Abstract
Neutrophils are the first responders to sites of inflammation when the intestinal epithelial barrier is breached and the gut microbiota invade. Despite current efforts in understanding the role of neutrophils in intestinal homeostasis, the complex interactions between neutrophils and intestinal epithelial cells (IECs) is still not well characterized. Here, we demonstrate that neutrophils enhanced production of amphiregulin (AREG), a member of EGFR ligand family, by IECs, which promoted IEC barrier function and tissue repair. Depletion of neutrophils resulted in more severe colitis in mice due to decreased AREG production by IECs upon dextran sodium sulfate (DSS) insult. Administration of AREG restored epithelial barrier function and ameliorated colitis. Furthermore, neutrophil derived TGFβ promoted AREG production by IECs. Mechanistically, TGFβ activated MEK1/2 signaling, and inhibition of MEK1/2 abrogated TGFβ-induced AREG production by IECs. Collectively, these findings reveal that neutrophils play an important role in the maintenance of IEC barrier function and homeostasis.
Introduction
As the largest mucosal surface in the body, the intestinal epithelium serves as a critical relay station between microbiota and mucosal immune cells. Multiple pattern recognition receptors allow intestinal epithelial cells (IECs) to sense microbes and translate the signals to mucosal immunity(1). Meanwhile, the thick layer of mucus and deep invaginated intestinal crypt structure buffer microbial signals to prevent excessive antigen exposure and subsequent over activation of the immune system(2). The capacity of IECs for rapid self-renewal protects the host from continuous exposure to microbial stimuli and environmental insults(3). On the other hand, tissue-resident immune cells closely interact with IECs to support barrier function and regulate luminal microbiota. We have previously reported that Th17 cells upregulate polymeric Ig receptor (pIgR) on IECs as a way to promote intestinal IgA responses to microbiota, thus, contributing to the maintenance of intestinal homeostasis(4). Accumulating evidence also shows that innate lymphoid cells (ILCs) are capable of producing IL-22 and amphiregulin (AREG) to exert beneficial effects on IECs(5, 6). Dysfunction of IECs has detrimental effects on the host, resulting in increased bacterial translocation and risk of developing inflammatory diseases, including Inflammatory Bowel Disease (IBD). It has been reported that individuals with altered IEC gene expression are more susceptible to IBD(7). Moreover, it has been shown that patients with IBD have increased intestinal permeability, much of which was attributed to compromised IEC barrier function(8). However, the cells and factors that regulate IEC function are still not well understood.
Emerging evidence has demonstrated that AREG plays an important role in the regulation of intestinal homeostasis(9). As a member of the epidermal growth factor family, AREG is essential in regulating cell differentiation and proliferation. It has been reported that AREG-deficient (AREG−/−) mice spontaneously develop gastric tumors(10). In the context of intestinal injury after dextran sodium sulfate (DSS) administration, AREG−/− mice develop more severe colitis compared to wild-type B6 (WT) mice, which suggests a crucial role for AREG in wound healing and tissue repair(5). Additionally, AREG is thought to play a role in type 2-mediated immune resistance and tolerance. Lung resident Th2 cells and group 2 ILCs produce AREG to promote tissue homeostasis following infection of influenza virus and nematodes, allowing for increased barrier protection (11, 12). Regulatory T cells (Treg) have also been identified as an important source of AREG during early influenza infection(13), and AREG has been reported to promote the suppressive capacity of Treg during inflammation(14). Among various hematopoietic and non-hematopoietic AREG producers, IECs remain a critical source for both paracrine and autocrine AREG responses. Within the lung, epithelial-derived AREG can enhance barrier defense against pathogens(15). Despite these advances, relatively little is known about the factors that regulate IEC production of AREG and the functional significance of IEC-derived AREG in regulating intestinal immune responses.
The association between massive infiltration of neutrophils into the intestines and compromised IEC function found in IBD patients suggests a central role for dysregulated neutrophil-IEC interaction in the pathogenesis of IBD(16). Neutrophils are the most abundant leukocytes in circulation and are pertinent in responding to microbial invasion at epithelial surfaces. At sites of microbial invasion, neutrophils perform several functions to control inflammation, including direct phagocytosis of invading pathogens, formation of neutrophil extracellular traps (NETs), and production of matrix metalloproteases (MMPs), elastase, and other proteolytic enzymes(17). Neutrophils are also potent cytokine producers. We have previously reported that neutrophils protect the intestines from inflammation, and neutrophil-derived IL-22 ameliorated colitis by promoting epithelial integrity(18, 19). In addition, IL-22-producing neutrophils have been shown to enhance IEC antimicrobial peptide production, which aids in barrier defense(20). Moreover, the infiltration of neutrophils can rapidly deplete O2 levels in the microenvironment via reactive oxygen species (ROS) production, which also contributes to barrier protection by stabilizing IEC hypoxia-inducible factor (HIF) expression(21). It has been shown that dysregulated neutrophil transepithelial migration results in altered expression of tight junction proteins in IECs, and neutrophil-derived pro-inflammatory mediators further affect IEC viability(16, 22). Hence, proper crosstalk between IECs and neutrophils is crucial for maintaining a delicate balance of local immune response and intestinal homeostasis. However, it is still unknown if neutrophils regulate intestinal epithelial function and intestinal homeostasis through regulating IEC AREG expression.
In the current study, we demonstrated that neutrophils induce AREG production from IECs through production of TGFβ. Upon DSS insult, depletion of neutrophils results in more severe colitis due to decreased AREG production by IECs. Administration of AREG to mice after depletion of neutrophils alleviates intestinal injury. Thus, our study demonstrates that neutrophils augment epithelial protection by promoting AREG production by IECs and reveals a critical pathway of neutrophil-mediated tissue protection.
Materials and Methods
Mice
Specific pathogen free C57BL/6 (B6) mice, which were norovirus and Helicobacter free, were obtained from the Jackson Laboratory, and bred and maintained in the Animal Facilities at the University of Texas Medical Branch (UTMB). Both male and female mice were used. All experiments were reviewed and approved by the Institutional Animal Care and Use Committees of UTMB.
Reagents
Neutralizing antibody against Ly6G (1A8) was purchased from Bio × Cell. Recombinant AREG was purchased from Leinco Tecnologies. Thioglycollate broth and percoll were purchased from Sigma-Aldrich. Culture medium RPMI 1640, HEPES, penicillin/streptomycin, β-Mercaptoethanol (β-ME), sodium pyruvate, L-glutamine and ITS were purchased from Life Technologies. HBSS and DMEM were purchased from Corning. DMEM/F12 media (12634–010), L-Glutamine (25030) and B27 (12587–010) were purchased from Invitrogen. Mouse recombinant cytokines were purchased from Biolegend. Retinoic acid was purchased from Sigma-Aldrich. Matrigel was purchased from BD Bioscience. Recombinant EGF (2028-EG), Noggin (1967-NG/CF), R-spondin (3474-RS), Wnt3a (35036-WN/CF) and N2 supplement (AR009) were purchased from R&D Systems. SMARTpool siRNAs specific for murine MEK1, MEK2 and non-targeting siRNA were purchased from Dharmacon. Inhibitor U0126 and PD98059 were purchased from Promega. Western blot antibodies against phosphorylated ERK1/2, β-actin, and anti-rabbit secondary antibody conjugated with HRP were purchased from Cell Signaling Technology.
