 1.3 | 1.9 | 2.6 | 7.6 | |
| WT-3 | 7.3 | 24.0 | 1.5 | 2.3 | 1.1 | 5.4 | |
| CD1d-KO-1 | 14.5 | 32.5 | 1.1 | 1.8 | 3.4 | 10.1 | |
| CD1d-KO-2 | 9.2 | 18.8 | 1.7 | 2.3 | 2.0 | 9.8 | |
| 2 | WT-1 | 15.7 | 18.7 | 1.0 | 1.7 | 0.6 | 1.3 |
| WT-2 | 16.4 | 16.7 | 1.1 | 1.4 | 0.6 | 1.5 | |
| WT-3 | 6.0 | 12.3 | 1.1 | 1.3 | 2.4 | 2.5 | |
| CD1d-KO-1 | 18.6 | 21.2 | 0.9 | 1.5 | 0.6 | 1.8 | |
| CD1d-KO-2 | 6.7 | 13.8 | 1.2 | 1.5 | 2.5 | 3.0 | |
| 3 | WT-1 | 14.7 | 30.6 | 1.9 | 1.6 | 1.0 | 1.6 |
| WT-2 | 19.4 | 32.2 | 1.7 | 1.4 | 0.9 | 1.4 | |
| CD1d-KO-1 | 40.0 | 35.0 | 1.6 | 1.8 | 1.6 | 1.5 | |
Data from representative individual mice are shown. CD69+ recently activated cells are shown as percentages of each lymphocyte population (100%).
Increased susceptibility of CD1d-KO splenocytes to reinfection with EMCV.
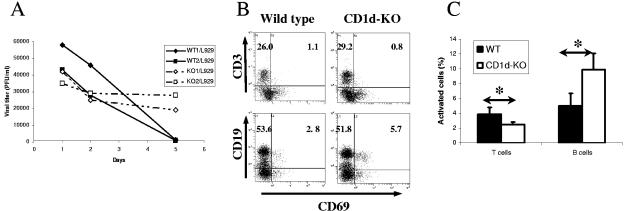
It was clear that EMCV infection of C57BL/6 mouse splenocytes in vitro is not productive, and it is likely that the number of primary splenocyte cells susceptible to productive EMCV infection in such cultures is relatively low. However, it is also possible that infection may be partially aborted by the immediate immune response, which could be related to NK-cell activation. In either case, the effects observed during the previous experiments using a single infection cycle in vitro may less-fully reflect infection defense mechanisms existing in vivo. Moreover, it has been shown that resolution of EMCV infection in vivo is substantially delayed in CD1d-KO mice (21, 22). To mimic the situation that exists during continuous viral infection, we first subjected EMCV-treated splenocyte cultures to reinfection with EMCV 4 to 6 days after the initial infection. This resulted in decreased EMCV recovery in the wild-type cultures relative to that in CD1d-KO splenocytes (Fig. 2A). Inhibition of viral replication was reflected in differential IFN-γ production in these cultures. Wild-type cultures exhibited increased production of IFN-γ in response to reinfection with EMCV, with hardly any additional IFN-γ production observed in CD1d-KO cultures (Fig. 2B).
FIG. 2.
EMCV reinfection of wild-type and CD1d-KO mouse splenocytes. (A) EMCV replication in vitro: reinfection. Splenocytes from wild-type and CD1d-KO C57BL/6 mice were infected by EMCV using a MOI of 1.0 and reinfected with the same dose at day 5 after initial infection. (B) IFN-γ production after EMCV reinfection. Cytokine levels immediately before reinfection by EMCV (MOI = 1.0) and 48 h after are shown. Results from splenocyte cultures from representative individual mice (wild-type or CD1d-KO) are shown. (C) Levels of activation of NK, T, and B cells after EMCV reinfection. Splenocyte cultures from wild-type and CD1d-KO mice infected with EMCV at inception were reinfected with 1 MOI of EMCV at day 5. Sample FACS profiles and absolute percentages of inactive and activated (CD69+) NK1.1+ CD3− NK cells, CD3+ T cells, CD3+ CD8+ double-positive CD8 T cells, and CD19+ B cells are shown. (D) Activation of leukocytes after EMCV reinfection. Mean percentages (± SD) of activated cells for each subtype 24 h after reinfection are shown. *, P < 0.05.
As shown in Fig. 2C, before reinfection on day 5, both wild-type and CD1d-KO cultures exhibit considerable NK-cell activation, substantial T-cell activation (including both CD4+ and CD8+), and little if any activation of B cells (absolute B-cell numbers in the cultures declined as expected due to lack of B-cell growth factor, whereas both NK cells and T cells persisted beyond 7 days in the presence of IL-2). Reinfection led to additional stimulation of NK cells in both wild-type and CD1d-KO cultures. Interestingly, however, stronger additional T-cell stimulation was observed in wild-type cultures, especially among CD8+ T cells (Fig. 2C and D). In fact, minimal stimulation was seen in T cells from CD1d-KO mice, whereas ∼50% of wild-type cells were activated (and ∼25% of total CD8+ cells). No additional activation of B cells was seen in either type of culture (Fig. 2C and D).
Increased susceptibility of CD1d-KO splenocyte cultures to infection with EMCV in presence of susceptible L929 cells.
Another system which may provide for an additional round of infection with EMCV in vitro is coculture of splenocytes with susceptible L929 cells. L929 is a mouse fibroblast cell line capable of rapid productive infection with EMCV. Thus, splenocytes infected with EMCV in the presence of L929 cells will be exposed to multiple rounds of virus infection—by the original virus stock and by virus released from EMCV-infected L929 cells. L929 cells are lysed by EMCV after overnight incubation; therefore, one may assume that such a system will provide for no more than one or two additional viral infectious rounds.
