Abstract
We previously reported that alymphoplasia (aly/aly) mice, which have a natural loss-of-function mutation in the Nik gene, which encodes a kinase essential for the processing of p100 to p52 in the alternative nuclear factor-κB (NF-κB) pathway, show mild osteopetrosis with an increase in several parameters of bone formation: bone formation rate, mineral apposition rate, and osteoblast number. We therefore investigated the molecular mechanisms triggered by the alternative NF-κB pathway in the regulation of osteoblast differentiation using primary osteoblasts (POB) prepared from aly/aly mice. Alkaline phosphatase (ALP) activity and mineralization induced by the presence of β-glycerophosphate and ascorbic acid were enhanced in POB from aly/aly compared with wild-type (WT) mice. Furthermore, osteoblastic differentiation induced by bone morphogenetic protein 2 (BMP2), as shown by ALP activity, mRNA expression of osteocalcin, Id1, Osterix and Runx2, and Sma- and Mad-related protein (Smad)1/5/8 phosphorylation, was also enhanced in POB from aly/aly mice. The ectopic bone formation in vivo that was induced by BMP2 was enhanced in aly/aly mice compared with controls. Transfection of a mutant form of p100, p100ΔGRR, which cannot be processed to p52, stimulated ALP activity and Smad phosphorylation. In contrast to p100ΔGRR, overexpression of p52 inhibited these events. Both BMP2-induced ALP activity and Smad phosphorylation were reduced in POB from p100-deficient mice, which carry a homozygous deletion of the COOH-terminal ankyrin repeats of p100 but still express functional p52 protein. p52 and p100ΔGRR interacted with a BMP receptor, ALK2, in overexpressed COS7 cells and changed the ALK2 protein levels in opposite directions: p52 reduced ALK2 and p100 increased it. Thus, the alternative the NF-κB pathway via the processing of p52 from p100 negatively regulates osteoblastic differentiation and bone formation by modifying BMP activity.
Although bone appears to be a static tissue, it is characterized by a dynamic turnover known as bone remodeling, which maintains bone volume and calcium homeostasis throughout life (1, 2). At remodeling sites, osteoblasts compose new bone, and osteoclasts resorb existing bone. Osteoblasts differentiate from mesenchymal stem cells, and the differentiation processes are coordinately and dynamically controlled by specific signal transduction pathways, either directly or indirectly. Several cytokines and hormones, such as bone morphogenetic protein (BMP), TGF-β, Wnt, hedgehog, fibroblast growth factors, and estrogen regulate mesenchymal cell differentiation by stimulating intracellular signaling pathways (3, 4). Specific intracellular signaling molecules are activated in response to their ligands through phosphorylation, ubiquitination, protein-protein interactions, and conformational changes. These molecules, in turn, affect specific transcription factors by up-regulating their transcriptional activity and/or by translocating into the nucleus. These signaling pathways also engage in cross talk, forming complex network systems. Two well-known examples of such transcription factors are Runx2 and Osterix. The deletion of either of these transcription factors in mice results in a drastic phenotype in which the cartilaginous skeleton fails to ossify, leading to a complete lack of bone with less ossification of cartilaginous skeleton (1–4).
The transcription factor nuclear factor-κB (NF-κB) regulates the expression of a wide variety of genes that are involved in immune and inflammatory responses, proliferation, tumorigenesis, and survival (5–7). The NF-κB family includes p65 (RelA), RelB, cRel, NF-κB1, and NF-κB2. These members can homodimerize and heterodimerize in numerous combinations; the predominant cellular species are p50:p65, p50:cRel, and NF-κB2:RelB. Each of these dimers is bound in the cytoplasm by the inhibitor of κB (IκB), which prevents the nuclear translocation and transcriptional activation potential of the NF-κB complex. NF-κB1 and NF-κB2 are synthesized as large precursors, p105 and p100, respectively, and they have long COOH-terminal domains that contain multiple ankyrin repeats, rendering these precursors functionally similar to the inhibitor IκB. The inhibitory effect of p105 and p100 is relieved on being processed into p50 and p52, respectively (5–7). Research in recent years has defined two distinct NF-κB activation pathways, termed the “classical” and “alternative” NF-κB signaling pathways (5–7). Classical NF-κB activation is based on inducible IκB degradation. This pathway can be rapidly and transiently activated by a large variety of substances, such as mitogens, cytokines, and microbial components. Activation is dependent on a specific IκB kinase (IKK) composed of two catalytic subunits, IKKα (IKK1) and IKKβ (IKK2), and the regulatory subunit NEMO (IKKγ). Upon activation, IKK phosphorylates specific serines in the IκB proteins, triggering their ubiquitination and degradation by the proteasome, thus allowing the NF-κB dimers to move to the nucleus to regulate gene expression.
In contrast, the alternative NF-κB signaling pathway is activated by a select group of TNF receptors, such as CD40, lymphotoxin-β receptor, and receptor activator of NF-κB. Activation of NF-κB-inducing kinase (NIK) results in activation of IKKα homodimers and processing of the p100 precursor to p52. The processing of p105 to p50 occurs constitutively, whereas the processing of p100 to p52 is stimuli dependent. Removal of the ankyrin repeats from p100 reduces its IκB-like activity, and the processing of p100 allows nuclear translocation of RelB, which may heterodimerize with either p52 or p50 for subsequent gene transcription (5–8).
Alymphoplasia (aly/aly) mice have a natural loss-of-function mutation in the gene encoding Nik (9), which is an essential kinase for the processing of p100 to p52 in the alternative NF-κB pathway. The alternative NF-κB signaling pathway is inhibited downstream of NIK in these mice (8, 10, 11). Therefore, aly/aly mice are useful for understanding the physiological role of the alternative NF-κB pathway in tissue development and regeneration. We have recently shown that aly/aly mice have mild osteopetrosis with increases in several bone formation parameters: bone formation rate, mineral apposition rate, and osteoblast number. In contrast, p100-deficient mice, which carry a homozygous deletion of the COOH-terminal ankyrin repeats of p100 but still express functional p52 protein, mirror precisely the bone phenotype of aly/aly mice: osteopenia with a decreased number of osteoblasts (10), suggesting that alternative NF-κB signaling regulates osteoblast differentiation. The roles of p100 and p52 in regulating osteoblast differentiation and bone formation are, however, not clear. In this study, we examined the role of the NF-κB alternative pathway on osteoblastic differentiation in vitro and bone formation in vivo using aly/aly mice.