Neutrophil isolation
Neutrophils were collected from the peritoneal cavity as previously described(18). Briefly, peritoneal cells were collected by lavage with 10ml PBS 5% FBS 5 h after 1ml 3% thioglycollate broth i.p. injection. Neutrophils were separated from other cell types by using 50% Percoll. After spinning for 20 min at 260 g, the neutrophil pellet was collected. Neutrophil purity was >90% as tested by flow cytometry after CD11b and Ly6G staining. Neutrophil supernatant was harvested after 24 h of culture in complete culture media.
Enteroid culture
After cleaning, small intestines were removed from the euthanized mice, cut into 0.5-cm pieces, and rocked in a Falcon tube with ice cold PBS for 15 min at 4°C. The intestinal tissues were treated with 2 mM EDTA for 30 min at 4 °C. The tissues were then transferred into a new tube with 5 ml cold PBS containing 43.3 mM sucrose and 54.9 mM sorbitol. After shaking for 2 min, the tissues were filtered through a 70-μm cell strainer and rinsed with 5 ml shaking buffer. Supernatant was collected and centrifuged at 150 g for 10 min at 4 °C. The resulting pellet containing detached crypts was re-suspended gently in Matrigel with 0.5 μg/ml recombinant EGF, 1 μg/ml recombinant Noggin, 5 μg/ml recombinant R-spondin and 1 μg/ml recombinant Wnt3a. Next, 50μl Matrigel with 500 crypts was plated in each well of the pre-warmed (37 °C) 24-well plate. After polymerization of Matrigel for 30 min, 500 μl of pre-warmed advanced DMEM/F12 media with 2 mM L-Glutamine, 1% penicillin/streptomycin, 10 μM HEPES, 1 × N2 supplement and 1 × B27 was gently added to each well. Enteroids were used after 5 days of culture and 250 μl of media was replaced with neutrophil supernatant for microarray study. For enteroid extraction, media was removed and the matrigel was gently washed with ice cold PBS. Following washing, 1 ml cold PBS was added to each well, and the matrigel was mechanically dissociated using a pipette tip. The dissociated matrigel was collected and centrifuged for 8min at 350g. The resulting enteroid pellet was collected for subsequent studies.
Microarray analysis
RNA expression analysis was conducted using Affymetrix Mouse transcriptome 1.0 assay kit (Affymetrix, PN 902919). Total RNA was isolated using the TRIzol method (Invitrogen) and RNA quality and quantity was analyzed by the Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) and only RNA with a RNA integrity number (RIN) >7.0 were included in the microarray analysis (MA). Gene expression analysis (GEA) was done using Affymetrix GeneChip Mouse transcriptome array 1.0, which evaluates the expression of more than 66,100 different genes. Total RNA sample processing, labeling and hybridization were performed using the Affymetrix GeneChip WT PLUS with the WT Terminal Labeling Kit, according to the manufacturer’s guidelines (Affymetrix). Scanning and data extraction of the microarray were followed by the transformation of fluorescence data into CEL files employing the Affymetrix GeneChip Command Console (AGCC) software. Microarray expression data was further analyzed using Transcriptome Analysis Console (TAC) 4.0 Software. Microarray data has been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-6179.
Epithelial cell culture
Mouse small intestinal epithelium (MSIE) cells, a conditionally immortalized epithelial cell line established from the intestines of wild-type mice(23), were cultured in RPMI 1640 medium with 5 U/ml murine IFNγ, ITS (5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenous acid), 100 U/ml penicillin/streptomycin, and 5% FBS at 33°C and 5% CO2. After reaching 80% confluence, cells were starved in RPMI 1640 medium with 100 U/ml penicillin/streptomycin and 0.5% FBS at 37°C for 16 h before subsequent experiments. All subsequent experiments were performed at 37°C. Caco-2 cells were cultured in DMEM supplemented with 10% FBS at 37°C and 5% CO2.
siRNA transfection
siRNA transfection of MSIE cells was performed by using Lipofectamine RNAiMAX transfection reagent according to the manufacturer’s instructions. 2 × 105 MSIE cells were incubated with 30 pmol siRNA and 3 μl Lipofectamine RNAiMAX transfection reagent in OPTI-MEM medium (1 ml per well) for 6 h, followed by 1 ml normal medium per well for 24 h at 33°C and 5% CO2. MSIE cells were then cultured in medium containing only 0.5% FBS for 16 h at 37°C and 5% CO2 before treatment. Transfection efficiency was determined at 24 h post transfection (Figure S3A).
Knockout of MEK1 by using CRISPR
LentiCRISPR vector (Addgene, Cambridge, USA, plasmid#52961) established by the Zhang lab (Sanjana NE, Pubmed 25075903) was used to create knockouts of MEK1. The design and cloning of the target gRNA sequences was performed as recommended by the Zhang lab GeCKO website (http://www.genome-engineering.org/gecko/). Briefly, the suitable target sites for gRNA sequence design against MEK1 were identified using CRISPR design tool software at http://crispr.mit.edu. Cas9-target sites for the indicated genes were designed in http://crispr.genome-engineering.org. Then, synthetic gRNA (ITN) containing target sites were sub-cloned into the lentiCRISPR vector. The newly constructed lentiCRISPR plasmids were then transfected into MSIE cells. After antibiotic positive selection, cells were established as a stable cell line. Transfection efficiency was determined at 24 h post transfection via qRT-PCR (Figure S3C). Guide RNA oligo sequences for lentiCRISPR are listed in Supplementary Table S1.
Quantitative Real-time PCR
RNA was extracted from homogenized tissue or cultured cells with TRI Reagent (Molecular Research Center) and followed by cDNA synthesis with qScript cDNA Supermix (Quanta BioSciences). Quantitative PCR was performed with SYBR Green Gene Expression Assays. Predesigned primers were ordered from Life Technologies and normalized against Gapdh mRNA expression. The specific primer sequences are listed in Supplementary Table S1.
Dextran sodium sulfate model of colitis and neutrophil depletion
1.5–2% dextran sodium sulfate (DSS) w/v (Gojira FC) was dissolved into drinking water and administered ad libitum for 7 days, followed by 3 days of water without DSS. Body weights were monitored daily. For neutrophil depletion, Ly6G-depleting antibody(1A8) (4 mg/kg) or control antibody were administered via intraperitoneal injection (i.p.) to DSS-treated mice every 3 days. Mice were sacrificed by using carbon dioxide asphyxiation on day 10.
