Abstract
Mitochondria function as platforms for bioenergetics, nutrient metabolism, intracellular signaling, innate immunity regulators, and modulators of stem cell activity. Thus, the decline in mitochondrial functions causes or correlates with diabetes mellitus and many aging-related diseases. Upon stress or damage, the mitochondria elicit a series of adaptive responses to overcome stress and restore their structural integrity and functional homeostasis. These adaptive responses to low-level or transient mitochondrial stress promote health and resilience to upcoming stress. Beneficial effects of low-grade mitochondrial stress, termed mitohormesis, have been observed in various organisms, including mammals. Accumulated evidence indicates that treatments boosting mitohormesis have therapeutic potential in various human diseases accompanied by mitochondrial stress. Here, we review multiple cellular signaling pathways and interorgan communication mechanisms through which mitochondrial stress leads to advantageous outcomes. We also discuss the relevance of mitohormesis in obesity, diabetes, metabolic liver disease, aging, and exercise.
Keywords: Mitochondria, stress, hormesis, obesity, diabetes, aging
Graphical Abstract
INTRODUCTION
Mitochondria are crucial organelles for cellular energy generation. In addition, the mitochondria modulate various cellular functions, such as the biosynthesis of metabolic intermediates, cellular signaling, cell death, cellular proliferation, and calcium homeostasis.1,2 In regard to nutrient metabolism, the mitochondria are important as the tricarboxylic acid cycles and fatty acid oxidation occur in the mitochondrial matrix. As precise nutrient metabolism largely relies on mitochondrial functions, mitochondrial dysfunction has been linked to metabolic disorders, such as type 2 diabetes. A reduction in mitochondrial fatty acid oxidation culminates in the accumulation of toxic lipid metabolic intermediates, such as medium-chain fatty acids, long-chain fatty acids, and carnitine derivatives, activating signaling pathways that disrupt insulin signaling.3 On the other hand, reactive oxygen species (ROS) are produced as a byproduct of mitochondrial oxidative phosphorylation. Excessive production of mitochondrial ROS damages proteins, membranes, and DNA. Such mitochondrial oxidative stress has been proposed to be associated with various diseases, such as diabetes, cancer, and neurodegenerative diseases, including Alzheimer’s and Parkinson’s diseases.4,5,6,7 Moreover, impairment of mitochondrial activity is associated with aging.7,8,9
Mitochondria face various external and internal stresses, including reduced mitochondrial electron transport, limited nutrient supply, hypoxia, hypothermia or heat, exposure to xenobiotics and toxins, and alteration of signaling pathways, such as the insulin and IGF-1 pathways, which culminate in mitochondrial dysfunction and defective ATP generation.10 In studies of lower organisms, such as yeast, worm, and fly, many researchers repeatedly observed a phenomenon where the inhibition of mitochondrial respiration extended life span and retarded aging.11 This beneficial effect of mitochondrial stress is termed mitohormesis, a compound word for mitochondria and hormesis. “Hormesis” means a biphasic response to toxins and stressors characterized by a low-dose response opposite to that observed at a high dose. The concept of hormesis was first discussed by the Swiss physician Paracelsus in the 16th century, who stated, “Only the dose makes the poison.”12 The term “mitohormesis” appeared in 2006 as a theory to define hormetic responses that promote health and vitality induced by sublethal mitochondrial stress.13 Subsequently, accumulated evidence has demonstrated that low-level mitochondrial stress is advantageous rather than harmful to organismal health (Fig. 1).10,14 In this review, we outline current knowledge of mitohormesis from the perspectives of metabolism, longevity, and aging.
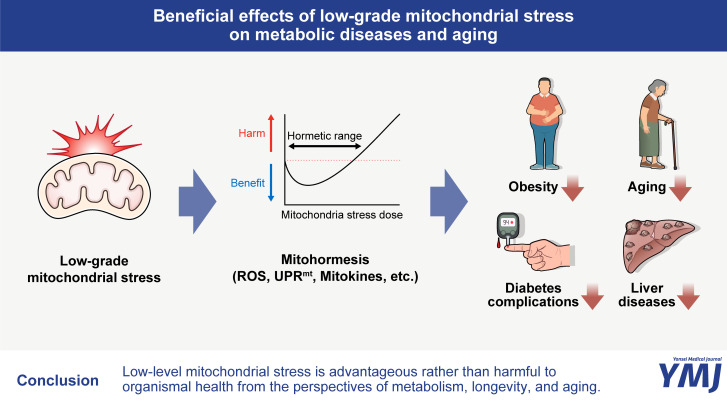
Fig. 1. Beneficial effects of low-grade mitochondrial stress on metabolic diseases and aging. Repeated exposure to low levels of mitochondrial stress induces a wide range of cytosolic and nuclear adaptive responses, which contribute to building resilience against higher levels of mitochondrial stress. These mitohormetic responses have been demonstrated to confer several health benefits, including the reduction of obesity and diabetes complications, delayed aging, and improved outcomes in the management of liver diseases. ROS, reactive oxygen species; UPRmt, mitochondrial unfolded protein response.
MECHANISMS UNDERLYING MITOHORMESIS
Repeated exposure to low levels of mitochondrial stress induces a wide range of cytosolic and nuclear adaptive responses that build resilience against higher levels of mitochondrial stress. The mechanisms and mediators of these mitohormetic responses have been extensively studied in lower model organisms and are now being revealed in mammalian cells (Fig. 2).
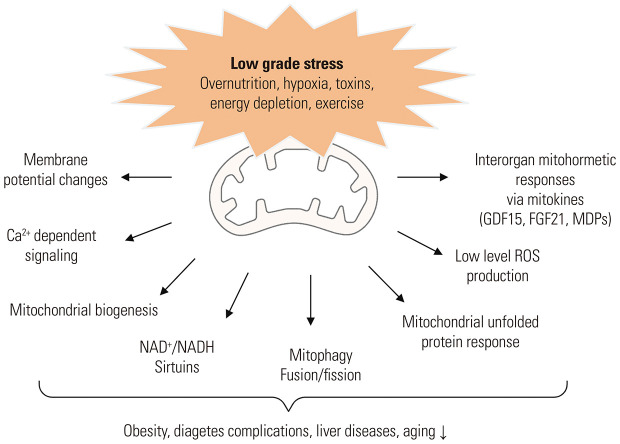
Fig. 2. Molecular and cellular mechanisms of mitohormesis. Low-grade, multiple mitochondrial stresses induce mitohormetic responses that offer protection from metabolic diseases (obesity, diabetes, and liver diseases) and aging-related pathologies. These adaptive responses include ROS production, mitochondria biogenesis and dynamics (mitophagy, mitochondrial fusion and fission), alterations in mitochondrial membrane potential, activation of cytosolic Ca2+-dependent signaling pathways, mitochondrial unfolded protein responses, and inter-organ mitochondria stress response via the release of mitokines. ROS, reactive oxygen species; MDPs, mitochondria-derived peptides.
Mitochondrial retrograde signaling
Mitochondria are thought to originate from freely-moving bacteria that succeeded in symbiosis with pre-eukaryotic cells billions of years ago.15 During endosymbiotic evolution, mitochondria transferred genetic materials to the nucleus of host cells. As a result, most (more than 1000) mitochondrial proteins are encoded by the nuclear DNA (nDNA), and mitochondrial DNA (mtDNA) only encodes 13 electron transport chain (ETC) proteins 2 rRNA and 22 tRNA molecules.16 Thus, mitonuclear communication and precise nuclear adaptive responses are essential for a successful recovery from perturbations in mitochondrial functions. An early study in the yeast Saccharomyces cerevisiae has shown that mitochondrial genetic deficits induce coordinated and complex nuclear responses that alter more than 40 genes.17 Similar retrograde signaling to induce coordinated nuclear responses has been reported in mammalian cells with mtDNA 3243 mutations associated with mitochondrial encephalopathy with lactic acidosis and stroke-like episodes.18 If so, how do damaged mitochondria signal to the cytosol and the nucleus? In yeast, leucine zipper transcriptional factors Rtg1, Rtg2, and Rtg3 mediate retrograde signaling from the mitochondria to the nucleus.19 Under normal conditions, these proteins are imported from the cytosol to the mitochondria where they are degraded. However, under mitochondrial stress, these proteins no longer enter the mitochondria. As a result, they accumulate in the cytosol and traffic to the nucleus, triggering transcriptional programs implicated in mitohormetic responses.20,21 In mammalian cells, signaling molecules and events appear to inform the cytosolic signaling system about mitochondrial stress.22 The most attractive signal is mitochondrial ROS, as its levels increase under most mitochondrial stress conditions.11 Whereas ROS at high levels is undoubtedly detrimental, ROS at low levels acts as a signaling molecule in numerous signaling pathways.23 In yeast, mitochondrial stress, induced by glucose restriction or reduced insulin/IGF1 signaling and target of rapamycin signaling, releases mitochondrial ROS and extends life span. These mitohormetic responses are blocked by antioxidant treatment, proving a critical role for mitochondrial ROS.24,25,26
Another activating signal may involve a reduction in mitochondrial membrane potential, which is commonly observed in dysfunctional mitochondria. Mitochondrial membrane depolarization can induce recruitment and activation of signaling molecules, for example, phosphatase and tension homolog-induced kinase (PINK)/Parkin in the mitochondrial autophagy (mitophagy) process.27 Another possible mediator is calcium, as the mitochondria are involved in cellular calcium homeostasis through the uptake and release of calcium. As such, mitochondrial disturbances may increase cytosolic calcium levels and modulate the activities of transcriptional factors through activation of calcium-dependent kinases and phosphatases.28 The altered mitochondrial activity could change the amount of metabolic intermediates, such as NAD+/NADH. As NAD+ is known as an obligate cofactor of important enzymes, including sirtuins and poly-ADP-ribose polymerase, altered NAD+ levels can lead to broad biological outcomes as a part of adaptation to mitochondrial disturbances.29,30 Nuclear sirtuin SIRT1 and mitochondrial sirtuin SIRT3 play an important role in mitochondrial stress defense via the deacetylation of dozens of nuclear, cytosolic, and mitochondrial proteins.31,32
Mitochondrial biogenesis, dynamics, and mitophagy
One of adaptive responses to mitochondrial dysfunction is the generation of new mitochondria, called mitochondrial biogenesis, which aims to reestablish healthy mitochondrial mass. A master regulator of this process is the transcriptional coactivator, peroxisome proliferator activated-receptor γ coactivator-1-α (PGC1α).33 PGC1α stimulates the transcription of nDNA-encoded mitochondrial enzymes and ETC proteins by acting as a coactivator of nuclear respiratory factor 1 (NRF1).33 Activation of the PGC1α-NRF1 axis promotes mtDNA transcription and replication by producing mitochondrial transcription factor A (mtTFA).33 Stimulation of mitochondrial biogenesis inevitably increases mitochondrial respiration and ROS production; PGC1α simultaneously stimulates the transcription of antioxidant enzymes, such as superoxide dismutase (SOD) and catalase, to buffer mitochondrial oxidative stress by acting as a nuclear factor erythroid-2 related factor-2 (NRF2) coactivator.34 On the other hand, dysfunctional and damaged mitochondria need to be removed by mitophagy to maintain functional mitochondria pool. As mentioned above, a major driving factor in this process is a reduction in the mitochondrial membrane potential, which hampers mitochondrial import of PINK1 and accumulates it on the outer mitochondrial membranes (OMM). PINK1 recruits E3 ubiquitin ligase Parkin onto the OMM, and Parkin, in turn, ubiquitinates OMM proteins and triggers mitophagy.35
The mitochondria constantly change their shapes through mitochondrial fusion and fission in response to the ever-changing cellular milieu, and these events are called mitochondrial dynamics.36 Mitochondrial fusion results in more interconnected networks between neighboring mitochondria, and it helps recover from mitochondrial dysfunction by allowing the exchange of DNA and metabolites and promoting complementation between damaged mitochondria.37 Mitochondrial fission facilitates the removal of dysfunctional mitochondria by mitophagy and the generation of new mitochondria.37 Mitochondrial dynamics is an efficient way to change mitochondrial functions to meet bioenergetic needs.
