Summary
Background
Sabin inactivated and bivalent oral poliovirus vaccine (sIPV, bOPV) were commonly used in China since 2016. We conducted an open-label, randomised, controlled phase 4 trial to assess immune persistence following sequential immunisation with sIPV or bOPV, and immunogenicity and safety of a booster dose of poliovirus vaccine in children aged 4 years.
Methods
Participants from a previous clinical trial with three different sequential schedules with sIPV (I) or bOPV (B) at ages 2, 3, and 4 months (Groups I–B–B, I–I–B, I–I–I) in 2017 were followed-up. The children were further divided into five subgroups after sIPV was given for Group I–B–B, and sIPV or bOPV randomly given for Group I–I–B and Group I–I–I (128 children in Groups I–B–B–I, 60 in Group I–I–B–B, 64 in Group I–I–B–I, 68 in Group I–I–I–B, 67 in Group I–I–I–I). Immune persistence and immunogenicity were assessed by measuring poliovirus type-specific antibodies, and safety were analysed in all children who received the booster dose.
Findings
Between Dec 5, 2020 and Jun 30, 2021, we respectively enrolled 381 participants in the immune persistence analysis, and 352 participants in per protocol (PP) analysis of the immunogenicity of the booster immunisation. Seropositivity rates of antibodies against poliovirus types 1 and 3 were all >90% four years after primary immunisation, while for poliovirus type 2 were 46.83%, 75.41%, and 90.23% (χ2 = 60.948, P < 0.001) for Groups I–B–B, I–I–B, and I–I–I, respectively. After the booster dose, seropositivity rates were 100% for all three serotypes in Group I–B–B–I, I–I–B–I and I–I–I–I; In Group I–I–B–B and I–I–I–B, the seropositivity rates for types 1 and 3 were all 100%, for type 2 were 92.59% and 98.46%. The geometric mean titres (GMTs) against poliovirus 1 and 3 were all high in five groups (>1860.73), and the GMTs against type 2 were significantly lower in groups booster with bOPV: Group I–I–B–B (50.60) and Group I–I–I–B (247.84). There was no significant difference in seropositivity rates or GMTs for all three serotypes (P > 0.05) between Group I–I–B–I and I–I–I–I. No serious adverse events occurred during the study.
Interpretation
Our findings suggest that at least two sIPV doses are needed in the current routine poliovirus immunisation schedule, and schedules containing 3 or 4 doses of sIPV provide better protection against poliovirus type 2 than the current sIPV-sIPV-bOPV-bOPV schedule in China.
Funding
Medical and Health Science and Technology of Zhejiang Province (2021KY118). This trial was registered with ClinicalTrials.gov (NCT04576910).
Keywords: Poliovirus, Sabin strain, Persistence immunity, Booster dose, Immunogenicity
Research in context.
Evidence before this study
A primary study on immunogenicity of three different Sabin IPV and bOPV sequential schedules was completed by our study group in 2018, and the results showed that all schedules were highly immunogenic against poliovirus types 1 and 3 one month after the 3rd dose of primary polio vaccination. Sequential schedules “sIPV-bOPV-bOPV” and “sIPV-sIPV-bOPV” demonstrated higher sero-protection rate against serotypes 1 and 3 than the “3sIPV” schedule. Only one dose of Sabin IPV is insufficient to protect children against poliovirus type 2 in the “sIPV-bOPV-bOPV” schedule.
We searched PubMed for all English-language papers published from Jan 1, 1978 to Sep 30, 2022, with the terms “inactivated polio vaccine”, “oral polio vaccine”, “bivalent oral polio vaccine”, “IPV”, “OPV”, “bOPV”, “Sabin”, “Booster”,“ Persistence ”, “Sequential schedule”. We identified several studies that assessed the antibody persistence of different schedules of poliovirus vaccines.
An Italian retrospective cohort study evaluated long-term immunogenicity of OPV among population fully vaccinated with four doses of OPV or IPV. A study in China compared antibody persistence among four groups of infants aged 18 months who were previously administered with different primary schedules at 2, 3, and 4 months of age, respectively (IPV-OPV-OPV, IPV-IPV-OPV, IPV-IPV-IPV, OPV-OPV-OPV). A similar study in China compared immunogenicity at 14 months after 3-dose primary series administered using Salk IPV and OPV (IPV-OPV-OPV, IPV-IPV-OPV, OPV-OPV-OPV). One study in Panama reported the persistence of seroprotective antibodies against poliovirus in 4-year-old children who previously received four doses of Salk IPV-Al (a reduced dose, aluminium hydroxide (Al(OH)3)-adjuvanted IPV vaccine) at 2, 3, 4 and 15–18 months of age.
Sabin IPV containing vaccine was first licensed in Japan (sIPV-containing diphtheria-tetanus-acellular pertussis combination vaccines, DTP-sIPV) in Nov 2012, and the single antigen vaccine (sIPV) was first produced in China in Jan 2015, respectively. Therefore, reports on the immunity induced by sIPV, especially the antibody persistence, are limited. A cross-sectional survey in Chongqing, China, reported GMT levels with different primary sequential poliovirus vaccination schedules (administered at 2, 3, 4 months of age) 5–11 months after completing the primary vaccination, but only one study group (sIPV-bOPV-bOPV) in this research was consistent with ours, and booster vaccination was not considered. A surveillance study in Japan compared the immunity induced by four doses of DTP-sIPV or Salk IPV; however, no booster dose of sIPV was included.
In general, none of these studies had administered sIPV or bOPV as booster dose after primary sequential schedules. It is very important for policy makers making to know the long-term persistence of primary sequential schedules and the immunogenicity of the booster dose, as so to better cope with the critical current polio situation.
Added value of this study
To our knowledge, this is the first study to assess the persistence of polio antibodies of three different primary immunisation schedules containing Sabin IPV, each of which started with Sabin IPV at age 2 months and received one, two, or no dose of bOPV. In addition, we are the first to report data about the immunogenicity and safety of a booster dose of polio vaccine with Sabin IPV or bOPV at 4 years old. The persistence results showed that the seropositivity rates and GMTs against types 1 and 3 remained high among the three groups, while the GMTs against poliovirus type 2 in children vaccinated with one dose of sIPV was relatively lower. The immunogenicity results demonstrate that the booster of sIPV could significantly increase the antibody level of type 2 and the immunogenicity was better than the boost immunization with bOPV in children who previously received sIPV-sIPV-bOPV. All groups exhibited good safety and no vaccine-related serious adverse events were reported. We believe that two or three doses of Sabin IPV in a bOPV-containing sequential schedules are needed to protect against poliovirus, especially for type 2.