Results
Alternative NF-κB signaling regulates osteoblast differentiation in vitro
We previously performed bone histomorphometric analyses of bone formation parameters in WT and aly/aly mice. Enhanced bone formation with increased BMD was observed in aly/aly mice compared with WT mice (10, 11), suggesting that loss of the alternative NF-κB signaling pathway increases osteoblastogenesis.
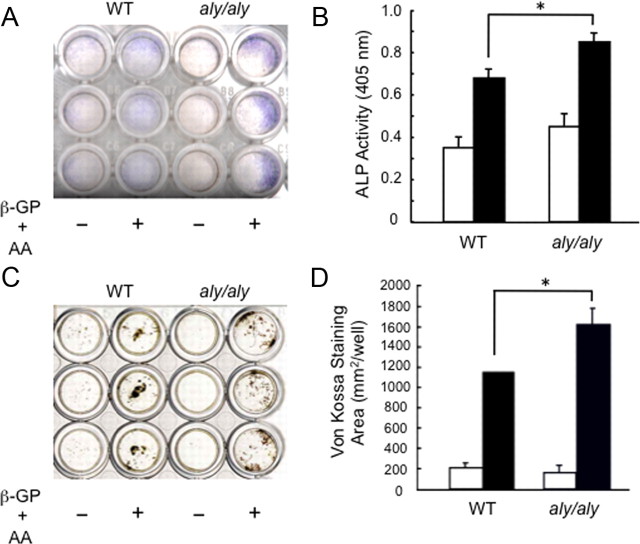
Therefore, we prepared primary osteoblasts (POB) from the calvariae of 1-d-old WT and aly/aly mice to examine the role of the alternative NF-κB signaling pathway in osteoblast differentiation in vitro. The number of alkaline phosphatase (ALP)-positive cells and ALP activity were higher in POB from aly/aly compared with WT mice in the presence of β-glycerophosphate and ascorbic acid (Fig. 1, A and B). Moreover, von Kossa staining clearly showed enhanced mineralization in POB from aly/aly mice (Fig. 1, C and D).
Fig. 1.
The alternative NF-κB signaling pathway regulates osteoblast differentiation in vitro. POB from calvariae of 1-d-old WT or aly/aly mice were cultured in the presence (closed column) or absence (open column) of β-glycerophosphate (β-GP, 10 mm) and ascorbic acid (AA, 50 μg/ml) for 7 d. A, Cells were stained for ALP activity. B, Cells were fixed with an acetone-ethanol mixture and incubated with a substrate solution. ALP activity was then determined. The data are means ± sd (n = 3), *, P < 0.01. C, von Kossa staining of calvaria cultures in the presence (closed column) or absence (open column) of β-glycerophosphate and ascorbic acid for 14 d. D, The stained area was measured. The data are means ± sd (n = 3), *, P < 0.01.
BMP2-induced osteoblast differentiation is enhanced in aly/aly POB
We examined the effect of the alternative NF-κB signaling pathway on BMP2-induced osteoblastic differentiation using the method reported previously (12). The increase in ALP activity seen by adding 50 or 100 ng/ml BMP2 was higher in POB from aly/aly than in WT mice (Fig. 2A). BMP2-induced osteocalcin mRNA expression was also increased in POB from aly/aly mice (Fig. 2B). In the presence of BMP2, the mRNA expression of transcription factors involved in osteoblast differentiation, such as Id1, Osterix, and Runx2, also increased more in POB from aly/aly mice compared with those from WT mice (Fig. 2C). These results suggest that loss of the alternative NF-κB signaling pathway via loss of processing of p100 to p52 positively regulates osteoblastogenesis.
Fig. 2.
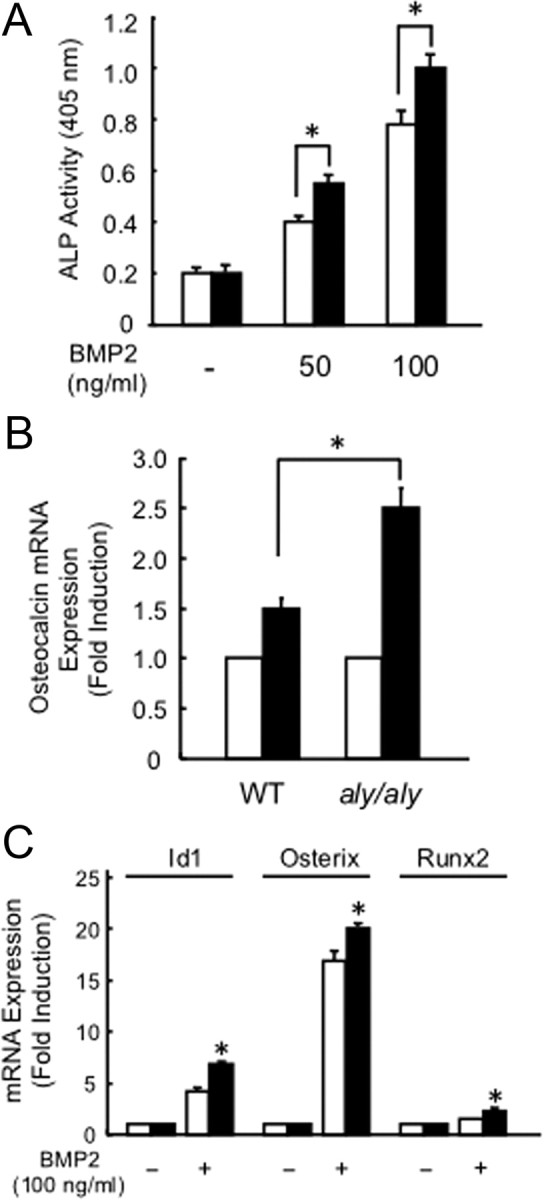
BMP2-induced osteoblast differentiation is enhanced in POB from aly/aly mice. A, POB from calvariae of 1-d-old WT (open column) or aly/aly mice (closed column) were treated with BMP2 (0, 50, 100 ng/ml) for 72 h. The cells were fixed with an acetone-ethanol mixture and incubated with a substrate solution. ALP activity was then determined. The data are means ± sd (n = 3), *, P < 0.01. B, POB were treated with (closed column) or without (open column) BMP2 (100 ng/ml) for 72 h. Total RNA was isolated and the expression level of osteocalcin relative to glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was measured by quantitative real-time PCR analysis. The data are means ± sd (n = 3), *, P < 0.01. C, POB from calvariae of 1-d-old WT (open column) or aly/aly (closed column) mice were treated with or without BMP2 (100 ng/ml) for 24 h. Total RNA was isolated, and the expression levels of Id1, Osterix, or Runx2 relative to GAPDH were measured by quantitative real-time PCR analysis. The data are means ± sd (n = 3), *, P < 0.01.