Mitochondrial unfolded protein responses (UPRmt)
The UPRmt is a mitohormetic response to mitochondrial proteostatic stress, which is equivalent to the UPR to endoplasmic reticulum stress.38,39,40 When unfolded or misfolded proteins accumulate in the mitochondria, mitochondrial proteases degrade these proteins into peptides of various sizes. Fragmented peptides then enter the cytosol and activate transcriptional programs via cytosolic signaling events. Representative transcription factors involved in UPRmt are activating transcription factors associated with stress-1 in Caenorhabditis elegans, c-Jun N-terminal kinase, and activating transcription factor 4 in mammals. As a result of UPRmt transcriptional responses, mitochondrial heat shock proteins, chaperones, proteases, and ETC proteins are produced to resolve mitochondrial proteotoxic stress.22 Mitochondrial ETC complexes, except for complex II, comprise proteins encoded by nDNA and mtDNA. Therefore, appropriate stoichiometric ratios between nDNA-and mtDNA-encoded ETC proteins are critical for mitochondrial respiration.41 Thus, the UPRmt response can be induced by a stoichiometric imbalance of nDNA-encoded and mtDNA-encoded ETC proteins, which is called mitonuclear protein imbalance.41,42
Multiple studies have demonstrated that the UPRmt has survival and health benefits.38,39,40 Mitochondrial stress induces UPRmt in neighboring cells or remote organs; this is called non-cell-autonomous UPRmt.43 For instance, knocking down ETC components in the nervous system can trigger UPRmt responses in the intestine and extend the life span of C. elegans.43 Beyond UPRmt, mitochondrial stress elicits multiple axes of adaptive responses called mitochondrial integrated stress responses (ISRmt), which include transcriptional upregulation of fibroblast growth factor 21 (FGF21) and growth and differentiation factor 15 (GDF15), reprograming of biosynthetic pathways (imbalance of folate-driven one-carbon metabolism, aberrant nucleotide synthesis, induction of de novo serine and glutathione synthesis), and mitochondrial biogenesis.44
Mitokines
Upon mitochondrial stress, cells release biological molecules called mitokines, which signal local mitochondrial stress to distant cells and organs. Representative mitokines are GDF15, FGF21 (nDNA-encoded), and several mitochondrial-derived peptides (MDPs, mtDNA-encoded). These mitokines have beneficial effects on metabolism and health; and therefore, they are considered a potential mediator of non-cell-autonomous mitohormetic responses.
GDF15
GDF15 is a secretory protein that belongs to the transforming growth factor beta superfamily.45 GDF15 production increases under mitochondrial stress as a part of ISR46 and leads to favorable metabolic outcomes, supporting the role of GFD15 as a mitokine.47 Several mouse models of muscle mitochondrial stress have shown increased muscle GDF15 expression and plasma GDF15 concentrations.46,48 These data suggest that under mitochondrial stress, skeletal muscle cells secrete GDF15 into the bloodstream. In mice, skeletal muscle deficiency of the mitochondrial ribosomal protein CRIF1 alleviates high-fat diet (HFD)-induced obesity and insulin resistance. These beneficial metabolic effects are mediated by skeletal muscle-released GDF15, which acts on adipose tissue and the liver to stimulate lipolysis and fatty acid oxidation.46 In addition, the administration of GDF15 can suppress food intake and obesity in HFD-fed mice and primates with spontaneous obesity by acting on the brainstem GFRAL receptor,49 although it is unclear whether this anorexic anti-obesity effect of GDF15 is relevant to mitochondrial stress. In the liver, GDF15 expression levels are elevated in chronic liver disorders, such as hepatitis, advanced cirrhosis, and hepatocellular carcinoma.50,51,52,53,54 The loss of GDF15 worsens hepatic inflammation and fibrosis induced by carbon tetrachloride (CCl4), alcohol, and fat-rich diets. Consistently, recombinant GDF15 therapy or genetic overexpression of GDF15 improves hepatic inflammatory and fibrotic phenotypes.55,56,57 As CCl4 and alcohol and fat-rich diets have been shown to induce mitochondrial stress,55,56,57 these results may support the mitohormetic effect of GDF15 in the liver. A recent paper reported that GDF15 has cardioprotective effects in a large-dose isoproterenol-induced heart failure mouse model through mitochondrial actions.58 In contrast, the production of high levels of GDF15 by cancer cells causes anorexia and cachexia,59 demonstrating the dose-dependent opposing effects (mitohormesis) of GDF15.
GDF15 has also been reported to possess anti-inflammatory properties. In a study involving mice with macrophage-specific mitoribosomal stress, reduced GDF15 expression was associated with an increase in pro-inflammatory type 1 immune responses.60 Conversely, increased GDF15 expression coincided with an increase in M2 polarization of macrophages in the adipose tissue of CRIF1-deleted mice.61 Additionally, a study by Luan, et al.62 demonstrated that the GDF15 expression in humans and mice increased during inflammatory conditions induced by bacterial and viral infections. They further revealed that GDF15 induced cardioprotective effects during acute inflammation. These findings collectively suggest that GDF15 may exert anti-inflammatory effects.
In a study involving transgenic mice overexpressing NAG-1/GDF15, female mice exhibited an extended life span, accompanied by improved metabolic parameters, including reduced body weight, enhanced glucose tolerance, and insulin sensitivity, as well as lower serum levels of insulin and IGF-1. Notably, the longevity-enhancing effect was more pronounced in mice fed a HFD.63 Hence, it is plausible that raising the GDF15 levels may extend a healthy life span, particularly in conditions of overnutrition.
FGF21
FGF21 is a liver-derived hormone that improves diabetes, insulin resistance, obesity, and hepatic steatosis in rodents,64,65 rhesus monkeys,66 and humans67 by acting through FGF receptor-1c and the coreceptor β-Klotho.68 FGF21 is also produced in other metabolic organs, such as brown and white adipose tissues and skeletal muscle, and its expression levels are upregulated under metabolic stress conditions induced by starvation,69 amino acid depletion,70 ketogenic diet,71 overnutrition or obesity,72 cold exposure,73 and exercise,74 all of which accompany mitochondrial stress. Multiple mouse models of mitochondrial stress have demonstrated an upregulation of FGF21 as a protective mechanism against mitochondrial injury and metabolic dysregulation.61,70,75,76,77,78,79,80 Mice with accumulation of dysfunctional mitochondria in the liver and skeletal muscle due to an autophagy deficit have shown obesity resistance, and FGF21 mediates this anti-obesity effect.70 Mitochondrial myopathy, caused by replicative mitochondrial helicase twinkle mutations, stimulates skeletal muscle FGF21 production and secretion, which induces starvation-like metabolic phenotypes in the liver and adipose tissues as well as systemic stress responses, such as weight loss.75,76 Similarly, in various models characterized by disrupted mitochondrial dynamics in the skeletal muscle,77 BAT,78 and liver,79 the secretion of FGF21 has shown protective effects against diet-induced obesity (DIO) and improved metabolic phenotypes. Genetic impairment of adipocytes’ oxidative phosphorylation (OxPhos) function also protects mice from DIO and insulin resistance, with the ablation of FGF21 in this model resulting in increased body weight, adiposity, and hepatic steatosis after 8 weeks on a HFD.61 Moreover, FGF21 lowers blood glucose levels by promoting glucose uptake in the muscle, heart, and brown and white adipose tissue.76 The anti-diabetic drug metformin is known to inhibit ETC complex 1,81 and the anti-diabetic effect of metformin is mediated by FGF21.82 These data suggest that FGF21 serves as a downstream mediator of the beneficial outcomes of various mitochondrial stresses.
Blood FGF21 concentrations are robustly elevated in patients with skeletal muscle respiratory chain deficits but not in those with non-mitochondrial myopathy.83 These findings indicate the diagnostic value of serum FGF21 as a biomarker of mitochondrial diseases.