Implications of all the available evidence
Our results provide supportive evidence for countries who use bOPV and sabin IPV in their routine polio vaccination schedules. Since Jan 2020, the sIPV-sIPV-bOPV-bOPV vaccination schedule has been adopted as the national immunisation strategy in China. A schedule comprising two or three doses of Sabin IPV will ensure infants have adequate protection against type 2 poliovirus after the withdrawal of all type 2-containing live vaccines, as recommended by SAGE. We believe our data will help national, regional, and global policy makers, especially in low-income and middle-income countries, to optimize the immunisation schedule to help achieve polio eradication.
Introduction
Epidemics of polio caused by poliovirus occurred annually in the early 20th century.1 Since the World Health Assembly (WHA) resolved to reach the goal of eradicating polio in 1988, the number of wild polio cases declined, from more than 340,000 cases per year to six in the last year.2 While the oral poliovirus vaccine (OPV) has played an important role on the success of globally polio control, on rare occasions, it causes either vaccine-associated polio paralysis (VAPP) in vaccinated children, or cases caused by vaccine-derived polioviruses (VDPVs).3 As wild-type poliovirus (WPV) will be eradicated in the near future, the disadvantages of OPV are becoming more pronounced, and the number of cases caused by VAPP and VDPV is more than the number caused by WPV.4 The implementation of polio switch strategy of withdrawal trivalent OPV (tOPV) and using bivalent types 1 and 3 OPV (bOPV) was happened in April 2016, after type 2 WPV was eradicated.5 However, outbreaks of circulating VDVP 2 (cVDPV2) become a threat against public health because the number of cases caused by cVDPV2 exceeded the number of cases caused by WPV1 since 2017 annually.6 Recently, in the United States, there was a case of VDPV2 reported. The VDPV2 isolated from the case was genetically linked to environmental samples in Israel and New York, as well as in London.7 This means that every country should maintain high population immunity level to ensure high immunity against poliovirus type 2.
The eradication of WPV and VDPV requires the global withdrawal of tOPV and replacement with IPV, because IPV has the advantage of containing inactivated virus with no risk of emergence from VDPV or VAPP compared with OPV. Furthermore, in China, the polio immunisation schedule was switched from one IPV dose and three bOPV doses, to two IPV doses (2, 3 months of age), with two bOPV doses (4 months and 4 years old) in 2020.8 Most of the IPV used in China, the Sabin-strain IPV (sIPV), are produced by Chinese manufacturers, using Sabin strain with a lower biosafety risk and low price compared to Salk-strain IPV, and for developing countries it is deemed more suitable.9 Therefore, at the final stage of polio eradication globally, it will play very important role.
It is very important to understand the persistence and compare the effects of different schedules on this stage of polio eradication. French and Swedish studies found high persistence in polio-neutralising antibodies 4–5 years after three-four doses of Salk-strain IPV in the polio vaccine recipients.10,11 Some developed countries, for example, the United States and France choose to administer a booster vaccination against polio when children reach 4–6 years old not 18 months of age, and Salk-strain IPV was used there for many years.12 Our current age of 4 years at booster immunisation is based on past experience or procedural regulations, and it is also hard to compare the polio immunization schedule in use in China at the time with the "1 dose after 14/18 weeks" schedule recommended by WHO, we should do some research to further evaluate our sequential immunisation program.13
In 2017, we assessed the immunogenicity along with the safety of different primary polio immunisation sequential schedules of sIPV or bOPV in three groups which were one dose of sIPV followed by two doses of bOPV, two doses of sIPV followed by one dose of bOPV, or three doses of sIPV administrated at 2, 3, and 4 months.14 Until now, there has been no evidence of the antibody persistence for different sequential schedule histories by sIPV or bOPV; furthermore, immunogenicity after the booster dose of polio vaccination by sIPV or bOPV is unknown. These results are important for a better evaluation of the sequential immunisation programmes using different polio vaccines, as well as for the development of booster immunisation strategies in the polio eradication phase. In this clinical trial, we explored and evaluated after following up the original participants, who were 4 years old, and evaluated the immunogenicity along with the safety of different polio booster vaccine.
Methods
Study design
This follow-up, open-label, randomised, controlled phase 4 trial was conducted in Zhejiang Province, China where the participants were previously included in a randomised controlled trial evaluating the immunogenicity and safety about three sequential polio vaccination schedules using sIPV and bOPV: sIPV-bOPV-bOPV (I–B–B), sIPV-sIPV-bOPV (I–I–B), or sIPV-sIPV-sIPV (I–I–I) at ages 2, 3, and 4 months, from 2016 to 2017; who could be followed-up and enrolled when aged between 48 and 51 months; and without any vaccination within 2 weeks before enrolment. Exclusion criteria were completing four doses of polio vaccine, allergy to the vaccine component, and any situation that may interfere immune response. If participants could not be followed up were excluded.
The ethics committee of the Zhejiang Provincial Centre for Disease Control and Prevention (CDC) approved the study protocol, and the study was conducted in accordance with the Declaration of Helsinki. Every participant's guardian completed the consent forms before the enrolment.
Randomisation and masking
Considering the implementation of the polio immunisation strategy of two doses IPV follewed by two doses of bOPV in China since January 2020, children previously vaccinated with only one dose of IPV should receive an additional dose of IPV. Accordingly, participants in Groups I–B–B, who were vaccinated with one dose of sIPV and two doses of bOPV, were not randomly assigned; they all received a booster dose of sIPV (Group I–B–B–I). Participants in Groups I–I–B, who were vaccinated with two doses of sIPV followed with one dose of bOPV, randomly assigned (1:1) to vaccinate with one dose of bOPV (Group I–I–B–B) or sIPV (Group I–I–B–I). Participants in Groups I–I–I, who had received three doses of sIPV, were randomly assigned (1:1) to vaccinate with one dose of bOPV (Group I–I–I–B) or sIPV (Group I–I–I–I). Randomisation was performed by an independent statistician using block randomisation (block size four) and stratified by study site (Longyou and Chunan Counties). The randomisation assignment number was placed in envelopes, local investigators could not know the randomisation information until they enrolled a participant. The allocation was unblinded, as both vaccinators and participants knew the group assignments considering the formulation and route of immunisation; however, the laboratory technician and statistician were unknow about which children were vaccinated with which vaccines.
