Abstract
Background.
Immune-checkpoint-inhibitors (ICIs) have dramatically improved clinical outcomes in multiple cancer types and are increasingly being used in early disease settings and in combinations. However, ICIs can also cause severe or even fatal immune-mediated adverse-events (irAE). Here, we identify and characterize significant cardiovascular irAE (CV-irAEs) associated with ICIs.
Methods.
We used VigiBase, the WHO’s global Individual-Case-Safety-Report database to identify drug-AE related to ICIs (n:31,321) and related to other drugs (n:16,343,451) through 01/2018. We evaluated the association between ICI and CV events using Reporting-Odds-Ratio (ROR) and Information-Component (IC). IC is an indicator value for disproportionate Bayesian reporting that compares observed and expected values to find drug-AE associations. IC025 is the lower-end of IC 95% credibility-interval and an IC025>0 is considered statistically significant.
Findings.
Using this agnostic approach, we identified multiple CV entities over-reported after ICI treatment compared to the entire database. ICI treatment was associated with higher reporting of myocarditis (n:122, ROR: 11.21 [9.36–13.43], IC025:3.2), pericardial diseases (n:95, ROR: 3.8 [3.08–4.62], IC025:1.63), and vasculitis (n:82, ROR: 1.56 [1.25–1.94], IC025:0.03), including temporal-arteritis (n:18, ROR: 12.99 [8.12–20.77], IC025:2.59). These CV-irAE affected mostly men (58–67%), with a wide age range (20–90 years) and occurred early after ICI administration (40–80% within one month of first ICI administration). Pericardial disorders were reported more often in patients with lung cancer (56.3%) whereas myocarditis and vasculitis were more commonly reported in patients with melanoma (40.7% and 60%, respectively; p<0.001). Vision was impaired in 27.8% of temporal-arteritis cases. CV-irAE were serious in the majority of cases (>80%), with fatalities occurring in 50% of myocarditis cases, 21.1% of pericardial disorders and 6.1% of vasculitis (p<0.0001). Among myocarditis cases, fatality was most frequent in ICI combination therapy compared to ICI monotherapy (65.6% vs. 44.4%, p:0.04).
Interpretation.
ICI may lead to severe and disabling inflammatory CV-irAEs early during therapy. Besides life-threatening myocarditis, these toxicities include pericardial disorders, as well as temporal arteritis with a risk for blindness.
Keywords: immune checkpoint inhibitors, oncology, cardiology, myocarditis, vasculitis, pericarditis, cardio-oncology
Introduction
Immune-checkpoint inhibitors (ICIs) have improved clinical outcomes in multiple cancer types and are now a mainstay of cancer treatment.1 ICIs include programmed cell death-protein 1 inhibitors (anti-PD-1 antibodies: nivolumab, pembrolizumab); programmed cell death-ligand 1 inhibitors (anti-PD-L1 antibodies: atezolizumab, avelumab, durvalumab) and cytotoxic T-lymphocyte–associated antigen 4 inhibitors (anti-CTLA-4 antibodies: ipilimumab, tremelimumab).2 Combination ICI therapy (for example, ipilimumab and nivolumab) has demonstrated particular efficacy in melanoma, renal cell carcinoma and lung cancer.1 ICIs can also cause immune-mediated adverse events (irAE), in some cases leading to severe or even fatal complications.[3] Recently, there have been increased reports of myocarditis associated with ICI treatment.[3–6] It remains unclear, however, if ICI are associated with other immune mediated cardiovascular adverse events (CV-irAE). Moreover, the characteristics, timing, and outcomes of myocarditis and other possible ICI immune related CV-irAE are unknown. Defining these ICI-associated toxicities is a critical issue for patient safety, especially given that ICI are combined with other agents which have their own CV toxicities. Here, we used VigiBase, the World Health Organization’s (WHO) global database of individual case safety reports (ICSRs) to further characterize these CV-irAE.[7]
Material and Methods
CV-irAE over-reporting signal detection using the World health organization (WHO) pharmacovigilance database of individual case safety reports (ICSRs), VigiBase[7]
Study design and data sources
The study is a disproportionality analysis based on adverse drug reactions reported within VigiBase, the WHO global deduplicated ICSRs database, originating from more than 130 countries.7 VigiBase is managed by Uppsula monitoring center (UMC) and contains more than 16 million ICSRs submitted by national pharmacovigilance centers since 1967. These reports originate from different sources such as healthcare professionals, patients, and pharmaceutical companies and are generally notified post-marketing. The use of confidential electronically processed patient data was approved by the French National Commission for Data Protection and Liberties (Commission Nationale de l’Informatique et des Libertés; reference: 1922081).
Procedures
This observational retrospective study included all CV-irAE classified by group queries according to the Medical Dictionary for Drug Regulatory Activities (MedDRA) detailed in Appendix page 1, between inception in November 14, 1967 and January 02, 2018. CV-irAE specifically considered in the analysis were those notified as suspected to be induced by ICI. Each report contains general administrative information (country of origin, date of reporting, reporter qualification), patient characteristics (sex, age), drugs (indication for the drug, start and end dates, dosage regimen, route of administration), reactions/events (reported terms, MedDRA classification terms, onset date, end date, seriousness, final outcome). ICI studied were anti-PD-1 antibodies (nivolumab, pembrolizumab), anti-PD-L1 antibodies (atezolizumab, avelumab, durvalumab) and anti-CTLA-4 antibodies (ipilimumab, tremelimumab). A severe AE was defined as such when causing death, being life-threatening, requiring hospitalization (initial or prolonged), leading to persistent or significant disability, congenital anomaly, birth defect, or to any other medically important conditions. Of note, First reports of ICSRs associated with ICI started in 2008.
Statistical analysis
VigiBase allows for disproportionality analysis (also known as case/non-case analysis), which we used to study if suspected drug-induced CV events were differentially reported with ICI as compared to CV events reported in the entire database with all the suspected drug-induced adverse reactions. Disproportionality analysis was also used to compare CV toxicities with different ICI regimens: anti-CTLA-4 monotherapy versus anti-PD-1/PD-L1 monotherapy versus combination ICI therapy (anti-CTLA-4 and anti-PD-1/PD-L1 combination therapy). Disproportionality analysis compares the proportion of selected specific adverse-drug-reaction reported for a single or a group of drugs (e.g., ICI) with the proportion of the same adverse-drug-reaction for a control group of drugs (e.g., full database). The denominator in these analyses is the overall adverse-drug-reactions reported for each group of drugs. If the proportion of an adverse-drug-reaction is greater in patients exposed to a group of drug (cases) than in patients not exposed to this drug (non-cases), this suggests an association between the specific drug and the reaction and is a potential signal for safety. Disproportionality can be either calculated by the information component (IC) or reporting odds-ratio (ROR) when using full database as comparator, and only ROR when using different drug regimen subgroups as comparators.
Calculation of the IC, using a Bayesian confidence propagation neural network, was specifically developed and validated by UMC as a flexible, automated indicator value for disproportionate reporting that compares observed and expected drug-AE associations to find new drug-AE signals with identification of probability difference from the background data (full database).8 Probabilistic reasoning in intelligent systems (information theory) has been proven effective to manage large data sets, is robust in handling incomplete data, and may be used with complex variables. Information theory tool is ideal for finding drug-AE combinations with other variables, which are highly associated compared to the generality of the stored data.8 Several examples with IC have been first validated showing the power of the technique to find signals very early after drug approval (e.g. captopril ± coughing) and to avoid false positives where a common drug and a common AE association occur in the database, only because the drug is widely used and the AE frequently reported (e.g. digoxin ± acne; digoxin ± rash).8,9
The statistical formula is as follows,
| (1) |
| (2) |
Nexpected: the number of case reports expected for the drug-adverse effect combination
Nobserved: the actual number of case reports for the drug- adverse effect combination
Ndrug: the number of case reports for the drug, regardless of adverse effects
Neffect: the number of case reports for the adverse effect, regardless of drug
Ntotal: the total number of case reports in the database
IC025 is the lower end of a 95% credibility interval for the Information Component. A positive IC025 value (>0) is the traditional threshold used in statistical signal detection at UMC.8,9 IC025 have only been validated for comparison of a drug versus full database and cannot be used to compare disproportionate reporting within different ICI regimens. Disproportionality for CV-irAE reporting as a function of variable ICI regimen was estimated by calculating the ROR Chi-square (Graphpad Prism 7), described elsewhere and detailed in Appendix page 2.10–12 The lower end of ROR 95% confidence interval (CI) ≥1 is the threshold used for significant statistical signal detection.