Histopathological assessment
At necropsy, colons were harvested and Swiss rolls prepared. Colons were fixed in 10% buffered formalin and paraffin embedded. 5μm sections were sliced, stained with H&E, and blindly scored by an experienced pathologist. Histological scoring was performed using a modification of scoring system(24). Briefly, longitudinal sections were examined for epithelial damage based on hyperplasia, crypt degeneration, and loss; goblet cell loss; crypt exudate; LP and submucosal inflammatory cell accumulation; submucosal edema; mucosal ulceration; and transmural inflammation. Each lesion component was scored for intensity and extent. Intensity was scored either a 0, 1, 2, or 3 for absent, mild, moderate, or severe inflammation while extent was scored a 1, 2, 3, or 4 corresponding to 25, 50, 75, or 100% of the total tissue effected. The total lesion severity score was calculated by summing the extent and intensity scores for each individual lesion component.
Isolation of Primary IECs
After cleaning, small intestines and large intestines were removed from euthanized mice, cut into small pieces, and rinsed thoroughly with cold PBS. After 40 min incubation with 5mM EDTA in HBSS buffer containing 5% FBS at 37°C, IECs were collected by passing the supernatant through a 100 μM cell strainer (BD Falcon). After washing with PBS, IECs were then underlayed with a solution of 20% percoll and 40% percoll. After spinning for 20min at 830 g and 25°, IECs were collected at the 20/40 interface.
Ex Vivo Colonic Tissue Cultures
After cleaning, two colonic punctures (2mm) were obtained from the proximal and distal colon in each mouse. Tissue segments were cultured in 1640 RPMI medium supplemented with 100 U/ml penicillin/streptomycin, 10% FBS, 100 mM sodium pyruvate, 10 mM HEPES and 50 μM 2-Mercaptoethanol in a 24-well culture plate for 24 h at 37 °C and 5% CO2.
Statistics
Statistical significance was calculated with GraphPad (Prism 6.0) using paired or unpaired Student’s t tests, and one-way ANOVA for multiple comparisons. The Mann–Whitney U test was used for assessing pathology scores. Where appropriate, mean ± SEM is represented on each graph. A p value < 0.05 was considered statistically significant and shown as an asterisk (*).
Results
Neutrophils induce AREG expression in IECs
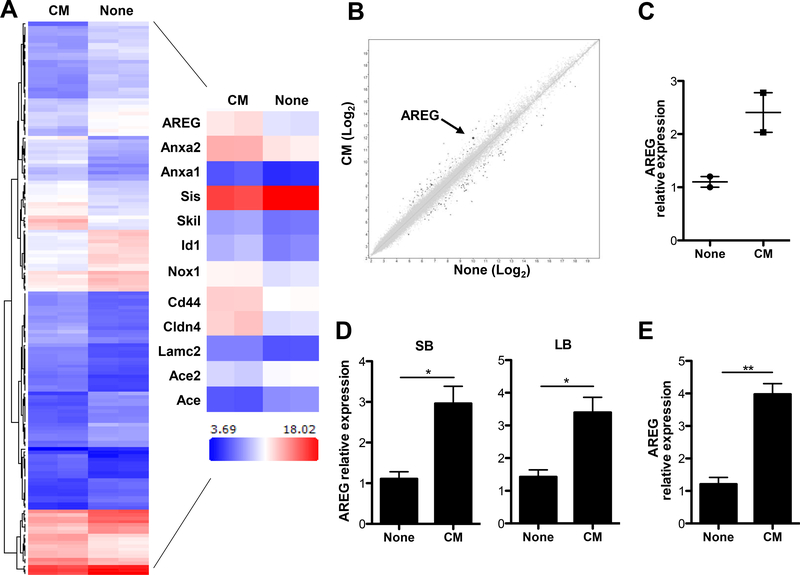
Our previous studies have demonstrated that neutrophils protected the intestines from inflammation and depletion of functional neutrophils in mice led to increased intestinal permeability upon dextran sodium sulfate (DSS) insult(18). As IECs serve as the first line of defense against gut bacterial invasion, we hypothesized that tissue-infiltrating neutrophils could directly regulate IEC functions as a way to restore intestinal homeostasis. To investigate whether neutrophils regulate IEC functions, we utilized intestinal epithelial enteroids, a 3-dimensional primary culture system for mouse intestinal epithelial cells. It is practically hard to isolate neutrophils from intestines due to the short half-life of neutrophils, as it requires a long processing time, and most neutrophils isolated from intestines would have died by the end of preparation. Thus, we utilized peritoneal neutrophils, as they are readily isolated in large quantities(18). We first cultured peritoneal neutrophils for 24 h and then collected the supernatants to serve as neutrophil conditioned media. Next, we cultured the enteroids in the presence or absence of conditioned media for 6 h. RNA was extracted for microarray analysis using Affymetrix Mouse Gene 1.0 ST microarrays. Using the criteria of >2-fold increase or decrease, we observed 579 genes with significantly altered expression between the two groups of enteroids (Figure 1A). To better understand the functional interaction of these genes, we analyzed the 579 probes using Cytoscape software. Data analysis revealed that neutrophil conditioned media differentially regulated genes that are pertinent for IEC turnover and barrier function. The heat map in Figure 1A demonstrates the differentially expressed transcripts associated with IEC proliferation (AREG, Anxa1 and Anxa2), differentiation (AREG, Skil, Id1, Cd44 and Nox1), and junction formation (Cldn4, Lamc2, Ace2 and Ace). Compared to control, enteroids treated with neutrophil conditioned media had increased AREG transcripts (Figure 1A-B). We further verified these findings with quantitative real-time PCR (Figure 1C). Additionally, neutrophil conditioned media upregulated AREG transcription in epithelial cells from both small and large intestines (Figure 1D), as well as mouse small intestinal epithelium (MSIE) cells (Figure 1E), a conditionally immortalized epithelial cell line established from the intestines of normal mice(23),. Taken together, these results demonstrated that neutrophils induce IEC production of AREG through production of soluble factors.
Figure 1. Neutrophil conditioned media induces AREG expression by IECs.
Intestinal enteroids were cultured with or without neutrophil conditioned media for 6 h followed by microarray analysis. (A) Hierarchical clustering of genes that were differentially (|Fold change| > 2) expressed between the two groups (n = 2). Heat map of some differentially expressed probes between the two groups (n = 2). (B) Scatterplot displaying the log2 fold change in expression between the two groups (n = 2). (C) mRNA expression levels of AREG in the enteroids were measured by qRT-PCR and normalized to GAPDH. (D) Primary IECs from either small intestines (SB) or large intestines (LB) and treated with or without neutrophil conditioned media for 6 h, and mRNA expression level of AREG measured by qRT-PCR and normalized to GAPDH. (E) mRNA expression level of AREG in the MSIE cells treated with neutrophil conditioned media (CM) for 6 h. Data are presented as mean ± SEM of three independent experiments. CM, neutrophil conditioned media; None, media alone. *P < 0.05, **P < 0.01 Student’s t test.