BMP2-induced Sma- and Mad-related protein (Smad) 1/5/8 phosphorylation is enhanced in aly/aly POB without affecting expression levels of BMP receptors and Smad proteins
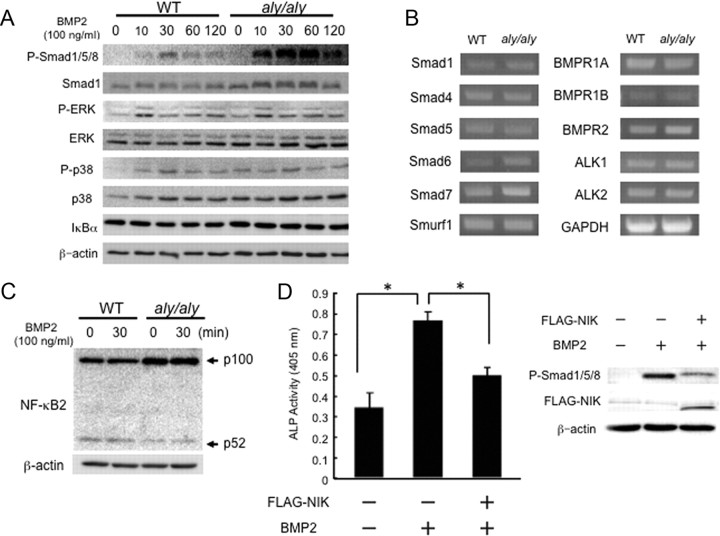
Phosphorylation of Smad1/5/8 upon BMP stimulation is a key step in BMP signal transduction (13, 14). As shown in Fig. 3A (top panel), the Smad1/5/8 phosphorylation induced by BMP2 was enhanced in POB from aly/aly compared with WT mice even though total Smad1 level was constant. The phosphorylation levels of ERK and p38, and the IκBα levels were equivalent between WT and aly/aly cells (Fig. 3A). RT-PCR analysis indicated that the expression levels of critical molecules involved in BMP signaling, including Smad and BMP receptors, were not affected in POB from aly/aly mice compared with WT mice (Fig. 3B). The protein levels of p100 were higher in aly/aly cells due to the lack of processing to p52 significantly and adding BMP2 to WT and aly/aly cells did not change the levels of p100 or p52 (Fig. 3C). The overexpression of FLAG-tagged NIK suppressed BMP2-induced ALP activity and Smad1/5/8 phosphorylation (Fig. 3D).
Fig. 3.
BMP2/Smad signaling is enhanced in POB from aly/aly mice. A, POB from calvariae of 1-d-old WT or aly/aly mice were cultured in the presence of BMP2 (100 ng/ml) for the indicated times. Total cell lysates were immunoblotted with antiphosphorylated Smad1/5/8, Smad1, phosphorylated ERK, ERK, phosphorylated p38, and p38 antibodies. Anti-β-actin was used as a loading control. B, Total RNA was prepared and then the expression levels of R-Smad (Smad1 and Smad5), Co-Smad (Smad4), I-Smad (Smad6 and Smad7), Smurf1, BMP receptors (BMPR1A, BMPR1B, BMPR2, ALK1, and ALK2), and GAPDH mRNA were analyzed using RT-PCR. C, POB were treated with or without BMP2 (100 ng/ml) for 30 min. Total cell lysates were immunoblotted with anti-NF-κB2 antibodies. Anti-β-actin was used as a loading control. D, POB from aly/aly mice were transfected with or without FLAG-tagged NIK for 24 h. The cells were further cultured for 72 h in the presence of BMP2 (100 ng/ml). ALP activity was measured. The data are means ± sd (n = 3), *, P < 0.01. After transfection, cells were treated with or without BMP2 (100 ng/ml) for 1 h. Smad 1/5/8 phosphorylation was determined by immunoblotting.
BMP2-induced ectopic bone formation is enhanced in aly/aly mice in vivo
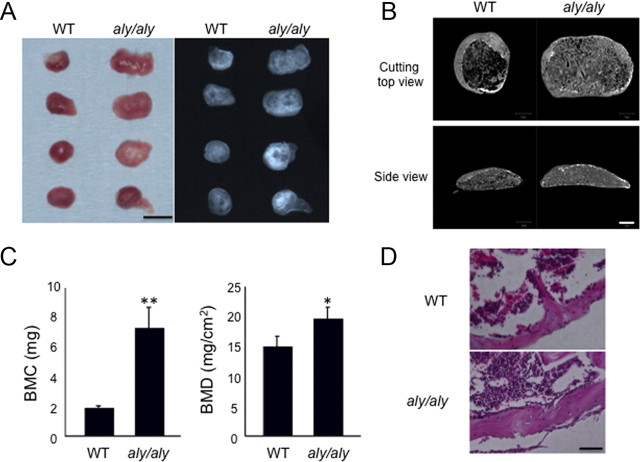
We next examined BMP2-induced ectopic bone formation in aly/aly mice. Ectopic bones formed in aly/aly mice were enlarged and had enhanced radiopaques compared with WT mice (Fig. 4A). The μCT analysis showed a thicker outer bone filled with larger numbers of trabecular bone in aly/aly mice than WT mice (Fig. 4B). The bone mineral content (BMC) and bone mineral density (BMD) of these ectopic bones were significantly increased in aly/aly mice compared with WT mice (Fig. 4C). With hematoxylin/eosin (H&E) staining of sections of formed ectopic bones, we observed thicker cortical bone and reduced bone marrow area in aly/aly mice (Fig. 4D). These results further confirmed our hypothesis that the lack of alternative NF-κB signaling pathway increases bone formation.