Characteristics of cases were described in terms of means (± standard deviation) or medians (with interquartile range) for quantitative variables, and in terms of effective and proportion for qualitative ones.
Results
CV-irAE signal detected using WHO’s global database of ICSRs
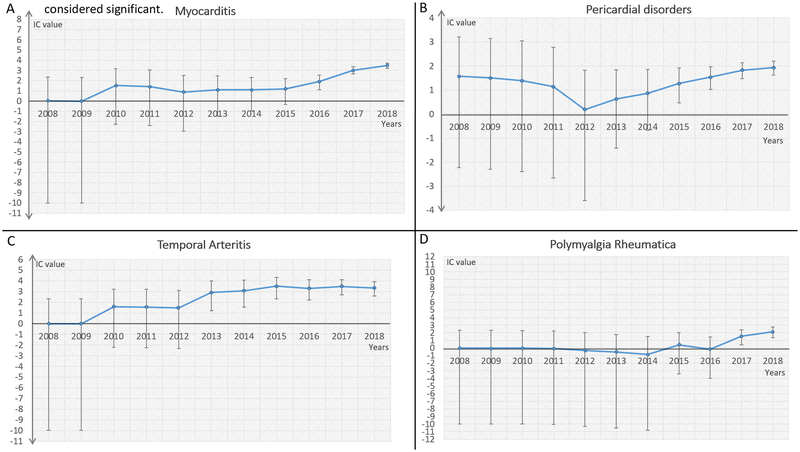
Given the considerable overlap of symptoms for various CV disease etiologies as well as redundancies in reporting cardiac complications associated with oncologic therapies, we broadly categorized cardiac and vascular disease entities in the MedDRA Classification (Version 20.1) based on underlying pathophysiology (Appendix page 1). CV-AE reporting was compared to total AE (rate) in ICI subgroup (n: 31,321) versus the entire database (n: 16,343,451 for IC calculation, and n: 12,455,401 for ROR calculation). All patients were included for these analysis. IC calculation was based on all drugs and all events in VigiBase (from November 14, 1967 to January 02, 2018, as validated elsewhere),8,9 while ROR calculation was applied to all drugs and all events after the year of first AE report associated with ICI in VigiBase (from January 01, 2008 to January 02, 2018). Details concerning number of CV-irAE by different immunotherapy regimen and CV grouping categories are available in Tables 1 and 2. Using this agnostic approach, we identified four broad CV entities where CV reporting was significantly increased after ICI treatment as compared to the entire database. ICI treatment was associated with higher reporting of myocarditis (ROR: 11.21 [9.36–13.43], IC025: 3.2, Figure 1), pericardial diseases (ROR: 3.8 [3.08–4.62], IC025: 1.63, Figure 1), supraventricular arrhythmias (ROR: 1.72 [1.51–1.97], IC025: 0.56, Figure 1) and vasculitis (ROR: 1.56 [1.25–1.94], IC025: 0.03). Further interrogation of vasculitis reporting revealed that this signal was driven primarily by temporal arteritis (ROR: 12.99 [8.12–20.77], IC025: 2.59, Figure 1) and polymyalgia rheumatica (ROR: 5.13 [3.13–8.40], IC025: 1.33, Figure 1). Other cardiac disease conditions, including ischemia, heart failure, and valvular disorders were not over-reported in this population (Table 1). IC values and their 95% credibility interval over time for myocarditis, pericardial disorders, temporal arteritis and polymyalgia rheumatica are represented in Figure 1.
Table 1. Information component (IC) and its 95% credibility interval lower endpoint (IC025) comparing cardiovascular immune related adverse events (CV-irAE) associated to overall immunotherapy vs. full database from VigiBase (from inception in 1967 to 01/2018).
A positive IC025 value (>0) is the traditional threshold used in statistical signal detection with VigiBase. Results that are statistically significant are in bold.
| Overall immunotherapy | Full database (starting 1967) | IC / IC025 | ||
|---|---|---|---|---|
| Total number of ICSRs available | 31,321 | 16,343,451 | ||
| Number of ICSRs by CV-irAE subgroups | ||||
| Myocarditis | 122 (0.39%) | 5,515 (0.03%) | 3.47/3.2 | |
| Pericardial diseases | 95 (0.3%) | 12,800 (0.08%) | 1.93/1.63 | |
| Cardiac supra-ventricular arrhythmias | 222 (0.71%) | 68,597 (0.42%) | 0.75/0.56 | |
| Vasculitis | 82 (0.26%) | 33,289 (0.2%) | 0.36/0.03 | |
| - Polymyalgia rheumatica | 16 (0.05%) | 1709 (0.01%) | 2.12/1.33 | |
| Heart Failure | 225 (0.72%) | 142,502 (0.87%) | −0.28/−0.47 | |
| Cerebral hemorrhage | 250 (0.8%) | 179,621 (1.1%) | −0.46/−0.65 | |
| Endocardial disorders | 8 (0.03%) | 3,149 (0.02%) | 0.38/−0.79 | |
| Hemorrhage (clinical events) | 1,023 (3.27%) | 875,398 (5.36%) | −0.71/−0.80 | |
| Cerebral arterial ischemia | 195 (0.62%) | 161,618 (0.99%) | −0.67/−0.88 | |
| Cardiac conductive disorders | 37 (0.12%) | 26,008 (0.16%) | −0.42/−0.93 | |
| Myocardial infarction | 167 (0.53%) | 163,908 (1%) | −0.91/−1.14 | |
| Biological hemostatic disorders favoring hemorrhage | 135 (0.43%) | 136,474 (0.84%) | −0.95/−1.21 | |
| Arterial systemic ischemia | 203 (0.65%) | 215,741 (1.32%) | −1.02/−1.23 | |
| Cardiac death or shock | 136 (0.43%) | 144,825 (0.89%) | −1.03/−1.28 | |
| Hypertension and related end-organ damages | 198 (0.63%) | 239,232 (1.46%) | −1.2/−1.42 | |
| Vascular neoplasm | 4 (0.01%) | 2,687 (0.02%) | −0.33/−2.06 | |
| Torsade de pointes / Long QT | 22 (0.07%) | 31,642 (0.19%) | −1.44/−2.11 | |
| Cardiac ventricular arrhythmias | 22 (0.07%) | 33,504 (0.2%) | −1.52/−2.19 | |
| Pulmonary hypertension and related cardiac involvement | 17 (0.05%) | 30,718 (0.19%) | −1.76/−2.53 | |
| Cardiac valve disorders | 2 (0.01%) | 25,500 (0.16%) | −4.3/−6.89 | |
| Dyslipidemia | 20 (0.06%) | 64,555 (0.39%) | −2.6/−3.3 | |
Overall immunotherapy: Any individual case safety reports related to nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, ipilimumab or tremelimumab.
Table 2. Reporting Odds-Ratio (ROR) and its 95% confidence interval (CI), comparing selected cardiovascular adverse events (detected as signals) in overall immunotherapy (IMU) vs. full database (full); combined immunotherapy (COMB) vs. monoimmunotherapy (MONO); mono-immunotherapy with anti-PD-1/PD-L1 (PD1) vs. mono-immunotherapy with anti-CTLA-4 (CTLA4) from VigiBase (time period: 01/2008 to 01/2018).