MDPs
Stressed mitochondria produce and release small peptides from their mtDNAs called MDPs. Thus far, eight MDPs have been identified, most of which have beneficial roles in various aspects of health concerns.84 Humanin was the first identified human MDP having neuro-protective effects in an Alzheimer’s disease model, and is derived from a small cryptic open reading frame (ORF) within the mitochondrial 16S rRNA called MT-RNR2.85,86 Humanin treatment in rodents has shown positive effects on their glucose metabolism by improving glucose-stimulated insulin secretion and sensitivity.87,88 Subsequently, six small humanin-like peptides (SHLP) have been identified as MDPs, which are encoded in the ORFs within the same gene in which humanin is located.89 SHLP-2 and 3 have similar neuro-protective effects as those of humanin, and they improve mitochondrial metabolism and reduce apoptosis and ROS production.89,90 SHLP-2 and 3 act as insulin sensitizers by enhancing insulin-activated Akt signaling; thus, central and systemic treatment with both peptides enhances the glucose-lowering effects of insulin.89 In addition, SHLP-2 and 3 promote adipocyte differentiation in 3T3-L1 preadipocytes.89
A novel MDP named MOTS-c was recently identified.91 By inhibiting the folate cycle at the level of 5-methyl-tetrahydrofolate (5Me-THF), MOTS-c increased the cellular levels of 5-aminoimidazole-4-carboxamide ribonucleotide, an activator of AMPK, in skeletal muscle cells,91 leading to AMPK-mediated improvement of glucose and fatty acid metabolism. MOTS-c is directly involved in mitonuclear communication upon metabolic stress, as it traffics to the nucleus and elicits adaptive nuclear transcriptional programs.92 A 7-day MOTS-c infusion significantly increased glucose clearance and the insulin-stimulated glucose disposal rate in mice through increased glucose transporter 4 expression and translocation to the plasma membrane.91 Moreover, 8-week MOTS-c administration alleviated obesity and insulin resistance induced by the consumption of an HFD, aging, and ovariectomy.91,93 Following studies have demonstrated multiple beneficial effects of MOTS-c on bone biology, inflammation, pain, vascular calcification, and myocardial remodeling, where these actions appeared to be largely mediated via AMPK activation.94,95,96,97
Inter-organ mitohormetic responses
Mitochondrial stress in one organ elicits adaptive stress responses in the remote organs, and these inter-organ mitochondria stress responses have been shown to be beneficial for metabolic health and longevity (Fig. 3).
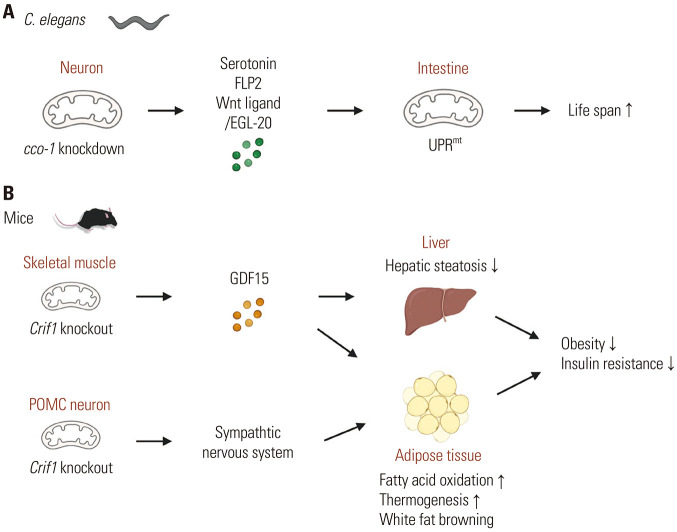
Fig. 3. Examples of inter-organ mitohormetic responses. (A) In C. elegans, deletion of mitochondrial respiration component cytochrome c oxidase-1 (cco-1) in neurons extends life span by the induction of mitochondria unfolded protein responses (UPRmt) in the intestine. Secretory factors released by stressed neurons (serotonin, FLP-2, and Wnt ligand/EGL-20) mediate these inter-organ mitochondria stress responses. (B) In mice, deletion of mitochondrial ribosomal protein CRIF1 in the skeletal muscles and hypothalamic POMC neurons improves obesity, insulin resistance, and hepatic steatosis by enhancing fatty acid oxidation and lipolysis in the adipose tissue and liver. These interorgan communication in mitochondrial stress responses occur by means of mitokine GDF15 and the sympathetic nervous system.
Mitohormetic response between peripheral metabolic organs
Outcomes of peripheral organ-specific mitochondrial stress have been extensively studied using mice models without mitochondrial ribosomal protein CRIF1 in a tissue-specific manner.46,60,61,98,99,100,101 Muscle-specific CRIF1 knockout mice have exhibited healthier metabolic phenotypes, especially in HFD conditions. They are markedly protected from HFD-induced obesity, glucose intolerance, insulin resistance, and hepatic steatosis.46 These favorable metabolic outcomes are largely mediated by GDF15, which acts on the liver and adipose tissues and reduces fat overload via enhanced fatty acid oxidation and lipolysis.46 Likewise, liver-specific CRIF1 knockout mice are less obese, more insulin-sensitive in HFD-fed conditions, and show higher energy expenditure and adipose tissue thermogenesis.98 FGF21 and GDF15 mediate these effects with differential effects: GDF15 reduces body weight, fat mass, and hepatic steatosis, whereas FGF21 stimulates energy expenditure and thermogenesis.98 In contrast, mice with CRIF1 depletion in adipose tissues, pancreatic β-cells, and macrophages do not induce mitohormetic responses, and they show adverse metabolic phenotypes.60,61,102 CRIF1 deficiency in adipose tissues and macrophages aggravates HFD-induced insulin resistance and glucose intolerance through enhanced inflammatory responses to fat-rich diets in adipose tissue and macrophages.60,61 Mitochondrial stress, caused by CRIF1 deficiency, in β-cells disrupts the first phase of insulin secretion and facilitates islet failure in response to HFD, and this islet phenotype mimics early-stage type 2 diabetes in humans.99,102 CRIF1 depletion in the cardiovascular system also culminates in harmful outcomes.101,103 The findings from CRIF1 knockout models have demonstrated that mitohormetic responses occur in an organ-specific manner.
In agreement with CRIF1-knockout models, several models with muscle mitochondrial stress manifest mitohormetic phenotypes.75,76 Muscle-specific mtDNA depletion induces beneficial metabolic outcomes, including liver lipolysis, inguinal subcutaneous white adipose tissue browning (conversion of white adipocytes to thermogenic brown adipocyte-like cells), weight loss, and increased glucose uptake in the muscle, heart, hippocampus, and white adipose tissue.75,76 As a result, these mice manifest small adipocyte sizes, low fat content in the liver, and obesity resistance to HFD.75,76 Similarly, muscle mitochondrial uncoupling protein UCP1-transgenic mice have shown increased whole-body energy expenditure and thermogenesis and decreased white adipose tissue content.104,105 Moreover, they have displayed increased respiratory quotient levels during the nighttime, indicative of increased overall glucose oxidation.106 Therefore, the beneficial effects of muscle mitochondrial stress on the liver and adipose tissue metabolism are reproducible and evident, especially under the circumstances of fat overconsumption.
Mitohormetic responses between the brain and peripheral metabolic organs
The first evidence of a mitohormetic response between the brain and peripheral metabolic organs came from the study of C. elegans.43 In this study, a gene encoding cytochrome c oxygenase-1 (cco-1), a component of ETC-complex 4, was deleted in a tissue-specific manner. They reported that neuronal and intestinal cco-1 knockout increased life span, whereas muscle cco-1 knockout reduced it. Furthermore, they observed that neuronal cco-1 knockout induces the UPRmt response in the intestine. Moreover, the favorable effect of neuronal cco-1 knockout on life span depends on the intestinal UPRmt response.43 Several secretory factors mediating inter-organ UPRmt responses have been proposed. In the worm C. elegans, neuronal secretory factors, such as the neurotransmitter serotonin and the neuropeptide FLP2, can mediate this UPRmt.107,108 In addition, it has been shown that Wnt signaling mediates the neuron-to-periphery mitochondrial stress response: neurons with mitochondrial stress secrete Wnt ligand/EGL-20, which activates Wnt signaling and UPRmt in the intestine.109 Intriguingly, neuronal mitochondrial stress can be transmitted to the offspring for multiple generations via germline UPRmt, extending the offspring’s life span in C. elegans.110
The hypothalamus is a pivotal organ governing body weight and whole-body metabolic processes.111,112 Proopiomelanocortin (POMC)-producing neurons residing in the hypothalamic arcuate nucleus are the most critical neurons for maintaining normal body weight. POMC neuronal activity is strongly linked to mitochondria; POMC neuronal activity can be upregulated by mitochondrial-derived ROS in mice.113 Moreover, mitochondrial dynamics (i.e., fusion and fission) in POMC neurons are essential for maintaining whole-body energy and glucose homeostasis under altered metabolic conditions.114,115 It has been shown that mice with POMC neuronal mitochondrial stress by deleting the Crif1 gene had normal body weight in the chow diet-fed condition, but they gained less weight in HFD-fed conditions.116 This anti-obesity phenotype was related to increased thermogenesis in the interscapular brown adipose tissue and inguinal white adipose tissue via sympathetic nervous system-mediated adipose tissue UPRmt responses.116
Conversely, mitochondrial stress in peripheral metabolic organs can influence the brain’s functions. For instance, under mitochondrial stress, skeletal muscle releases GDF15 in a circadian-dependent manner, and GDF15 induces daytime-restricted anorexia through brainstem GFRAL signaling.48,117 In the same model, GDF15 induces anxiety-like behavior via the activation of the hypothalamic corticotrophin-releasing hormone.117 Mitokine FGF21 also signals mitochondrial stress to the brain.76 In the animal model of mitochondrial myopathy, muscle-derived FGF21 increases glucose uptake along with mitochondrial changes in the dorsal hippocampus CA2 region, although neurological manifestation of this action remains unknown. In addition, FGF21 acts on the hypothalamus to induce weight loss via hypothalamic β-Klotho signaling.118
MITOHORMESIS AND DISEASES
Obesity
Excessive nutrient supply increases mitochondrial ROS formation as a byproduct of mitochondrial respiration.119,120 These mechanisms trigger obesity-associated insulin resistance and glucose intolerance.121 Meanwhile, mitochondria upregulate their biogenesis and antioxidant defense upon increased metabolic demand and ROS production. Increased mitochondrial contents have been observed in the skeletal muscle of rodent models of obesity. Mitochondrial ROS release appears critical in this hormetic response, as mitochondria-targeted antioxidants prevent an increase in muscle mitochondrial biogenesis in obesity.122
A redox-sensitive transcription factor, NRF2, serves as a major mediator of antioxidant defense under nutrient oversupply. Upon exposure to oxidative stress, NRF2 is translocated to the nucleus and binds the promoters of genes encoding proteins implicated in antioxidant pathways and other cellular defenses to stimulate their expression.123 In the HFD-fed mice model, the nuclear content of NRF2 was reduced in the adipose tissue, along with reduced production of antioxidant enzymes, such as heme oxygenase-1 (HO-1) and SOD, and increased protein oxidation.124 Chronic administration of the NRF2 activator oltipraz in these HFD-fed mice prevented weight gain and impaired insulin signaling and glucose disposal in the adipose tissues,124 indicating a protective role of NRF2 in obesity and related metabolic complications.