AREG protects against DSS-induced colitis
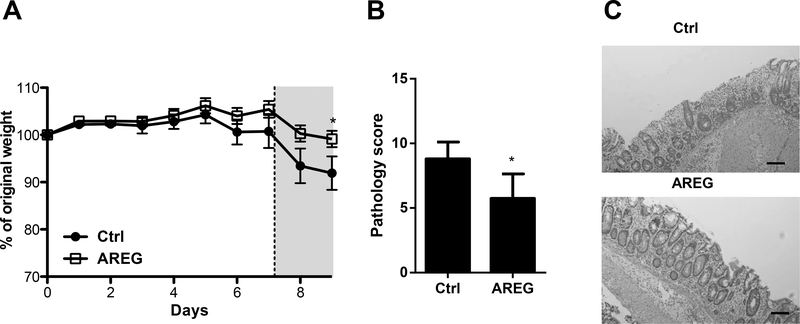
AREG is a growth factor capable of inducing proliferation, differentiation, and maturation of a variety of mesenchymal and epithelial cells(25). Previous studies have also detailed the importance of AREG in the development of intestinal epithelial structure and integrity(10, 26). Therefore, we sought to determine if administration of exogenous recombinant AREG conferred epithelial protection in the DSS colitis model. WT mice were fed DSS in drinking water, and recombinant AREG was administered to one group of the mice every 2 days. Consistent with previous reports(10, 26), after 7 days of DSS exposure, followed by 3 days water without DSS, mice that received exogenous AREG exhibited less severe systemic disease as evidenced by less weight loss as compared to mice fed DSS without AREG treatment (Figure 2A). Consistent with this finding, histopathological assessment showed that AREG treatment protected against the development of severe colitis (Figures 2B and C). Taken together, these data indicated that AREG contributes to the maintenance of intestinal homeostasis.
Figure 2. AREG protects the intestinal epithelium from DSS-induced injury.
WT mice were administered 2% DSS in drinking water for 7 days, followed by 3 days of water. One group of mice received 15μg of AREG every 2 days. (A) Relative body weight change. (B) Blinded histopathological scoring. (C) Representative images of H&E-stained colon sections. Scale bar, 100 μm. N = 4 per group per experiment. Data are representative of 3 independent experiments; *p<0.05 Student’s t test.
Neutrophils confer protection against colitis through induction of AREG by IECs
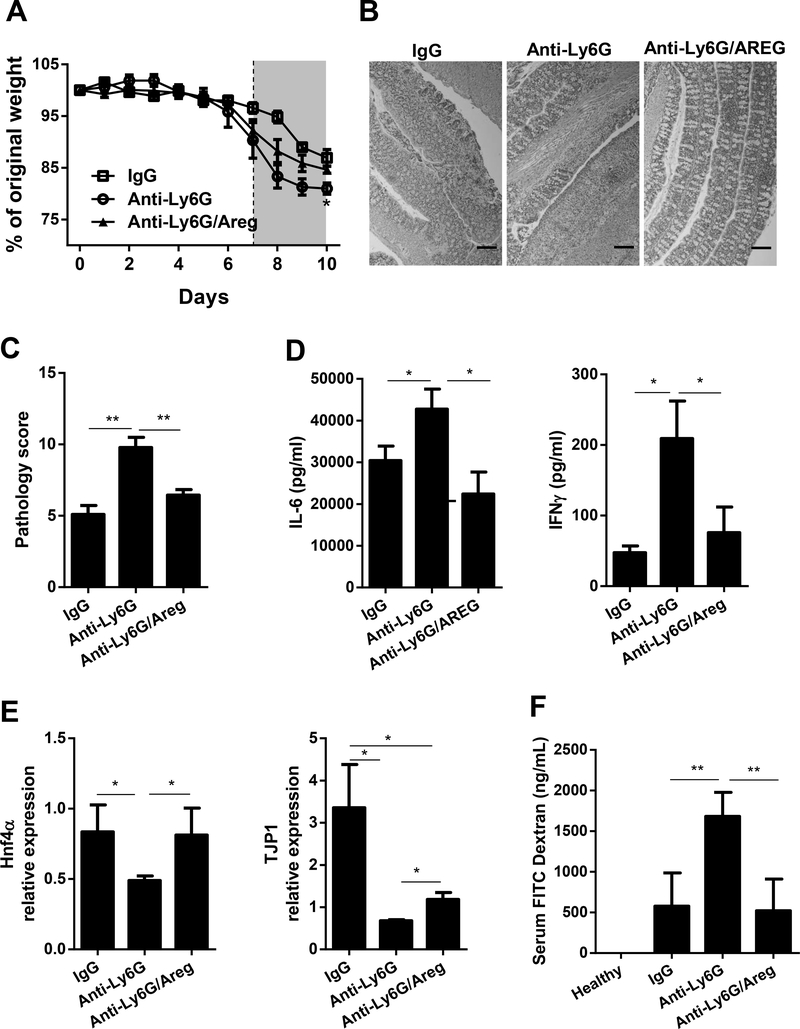
It has been shown that AREG has a potent effect on maintaining epithelial barrier integrity and repair. To determine whether neutrophils protect mice from intestinal inflammation through inducing IEC production of AREG, we treated WT mice with either IgG control or Ly6G-specific neutralizing antibody to deplete neutrophils (Figure S1). Mice were then fed DSS and treated with or without exogenous AREG. As shown in Figure 3, neutrophil-depleted mice experienced more weight loss compared to control IgG-treated mice. In contrast, neutrophil-depleted mice that received AREG treatment experienced less weight loss compared to anti-Ly6G-treated mice without treatment of AREG, experiencing similar weight loss to that of IgG-treated mice (Figure 3A). Histologically, neutrophil-depleted mice developed more severe colitis compared with IgG-treated mice, characterized by increased infiltrates and higher pathology scores (Figure 3B and C). Interestingly, AREG-supplementation resulted in less severe colitis in anti-ly6G-treated mice, as evidenced by decreased infiltrates at sites of inflammation and lower pathology scores (Figure 3B and C). Moreover, ex vivo culture of colonic tissues from anti-Ly6G treated mice that received AREG showed decreased production of pro-inflammatory cytokines including, IL-6, and IFNγ, as compared to anti-Ly6G-treated mice without treatment of AREG (Figure 3D), suggesting that AREG can, at least partially, compensate for the loss of neutrophils. Importantly, neutrophil depletion decreased IEC expression of two genes which play a critical role in epithelial integrity, HNF4α and TJP1, which were partially restored with AREG treatment (Figure 3E). To investigate whether AREG regulates epithelial barrier permeability, we fed mice FITC-dextran and examined the serum for fluorescence. Higher serum FITC-dextran concentrations were observed in neutrophil-depleted mice as compared with IgG-treated mice, and AREG treatment decreased the serum FITC-dextran concentrations in anti-Ly6G-treated mice (Figure 3F). Taken together, these data indicate that neutrophils play a protective role in intestinal inflammation at least partially through AREG.
Figure 3. Neutrophils confer protection against colitis through induction of AREG by IECs.
WT mice were administered 2% DSS in drinking water for 7 days, followed by 3 days of water. The mice were given Ly6G-depleting antibody i.p. every 3 days. One group of mice received AREG every 2 days and the other group received PBS. One group of mice treated with DSS were administrated with IgG to serve as control. (A) Relative change in body weight. (B) Representative images of H&E-stained colon sections. Scale bar, 100 μm. (C) Blinded histopathological scoring. (D) Protein level of inflammatory cytokines in colonic culture supernatant. (E) mRNA expression level of HNF4α, TJP1 in isolated IECs. (F) FITC-dextran level in plasma was determined. n = 4 per group. Data are representative of 3 independent experiments; ND, not detectable; *P < 0.05, **P < 0.01, ***P < 0.001 unpaired Student’s t test, one-way ANOVA, nonparametric Mann–Whitney U test.