Significant over-reporting are in bold after Bonferroni-adjustment for multiple tests within immunotherapy subgroups (p≤(0.05/10 tests)=> p≤0.005). First reports of ICSRs associated with ICI started in 2008.
| Overall immunotherapy (IMU; n: 31,321) | |||||||
|---|---|---|---|---|---|---|---|
| MONO (n: 28,909) | COMB (n: 2,412) | ||||||
| MONO-PD1 (n: 20,643) | MONO-CTLA4 (n: 8,266) | ||||||
| Number of ICSRs by CV-ADR subgroup | |||||||
| Myocarditis | 84 (0.41%) | 6 (0.07%) | 32 (1.3%) | 4,454 (0.04%) | 5.62 [2.46–12.88] | 4.31 [2.86–6.38] | 11.21 [9.36–13.43] |
| Pericardial diseases | 74 (0.36%) | 13 (0.16%) | 8 (0.33%) | 10,009 (0.08%) | 2.28 [1.27–4.12] | 1.1 [0.53–2.24] | 3.8 [3.08–4.62] |
| Vasculitis | 56 (0.27%) | 18 (0.22%) | 8 (0.33%) | 20,987 (0.2%) | 1.25 [0.73–2.12] | 1.3 [0.62–2.67] | 1.56 [1.25–1.94] |
| Number of ICSRs in vasculitis-ADR subgroup | |||||||
| Temporal arteritis | 7 (0.03%) | 10 (0.12%) | 1 (0.04%) | 568 (<0.01%) | 0.28 [0.11–0.74] | 0.71 [0.07–3.94] | 12.99 [8.12–20.77] |
| Polymyalgia rheumatica | 14 (0.07%) | 1 (0.01%) | 1 (0.04%) | 1254 (0.01%) | 5.61 [0.74–42.66] | 0.8 [0.08–4.62] | 5.13 [3.13–8.40] |
Overall immunotherapy (IMU): Any individual case safety report related to nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, ipilimumab or tremelimumab.
Mono immunotherapy (MONO):
- Anti PD-1/PD-L1 monotherapy: any of nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab used alone (MONO-PD1)
- Anti CTLA-4 monotherapy: ipilimumab or tremelimumab alone (MONO-CTLA4)
Combination immunotherapy (COMB):
Any individual case safety report related to at least one drug from Anti PD-1/PD-L1 inhibitors combined to an Anti CTLA-4
Abbreviations: ICSRs, individual case safety reports
Figure 1.
Information component (IC) and its 95% credibility interval (error bar, IC025 and IC975) over time for myocarditis (A), pericardial disorders (B), temporal arteritis (C), and polymyalgia rheumatica (D). Lower end of IC 95% credibility interval >0 is considered significant.
Reported supraventricular arrhythmias on ICI (n: 222, Appendix page 3) were overwhelmingly associated with other concurrent irAE such as gastrointestinal disorders (93 (41.9%) of 222; mainly colitis and diarrhea leading to dehydration and electrolyte disorders), other cardiac conditions (69 (31.1%) of 222, mainly cardiac dysfunction, cardiac ischemia and pericardial disorders), endocrine disorders (66 (29.7%) of 222; mainly thyroid abnormalities), and neurologic disorders (28 (12.6%) of 222, including strokes and encephalitis). Since supraventricular arrhythmias are common in the cancer population and usually co-occurred with other irAE, we suspect that they were secondary to concurrent irAE complications rather than ICI treatment itself. Thus, we focused on the other CV-irAE (myocarditis, pericardial diseases and vasculitis) to determine if there was an increased signal for toxicity with various ICI regimens (Table 2) as well as to describe clinical characteristics associated with each toxicity.
We asked whether each CV-irAE was more common with anti-CTLA-4 monotherapy compared to anti-PD-1/PD-L1 monotherapy or combination ICI therapy compared to monotherapy (Table 2). ICI-associated myocarditis was over-reported for anti-PD-1/PDL1 vs. anti-CTLA-4 monotherapy (ROR: 5.62 [2.46–12.88]) and with combination vs. mono immunotherapy (ROR: 4.31 [2.86–6.38]). ICI-associated pericardial disorders were over-reported for anti-PD-1/PD-L1 vs. anti-CTLA-4 monotherapy (ROR: 2.28 [1.27–4.12]). On the other hand, temporal arteritis was over-reported for anti-CTLA-4 monotherapy versus anti-PD-1/PD-L1 monotherapy (ROR: 3.57 [1.34–9.33]).
Characteristics of patients, timing, and outcomes of CV-irAE.
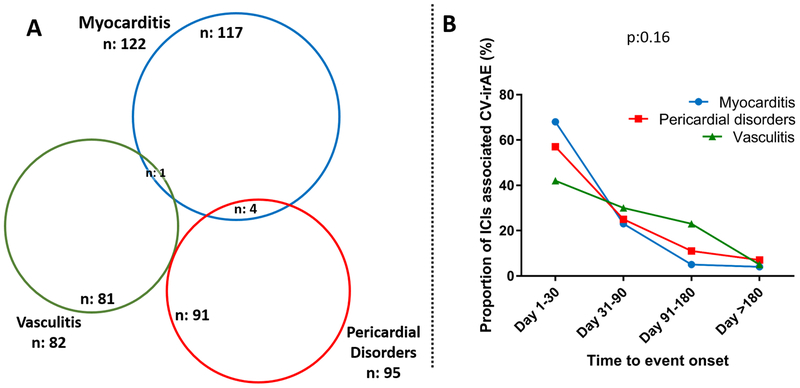
Given that ICI-associated toxicities are novel clinical entities and represent new challenges for the clinician, we extracted further data to better characterize clinical characteristics of ICI-associated myocarditis (n: 122), pericardial disorders (n: 95), and vasculitis (n: 82 which included polymyalgia rheumatica n: 16 and temporal arteritis n: 18) (Tables 3 and 4). Cases of myocarditis, pericardial disorders and vasculitis were rarely overlapping (Figure 2). Diagnosis of each was made from across the globe, mainly by healthcare professionals with an increasing rate over time with most of the cases reported in 2017 and 2018. CV-irAE affected mostly men (58–67%), with a wide age range (20–90 years old). All cancer types where ICI have been utilized were affected, with melanoma, lung cancer and renal cell carcinoma being the most common (Tables 3–4). Pericardial disorders were more likely to be reported in patients with lung cancer whereas myocarditis and vasculitis were more commonly observed in patients with melanoma (p<0.001, Tables 3–4). These CV-irAE occurred early after ICI administration, as soon as after the first ICI dose, with a median time to onset of 30 days (IQR: 18–60 days) for myocarditis, 30 days (IQR: 8.5–90 days) for pericardial disorders and 55 days (IQR: 21–98 days) for vasculitis. There was no significant difference in time to onset between these CV-irAE (Figure 2, p: 0.16). CV-irAE were serious in the majority of cases (>80%), with fatalities occurring in 61 (50%) of 122 myocarditis cases, 20 (21.1%) of 95 pericardial disorders and 5 (6.1%) of 82 vasculitis (Chi-2 test for fatality rates between these latter conditions; p<0.0001). Among myocarditis cases, there were 21 deaths among 32 ICI combination therapy (65.6%) compared to 40 (44.4%) of 90 ICI monotherapy (p: 0.04,). Myocarditis was frequently associated with myositis, cardiac arrhythmias, heart failure, myasthenia gravis-like symptoms, pneumonitis and rarely pericardial disorders. Pericardial disorders were frequently associated with pulmonary disorders (mainly pleural effusions and pneumonitis) and thyroid dysfunction. Vasculitis was frequently associated with concurrent dermatologic and rheumatologic conditions (mainly arthritis) and ophthalmologic conditions (leading to impaired vision in 5 (27.8%) of 18 temporal arteritis cases). More specific details and proportion concerning characteristics of concurrent irAE associated with these CV-irAE and of ICI associated temporal arteritis and polymyalgia rheumatica are provided in Table 4.
Table 3.