Another example showing the anti-obesity effect of the mitohormetic response involved mice lacking caseinolytic peptidase P (ClpP), which is a mitochondrial protease comprising mitochondrial retrograde signaling and UPRmt. In chow diet-fed conditions, these mice showed reduced adiposity and improved insulin sensitivity due to increased whole-body energy expenditure and enhanced mitochondrial biogenesis in the white adipose tissue.125 Moreover, they resisted HFD-induced obesity, glucose intolerance, and insulin resistance despite unaltered food intake.125 These paradoxical anti-obesity effects of ClpP deficiency may be related to compensatory mitohormetic responses. In addition, the mitokines mentioned above, such as FGF21, GDF15, and MOTS-c, had anti-obesity effects, especially in mice undergoing overnutritional stress.121 All this evidence suggests that mitohormetic responses counteract the development and aggravation of obesity.
Diabetes and cardiovascular complications
The causative role of excessive mitochondrial ROS production in diabetic vascular complications has been widely accepted,126 whereas interventions with antioxidants have failed to stop the progression of diabetic complications.127,128 Moreover, recent evidence suggests that reduced mitochondrial superoxide production may harm diabetic complications.127,128 Mitochondrial oxidative phosphorylation, ATP production, and superoxide production in response to excessive glucose exposure or nutrient stress, decrease in the organs prone to diabetic complications.129 Moreover, restoring mitochondrial function and superoxide production improves renal, cardiovascular, and neuronal dysfunction in diabetic mice. A high glucose-induced reduction in mitochondrial ETC activity and ATP/superoxide production might be an adaptive response to high levels of cellular ATP and superoxide. However, in the long run, it may cause a persistent reduction of mitochondrial function and biogenesis, leading to dysfunction and damage of target tissues involved in diabetic complications.129
AMP-activated protein kinase (AMPK) is a cellular energy sensor activated by low-energy stress, and it helps in recovery from cellular energy deficit. AMPK activity is enhanced under various mitochondrial stresses, which is a key event in eliciting mitohormetic effects. Increasing evidence suggests that inappropriately low AMPK activity may be related to diabetic complications. Humans and mice with diabetic nephropathy displayed low renal AMPK activities,130,131,132 and a similar decline was observed in the heart, nerves, and retina of diabetic animal models.133,134,135,136,137 Furthermore, genetic and chemical activation of AMPK improved albuminuria and reduced mesangial matrix expansion in the kidney of diabetic mice, along with an increase in mitochondrial ETC activity, PGC1α expression levels, mitochondrial content, and superoxide production.130,131,138 In the streptozotocin-induced diabetic rat model, AMPK activity was reduced in dorsal root ganglion neurons, and chronic treatment with the AMPK agonist resveratrol improved diabetic sensory neuropathy via the enhancement of mitochondrial function.133 In agreement with this, mitochondrial biogenesis and AMPK activity were decreased in the diabetic heart. Altogether, inadequate mitohormetic responses, including low AMPK activation under chronic hyperglycemic stress, may promote diabetic complications. In contrast, metformin-induced AMPK activation improved cardiac function in a mouse model of diabetic cardiomyopathy.139 Another AMPK activator berberine has been demonstrated to diminish myocardial ischemic-reperfusion injury in diabetic rats by triggering mitohormetic responses.140 Notably, berberine accumulates inside mitochondria, mildly inhibiting ETC activity, which in turn results in a moderate increase in ROS, consequently inducing mitohormetic responses.141 Altogether, inadequate mitohormetic responses, including low AMPK activation under chronic hyperglycemic stress, may promote diabetic complications. Therefore, a therapeutic strategy to promote mitohormesis may potentially alleviate the progression of diabetic complications.
Liver diseases
Cumulative evidence suggests that mitochondrial dysfunction underpins various liver diseases, ranging from fatty liver disease to hepatocellular carcinoma.142 In contrast, emerging evidence suggests that low-grade mitochondrial stress and its adaptive stress responses could attenuate the progression of non-alcoholic fatty liver diseases (NAFLD), hepatic fibrosis, and alcoholic liver injury.53,143 Upon various toxic injuries, UPRmt may be critical for the functional recovery of hepatocytes by producing mitochondrial chaperones and proteases. Heat shock protein 60 (Hsp60) is a mitochondrial chaperone implicated in NAFLD/non-alcoholic steatohepatitis (NASH), chronic hepatitis, and liver cancer.144 Hsp60 is a primary defense mechanism against mitochondrial proteotoxic damage during alcoholic liver damage.145 Rodents lacking Hsp60 in the liver are prone to acute hepatic injury and cholangiocellular tumorigenesis.146 Likewise, liver-specific deletion of mitochondrial chaperone prohibitin 1 promotes spontaneous liver injury, inflammation, fibrosis, and the development of hepatocellular carcinoma in mice.147,148
Similar to chaperones, mitochondrial proteases have protective roles in liver disease. Mitochondrial protease LON protease (LONP1) maintains mitochondrial homeostasis by degrading damaged mitochondrial matrix proteins. LONP1 depletion in human hepatocytes impairs insulin signaling and increases hepatic gluconeogenesis, whereas LONP1 overexpression improves cholesterol- and palmitate-induced insulin resistance.149 In contrast, deficiency of another mitochondrial protease, ClpP, protects against HFD-triggered fatty liver.125 The paradoxically beneficial effect might result from a compensatory mitohormetic response to ClpP deficiency.
Mitokines serve as mitohormetic mediators in metabolic liver diseases. FGF21 stimulates fatty acid oxidation in the liver;71 and as a result, FGF21 administration inhibits hepatic steatosis and increases hepatic insulin sensitivity in diet-induced obese mice.65 Moreover, FGF21 treatment ameliorates alcohol-induced fatty liver and hepatic inflammation by activating the AMPK-SIRT1 pathway and reducing ROS production.150,151 Another mitokine, GDF15, is closely associated with metabolic liver diseases. GDF15 has been proposed as a serum biomarker for predicting liver pathologies in humans, including NAFLD, NASH, and advanced liver fibrosis.152 Furthermore, treatment with recombinant GDF15 decreases liver fat accumulation and slows down the progression of NAFLD in HFD-fed mice.46,52
Aging
Aging accompanies a decline in mitochondrial functions, which has long been suspected of causing aging-related organ dysfunction and diseases. One potential mitochondrial mechanism of aging is mtDNA mutation. Due to constant replication independent of cell cycles and inefficient DNA repair, mtDNA mutations are prone to accumulate throughout a person’s lifetime. Thus, the level of heteroplasmy, which is the coexistence of normal and mutant mtDNA in the same cell, increases with age, and above a certain threshold, it might deteriorate mitochondrial functions and accelerate aging as seen in mice with a mtDNA proof-reading defect.153 Nevertheless, the heteroplasmy level in aged human muscle cells rarely reaches the threshold level to affect ETC functions, arguing against a significant contribution of mtDNA mutations to normal aging.154
Chronic inflammation is a pathologic feature of aging, termed inflammaging. mtDNA is released into the cytosol and extracellular space upon mitochondrial damage. Cytosolic and extracellular mtDNA act as a damage-associated molecular pattern to activate inflammation signaling pathways, such as NLRP3-inflammasome, cGAS-STING-IRF3, and endosome toll-like receptor 9 signaling. Indeed, it is worth noting that the treatment of FGF21 leads to a reduction in NLRP3 inflammasome activation in human macrophages.155 The amount of circulating mtDNA increases gradually with age and correlates with that of serum inflammatory markers,156 implying a potential role of extracellular mtDNA in age-related innate immune activation.157
Aging seems to be associated with alterations in mitohormetic pathways. A representative mitohormetic response, UPRmt, is deregulated in several human age-related diseases, such as sarcopenia and Alzheimer’s disease, whilst the UPRmt pathway positively modulates stem cell functions in aging.158 Increasing evidence has shown that the aging process can adversely impact stem cells; stem cells’ abilities to self-renew and differentiate into various cell types decline with aging.159 A reduction in the nicotinamide adenine dinucleotide NAD+, a critical cofactor of multiple metabolic reactions, is associated with stem cell dysfunction, and a reversal of NAD+ depletion improves mitochondrial and stem cell functions and enhances life span in mice via mechanisms involving UPRmt.160
Mitochondrial membrane dynamics, i.e., fusion and fission, are linked to aging. In worms, fragmentation of the mitochondrial network is observed with aging. In contrast, various longevity pathways are associated with increased mitochondrial fusion, as mitochondrial fusion is essential for maintaining mtDNA stability by diluting mtDNA mutations.161 In mice, both mitochondrial fusion and fission are impaired with age. Inhibition of either pathway accelerates mitochondrial senescence in the heart.162 These findings suggest that imbalanced mitochondrial fusion and fission may contribute to aging. Evidence also suggests that mitophagy, which is the autophagic removal of damaged or dysfunctional mitochondria, is essential for life span expansion in several long-lived model organisms.163 Mitophagy decline occurs in several tissues of aged mice, contributing to OxPhos dysfunction and aging-related phenotypes. Furthermore, mitophagy modulators, such as urolithin A, NAD+ enhancers, and spermidine, mitigate aging-related diseases.164,165,166
MITOHORMESIS AND HEALTH
Exercise
Exercise training enhances muscle strength and endurance. Exercise offers metabolic advantages by increasing oxygen consumption, insulin sensitivity, and fatty acid oxidation,167 all of which are strongly related to mitochondrial functions.168,169,170,171 Exercise exerts stress on the body as it depletes stored energy, thereby inducing mitochondrial stress responses.172,173 Therefore, it may be a powerful and simple way to induce mitohormesis. The mechanisms of exercise-induced mitohormesis are similar to the general mechanisms of mitohormesis, and were extensively reviewed in our recent paper.172 In brief, ROS generated from the mitochondria of contracting muscles is thought to be a critical mediator in exercise-induced mitohormetic effects, as antioxidant treatment significantly attenuates the health-promoting effects of exercise.174,175 Interestingly, exercise training induces ROS production in exercise-unrelated organs. For instance, moderate-intensity running exercise for 2 weeks induces mitochondrial stress and ROS production in the hypothalamus of mice.116 Intra-hypothalamic antioxidant treatment during exercise training obliterates exercise-induced thermogenesis and UPRmt in adipose tissues, implying that ROS generation in hypothalamic neurons is a critical signaling event for systemic metabolic adaptation to exercise training.