Procedures
Each participant information of birth, sex, weight, and height was recorded in the questionnaire by the physician. The sIPV(0.5 mL/dose, lot number: 202003016) and bOPV(1.0 mL/dose, lot number: 202001004) were manufactured by Beijing Institute of Biological Products Co., Ltd. The two kinds of vaccines were used for national immunisation in China. The Zhejiang Provincial CDC purchased research vaccines and delivered to vaccination centres. The sIPV and bOPV vaccines were stored at 2–8 °C and −20 °C, respectively.
Approximately 1.5 mL of blood sample was collected before (day 0) and after (days 28–60) the booster dose. The China National Institutes for Food and Drug Control (NIFDC) measured the antibody titres by using a standardised Sabin strain-based neutralisation assay, according to the method the World Health Organisation (WHO) recommended. Seropositivity was defined as a neutralisation antibody titre of 1:8 or higher, while the titre <1:8 means seronegative. Seroconversion was defined as the change from seronegative to seropositive or at least a 4-fold increase in poliovirus type-specific antibody concentrations.
After the booster dose of polio vaccine, adverse events were recorded by the study staff at the trial site for 30 min, and at home on days 3 and 30, through active surveillance visits. Diary cards were provided to the participants' parents (s) or guardians (s), to record any adverse events within 30 days. Parents observed any vaccine-related or serious adverse events could contact study physician.
Outcomes
We performed a persistence analysis on all participants enrolled at age 4 who met the inclusion/exclusion criteria. Per-protocol (PP) population included participants who had serum results and immunogenicity assessment for the booster dose of polio vaccine. We did safety assessment by recording the number of important medical events and serious adverse events of the participants who received the fourth dose and experienced at least one safety assessment according to the study protocol.
The primary outcomes were the seropositivity rates of three poliovirus-neutralizing antibodies before and after booster immunisation. The secondary outcomes were the geometric mean titres (GMTs) for three types of antibodies before and after the fourth dose of polio vaccine and the safety of the booster dose.
Statistical analysis
We reported the baseline characteristics using descriptive statistics and summarised continuous variables using mean ± standard deviation (SD), categorical variables using n (%). The persistence evaluation of different sequential primary schedules was done among three previous groups, and immunogenicity evaluation was done in the per-protocol population among five groups. The seropositivity rates were compared using Fisher's exact test or Chi-square (χ2) test across all groups and between any two groups, the 95% confidence intervals (CIs) were calculated by the Clopper-Pearson method. GMTs with 95% CIs were used to describe the antibody titres and were compared by analysis of variance and the generalised linear model to determine differences across all groups and between any two groups.
Adverse events on different days after vaccination were shown as n (%). Solicited systemic adverse events included irritation or depression, fever, vomiting, anorexia, diarrhoea, lethargy, and acute anaphylaxis. Local adverse events included induration, pain, swelling, redness, local rash, and pruritus. Adverse events due to other factors considered to be causally related to the vaccine were judged by the investigators.
SAS software (SAS Institute Inc., Cary, USA) (version 9.4) was used in the data analysis. Statistical significance was set at P < 0.05. The trial had been registered with ClinicalTrials.gov (NCT04576910).
Role of the funding source
The Zhejiang Provincial CDC led the study design, data collection, data analysis, data interpretation, and writing of the report. The China National Biotec Group Company contributed to the study supervision and writing of the report. The corresponding author had full access to all data in the study, and had final responsibility for the decision to submit for publication.
Results
Demographic characteristics
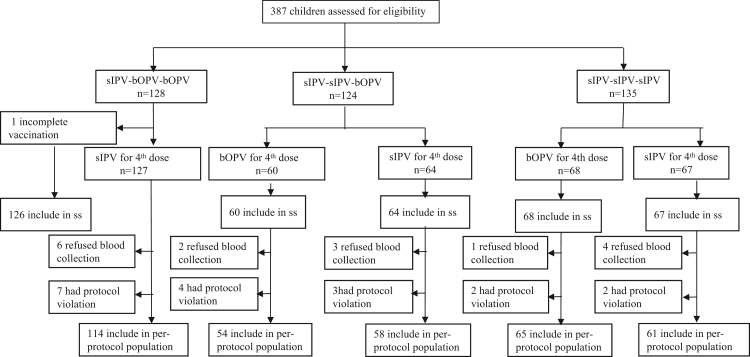
The participants were based on our previous polio vaccine sequential clinical trial cohort, and 387 children were followed up and enrolled in this study. A total of 381 participants were enrolled in the immune persistence analysis (126, 122, and 133, in groups I–B–B, I–I–B, and I–I–I, respectively), and 352 (114, 54, 58, 65 and 61 in groups I–B–B–I, I–I–B–B, I–I–B–I, I–I–I–B, and I–I–I–I, respectively) in the per protocol (PP) dataset to analyse the immunogenicity of the boost immunisation. Safety observations were performed including 386 participants (127, 60, 64, 68, and 67 in groups I–B–B–I, I–I–B–B, I–I–B–I, I–I–I–B, and I–I–I–I, respectively) after vaccination, to evaluate safety (Fig. 1).
Fig. 1.
Trial profile. Enrolled subjects were assigned to one of the two booster vaccination groups. SS = safety set. PPS = Per-protocol dataset. bOPV = bivalent types 1 and 3 oral polio vaccine. sIPV = Sabin strain inactivated polio vaccine.
As shown in Table 1, the children's mean ages in groups I–B–B–I, I–I–B–B, I–I–B–I, -I-I-I-B, and I–I–I–I in the PP dataset for immunogenicity of the boost immunisation were 48.63, 48.70, 48.64, 48.79, and 48.69 months. For the immune persistence analysis, the participants’ mean ages in groups I–B–B, I–I–B, and I–I–I were 48.63, 48.67, and 48.75 months, respectively. The demographic characteristics of enrolled children in the immune persistence analysis see Supplementary Table S1.