Details concerning patients with ICI associated myocarditis (n: 122) or pericardial disorders (n: 95) collected from VigiBase (last accessed: 01/2018).
| Myocarditis | Pericardial disorders | |||
|---|---|---|---|---|
| Characteristics | n (%) | Data available, n (%) | n (%) | Data available, n (%) |
| Reporting region | 122 (100.0) | 95 (100.0) | ||
| Americas | 58 (47.5) | 47 (49.4) | ||
| Europe | 49 (40.2) | 38 (40.0) | ||
| Australia | 2(1.6) | 1 (1.1) | ||
| Asia | 13 (10.7) | 9(9.5) | ||
| Reporters | 119 (97.5) | 94 (98.9) | ||
| Healthcare professional | 101 (84.9) | 77 (81.9) | ||
| Non-healthcare professional | 18 (15.1) | 17 (18.1) | ||
| Reporting year | 122 (100.0) | 95 (100.0) | ||
| 2018 | 35 (28.7) | 13 (13.7) | ||
| 2017 | 72 (59.0) | 49 (51.6) | ||
| 2016 | 8(6.6) | 15 (15.8) | ||
| 2015 | 5(4.1) | 13 (13.7) | ||
| 2013–2014 | 2(1.6) | 5(5.3) | ||
| Gender | 117 (95.9) | 95 (100.0) | ||
| Male | 78 (66.7) | 57 (60.0) | ||
| Female | 39 (33.3) | 38 (40.0) | ||
| Age at onset, mean ± SD, years | 66.4 ± 12.7 | 99 (81.1) | 59.5 ± 11.8 | 81 (85.3) |
| [min-max] | [20–90] | [22–82] | ||
| Suspected Drugs* | 122 (100.0) | 95 (100.0) | ||
| Only ICI | 114 (93.4) | 88 (92.6) | ||
| ICI + 1 other drug | 4(3.3) | 4(4.2) | ||
| ICI + ≥2 other drugs | 4(3.3) | 3(3.2) | ||
| Drugs | 122 (100.0) | 95 (100.0) | ||
| Monotherapy with Anti PD-1/PD-L1 | 84 (68.9) | 74 (77.9) | ||
| - Nivolumab | 58 (47.5) | 42 (44.2) | ||
| - Pembrolizumab | 22 (18.0) | 21 (22.1) | ||
| - Atezolizumab | 2 (1.6) | 9 (9.5) | ||
| - Avelumab | 1 (0.8) | 1 (1.1) | ||
| - Durvalumab | 1 (0.8) | 1 (1.1) | ||
| Monotherapy with Anti CTLA-4 | 6(4.9) | 13 (13.7) | ||
| - Ipilimumab | ||||
| Combination therapy | 32 (26.2) | 8 (8.4) | ||
| - Nivolumab + Ipilimumab | 30 (24.6) | 8 (8.4) | ||
| - Pembrolizumab + Ipilimumab | 1 (0.8) | 0 (0.0) | ||
| - Tremelimumab + Durvalumab | 1 (0.8) | 0 (0.0) | ||
| Drug dosing | ||||
| Ipilimumab | 23 (62.7) | 12 (57.1) | ||
| - 1–3 mg/kg | 19 (73.1) | 11 (91.7) | ||
| - 5 mg/kg | 1 (3.8) | 1 (8.3) | ||
| - 10 mg/kg | 3 (11.5) | 0 (0.0) | ||
| Nivolumab | 49 (55.7) | 38 (76.0) | ||
| - 1–2 mg/kg | 16 (32.7) | 7 (18.4) | ||
| - ≥ 3 mg/kg | 33 (67.3) | 31 (81.6) | ||
| Pembrolizumab | 11 (47.8) | 16 (76.2) | ||
| - 2 mg/kg | 6 (54.5) | 4 (25.0) | ||
| - 3 mg/kg | 5 (45.5) | 12 (75.0) | ||
| Number of ICI administration before onset, median [IQR], | 1 (1–2.75) | 21 (17.2) | 2(1–6) | 9(9.5) |
| [min-max] | [1–8] | [1–10] | ||
| Time to irAE onset, days: | 44 (36.1) | 28 (29.5) | ||
| Median, [IQR] | 30 [18–60] | 30 [8.5–90] | ||
| [min-max] | [1–240] | [0–330] | ||
| Severe AE | 102 (83.6) | 122 (100.0) | 77 (81.0) | 95 (100.0) |
| Death | 61 (50.0) | 122 (100.0) | 20 (21.1) | 95 (100.0) |
| Malignant Neoplasm Progression | 8(6.6) | 122 (100.0) | 11 (11.6) | 95 (100.0) |
| Indications** | 103 (84.4) | 87 (91.6) | ||
| Malignant melanoma | 42 (40.7) | 16 (18.4) | ||
| Lung cancer | 33 (32.1) | 49 (56.3) | ||
| Renal cell carcinoma | 11 (10.7) | 6 (7.0) | ||
| Gastrointestinal cancer | 5(4.9) | 1 (1.1) | ||
| Mesothelioma | 4(3.9) | 1 (1.1) | ||
| Hematologic cancer and lymphoma | 2(1.9) | 3(3.5) | ||
| Thymic cancer | 2 (1.9) | 0 (0.0) | ||
| Urothelial cancer | 2 (1.9) | 2(2.3) | ||
| Malignant neoplasm non specified | 2 (1.9) | 2 (2.3) | ||
| Squamous cell carcinoma of head and neck | 0 (0.0) | 2 (2.3) | ||
| Glioblastoma | 0 (0.0) | 1 (1.1) | ||
| Breast cancer | 0 (0.0) | 4(4.6) | ||
| Concurrent irAE | 122 (100.0) | 95 (100.0) | ||
| None (lone) | 36 (29.5) | 35 (36.8) | ||
| Gastro-intestinal disorders (any) | 19 (15.6) | 10 (10.5) | ||
| - Hepatitis / hepatic failure / Abnormal liver enzymes | 13 (10.7) | 6 (6.3) | ||
| - Colitis / diarrhea / gastroenteritis / enteritis / Dehydration | 9 (7.4) | 4 (4.2) | ||
| - Gastro-intestinal bleeding | 1 (0.8) | 0 (0.0) | ||
| - Electrolyte disorders | 1 (0.8) | 1 (1.1) | ||
| Endocrino-metabolic disorders (any) | 6 (4.9) | 6 (6.3) | ||
| - Hypophysitis | 3 (2.5) | 0 (0.0) | ||
| - Thyroid disorders | 3 (2.5) | 5(5.3) | ||
| - Adrenal insufficiency | 1 (0.8) | 3 (3.2) | ||
| - Diabetes mellitus | 1 (0.8) | 0 (0.0) | ||
| Pulmonary disorders (any) | 16 (13.1) | 38 (40.0) | ||
| - Pneumonitis / Pneumonia | 13 (10.7) | 8 (8.4) | ||
| - Pleural effusion | 3 (2.5) | 22 (23.2) | ||
| - Respiratory failure / respiratory disorder | 2(1.6) | 4(4.2) | ||
| - Pulmonary embolism | 0 (0.0) | 4 (4.2) | ||
| - Pulmonary hypertension | 0 (0.0) | 1 (1.1) | ||
| Cardiovascular disorders (any) | 49 (40.2) | 14 (14.7) | ||
| - Arrhythmia | 23 (18.9) | 6 (6.3) | ||
| - Cardiac failure or shock / pulmonary edema | 19 (15.6) | 3 (3.2) | ||
| - Cardiac arrest | 11 (9) | 4 (4.2) | ||
| - Myocardial infarction / coronary artery disease | 5 (4.1) | 1 (1.1) | ||
| - Myocarditis | --- | 4 (4.2) | ||
| - Pericarditis / pericardial effusion / cardiac tamponade | 4 (3.3) | --- | ||
| - Vasculitis | 1 (0.8) | 0 (0.0) | ||
| Renal disorders (any) | 4 (3.3) | 8 (8.4) | ||
| - Nephritis | 2 (1.6) | 1 (1.1) | ||
| - Acute kidney injury / renal failure | 2 (1.6) | 7 (7.4) | ||
| Hematologic disorders (any) | 2 (1.6) | 0 (0.0) | ||
| - Leukopenia | 1 (0.8) | 0 (0.0) | ||
| - Thrombocytopenia | 1 (0.8) | 0 (0.0) | ||
| Musculoskeletal disorders (any) | 34 (27.9) | 5(5.3) | ||
| - Myositis / Rhabdomyolysis | 31 (25.4) | 3 (3.2) | ||
| - Arthralgia / Rheumatoid arthritis / Joint inflammation | 3(2.5) | 3 (3.2) | ||
| Neurologic disorders (any) | 15 (12.3) | 1 (1.1) | ||
| - Myasthenia gravis | 13 (10.7) | 1 (1.1) | ||
| - Encephalitis / Meningitis | 2(1.6) | 0 (0.0) | ||
| - Stroke (ischemic or hemorrhagic) | 1 (0.8) | 0 (0.0) | ||
| Dermatologic disorders (any) | 3 (2.5) | 3(3.2) | ||
| - Unspecified rash / Purpura / Pruritis | 1 (0.8) | 3(3.2) | ||
| - Steven Johnson Syndrome | 1 (0.8) | 0 (0.0) | ||
| - Pemphigus | 1 (0.8) | 0 (0.0) | ||
| - Lichen planus | 1 (0.8) | 0 (0.0) | ||
Other concomitant reported suspected medications for myocarditis were carboplatin (n: 3), axitinib (n: 1), bendamustine (n: 1), cetuximab (n: 1), cisplatin (n: 1), cyclophosphamide (n: 1), dabrafenib (n: 1), etinostat (n: 1), etoposide (n: 1), gemcitabine (n: 1), niraprib (n: 1), obinutuzumab (n: 1), paclitaxel (n: 2), temozolomide (n: 1), trametinib (n: 1).