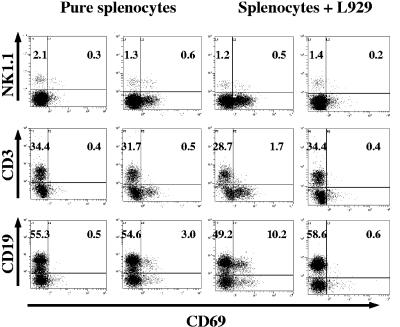
Not surprisingly, wild-type mouse splenocytes responded strongly to the EMCV infection in coculture with L929 cells, while coincubation with mock-infected L929 did not have any effect. In Fig. 3 it can be seen that the major subsets of immune cells (mostly B but also T cells) were rapidly and relatively strongly activated by EMCV infection in the presence of L929 cells beyond the activation observed during EMCV infection of pure splenocyte cultures. This was especially true for activation of B cells, which, as shown in Fig. 1C and 2C, served as an early marker of primary splenocyte EMCV infection in vitro. A similar phenomenon has been reported for in vitro splenocyte infection by another unrelated virus, murine herpesvirus 68 (52).
FIG. 3.
Levels of activation of NK, T, and B cells after EMCV infection of wild-type mouse splenocytes in the presence of L929 cells. Cells were infected with EMCV (MOI = 1.0). Sample FACS profiles and absolute percentages of inactive and activated (CD69+) NK1.1+ CD3− NK cells, CD3+ T cells, and CD19+ B cells are shown 24 h after infection. Mock-infected samples are shown in panels on the far left and far right; virus-infected samples are shown in the inner panels for easy comparison.
Titration of EMCV showed that although no clear difference in viral replication between L929-mixed CD1d-KO and wild-type cultures could be observed early in the infection, markedly higher levels of EMCV were maintained at the later time points in L929-mixed CD1d-deficient cultures (Fig. 4A). However, no significant differences in IFN-γ production between knockout- and wild-type-derived splenocytes were detected after primary infection of mixed L929/splenocyte cultures (not shown).
FIG. 4.
Primary EMCV infection of wild-type and CD1d-KO mouse splenocytes coincubated with L929 cells. (A) EMCV replication in vitro. Wild-type and CD1d-KO splenocyte/L929 cocultures were infected with EMCV (MOI = 1.0). Data for four independent cultures are shown. (B) Levels of activation of T and B cells after primary EMCV infection in vitro. Sample FACS profiles and absolute percentages of inactive and activated (CD69+) NK1.1+ CD3− NK cells, CD3+ T cells, and CD19+ B cells are shown 48 h after infection. (C) Activation of T and B cells after EMCV infection in vitro. Mean percentages (± SD) of activated B and T cells 48 h after infection are shown. *, P < 0.05.
Immune cell activation observed at day 1 after initiation of EMCV infection in such mixed cultures also showed no difference between wild-type and CD1d-KO cultures (not shown). However, on day 2 the T-cell response in CD1d-KO culture was somewhat less, while B-cell activation was markedly greater (Fig. 4B and C), unlike in primary infection, potentially reflecting subsequently higher levels of viral persistence (Fig. 4A).
More marked differences in the T-cell activation profile of wild-type and CD1d-KO splenocytes emerged when these L929-mixed cultures were subjected to an additional round of infection with exogenous EMCV, which was also seen with both major T-cell populations. Differences in CD8+ cells activation in wild-type and CD1d-KO splenocytes were most pronounced (Fig. 5A and B), although CD4+ cells were also differently activated (not shown). This was closely matched by levels of IFN-γ production, which were again markedly higher in wild-type-derived cultures after an additional round of infection, while decreasing in CD1d-KO-derived cultures (Fig. 5C). Even more-pronounced differences after reinfection of wild-type and CD1d-deficient L929/splenocyte mixed cultures were observed in relation to IFN-α production (Fig. 5D). Therefore, stronger activation of T cells and higher levels of cytokine production in these reinfected wild-type cultures were reciprocally related to levels of viral growth in vitro (Fig. 4 and 5).
FIG. 5.
EMCV reinfection of wild-type and CD1d-KO mouse splenocytes coincubated with L929 cells. (A) Levels of activation of CD8+ T cells after EMCV reinfection. Sample FACS profiles and absolute percentages of inactive and activated (CD69+) CD8+ T cells are shown 24 h after reinfection with EMCV (MOI = 1.0). (B) Activation of CD8+ T cells after EMCV reinfection. Mean percentages (± SD) of activated cells 24 h after reinfection are shown. *, P < 0.05. (C) IFN-γ production after EMCV reinfection. Cocultures were infected with 1 MOI per splenocyte. Reinfection was done on day 7. Results from splenocyte/L929 cultures from representative individual mice (wild-type or CD1d-KO) are shown. (D) IFN-α production after EMCV infection and reinfection. Cocultures were infected as described and IFN-α level measured after initial infection and reinfection. Background IFN-α levels from uninfected cultures set up in parallel are subtracted.
CD1d MAb increased susceptibility of WT splenocyte cultures to EMCV infection.
L929-mixed CD1d-KO splenocyte cultures consistently showed higher levels of EMCV replication compared to wild-type-derived cultures at the later time points (Fig. 6A), which was manifested especially clearly after reinfection of such cultures (Fig. 6A and B). Notably, wild-type-derived mixed cultures treated with soluble anti-CD1d antibody maintained markedly higher levels of viral replication (200 to 600% after MAb addition), while anti-CD1d had little if any specific effect on CD1d-KO cultures (Fig. 6A and B). When anti-CD1d antibody was added to wild-type splenocytes concurrently with primary infection, EMCV titers were higher than the levels of viral replication in CD1d-KO-derived cultures during primary infection and still higher than those in wild-type cultures after reinfection of previously MAb-treated cultures (Fig. 6A). When anti-CD1d antibody was freshly added only upon reinfection (to the previously untreated wild-type and CD1d-deficient cultures), the increase of viral titers in reinfected wild-type cultures was most pronounced (Fig. 6B).
FIG. 6.
EMCV replication in wild-type and CD1d-KO mouse splenocytes coincubated with L929 cells in the presence of anti-CD1d antibody. Splenocyte/L929 cocultures were twice (days 0 and 5) infected with EMCV (MOI = 1.0) and treated with soluble anti-CD1d/isotype MAbs either concurrently with initial infection (A) or with reinfection (B).