Table 1.
Demographic characteristics of enrolled children in per protocol (PP) analyses.
| sIPV-2bOPV-sIPV | 2sIPV-bOPV-bOPV | 2sIPV-bOPV-sIPV | 3sIPV-bOPV | 4sIPV | |
|---|---|---|---|---|---|
| N | 114 | 54 | 58 | 65 | 61 |
| Age (months) | |||||
| mean ± SD | 48.63 ± 0.58 | 48.70 ± 0.58 | 48.64 ± 0.6 | 48.79 ± 0.68 | 48.69 ± 0.62 |
| Gender | |||||
| Male [n (%)] | 57 (50) | 24 (44.44) | 28 (48.28) | 36 (55.38) | 34 (55.74) |
| Weight(kg) | |||||
| mean ± SD | 16.90 ± 3.23 | 16.69 ± 2.43 | 17.16 ± 3.22 | 16.67 ± 2.67 | 17.36 ± 3.36 |
| Height(cm) | |||||
| mean ± SD | 103.14 ± 4.64 | 102.28 ± 4.53 | 102.79 ± 3.96 | 103.40 ± 4.15 | 103.72 ± 3.62 |
sIPV = Sabin inactivated poliovirus vaccine; bOPV = bivalent oral polio vaccine; SD = standard deviation.
Immune persistence
The seropositivity rates against poliovirus type 1 were all above 99% in the three groups, 4 years after the primary polio vaccine immunisation (100%, 99.18%, and 100% for groups I–B–B, I–I–B, and I–I–I; respectively; Fisher's exact probability test, P = 0.320). There was a statistically significant difference in seropositivity rates against poliovirus type 2 (46.83%, 75.41%, and 90.23% for groups I–B–B, I–I–B, and I–I–I, respectively; χ2 = 60.948, P < 0.001). The seropositivity rates against poliovirus type 3 were all above 90%, and the difference was statistically significant (100%, 99.18%, and 93.23% in groups I–B–B, I–I–B, and I–I–I; Fisher's exact probability test, P < 0.001). The immune persistence analysis in the three groups was in Supplementary Table S2.
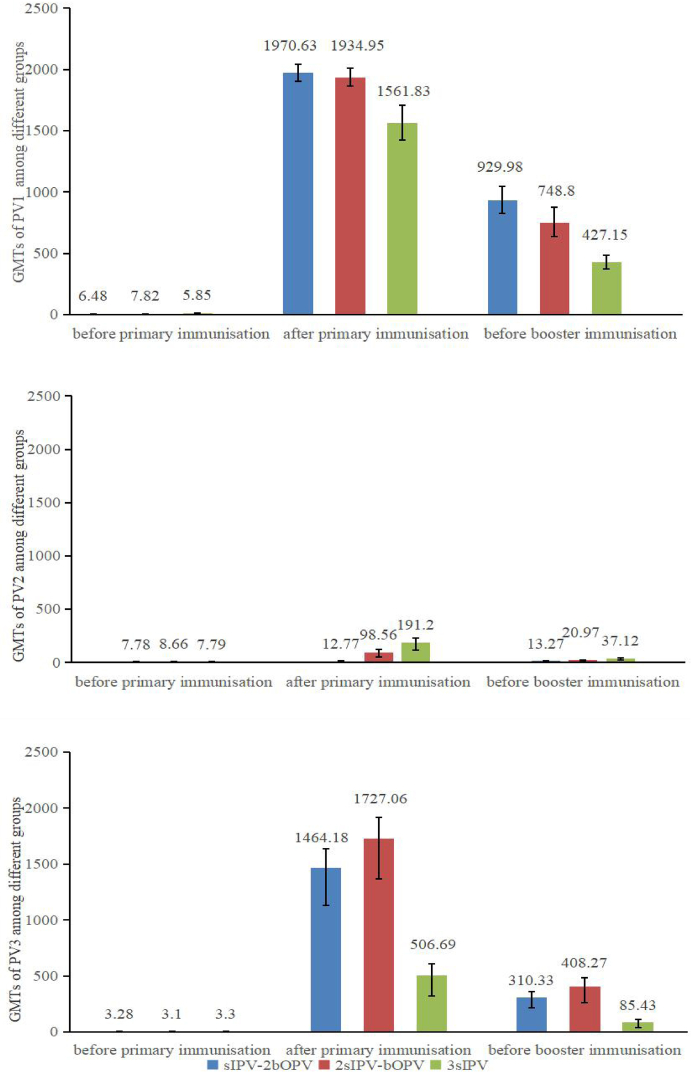
Fig. 2 shown the GMTs among three groups with different primary polio program. Similarly, higher GMTs of antibodies against poliovirus types 1 and 3 were found in participants with sequential schedules of sIPV and bOPV vaccines (929.98, 748.80, and 427.15 for type 1 in groups I–B–B, I–I–B, and I–I–I; 310.33, 408.27, and 85.43, for type 3, respectively; all P < 0.001). The GMTs of antibodies against poliovirus type 2 were 13.27, 20.97, and 37.12, in groups I–B–B, I–I–B, and I–I–I, respectively, and the difference was statistically significant among the three groups (F = 18.430, P < 0.001).
Fig. 2.
GMTs of antibodies against PV1/PV2/PV3 among three groups with different primary polio vaccination schedules. GMT = geometric mean titre; PV1 = polivirus type 1; PV2 = polivirus type 2; PV3 = polivirus type 3.
The pairwise comparison showed significantly higher seropositivity rates against poliovirus type 3, and higher GMTs against poliovirus types 1 and 3 in the sequential groups than in the sIPV only group (Supplementary Table S3).
Immunogenicity of the booster vaccination
For group I–B–B–I, against poliovirus types 1, 2, and 3, the seroconversion rates were 87.72%, 92.11%, and 98.25%, respectively; the seropositivity rates were all 100%; and the GMTs of antibodies were 12568.55, 1112.70, and 10955.87, respectively.