Other concomitant reported suspected medications for pericardial disorders were paclitaxel (n: 3), carboplatin (n: 2), dabrafenib (n: 1), etinostat (n: 1), niraprib (n: 1), temozolomide (n: 1), trametinib (n: 1).
Of the 42 ICI-associated myocarditis cases in melanoma patients, 6 were treated with anti-CTLA-4 monotherapy, 15 with anti-PD-1 or anti-PD-L1 monotherapy, and 21 with a combination of an anti-CTLA-4 plus an anti-PD-1 or an anti-PD-L1 therapy. Of the 33 ICI-associated myocarditis cases in lung cancer patients, 27 were treated with anti-PD-1 or anti-PD-L1 monotherapy, and 6 were treated with a combination of an anti-CTLA-4 plus an anti-PD-1 or anti-PD-L1 therapy. Of the 16 ICI-associated pericardial disorders in melanoma patients, anti-CTLA-4 monotherapy, anti-PD-1 or anti-PDL-L1 monotherapy, and combination therapy were used in 4, 8, 4 patients, respectively. In total, 49 cases of ICI-associated pericardial disorders was seen in lung cancer patients of which 48 were treated with anti-PD-1 or anti-PD-L1 monotherapy, and 1 with combination therapy.
Abbreviations: CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ICI, immune checkpoint inhibitor; IQR, interquartile range; irAE, immune related adverse event; [min-max], minimum-maximum; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; SD, standard deviation
Table 4.
Details concerning patients with ICI associated temporal arteritis (n: 18), polymyalgia rheumatica (n: 16) or vasculitis (n: 82) collected from VigiBase (last accessed: 01/2018).
| Temporal arteritis | Polymyalgia Rheumatica | Vasculitis | ||||
|---|---|---|---|---|---|---|
| Characteristics | n (%) | Data availability, n (%) | n (%) | Data availability, n (%) | n (%) | Data availability, n (%) |
| Reporting region | 18 (100.0) | 16 (100.0.0) | 82 (100.0) | |||
| Americas : | 9 (50.0) | 4 (25.0) | 33 (40.2) | |||
| Europe | 8 (44.4) | 6 (37.5) | 40 (48.8) | |||
| Asia | 1 (5.6) | 6 (37.5) | 9 (11.0) | |||
| Reporters | 18 (100.0) | 16 (100.0) | 81 (98.8) | |||
| Healthcare professional | 17 (94.4) | 15 (93.8) | 73 (90.1) | |||
| Non-healthcare professional | 1 (5.6) | 1 (6.2) | 8(9.9) | |||
| Reporting year | 18 (100.0) | 16 (100.0) | 82 (100.0) | |||
| 2018 (thru February the 16th 2018) | 2 (11.1) | 0 (0.0) | 2 (2.4) | |||
| 2017 | 5 (27.8) | 12 (75.0) | 44 (53.7) | |||
| 2016 | 4 (22.2) | 3 (18.8) | 17 (20.7) | |||
| 2015 | 4 (22.2) | 0 (0.0) | 10 (12.2) | |||
| 2012–2014 | 3 (16.7) | 1 (6.2) | 9 (11.0) | |||
| Gender | 17 (94.5) | 15 (93.8) | 77 (93.9) | |||
| Male | 9 (52.9) | 10 (66.7) | 45 (58.4) | |||
| Female | 8 (47.1) | 5 (33.3) | 32 (41.6) | |||
| Age at onset, mean ± SD, years | 72.93 ± 8.1 | 14 (77.8) | 75.54 ± 6.4 | 13 (81.3) | 68.24 ± 11.8 | 67 (81.7) |
| [min-max] | [60–83] | [63–88] | [31–88] | |||
| Suspected Drugs* | 18 (100.0) | 16 (100.0) | 82 (100.0) | |||
| Only ICI | 14 (77.8) | 16 (100) | 71 (86.6) | |||
| ICI + 1 other drug | 2 (11.1) | 0 (0.0) | 7(8.5) | |||
| ICI + ≥2 other drugs | 2 (11.1) | 0 (0.0) | 4(4.9) | |||
| Drugs | 18 (100.0) | 16 (100.0) | 82 (100.0) | |||
| Monotherapy with Anti PD1/PD-L1 | 7 (38.9) | 14 (87.6) | 56 (68.3) | |||
| - Nivolumab | 3 (16.7) | 11 (68.8) | 33 (40.3) | |||
| - Pembrolizumab | 4 (22.2) | 3 (18.8) | 20 (24.4) | |||
| - Durvalumab | 0 (0.0) | 0 (0.0) | 1 (1.2) | |||
| - Atezolizumab | 0 (0.0) | 0 (0.0) | 2 (2.4) | |||
| Monotherapy with Anti CTLA-4 | 10 (55.5) | 1 (6.2) | 18 (22.0) | |||
| - Ipilimumab | ||||||
| Combination therapy | 1 (5.6) | 1 (6.2) | 8(9.7) | |||
| - Nivolumab + Ipilimumab | 1 (5.6) | 1 (6.2) | 7(8.5) | |||
| - Pembrolizumab + Ipilimumab | 0 (0.0) | 0 (0.0) | 1 (1.2) | |||
| Drug dosing | ||||||
| Ipilimumab | 7 (63.6) | 1 (50.0) | 16 (61.5) | |||
| - 1–3 mg/kg | 5 (71.4) | 1 (100.0) | 12 (75.0) | |||
| - 10 mg/kg | 2 (28.6) | 0 (0.0) | 4 (25.0) | |||
| Nivolumab | 2 (50.0) | 7 (58.3) | 23 (57.5) | |||
| - 3 mg/kg | 2 (100.0) | 7 (100.0) | 23 (100.0) | |||
| Pembrolizumab | 2 (50.0) | 3 (100.0) | 13 (61.9) | |||
| - 2 mg/kg | 1 (50.0) | 3 (100.0) | 9 (69.2) | |||
| - 3 mg/kg | 1 (50.0) | 0 (0.0) | 4 (30.8) | |||
| Number of ICI administration before onset, | 7 (38.9) | 7 (43.8) | 39 (47.6) | |||
| Median, [IQR] | 2 [2–5] | 6 [5–12] | 3 [1–7] | |||
| [min-max] | [1–7] | [2–12] | [1–31] | |||
| Time to irAE onset, days: | 5 (27.8) | 8 (50.0) | 39 (47.6) | |||
| Median, [IQR] | 21 [21–98] | 77 [59–153] | 55 [21–98] | |||
| [min-max] | [21–131] | [20–168] | [1–542] | |||
| Severe AE | 11 (68.8) | 16 (88.9) | 7 (43.8) | 16 (100.0) | 47 (61.8) | 76 (92.7) |
| Death | 0 (0.0) | 18 (100.0) | 0 (0.0) | 16 (100.0) | 5 (6.1) | 82 (100.0) |
| Malignant neoplasm progression | 0 (0.0) | 18 (100.0) | 0 (0.0) | 16 (100.0) | 1 (1.2) | 82 (100.0) |
| Indications** | 13 (72.3) | 13 (81.25) | 70 (85.3) | |||
| Malignant melanoma | 10 (76.9) | 9 (69.2) | 42 (60.0) | |||
| Lung cancer | 2 (15.4) | 3 (23.1) | 17 (24.4) | |||
| Squamous cell carcinoma of head and neck | 1 (7.7) | 0 (0) | 4 (5.7) | |||