Muscle-derived mitokines FGF21 and GDF15 may mediate exercise-induced mitohormetic responses. Exercise-induced mitochondrial stress increases skeletal muscle expression levels of both factors,74,176 and these mitokines are released into the systemic circulation to stimulate adipose tissue thermogenesis, lipolysis, and fatty acid oxidation, leading to the alleviation of obesity and obesity-related metabolic complications.47,177,178 MDPs are also engaged in the mitohormetic effects of exercise. In humans, acute high-intensity endurance exercise increases plasma concentrations of humanin, SHLP-6, and MOTS-c, as well as the skeletal muscle expression of humanin and MOTS-c,179,180,181 although exercise-induced changes in MDPs might depend on the types and durations of exercise. Among MDPs, MOTS-c is most relevant to exercise. While exercise affects MOTS-c expression, MOTS-c controls exercise performance. Chronic MOTS-c treatment increases muscle force and stride length, along with increased lean mass and decreased fat mass. As a result, it enhances physical activity and health span in young, middle-aged, and old mice.180 In addition, MOTS-c is produced in the hypothalamus during exercise training, and similar to ROS, hypothalamic MOTS-c mediates exercise-induced adipose tissue thermogenesis and the anti-obesity effects of exercise.116 Based on these results, MOTS-c may be a promising target as an exercise mimetic or physical performance enhancer.
CONCLUDING REMARKS
Although this review focused on the beneficial aspects of the mitochondrial stress response, mitochondrial stress and the resultant mitochondrial dysfunction can threaten normal cellular functions and organismal health. If so, what factors of mitochondrial stress determine beneficial or adverse consequences? First, the stress level may be important, as indicated by the concept of hormesis. Second, the spatiotemporal aspect of stress may affect the ability to trigger the mitohormetic response. For example, in C. elegans, mitochondrial stress in the larval stages extends life span. In contrast, the same stress exerted in the adult stage has no such effect.43 Hence, mitochondrial perturbations in early life might have lifelong positive impacts on health and longevity. In addition, the longevity effect of UPRmt occurs in a tissue-specific manner, as ETC dysfunction in neurons and intestines, but not muscle, increases longevity.43 Third, chronic mitochondrial stress appears to be harmful. UPRmt enhances the stability of mtDNA mutants, leading to the propagation of mtDNA mutations in C. elegans.182 Fourth, the capacity for mitochondrial stress resistance might be inherited. In worms, neuronal mitochondrial stress can be maternally transmitted for more than 50 generations and extend lifespan of the descendants.110
Given the benefit of mitohormesis on energy metabolism and obesity, it is worth to conduct clinical trials to test the therapeutic potential of mitohormesis inducers in overnutrition-associated metabolic disorders. In this aspect, regular, moderate-intensity exercise may be an effective and safe way to induce mitohormetic effects in many tissues.172 In addition, mitokines, such as GDF15, FGF21, and MOTS-c, and their modified analogues could be used for this purpose. The favorable effect of mitochondrial stress on longevity has been largely observed in lower organisms; however, emerging evidence suggests that agents eliciting mild mitochondrial stress (metformin) and those inducing mitonuclear protein imbalance (resveratrol and NAD+ precursors nicotinamide ribose and nicotinamide mononucleotide) may have the potential to delay aging and extend life span in mammals.157 Therefore, a deeper understanding of mitohormesis and the development of mitohormesis-targeting agents will lead us to a new therapeutic avenue for many human diseases associated with obesity and aging.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT of Korea (2020R1A2C3004843, 2022M3E5E8017213, 2022R1C1C1012590, and 2022R1C1C2007378).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Se Hee Min, Gil Myoung Kang, and Min-Seon Kim.
- Investigation: all authors.
- Supervision: Min-Seon Kim.
- Writing—original draft: Se Hee Min and Min-Seon Kim.
- Writing—review & editing: all authors.
- Approval of final manuscript: all authors.
References
- 1.Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab. 2012;26:711–723. doi: 10.1016/j.beem.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keipert S, Lutter D, Schroeder BO, Brandt D, Ståhlman M, Schwarzmayr T, et al. Endogenous FGF21-signaling controls paradoxical obesity resistance of UCP1-deficient mice. Nat Commun. 2020;11:624. doi: 10.1038/s41467-019-14069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wajner M, Amaral AU. Mitochondrial dysfunction in fatty acid oxidation disorders: insights from human and animal studies. Biosci Rep. 2015;36:e00281. doi: 10.1042/BSR20150240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147:2643–2649. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- 5.Ristow M. Oxidative metabolism in cancer growth. Curr Opin Clin Nutr Metab Care. 2006;9:339–345. doi: 10.1097/01.mco.0000232892.43921.98. [DOI] [PubMed] [Google Scholar]
- 6.Fukui H, Moraes CT. The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci. 2008;31:251–256. doi: 10.1016/j.tins.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 10.Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS) Dose Response. 2014;12:288–341. doi: 10.2203/dose-response.13-035.Ristow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaleas SN, Laios K, Tsoucalas G, Androutsos G. Theophrastus Bombastus Von Hohenheim (Paracelsus) (1493-1541): the eminent physician and pioneer of toxicology. Toxicol Rep. 2021;8:411–414. doi: 10.1016/j.toxrep.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “mitohormesis” for health and vitality. Med Hypotheses. 2006;66:832–843. doi: 10.1016/j.mehy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Sagan L. On the origin of mitosing cells. J Theor Biol. 1967;14:255–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 16.Sharma MR, Koc EC, Datta PP, Booth TM, Spremulli LL, Agrawal RK. Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell. 2003;115:97–108. doi: 10.1016/s0092-8674(03)00762-1. [DOI] [PubMed] [Google Scholar]
- 17.Epstein CB, Waddle JA, Hale W, 4th, Davé V, Thornton J, Macatee TL, et al. Genome-wide responses to mitochondrial dysfunction. Mol Biol Cell. 2001;12:297–308. doi: 10.1091/mbc.12.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chae S, Ahn BY, Byun K, Cho YM, Yu MH, Lee B, et al. A systems approach for decoding mitochondrial retrograde signaling pathways. Sci Signal. 2013;6:rs4. doi: 10.1126/scisignal.2003266. [DOI] [PubMed] [Google Scholar]
- 19.Jazwinski SM, Kriete A. The yeast retrograde response as a model of intracellular signaling of mitochondrial dysfunction. Front Physiol. 2012;3:139. doi: 10.3389/fphys.2012.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothermel BA, Shyjan AW, Etheredge JL, Butow RA. Transactivation by Rtg1p, a basic helix-loop-helix protein that functions in communication between mitochondria and the nucleus in yeast. J Biol Chem. 1995;270:29476–29482. doi: 10.1074/jbc.270.49.29476. [DOI] [PubMed] [Google Scholar]
- 21.Rothermel BA, Thornton JL, Butow RA. Rtg3p, a basic helixloop-helix/leucine zipper protein that functions in mitochondrial-induced changes in gene expression, contains independent activation domains. J Biol Chem. 1997;272:19801–19807. doi: 10.1074/jbc.272.32.19801. [DOI] [PubMed] [Google Scholar]
- 22.Arnould T, Michel S, Renard P. Mitochondria retrograde signaling and the UPRmt: where are we in mammals? Int J Mol Sci. 2015;16:18224–18251. doi: 10.3390/ijms160818224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, et al. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011;13:668–678. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.da Cunha FM, Torelli NQ, Kowaltowski AJ. Mitochondrial retrograde signaling: triggers, pathways, and outcomes. Oxid Med Cell Longev. 2015;2015:482582. doi: 10.1155/2015/482582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dölle C, Rack JG, Ziegler M. NAD and ADP-ribose metabolism in mitochondria. FEBS J. 2013;280:3530–3541. doi: 10.1111/febs.12304. [DOI] [PubMed] [Google Scholar]
- 30.Houtkooper RH, Auwerx J. Exploring the therapeutic space around NAD+ J Cell Biol. 2012;199:205–209. doi: 10.1083/jcb.201207019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang BL. Sirt1 and the mitochondria. Mol Cells. 2016;39:87–95. doi: 10.14348/molcells.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bugga P, Alam MJ, Kumar R, Pal S, Chattopadyay N, Banerjee SK. Sirt3 ameliorates mitochondrial dysfunction and oxidative stress through regulating mitochondrial biogenesis and dynamics in cardiomyoblast. Cell Signal. 2022;94:110309. doi: 10.1016/j.cellsig.2022.110309. [DOI] [PubMed] [Google Scholar]
- 33.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1α. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 34.Gureev AP, Shaforostova EA, Popov VN. Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front Genet. 2019;10:435. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickles S, Vigié P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu R, Lendahl U, Nistér M, Zhao J. Regulation of mammalian mitochondrial dynamics: opportunities and challenges. Front Endocrinol (Lausanne) 2020;11:374. doi: 10.3389/fendo.2020.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 2018;19:109–120. doi: 10.1038/nrm.2017.110. [DOI] [PubMed] [Google Scholar]