Increased susceptibility of immune-subset-depleted splenocyte cultures to EMCV infection.
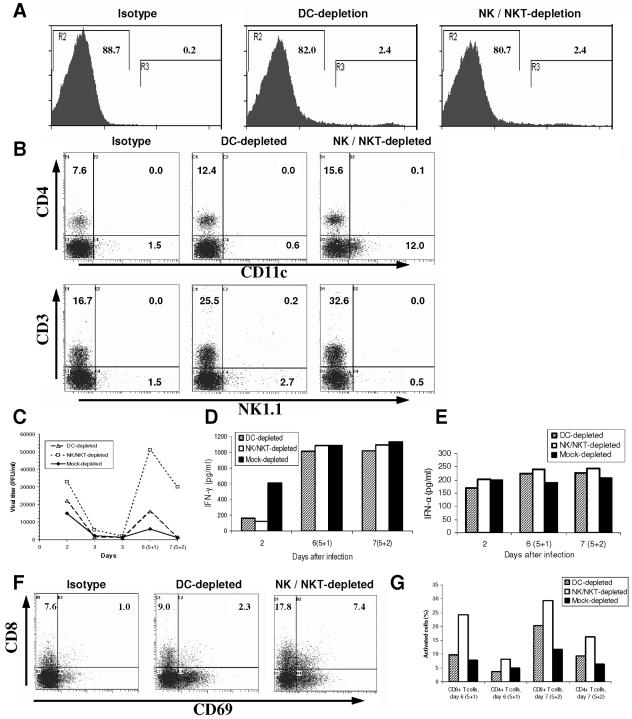
Since CD1d-KO splenocytes demonstrated a role for CD1d in resistance to EMCV replication in vitro (Fig. 1 to 5), we next determined the contribution of distinct immune subsets. We used high-speed FACS sorting, thereby eliminating concern of any residual undepleted cells and of detection of positively purified cells (blocking by depleting MAb) when using magnetic beads. Even with soluble fluorescence-conjugated MAb, it was also important to avoid stimulating residual cells by antibody cross-linking through multiparameter sorting (e.g., using CD3/NK1.1, leaving single positive NK cells and conventional T cells both activated by MAb cross-linking). Therefore, positive depletion of the two subsets tested was employed (i.e., FACS sorting only negative cells) (Fig. 7A), without use of either multiparameter staining (overlapping with other retained subsets) or negative depletion (leaving lineage-specific MAbs on most cells). Therefore, for DC we employed CD11c and the DC-specific marker 33D1 (61), thereby determining whether loss of all DC subsets (Fig. 7A and B) was critical in the antiviral response.
FIG. 7.
EMCV reinfection of immune cell-depleted splenocytes cocultured with L929 cells. Splenocytes were depleted with anti-DC MAb CD11c and 33D1 (61) or anti-NK/NKT-cell MAb (NK1.1 and Ly49b) by high-speed FACS (MoFlo). Splenocyte/L929 cocultures were infected with EMCV (MOI = 1.0) on days 0 and 5. (A) Splenocyte immune cell depletion. Splenocytes were stained with MAb to DC or NK/NKT, and negative cells (gate “R2”) were collected from high-speed FACS sorting with the percentage shown. (B) Levels of splenocyte immune cell subsets following depletion and EMCV infection. Representative FACS profiles of splenocyte immune cell subsets following depletions and EMCV infection. Upper panels: stained for CD4 and DC. Lower panels: stained for NK, NKT, and T cells. Cultures were infected with EMCV for 48 h. (C) EMCV replication in immune cell-depleted splenocytes. Viral titers shown are from individual representative cultures. (D) IFN-γ production after EMCV reinfection of immune cell-depleted splenocytes. Results of individual representative cultures from same experiment as that shown in panel A. (E) IFN-α production after EMCV reinfection of immune cell-depleted splenocytes. Results of individual representative cultures from same experiment as that shown in panel A. Background IFN-α levels from uninfected cultures were subtracted. (F) Levels of activation of CD8+ T cells after EMCV reinfection of immune cell-depleted splenocytes. Sample FACS profiles and absolute percentages of inactive and activated (CD69+) CD8+ T cells are shown at peak (48 h) after reinfection with EMCV from the same experiment as that shown in panel A. (G) Activation of CD4+ and CD8+ T cells after EMCV reinfection of immune cell-depleted splenocytes. Percentages of activated T cells within CD4+ and CD8+ subsets 24 and 48 h after reinfection from same experiment as that shown in panel A are shown.
We have shown that unlike the case with CD1 KO mice (22), loss of invariant NKT cells alone (Jα18 KO mice) is not sufficient to impair the antiviral response in vivo (21), and there is currently no way to only deplete both invariant and “noninvariant” CD1d-dependent T cells. Nearest approaches either also deplete NK cells or activate NKT cells first, thereby changing the immune cell environment (4, 11, 15, 25, 29, 47, 58). Hence, we employed two different MAb (NK1.1 and Ly49b) to efficiently deplete both NK and NKT populations (Fig. 7A and B).
Effective depletion of either DC or NK/NKT cells was further documented in the corresponding cultures post-EMCV infection (Fig. 7B). Following infection there was a small increase in CD11c and NK1.1 expression (to 0.6% and 0.5%, respectively, from undetectable), as expected for these activation markers. More interestingly, in the NK/NKT-cell-depleted cultures, CD11c+ cells markedly increased (Fig. 7B) (to 12.0% versus 1.5% for undepleted and 0.6% for DC depleted). Since very few of these cells coexpressed CD4, most of these appear to be DC. CD4+ cells also increased by twofold in both depleted culture types. Whether each cell type proliferated in response to EMCV or was selectively spared from virus-mediated cell death in the context of the corresponding depletions is unclear. Significantly, however, the large numbers of DC-like cells were apparently unable to compensate for the profound effect of lack of NK/NKT cells on viral responses in these cultures.