TGFβ induces AREG production in IECs
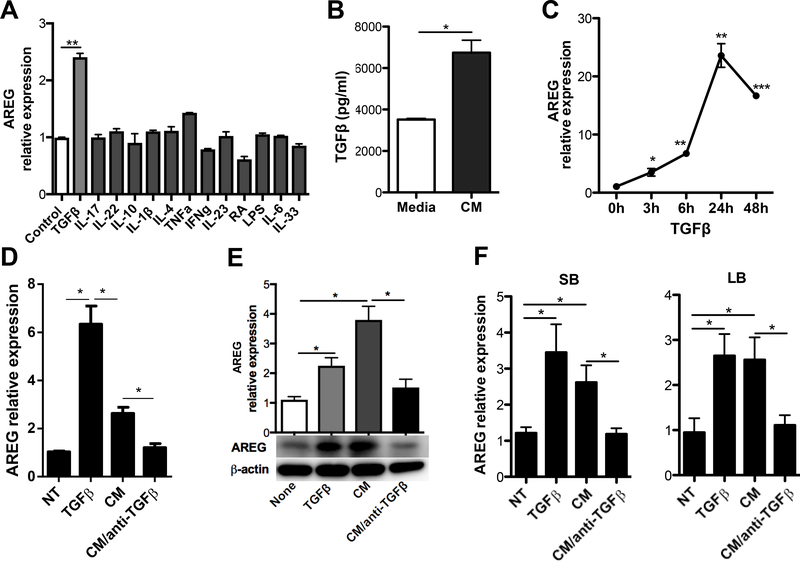
To identify the factors that potentially induce IEC AREG production, we treated MSIE cells with different cytokines, including TGF-β1 (hereafter referred to as TGFβ), IL-17, IL-22, IL-10, IL-1β, IL-4, TNFα, IFNγ, and IL-6 for 6 h(19, 27–31). We also treated MSIE cells with retinoic acid (RA), lipopolysaccharide (LPS), and IL-33, which were previously reported as capable of inducing AREG expression in different cells(5, 32, 33). Among all the candidates, only TGFβ induced AREG expression in MSIE cells (Figure 4A). Consistently, we observed increased AREG expression in primary IECs treated with TGFβ, but no other cytokines tested (Figure S2A), indicating that TGFβ is an inducer of AREG in IECs. Next, we performed ELISA analysis and confirmed the presence of TGFβ in conditioned neutrophil media (Figure 4B). To determine the kinetics of TGFβ induction of AREG in IEC, we treated the MSIE cells with TGFβ and measured AREG expression with respect to time over the course of 48 h. The increase in AREG expression started as early as 3 h post-treatment with TGFβ and peaked at 24 h before declining (Figure 4C). To determine whether TGFβ mediated neutrophil conditioned media induction of AREG by IECs, we pre-treated neutrophil conditioned media with anti-TGFβ antibody, or control antibody, to neutralize TGFβ before applying to cultures of MSIE cells. Addition of anti-TGFβ antibody abrogated the effect of neutrophil conditioned media in the induction of AREG by MSIE cells at both RNA and protein levels (Figure 4D-E). These results were replicated using primary IECs from both the small and large intestines, suggesting that neutrophil-derived TGFβ contributes to the AREG production by IECs (Figure 5F).
Figure 4. TGFβ induces AREG production in IEC.
(A) MSIE cells were treated with different cytokines/factors for 6 h, and mRNA expression level of AREG was measured by qRT-PCR and normalized against GAPDH. (B) Level of TGFβ was determined in neutrophil conditioned media versus media control. (C) mRNA expression level of AREG in the MSIE cells treated with 20 μg/ml TGFβ over time, determined by qRT-PCR and normalized against GAPDH. (D and E) Neutrophil conditioned media was pre-treated with 10 μg/ml anti-TGFβ antibody and then added into MSIE cell cultures. (D) mRNA expression level (24 h) and (E) protein level of AREG (24 h) were measured by qRT-PCR and Western blot, respectively. (F) mRNA expression level of AREG in primary IECs isolated from either SB or LB. Data are presented as mean ± SEM of three independent experiments. CM, neutrophil conditioned media; None, media alone. *P < 0.05, **P < 0.01, ***P < 0.001 Student’s t test, one-way ANOVA.
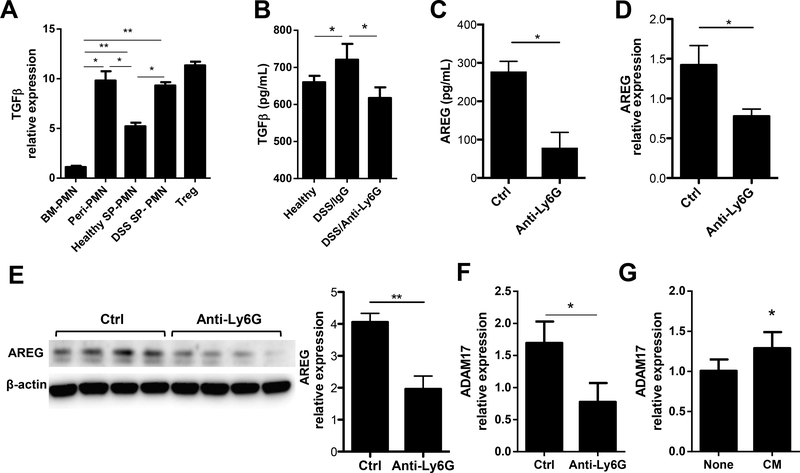
Figure 5. Neutrophils produce TGFβ which regulates IEC production of AREG in the setting of inflammation.
(A) mRNA expression level of TGFβ in bone marrow, peritoneal cavity, spleen neutrophils, with Treg as positive control. (B) Protein levels of TGFβ in the colonic tissue culture were analyzed by ELISA. (C) Soluble AREG was measured in colonic culture supernatant via ELISA. (D and E) IECs were isolated from the colon of mice, mRNA expression level (G) and protein level (E) of AREG were measured in IECs. (F) mRNA expression level of ADAM17 in the colonic IECs of mice. (G) Intestinal enteroids were cultured with or without neutrophil conditioned media for 6 h, mRNA expression levels of ADAM17 in the enteroids were measured by qRT-PCR and normalized to GAPDH. Data are representative of 3 independent experiments; PMN, neutrophil; *P < 0.05, **P < 0.01 unpaired Student’s t test, one-way ANOVA, nonparametric Mann–Whitney U test.