- 39.Mottis A, Herzig S, Auwerx J. Mitocellular communication: shaping health and disease. Science. 2019;366:827–832. doi: 10.1126/science.aax3768. [DOI] [PubMed] [Google Scholar]
- 40.Qureshi MA, Haynes CM, Pellegrino MW. The mitochondrial unfolded protein response: signaling from the powerhouse. J Biol Chem. 2017;292:13500–13506. doi: 10.1074/jbc.R117.791061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suhm T, Kaimal JM, Dawitz H, Peselj C, Masser AE, Hanzén S, et al. Mitochondrial translation efficiency controls cytoplasmic protein homeostasis. Cell Metab. 2018;27:1309–1322.e6. doi: 10.1016/j.cmet.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan NA, Nikkanen J, Yatsuga S, Jackson C, Wang L, Pradhan S, et al. mTORC1 regulates mitochondrial integrated stress response and mitochondrial myopathy progression. Cell Metab. 2017;26:419–428.e5. doi: 10.1016/j.cmet.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-β superfamily. Proc Natl Acad Sci U S A. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung HK, Ryu D, Kim KS, Chang JY, Kim YK, Yi HS, et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol. 2017;216:149–165. doi: 10.1083/jcb.201607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johann K, Kleinert M, Klaus S. The role of GDF15 as a myomitokine. Cells. 2021;10:2990. doi: 10.3390/cells10112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ost M, Igual Gil C, Coleman V, Keipert S, Efstathiou S, Vidic V, et al. Muscle-derived GDF15 drives diurnal anorexia and systemic metabolic remodeling during mitochondrial stress. EMBO Rep. 2020;21:e48804. doi: 10.15252/embr.201948804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017;23:1150–1157. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Chi X, Gong Q, Gao L, Niu Y, Chi X, et al. Association of serum level of growth differentiation factor 15 with liver cirrhosis and hepatocellular carcinoma. PLoS One. 2015;10:e0127518. doi: 10.1371/journal.pone.0127518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee ES, Kim SH, Kim HJ, Kim KH, Lee BS, Ku BJ. Growth differentiation factor 15 predicts chronic liver disease severity. Gut Liver. 2017;11:276–282. doi: 10.5009/gnl16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim KH, Kim SH, Han DH, Jo YS, Lee YH, Lee MS. Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Sci Rep. 2018;8:6789. doi: 10.1038/s41598-018-25098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung HK, Kim JT, Kim HW, Kwon M, Kim SY, Shong M, et al. GDF15 deficiency exacerbates chronic alcohol- and carbon tetrachloride-induced liver injury. Sci Rep. 2017;7:17238. doi: 10.1038/s41598-017-17574-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsiao EC, Koniaris LG, Zimmers-Koniaris T, Sebald SM, Huynh TV, Lee SJ. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol. 2000;20:3742–3751. doi: 10.1128/mcb.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knockaert L, Berson A, Ribault C, Prost PE, Fautrel A, Pajaud J, et al. Carbon tetrachloride-mediated lipid peroxidation induces early mitochondrial alterations in mouse liver. Lab Invest. 2012;92:396–410. doi: 10.1038/labinvest.2011.193. [DOI] [PubMed] [Google Scholar]
- 56.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, et al. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Mei Z, Jia X, Song H, Liu J, Tian X. Cardioprotective effect of growth differentiation factor 15 against isoproterenol-induced cardiomyocyte apoptosis via regulation of the mitochondrial fusion. Cardiol Discov. 2022;2:89–96. [Google Scholar]
- 59.Borner T, Shaulson ED, Ghidewon MY, Barnett AB, Horn CC, Doyle RP, et al. GDF15 induces anorexia through nausea and emesis. Cell Metab. 2020;31:351–362.e5. doi: 10.1016/j.cmet.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jung SB, Choi MJ, Ryu D, Yi HS, Lee SE, Chang JY, et al. Reduced oxidative capacity in macrophages results in systemic insulin resistance. Nat Commun. 2018;9:1551. doi: 10.1038/s41467-018-03998-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi MJ, Jung SB, Lee SE, Kang SG, Lee JH, Ryu MJ, et al. An adipocyte-specific defect in oxidative phosphorylation increases systemic energy expenditure and protects against diet-induced obesity in mouse models. Diabetologia. 2020;63:837–852. doi: 10.1007/s00125-019-05082-7. [DOI] [PubMed] [Google Scholar]
- 62.Luan HH, Wang A, Hilliard BK, Carvalho F, Rosen CE, Ahasic AM, et al. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell. 2019;178:1231–1244.e11. doi: 10.1016/j.cell.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Chrysovergis K, Kosak J, Kissling G, Streicker M, Moser G, et al. hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling. Aging (Albany NY) 2014;6:690–704. doi: 10.18632/aging.100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 65.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 67.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Kilkenny DM, Rocheleau JV. The FGF21 receptor signaling complex: Klothoβ, FGFR1c, and other regulatory interactions. Vitam Horm. 2016;101:17–58. doi: 10.1016/bs.vh.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 69.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 71.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPA-Ralpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 73.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One. 2013;8:e63517. doi: 10.1371/journal.pone.0063517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tyynismaa H, Carroll CJ, Raimundo N, Ahola-Erkkilä S, Wenz T, Ruhanen H, et al. Mitochondrial myopathy induces a starvationlike response. Hum Mol Genet. 2010;19:3948–3958. doi: 10.1093/hmg/ddq310. [DOI] [PubMed] [Google Scholar]
- 76.Forsström S, Jackson CB, Carroll CJ, Kuronen M, Pirinen E, Pradhan S, et al. Fibroblast growth factor 21 drives dynamics of local and systemic stress responses in mitochondrial myopathy with mtDNA deletions. Cell Metab. 2019;30:1040–1054.e7. doi: 10.1016/j.cmet.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 77.Pereira RO, Tadinada SM, Zasadny FM, Oliveira KJ, Pires KMP, Olvera A, et al. OPA1 deficiency promotes secretion of FGF21 from muscle that prevents obesity and insulin resistance. EMBO J. 2017;36:2126–2145. doi: 10.15252/embj.201696179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pereira RO, Marti A, Olvera AC, Tadinada SM, Bjorkman SH, Weatherford ET, et al. OPA1 deletion in brown adipose tissue improves thermoregulation and systemic metabolism via FGF21. Elife. 2021;10:e66519. doi: 10.7554/eLife.66519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang L, Ishihara T, Ibayashi Y, Tatsushima K, Setoyama D, Hanada Y, et al. Disruption of mitochondrial fission in the liver protects mice from diet-induced obesity and metabolic deterioration. Diabetologia. 2015;58:2371–2380. doi: 10.1007/s00125-015-3704-7. [DOI] [PubMed] [Google Scholar]
- 80.Fu T, Xu Z, Liu L, Guo Q, Wu H, Liang X, et al. Mitophagy directs muscle-adipose crosstalk to alleviate dietary obesity. Cell Rep. 2018;23:1357–1372. doi: 10.1016/j.celrep.2018.03.127. [DOI] [PubMed] [Google Scholar]
- 81.Fontaine E. Metformin-induced mitochondrial complex I inhibition: facts, uncertainties, and consequences. Front Endocrinol (Lausanne) 2018;9:753. doi: 10.3389/fendo.2018.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim KH, Jeong YT, Kim SH, Jung HS, Park KS, Lee HY, et al. Metformin-induced inhibition of the mitochondrial respiratory chain increases FGF21 expression via ATF4 activation. Biochem Biophys Res Commun. 2013;440:76–81. doi: 10.1016/j.bbrc.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 83.Suomalainen A, Elo JM, Pietiläinen KH, Hakonen AH, Sevastianova K, Korpela M, et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 2011;10:806–818. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merry TL, Chan A, Woodhead JST, Reynolds JC, Kumagai H, Kim SJ, et al. Mitochondrial-derived peptides in energy metabolism. Am J Physiol Endocrinol Metab. 2020;319:E659–E666. doi: 10.1152/ajpendo.00249.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bodzioch M, Lapicka-Bodzioch K, Zapala B, Kamysz W, Kiec-Wilk B, Dembinska-Kiec A. Evidence for potential functionality of nuclearly-encoded humanin isoforms. Genomics. 2009;94:247–256. doi: 10.1016/j.ygeno.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 86.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Aβ. Proc Natl Acad Sci U S A. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuliawat R, Klein L, Gong Z, Nicoletta-Gentile M, Nemkal A, Cui L, et al. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the β cell. FASEB J. 2013;27:4890–4898. doi: 10.1096/fj.13-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY) 2016;8:796–809. doi: 10.18632/aging.100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim SJ, Devgan A, Mehta HH, Cohen P. Mitochondrial-derived peptide, SHLP2, a novel protective factor in Parkinson’s disease. Innov Aging. 2019;3(Suppl 1):S838 [Google Scholar]
- 91.Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21:443–454. doi: 10.1016/j.cmet.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim KH, Son JM, Benayoun BA, Lee C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab. 2018;28:516–524.e7. doi: 10.1016/j.cmet.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu H, Wei M, Zhai Y, Li Q, Ye Z, Wang L, et al. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J Mol Med (Berl) 2019;97:473–485. doi: 10.1007/s00109-018-01738-w. [DOI] [PubMed] [Google Scholar]