Notably, splenocyte-L929 mixed cultures depleted of DC or NK and NKT cells maintained higher levels of viral replication in primary and secondary infection (Fig. 7C), most profoundly in secondary infection (2-fold for DC; 10-fold for NK/NKT cells), compared to mock-depleted cultures (Fig. 7C). Furthermore, systemic early IFN-γ levels were low (both with DC or NK/NKT-cell depletion) whereas IFN-α (DC only) was only modestly reduced (Fig. 7D and E), indicating that the majority of IFN-α in this infection was coming from other cell types.
T cells were found to be actually most active without both NK and NKT cells (Fig. 7F and G), rather than after DC depletion (modest increase, particularly in CD8+ cells but only at 2 days reinfection) (Fig. 7G). The responses were most pronounced for CD8+ T cells (Fig. 7F and G). Apparently, loss of NK/NKT cells so affected the response (notably, more than CD1d KO alone) that T cells actually became more activated. Therefore, loss of both NK and NKT cells, but not of all DC, severely impaired the antiviral response in vitro.
Increased susceptibility of CD1d-KO mice to infection with EMCV in vivo coupled with lower levels of lymphocyte activation and IFN-α production.
To determine whether viral replication and the immune response of CD1d-KO mice were similarly impacted by infection in vivo, mice were infected with EMCV-D as described previously (21, 22). Levels of virus and IFN-α and IFN-γ production in the sera were evaluated. Splenocytes were isolated from infected animals and the level of lymphocyte activation assessed ex vivo as in vitro. Representative results from three separate in vivo experiments are summarized in Fig. 8 and 9. The levels of EMCV-D in animal sera were found to be markedly higher in CD1d-KO mice (Fig. 8A). Interestingly, highest levels of CD8+ T-cell activation were found with least systemic viral load and vise versa, and viremia was inversely correlated with the levels of CD8+ T-cell activation with each individual animal (Fig. 8A, B, and C). Therefore, similarly to the above in vitro data, CD8+ T lymphocytes isolated from EMCV-infected wild-type animals showed uniformly higher activation than the equivalent cells isolated from EMCV-infected CD1d-KO mice (Fig. 8B and C). These differences were most pronounced at early points of infection when CD69 levels were highest (Fig. 8B and C) but were maintained until later points (not shown).
FIG. 8.
EMCV infection of wild-type and CD1d-KO mice in vivo. (A) EMCV replication in vivo. Animal sera drawn from wild-type and CD1d-KO C57BL/6 mice after 48 h of EMCV infection were used to determine viral titers. Data shown are from sera of each experimental group, which consisted of three wild-type (WT1 to WT3) and three CD1d-KO (CD1d-KO1 to CD1d-KO3) 4-week-old animals. (B) Levels of activation of CD8+ T cells after EMCV infection in vivo. FACS profiles and absolute percentages of inactive and activated (CD69+) CD8+ T cells are shown 48 h after infection with EMCV in vivo. Data are from the same animals as in panel A. (C) Activation of CD8+ T cells after EMCV infection in vivo. Percentages of activated cells 48 h after infection in vivo are shown. Data are from the same animals as in panel A.
FIG. 9.
Lymphocyte activation following EMCV infection of wild-type and CD1d-KO mice in vivo. (A) Levels of activation of T and B cells after EMCV infection in vivo. Sample FACS profiles and absolute percentages of inactive and activated (CD69+) CD4+ T cells and CD19+ B cells are shown 48 h after viral infection. Uninfected freshly isolated wild-type splenocytes are used as a control. (B) Activation of CD8+ and CD4+ T cells after EMCV infection in vivo. Percentages of activated cells 48 h after infection are shown. Data are summarized from several separate experimental groups for each data point. **, P < 0.01. Data are from the same animals as in panel A. (C) IFN-α production after EMCV infection in vivo. Levels of IFN-α were measured in the sera of experimental animals 48 h after viral infection. Background IFN-α levels from the sera of uninfected cultures assayed in parallel are subtracted. Data are from the same animals as in panel A.
The lower level of activation of immune cells in CD1d-KO EMCV-infected animals was also observed for both T cells and B cells (Fig. 9A and B). The overall level of T-cell activation was higher in EMCV-infected wild-type animals, both for CD8+ and CD4+ populations, and was consistently observed in different experimental groups (Fig. 8B and C and A and B). However, no difference of activation levels was seen for cells stained with DC marker CD11c or monocyte marker F4/80 (not shown). In addition, increased viremia did not correspond to any detectable changes in the levels of systemic IFN-γ (not shown), as previously found in vivo (22). In contrast, the levels of IFN-α were notably elevated in the sera of wild-type mice upon EMCV infection, compared to those of CD1d-KO animals, which displayed little if any systemic IFN-α (Fig. 9C), as with mock-infected control animals (not shown).
DISCUSSION
CD1d-restricted “NKT” cells are known to mediate resistance to a number of infections. However, their role in response to infectious disease differs from system to system depending on the nature of the infectious agent, genetic background, etc. CD1d-KO mice, which lack CD1d and consequently all CD1d-restricted T cells, are substantially more sensitive to infection with a natural murine pathogen, EMCV, than wild-type controls, whereas animals lacking only classic invariant NKT cells are not (21, 22). Therefore, we further investigated the contribution of the CD1d system to resistance against acute viral infection.
To dissect targets of the CD1d system in the response to EMCV, splenocytes from CD1d-KO mice were infected with EMCV in vitro. EMCV declined in titer but persisted in such cultures for over a week, inducing steadily increasing amounts of IFN-γ. Both wild-type and CD1d-KO splenocyte cultures showed a substantial resistance to EMCV infection (∼70% viable ± EMCV at 7 days), with steadily diminishing viral titer from day 1, although virus persisted in CD1d-KO cultures to a significantly higher degree. Similarly, it has been reported that primary mouse T cells are relatively EMCV resistant (42). At the same time, infection with EMCV in vitro resulted in strong stimulation of NK cells at comparable levels in both wild-type and CD1d-KO cultures. It is possible that NK cells are important for expeditious resolution of EMCV infection in vitro, as previously shown in vivo (63). Overall there was no marked difference detectable between T-, B-, and NK-cell activation during primary EMCV infection in wild-type and CD1d-KO splenocyte cultures. This corresponded to similar levels of IFN-γ production between these two groups. The finding that B cells were activated early in both WT and CD1d-KO cultures in vitro suggests that B cells might serve as indicators of viral infections, such effects not necessarily being limited to lymphotrophic viral agents (52, 53). However, while CD1d-KO mouse B-cell activation was greater in vitro, B-cell responses were deficient in vivo, as were T cells both in vivo and in vitro. This may reflect a limitation of the EMCV in vitro model.