For group I–I–B–B, against poliovirus type 1, 2, 3, the seroconversion rates were 46.30%, 29.63%, and 57.41% after booster vaccination; the seropositivity rates were 100%, 92.59%, and 100%; while the GMTs of antibodies were 5337.42, 50.60, and 1860.73, respectively. For group I–I–B–I, against poliovirus types 1, 2, and 3, the seroconversion rates were 94.83%, 100%, and 96.55% after booster vaccination with sIPV; seropositivity rates were all 100%; and the GMTs of antibodies were 11753.85, 3604.50, and 9931.75, respectively.
For participants with three sIPV primary immunisation, against poliovirus types 1, 2, and 3, the seroconversion rates were 98.46%, 61.54%, and 89.23% after booster vaccination with bOPV (group I–I–I–B); the seropositivity rates were all 100%; and the GMTs of antibodies were 12825.75, 247.84, and 5962.40, respectively. For group I–I–I–I, against poliovirus types 1, 2, and 3, the seroconversion rates were 98.36%, 100%, and 96.72% after booster vaccination with sIPV; the seropositivity rates were all 100%; and the GMTs of antibodies were 11957.62, 5518.18, and 8125.64, respectively. Immunogenicity of the booster vaccination were shown in Table 2 and Supplementary Fig. S1.
Table 2.
Anti-poliovirus antibodies before and after the booster vaccination by group.
| sIPV-2bOPV-sIPV(n = 114) | 2sIPV-bOPV-bOPV(n = 54) | 2sIPV-bOPV-sIPV(n = 58) | 3sIPV-bOPV(n = 65) | 4sIPV(n = 61) | χ2/F | P value | |
|---|---|---|---|---|---|---|---|
| Anti-poliovirus serotype 1 antibody | |||||||
| Pre-booster | |||||||
| Seropositive | 114 (100%) | 53 (98.15%) | 58 (100%) | 65 (100%) | 61 (100%) | – | 0.153 |
| GMT | 1026.73 | 976.31 | 650.7 | 426.69 | 441.61 | 16.568 | <0.001 |
| Post-booster | |||||||
| Seropositive | 114 (100.00%) | 54 (100.00%) | 58 (100.00%) | 65 (100.00%) | 61 (100.00%) | – | – |
| Seroconversion | 100 (87.72%) | 25 (46.30%) | 55 (94.83%) | 64 (98.46%) | 60 (98.36%) | 92.850 | <0.001 |
| GMT | 12568.55 | 5337.42 | 11753.85 | 12825.75 | 11957.62 | 12.072 | <0.001 |
| Anti-poliovirus serotype 2 antibody | |||||||
| Pre-booster | |||||||
| Seropositive | 48 (42.11%) | 42 (77.78%) | 43 (74.14%) | 58 (89.23%) | 57 (93.44%) | 72.278 | <0.001 |
| GMT | 11.26 | 26.95 | 17.56 | 42.62 | 33.76 | – | <0.001 |
| Post-booster | |||||||
| Seropositive | 114 (100.00%) | 50 (92.59%) | 58 (100.00%) | 64 (98.46%) | 61 (100.00%) | – | 0.002 |
| Seroconversion | 105 (92.11%) | 16 (29.63%) | 58 (100.00%) | 40 (61.54%) | 61 (100.00%) | 137.299 | <0.001 |
| GMT | 1112.70 | 50.60 | 3604.50 | 247.84 | 5518.18 | – | <0.001 |
| Anti-poliovirus serotype 3 antibody | |||||||
| Pre-booster | |||||||
| Seropositive | 114 (100.00%) | 53 (98.15%) | 58 (100.00%) | 61 (93.85%) | 56 (91.80%) | – | 0.002 |
| GMT | 300.26 | 422.03 | 345.30 | 75.07 | 90.11 | – | <0.001 |
| Post-booster | |||||||
| Seropositive | 114 (100.00%) | 54 (100.00%) | 58 (100.00%) | 65 (100.00%) | 61 (100.00%) | – | |
| Seroconversionn | 112 (98.25%) | 31 (57.41%) | 56 (96.55%) | 58 (89.23%) | 59 (96.72%) | 76.655 | <0.001 |
| GMT | 10955.87 | 1860.73 | 9931.75 | 5962.40 | 8125.64 | – | <0.001 |
sIPV = Sabin inactivated poliovirus vaccine; bOPV = bivalent oral polio vaccine; GMT = geometric mean titre.
There were no significant difference in seropositivity rates against poliovirus types 1 and 3 among five groups. The GMTs against poliovirus types 1, 2, and 3 in group I–I–B–B were all significantly lower than those in group I–I–B–I (all P < 0.05), and the GMT against poliovirus types 2 in group I–I–I–B was significantly lower than that in group I–I–I–I (P = 0.046). There was no significant difference in GMTs against poliovirus types 1, 2 and 3 between group I–I–B–I and I–I–I–I (all P > 0.05). Pairwise comparison of seropositive rates and GMTs between any two groups were shown in Supplementary Table S4.
Safety of the booster vaccination
The vaccines used in this study were well tolerated, and no deaths, no severe adverse events had been reported. For groups I–B–B–I, I–I–B–B, I–I–B–I, I–I–I–B, and I–I–I–I, the proportion of adverse events was 7.09%, 1.67%, 4.69%, 4.41%, and 14.93% (Table 3), there was no statistically significant difference among groups (P = 0.052). The most common adverse events were local site reactions including induration, redness, pain, and swelling. The proportions of adverse events in five groups were as follows: induration ≤2.99%, redness ≤1.57%, pain ≤1.57%, and swelling ≤0.79%, and there was no significant difference among five groups. No deaths or severe adverse events reported in the study.
Table 3.