- 94.Wei M, Gan L, Liu Z, Liu L, Chang JR, Yin DC, et al. Mitochondrial-derived peptide MOTS-c attenuates vascular calcification and secondary myocardial remodeling via adenosine monophosphate-activated protein kinase signaling pathway. Cardiorenal Med. 2020;10:42–50. doi: 10.1159/000503224. [DOI] [PubMed] [Google Scholar]
- 95.Yan Z, Zhu S, Wang H, Wang L, Du T, Ye Z, et al. MOTS-c inhibits osteolysis in the mouse calvaria by affecting osteocyte-osteoclast crosstalk and inhibiting inflammation. Pharmacol Res. 2019;147:104381. doi: 10.1016/j.phrs.2019.104381. [DOI] [PubMed] [Google Scholar]
- 96.Ming W, Lu G, Xin S, Huanyu L, Yinghao J, Xiaoying L, et al. Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem Biophys Res Commun. 2016;476:412–419. doi: 10.1016/j.bbrc.2016.05.135. [DOI] [PubMed] [Google Scholar]
- 97.Yin X, Jing Y, Chen Q, Abbas AB, Hu J, Xu H. The intraperitoneal administration of MOTS-c produces antinociceptive and anti-inflammatory effects through the activation of AMPK pathway in the mouse formalin test. Eur J Pharmacol. 2020;870:172909. doi: 10.1016/j.ejphar.2020.172909. [DOI] [PubMed] [Google Scholar]
- 98.Kang SG, Choi MJ, Jung SB, Chung HK, Chang JY, Kim JT, et al. Differential roles of GDF15 and FGF21 in systemic metabolic adaptation to the mitochondrial integrated stress response. iScience. 2021;24:102181. doi: 10.1016/j.isci.2021.102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hong HJ, Joung KH, Kim YK, Choi MJ, Kang SG, Kim JT, et al. Mitoribosome insufficiency in β cells is associated with type 2 diabetes-like islet failure. Exp Mol Med. 2022;54:932–945. doi: 10.1038/s12276-022-00797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagar H, Jung SB, Ryu MJ, Choi SJ, Piao S, Song HJ, et al. CR6-interacting factor 1 deficiency impairs vascular function by inhibiting the Sirt1-endothelial nitric oxide synthase pathway. Antioxid Redox Signal. 2017;27:234–249. doi: 10.1089/ars.2016.6719. [DOI] [PubMed] [Google Scholar]
- 101.Shin J, Lee SH, Kwon MC, Yang DK, Seo HR, Kim J, et al. Cardiomyocyte specific deletion of Crif1 causes mitochondrial cardiomyopathy in mice. PLoS One. 2013;8:e53577. doi: 10.1371/journal.pone.0053577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim YK, Joung KH, Ryu MJ, Kim SJ, Kim H, Chung HK, et al. Disruption of CR6-interacting factor-1 (CRIF1) in mouse islet beta cells leads to mitochondrial diabetes with progressive beta cell failure. Diabetologia. 2015;58:771–780. doi: 10.1007/s00125-015-3506-y. [DOI] [PubMed] [Google Scholar]
- 103.Kim S, Piao S, Lee I, Nagar H, Choi SJ, Shin N, et al. CR6 interacting factor 1 deficiency induces premature senescence via SIRT3 inhibition in endothelial cells. Free Radic Biol Med. 2020;150:161–171. doi: 10.1016/j.freeradbiomed.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 104.Adjeitey CN, Mailloux RJ, Dekemp RA, Harper ME. Mitochondrial uncoupling in skeletal muscle by UCP1 augments energy expenditure and glutathione content while mitigating ROS production. Am J Physiol Endocrinol Metab. 2013;305:E405–E415. doi: 10.1152/ajpendo.00057.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keipert S, Klaus S, Heldmaier G, Jastroch M. UCP1 ectopically expressed in murine muscle displays native function and mitigates mitochondrial superoxide production. Biochim Biophys Acta. 2010;1797:324–330. doi: 10.1016/j.bbabio.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 106.Klaus S, Rudolph B, Dohrmann C, Wehr R. Expression of uncoupling protein 1 in skeletal muscle decreases muscle energy efficiency and affects thermoregulation and substrate oxidation. Physiol Genomics. 2005;21:193–200. doi: 10.1152/physiolgenomics.00299.2004. [DOI] [PubMed] [Google Scholar]
- 107.Berendzen KM, Durieux J, Shao LW, Tian Y, Kim HE, Wolff S, et al. Neuroendocrine coordination of mitochondrial stress signaling and proteostasis. Cell. 2016;166:1553–1563.e10. doi: 10.1016/j.cell.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shao LW, Niu R, Liu Y. Neuropeptide signals cell non-autonomous mitochondrial unfolded protein response. Cell Res. 2016;26:1182–1196. doi: 10.1038/cr.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Q, Wu X, Chen P, Liu L, Xin N, Tian Y, et al. The mitochondrial unfolded protein response is mediated cell-non-autonomously by retromer-dependent Wnt signaling. Cell. 2018;174:870–883.e17. doi: 10.1016/j.cell.2018.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Q, Wang Z, Zhang W, Wen Q, Li X, Zhou J, et al. The memory of neuronal mitochondrial stress is inherited transgenerationally via elevated mitochondrial DNA levels. Nat Cell Biol. 2021;23:870–880. doi: 10.1038/s41556-021-00724-8. [DOI] [PubMed] [Google Scholar]
- 111.Waterson MJ, Horvath TL. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 2015;22:962–970. doi: 10.1016/j.cmet.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 112.Roh E, Song DK, Kim MS. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med. 2016;48:e216. doi: 10.1038/emm.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, Kim E, et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011;17:1121–1127. doi: 10.1038/nm.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramírez S, Gómez-Valadés AG, Schneeberger M, Varela L, Haddad-Tóvolli R, Altirriba J, et al. Mitochondrial dynamics mediated by mitofusin 1 is required for POMC neuron glucose-sensing and insulin release control. Cell Metab. 2017;25:1390–1399.e6. doi: 10.1016/j.cmet.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 115.Santoro A, Campolo M, Liu C, Sesaki H, Meli R, Liu ZW, et al. DRP1 suppresses leptin and glucose sensing of POMC neurons. Cell Metab. 2017;25:647–660. doi: 10.1016/j.cmet.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kang GM, Min SH, Lee CH, Kim JY, Lim HS, Choi MJ, et al. Mitohormesis in hypothalamic POMC neurons mediates regular exercise-induced high-turnover metabolism. Cell Metab. 2021;33:334–349.e6. doi: 10.1016/j.cmet.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Igual Gil C, Coull BM, Jonas W, Lippert RN, Klaus S, Ost M. Mitochondrial stress-induced GFRAL signaling controls diurnal food intake and anxiety-like behavior. Life Sci Alliance. 2022;5:e202201495. doi: 10.26508/lsa.202201495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Landry T, Laing BT, Li P, Bunner W, Rao Z, Prete A, et al. Central α-klotho suppresses NPY/AgRP neuron activity and regulates metabolism in mice. Diabetes. 2020;69:1368–1381. doi: 10.2337/db19-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lambertucci RH, Hirabara SM, Silveira Ldos R, Levada-Pires AC, Curi R, Pithon-Curi TC. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J Cell Physiol. 2008;216:796–804. doi: 10.1002/jcp.21463. [DOI] [PubMed] [Google Scholar]
- 121.Yi HS, Chang JY, Shong M. The mitochondrial unfolded protein response and mitohormesis: a perspective on metabolic diseases. J Mol Endocrinol. 2018;61:R91–R105. doi: 10.1530/JME-18-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jain SS, Paglialunga S, Vigna C, Ludzki A, Herbst EA, Lally JS, et al. High-fat diet-induced mitochondrial biogenesis is regulated by mitochondrial-derived reactive oxygen species activation of CaMKII. Diabetes. 2014;63:1907–1913. doi: 10.2337/db13-0816. [DOI] [PubMed] [Google Scholar]
- 123.Cheng X, Siow RC, Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelchlike ECH-associated protein 1 defense pathway. Antioxid Redox Signal. 2011;14:469–487. doi: 10.1089/ars.2010.3283. [DOI] [PubMed] [Google Scholar]
- 124.Yu Z, Shao W, Chiang Y, Foltz W, Zhang Z, Ling W, et al. Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected]( NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia. 2011;54:922–934. doi: 10.1007/s00125-010-2001-8. [DOI] [PubMed] [Google Scholar]
- 125.Bhaskaran S, Pharaoh G, Ranjit R, Murphy A, Matsuzaki S, Nair BC, et al. Loss of mitochondrial protease ClpP protects mice from diet-induced obesity and insulin resistance. EMBO Rep. 2018;19:e45009. doi: 10.15252/embr.201745009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, et al. Impact of mitochondrial ROS production on diabetic vascular complications. Diabetes Res Clin Pract. 2007;77(Suppl 1):S41–S45. doi: 10.1016/j.diabres.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 127.Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, et al. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;346:f10. doi: 10.1136/bmj.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 129.Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes. 2015;64:663–672. doi: 10.2337/db14-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, De-Cleves AE, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123:4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, et al. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285:37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292:F617–F627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 133.Roy Chowdhury SK, Smith DR, Saleh A, Schapansky J, Marquez A, Gomes S, et al. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012;135:1751–1766. doi: 10.1093/brain/aws097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yan W, Zhang H, Liu P, Wang H, Liu J, Gao C, et al. Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling contributing to increased vulnerability in diabetic heart. Basic Res Cardiol. 2013;108:329. doi: 10.1007/s00395-013-0329-1. [DOI] [PubMed] [Google Scholar]