Fig. 4.
BMP2-induced ectopic bone formation is enhanced in aly/aly mice in vivo. BMP2 (4 μg) was implanted sc to induce ectopic bone formation in WT or aly/aly mice. A, After 4 wk, implants were removed and examined by soft x-ray analysis. Bar, 5 mm. B, μCT reconstruction images of ectopic bone in WT or aly/aly mice. Bar, 1 mm. C, BMC and BMD of the ectopic bone were measured by dual-energy x-ray absorptiometry. *, P < 0.05; **, P < 0.01. D, Sections of implants at 4 wk were stained with H&E. Bar, 25 μm.
Accumulation of p100 stimulated both Smad phosphorylation and ALP activity induced by a constitutively activated BMP receptor
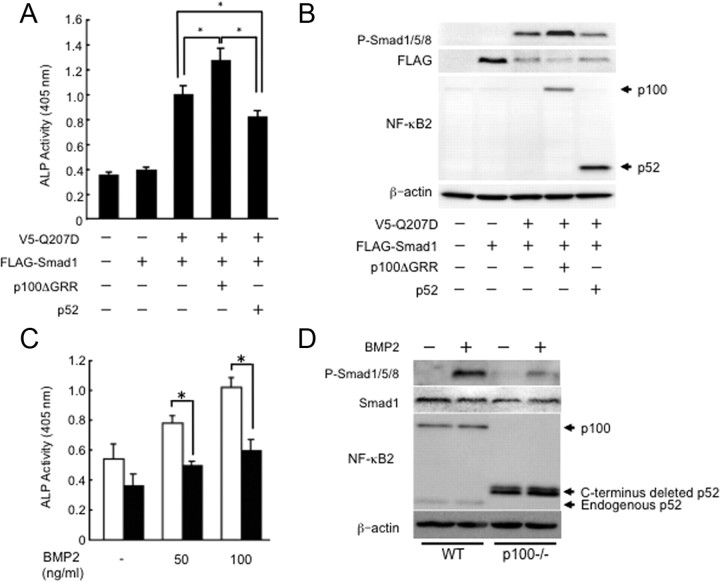
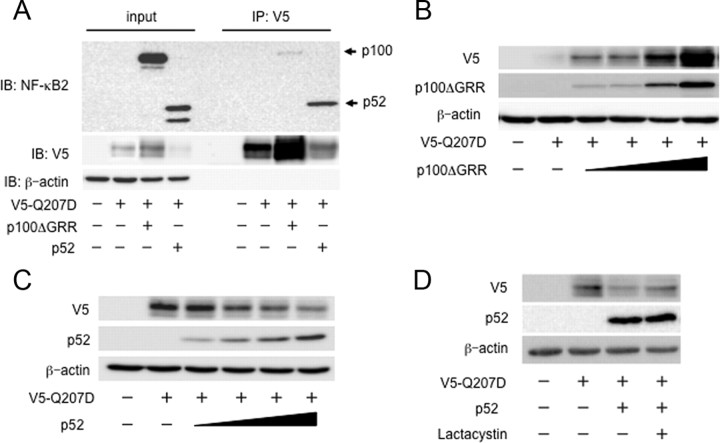
Our findings suggested that the alternative NF-κB signaling pathway via p52 processing from p100 negatively regulates osteoblast differentiation and bone formation. The roles of p100 and p52 in acceleration of osteoblast differentiation are still not clear. Thus, we examined the osteoblastic differentiation of POB prepared from Nfkb2−/− mice, which lack both p100 and p52 and show no obvious bone phenotype in vivo (11). Consistent with the previous findings in vivo, there were no differences in the BMP2-induced ALP activity or the Smad1/5/8 phosphorylation levels between Nfkb2−/− and WT POB (Supplemental Fig.1). A possible explanation is that the lack of p52 was compensated for by the p50 subunit in Nfkb2−/− POB (15). To further clarify the role of p100 without the ambiguity of p52, we transfected Nfkb2−/− POB with p100ΔGRR, which is not processed into p52 due to substitutions in the critical processing site. The ALP activity and Smad1/5/8 phosphorylation induced by cotransfection of Smad1 and a constitutively activated BMP receptor, ALK2 [ALK2 (Q207D)], was enhanced by overexpression of p100ΔGRR in Nfkb2−/− POB (Fig. 5, A and B). In contrast, overexpression of p52 together with ALK2 and Smad1 inhibited the events induced by BMP signaling in Nfkb2−/− POB (Fig. 5, A and B). We further confirmed the role of p52 on BMP2-induced osteoblast differentiation using POB from p100−/−mice, which carry a homozygous deletion of the COOH-terminal ankyrin repeats of p100 but still express functional p52 protein. The increase in ALP activity by adding 50 or 100 ng/ml BMP2 was lower in POB from p100−/− mice than in WT mice (Fig. 5C). The Smad1/5/8 phosphorylation was also decreased in p100−/− POB compared with WT mice, even though total Smad1 level was constant (Fig. 5D). Taken together these results suggest that p100 and p52 regulate BMP signaling positively and negatively, respectively.
Fig. 5.