| Renal carcinoma | 0 (0.0) | 1 (7.7) | 3 (4.3) | |||
| Melanoma + Multiple Myeloma | 0 (0.0) | 0 (0.0) | 1 (1.4) | |||
| Multiple Myeloma | 0 (0.0) | 0 (0.0) | 1 (1.4) | |||
| Colon Cancer - Metastatic | 0 (0.0) | 0 (0.0) | 1 (1.4) | |||
| T-Cell Lymphoma | 0 (0.0) | 0 (0.0) | 1 (1.4) | |||
| Concurrent irAE | 18 (100) | 16 (100) | 82 (100.0) | |||
| None (lone) | 7 (38.9) | 10 (62.5) | 30 (36.6) | |||
| Gastro-intestinal disorders (any) | 3 (16.7) | 2(12.5) | 9 (11.0) | |||
| - Colitis/ Enteritis / Diarrhea /Dehydration | 2 (11.1) | 2(12.5) | 7(8.5) | |||
| - Abdominal pain | 0 (0.0) | 1 (6.2) | 2 (2.4) | |||
| - Nausea/ Vomiting | 0 (0.0) | 1 (6.2) | 1 (1.2) | |||
| - Hepatic disorder | 1 (5.6) | 0 (0) | 1 (1.2) | |||
| Endocrino-metabolic disorders (any) | 3 (16.7) | 2 (12.5) | 7 (8.5) | |||
| - Fever | 1 (5.6) | 1 (6.2) | 3 (3.7) | |||
| - Thyroid disorders | 1 (5.6) | 0 (0) | 2 (2.4) | |||
| - Potassium imbalance | 0 (0.0) | 1 (6.2) | 1 (1.2) | |||
| - Adrenal insufficiency | 1 (5.6) | 0 (0) | 1 (1.2) | |||
| - Hypophysitis | 0 (0.0) | 1 (6.2) | 1 (1.2) | |||
| Dermatologic and Rheumatologic conditions (any) | 4 (22.2) | 4 (25.0) | 23 (28.0) | |||
| - Arthralgia/ Joint stiffness/ Arteritis | 2 (11.1) | 3 (18.8) | 9 (11.0) | |||
| - Rash / Purpura | 0 (0.0) | 0 (0) | 5 (6.1) | |||
| - Cutaneous vasculitis /Dermatitis | 0 (0.0) | 0 (0) | 4 (4.9) | |||
| - Skin discoloration/ Vitiligo | 0 (0.0) | 0 (0) | 3 (3.7) | |||
| - Myositis / Myasthenia gravis / Other | 2 (11.1) | 1 (6.2) | 3 (3.7) | |||
| - Pruritus | 0 (0.0) | 1 (6.2) | 1 (1.2) | |||
| - Skin ulcer | 0 (0.0) | 0 (0) | 1 (1.2) | |||
| - Skin infection | 0 (0.0) | 0 (0) | 1 (1.2) | |||
| Neurologic conditions (any) | 4 (22.2) | 1 (6.2) | 15 (18.3) | |||
| - Cerebral vasculitis | 0 (0.0) | 0 (0) | 4 (4.9) | |||
| - Ataxia/ Gait disturbance/ Vertigo | 0 (0.0) | 0 (0) | 4 (4.9) | |||
| - Headache | 3 (16.7) | 0 (0) | 3 (3.7) | |||
| - Decreased level of consciousness | 0 (0.0) | 0 (0) | 2 (2.4) | |||
| - Stoke (ischemic or hemorrhagic) | 0 (0.0) | 0 (0.0) | 2 (2.4) | |||
| - Encephalitis /meningitis | 0 (0.0) | 0 (0.0) | 2 (2.4) | |||
| - Hypoacousis | 0 (0.0) | 1 (6.2) | 1 (1.2) | |||
| - Unspecified neurologic disorder | 1 (5.6) | 0 (0.0) | 1 (1.2) | |||
| Ophthalmic conditions(any) | 5 (27.8) | 1 (6.2) | 10 (12.2) | |||
| - Blindness / impaired vision | 5 (27.8) | 1 (6.2) | 7 (8.5) | |||
| - Retinal hemorrhage/ retinal vasculitis | 1 (5.6) | 0 (0.0) | 2 (2.4) | |||
| - Optic neuritis | 1 (5.6) | 0 (0.0) | 1 (1.2) | |||
| - Diplopia | 1 (5.6) | 0 (0.0) | 1 (1.2) | |||
| - Ptosis | 1 (5.6) | 0 (0.0) | 1 (1.2) | |||
| - Uveitis | 0 (0.0) | 0 (0.0) | 1 (1.2) | |||
| Pulmonary conditions (any) | 1 (5.6) | 0 (0.0) | 5 (6.1) | |||
| - Pneumonia/ Pneumonitis | 1 (5.6) | 0 (0.0) | 4(4.9) | |||
| - Interstitial lung disease | 0 (0.0) | 0 (0.0) | 1 (1.2) | |||
| Hematologic conditions (any) | 1 (5.6) | 0 (0.0) | 6(7.3) | |||
| - Cytopenia | 1 (5.6) | 0 (0.0) | 3 (3.7) | |||
| - Anemia | 0 (0.0) | 0 (0.0) | 2 (2.4) | |||
| - Coagulopathy | 0 (0.0) | 0 (0.0) | 1 (1.2) | |||
| - Serum sickness syndrome | 0 (0.0) | 0 (0.0) | 1 (1.2) | |||
| Cardiac-vascular conditions (any) | 0 (0.0) | 0 (0.0) | 3 (3.7) | |||
| - Myocarditis | 0 (0.0) | 0 (0.0) | 1 (1.2) | |||
| - Myocardial infarction | 0 (0.0) | 0 (0.0) | 1 (1.2) | |||
| - Cardiac arrest | 0 (0.0) | 0 (0.0) | 1 (1.2) | |||
| - Deep vein thrombosis | 0 (0.0) | 0 (0.0) | 1 (1.2) | |||
Other concomitant reported suspected medications for vasculitis were allopurinol (n: 1), amoxicillinclavulanic acid (n: 1), bevacizumab (n: 1), ceritinib (n: 1), cisplatin (n: 1), dexamethasone (n: 1), duloxetine (n: 1), levothyroxine (n: 1), mupirocin (n: 1), paracetamol (n: 1), pemetrexed (n: 1), pomalidomide (n: 1), telmisartan (n: 1), temozolomide (n: 1), vemurafenib (n: 1), and unspecified drugs (n: 2).
Other concomitant reported suspected medications for temporal arteritis were bevacizumab (n: 1), duloxetine (n: 1), paracetamol (n: 1), temozolomide (n: 1), and vemurafenib (n: 1).
Of the 42 melanoma patients with ICI-associated vasculitis, 11 were treated with anti-CTLA-4 monotherapy, 21 with anti-PD-1 or anti-PD-L1 monotherapy, and 10 with combination of an anti-CTLA-4 plus an anti-PD-1 or an anti-PD-L1 therapy. All 17 vasculitis cases associated with ICI in lung cancer patients were treated with anti-PD-1 or anti-PD-L1 monotherapy.
Abbreviations: CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ICI, immune checkpoint inhibitor; IQR, interquartile range; irAE, immune related adverse event; [min-max], minimum-maximum; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; SD, standard deviation
Figure 2.
Overlap (A) and time to event onset (B) for selected ICI immune related cardiovascular adverse events (CV-irAE: myocarditis, pericardial disorders and vasculitis) in individual case safety reports from VigiBase (last accessed: 01/2018).