Adverse events after the booster dose.
| sIPV-2bOPV-sIPV(n = 114) |
2sIPV-bOPV-bOPV(n = 54) |
2sIPV-bOPV-sIPV(n = 58) |
3sIPV-bOPV(n = 65) |
4sIPV(n = 61) |
P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of AEFI Case | Case Reporting Rate (%) | No. of AEFI Case | Case Reporting Rate (%) | No. of AEFI Case | Case Reporting Rate (%) | No. of AEFI Case | Case Reporting Rate (%) | No. of AEFI Case | Case Reporting Rate (%) | ||
| Overall | 9 | 7.09 | 1 | 1.67 | 3 | 4.69 | 3 | 4.41 | 10 | 14.93 | 0.052 |
| Vaccine-related | 7 | 5.51 | 1 | 1.67 | 3 | 4.69 | 3 | 4.41 | 10 | 14.93 | 0.043 |
| Vaccine non-related | 2 | 1.57 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.49 | 0.767 |
| Solicited adverse events | 6 | 4.72 | 1 | 1.67 | 0 | 0 | 1 | 1.47 | 6 | 8.96 | 0.052 |
| Vaccine-related | 4 | 3.15 | 1 | 1.67 | 0 | 0 | 1 | 1.47 | 6 | 8.96 | 0.050 |
| Vaccine non-related | 2 | 1.57 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.49 | 0.767 |
| Non-solicited adverse events | 4 | 3.15 | 0 | 0 | 3 | 4.69 | 2 | 2.94 | 4 | 5.97 | 0.399 |
| Vaccine-related | 3 | 2.36 | 0 | 0 | 3 | 4.69 | 2 | 2.94 | 4 | 5.97 | 0.309 |
| Vaccine non-related | 1 | 0.79 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.000 |
| Adverse events of grade 3 or higher | 1 | 0.79 | 1 | 1.67 | 0 | 0 | 0 | 0 | 0 | 0 | 0.552 |
| Vaccine-related | 1 | 0.79 | 1 | 1.67 | 0 | 0 | 0 | 0 | 0 | 0 | 0.552 |
| Vaccine non-related | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
sIPV = Sabin inactivated poliovirus vaccine; bOPV = bivalent oral polio vaccine.
Discussion
In our earlier study, we found that different primary schedules of sIPV and bOPV were highly immunogenic for types 1 and 3 poliovirus, whereas one dose of sIPV was insufficient against type 2 poliovirus.14 In this trial, we followed these children at 4 years of age to explore the persistence of different sequential primary schedules and assess the immunogenicity and safety of a fourth booster dose of polio vaccine with sIPV or bOPV. The results of this study indicated that the immune persistence 4 years after primary vaccination with sIPV and bOPV was high, while groups with one or two sIPV doses showed relative lower antibodies against poliovirus type 2. Five different schedules showed tolerated and highly immunogenic against 3 poliovirus types, and booster with sIPV at age 4 had higher seropositivity rates against poliovirus type 2 (Supplementary Table S4).
The persistence results showed that the seropositivity rates and GMTs of antibodies against poliovirus types 1 and 3 (seropositivity rates: 93.23%–100%, GMTs: 85.43–929.98) remained high 4 years after primary vaccination in all three groups. However, the groups with sequential primary schedules maintained higher GMTs against poliovirus types 1 and 3 compared to the IPV-only group in our study. These results are consistent with that observed one month later after primary immunisation among the three groups.14 A similar study showed that GMTs with sequential schedules of Salk IPV and tOPV were higher than Salk IPV only schedule.15 A review also revealed that sequential schedules with IPV and OPV might increase polio-neutralising antibodies compared to those of IPV alone.16 Therefore, the primary immunisation with sequential sIPV and bOPV can maintain high antibody levels to serotype 1 and 3 after 4 years, while the immunogenicity in group I–I–I against poliovirus type 2 was better than that in other two groups.
Our study indicated that the seropositivity rates of antibodies against poliovirus type 2 at 4 years of age were reduced to 46.83% in group I–B–B, which was significantly lower than those in other groups (75.41% in group I–I–B and 90.23% in group I–I–I; χ2 = 60.948, P < 0.001). These results are another indication that one or two doses of sIPV in the primary schedule are insufficient, especially the one dose group for poliovirus type 2 immunity. As reported in the review,16 the number of persons with polio type 2 protective humoral immunity and polio type 2 neutralising antibodies is probably lower with bOPV than with tOPV or IPV alone. Type 2 components were withdrawn from the tOPV, and antibodies against poliovirus type 2 were mainly from the sIPV in our study. Considering the threat of continuous outbreak of cVDPV2 in many countries and even China, two sIPV doses in the primary schedule are necessary, which now complies with the current polio vaccination programme in China.
In addition, our study also provides evidence that seropositivity rates of antibodies against three types of polioviruses (all >90%) remained high after 4 years in children who received three doses of sIPV at 2, 3, and 4 months and the immunogenicity in group I–I–I against poliovirus type 2 was better than that in other two groups. These findings may be helpful for countries using the IPV-only schedule like three doses of sIPV in the near future and preparing for future bOPV cessation following the global eradication as a dose-sparing strategy.
Booster vaccination with sIPV or bOPV can significantly increase the levels of antibodies. The seropositivity rates against three serotypes were all 100% after boosting with sIPV in group I–B–B. Therefore, the booster schedule of IPV for those with only one dose of IPV vaccination history, is effective, which also provides a guide for those countries using similar primary immunisation program in polio eradication. Meanwhile, our results showed that the GMTs against three poliovirus types in Group I–I–B–B were all significantly lower than those in Group I–I–B–I, which may indicate that the enhancement effect of the IPV may be better than that of attenuated vaccines for our current polio vaccine immunisation program. In addition, we found that the booster dose of bOPV after 2sIPV-bOPV resulted in a 14.81% increase in seropositivity rates against poliovirus type 2. This result may indicate the possible cross-protection from bOPV,17 which is consistent with research findings in Chilean infants.18 But the heterotypic protection may not sufficiently raise immunity against poliovirus type 2 and has been observed to be short lasting.19 For participants with three doses of IPV in the primary immunisation, the seropositivity against poliovirus types 1, 2, and 3 reached 98.46%–100% after booster with sIPV or bOPV. No significant difference was observed in the seropositivity rates of all three serotypes between groups I–I–I–B and I–I–I–I. GMTs against poliovirus types 1 and 3 were both high in these two groups, while GMTs against type 2 were significantly lower in Group I–I–I–B than in Group I–I–I–I. Meanwhile, the booster dose of bOPV after 3sIPV treatment resulted in a 9.23% increase in seropositivity against poliovirus type 2, which is similar to the results in Group I–I–B–B. The 4-dose sIPV program (2, 3, and 4 months of age, and at 4 years of age) in our study showed good immunity, but the long-term persistence after four dose vaccinations and the epidemiological effects need to be studied further in the future.