Accumulation of p100 enhanced the Smad phosphorylation and ALP activity induced by the constitutively activated BMP receptor. A, ALP activity was induced by overexpression of V5-tagged ALK2(Q207D) with FLAG-tagged Smad1 in the presence or absence of either p100ΔGRR or p52 in POB from calvariae of 1-d-old Nfkb2−/− mice. The data are presented as the mean ± sd (n = 3). *, P < 0.01. B, Phosphorylation of Smad1/5/8 was induced by overexpression of V5-tagged ALK2(Q207D) with FLAG-tagged Smad1 in the presence or absence of either p100ΔGRR or p52 in Nfkb2−/− POB. C, POB from calvariae of 1-d-old WT (open column) or p100−/− mice (closed column) were treated with BMP2 (0, 50, 100 ng/ml) for 72 h. The cells were fixed with an acetone-ethanol mixture and incubated with a substrate solution. ALP activity was then determined. The data are means ± sd (n = 3), *, P < 0.01. D, POB from calvariae of 1-d-old WT or p100−/− mice were cultured in the presence of BMP2 (100 ng/ml) for 1 h. Total cell lysates were immunoblotted with antiphosphorylated Smad1/5/8, anti-Smad1, and anti-NFκB2 antibodies. Anti-β-actin was used as a loading control.
p52 induced BMP receptor degradation by interacting BMP receptor
To examine whether p100 or p52 interacts with BMP receptors, we cotransfected either p100ΔGRR or p52 together with V5-tagged ALK2(Q207D) into COS7 cells, cell extracts were subjected to immunoprecipitation with anti-V5 antibody, followed by immunoblotting with anti-NF-κB2 antibodies. A larger amount of p52 interacted with ALK2(Q207D) compared with p100 ΔGRR (Fig. 6A). We noticed that the amounts of ALK2(Q207D) in the cell extracts were increased and decreased by the cotransfection with p100 ΔGRR and p52, respectively (Fig. 6A). This finding was confirmed in the cotransfection experiments of ALK2(Q207D) and increasing amounts of p100ΔGRR or p52. Again, p100ΔGRR and p52 increased and decreased the ALK2(Q207D) levels in the dose-dependent manners, respectively (Fig. 6, B and C). The p52-induced decrease of ALK2 was partially blocked by the lactacystin treatment, suggesting that, at least in part, a proteasome-dependent degradation is involved in these phenomena (Fig. 6D).
Fig. 6.
p52 induced BMP receptor degradation by interacting BMP receptor. A, COS7 cells were cotransfected with V5-tagged ALK2(Q207D) in the presence or absence of either p100ΔGRR or p52. Whole-cell lysates were immunoprecipitated (IP) with an anti-V5 antibody and immunoblotted (IB) with anti-NF-κB2 antibodies. POB from calvariae of 1-d-old NfκB2−/− mice were cotransfected with V5-tagged ALK2(Q207D) in the presence or absence of either p100ΔGRR (panel B) or p52 (panel C). Total cell lysates were immunoblotted with anti-V5 and anti-NFκB2 antibodies. Anti-β-actin was used as a loading control. D, POB from calvariae of 1-d-old NfκB2−/− mice were cotransfected with V5-tagged ALK2(Q207D) in the presence or absence of p52. Cells were treated 24 h after transfection with vehicle or with 50 μm lactacystin for 9 h. Total cell lysates were immunoblotted with anti-V5 and anti-NFκB2 antibodies. Anti-β-actin was used as a loading control.
Discussion
The importance of NF-κB in osteoblasts has recently been revealed. The inhibition of NF-κB in the mature osteoblasts of mice expressing a dominant-negative form of IKKβ showed increased BMD and bone volume due to the increased activity of osteoblasts (16). Furthermore, selective inhibition of NF-κB blocked the inhibitory effect of TNFα on BMP2-induced osteoblast differentiation and prevented bone loss in ovariectomized mice (17, 18). In the present study, we examined the role of the alternative NF-κB signaling pathway in osteoblast differentiation and bone formation using aly/aly mice, which do not process p100 into p52 due to a loss-of-function mutation in Nik. We found that both osteoblastic differentiation in vitro and ectopic bone formation in vivo were enhanced in aly/aly mice. Moreover, overexpression of p100 and p52 in Nfkb2−/− POB stimulated and suppressed osteoblastic differentiation, respectively. Finally, the ALP activity and Smad phosphorylation were decreased in POB from p100−/− mice, which lack functional p100 but still express functional p52, compared with WT POB. These findings suggest that p100 itself and p52 processed from p100 may act independently as an activator and an inhibitor of bone formation, respectively.
A previous study indicated that the accumulated p100 in aly/aly cells inhibited the alternative NF-κB pathway by preventing nuclear translocation and DNA binding of RelB through direct interactions (19). Moreover, p100-deficient mice revealed significantly reduced BMD (10), and previous study showed that the nuclear binding of p52 and RelB was strongly increased in tissues from p100−/− mice compared with WT control, whereas nuclear binding of p65 remained largely unchanged (20, 21). The bone phenotype in p100−/− mice was rescued by crossing with RelB-deficient mice, suggesting that the alternative NF-κB pathway may negatively regulate bone formation via cooperation of RelB with p52 that has been processed from p100 (10).
One of the mechanisms in stimulation of bone formation in aly/aly mice seemed to be an enhancement of intracellular signaling of BMP, which are potent cytokines that stimulate bone formation in vivo. In the present study, we found that phosphorylation of Smad1/5/8, which is a direct event induced by activation of the BMP receptor, was enhanced in aly/aly POB. The activation of a Smad-dependent pathway by overexpressing a constitutively activated Smad1 induced osteoblastic differentiation in C2C12 myoblasts (22). In contrast, the inhibition of BMP-specific Smad phosphorylation by chemical inhibitors suppressed osteoblastic differentiation of C2C12 cells in vitro in response to BMP stimulation and inhibited heterotopic bone formation in vivo (23, 24). A physical interaction between p52 and TGF-β-specific Smad3 was enhanced in response to TGF-β signaling (25). We found that p52 interacted with BMP receptor, ALK2, and reduced its protein levels via a proteasome pathway. In contrast, p100ΔGRR increased the ALK2 levels in a dose-dependent manner. These findings may explain, at least in part, molecular mechanisms of the enhancement of BMP activity in aly/aly mice, because these mice lack p52 and have larger amounts of p100 than WT mice. However, additional studies will be required to elucidate the molecular mechanisms of positive and negative regulation of osteoblast differentiation and bone formation by p100 and p52-RelB, respectively.