Discussion
We report the largest-to-date and most extensive clinical characterization of CV-irAEs associated with ICI through analysis of individualized reportable events from the WHO pharmacovigilance database. The results show significant incidence of myocarditis, pericardial disorders and vasculitis-related disorders with ICI, suggesting that CV-irAEs may be underrepresented in published literature.
ICIs are now widely used for the treatment of many cancers including melanoma, lung cancer, renal cell and urothelial cancer, as early clinical trials have demonstrated significant improvement in clinical outcomes.1 IrAEs, which include colitis, hepatitis, pneumonitis, hypophysitis, thyroiditis, have emerged as the most clinically relevant complications of ICI.13 In the CV system, although myocarditis was rarely reported in early clinical trials with anti CTLA-4 and anti PD-1 therapy, there is an increasing number of published case reports and case series of ICI-myocarditis.3,5,6 These case series have demonstrated a variable presentation of ICI-associated myocarditis.6,14 However, the broad spectrum of acute CV-irAEs are unknown. Here, we report pericardial disease and vasculitis as additional possible CV toxicity considerations for clinicians involved in the care of cancer patients treated with immunotherapies. These CV-irAEs were predominantly affecting men, which is consistent with the over-representation of men treated by ICI in clinical settings.15,16 The majority of irAE reports associated with ICI retrieved in VigiBase were from non-clinical trial (standard of care) settings suggesting that the increased reporting over time were not strictly due to increased monitoring as part of clinical trials, but rather expansion of ICI indications and increased awareness from treating healthcare professionals during post-marketing surveillance.
Our report includes the identification of 122 patients with ICI-associated myocarditis, the largest such collection of cases to date. In these patients, severe myocarditis can occur as early as after the first ICI dose with a median time of 30 days after initial exposure to ICI. Myocarditis portends poor outcomes with 50% of cases resulting in fatality. ICI-associated myocarditis was associated with various ICI and many different cancers, with combination ICI treatment as the only cancer-related risk factor. These findings are in line with the other published case series also reporting early onset and high fatality rate of ICI-associated myocarditis.3,5,6 Identification of patients at higher risk of ICI-associated myocarditis will be important since cardiovascular screening (for example, baseline and early follow-up screening with an ECG or cardiac biomarkers) may be considered in these patients. In addition, since the diagnosis of myocarditis can be difficult clinically, the identification of concomitant irAEs (such as myositis) and cardiac presentations (heart failure and arrhythmias) from our report may additionally help the clinician diagnose ICI-associated myocarditis. The mechanisms of ICI-associated myocarditis have biological plausibility. Genetic deletion of CTLA-4 and PD-1 can cause autoimmune myocarditis in preclinical models. Moreover, early histological analysis in ICI-associated myocarditis in humans confirmed infiltration of T-cell lymphocytes (both CD4+ and CD8+ T-cells) and macrophages, suggesting immune infiltration as the main pathophysiological driver.3,17,18 As such, early initiation of high-dose glucocorticoids are a mainstay of myocarditis treatment (as with other irAE), although the addition of other T-cell directed therapies tacrolimus or anti-thymoglobulin globulin, such as what is used in patients with heart transplants with cellular rejection may be considered.3,19–21 Importantly, we describe clinical characteristics of these novel cardiovascular clinical syndromes associated with ICI. Our analysis reveals pericardial disorders (pericarditis, pericardial effusion and tamponade) are also associated with ICIs. In the literature, there have been only 6 published case reports of ICI-associated pericardial disease.22 However, we observed over-representation of pericardial disorders, particularly in patients receiving anti-PD1/PDL-1 therapy for lung cancer. The disease-specific effects of lung cancer in this observation are unclear. One plausible hypothesis is the proposed synergistic effects of radiation and immunotherapies which may be at play in lung cancer patients. Radiation’s possible ability to prime an endogenous antigen-specific immune response has been used a rationale for combining radiation and immunotherapies for synergistic effects.23 In this regard, it is plausible that patients with cancer who receive ICI following irradiation to the thoracic area may be more prone to pericardial disorders, exposing potential shared antigens to T-cell recognition. Conversely, the signal identified here for pericardial disorders might be also driven by over-reporting of such pericardial disorders in general in cancer patients, since pericardial disorders are common complications of some malignancies.24
We also observed vasculitis disorders associated with ICIs, particularly temporal arteritis (TA) and polymyalgia rheumatica (PMR), which have been previously described only in case reports.25 Here, we describe the characteristics of affected patients. TA and PMR are entities mainly affecting older Caucasian populations. While TA and PMR are associated with various cancer types, there is over-reporting with ipilimumab monotherapy treatment. Deposition of immune complexes in affected arteries are believed to be responsible for TA whereas a milder and immune-systemic reaction may result in PMR.26 Several lines of evidence support a major role of PD-1 and CTLA-4 pathways in the pathophysiology of vasculitis. Single-nucleotide polymorphisms in the genes encoding PD-1 and CTLA-4 have been associated with T-cell hyperactivity at a vascular level in patients presenting various form of vasculitis.27 Use of abatacept, a fusion protein of the extracellular domain of CTLA-4 and modified fragment crystallizable portion of human immunoglobulin-G1, has demonstrated efficacy in TA treatment.28 High levels of inflammatory markers that recruit CD4+ T-cells, macrophages and multinucleated giant cells have been demonstrated in the medium to large arteries of TA-affected patients secondary to a decrease in PD-1 expression and transcription.29 Therefore, it is likely that an immune environment favoring emergence of vasculitis is reproduced in the setting of ICI use. Ophthalmic events are considered an emergency in TA due to the risk of permanent blindness and can occur in 15–20 % of affected patients.30 Concordantly, our study observed that approximately one third of TA reports were accompanied by visual impairment and blindness.
Limitations
Several limitations need to be recognized for VigiBase analysis. Pharmacovigilance analysis allow for signal detection and generate hypothesis which need to be replicated ideally by prospective studies. Replication of disproportionality analysis in other smaller size continental pharmacovigilance databases such as European Medicine Agency or US. Food and Drug Administration AE Reporting system (FAERS) is biased by an important ICSRs overlap between databases. Alternatively, some cases of suspected drug-induced CV-irAEs are likely not reported to the national drug authorities, and therefore not submitted to VigiBase. However, a major strength is that VigiBase aggregates ICSRs collected from over 130 countries, which enables better identification of these rare AE and broader generalization of our findings. Level of causality attributed to ICI was high based on extrinsic data, chronology, symptomatology and known ICI mechanisms of action. Another limitation of Vigibase is that sources of reports are non-homogeneous, and there is limited possibility of verification of the clinical, laboratory tests or radiological findings justifying the reported diagnosis, nor completeness of reporting for age, drug dosing, time to onset, comorbid conditions and concomitant drugs. The exact denominator of patients exposed to the different ICI regimen cannot be evaluated. Instead, total number of ICSRs for each group of drug is used as denominator for this kind of disproportionality analysis in pharmacovigilance databases for signal detection.10,11 The volume of reports for a particular medicinal product may be influenced by the extent of use of the product, publicity, the nature of the reactions and other factors such as competition bias. The value of disproportionality reporting (as the ROR) for several CV-AE and culprit drugs has already been demonstrated in various settings; nevertheless, there is still a risk that comparisons of disproportionality between medicinal products in pharmacovigilance databases may be misleading. As an example of another validated association, over-reporting for long-QT and Torsade-de-Pointes has been associated with the extent of IKr drug-induced blockade using VigiBase;10 or with identification of over-reporting for long-QT and Torsade-de-Pointes on selective-estrogen receptor modulators vs. aromatase inhibitors in women using European pharmacovigilance database.[12]
Clinical trials are mandatory to establish efficacy but may not allow definitive conclusions on drug safety in part due to selected populations and limited power to detect balances in rare adverse events. Spontaneous notifications remain the cornerstone for ADR evaluation despite their limitations. Disproportionality analysis in pharmacovigilance databases is an important method to detect signals in drug safety research and post-marketing surveillance. Herein, we use this analysis to identify several cardiovascular complications, specifically myocarditis, pericardial disorders, and vasculitis, associated with immune checkpoint inhibitors. These types of analyses may identify other uncommon but clinically-significant complications.