Since it appeared possible that EMCV infection of splenocytes in vitro was partially blocked by virus-cell interaction factors, such as primary cell susceptibility (viral entry, replication, release, etc.) as well as in vitro immune responses, we attempted to more closely mimic infection in vivo by reinfecting cultures with EMCV several days after primary infection. Interestingly, there was again a noticeable difference in viral persistence, with CD1d-KO cultures showing higher levels of virus, consistent with increased virus-mediated disease observed during CD1d-KO mouse infection in vivo (21, 22). Viral expression was inversely correlated with IFN-γ levels, especially late in the infection process. Moreover, CD4+ and CD8+ T-cell activation was markedly stronger during secondary infection of wild-type cultures. Thus, it appears that CD1d can control the resistance to viral infection in vitro and that this mechanism could function through activation of conventional T cells.
If the difference in EMCV titer between wild-type and CD1d-KO splenocyte infections observed after reinfection was related to processes reflecting multicycle infection, similar to the in vivo situation, then addition of the productively EMCV-infectible mouse cell line L929 would be expected to induce similar effects. Such a difference was indeed observed in mixed splenocyte/L929 cultures, with even the resolution of primary EMCV infection being delayed in such mixed CD1d-KO cultures compared to results with the wild type. Moreover, upon reinfection with EMCV, CD1d-KO cultures showed lower levels of T-cell activation and IFN-α and -γ production and higher levels of viral replication. Anti-CD1d MAb, which could inhibit CD1d-directed immune responses, markedly enhanced EMCV proliferation in L929/mixed wild-type-derived cultures, to levels similar to those generated in CD1d-KO-derived L929/mixed splenocyte cultures.
The ability to mirror in vivo effects of acute viral infection in vitro permitted us to begin to investigate contributing immune cell subsets. Unfortunately, it is not currently known how to selectively deplete both invariant and noninvariant NKT populations (some of which are indeed NK1.1 negative [4, 14, 16, 20, 25, 29]) without also depleting NK cells and avoiding activation of depleted cells prior to their depletion and/or of at least some remaining cells. Also, Jα18 KO mice are as resistant to EMCV-D as WT mice (21). Thus, to further initially exploit the in vitro infection system, we determined the impact of removal of all DC subsets or of NK and NKT. Interestingly, depletion of DC significantly but only modestly reduced viral clearance. A much more profound defect was seen when both NK and NKT cells were depleted. This effect was also broadly consistent with but greater than CD1d blocking or genetic loss of CD1d (CD1d-KO), where T-cell responses were dampened. The current data support previous work that NK cells limit early EMCV replication in vivo and in vitro (22, 63). Apparently absence of NK as well as NKT cells caused overwhelming viral infection, leading in this case to greater residual immune activation. Notably, although early (2 day) IFN-α was slightly lower from DC-depleted cultures, there was no defect later, suggesting that DC subsets are not the only or even major source of EMCV-induced IFN-α. While plasmacytoid DC are well known as the major source of immediate IFN-α for certain antiviral and other responses, this is now known to not always be the case, and other cells can also rapidly contribute (65). It may well be that most cells each contribute modestly to IFN-α production, as can occur in many virus infections. Interestingly, depletion of either DC or NK/NKT cells substantially inhibited early IFN-γ production, suggesting DC-NK/NKT-cell interactions are important in primary anti-EMCV cytokine responses, as also recently shown for other viral infections (10, 65). T-cell activation was actually greatest in the absence of NK/NKT. This may reflect a mechanism distinct from the defect in T-cell responses of CD1d-KO mice, since viral replication was overwhelming. Alternatively, CD1d-dependent T cells which lack the NK markers used may contribute to this priming. Such CD1d-reactive CD161-negative cells have been described (4, 14, 16, 20, 25, 29). Taken together, previous and current data demonstrate that both NK and NKT cells can contribute critically to even secondary anti-EMCV responses, whereas, perhaps surprisingly, no DC appear to be essential for either cytokine or CD8 T-cell responses to EMCV infection in vitro. In the future, it will be possible to combine such cell type depletions and additions from appropriate genetically deficient animals to identify all mechanistic contributions to control of virus replication in this and similar in vitro systems.
EMCV infection in vivo demonstrated differences between wild-type and CD1d-KO animals similar to those in vitro. The levels of viremia in the latter were higher, which was inversely correlated with the production of IFN-α. Levels of lymphocyte activation, notably of both CD4+ and CD8+ T cells, were also impaired in CD1d-KO EMCV-infected mice compared to those in wild-type animals. Consistent with the observations made in the present study, it was reported previously that in vivo resistance to EMCV infection is dependent on both CD4+ and CD8+ T cells (43, 44).
It is thought that a critical role of CD1d-restricted T cells in EMCV infection resolution in vivo is mediated through activation of NK cells (22), apparently via induction of APC IL-12, intimately connected with IFN-γ production (18, 19). The data reported here suggest an additional level of resistance. It is plausible to suggest that the NK-cell response to viral infection in vitro may be less dependent on CD1d-restricted T cells and that EMCV infection is capable of self-resolving in a single-cycle/nonproductive round, possibly partly via IFN-γ-dependent mechanisms. However, when a multicycle infection, more reflective of the in vivo situation, was established in vitro, CD1d-deficient cultures were at a greater disadvantage. These cultures supported higher viral levels, synthesized less IFN-γ and IFN-α, and showed lack of T cell activation at different points during the infection process, particularly following reinfection. A similar effect of suppressed immune activation in CD1d-KO animals was observed upon EMCV infection in vivo, which was coupled with reduced levels of IFN-α production.