We have previously reported that activation of the classical NF-κB pathway induced by TNFα inhibited BMP2-induced osteoblastic differentiation (17). Activation of the classical NF-κB pathway had no effect on the phosphorylation of Smad1/5/8 or on the nuclear translocation of the Smad1-Smad4 complex. Although TNFα failed to affect receptor-dependent formation of the Smad1-Smad4 complex, p65, a main subunit of classical NF-κB pathway, associated with the complex. TNFα suppressed the DNA binding of Smad proteins to the target genes. The NF-κB inhibitor, BAY11–7082, removed repression of Smad binding to the DNA. These results suggest that activation of the classical NF-κB pathway inhibits BMP signaling by interfering with DNA binding of Smad. In contrast, mice harboring a novel mutation in Nfkb2(Nfkb2Lym1/Lym1), which encodes a nonprocessible form of p100, have a similar bone phenotype as aly/aly mice (26), strongly suggesting that the classical NF-κB pathway involves inflammatory bone metabolism whereas the alternative NF-κB pathway, particularly the processing of p52 from p100, regulates physiological bone metabolism. Taken together, we clearly showed that the classical and alternative NF-κB pathways have distinct roles in modulating BMP/Smad signaling, i.e. inhibiting BMP-induced Smad DNA binding and Smad1/5/8 phosphorylation, respectively.
Recently, drugs such as bisphosphonate have been used as osteoporotic therapeutics, which is problematic because bisphosphonate induces necrosis of the jaw bone as its side effect (27–29). Although specific inhibitors of the classical NF-κB pathway seem to be efficient in preventing bone loss (16–18), mice deficient in p65, which is the main subunit of the pathway, are embryonic lethal (30), suggesting that inhibition of the classical NF-κB pathway may result in life-compromising side effects. However, the inhibition of the alternative NF-κB pathway due to the Nik mutation in aly/aly mice did not lead to lethality. Therefore, the alternative NF-κB signaling pathway may be a novel therapeutic target for diseases accompanied by bone loss by inducing high bone mass.
Materials and Methods
Animals
Mice (8 wk old) heterozygous for aly (aly/+) were purchased from Nippon CLEA (Tokyo, Japan). Heterozygous males and females were bred to produce homozygous aly/aly mice. The genotypes of the offspring were screened using PCR. Nfkb2−/− and p100−/− mice were obtained from the Leibniz-Institute for Age Research-Fritz-Lipmann-Institute (Jena, Germany). All mice were maintained at the Animal Resource Center. Experimental procedures were approved by the Animal Care and Use Committee of Kyushu Dental College (approval no. 10-013).
Reagents
Purified recombinant human BMP2 was provided by Wyeth Pharmaceuticals (Madison, NJ). Anti-IκBα (sc-371), antiphosphorylated p38 (sc-7973), anti-p38 (sc-7149), and antiphosphorylated ERK (sc-7383) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-p100/p52 (catalog no. 4882), antiphosphorylated Smad1/5/8 (catalog no. 9511), anti-Smad1 (catalog no. 9743), and anti-ERK (catalog no. 9102) antibodies were purchased from Cell Signaling Technology (Beverly, MA). The anti-β-actin (clone: AC-15) and anti-FLAG (M5) antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Lactacystin was purchased form Calbiochem (San Diego, CA).
ALP activity and staining
POB were prepared from the calvariae of 1-d-old WT, aly/aly, Nfkb2−/−, and p100−/− mice by digestion with 0.1% collagenase (Wako, Osaka, Japan) and 0.2% dispase (Godo Shusei, Tokyo, Japan). POB were seeded the day before treatment at a density of 1.0 × 104 cells per well in 96-well plates with α-MEM containing 10% fetal bovine serum (FBS). The cells were cultured with induction medium for osteoblast differentiation [β-glycerophosphate (10 mm) and ascorbic acid (50 μg/ml)] for 7 d or treated with BMP2 (100 ng/ml) for 72 h. After treatment, the cells were fixed with an acetone/ethanol mixture (50:50, vol/vol) and then incubated with a substrate solution (0.1 m diethanolamine, 1 mm MgCl2, and 10 mg/ml p-nitrophenyl phosphate). Reactions were stopped by adding 5 m NaOH, and the absorbance was measured at 405 nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA). Histochemical measurements of ALP enzyme activities were performed as described previously (12).
von Kossa staining
POB were seeded the day before treatment at a density of 1.0 × 105 cells per well in 24-well plates with α-MEM containing 10% FBS. The cells were treated with induction medium for osteoblast differentiation for 14 d. Cells were fixed with 10% (vol/vol) formalin in PBS for 30 min and washed three times using deionized water. Cells were stained with freshly prepared 5% (vol/vol) silver nitrate solution for 30 min and washed three times using deionized water. Next, cells were developed with fresh 5% (vol/vol) sodium carbonate in 25% (vol/vol) formalin for at least 5 min for mineral and matrix staining. After washing three times with deionized water, cells were fixed with 5% (vol/vol) sodium thiosulfate for 2 min to remove unreacted silver nitrate. Finally, cells were washed three times using deionized water and air dried.
Western blot analysis
Cells were lysed in TNT buffer (20 mm Tris-HCl, pH 7.5; 200 mm NaCl, 1% Triton X-100, and 1 mm dithiothreitol) containing protease inhibitors and phosphatase inhibitors (Roche, Basel, Switzerland). The lysates were resolved by 10% SDS-PAGE, transferred to Immobilon-P membranes (Millipore Corp., Billerica, MA), and immunoblotted with individual antibodies. Then, membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology). The immunoreactive proteins were visualized using ECL (Amersham Pharmacia Biotech, Piscataway, NJ) and were analyzed with a Luminescent image analyzer (Fujifilm, Tokyo, Japan).