Neutrophils produce TGFβ which regulates IEC production of AREG in the setting of inflammation
To determine if neutrophils produce TGFβ, which possibly regulates IEC production of AREG in the setting of inflammation, neutrophils were isolated to measure the expression of TGFβ. Because neutrophils are extremely fragile and it is almost practically impossible to isolate purified neutrophils from intestinal lamina propria, as almost all neutrophils are dead after long process time (normally over 12 h), we isolated neutrophils from the bone marrow, peritoneal cavity, and spleen of mice and measured TGFβ expression by qRT-PCR. As it has been well-established that T regulatory (Treg) cells produce high levels of TGFβ(34), we also generated Treg cells from CD4+ T cells to serve as positive controls. As shown in Figure 5A, splenic neutrophils from DSS fed mice expressed TGFβ at a similar level to peritoneal neutrophils and Treg cells, which is much greater than splenic neutrophils from healthy wild-type mice. Moreover, ex vivo colonic organ culture showed higher TGFβ levels in IgG-treated mice after DSS administration, which was decreased in neutrophil-depleted mice (Figure 5B). Interestingly, lower levels of soluble AREG were detected in the supernatants of ex vivo colon cultures in neutrophil-depleted mice as compared with control mice (Figure 5C). Consistently, primary IECs isolated from neutrophil-depleted mice showed decreased expression of AREG at both RNA and protein levels (Figures 5D and E). It has been shown that AREG is first expressed as a transmembrane precursor in the cells(35). Shedding of AREG requires ADAM17, a TNFα converting enzyme found on IECs(36). We found that depletion of neutrophils resulted in decreased ADAM17 in the IECs of mice upon DSS insult (Figure 5F). Furthermore, we cultured enteroids in the presence or absence of neutrophil-conditioned media for 6 h, and ADAM17 expression was increased with neutrophil conditioned media as compared to controls. Together, these data indicated that neutrophils induce IEC expression of AREG and ADAM17, which sheds AREG, thus promoting AREG production by IECs. It also suggests a role for TGFβ-producing neutrophils in a AREG-dependent protective pathway in the intestines.
TGFβ induction of AREG in IECs is MEK1/2-dependent
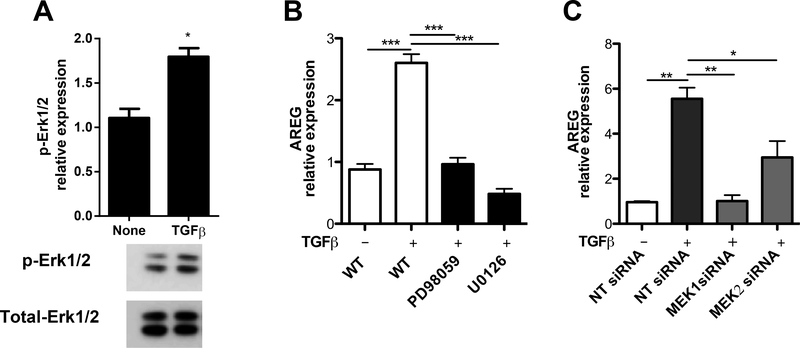
Lastly, we investigated the mechanisms underlying TGFβ-induced AREG production by IECs. Previous studies have suggested a positive feedback loop in TGFβ signaling through upregulating the expression of TGFβ receptors in different cell types (37, 38). We first set out to assess whether the phenomenon was consistent in IECs. However, we observed no significant change in the expression of either TGFβ type I receptor (TGFβ-RI) or TGFβ type II receptor (TGFβ-RII) in MSIE cells with TGFβ treatment (data not shown). In addition to activating the cascade of SMADs, it is known that the activation of TGFβ receptor complex can also lead to the subsequent activation of mitogen activated protein (MAP) kinase pathways(39). We then sought to determine if MEK-ERK signaling regulates AREG production in IECs. Western blot analysis revealed that TGFβ activated ERK1/2 in MSIE cells, as evidenced by increased phosphorylation of ERK1/2 after treatment with TGFβ (Figure 6A). To determine if activation of ERK mediates TGFβ-induction of AREG in IECs, we used two selective inhibitors of an ERK-activating enzyme MEK, PD98059 and U0126, which are small molecules capable of readily crossing the cell membrane to block ERK activation. We showed that addition of PD98059 and U0126 greatly decreased AREG expression induced by TGFβ (Figure 6B). To further confirm these results, we used MEK siRNA, which specifically inhibits the expression of MEK. Transfection of MSIE cells with MEK1 or MEK2 siRNA decreased expression of ERK-activating enzymes (Figure S3A). Additionally, transfection with either MEK1 or MEK2 siRNA decreased AREG expression in TGFβ-treated MSIE cells compared to non-targeting controls (Figure 6C). Furthermore, CRISPR knockout of MEK1 caused a marked decrease in AREG production induced by TGFβ in MSIE cells as compared to WT controls (Figure S3B). Taken together, these data indicated that MEK1/2 is required for TGFβ-induction of AREG production by IECs.
Figure 6. TGFβ induction of AREG in IECs is MEK1/2-dependent.
MSIE cells were treated with or without TGFβ. (A) The phosphorylated ERK1/2 (pERK1/2) was determined by Western blot (1 h). (B and C) MEK1/2 was inhibited or knockdown in MSIE cells in the presence of TGFβ. mRNA expression level of AREG was measured by qRT-PCR in the MSIE cells treated with (B) specific inhibitors or (C) siRNA. Data are presented as mean ± SEM of three independent experiments; NT, no treatment; NT siRNA, non-targeting siRNA. *P < 0.05, **P < 0.01, ***P < 0.001 one-way ANOVA.
Discussion
Tightly regulated intestinal barrier function is crucial in the maintenance of intestinal homeostasis. In this report, we demonstrate that neutrophils protect the intestines from inflammation, at least partially, through the induction of AREG by IECs. Administration of exogenous AREG mitigated tissue damage following depletion of neutrophils upon DSS challenge by restoring epithelial barrier function. Our findings, thus, reveal a previously unappreciated immune interaction between neutrophils and IECs in maintenance of intestinal homeostasis.
Despite many advances made in studying the interaction between epithelial cells and immune cells, the interplay between IECs and neutrophils remains poorly defined. We previously reported that IL-22-producing neutrophils enhance intestinal barrier integrity in the context of colitis, suggesting that neutrophil-IEC crosstalk plays a critical role during the inflammatory state(18). To further delineate the mechanisms underlying neutrophil regulation of IEC, we use intestinal epithelial enteroids, which recapitulate the diverse composition and complexity of intestinal epithelial cells. Analysis of microarray data showed that neutrophil conditioned media-treated enteroids were enriched in mRNA responsible for wound healing and junction formation, indicating a role for neutrophils in tissue repair. We also observed an increase in mRNA encoding proteins for IEC proliferation and differentiation; AREG is essential in both processes(40). We confirmed that neutrophil conditioned media induced AREG expression by MSIE cells, a mouse small intestinal epithelial cell line.