Booster immunisation with either the bOPV or sIPV vaccine in different primary sequential groups showed a very good safety profile, which is consistent with previous studies.14,20 The adverse events in the study were mild and temporary. The occurrence of induration, redness, pain, and swelling reported among the five study groups were not different. The most common reported adverse events were local site reactions. In the study there were no deaths and no severe adverse events reported.
WPV is still endemic in Afghanistan and Pakistan which are the neighboring countries of our country, and the threat of poliovirus transmission has never ceased in China.21, 22, 23 Administration of IPV followed with bOPV reduces the risk of VAPP, while intestinal mucosal immunity keep high levels conferring by bOPV, it is the main potential benefit.24 Combined with the results of our study, the sequential vaccination regimen of sIPV and bOPV vaccines is still appropriate for China at the present stage. The persistence and immunogenicity results revealed that sIPV is more suitable for booster vaccination in children in China who have completed I–I–B as their primary immunisation. Group I–I–B–I seems better than group I–I–B–B in GMT against poliovirus type 2 considering the threat of VDPV2 at this stage, but whether transferring the polio vaccine program in China from I–I–B–B to I–I–B–I must consider many other factors like vaccine supply capacity, increased expenditure, and acceptance, etc. For the long-term consideration of the polio endgame,6 the tendency is gradually reducing the use of live attenuated vaccines when WPV is completely eliminated in the future, someday we will use all sIPV schedule at the final stages of polio eradication, our results support that schedule I–I–I–I may suitable for that stage in China and other similar countries which also using sIPV. On the other hand, novel OPV (nOPV) may play the role of a “back-up” vaccine to stimulate the mucosal immunity in preventing poliovirus transmission, with no risk of cVDPV.25 In addition to our research, as reported by Yamin Wang,26 the 2-dose schedule (4 month, 8–11 month) of sIPV provided same immunogenicity as 3-dose schedule (2, 3, 4 months) of sIPV which may considered as a dose-sparing strategy in the future. A comparative assessment of Salk-strain IPV and fIPV done in India demonstrated the superiority of 2 fIPV doses over 1 full-dose IPV,27 we believe that the results of our study combined with similar studies will be helpful for China polio eradication policy makers and other countries worldwide, especially at the final stage.
Limitations
Firstly, a certain percentage of participants were lost to follow-up due to the 4 years of follow up, the sample size for this trial depends on the follow-up rate of the previous trial. Secondly, for primary group I–B–B, we did not vaccinate them with bOPV as the booster dose because this schedule was unlikely to be the candidate strategy for poliovirus immunisation programmes in China since 2020.
Conclusions
Our study improves the understanding of different sIPV-bOPV sequential schedules, which is important to inform immunisation strategies for the next phase of polio eradication. The results indicated that high immune persistence against poliovirus types 1 and 3 was maintained four years after primary immunisation using different sequential schedules, while lower antibodies against poliovirus type 2 were found in children with one or two sIPV dose groups. We demonstrated that schedules containing three sIPV doses or with booster dose by sIPV at age 4 stimulate higher seropositivity rates against type 2 poliovirus. Our findings suggest that at least two doses of sIPV are needed in the current routine poliovirus immunisation schedule, and schedules containing 3 or 4 doses of sIPV provided better protection against poliovirus type 2 than the current schedule of sIPV-sIPV-bOPV-bOPV in China. Schedule containing 4 doses of sIPV can be adopted after OPV permanently withdrawal in the near future.
Contributors
Xuewen Tang supervised the data collection and verified the results. Hanqing He conceptualized the study and interpreted the results. YZ, RY, YL, ZYY, GPZ, ZBC and JJ coordinated study participant recruitment and study implementation. SYW、HPC、LYL、HW、XJZ contributed to study supervision. XD, YZ contributed to data analysis. XWT, YHX, HPC, XMY and HQH drafted the original version of the manuscript. All authors critically reviewed and approved the final version. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
De-identified data will be made available upon approval by researchers, with relevant agreements and approvals. Requests should be considered by request to the corresponding author.
Declaration of interests
XMY, YHX, HPC, LYL and SYW are employees of the China National Biotec Group Company Limited. HW and XJZ are employees of the Beijing Institute of Biological Products Company Limited. All other authors declare no competing interests.
Acknowledgments
We thank the study participants who involved in this study. This study was funded by the Medical Health Science and Technology of Zhejiang Province, China.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100725.
Contributor Information
Xiaoming Yang, Email: yangxiaoming@sinopharm.com.
Hanqing He, Email: hanqinghe@cdc.zj.cn.