Real-time RT-PCR
Total RNA from WT or aly/aly POB was prepared with TRIzol (Invitrogen, Carlsbad, CA) and was amplified by Superscript II and Taq polymerase (Invitrogen). Real-time PCR was performed using SYBR Green PCR master mix on the 7300 Real-time PCR system (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Samples were matched to a standard curve generated by amplifying serially diluted products under the same PCR conditions. Glyceraldehyde-3-phosphate dehydrogenase expression served as an internal control. Primer sequences were described previously (31).
Ectopic bone formation assay
The effects of BMP2-induced bone formation in vivo were examined by the ectopic bone formation assay in WT and aly/aly mice. A collagen sponge disk (6 mm diameter, 1 mm thickness) made from commercially available bovine collagen sheets (Helistat, Integra Neuro-Sciences, Plainsboro, NJ) was blotted with BMP2 (4 μg), freeze dried, and kept at −20 C until implantation into mice (32). All procedures were carried out under sterile conditions. Mice were anesthetized by diethyl ether gas inhalation, and collagen pellets were surgically implanted into dorsal muscle pouches (two pellets per animal) of both WT and aly/aly mice (4 wk old). Mice were euthanized 4 wk after surgery, and the implants were harvested and processed for histological analysis. Samples were fixed in PBS-buffered glutaraldehyde (0.25%)-formalin (4%) fixative (pH 7.4) for 2 d at 4 C and washed with PBS for further studies. The BMC and BMD of the ectopic bone were measured by dual-energy x-ray absorptiometry (DCS-600R; Aloka, Tokyo, Japan). Three-dimensional reconstruction images of proximal tibias were obtained by microfocal computed tomography (μCT) (ScanXmate-E090; Comscan, Kanagawa, Japan) as previously described (10, 11). Sections of the ectopic bone from each group were stained with H&E after which sections were examined by light microscopy.
Transfection
Plasmids encoding WT murine Smad, the constitutively active BMP type I receptor ALK2(Q207D), p100ΔGRR, and p52 have been described previously (11, 20). POB from Nfkb2−/− mice or COS7 cells were transfected with plasmids using Genejuice (Merck, Darmstadt, Germany) transfection reagent.
Immunoprecipitation
Cells were lysed in TNT buffer (20 mm Tris-HCl, pH 7.5; 200 mm NaCl; 1% Triton X-100; 1 mm dithiothreitol) containing protease inhibitors (Roche, Basel, Switzerland). For coprecipitation experiments, whole-cell extracts were incubated for 6 h at 4 C with anti-V5, antibody coupled to A/G-Sepharose beads. The immune complex was extensively washed with TNT buffer, after which the samples were boiled and analyzed by immunoblotting with anti-NF-κB2 antibodies.
Statistical analysis
Comparisons were made using factorial ANOVA. When significant F values were detected, Fisher's PLSD post hoc test was performed to compare each of the groups. The data were expressed as the mean ± sd; values of P < 0.05 were considered significant.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan [Grant 22592042 (to M.Z.) and Grant 23390424 (to E.J.)] and a grant-in-aid from Kyushu Dental College Internal Grants (to H.F. and E.J.).
Disclosure Summary: The authors have no conflicts to disclose.
Footnotes
- ALP
- Alkaline phosphatase
- BMC
- bone mineral content
- BMD
- bone mineral density
- BMP
- bone morphogenetic protein
- μCT
- micro-computed tomography
- H&E
- hematoxylin/eosin
- IKK
- IκB kinase
- IκB
- inhibitor of κB
- NF-κB
- nuclear factor-κB
- NIK
- NF-κB-inducing kinase
- POB
- primary osteoblasts
- Smad
- Sma- and Mad-related protein
- WT
- wild-type.
References
- 1. Aubin JE , Triffitt JT. 2002. Mesenchymal stem cells and osteoblast differentiation. In: , Bilezikian JP , Raisz LG , Rodan GA, eds. Principles of bone biology. 2nd ed San Diego: Academic Press; 59–81 [Google Scholar]
- 2. Mundy GR. 1996. Regulation of bone formation by bone morphogenetic proteins and other growth factors. Clin Orthop Relat Res 324:24–28 [DOI] [PubMed] [Google Scholar]
- 3. Yamaguchi A , Komori T , Suda T. 2000. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev 21:393–411 [DOI] [PubMed] [Google Scholar]
- 4. Nishimura R , Hata K , Ikeda F , Ichida F , Shimoyama A , Matsubara T , Wada M , Amano K , Yoneda T. 2008. Signal transduction and transcriptional regulation during mesenchymal cell differentiation. J Bone Miner Metab 26:203–212 [DOI] [PubMed] [Google Scholar]
- 5. Ghosh S , Karin M. 2002. Missing pieces in the NF-κB puzzle. Cell 109:S81–S96 [DOI] [PubMed] [Google Scholar]
- 6. Vallabhapurapu S , Karin M. 2009. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol 27:693–733 [DOI] [PubMed] [Google Scholar]
- 7. Baker RG , Hayden MS , Ghosh S. 2011. NF-κB, inflammation, and metabolic disease. Cell Metab 13:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiao G , Harhaj EW , Sun SC. 2001. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol Cell 7:401–409 [DOI] [PubMed] [Google Scholar]
- 9. Shinkura R , Kitada K , Matsuda F , Tashiro K , Ikuta K , Suzuki M , Kogishi K , Serikawa T , Honjo T. 1999. Alymphoplasia is caused by a point mutation in the mouse gene encoding NF-κB-inducing kinase. Nat Genet 22:74–77 [DOI] [PubMed] [Google Scholar]