AREG acts as an EGFR ligand, and has been shown to be crucial in protection against intestinal inflammation(5, 40). We showed that the increased levels of AREG corresponded with improved disease outcomes in the DSS-induced colitis model. Moreover, additional exogenous AREG administration ameliorated disease and significantly improved the integrity of the epithelial barrier. Given that IBD is rooted in chronic dysregulated immune responses against gut microbiota, any initial contact with the microbiota by the immune system would lead to overwhelming inflammation that perpetuates itself into a chronic diseased state. As such, the key for preventing aberrant immune activation is to possess a strong intact epithelial barrier to prevent penetration by the microbiota and leave the immune system quiescent. We speculate that deficiency in AREG production leads to a less robust epithelial barrier, thereby conferring susceptibility to IBD. In supporting this notion, a recent report using transcriptomic microarray analysis demonstrated an increased intestinal ERRFI1 in patients with either Crohn’s Disease or Ulcerative Colitis. ERRFI1 mediates inhibition of the EGFR, thereby blocking signaling of AREG and other EGF family members(41). Interestingly, IECs themselves also express EGFR(42). As accumulating evidence has shown that AREG enhances epithelial function(5, 40), we thus treated MSIE cells with exogenous AREG to investigate if AREG can further amplify its own production. However, there was no evidence for a change in expression of AREG in IECs (Figure S2B).
Consistent with our previous finding using the T cell-induced colitis model(18), depletion of neutrophils led to more severe inflammation in a DSS colitis model. This data reinforces the idea that innate immune components, especially neutrophils, are indispensable regulators for resolving inflammation. We found that expression of TGFβ was impaired in the intestines of mice receiving neutrophil-depleting treatment. Similar to lung epithelium, we demonstrated that TGFβ induced robust expression of AREG in IECs. However, in comparison to other cell types (including innate lymphoid cell(5), Treg(13), cholangiocyte(32), epidermal cell(33) or fibroblast(43, 44), TGFβ remains the only inducer among the cytokines/factors tested capable of eliciting AREG production in IECs. Although the first report of neutrophil production of TGFβ dates back to 1989(45), attention has been mainly focused on neutrophil-derived products that facilitate TGFβ activation(46). Our data provide new evidence that neutrophils increase production of TGFβ under an inflammatory state. Correlation between impaired tissue TGFβ levels and decreased IEC production of AREG revealed a previously unappreciated role for neutrophil regulation of barrier function.
As an essential regulator of epithelial integrity, HNF4α has been identified as a susceptibility gene that confers risk of IBD(47). We demonstrated that depletion of neutrophils impaired HNF4α in colonic tissue(18), whereas exogenous AREG treatment rescued HNF4α expression in the colon of neutrophil-depleted mice, further restoring epithelial permeability. Additionally, neutrophil conditioned media induced Cldn4 encoding tight junction protein and Lamc2 encoding extracellular matrix glycoproteins in enteroids. As reported, direct contact with IECs prevents neutrophil apoptosis(48). The question of whether direct physical interaction between neutrophils and IECs could also promote IEC barrier function warrants future investigation.
ADAM17, a transmembrane protease found on the surface of neutrophils, regulates shedding of various substrates including AREG(49). Our finding that neutrophil depletion impaired intestinal epithelial cells expression of ADAM17 indicates a potential protective role for infiltrating neutrophils in the regulation of inflammation through localized AREG induction. Furthermore, neutrophil-facilitated AREG production is not only limited to epithelial origin, but other cellular sources as well. Given the chemotactic effect of IECs on neutrophils under mucosal injury(50, 51), it is intriguing to speculate that IECs may recruit and retain neutrophils at the inflammatory site as a way to increase local TGFβ levels and promote AREG secretion during rampant inflammation. Such an occurrence would be consistent with the role of neutrophils in maintaining and/or restoring intestinal homeostasis.
In summary, our studies demonstrated a novel pathway of neutrophil regulation of intestinal inflammation through induction of AREG by IECs, which is dependent on TGFβ. As TGFβ has been considered to be one of the most important anti-inflammatory cytokines, our data provides a novel anti-inflammatory function of TGFβ in the intestines through induction of IEC production of AREG.
Supplementary Material
Acknowledgments:
We appreciate Dr. Linsey Yeager of The University of Texas Medical Branch for proofreading the manuscript.
Grant Support: This work was supported by NIH grants DK098370, DK105585, and DK112436, and John Sealy Memorial Endowment Fund (YC). FC is a recipient of the J.W. McLaughlin Predoctoral Fellowship, UTMB.
Abbreviations:
- AREG
amphiregulin
- IEC
intestinal epithelial cells
- HNF4
hepatocyte nuclear factor 4
- ADAM17
ADAM metallopeptidase domain 17
- (MSIE)
mouse small intestinal epithelium
- (DSS)
dextran sodium sulfate
- (IBD)
Inflammatory Bowel Disease
Footnotes
Disclosures: The authors report no financial conflict of interests.
References:
- 1.Peterson LW, and Artis D. 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14: 141–153. [DOI] [PubMed] [Google Scholar]
- 2.Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, and Stappenbeck TS. 2016. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 165: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Flier LG, and Clevers H. 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–260. [DOI] [PubMed] [Google Scholar]
- 4.Cao AT, Yao S, Gong B, Elson CO, and Cong Y. 2012. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol 189: 4666–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, and Artis D. 2015. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A 112: 10762–10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonnenberg GF, and Artis D. 2012. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity 37: 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCole DF 2014. IBD candidate genes and intestinal barrier regulation. Inflamm Bowel Dis 20: 1829–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, and Wells JM. 2014. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol 14: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaiss DMW, Gause WC, Osborne LC, and Artis D. 2015. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 42: 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam KT, Lee HJ, Mok H, Romero-Gallo J, Crowe JE Jr., Peek RM Jr., and Goldenring JR. 2009. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology 136: 1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, and Artis D. 2011. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, and Mosmann TR. 2006. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science 314: 1746. [DOI] [PubMed] [Google Scholar]
- 13.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, and Rudensky AY. 2015. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 162: 1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M, van PM Bergen en Henegouwen, Roovers RC, Coffer PJ, and Sijts AJ. 2013. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 38: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall OJ, Limjunyawong N, Vermillion MS, Robinson DP, Wohlgemuth N, Pekosz A, Mitzner W, and Klein SL. 2016. Progesterone-Based Therapy Protects Against Influenza by Promoting Lung Repair and Recovery in Females. PLoS Pathog 12: e1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier BM, and Parkos CA. 2012. The role of neutrophils during intestinal inflammation. Mucosal Immunol 5: 354–366. [DOI] [PubMed] [Google Scholar]