- 135.Zou MH, Xie Z. Regulation of interplay between autophagy and apoptosis in the diabetic heart: new role of AMPK. Autophagy. 2013;9:624–625. doi: 10.4161/auto.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu X, Zhang L, Yang X, Huang H, Huang Z, Shi L, et al. Salvianolic acid A protects the peripheral nerve function in diabetic rats through regulation of the AMPK-PGC1α-Sirt3 axis. Molecules. 2012;17:11216–11228. doi: 10.3390/molecules170911216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kubota S, Ozawa Y, Kurihara T, Sasaki M, Yuki K, Miyake S, et al. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Invest Ophthalmol Vis Sci. 2011;52:9142–9148. doi: 10.1167/iovs.11-8041. [DOI] [PubMed] [Google Scholar]
- 138.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu DQ, Chen SP, Sun J, Wang XM, Chen N, Zhou YQ, et al. Berberine protects against ischemia-reperfusion injury: a review of evidence from animal models and clinical studies. Pharmacol Res. 2019;148:104385. doi: 10.1016/j.phrs.2019.104385. [DOI] [PubMed] [Google Scholar]
- 141.Zhu X, Wei Y, Yang B, Yin X, Guo X. The mitohormetic response as part of the cytoprotection mechanism of berberine: berberine induces mitohormesis and mechanisms. Mol Med. 2020;26:10. doi: 10.1186/s10020-020-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nassir F, Ibdah JA. Role of mitochondria in alcoholic liver disease. World J Gastroenterol. 2014;20:2136–2142. doi: 10.3748/wjg.v20.i9.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190–1204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cappello F, Marino Gammazza A, Palumbo Piccionello A, Campanella C, Pace A, Conway de Macario E, et al. Hsp60 chaperonopathies and chaperonotherapy: targets and agents. Expert Opin Ther Targets. 2014;18:185–208. doi: 10.1517/14728222.2014.856417. [DOI] [PubMed] [Google Scholar]
- 145.Rakonczay Z, Jr, Boros I, Jármay K, Hegyi P, Lonovics J, Takacs T. Ethanol administration generates oxidative stress in the pancreas and liver, but fails to induce heat-shock proteins in rats. J Gastroenterol Hepatol. 2003;18:858–867. doi: 10.1046/j.1440-1746.2003.03076.x. [DOI] [PubMed] [Google Scholar]
- 146.Yuan D, Huang S, Berger E, Liu L, Gross N, Heinzmann F, et al. Kupffer cell-derived Tnf triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell. 2017;31:771–789.e6. doi: 10.1016/j.ccell.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ko KS, Tomasi ML, Iglesias-Ara A, French BA, French SW, Ramani K, et al. Liver-specific deletion of prohibitin 1 results in spontaneous liver injury, fibrosis, and hepatocellular carcinoma in mice. Hepatology. 2010;52:2096–2108. doi: 10.1002/hep.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fan W, Yang H, Liu T, Wang J, Li TW, Mavila N, et al. Prohibitin 1 suppresses liver cancer tumorigenesis in mice and human hepatocellular and cholangiocarcinoma cells. Hepatology. 2017;65:1249–1266. doi: 10.1002/hep.28964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee HJ, Chung K, Lee H, Lee K, Lim JH, Song J. Downregulation of mitochondrial lon protease impairs mitochondrial function and causes hepatic insulin resistance in human liver SK-HEP-1 cells. Diabetologia. 2011;54:1437–1446. doi: 10.1007/s00125-011-2074-z. [DOI] [PubMed] [Google Scholar]
- 150.Zhu S, Ma L, Wu Y, Ye X, Zhang T, Zhang Q, et al. FGF21 treatment ameliorates alcoholic fatty liver through activation of AMPKSIRT1 pathway. Acta Biochim Biophys Sin (Shanghai) 2014;46:1041–1048. doi: 10.1093/abbs/gmu097. [DOI] [PubMed] [Google Scholar]
- 151.Liu Y, Zhao C, Xiao J, Liu L, Zhang M, Wang C, et al. Fibroblast growth factor 21 deficiency exacerbates chronic alcohol-induced hepatic steatosis and injury. Sci Rep. 2016;6:31026. doi: 10.1038/srep31026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Koo BK, Um SH, Seo DS, Joo SK, Bae JM, Park JH, et al. Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver Int. 2018;38:695–705. doi: 10.1111/liv.13587. [DOI] [PubMed] [Google Scholar]
- 153.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 154.Vincent AE, Rosa HS, Pabis K, Lawless C, Chen C, Grünewald A, et al. Subcellular origin of mitochondrial DNA deletions in human skeletal muscle. Ann Neurol. 2018;84:289–301. doi: 10.1002/ana.25288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim ER, Kim SR, Cho W, Lee SG, Kim SH, Kim JH, et al. Short term isocaloric ketogenic diet modulates NLRP3 inflammasome via B-hydroxybutyrate and fibroblast growth factor 21. Front Immunol. 2022;13:843520. doi: 10.3389/fimmu.2022.843520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, et al. Circulating mitochondrial DNA increases with age and is a familiar trait: implications for “inflamm-aging”. Eur J Immunol. 2014;44:1552–1562. doi: 10.1002/eji.201343921. [DOI] [PubMed] [Google Scholar]
- 157.Lima T, Li TY, Mottis A, Auwerx J. Pleiotropic effects of mitochondria in aging. Nat Aging. 2022;2:199–213. doi: 10.1038/s43587-022-00191-2. [DOI] [PubMed] [Google Scholar]
- 158.Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature. 2017;552:187–193. doi: 10.1038/nature25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ahmed AS, Sheng MH, Wasnik S, Baylink DJ, Lau KW. Effect of aging on stem cells. World J Exp Med. 2017;7:1–10. doi: 10.5493/wjem.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 161.Chaudhari SN, Kipreos ET. Increased mitochondrial fusion allows the survival of older animals in diverse C. elegans longevity pathways. Nat Commun. 2017;8:182. doi: 10.1038/s41467-017-00274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Song M, Franco A, Fleischer JA, Zhang L, Dorn GW., 2nd Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab. 2017;26:872–883.e5. doi: 10.1016/j.cmet.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol. 2018;19:579–593. doi: 10.1038/s41580-018-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci. 2019;22:401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fang EF, Hou Y, Lautrup S, Jensen MB, Yang B, SenGupta T, et al. NAD+ augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat Commun. 2019;10:5284. doi: 10.1038/s41467-019-13172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schwarz C, Stekovic S, Wirth M, Benson G, Royer P, Sigrist SJ, et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging (Albany NY) 2018;10:19–33. doi: 10.18632/aging.101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pengam M, Amérand A, Simon B, Guernec A, Inizan M, Moisan C. How do exercise training variables stimulate processes related to mitochondrial biogenesis in slow and fast trout muscle fibres? Exp Physiol. 2021;106:938–957. doi: 10.1113/EP089231. [DOI] [PubMed] [Google Scholar]
- 168.Russell AP, Foletta VC, Snow RJ, Wadley GD. Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim Biophys Acta. 2014;1840:1276–1284. doi: 10.1016/j.bbagen.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 169.Kim KB, Kim K, Kim C, Kang SJ, Kim HJ, Yoon S, et al. Effects of exercise on the body composition and lipid profile of individuals with obesity: a systematic review and meta-analysis. J Obes Metab Syndr. 2019;28:278–294. doi: 10.7570/jomes.2019.28.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huertas JR, Casuso RA, Agustín PH, Cogliati S. Stay fit, stay young: mitochondria in movement: the role of exercise in the new mitochondrial paradigm. Oxid Med Cell Longev. 2019;2019:7058350. doi: 10.1155/2019/7058350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab. 2004;287:E857–E862. doi: 10.1152/ajpendo.00459.2003. [DOI] [PubMed] [Google Scholar]
- 172.Yoon TK, Lee CH, Kwon O, Kim MS. Exercise, mitohormesis, and mitochondrial ORF of the 12S rRNA type-C (MOTS-c) Diabetes Metab J. 2022;46:402–413. doi: 10.4093/dmj.2022.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Merry TL, Ristow M. Mitohormesis in exercise training. Free Radic Biol Med. 2016;98:123–130. doi: 10.1016/j.freeradbiomed.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 174.Gomez-Cabrera MC, Borrás C, Pallardó FV, Sastre J, Ji LL, Viña J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Laurens C, Parmar A, Murphy E, Carper D, Lair B, Maes P, et al. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight. 2020;5:e131870. doi: 10.1172/jci.insight.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim KH, Lee MS. FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab J. 2014;38:245–251. doi: 10.4093/dmj.2014.38.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim KH, Lee MS. GDF15 as a central mediator for integrated stress response and a promising therapeutic molecule for metabolic disorders and NASH. Biochim Biophys Acta Gen Subj. 2021;1865:129834. doi: 10.1016/j.bbagen.2020.129834. [DOI] [PubMed] [Google Scholar]
- 179.Woodhead JST, D’Souza RF, Hedges CP, Wan J, Berridge MV, Cameron-Smith D, et al. High-intensity interval exercise increases humanin, a mitochondrial encoded peptide, in the plasma and muscle of men. J Appl Physiol (1985) 2020;128:1346–1354. doi: 10.1152/japplphysiol.00032.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reynolds JC, Lai RW, Woodhead JST, Joly JH, Mitchell CJ, Cameron-Smith D, et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat Commun. 2021;12:470. doi: 10.1038/s41467-020-20790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.von Walden F, Fernandez-Gonzalo R, Norrbom J, Emanuelsson EB, Figueiredo VC, Gidlund EK, et al. Acute endurance exercise stimulates circulating levels of mitochondrial-derived peptides in humans. J Appl Physiol (1985) 2021;131:1035–1042. doi: 10.1152/japplphysiol.00706.2019. [DOI] [PubMed] [Google Scholar]
- 182.Lin YF, Schulz AM, Pellegrino MW, Lu Y, Shaham S, Haynes CM. Maintenance and propagation of a deleterious mitochondrial genome by the mitochondrial unfolded protein response. Nature. 2016;533:416–419. doi: 10.1038/nature17989. [DOI] [PMC free article] [PubMed] [Google Scholar]