Thus, it is plausible that CD1d-restricted T cells and/or CD1d-expressing APC serve as important mediators of antiviral T-cell responses, especially after the rapid initial productive viral infection round. Furthermore, their role may be essential for the eventual suppression of viral replication, since T-cell-mediated responses were previously shown to confer protection against EMCV infection in vivo (43, 44). The differences between wild-type and CD1d-KO mouse resistance are greatest at early-to-intermediate stages of the infectious cycle, consistent with rapid innate immune-like effects attributed to the CD1d system. In addition to antiviral effects of CD1d-restricted T cells, CD1d antibody cross-linking results in stimulation of CD1d+ APC to produce substantial amounts of proinflammatory IL-12 (62). Whether this reflects a physiological effect of CD1d-restricted T cells or as-yet-undefined ligands remains to be seen.
A differing set of observations has been made for LMCV infection, where the CD1d system does not play a critical role in the control of viral replication in vivo, and in fact, cytokine production is enhanced and viral RNA is cleared faster in CD1-deficient animals (48, 51). Furthermore, long-term loss of NKT cells is observed during LMCV infection, which proceeds in a CD1d-independent manner, and is likely a normal component of the host postviral response (37). Yet another situation is found in respiratory syncytial virus infection (33). In this case, a delay of viral clearance was observed in CD1d-deficient animals, but the effect was dependent on a number of factors, including the genetic background, which were important for both the resolution of infection and associated immunopathogenesis.
Interestingly, the link between NK-cell-dependent innate responses and T-cell adaptive responses has been established in a nonviral model. Specifically, tumor cell lines in which the surface expression of MHC class I is downregulated have been shown to activate NK cells, which then leads to the induction of CD8+-dependent memory response via priming of DC (39).
Collectively, there is evidence for various roles of the CD1d-“NKT” system in resolution of various human and model viral infections. The range of CD1d involvement in disease caused by each viral pathogen presumably reflects differences in antiviral strategies employed during the course of different model infections, with the resolution of picornavirus (as here), hepadnavirus, and herpesvirus infections seemingly most dependent on the CD1d system. Reciprocally, the recent findings of specific down-regulation of CD1d by Kaposi’s sarcoma-associated herpesvirus and HIV support the importance of CD1d in resistance to human viral pathogens (17, 31, 49).
Acknowledgments
We particularly thank Nancy Bigley (Dayton, OH) for providing the initial EMCV-D stock and advice, as well as Michael Brenner, Brian Wilson, and other colleagues. We also thank Vasilis Toxavidis and John Tigges of the BIDMC FACS Core for expert high-speed FACS sorting. We also thank Kirin, Inc., Japan, for kindly providing α-GalCer.
The work was supported by NIH R01 DK 066917 (M.A.E.) and NIH R01 42955 (S.P.B.).
REFERENCES
- 1.Ashkar, A. A., and K. L. Rosenthal. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek, H. S., and J. W. Yoon. 1990. Role of macrophages in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J. Virol. 64:5708-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron, J. L., L. Gardiner, S. Nishimura, K. Shinkai, R. Locksley, and D. Ganem. 2002. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 16:583-594. [DOI] [PubMed] [Google Scholar]
- 4.Behar, S. M., and S. Cardell. 2000. Diverse CD1d-restricted T cells: diverse phenotypes, and diverse functions. Semin. Immunol. 12:551-560. [DOI] [PubMed] [Google Scholar]
- 5.Behar, S. M., T. A. Podrebarac, C. J. Roy, C. R. Wang, and M. B. Brenner. 1999. Diverse TCRs recognize murine CD1. J. Immunol. 162:161-167. [PubMed] [Google Scholar]
- 6.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendelac, A., O. Lantz, M. E. Quimby, J. W. Yewdell, J. R. Bennink, and R. R. Brutkiewicz. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science 268:863-865. [DOI] [PubMed] [Google Scholar]
- 8.Bendelac, A., M. N. Rivera, S. H. Park, and J. H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535-562. [DOI] [PubMed] [Google Scholar]
- 9.Benton, K. A., J. A. Misplon, C.-Y. Lo, R. R. Brutkiewicz, S. A. Prasad, and S. L. Epstein. 2001. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or γδ T cells. J. Immunol. 166:7437-7445. [DOI] [PubMed] [Google Scholar]
- 10.Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 11.Brigl, M., and M. B. Brenner. 2004. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22:817-890. [DOI] [PubMed] [Google Scholar]
- 12.Brossay, L., N. Burdin, S. Tangri, and M. Kronenberg. 1998. Antigen-presenting function of mouse CD1: one molecule with two different kinds of antigenic ligands. Immunol. Rev. 163:139-150. [DOI] [PubMed] [Google Scholar]
- 13.Brossay, L., M. Chioda, N. Burdin, Y. Koezuka, G. Casorati, P. Dellabona, and M. Kronenberg. 1998. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brossay, L., S. Tangri, M. Bix, S. Cardell, R. Locksley, and M. Kronenberg. 1998. Mouse CD1-autoreactive T cells have diverse patterns of reactivity to CD1+ targets. J. Immunol. 160:3681-3688. [PubMed] [Google Scholar]
- 15.Brutkiewicz, R. R., Y. Lin, S. Cho, Y. K. Hwang, V. Sriram, and T. J. Roberts. 2003. CD1d-mediated antigen presentation to natural killer T (NKT) cells. Crit. Rev. Immunol. 23:403-419. [DOI] [PubMed] [Google Scholar]