Appendix A. Supplementary data
References
- 1.Donlan A.N., Petri W.A., Jr. Mucosal immunity and the eradication of polio. Science. 2020;368(6489):362–363. doi: 10.1126/science.abb8588. PMID: 32327582; PMCID: PMC7821416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Polio Eradication Initiative≥Data and monitoring > Polio this week > Wild poliovirus list [Internet] 2022. https://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/ [cited 2022 May 7]
- 3.Manish P., Stephen C. Addressing the challenges and opportunities of the polio endgame: lessons for the future. J Infect Dis. 2017;216(suppl_1):S1. doi: 10.1093/infdis/jix117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel M., Zipursky S., Orenstein W., et al. Polio endgame: the global introduction of inactivated polio vaccine. Expert Rev Vaccines. 2015;14(5):749–762. doi: 10.1586/14760584.2015.1001750. [DOI] [PubMed] [Google Scholar]
- 5.Garon J., Seib K., Orenstein W.A., et al. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccines. 2016:1–16. doi: 10.1586/14760584.2016.1140041. [DOI] [PubMed] [Google Scholar]
- 6.Polio Eradication Strategy 2022–2026: Executive summary. World Health Organization; Geneva: 2021. Licence CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 7.2022. https://polioeradication.org/news-post/report-of-polio-detection-in-united-states/
- 8.Wen N., Su Q., An Z., et al. Considerations and suggestions for polio vaccination strategies in China. J Vaccine Immun. 2018;3:349–353. [Google Scholar]
- 9.Okayasu H., Sein C., Hamidi A., Bakker W.A.M., Sutter R.W. Development of inactivated poliovirus vaccine from Sabin strains: a progress report. Biologicals. 2016;44:581–587. doi: 10.1016/j.biologicals.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson R.M., Claesson B.A., Fagerlund E., Knutsson N., Lundin C. Antibody persistence in five-year-old children who received a pentavalent combination vaccine in infancy. Pediatr Infect Dis J. 2002;21(6):535–541. doi: 10.1097/00006454-200206000-00011. PMID: 12182378. [DOI] [PubMed] [Google Scholar]
- 11.Langue J., Matisse N., Pacoret P., et al. Persistence of antibodies at 5-6 years of age for children who had received a primary series vaccination with a pentavalent whole-cell pertussis vaccine and a first booster with a pentavalent acellular pertussis vaccine: immunogenicity and tolerance of second booster with a tetravalent acellular vaccine at 5-6 years of age. Vaccine. 2004;22(11–12):1406–1414. doi: 10.1016/j.vaccine.2003.10.026. PMID: 15063563. [DOI] [PubMed] [Google Scholar]
- 12.Robinson C.L., Advisory committee on immunization practices (ACIP) ACIP child/adolescent immunization work group Advisory committee on immunization practices recommended immunization schedules for persons aged 0 through 18 Years--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(4):86–87. doi: 10.15585/mmwr.mm6504a4. PMID: 26845283. [DOI] [PubMed] [Google Scholar]
- 13.Guo X., Li Z., Yang J.P., et al. Enlightment of routine vaccination under the prevention and control of COVID-19 based on the circulating event of type Ⅲ vaccine-derived poliovirus in Shanghai. Zhonghua Yufang Yixue Zazhi. 2021;55(12):1377–1382. doi: 10.3760/cma.j.cn112150-20210809-00772. Chinese PMID: 34963232. [DOI] [PubMed] [Google Scholar]
- 14.He H., Wang Y., Deng X., et al. Immunogenicity of three sequential schedules with Sabin inactivated poliovirus vaccine and bivalent oral poliovirus vaccine in Zhejiang, China: an open-label, randomised, controlled trial. Lancet Infect Dis. 2020;20(9):1071–1079. doi: 10.1016/S1473-3099(19)30738-8. Epub 2020 May 19. PMID: 32442523. [DOI] [PubMed] [Google Scholar]
- 15.Lu L., Li X., Zhang H., et al. Immunogenicity and persistence from different 3-dose schedules of live and inactivated polio vaccines in Chinese infants. Vaccine. 2015;33(36):4653–4658. doi: 10.1016/j.vaccine.2014.08.091. Epub 2015 Feb 11. PMID: 25681659. [DOI] [PubMed] [Google Scholar]
- 16.Ciapponi A., Bardach A., Rey Ares L., et al. Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis. Cochrane Database Syst Rev. 2019;12(12):CD011260. doi: 10.1002/14651858.CD011260.pub2. PMID: 31801180; PMCID: PMC6953375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaby P., Nielsen S., Fisker A.B., et al. Stopping oral polio vaccine (OPV) after defeating poliomyelitis in low- and middle-income countries: harmful unintended consequences? Review of the nonspecific effects of OPV. Open Forum Infect Dis. 2022;9(8):ofac340. doi: 10.1093/ofid/ofac340. PMID: 35937644; PMCID: PMC9348612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Ryan M., Bandyopadhyay A.S., Villena R., et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect Dis. 2015;15(11):1273–1282. doi: 10.1016/S1473-3099(15)00219-4. [DOI] [PubMed] [Google Scholar]
- 19.Wright P.F., Wieland-Alter W., Ilyushina N.A., et al. Intestinal immunity is a determinant of clearance of poliovirus after oral vaccination. J Infect Dis. 2014;209:1628–1634. doi: 10.1093/infdis/jit671. [DOI] [PubMed] [Google Scholar]
- 20.Lv H., Pan X., Liang H., et al. A comparison with adverse events following immunization associated with sabin-strains and salk-strains inactivated polio vaccines in Zhejiang Province, China. Vaccines. 2022;10(2):319. doi: 10.3390/vaccines10020319. PMID: 35214777; PMCID: PMC8874468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu J., Yang Y., Huang L., et al. Immunogenicity and safety evaluation of bivalent types 1 and 3 oral poliovirus vaccine by comparing different poliomyelitis vaccination schedules in China: a randomized controlled non-inferiority clinical trial. Hum Vaccin Immunother. 2017;13:1–10. doi: 10.1080/21645515.2017.1288769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong S.Z., Zhu W.B. The role of Sabin inactivated poliovirus vaccine in the final phase of global polio eradication. Zhonghua Yufang Yixue Zazhi. 2016;50:1032–1035. doi: 10.3760/cma.j.issn.0253-9624.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Wright P.F., Connor R.I., Wieland-Alter W.F., et al. Vaccine-induced mucosal immunity to poliovirus: analysis of cohorts from an open-label, randomised controlled trial in Latin American infants. Lancet Infect Dis. 2016;16:1377–1384. doi: 10.1016/S1473-3099(16)30169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polio vaccines: WHO position paper – June 2022. 2022. http://www.who.int/wer Weekly epidemiological record. No 25 vol. 97, 277-300.
- 25.Konopka-Anstadt J.L., Campagnoli R., Vincent A., et al. Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization. NPJ Vaccines. 2020 Mar 20;5(1):26. doi: 10.1038/s41541-020-0176-7. PMID: 32218998; PMCID: PMC7083942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Xu Q., Jeyaseelan V., et al. Immunogenicity of two-dose and three-dose vaccination schedules with Sabin inactivated poliovirus vaccine in China: an open-label, randomized, controlled trial. Lancet Reg Health West Pac. 2021;10 doi: 10.1016/j.lanwpc.2021.100133. PMID: 34327346; PMCID: PMC8315596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad M., Verma H., Deshpande J., et al. Immunogenicity of fractional dose inactivated poliovirus vaccine in India. J Pediatric Infect Dis Soc. 2022;11(2):60–68. doi: 10.1093/jpids/piab091. PMID: 34791350; PMCID: PMC8865014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.