- 17.Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J, and Hartl D. 2015. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog 11: e1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen F, Cao A, Yao S, Evans-Marin HL, Liu H, Wu W, Carlsen ED, Dann SM, Soong L, Sun J, Zhao Q, and Cong Y. 2016. mTOR Mediates IL-23 Induction of Neutrophil IL-17 and IL-22 Production. J Immunol 196: 4390–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou G, Yu L, Fang L, Yang W, Yu T, Miao Y, Chen M, Wu K, Chen F, Cong Y, and Liu Z. 2017. CD177+ neutrophils as functionally activated neutrophils negatively regulate IBD. Gut 67: 1052–1063. [DOI] [PubMed] [Google Scholar]
- 20.Zindl CL, Lai JF, Lee YK, Maynard CL, Harbour SN, Ouyang W, Chaplin DD, and Weaver CT. 2013. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci U S A 110: 12768–12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, and Colgan SP. 2014. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40: 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wera O, Lancellotti P, and Oury C. 2016. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med 5:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehead RH, and Robinson PS. 2009. Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am J Physiol Gastrointest Liver Physiol 296: G455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iqbal N, Oliver JR, Wagner FH, Lazenby AS, Elson CO, and Weaver CT. 2002. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J. Exp. Med 195: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaiss DM, Gause WC, Osborne LC, and Artis D. 2015. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 42: 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troyer KL, Luetteke NC, Saxon ML, Qiu TH, Xian CJ, and Lee DC. 2001. Growth retardation, duodenal lesions, and aberrant ileum architecture in triple null mice lacking EGF, amphiregulin, and TGF-alpha. Gastroenterology 121: 68–78. [DOI] [PubMed] [Google Scholar]
- 27.Wright HL, Moots RJ, and Edwards SW. 2014. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol 10: 593–601. [DOI] [PubMed] [Google Scholar]
- 28.Naegelen I, Beaume N, Plancon S, Schenten V, Tschirhart EJ, and Brechard S. 2015. Regulation of Neutrophil Degranulation and Cytokine Secretion: A Novel Model Approach Based on Linear Fitting. J Immunol Res 2015: 817038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tecchio C, Micheletti A, and Cassatella MA. 2014. Neutrophil-derived cytokines: facts beyond expression. Front Immunol 5: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandt E, Woerly G, Younes AB, Loiseau S, and Capron M. 2000. IL-4 production by human polymorphonuclear neutrophils. J Leukoc Biol 68: 125–130. [PubMed] [Google Scholar]
- 31.Deguine J, Wei J, Barbalat R, Gronert K, and Barton GM. 2017. Local TNFR1 Signaling Licenses Murine Neutrophils for Increased TLR-Dependent Cytokine and Eicosanoid Production. J Immunol 198: 2865–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trussoni CE, Tabibian JH, Splinter PL, and O’Hara SP. 2015. Lipopolysaccharide (LPS)-Induced Biliary Epithelial Cell NRas Activation Requires Epidermal Growth Factor Receptor (EGFR). PLoS One 10: e0125793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rittie L, Varani J, Kang S, Voorhees JJ, and Fisher GJ. 2006. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol 126: 732–739. [DOI] [PubMed] [Google Scholar]
- 34.Tang Q, and Bluestone JA. 2008. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nature immunology 9: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sternlicht MD, and Sunnarborg SW. 2008. The ADAM17-amphiregulin-EGFR axis in mammary development and cancer. J Mammary Gland Biol Neoplasia 13: 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra HK, Ma J, and Walcheck B. 2017. Ectodomain Shedding by ADAM17: Its Role in Neutrophil Recruitment and the Impairment of This Process during Sepsis. Front Cell Infect Microbiol 7: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauge C, Cauvard O, Leclercq S, Galera P, and Boumediene K. 2011. Modulation of transforming growth factor beta signalling pathway genes by transforming growth factor beta in human osteoarthritic chondrocytes: involvement of Sp1 in both early and late response cells to transforming growth factor beta. Arthritis Res Ther 13: R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menke A, Geerling I, Giehl K, Vogelmann R, Reinshagen M, and Adler G. 1999. Transforming growth factor-beta-induced upregulation of transforming growth factor-beta receptor expression in pancreatic regeneration. Biochim Biophys Acta 1449: 178–185. [DOI] [PubMed] [Google Scholar]
- 39.Moustakas A, and Heldin CH. 2016. Mechanisms of TGFbeta-Induced Epithelial-Mesenchymal Transition. J Clin Med 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao J, and Sheng H. 2010. Amphiregulin promotes intestinal epithelial regeneration: roles of intestinal subepithelial myofibroblasts. Endocrinology 151: 3728–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raine T, Liu JZ, Anderson CA, Parkes M, and Kaser A. 2015. Generation of primary human intestinal T cell transcriptomes reveals differential expression at genetic risk loci for immune-mediated disease. Gut 64: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frey MR, Golovin A, and Polk DB. 2004. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem 279: 44513–44521. [DOI] [PubMed] [Google Scholar]
- 43.Liu FL, Wu CC, and Chang DM. 2014. TACE-dependent amphiregulin release is induced by IL-1beta and promotes cell invasion in fibroblast-like synoviocytes in rheumatoid arthritis. Rheumatology (Oxford) 53: 260–269. [DOI] [PubMed] [Google Scholar]
- 44.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, and Fujiyama Y. 2005. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129: 969–984. [DOI] [PubMed] [Google Scholar]
- 45.Grotendorst GR, Smale G, and Pencev D. 1989. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol 140: 396–402. [DOI] [PubMed] [Google Scholar]
- 46.Granot Z, and Jablonska J. 2015. Distinct Functions of Neutrophil in Cancer and Its Regulation. Mediators Inflamm 2015: 701067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Consortium UIG, Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H, Nimmo ER, Massey D, Blaszczyk K, Elliott T, Cotterill L, Dallal H, Lobo AJ, Mowat C, Sanderson JD, Jewell DP, Newman WG, Edwards C, Ahmad T, Mansfield JC, Satsangi J, Parkes M, Mathew CG, Wellcome C, Trust Case Control Donnelly P, Peltonen L, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Craddock N, Deloukas P, Duncanson A, Jankowski J, Markus HS, Mathew CG, McCarthy MI, Palmer CN, Plomin R, Rautanen A, Sawcer SJ, Samani N, Trembath RC, Viswanathan AC, Wood N, Spencer CC, Barrett JC, Bellenguez C, Davison D, Freeman C, Strange A, Donnelly P, Langford C, Hunt SE, Edkins S, Gwilliam R, Blackburn H, Bumpstead SJ, Dronov S, Gillman M, Gray E, Hammond N, Jayakumar A, McCann OT, Liddle J, Perez ML, Potter SC, Ravindrarajah R, Ricketts M, Waller M, Weston P, Widaa S, Whittaker P, Deloukas P, Peltonen L, Mathew CG, Blackwell JM, Brown MA, Corvin A, McCarthy MI, Spencer CC, Attwood AP, Stephens J, Sambrook J, Ouwehand WH, McArdle WL, Ring SM, and Strachan DP. 2009. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet 41: 1330–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sumagin R, Brazil JC, Nava P, Nishio H, Alam A, Luissint AC, Weber DA, Neish AS, Nusrat A, and Parkos CA. 2016. Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol 9: 1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carnet O, Lecomte J, Masset A, Primac I, Durre T, Maertens L, Detry B, Blacher S, Gilles C, Pequeux C, Paupert J, Foidart JM, Jerusalem G, Cataldo D, and Noel A. 2015. Mesenchymal Stem Cells Shed Amphiregulin at the Surface of Lung Carcinoma Cells in a Juxtacrine Manner. Neoplasia 17: 552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohtsuka Y, Lee J, Stamm DS, and Sanderson IR. 2001. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut 49: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuerer-Maly CC, Eckmann L, Kagnoff MF, Falco MT, and Maly FE. 1994. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology 81: 85–91. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.