- 16.Chiu, Y. H., J. Jayawardena, A. Weiss, D. Lee, S. H. Park, A. Dautry-Varsat, and A. Bendelac. 1999. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 189:103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho, S., K. S. Knox, L. M. Kohli, M. A. Exley, and R. R. Brutkiewicz. 2005. Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology 337:242-252. [DOI] [PubMed] [Google Scholar]
- 18.Curiel, R. E., K. M. Mason, T. D. Dryden, M. J. Maurer, and N. J. Bigley. 1998. Cytokines produced early in picornavirus infection reflect resistance or susceptibility to disease. J Interferon Cytokine Res. 18:587-596. [DOI] [PubMed] [Google Scholar]
- 19.Curiel, R. E., M. H. Miller, R. Ishikawa, D. C. Thomas, and N. J. Bigley. 1993. Does the gender difference in interferon production seen in picornavirus-infected spleen cell cultures from ICR Swiss mice have any in vivo significance? J. Interferon Res. 13:387-395. [DOI] [PubMed] [Google Scholar]
- 20.Eberl, G., R. Lees, S. T. Smiley, M. Taniguchi, M. J. Grusby, and H. R. MacDonald. 1999. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 162:6410-6419. [PubMed] [Google Scholar]
- 21.Exley, M. A., N. J. Bigley, O. Cheng, S. M. Tahir, S. T. Smiley, Q. L. Carter, H. F. Stills, M. J. Grusby, Y. Koezuka, M. Taniguchi, and S. P. Balk. 2001. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J. Leukoc. Biol. 69:713-718. [PubMed] [Google Scholar]
- 22.Exley, M. A., N. J. Bigley, O. Cheng, A. Shaulov, S. M. Tahir, Q. L. Carter, J. Garcia, C. Wang, K. Patten, H. F. Stills, F. W. Alt, S. B. Snapper, and S. P. Balk. 2003. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology 110:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Exley, M., J. Garcia, S. P. Balk, and S. Porcelli. 1997. Requirements for CD1d recognition by human invariant Valpha24+ CD4−CD8− T cells. J. Exp. Med. 186:109-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Exley, M., J. Garcia, S. B. Wilson, F. Spada, D. Gerdes, S. M. Tahir, K. T. Patton, R. S. Blumberg, S. Porcelli, A. Chott, and S. P. Balk. 2000. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 100:37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Exley, M. A., and M. J. Koziel. 2004. To be or not to be NKT: natural killer T cells in the liver. Hepatology 40:1033-1040. [DOI] [PubMed] [Google Scholar]
- 26.Exley, M. A., S. M. Tahir, O. Cheng, A. Shaulov, R. Joyce, D. Avigan, R. Sackstein, and S. P. Balk. 2001. A major fraction of human bone marrow lymphocytes are Th2-like CD1d-restricted T cells that can suppress mixed lymphocyte responses. J. Immunol. 167:5531-5534. [DOI] [PubMed] [Google Scholar]
- 27.Fuss, I. J., F. Heller, M. Boirivant, F. Leon, M. Yoshida, S. Fichtner-Feigl, Z. Yang, M. Exley, A. Kitani, R. S. Blumberg, P. Mannon, and W. Strober. 2004. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Investig. 113:1490-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glass, W. G., K. Subbarao, B. Murphy, and P. M. Murphy. 2004. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 173:4030-4039. [DOI] [PubMed] [Google Scholar]
- 29.Godfrey, D. I., and M. Kronenberg. 2004. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Investig. 114:1379-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grubor-Bauk, B., A. Simmons, G. Mayrhofer, and P. G. Speck. 2003. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J. Immunol. 170:1430-1434. [DOI] [PubMed] [Google Scholar]
- 31.Hegde, N. R., and D. C. Johnson. 2005. A seek-and-hide game between CD1-restricted T cells and herpes viruses. J. Clin. Investig. 115:1146-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hobbs, J. A., S. Cho, T. J. Roberts, V. Sriram, J. Zhang, M. Xu, and R. R. Brutkiewicz. 2001. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J. Virol. 75:10746-10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, T. R., S. Hong, L. Van Kaer, Y. Koezuka, and B. S. Graham. 2002. NK T cells contribute to expansion of CD8(+) T cells and amplification of antiviral immune responses to respiratory syncytial virus. J. Virol. 76:4294-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joyce, S. 2000. Natural T cells: cranking up the immune system by prompt cytokine secretion. Proc. Natl. Acad. Sci. USA 97:6933-6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakimi, K., L. G. Guidotti, Y. Koezuka, and F. V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy, O., J. S. Orange, P. Hibberd, S. Steinberg, P. LaRussa, A. iWeinberg, S. B. Wilson, A. Shaulov, G. Fleisher, R. S. Geha, F. A. Bonilla, and M. Exley. 2003. Disseminated varicella infection due to the vaccine strain of varicella-zoster virus, in a patient with a novel deficiency in natural killer T cells. J. Infect. Dis. 188:948-953. [DOI] [PubMed] [Google Scholar]
- 37.Lin, Y., T. J. Roberts, C. R. Wang, S. Cho, and R. R. Brutkiewitz. 2005. Long-term loss of canonical NKT cells following an acute virus infection. Eur. J. Immunol. 35:879-889. [DOI] [PubMed] [Google Scholar]
- 38.Loh, J., D. T. Chu, A. K. O'Guin, W. M. Yokoyama, and H. W. Virgin IV. 2005. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J. Virol. 79:661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mocikat, R., H. Braumüller, A. Gumy, O. Egeter, H. Ziegler, U. Reusch, A. Bubeck, J. Louis, R. Mailhammer, G. Riethmüller, U. Koszinowski, and M. Röcken. 2003. Natural killer cells activated by MHC class ILow targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 19:561-569. [DOI] [PubMed] [Google Scholar]
- 40.Motsinger, A., A. Azimzadeh, A. K. Stanic, R. P. Johnson, L. Van Kaer, S. Joyce, and D. Unutmaz. 2003. Identification and simian immunodeficiency virus infection of CD1d-restricted macaque natural killer T cells. J. Virol. 77:8153-8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motsinger, A., D. W. Haas, A. K. Stanic, L. Van Kaer, S. Joyce, and D. Unutmaz. 2002. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J. Exp. Med. 195:869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neal, Z. C., J. S. Harms, M. R. Hill, and G. A. Splitter. 2002. Encephalomyocarditis and Mengo viruses productively infect murine T-lymphocyte cell lines but not fresh ex vivo derived T lymphocytes. Viral Immunol. 15:155-163. [DOI] [PubMed] [Google Scholar]