- 10. Soysa NS , Alles N , Weih D , Lovas A , Mian AH , Shimokawa H , Yasuda H , Weih F , Jimi E , Ohya K , Aoki K. 2010. The pivotal role of the alternative NF-κB pathway in maintenance of basal bone homeostasis and osteoclastogenesis. J Bone Miner Res 25:809–818 [DOI] [PubMed] [Google Scholar]
- 11. Maruyama T , Fukushima H , Nakao K , Shin M , Yasuda H , Weih F , Doi T , Aoki K , Alles N , Ohya K , Hosokawa R , Jimi E. 2010. Processing of the NF-κB2 precursor, p100, to p52 is critical for RANKL-induced osteoclast differentiation. J Bone Miner Res 25:1058–1067 [DOI] [PubMed] [Google Scholar]
- 12. Katagiri T , Yamaguchi A , Komaki M , Abe E , Takahashi N , Ikeda T , Rosen V , Wozney JM , Fujisawa-Sehara A , Suda T. 1994. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127:1755–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Massagué J , Seoane J , Wotton D. 2005. Smad transcription factors. Genes Dev 19:2783–2810 [DOI] [PubMed] [Google Scholar]
- 14. Katagiri T , Suda T , Miyazono K. 2008. The bone morphogenetic proteins. In: , Derynck R , Miyazono K, eds. The TGF-β family. New York: Cold Spring Harbor Monograph; 121–149 [Google Scholar]
- 15. Yilmaz ZB , Weih DS , Sivakumar V , Weih F. 2003. RelB is required for Peyer's patch development: differential regulation of p52-RelB by lymphotoxin and TNF. EMBO J 22:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang J , Wang Z , Tang E , Fan Z , McCauley L , Franceschi R , Guan K , Krebsbach PH , Wang CY. 2009. Inhibition of osteoblastic bone formation by nuclear factor-κB. Nat Med 15:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamazaki M , Fukushima H , Shin M , Katagiri T , Doi T , Takahashi T , Jimi E. 2009. TNFα represses BMP signaling by interfering with the DNA binding of Smads through the activation of NF-κB. J Biol Chem 284:35987–35995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alles N , Soysa NS , Hayashi J , Khan M , Shimoda A , Shimokawa H , Ritzeler O , Akiyoshi K , Aoki K , Ohya K. 2010. Suppression of NF-κB increases bone formation and ameliorates osteopenia in ovariectomized mice. Endocrinology 151:4626–4634 [DOI] [PubMed] [Google Scholar]
- 19. Ramakrishnan P , Wang W , Wallach D. 2004. Receptor-specific signaling for both the alternative and the canonical NF-κB activation pathways by NF-κB-inducing kinase. Immunity 21:477–489 [DOI] [PubMed] [Google Scholar]
- 20. Ishikawa H , Carrasco D , Claudio E , Ryseck RP , Bravo R. 1997. Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-κB2. J Exp Med 186:999–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo F , Tänzer S , Busslinger M , Weih F. 2008. Lack of nuclear factor-κB2/p100 causes a RelB-dependent block in early B lymphopoiesis. Blood 112:551–559 [DOI] [PubMed] [Google Scholar]
- 22. Kokabu S , Nojima J , Kanomata K , Ohte S , Yoda T , Fukuda T , Katagiri T. 2010. Protein phosphatase magnesium-dependent 1A-mediated inhibition of BMP signaling is independent of Smad dephosphorylation. J Bone Miner Res 25:653–660 [DOI] [PubMed] [Google Scholar]
- 23. Yu PB , Deng DY , Lai CS , Hong CC , Cuny GD , Bouxsein ML , Hong DW , McManus PM , Katagiri T , Sachidanandan C , Kamiya N , Fukuda T , Mishina Y , Peterson RT , Bloch KD. 2008. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med 14:1363–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukuda T , Kohda M , Kanomata K , Nojima J , Nakamura A , Kamizono J , Noguchi Y , Iwakiri K , Kondo T , Kurose J , Endo K , Awakura T , Fukushi J , Nakashima Y , Chiyonobu T , Kawara A , Nishida Y , Wada I , Akita M , Komori T , Nakayama K , Nanba A , Maruki Y , Yoda T , Tomoda H , et al. 2009. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem 284:7149–7156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. López-Rovira T , Chalaux E , Rosa JL , Bartrons R , Ventura F. 2000. Interaction and functional cooperation of NF-κB with Smads. Transcriptional regulation of the junB promoter. J Biol Chem 275:28937–28946 [DOI] [PubMed] [Google Scholar]
- 26. Tucker E , O'Donnell K , Fuchsberger M , Hilton AA , Metcalf D , Greig K , Sims NA , Quinn JM , Alexander WS , Hilton DJ , Kile BT , Tarlinton DM , Starr R. 2007. A novel mutation in the Nfkb2 gene generates an NF-κB2 “super repressor.” J Immunol 179:7514–7522 [DOI] [PubMed] [Google Scholar]
- 27. Pogrel MA. 2004. Bisphosphonates and bone necrosis. J Oral Maxillofac Surg 62:391–392 [DOI] [PubMed] [Google Scholar]
- 28. Dannemann C , Grätz KW , Riener MO , Zwahlen RA. 2007. Jaw osteonecrosis related to bisphosphonate therapy: a severe secondary disorder. Bone 40:828–834 [DOI] [PubMed] [Google Scholar]
- 29. Allen MR , Burr DB. 2008. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J Oral Maxillofac Surg 66:987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beg AA , Sha WC , Bronson RT , Ghosh S , Baltimore D. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167–170 [DOI] [PubMed] [Google Scholar]
- 31. Hirata S , Kitamura C , Fukushima H , Nakamichi I , Abiko Y , Terashita M , Jimi E. 2010. Low-level laser irradiation enhances BMP-induced osteoblast differentiation by stimulating the BMP/Smad signaling pathway. J Cell Biochem 111:1445–1452 [DOI] [PubMed] [Google Scholar]
- 32. Zhao B , Katagiri T , Toyoda H , Takada T , Yanai T , Fukuda T , Chung UI , Koike T , Takaoka K , Kamijo R. 2006. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem 281:23246–23253 [DOI] [PubMed] [Google Scholar]