While the incidence of these CV-irAE events cannot be determined using VigiBase, we can provide the following data. First, objective responses occurred in 15–60% of patients with metastatic solid cancers treated with anti-PD-1/PD-L1 agents (including 10–40% durable responses), depending on the cancer type.23,24 Second, initial estimates suggested a rate of myocarditis ranging from 0.06% to 0.3%,8 although more recent studies in the setting of enhanced awareness have demonstrated up to 1.1% incidence.13 Pericardial and vascular disorders have been sporadically reported and thus the incidence is essentially unknown. Third, the risk of ICI induced fatal toxicities has not been studied in detail although our group have shown fatality rates ranging from 0.3% (anti-PD-1/PD-L1 monotherapy) to 1.2% (combination PD-1/CTLA-4 blockade); 17% of these fatalities were cardiac in nature (unpublished data; authors).
Supplementary Material
Research in context.
Evidence before this study.
We searched Pubmed up to May 3, 2018 using the terms “PD-1/PD1/PD-L1” OR “CTLA-4/CTLA4” AND “myocarditis” OR “cardiac” and found 522 manuscripts. Studies with relevant clinical data were limited to case reports and small case series reporting the clinical features of myocarditis. Immune checkpoint inhibitor induced myocarditis tends to occur earlier on therapy, with combination immunotherapy, and result in high fatality rates.
Added value of this study.
To our knowledge, we report the first large scale analysis linking specific cardiovascular complications with immune checkpoint inhibitors: myocarditis, pericardial diseases, vasculitis and supraventricular arrhythmias. These adverse events occurred early after immune checkpoint inhibitor administration, as soon as after the first dose, and carried a particularly high fatality rate for myocarditis.
Implications of all the available evidence.
Severe cardiac events related to myocarditis, pericardial disease, and vasculitis occur in patients exposed to immune checkpoint inhibitors. These events should be considered in patient care and in combination clinical trial designs.
Acknowledgments and research support:
The supplied data from VigiBase come from a variety of sources. The likelihood of a causal relationship is not the same in all reports. The information does not represent the opinion of the World Health Organization (WHO).
This study was supported by The Cancer ITMO of the French National Alliance for Life and Health Sciences (AVIESAN): “Plan Cancer 2014–2019” (JES, BLV) by NIH/NCI K23 CA204726 (DBJ), the James C. Bradford Jr. Melanoma Fund (DBJ), and the Melanoma Research Foundation (DBJ).
Funding. The Cancer ITMO of the French National Alliance for Life and Health Sciences (AVIESAN): “Plan Cancer 2014–2019”; NIH/NCI K23 CA204726; the James C. Bradford Jr. Melanoma Fund; the Melanoma Research Foundation. Funding sources had no role in study design, collection, analysis or interpretation of the data, neither in the writing of the manuscript nor the decision to submit it for publication. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: JMB has a patent Prediction of PD-1 outcome by HLA-DR expression pending. DBJ reports personal fees from Array Biopharma, grants and personal fees from Incyte, grants and personal fees from BMS, personal fees from Merck, outside the submitted work. MPB reports grants from Pfizer, grants and personal fees from AstraZeneca, grants and personal fees from Merck, grants and personal fees from Amgen, grants from MedImmune, personal fees from Bayer, personal fees from Janssen, outside the submitted work. JPS reports personal fees from Roche, personal fees from MSD, personal fees from BMS, personal fees from AztraZeneca, personal fees from Pfizer, outside the submitted work. AG reports personal fees from PFIZER, outside the submitted work. JJM reports grants and other from Pfizer, grants and other from Bristol Myers Squibb, other from Novartis, other from Regeneron, other from Myokardia, other from Ipsen, other from Takeda, outside the submitted work. The other authors have no conflict of interest. We would like to thank the custom searches team at UMC research section, without whom this study would not have been possible.
Bibliography.
- 1.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015; 33(17): 1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359(6382): 1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016; 375(18): 1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018; 391(10124): 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escudier M, Cautela J, Malissen N, et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation 2017; 136(21): 2085–7. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol 2018; 71(16): 1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindquist M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Drug Information Journal 2008; 42(5): 409–19. [Google Scholar]
- 8.Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 1998; 54(4): 315–21. [DOI] [PubMed] [Google Scholar]
- 9.Noren GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res 2013; 22(1): 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bruin ML, Pettersson M, Meyboom RH, Hoes AW, Leufkens HG. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur Heart J 2005; 26(6): 590–7. [DOI] [PubMed] [Google Scholar]
- 11.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf 2004; 13(8): 519–23. [DOI] [PubMed] [Google Scholar]
- 12.Grouthier V, Lebrun-Vignes B, Glazer AM, et al. Increased long QT and torsade de pointes reporting on tamoxifen compared with aromatase inhibitors. Heart 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018; 378(2): 158–68. [DOI] [PubMed] [Google Scholar]
- 14.Varricchi G, Marone G, Mercurio V, Galdiero MR, Bonaduce D, Tocchetti CG. Immune Checkpoint Inhibitors and Cardiac Toxicity: An Emerging Issue. Curr Med Chem 2018; 25(11): 1327–39. [DOI] [PubMed] [Google Scholar]
- 15.Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. The Lancet Oncology 2018; 19(6): 737–46. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Ju Q, Jia K, et al. Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors). Int J Cancer 2018; 143(1): 45–51. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001; 291(5502): 319–22. [DOI] [PubMed] [Google Scholar]
- 18.Varricchi G, Galdiero MR, Tocchetti CG. Cardiac Toxicity of Immune Checkpoint Inhibitors: Cardio-Oncology Meets Immunology. Circulation 2017; 136(21): 1989–92. [DOI] [PubMed] [Google Scholar]
- 19.Arangalage D, Delyon J, Lermuzeaux M, et al. Survival After Fulminant Myocarditis Induced by Immune-Checkpoint Inhibitors. Ann Intern Med 2017; 167(9): 683–4. [DOI] [PubMed] [Google Scholar]
- 20.Nasr F, El Rassy E, Maalouf G, et al. Severe ophthalmoplegia and myocarditis following the administration of pembrolizumab. Eur J Cancer 2018; 91: 171–3. [DOI] [PubMed] [Google Scholar]
- 21.Frigeri M, Meyer P, Banfi C, et al. Immune Checkpoint Inhibitor-Associated Myocarditis: A New Challenge for Cardiologists. Can J Cardiol 2018; 34(1): 92 e1–e3. [DOI] [PubMed] [Google Scholar]
- 22.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387(10027): 1540–50. [DOI] [PubMed] [Google Scholar]
- 23.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015; 16(13): e498–509. [DOI] [PubMed] [Google Scholar]
- 24.Imazio M, Demichelis B, Parrini I, et al. Relation of acute pericardial disease to malignancy. Am J Cardiol 2005; 95(11): 1393–4. [DOI] [PubMed] [Google Scholar]
- 25.Tocut M, Brenner R, Zandman-Goddard G. Autoimmune phenomena and disease in cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev 2018. [DOI] [PubMed] [Google Scholar]
- 26.Hid Cadena R, Abdulahad WH, Hospers GAP, et al. Checks and Balances in Autoimmune Vasculitis. Front Immunol 2018; 9: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamesh L, Heward JM, Williams JM, et al. CT60 and +49 polymorphisms of CTLA 4 are associated with ANCA-positive small vessel vasculitis. Rheumatology (Oxford) 2009; 48(12): 1502–5. [DOI] [PubMed] [Google Scholar]
- 28.Langford CA, Cuthbertson D, Ytterberg SR, et al. A Randomized, Double-Blind Trial of Abatacept (CTLA-4Ig) for the Treatment of Giant Cell Arteritis. Arthritis Rheumatol 2017; 69(4): 837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe R, Zhang H, Berry G, Goronzy JJ, Weyand CM. Immune checkpoint dysfunction in large and medium vessel vasculitis. Am J Physiol Heart Circ Physiol 2017; 312(5): H1052–H9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvarani C, Cimino L, Macchioni P, et al. Risk factors for visual loss in an Italian population-based cohort of patients with giant cell arteritis. Arthritis Rheum 2005; 53(2): 293–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.