- 43.Neal, Z. C., and G. A. Splitter. 1995. Picornavirus-specific CD4+ T lymphocytes possessing cytolytic activity confer protection in the absence of prophylactic antibodies. J. Virol. 69:4914-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neal, Z. C., and G. A. Splitter. 1998. Protection against lethal encephalomyocarditis virus infection in the absence of serum-neutralizing antibodies. J. Virol. 72:8052-8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nichols, K. E., J. Hom, S. Y. Gong, A. Ganguly, C. S. Ma, J. L. Cannons, S. G. Tangye, P. L. Schwartzberg, G. A. Koretzky, and P. L. Stein. 2005. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat. Med. 11:340-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasquier, B., L. Yin, M. C. Fondanèche, F. Relouzat, C. Bloch-Queyrat, N. Lambert, A. Fischer, G. de Saint-Basile, and S. Latour. 2005. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J. Exp. Med. 201:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porcelli, S. A., and R. L. Modlin. 1999. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297-329. [DOI] [PubMed] [Google Scholar]
- 48.Roberts, T. J., Y. Lin, P. M. Spence, L. Van Kaer, and R. R. Brutkiewicz. 2004. CD1d1-dependent control of the magnitude of an acute antiviral immune response. J. Immunol. 172:3454-3461. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez, D. J., J. E. Gumperz, and D. Ganem. 2005. Regulation of CD1d expression and function by a herpesvirus infection. J. Clin. Investig. 115:1369-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sköld, M., and S. M. Behar. 2003. Role of CD1d-restricted NKT cells in microbial immunity. Infect. Immun. 71:5447-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spence, P. M., S. Venkataran, L. van Kaer, J. A. Hobbs, and R. R. Brutkiewicz. 2001. Generation of cellular immunity to lymphocytic choriomeningitis virus is independent of CD1d1 expression. Immunology 104:168-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevenson, P. G., and P. C. Doherty. 1999. Non-antigen-specific B-cell activation following murine gammaherpesvirus infection is CD4 independent in vitro but CD4 dependent in vivo. J. Virol. 73:1075-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takamatsu, H., M. S. Denyer, C. Oura, A. Childerstone, J. K. Andersen, L. Pullen, and R. M. Parkhouse. 1999. African swine fever virus: a B cell-mitogenic virus in vivo and in vitro. J. Gen. Virol. 80:1453-1461. [DOI] [PubMed] [Google Scholar]
- 54.Taniguchi, M., M. Harada, S. Kojo, T. Nakayama, and H. Wakao. 2003. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21:483-513. [DOI] [PubMed] [Google Scholar]
- 55.Unutmaz, D. 2003. NKT cells and HIV infection. Microb. Infect. 5:1041-1047. [DOI] [PubMed] [Google Scholar]
- 56.van der Vliet, H. J., B. M. von Blomberg, M. D. Hazenberg, N. Nishi, S. A. Otto, B. H. van Benthem, M. Prins, F. A. Claessen, A. J. van den Eertwegh, G. Giaccone, F. Miedema, R. J. Scheper, and H. M. Pinedo. 2002. Selective decrease in circulating V alpha 24+V beta 11+ NKT cells during HIV type 1 infection. J. Immunol. 168:1490-1495. [DOI] [PubMed] [Google Scholar]
- 57.van der Vliet, H. J., J. W. Molling, B. M. von Blomberg, W. Kolgen, A. G. Stam, T. D. de Gruijl, C. J. Mulder, H. L. Janssen, N. Nishi, A. J. van den Eertwegh, R. J. Scheper, and C. J. van Nieuwkerk. 2005. Circulating Valpha24+Vbeta11+ NKT cell numbers and dendritic cell CD1d expression in hepatitis C virus infected patients. Clin. Immunol. 114:183-189. [DOI] [PubMed] [Google Scholar]
- 58.van der Vliet, H. J., J. W. Molling, B. M. von Blomberg, N. Nishi, W. Kolgen, A. J. van den Eertwegh, H. M. Pinedo, G. Giaccone, and R. J. Scheper. 2004. The immunoregulatory role of CD1d-restricted natural killer T cells in disease. Clin. Immunol. 112:8-23. [DOI] [PubMed] [Google Scholar]
- 59.van Dommelen, S. L., and M. A. Degli-Esposti. 2004. NKT cells and viral immunity. Immunol. Cell Biol. 82:332-341. [DOI] [PubMed] [Google Scholar]
- 60.van Dommelen, S. L., H. A. Tabarias, M. J. Smyth, and M. A. Degli-Esposti. 2003. Activation of natural killer (NK) T cells during murine cytomegalovirus infection enhances the antiviral response mediated by NK cells. J. Virol. 77:1877-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Voorhis, W. C., R. M. Steinman, L. S. Hair, J. Luban, M. D. Witmer, S. Koide, and Z. A. Cohn. 1983. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J. Exp. Med. 158:126-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yue, S., A. Shaulov, R. Wang, S. Balk, and M. Exley. 2005. CD1d ligation on human monocytes directly signals rapid NF-(kappa)B activation and production of bioactive IL-12. Proc. Natl. Acad. Sci. USA 102:11811-11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White, L. L., and R. A. Smith. 1990. D variant of encephalomyocarditis virus (EMCV-D)-induced diabetes following natural killer cell depletion in diabetes-resistant male C57BL/6J mice. Viral Immunol. 3:67-76. [DOI] [PubMed] [Google Scholar]
- 64.Zeng, D., G. Gazit, S. Dejbakhsh-Jones, S. P. Balk, S. Snapper, M. Taniguchi, and S. Strober. 1999. Heterogeneity of NK1.1+ T cells in the bone marrow: divergence from the thymus. J. Immunol. 163:5338-5345. [PubMed] [Google Scholar]
- 65.Zitvogel, L., M. Terme, C. Borg, and G. Trinchieri. 2006. Dendritic cell-NK cell cross-talk: regulation and physiopathology. Curr. Top. Microbiol. Immunol. 298:157-174. [DOI] [PubMed] [Google Scholar]